Highlights
-
•
A multi-variable regression analysis of data for hospitalized patients with coronavirus disease 2019 was undertaken.
-
•
The likelihood of death and cycle threshold (Ct) values at admission were studied.
-
•
Adjustments were made for known clinical risk factors for the disease.
-
•
Lower Ct values were associated with poorer outcomes in hospitalized patients.
Keywords: COVID-19, Respiratory infection, Viral infection
Abstract
This single-centre observational study demonstrated that lower cycle threshold (Ct) values (indicating higher viral loads) on admission to hospital were associated with poorer outcomes in unvaccinated, hospitalized patients with coronavirus disease 2019 (COVID-19). Demographic and outcome data were collected prospectively for all adult patients who tested positive for severe acute respiratory syndrome coronavirus-2 on admission to the University Hospitals North Midlands NHS Trust between 1 February and 1 July 2020. Nasopharyngeal swab samples were obtained, and a valid Ct value was determined for all patients using the Viasure reverse transcription polymerase chain reaction assay, validated by Public Health England, on admission to hospital. Multi-variable logistic regression results based on data from 618 individuals demonstrated a significant inverse relationship between the odds of death and Ct values (adjusted odds ratio 0.95, 95% confidence interval 0.92–0.98, P=0.001). The association remained highly significant after adjusting for known clinical risk factors for COVID-19
Introduction
Clinicians need to identify patients with coronavirus disease 2019 (COVID-19) with a higher risk of poor outcome and mortality at an early stage of hospital admission. This prospective, observational cohort study was conducted at a UK tertiary care hospital to determine the relationship between the likelihood of death and cycle threshold (Ct) values in an unvaccinated UK population with COVID-19 on admission to hospital. Statistical adjustment was made for other known risk factors associated with poor outcome. Ct values are semi-quantitative values which are inversely proportional to the viral load in a reverse transcription polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
Methods
Demographic and outcome data were collected prospectively for all adult patients who tested positive for SARS-CoV-2 on admission to the University Hospitals North Midlands (UHNM) NHS Trust between 1 February and 1 July 2020 using the ISARIC World Health Organization (WHO) Clinical Characterisation Protocol (Docherty et al., 2020). Nasopharyngeal swab samples were obtained, and valid Ct values were determined for all patients using the Viasure reverse transcription PCR assay, validated by Public Health England, on admission to hospital. Ct values <38 were considered positive. Viasure targets the ORF1ab (Target 1) and N genes (Target 2) of the SARS-CoV-2 viral genome (Public Health England, 2020). Target 1 was chosen as the primary exposure of interest as this is specific to the SARS-CoV-2 viral genome. Ct values were combined with ISARIC data for statistical analysis.
Multi-variable logistic regression was used to determine the association between Ct value and death within 28 days of a positive SARS-CoV-2 PCR test. Adjustments were made for age, gender, ethnicity, obesity, cardiovascular disease, chronic pulmonary disease, chronic kidney disease and diabetes. These covariates were selected a priori based on current understanding of the factors predicting poor outcomes in hospital cases of COVID-19 (Knight et al., 2020). Statistical significance was assessed at the 5% significance level and results are presented as odds ratios and 95% confidence intervals (CI). Analyses were conducted in R Version 4.0.1.
Results
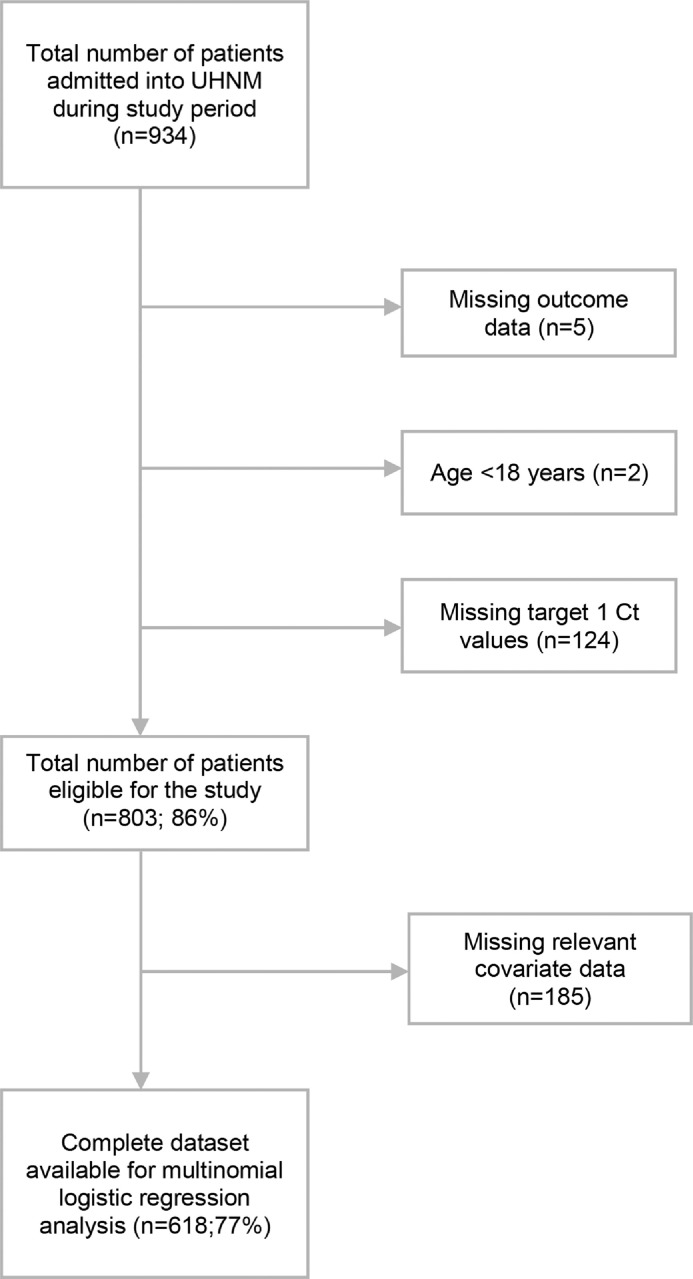
In total, 803 SARS-CoV-2-positive adults (>18 years of age) with a valid Ct value determined on admission to hospital were eligible for inclusion in this study, and were followed-up for a period of up to 28 days (Figure 1). The median age was 77 years (range 19–100 years), 55% were male and 91% were of white ethnicity. Thirty-five percent of participants had a history of cardiovascular disease, 18% had chronic pulmonary disease, 19% had chronic kidney disease, 14% were asthmatic, 13% had either complicated or uncomplicated diabetes, and 7% were obese. The median Ct value for Target 1 was 25.8 (interquartile range 21.3–30.0). In total, 285 of 803 patients (35.5%) died within 28 days of a positive SARS-CoV-2 PCR result.
Figure 1.
Flow diagram illustrating how the final study sample size was determined. UHNM, University Hospitals North Midlands NHS Trust; Ct, cycle threshold.
Multi-variable logistic regression results based on data from 618 (77.0%) individuals with complete covariate information are presented in Table 1. There was a significant inverse relationship between the probability of death and Ct value [adjusted odds ratio (aOR) 0.95, 95% CI 0.92–0.98; P=0.001]. The only other variables that were significantly associated with mortality were age (aOR 1.05, 95% CI 1.03–1.06; P<0.001) and diabetes (aOR 1.73, 95% CI 1.01–2.97; P=0.044).
Table 1.
Multi-variable logistic regression results based on complete case analysis.
| Death |
Death or continuous hospitalization |
|||
|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value |
| Age (years) | 1.05 (1.03–1.06) | <0.001 | 1.04 (1.02–1.05) | <0.001 |
| Male | 1.43 (1.00–2.07) | 0.052 | 1.2 (0.85–1.69) | 0.308 |
| Non-white | 0.66 (0.15–2.21) | 0.539 | 0.77 (0.23–2.27) | 0.65 |
| Cardiovascular disease | 1.00 (0.68–1.47) | 0.992 | 0.95 (0.65–1.39) | 0.799 |
| Chronic pulmonary disease | 1.17 (0.75–1.82) | 0.475 | 1.17 (0.76–1.82) | 0.469 |
| Chronic kidney disease | 1.22 (0.78–1.91) | 0.382 | 1.51 (0.97–2.36) | 0.071 |
| Diabetes | 1.68 (1.03–2.75) | 0.037 | 1.35 (0.83–2.22) | 0.227 |
| Obesity | 1.33 (0.68–2.53) | 0.39 | 1.1 (0.59–2.05) | 0.764 |
| Cycle threshold | 0.95 (0.92–0.98) | 0.001 | 0.94 (0.91–0.97) | <0.001 |
| Constant | 0.01 (0.00–0.04) | <0.001 | 0.05 (0.02–0.12) | <0.001 |
CI, confidence interval.
Sensitivity analyses carried out after imputing missing data produced results consistent with the complete case analysis for both outcomes. Similar results were obtained when Target 2 was used in the analysis instead of Target 1 (data available on request).
In this prospective, observational study carried out in a tertiary UK hospital, lower Ct values (indicating higher viral loads) were associated with poorer outcomes in hospitalized patients with COVID-19. This association remained highly significant after adjusting for known clinical risk factors for the disease. High viral loads are associated with adverse outcomes in human immunodeficiency virus and Ebola infections (Fitzpatrick et al., 2015; Li et al., 2015). There have been reports that higher viral loads are associated with adverse outcomes in patients with COVID-19 in China, Taiwan and Brazil (Choudhuri et al., 2020; Faíco-Filho et al., 2020; Huang et al., 2020; Liu et al., 2020; Yu et al., 2020), but none from the UK.
Discussion
The main strength of this study is that it used a validated, generalizable dataset for analysis. Ct values were measured by validated methods. Robust statistical methods were used to account for interactions and missing data. However, the study has several limitations. It was limited by its relatively small size. Therefore, the analysis was restricted to eight covariates known to be associated with poorer outcomes. Whilst this reduced the risk of confounding due to multiple hypothesis testing, it did not allow exploration of the data to determine whether other measurements, such as admission cardiovascular status, oxygen saturation and liver failure or need for mechanical ventilation were associated with death. However, a logistic regression model incorporating all of these measurements did not show any significant associations. Moreover, the data are limited to a single tertiary care centre in the UK, and the population characteristics may vary in relation to other areas. It is also unclear if these associations will remain significant after accounting for therapeutic agents (e.g. dexamethasone, anti-IL-6 treatment) in COVID-19 management. These findings suggest that Ct values may have the potential to help clinicians identify patients at high risk of mortality from COVID-19 at the point of hospital admission. It is suggested that the analysis should be repeated using a larger, multi-centre dataset across different time periods.
Acknowledgments
Acknowledgments
This work uses data provided by patients and collected by the NHS as part of their care and support #DataSavesLives.
Conflict of interest statement
None declared.
Funding
No external funding was sought for this analysis. ISARIC4C is supported by grants from the National Institute for Health Research (Award CO-CIN-01) and the Medical Research Council (Grant MC_PC_19059).
Ethical approval
Health Research Authority approval for this study and analysis was issued by the London–Chelsea Research Ethics Committee (REC Reference: 20/HRA/3967) on 10 November 2020. Ethical approval for data collection by ISARIC4C in England was given by the South Central–Oxford C Research Ethics Committee in England (Reference 13/SC/0149). The ISARIC WHO CCP-UK study was registered at https://www.isrctn.com/ISRCTN66726260 and designated an Urgent Public Health Research Study by the National Institute for Health Research.
Author contributions
TK and JW conceived this study and analysis. MGS, JKB and ISARIC4C investigators [https://isaric4c.net/about/authors/] conceived the ISARIC WHO CCP-UK study. TK, JW, LD, AA, WC and CT contributed to the study design. TK and JW obtained ethical approval for this study and analysis. AA, JW and ISARIC4C investigators collected data for the study. FA conducted the primary statistical analysis. All authors contributed to writing and editing the final manuscript.
References
- Choudhuri J, Carter J, Nelson R, Skalina K, Osterbur-Badhey M, Johnston A. SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L. ISARIC4C investigators. features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faíco-Filho KS, Passarelli VC, Bellei N. Is higher viral load in SARS-CoV-2 associated with death? Am J Trop Med Hyg. 2020;103:2019–2021. doi: 10.4269/ajtmh.20-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick G, Vogt F, Moi Gbabai OB, Decroo T, Keane M, De Clerck H. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Médecins Sans Frontières Ebola case management centre, Kailahun, Sierra Leone, June–October 2014. J Infect Dis. 2015;212:1752–1758. doi: 10.1093/infdis/jiv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JT, Ran RX, Lv ZH, Feng LN, Ran CY, Tong YQ. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:2158–2166. doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM. ISARIC4C investigators. risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. Erratum in: BMJ 2020;371:m4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England . PHE; London: 2020. Rapid assessment of the (ProLab/Certest) ViaSure SARS-CoV-2 real time PCR detection kit (VS-NCO296T)https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/889340/Rapid_assessment_ViaSure_SARS-CoV-2_Real_Time_PCR_detection_Kit_VS-NCO296T.pdf Available at. (accessed April 2021) [Google Scholar]
- Yu X, Sun S, Shi Y, Wang H, Zhao R, Sheng J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care. 2020;24:170. doi: 10.1186/s13054-020-02893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]