Abstract
Background
The coronavirus disease Rapid Antigen Testing Expansion Program (Program) employed a drive-thru model to maximize pharmacy staff and the public’s safety.
Objectives
To quickly design, implement, and disseminate a pharmacy-based point-of-care testing program during a public health crisis.
Practice description
Community pharmacies in Idaho were engaged in the state’s public health efforts to boost severe acute respiratory syndrome coronavirus 2 testing statewide. Geographic location was a major recruitment factor. Two recruitment periods were held to extend the Program’s reach into more remote underserved communities.
Practice innovation
Program and pharmacy staff developed workflows and materials in an iterative process. Pharmacies received testing supplies. Program staff created e-Care plans for documentation and reimbursement and designed a Web portal for state reporting of positive rapid antigen test results.
Evaluation methods
Testing data (pharmacy location, patient insurance status, test type and results, number of submitted Medicaid claims) were captured in an online form.
Results
From September to December 2020, 13 pharmacies opted into a drive-thru, rapid antigen point-of-care testing and nasal swab for offsite testing program. A total of 2425 tests were performed. Approximately 29.4% of point-of-care tests were positive, and 70.6% required backup polymerase chain reaction confirmatory analysis. Patient insurance breakdown was 72.1% private, 8% Medicare, 11.4% Medicaid, and 8.5% uninsured. On average, pharmacies tested patients an average of 2.3 hours/day and 2.6 days/week. As a group, they provided 77.5 hours of testing per week statewide. Program pharmacies accounted for an average of 5.1% of testing across the entire state at the end of December 2020.
Conclusion
Independent community-based pharmacies should be considered as partners in public health initiatives.
Key Points.
Background
-
•
COVID-19 is a contagious disease that is a public health threat.
-
•
Community pharmacies are accessible points of access to health care, many community pharmacists and technicians are trained in point-of-care testing, and such testing is within the clinical abilities of community pharmacists.
-
•
Multiple accurate point-of-care tests to detect SARS-CoV-2 are available, but sustainable workflows have not been identified for community pharmacies.
Findings
-
•
Rapid implementation and scale of testing for coronavirus disease in partnership with community pharmacies located across a largely rural state is feasible.
-
•
Shared resources and incentives supporting workflow implementation are helpful.
-
•
Centralized communication and support are important to success of widespread implementation.
Background
The coronavirus disease (COVID-19) pandemic poses particular challenges to rural states, which have lower health care capacity, hindering access to laboratory testing and health services. Idaho’s expanded scope of pharmacy practice allows pharmacists to independently provide immunization services, prescribe short-term or bridge medications, and provide disease testing via Clinical Laboratory Improvement Amendments (CLIA)–waived devices.1
Pharmacists in independently owned, community-based pharmacies are often the closest available health care providers to rural residents. To maximize access to testing, pharmacies were included in the expansion of the state’s COVID-19 testing efforts from September to December 2020. The COVID-19 Rapid Antigen Testing Expansion Program (Program) was sponsored by the Idaho Board of Pharmacy and administered by the Idaho State University College of Pharmacy (ISU). The Program presented an opportunity to quickly increase Idaho’s existing testing infrastructure and capacity, as well as to reduce the volume of samples requiring laboratory analysis. Moreover, it also was designed to be as contactless as possible to avoid endangering pharmacy staff and spreading the virus. A drive-thru model, with patients staying in their vehicles, was determined to be the best approach.
State law divides Idaho into 7 public health districts to ensure that all residents have access to essential public health services, which includes pharmacy services.2 Approximately 33% of Idahoans live in one of the state’s 21 rural-designated counties—those with population centers of 7500 or fewer people—and these areas are medically underserved with reduced health care access.3 Therefore, Program staff targeted recruitment of pharmacies in rural areas (population <2500) or urban clusters (population centers <50,000) across all public health districts.4 Independent pharmacies and small regional chains were prioritized for recruitment and Program participation because of their ability to quickly and effectively implement new workflows, requiring few internal and external approvals to implement program change. ISU engaged with a network of clinically integrated community-based pharmacies across Idaho, known as Community Pharmacy Enhanced Services Network of Idaho (CPESN-ID). CPESN-ID pharmacies were optimal sites for Program implementation because they all had established workflows that could be adapted to support testing in a pandemic. As members of CPESN, these pharmacies all have access to support for practice change in the form of written guidance, virtual and in-person meetings, and CPESN-specific trainings. These pharmacies also had experience in delivery of enhanced services, such as comprehensive pharmacy care management, medication synchronization, and administration of vaccines.5
Objective
The purpose of the Program was to rapidly implement a statewide COVID-19 pharmacy-based testing program. The intent was to create an easily replicable program for others seeking to develop programs with independently owned or other community-based pharmacies, especially during a public health emergency.
Practice description
Recruitment
CPESN-ID alerted its 19 member pharmacies statewide of the opportunity to participate in the Program via a standing network-wide e-mailed newsletter and 2 topic-specific recruitment e-mails sent over the course of 2 weeks in August 2020. To participate in the Program, pharmacies were asked to consider current workflow, staffing, volume of business, and considerations unique to their businesses that could influence feasibility, such as parking lot capacity and traffic flow, location, and available space for testing separate from current pharmacy customers. Pharmacies were requested to opt in or out of the Program through an online form by the end of August; nonresponsive pharmacies were followed up with via phone. Participating pharmacies were offered incentives in exchange for meeting participation requirements (Table 1 ).
Table 1.
Program participation incentives and requirements
| Incentives | Requirements |
|---|---|
|
|
Abbreviations used: POCT, point-of-care test; SARS-COV-2, severe acute respiratory syndrome coronavirus 2; CPESN-ID, Community Pharmacy Enhanced Services Network of Idaho; BD Veritor; Becton-Dickenson Veritor; IDHW, Idaho Department of Health and Welfare; PCR, polymerase chain reaction; PPE, personal protective equipment.
A second recruitment phase for non-CPESN pharmacies occurred beginning in November 2020 after launch and pilot of workflows in CPESN sites (roughly 6 weeks into the project). Non-CPESN pharmacies were identified using a list of community pharmacies registered with the Idaho Board of Pharmacy. Pharmacies with CLIA waivers in place were prioritized for initial contact, then an overlay of pharmacy location was used to identify geographic gaps in pharmacy-based COVID testing statewide (only pharmacy-based testing availability was formally mapped, but general access concerns communicated by health districts were considered). A priority list of 20 pharmacies was identified and contacted via e-mail with phone follow-up. Pharmacies were contacted via phone 3 times for this recruitment effort. They were required to do mock run-throughs of their proposed workflow changes, opt into the Program using the same opt-in form as used in the initial recruitment, and complete an attestation form indicating that they had worked through this process and identifying days and times to offer testing.
Practice innovation
Materials provided
All participating pharmacies were provided with 2 Becton-Dickenson Veritor (BD Veritor) Systems for rapid detection of severe acute respiratory syndrome coronavirus 2 (BD Veritor+) testing machines. They received a 3-month supply of rapid testing kits, nasal swab kits, and transport materials. All pharmacies were recognized as state testing providers and had access to public personal protective equipment (PPE) supplies.
Program communication
To promote Program communication, pharmacies were required to have at least 1 representative attend at least 1 of 2 weekly interactive check-in meetings, which were conducted via video conference. These meetings were designed to address concerns (collaboratively across pharmacies), disseminate information (e.g., information on rapidly changing public health guidance, updates on medical billing developments), and share pharmacy-identified lessons learned and best practice strategies. Pharmacies that could not attend weekly sessions were asked to contact Program staff via phone or e-mail to ensure that at least project-directed communication was maintained. Attendance, phone calls, and other contacts were recorded as a measure of participation. Feedback from participants was used to iteratively update Program materials. All Program materials were organized and maintained in a University-sponsored shareable online cloud storage system; materials could be viewed and downloaded for use or modification by anyone added to the online shared folder but could not be modified within the shared system.
Initial and ongoing workflow development
An initial draft workflow plan was developed by the Program team using state and Centers for Disease Control and Prevention (CDC) guidance to ensure inclusion of all broad components of the testing workflow, including patient intake, testing, and reporting. The workflow plan also provided testing teams with a shared infrastructure from which to build their formal workflows and targeted messaging for patient and provider communication. Contact information for all public health districts was collected by the project team and organized in an online spreadsheet. Patient education resources were adapted from CDC resources with guidance from public health contacts (e.g., form with CDC counseling and images embedded) to tailor materials to testing workflows. As an example, a one-page resource from CDC was combined with patient information into a single-page, double-sided tool to use for counseling patients on their test results. Resources to support billing the medical benefit were identified.
Patient triage guidance was developed and updated consistently throughout the Program to ensure alignment with both Federal- and state-level guidance, which changed frequently. Symptomatic individuals, as defined by the CDC (updated with changing guidance, e.g., days since symptom onset, presence and definition of fever) and the BD Veritor+ eligibility profile, were eligible for point-of-care test (POCT). All other individuals who did not meet these criteria were administered a nasal swab for polymerase chain reaction (PCR) analysis. Although patient triage was standardized, site-specific workflow was tailored by individual pharmacies based on available trained staff, space and pharmacy layout constraints, and other pharmacy-specific factors. Sample collection was performed by pharmacists, technicians, or interns in compliance with Idaho law. The flexibility of how workflows were adapted was important owing to the differences between individual independent pharmacies.
The anticipated time between receipt of testing supplies and rollout of testing was roughly 2 weeks. Pharmacies were tasked initially with tailoring the workflow materials to their specific needs and pharmacy organization. Ongoing communication between ISU Program staff and the primary pharmacy contacts was maintained via weekly Web-based meetings and as-needed telephone communication. Once pharmacies had piloted workflow (including training of all staff on appropriate PPE donning and doffing procedures and testing protocols), they were able to launch testing. Pharmacies were required to attest to piloting their workflow via an online form, which served as a safeguard to ensure that pharmacies had completed necessary groundwork to promote successful launch of testing.
When 80% of pharmacies reported readiness for testing, a statewide press release was created by Program staff and the project was presented to state public health district leadership. Pharmacy testing days and times were also included on a statewide testing website (outside of this project) that listed all available testing locations across the state as part of an effort to ensure that patients knew where to find COVID-19 testing.6 Public health districts were also apprised of the pharmacies and the hours of testing in their areas and disseminated the information to their respective communities.
Dissemination of information
A public-facing, open-access website was created 6 weeks into testing to facilitate rapid dissemination of workflow best practices identified from this program; the audience for this website was anyone interested in creating or streamlining community pharmacy–based testing for COVID-19 (https://covidrxpoct.org/). Click rates and click-through rates were tracked for the first 30 days of this website. Because materials could be downloaded and then shared, it is understood that the click rates would potentially underestimate actual dissemination, but sharing of information quickly was prioritized over tracking dissemination, so this disparity was determined to be acceptable.
Evaluation methods
Pharmacies documented patient care activities via their usual mechanism (e-Care plan software for CPESN pharmacies or other established systems for non-CPESN pharmacies). This information was communicated to patients’ primary care teams wherever possible. Pharmacies were required to submit daily reports to ISU project team members on testing using an online form. The measures reported included the following: number of POCTs performed, positive and negative rates of POCT, number of PCR samples collected, breakdown of patient insurance, and number of medical insurance claims submitted (or planned for submission). No patient identifiers were collected by ISU Program personnel. Pharmacies were also required to report patient-specific findings to the state-level public health agency via an online portal or, if the portal was not working, via a fax form to their local public health district. Pharmacies were thus in charge of all reporting of patient data. Last, pharmacies using PPE were required to complete an online form for the State of Idaho’s Office of Emergency Management to track PPE usage and ongoing needs; this form was the same as that used by all other health care providers in the state.
Results
Pharmacy recruitment
In August 2020, the Program team identified and reached out to 21 pharmacies with the capacity to perform enhanced services and to quickly implement a new workflow. Initially, 14 opted in to participate (66.7% initial opt-in). Two pharmacies dropped out before launching (both because of perceived community need), and 1 pharmacy did not participate at all despite having attested that they would, giving a final opt-in rate of 52.4%. During phase 2 of recruitment, 1 CPESN-ID pharmacy and 1 non-network pharmacy joined out of 20 contacted (10% acceptance rate), resulting in a total of 13 participating pharmacies (12 CPESN pharmacies and 1 non-CPESN pharmacy) from the final week in September–December 2020. The main reasons for pharmacies opting out of the Program were staff capacity, staff safety, and perceived need for service in their community.
Pharmacy characteristics
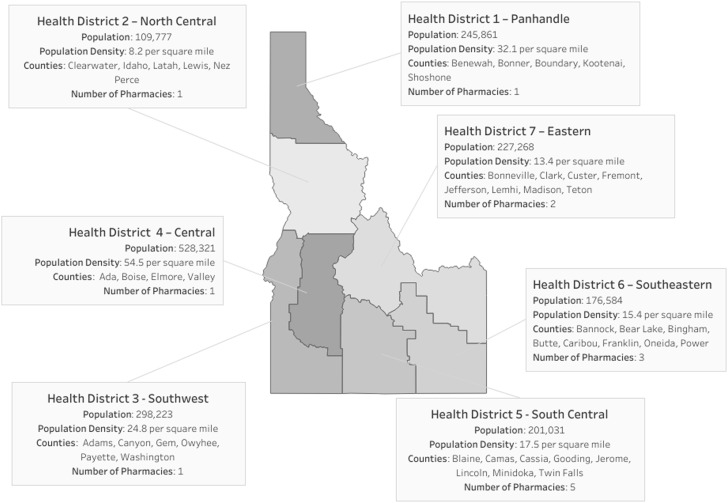
Aside from 3 in Pocatello (an urbanized area of 50,000 or more people) and 1 in Twin Falls (an urban cluster of 2500-49,999 people), the participating pharmacies were exclusively located in rural areas (<2500 people). All pharmacies had CLIA test waivers in place before enrollment in the Program. All but one health district were covered by the first phase of pharmacy recruitment. Coverage of all 7 public health districts was achieved during the second phase of recruitment. The geographic distribution of pharmacies by health districts (with corresponding counties and population) is presented in Figure 1 .
Figure 1.
Pharmacy coverage of Idaho's Public Health Districts.
A total of 6 workflow iterations were made and disseminated to pharmacies (see Table 2 ). Feedback and questions were responded to and revisions made to the workflow based on real-time data, with roughly 1 week between proposed revisions and published updates. All materials were disseminated at the same time, with initial pharmacies launching actual testing 2.5 weeks after dissemination and all pharmacies offering testing within 4 weeks of dissemination. The launch for the second wave of pharmacies was significantly shorter, with both pharmacies offering services within 2 weeks of material dissemination. Of participating pharmacies, 11 of 13 participated in weekly check-ins at least 75% of the time across the time period of their enrollment in the program.
Table 2.
Workflows developed
| Workflows adapted for program | |
|---|---|
| Appointment Scheduling (with triage) | Prearrival triage of patients to rapid antigen point-of-care-test or nasal swab collection for PCR analysis at point of scheduling |
| Pretesting and preparation | Mapping traffic flows and signage for drive-thru testing; preparing patient materials; setting up sample collection and storage stations; safe donning of PPE |
| Patient assessment | Final triage of scheduled and drop-in patients to testing modes; gathering patient consent |
| Contactless data collection | Instructions for patients to press their driver’s licenses, insurance information (or social security number if uninsured) against their car windows; photograph the information; manually enter it into the pharmacy scheduling software |
| Testing procedures | Pharmacy staff performed or directed nasal swabs; inserting trays into testing machine; patient counseling and education |
| Post-test disposal and sample delivery | Safe doffing of PPE; disposal of used testing materials and PPE; cold storage of samples for PCR; preparing samples for courier or mailing delivery |
| Billing and documentation | Credentialing as providers with Medicaid and private payers; submitting claims to payers; documenting point-of-care testing results to State Public Health via fax or Web portal; documenting all tests to CPESN-ID for grant fulfillment; ordering test materials from lab; submitting PPE utilization to statewide supply website |
Abbreviations used: CPESN-ID, Community Pharmacy Enhanced Services Network of Idaho; PCR, polymerase chain reaction; PPE, personal protective equipment.
Testing results
A total of 2425 (1424 POCT and 1001 PCR) patients were tested during the initial launch phase of the Program. The breakdown of testing by district is provided in Table 3 . Of the POCTs performed, 29.4% were positive and 70.6% required backup PCR testing for confirmation. On average, pharmacies tested patients 2.3 hours per day and 2.6 days per week. As a group, they provided 77.5 hours of testing per week statewide. From the September launch through the end of the period, pharmacies conducted an average of 119 POCTs and collected an average of 159 samples for PCR testing per week. The Program offered testing services Monday through Saturday. The range of weekly testing was 3 to 320; rural sites were more likely to have lower volumes of testing. Patient insurance breakdown was 72.1% private, 8% Medicare, 11.4% Medicaid, and 8.5% uninsured. Pharmacies in this program accounted for an average of 5.1% of testing across the entire state by the end of December 2020.
Table 3.
Testing by public health district
| District no. | POCT performed | POCT positive rate, % | PCR samples collected |
|---|---|---|---|
| 1 | 63 | 38.1 | 69 |
| 2 | 129 | 26.4 | 53 |
| 3 | 166 | 30.7 | 98 |
| 4 | 37 | 18.9 | 12 |
| 5 | 163 | 24.5 | 287 |
| 6 | 857 | 30.5 | 442 |
| 7 | 9 | 11.1 | 40 |
| Total | 1424 | 29.4 | 1001 |
Abbreviations used: PCR, polymerase chain reaction; POCT, point-of-care-testing.
PPE utilization
Participating pharmacies were advised to be conservative in their initial requests for PPE, and all subsequent requests were based on their usage rates calculated in the statewide PPE survey. Over the course of 3 months, pharmacies requested a combined total of 10,010 PPE items and received 3546 from their local public health district. The most requested items were nitrile gloves and N-95 masks.
Number of medical claims submitted
A total of 197 claims were submitted for payment of testing-related services (Evaluation and Management level 1 code, 99201) from medical insurance through December 2020. Of these, 69.5% were submitted to Medicaid and 30.5% to private insurance. The number of claims paid cannot be determined at the time of writing (May 2020) owing to the need for resubmission or lag time between submission and payment.
Public dissemination of program protocol
The program protocol was published via a unique, Program-specific website and shared by national pharmacy associations with their e-mail lists to encourage uptake. The 30-day click-through rate was 73.5%, with 200 unique visitors from 11 countries.
Challenges and resolutions
Table 4 presents challenges encountered during Program rollout and resolutions the Program team found. The intent of this table is not to be all-encompassing but rather to serve as a tool for other teams implementing similar projects in future.
Table 4.
Program problems and resolutions
| Problem | Resolution |
|---|---|
| Inconsistent policies and communication preferences among health districts | Program coordinator created a spreadsheet of contact information and policies for single point-of-reference for all pharmacies. |
| Inconsistent State-level and district-level data needs | Program subcontract to software engineer to create community pharmacy–friendly Web portal for data reporting; portal collected all state- and district-specific data in single place and reported via usual mechanism (used by labs) to streamline with overall reporting workflow used at the state. |
| Initially, no mechanism to report COVID-19 POCT results (onboarding delay of months) | While the portal was being created, interim fax reporting was used to ensure that districts had information specific to positive POCT findings. |
| Documentation of patient care | e-Care template that covered required elements of CPT code 99201 created for pharmacies to adapt to individual patient needs. |
| Variable PPE access among public health districts | District-specific approaches for requesting initial PPE determined and communicated to pharmacies. |
| Unmet initial PPE needs | District-specific policies linked in district spreadsheet for single point-of-access for pharmacies. |
| Ongoing PPE needs | Pharmacies integrated into usual practice for tracking PPE burn rate. |
| Need to offer PCR sample collection for negative or asymptomatic patients | Contracting with PCR laboratories in state to streamline transfer of pharmacy-collected samples to PCR facilities. |
Abbreviations used: PCR, polymerase chain reaction; POCT, point-of-care-testing; PPE, personal protective equipment; COVID-19, coronavirus disease 2019; CPT, Current Procedural Terminology.
Practice implications
This Program launch was successful at rapidly expanding access to testing for COVID-19 across the state of Idaho, especially in underserved areas with limited access to health care services and testing. The time-to-testing launch from initial workflow determination was less than a month for most pharmacies in the initial launch and roughly 2 weeks for pharmacies in the second phase. More patients than anticipated did not qualify for POCT and required swab collection for offsite PCR testing; this was due in part to the limitations of the POCT device and expanded testing requirements in the State (e.g., for return to work, travel) while the testing service was being offered. Testing was fairly balanced relative to population across the state; District 6 saw higher numbers because there were 3 testing sites in this district coupled with a relatively higher population.
An added layer of complexity uncovered through this project was communication with the 7 public health districts and State public health experts. Because of the rapidly changing nature of COVID-19, the alignment of communication across the state presented unique challenges; of note, the public health districts are all separate nonprofit entities. The Program team was in daily or weekly communication with representatives from the State and each public health district to ensure alignment of the initiative. This constant communication may be challenging for individual pharmacies to take on independently, but it was manageable in this project because ISU project team members served as communication liaisons and had dedicated time to support such efforts. Future work between public health entities and independent community pharmacies should ensure that clear lines of communication are established early on in the project.
A critical component of the Program was financial sustainability of pharmacy-provided services (in this instance, COVID testing, but in the future other services); these services are not reimbursed via the pharmacy benefit, the usual source of reimbursement for community pharmacies, but the medical benefit. To do this, establishment of public and private payer provider-level funding streams was determined critical to support community-based pharmacy testing beyond the grant. Although most demonstration or grant-funded projects do not involve seeking reimbursement for services, the Idaho Board of Pharmacy and Program team deemed it appropriate in this instance to facilitate long-term sustainability of the services, particularly for those patients who may be less likely to be able to afford testing after the project period ended. Pharmacists were recognized as providers under Idaho Medicaid in April 2020, so the requirement to submit at least 1 claim to the medical benefit per week for testing was reasonable and was designed to help catalyze pharmacies’ readiness to submit medical claims to all non-Medicare medical insurers. The expectation was that 100% of claims would be submitted to Medicaid, but 1 pharmacy shifted to submitting medical claims for services to private payers as well. The owner of the pharmacy in question also owns a pharmacy in Washington, where medical billing has been established for more payers for multiple years, so the owner was able to leverage pre-existing expertise in medical billing for this project. No other pharmacy submitted claims to private insurance during the project time period, although many were beginning to explore this option because of the establishment of medical billing via Medicaid.
This program demonstrated that the process of billing the medical benefit for health services provided to Medicaid patients is possible in independently owned community pharmacies, although it continues to present challenges related to the integration of pharmacies into established medical billing systems. Medicaid billing codes are almost exclusively geared toward physician practices, hospitals, and clinics, so submitting claims successfully is often a matter of trial and error. Claims are not approved or rejected in real time, and it can take pharmacies weeks to learn that the method they have been using results in rejections. Pharmacies with dedicated billing support staff may find these challenges somewhat easier to navigate, because they are often involved in the dispensing and billing of durable medical equipment, which typically follows pathways for reimbursement outside of the usual prescription reimbursement pathways. This project required pharmacies to navigate the relatively unexplored world of medical billing with an established payer that recognized pharmacists as providers; with the workflows established for medical billing processes, it is hoped that pharmacists will continue to explore opportunities for offering services that are reimbursed by the medical benefit.
This project has limitations that should be considered when interpreting the data presented and by those working to launch similar services. First, the pharmacy opt-in process precluded even geographic distribution of testing sites, resulting in a preponderance of sites in the southeastern section of the state. However, all public health districts, even those with low population density, had at least 1 participating pharmacy.
Second, pharmacies that participated in the Program were more likely to be CPESN leaders and were able to adapt and implement new workflows quickly; once workflows were established, the 2 additional pharmacies were able to launch the service on a very short timeline of 2 to 3 weeks. The rapid rollout of phase 2 pharmacies suggests that materials developed by pharmacy leaders were useful for launch; a consideration for future projects is to ensure pharmacy leaders are involved in initial workflow development. Despite existing workflow support, significantly more pharmacies opted out of participating than anticipated, suggesting a need for an alternative approach to recruitment for this sort of program. In addition, over 92% of pharmacies participating in this program were CPESN member pharmacies, which may influence the reproducibility of these services in non-CPESN pharmacies. Because CPESN regularly (before COVID-19) provides member pharmacies with structured support for practice change, it is possible that these pharmacies are more adept at adjusting to practice change than a non-CPESN pharmacy.
Third, another limitation is that access to data reported to the state via PCR was unavailable, so a true negative rate for patients presenting for this service cannot be determined. Finally, because only Medicaid claims were required to be submitted by each pharmacy per week (one/week), the sustainability of the service as it pertains to non-Medicaid claims remains in question. Furthermore, many claims remain pending at the conclusion of this project, so a true success rate for Medicaid claims cannot yet be determined.
Despite these limitations, the Program findings should be considered by community pharmacies and others weighing partnering with these entities in the launch of COVID-19 or other disease state testing programs. The pharmacies offered an accessible point for access to COVID-19 testing, thus increasing the capacity of the state to test for COVID-19. As noted earlier, because the testing protocols were designed to be as low contact as possible, with patients remaining in their vehicles, the strain on urgent care and emergency department facilities was potentially decreased as a result of this program.
Conclusion
Rapid scale of community pharmacy–based COVID-19 testing is feasible and might be considered as a tool to expand access to care for patients, particularly in rural areas. This Program was made possible because of in-place resources to support rapid rollout and expansion of testing; ongoing development and support of community-based testing services may be valuable to the work to respond to the COVID-19 pandemic. The Program team is open to sharing any and all materials related to this project with others interested in launching or expanding testing in their areas.
Acknowledgments
Idaho Board of Pharmacy, frontline pharmacy partners involved in this project, Idaho Coronavirus Financial Advisory Committee.
Biographies
Shanna K.O’Connor, PharmD, BCACP, Clinical Assistant Professor, Department of Pharmacy Practice, College of Pharmacy, Idaho State University, Pocatello, ID
Patricia Healey, MPH, Project Coordinator, Department of Pharmacy Practice, College of Pharmacy, Idaho State University, Pocatello, ID
Nicole Mark, BA, Project Coordinator, Department of Pharmacy Practice, College of Pharmacy, Idaho State University, Pocatello, ID
Jennifer L. Adams, PharmD, EdD, Associate Dean, Academic Affairs and Associate Professor, Department of Pharmacy Practice, College of Pharmacy, Idaho State University, Meridian, ID
Renee Robinson, PharmD, MPH, MSPharm, Associate Professor, Department of Pharmacy Practice, College of Pharmacy, Idaho State University, Pocatello, ID
Elaine Nguyen, PharmD, MPH, BCPS, BCACP, Assistant Professor, Department of Pharmacy Practice, College of Pharmacy, Idaho State University, Meridian, ID
Footnotes
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Funding: This work was supported by funding from the Idaho Board of Pharmacy, State of Idaho, and the CARES Act. The funder was not involved in the design or conduct of the project.
Previous presentation: Data from this project were presented at Idaho Society of Health-Systems Pharmacists’ Spring 2021 Meeting via a virtual podium presentation.
ORCIDShanna K. O’Connor: https://orcid.org/0000-0002-5123-0899
References
- 1.Idaho State Board of Pharmacy . Idaho State Board of Pharmacy; Boise, ID: 2020. Title 54 – professions, vocations & businesses: chapter 17 – pharmacists. In: Idaho Pharmacy Laws; p. 2.https://bop.idaho.gov/wp-content/uploads/sites/99/2020/07/BOP-Complete-Law-Book-2020.pdf Available at: [Google Scholar]
- 2.Idaho Statutes Title 39 health and safety: chapter 4: public health districts. https://legislature.idaho.gov/wp-content/uploads/statutesrules/idstat/Title39/T39CH4.pdf Available at:
- 3.Idaho Commerce & Labor Division of Commerce Profile of rural Idaho. https://www.uidaho.edu/-/media/UIdaho-Responsive/Files/president/direct-reports/mcclure-center/Idaho-at-a-Glance/IDG-Profile-of-Rural-Idaho.pdf Available at:
- 4.United States Census Bureau Urban and rural. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural.html Available at:
- 5.Community Pharmacy Enhanced Services Network Services available from CPESN network pharmacies. https://cpesn.com/payors/services-available-from-cpesn-network-pharmacies/ Available at:
- 6.State of Idaho One Idaho. https://one.idaho.gov/ Available at: