Significance
Stroke is the second leading cause of death and the most frequent cause of disability in adults. After stroke, most ischemic neurons die and a few neurons live, leading to brain dysfunction; yet, genes involved in both neuronal survival and death remain poorly understood. Here, we found that the activity-dependent transcription factor Npas4 is essential for acquisition of neuronal tolerance to ischemia. Moreover, a systematic search for Npas4-downstream genes identified Gem, which encodes Ras-related small GTPase that mediates neuroprotective effects of Npas4. Gem suppresses the membrane localization of voltage-gated Ca2+ channels to inhibit excess Ca2+ influx, thereby protecting neurons from excitotoxic death. Our findings suggest that Gem expression via Npas4 promotes neuroprotection and neuroplasticity in injured and healthy brains, respectively.
Keywords: neural activity–dependent, neuroprotection, neuroplasticity, ischemic stroke, Npas4
Abstract
Ischemic stroke, which results in loss of neurological function, initiates a complex cascade of pathological events in the brain, largely driven by excitotoxic Ca2+ influx in neurons. This leads to cortical spreading depolarization, which induces expression of genes involved in both neuronal death and survival; yet, the functions of these genes remain poorly understood. Here, we profiled gene expression changes that are common to ischemia (modeled by middle cerebral artery occlusion [MCAO]) and to experience-dependent activation (modeled by exposure to an enriched environment [EE]), which also induces Ca2+ transients that trigger transcriptional programs. We found that the activity-dependent transcription factor Npas4 was up-regulated under MCAO and EE conditions and that transient activation of cortical neurons in the healthy brain by the EE decreased cell death after stroke. Furthermore, both MCAO in vivo and oxygen-glucose deprivation in vitro revealed that Npas4 is necessary and sufficient for neuroprotection. We also found that this protection involves the inhibition of L-type voltage-gated Ca2+ channels (VGCCs). Next, our systematic search for Npas4-downstream genes identified Gem, which encodes a Ras-related small GTPase that mediates neuroprotective effects of Npas4. Gem suppresses the membrane localization of L-type VGCCs to inhibit excess Ca2+ influx, thereby protecting neurons from excitotoxic death after in vitro and in vivo ischemia. Collectively, our findings indicate that Gem expression via Npas4 is necessary and sufficient to promote neuroprotection in the injured brain. Importantly, Gem is also induced in human cerebral organoids cultured under an ischemic condition, revealing Gem as a new target for drug discovery.
Stroke is the second leading cause of death and the most frequent cause of disability in adults worldwide (1). Ischemic stroke initiates a complicated and highly interdigitated cascade of pathological events (including excitotoxicity, oxidase stress, inflammation, and apoptosis) and results in cellular damage and loss of neurological function (2–5). Because of the high energy demands of the brain, neurons are immediately depleted of energy by any impedance of cerebral blood flow, resulting in loss of resting membrane potential and uncontrolled glutamate release (3, 6). These insults trigger repetitive depolarization, called spreading depolarization, in neurons within the infarct area (6), leading to increased intracellular Ca2+ levels, production of inflammatory cytokines and growth factors, and transcription of immediate early genes (2, 4, 7). These ischemia-induced genes can activate both the neuroprotective program and pathogenic cascades, which culminate in apoptotic or necrotic cell death (2, 8). The observation that both protective and pathological cascades are coactivated by ischemia suggests that potentiation of protective signaling pathways may block the cytotoxic effects of ischemia; however, it remains unclear how to potentiate the neuroprotective program that is characteristic of ischemic responses.
In the healthy brain, external sensory stimulation, including an enriched environment (EE), induces neuronal Ca2+ transients and gene expression, leading to synaptic plasticity in neurons for learning and memory (9, 10). Furthermore, nuclear Ca2+/calmodulin signaling controls expression of neuroprotective genes in the healthy brain (11, 12). However, it remains unclear 1) how neuronal activity–regulated genes in the healthy brain are different from ischemia-regulated genes in the pathological brain and 2) whether induction of neuronal activity–regulated genes in the healthy brain affects neuroprotection after stroke. In this study, we found that transient activation of cortical neurons in the healthy brain decreases cell death after stroke (i.e., via activity-dependent ischemic tolerance). On the basis of a systematic search, we identified the activity-dependent transcription factor Npas4 (neuronal PAS domain protein 4), which is necessary for neurons to resist ischemia. Although Npas4 plays a neuroprotective role in ischemic stroke (13–15), it is unclear how it regulates this process at a molecular level. On the basis of another systematic search, we identified a molecule, Gem, which acts downstream of Npas4 and mediates neuroprotection; these findings point to a good therapeutic target for stroke.
Results
A Short Exposure to an EE Facilitates Neuroprotection after Stroke.
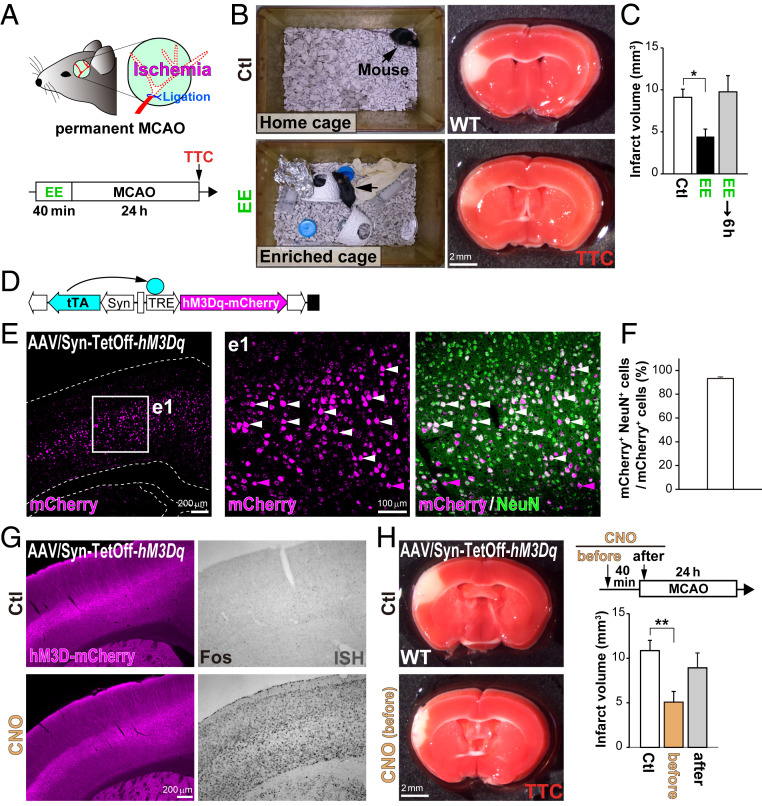
Rats housed in an enriched cage (provided with ladders, tubes, hiding places, etc.) for 1 mo show better functional outcomes after stroke than those housed under standard conditions (16). However, it remains unknown how an EE facilitates neuroprotection. To understand the relationship between EE and stroke, we performed middle cerebral artery occlusion (MCAO) in mice preexposed to an EE for 40 min (Fig. 1A). Intriguingly, even this short period of exposure to an EE was sufficient to decrease infarct volume (48.3 ± 9.8%) compared with that in the control group in the home cage (Fig. 1 B and C). However, imposing a 6-h interval between the EE exposure and MCAO surgery prevented this protective effect. To confirm the requirement of neural activity for neuroprotection, we utilized the Syn–TetOff system (17) with an adeno-associated virus (AAV) vector carrying the excitatory Designer Receptors Exclusively Activated by Designer Drugs (DREADD) gene, hM3Dq-mCherry, to be expressed in neocortical neurons (Fig. 1 D–F). Chemogenetic activation with an injection of clozapine N-oxide (CNO) induced Fos (activity-dependent gene) expression in these cortical neurons (Fig. 1G and SI Appendix, Fig. S1D). As expected, CNO injection at 40 min before MCAO significantly reduced the infarct volume (46.8 ± 11.0%) compared with that in the control, whereas CNO administered after MCAO did not (Fig. 1H). These results suggest that neural activation with either EE or chemogenetics before stroke is sufficient to protect neurons from ischemic death (activity-dependent ischemic tolerance).
Fig. 1.
A short exposure to an EE facilitates neuroprotection after stroke. (A) Schematic drawings of the MCAO surgery and the experimental timeline. TTC, 2,3,5-triphenyltetrazolium chloride. WT mice pre-exposed to an EE for 40 min show reduced volumes of cell death after stroke. (B and C) Living cells were stained with TTC 24 h after MCAO (B) to calculate the infarct volume (C). (D) Schematic drawing of the AAV/Syn–TetOff vector carrying hM3Dq-mCherry. The human synapsin promoter (Syn) drives expression of the tetracycline-controlled transactivator (tTA) gene specifically in neurons, and hM3Dq-mCherry is efficiently induced in the absence of Dox, as previously reported (16). (E and F) Expression of hM3Dq-mCherry in the neocortex. (E) AAV/Syn–TetOff–hM3Dq-mCherry was injected into the lateral ventricles of postnatal day 0 (P0) pups. (F) The ratio of NeuN+ neurons in mCherry+ cells that expressed hM3Dq in the neocortex. Immunohistochemistry was performed in sections of the neocortex using antibodies against red fluorescent protein (RFP; magenta) and NeuN (neuron; green). Enlarged images of the region enclosed by a white square in E are shown on the right (e1). White arrowheads indicate the double-positive cells (mCherry+ and NeuN+). (G) Activation of cortical neurons by DREADDs. Mice transfected with AAV/Syn–TetOff–hM3Dq-mCherry received i.p. injections with CNO, and immunohistochemistry was performed for mCherry (Left) and ISH for Fos (Right) in the neocortex 40 min after CNO. (H) MCAO surgery after activating cortical neurons with DREADDs. Living cells ware stained with TTC 24 h after MCAO. Note that preactivation of cortical neurons with DREADDs protects neurons from death after stroke. *P < 0.05, **P < 0.01 (one-way ANOVA with post hoc Tukey’s test in C and H).
Search for Genes Whose Expression Is Altered by Stroke and EE.
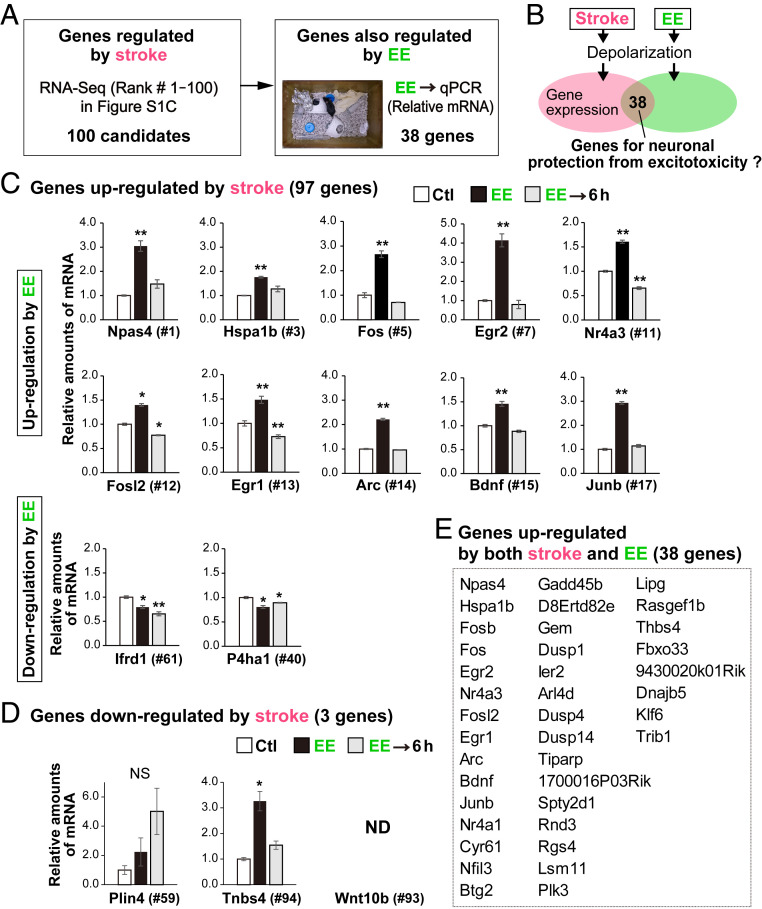
Preexposure to a brief period of ischemia (brief ischemia) induces a neuroprotective mechanism in which neurons acquire tolerance to ischemia (termed ischemic tolerance or ischemic preconditioning) (18, 19). We hypothesized that neural depolarization under both EE and brief ischemia conditions would trigger expression of common genes underlying the protective mechanism that blocks death induced by excitotoxicity (Fig. 2B). Thus, we systematically searched for genes whose expression would be affected by brief ischemia as well as by the EE (Fig. 2A). First, RNA-sequencing (RNA-Seq) analysis was performed on the control and ischemic sides of the neocortex 2 h after MCAO to identify ischemia-regulated genes (SI Appendix, Fig. S2C). Brief ischemia rapidly induced gene expression in the cortex: Most of the genes (97 of the top 100) were up-regulated on the ischemic side relative to expression on the control side of the cortex (SI Appendix, Fig. S2 B and C). Second, a qRT-PCR analysis for the top 100 genes identified in this primary screen was performed using mice exposed to an EE for 40 min, indicating that 38 genes were also up-regulated (Fig. 2 C–E). Among them, Npas4 was most highly expressed in neurons [neuronal nuclei (NeuN) positive] soon after ischemia (SI Appendix, Fig. S3) and was transiently induced by the EE (Figs. 2C and 3A). We confirmed that chemogenetic activation with CNO also induces Npas4 expression in cortical neurons (SI Appendix, Fig. S4 C and D).
Fig. 2.
Search for genes showing altered expression after stroke and exposure to EE. (A) Schematic drawing of genes up- and down-regulated by stroke and EE. The relative amounts of messenger RNA (mRNA) for the top 100 genes whose expression was altered by stroke according to the RNA-Seq analysis (SI Appendix, Fig. S2C) were quantified by qRT-PCR in mice with and without exposure to an EE (control [Ctl], home cage). (B) Brief ischemia and EE induce membrane depolarization in neurons; 38 genes were up-regulated in the cortex by both brief ischemia and EE. (C) Genes whose expression was up-regulated by stroke and up- or down-regulated by EE. (D) Genes whose expression was down-regulated by stroke and up- or down-regulated by EE. ND, not detected. (E) The 38 genes up-regulated simultaneously by stroke and EE. NS: not significant. *P < 0.05, **P < 0.01 (Student’s t test with Bonferroni correction compared to Ctl in C and D). Note that among the 97 genes up-regulated by stroke; expression of 38 genes was also up-regulated by EE. By contrast, expression of three genes down-regulated by stroke was not decreased by EE.
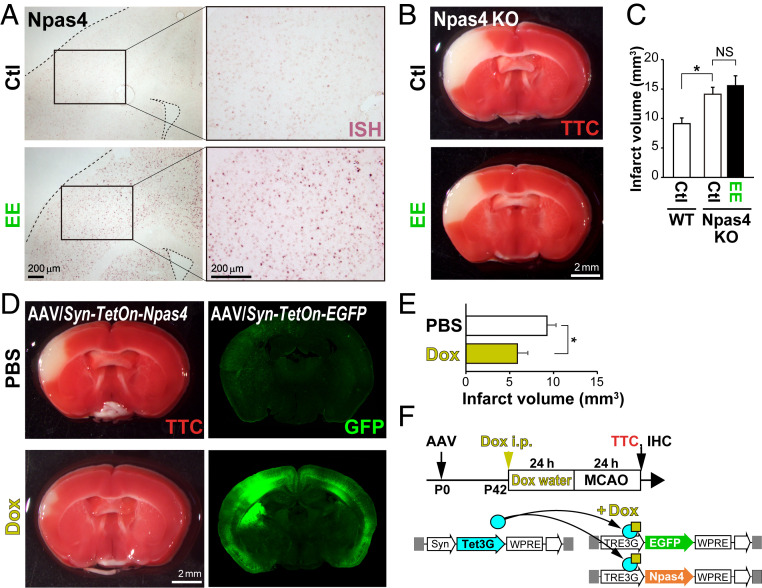
Fig. 3.
Overexpression of Npas4 via the AAV/Syn–TetOn system facilitates neuroprotection after stroke. (A) ISH shows Npas4 expression in the cortex after exposure to an EE. (B and C) Living cells in WT and Npas4 KO mice were stained with TTC 24 h after MCAO (B) to calculate the infarct volume (C). Note that preexposure to an EE is not protective against ischemia in Npas4 KO mice. (D–F) Schematic drawings of Npas4 expression by the AAV/Syn–TetOn system and the experimental timeline (F). Syn, synapsin promoter. AAV/Syn–Tet3G, AAV/TRE3G–EGFP, and AAV/TRE3G–Npas4 vectors were coinjected into the lateral ventricles of postnatal day 0 (P0) mouse pups; 42 d later, expression was induced with Dox, and MCAO was performed 24 h later. Living cells were stained with TTC 24 h after MCAO (D, Left) to calculate the infarct volume (E). Exogenous EGFP expression was confirmed by immunohistochemistry after TTC staining (D, Right). *P < 0.05; NS: not significant (one-way ANOVA with post hoc Tukey’s test in C and E).
To test the role of Npas4 in neuroprotection, we performed MCAO surgery in Npas4 knockout (KO) mice with or without EE exposure. Under non-EE conditions, infarct volumes were larger in Npas4 KO mice (155.0 ± 13.0%) than in wild-type (WT) mice (Fig. 3C), as previously reported (20). However, there was no difference in infarct volume between Npas4 KO mice exposed to EE and those kept in their home cages (Fig. 3 B and C). By contrast, Npas4 expression induced by doxycycline (Dox) administration using the AAV/Syn–TetOn system (SI Appendix, Fig. S5) significantly reduced the infarct volume after MCAO (56.6 ± 10.4%) relative to that in animals receiving phosphate-buffered saline (PBS) (Fig. 3 D–F). These in vivo studies demonstrated that Npas4 expression, induced just before stroke, is necessary and sufficient for activity-dependent ischemic tolerance.
Npas4 Expression Inhibits Excess Ca2+ Influx in Ischemic Neurons In Vitro.
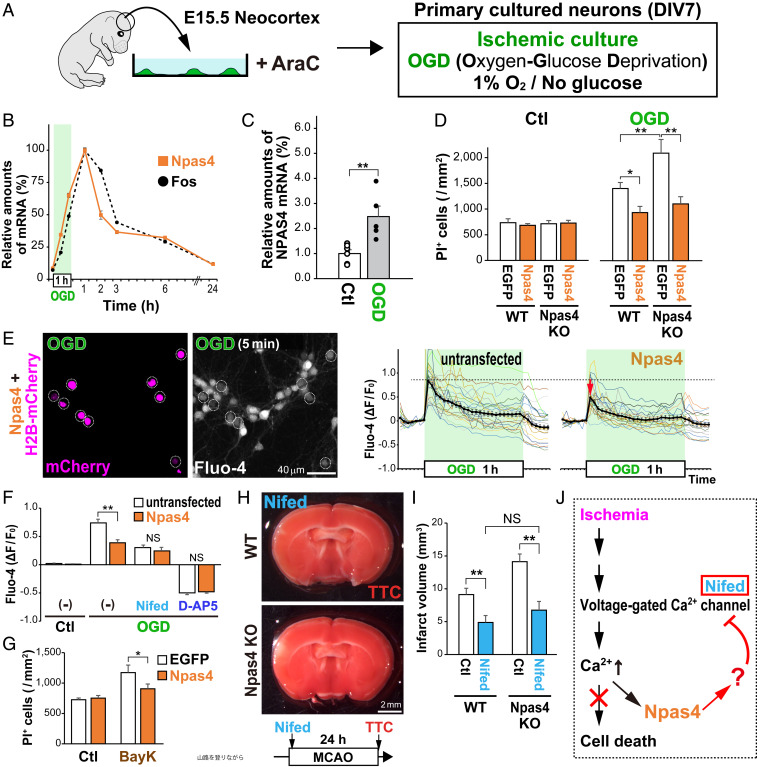
To identify the molecular mechanisms underlying the ability of Npas4 to protect neurons from death after stroke, we utilized an in vitro model of ischemia in which primary cultured cortical neurons (96.6 ± 0.3% MAP2+) were incubated under conditions of oxygen and glucose deprivation (OGD) (SI Appendix, Fig. S6 A–C). A 1-h treatment with OGD increased transcription of Npas4, which peaked 1 h after OGD before returning to baseline (Fig. 4B). To reveal whether expression of NPAS4 (the human homolog) is induced in an in vitro model of human ischemia, we generated cerebral organoids from human induced pluripotent stem cells. Interestingly, NPAS4 expression was increased markedly in human cerebral organoids 1 h after OGD (250 ± 40%; Fig. 4C), suggesting that both humans and mice share a common mechanism for Npas4 induction by ischemia. In the healthy mouse brain, Npas4 expression is induced by Ca2+ signaling in a sensory experience–dependent manner (21, 22). In ischemic neurons, membrane depolarization evoked by energy depletion activates N-methyl-d-aspartic acid (NMDA) receptors and L-type voltage-gated Ca2+ channels (VGCCs), resulting in abnormal Ca2+ influx (3, 23). Consistent with this, OGD-mediated increases in both Ca2+ influx (SI Appendix, Fig. S6E) and Npas4 expression (SI Appendix, Fig. S6F) in primary cortical neurons were inhibited either strongly (34.6 ± 1.9%; SI Appendix, Fig. S6E) (12.9 ± 2.8%; SI Appendix, Fig. S6F) by NMDA receptor antagonist d-(-)-2-amino-5-phosphonopentanoic acid (D-AP5) or significantly (78.2 ± 2.1%; SI Appendix, Fig. S6E) (66.5 ± 7.3%; SI Appendix, Fig. S6F) by L-type VGCC antagonist nifedipine (Nifed). The excess Ca2+ influx caused by OGD treatment induced death of primary neurons (24) (SI Appendix, Fig. S6 G and H). Npas4 was sufficient to block this effect, as WT primary neurons transfected with AAV/CMV–Npas4 at 24 h before OGD exhibited a decreased number of propidium iodide+ (PI+) dead cells after OGD (67.2 ± 7.0%) than those transfected with AAV/CMV–EGFP (Fig. 4D), consistent with the in vivo results (Fig. 3 D and E). Conversely, expression of Npas4 via AAV/CMV–Npas4 in Npas4 KO primary neurons was sufficient to block the increase in the number of PI+ dead cells (Fig. 4D). These in vitro studies revealed that Npas4 expression in primary neurons is both necessary and sufficient for protection against cell death.
Fig. 4.
Npas4 expression inhibits excess Ca2+ influx into ischemic neurons. (A) Schematic drawing of primary cultured neurons exposed to ischemia-like OGD. (B) qRT-PCR for relative amounts of Npas4 or Fos messenger RNA (mRNA) in primary neurons after 1 h of OGD. (C) qRT-PCR for relative amounts of NPAS4 mRNA 1 h after OGD in human brain organoid cultures generated from induced pluripotent stem cells. (D) Numbers of dead cells in either Npas4-overexpressing or Npas4-deficient neurons after OGD. Primary neurons prepared from WT or Npas4 KO embryonic cortexes were transfected with AAV/CMV–EGFP or AAV/CMV–Npas4 24 h before OGD. Dead cells were stained with PI 24 h after OGD. (E) Ca2+ imaging during OGD in primary neurons electroporated with plasmids carrying H2B-mCherry and Npas4. Dotted circles indicate H2B-mCherry–transduced cells (E, Left). Graphs on the right indicate Fluo-4 intensities (ΔF/F0) of Npas4-overexpressing neurons (H2B-mCherry– and Npas4-transfected; Npas4) relative to those of surrounding untransfected neurons (untransfected) during the 1-h OGD. (F) Graph shows Fluo-4 intensities of Npas4-overexpressing neurons relative to those of surrounding untransfected neurons 5 min after the onset of OGD in the presence or absence of Nifed (an inhibitor of L-type VGCCs) or D-AP5 (an inhibitor of the NMDA receptor). (G) Numbers of dead cells in Npas4-overexpressing and control neurons (transfected with AAV/CMV–EGFP and AAV/CMV–Npas4, respectively) after VGCCs were activated with BayK for 5 min. Dead cells were stained with PI 24 h after BayK treatment. (H and I) In vivo neuroprotective effects of Nifed in WT and Npas4 KO mice undergoing MCAO. Living cells were stained with TTC 24 h after MCAO (H) to calculate the infarct volume (I). (J) Schematic drawing of the deduced function for Npas4 in ischemic neurons. *P < 0.05, **P < 0.01, NS: not significant (Student’s t test in C and two-way ANOVA with post hoc Tukey’s test in D, F, G, and I).
Interestingly, Npas4 overexpression before OGD suppressed an increase in Ca2+ influx in primary cortical neurons (79.8 ± 3.1%; Fig. 4 E and F) similar to Nifed treatment (74.9 ± 2.5%; Fig. 4F); however, it did not reduce influx further (71.5 ± 3.5%; Fig. 4F). Furthermore, Npas4 overexpression reduced the number of dead primary neurons after excessive activation of L-type VGCCs with their agonist, Bay K8644 (BayK) (25) (77.2 ± 6.7%; Fig. 4G). These results suggest that Npas4 expression in primary neurons might directly or indirectly inhibit L-type VGCC function. To confirm observations in vivo, WT and Npas4 KO mice received intraperitoneal (i.p.) injections of Nifed soon after the MCAO surgery. It was reported previously that Nifed treatment at 2 d after MCAO markedly reduces infarct volume at 2 wk after MCAO (26). Consistent with this, Nifed reduced the acute infarct volume in WT mice (53.5 ± 11.6%; Fig. 4I). Intriguingly, the infarct volumes were not different between WT and Npas4 KO mice treated with Nifed (74.1 ± 14.6%; Fig. 4 H and I), although under the control conditions, the infarct volume was larger in Npas4 KO mice (155.0 ± 13.0%) than in WT mice (Fig. 3C). These results strongly suggest that Npas4 expression inhibits L-type VGCC function in vitro and in vivo.
Next, we investigated the relationship between Npas4 expression and NMDA receptor function. As shown in SI Appendix, Fig. S6E, D-AP5 strongly inhibited Ca2+ influx (34.6 ± 1.9%) in cultured neurons during OGD to a level comparable with that under the control condition, suggesting that activation of NMDA receptors is required to induce excessive Ca2+ influx in ischemic neurons (SI Appendix, Fig. S6I), as previously reported (27). Npas4 overexpression before OGD suppressed the increase in Ca2+ influx in primary cortical neurons (79.8 ± 3.1%; Fig. 4E), similar to D-AP5 treatment (28.8 ± 1.9%), but did not further reduce the Ca2+ influx (29.9 ± 1.5%; Fig. 4F). NMDA receptor activation enables Na+ and Ca2+ influx to further depolarize neurons (28), which in turn facilitates Ca2+ influx through voltage-gated Ca2+ channels (29) (SI Appendix, Fig. S6I). Although Npas4 inhibited L-type VGCCs (Fig. 4G), it was unknown whether it would also affect NMDA receptors (Fig. 4F). However, Npas4 overexpression did not prevent the death of primary neurons induced by NMDA (100.3 ± 4.9%; SI Appendix, Fig. S7A), suggesting Npas4 does not alter NMDA receptor function in cortical neurons. To confirm these observations in vivo, mice received i.p. injections of the NMDA receptor antagonist MK801 [because D-AP5 cannot cross the blood–brain barrier (30)] soon after MCAO. As expected, MK801 administration reduced the infarct volume similarly in WT and Npas4 KO mice (SI Appendix, Fig. S7 B and C). As NMDA receptor activation was required for Ca2+ influx in ischemic neurons (SI Appendix, Fig. S6E), inhibition by MK801 prevented cell death in Npas4 KO mice after MCAO (SI Appendix, Fig. S7 B and C).
Search for Npas4 Target Genes Conferring Protection against Ischemic Death.
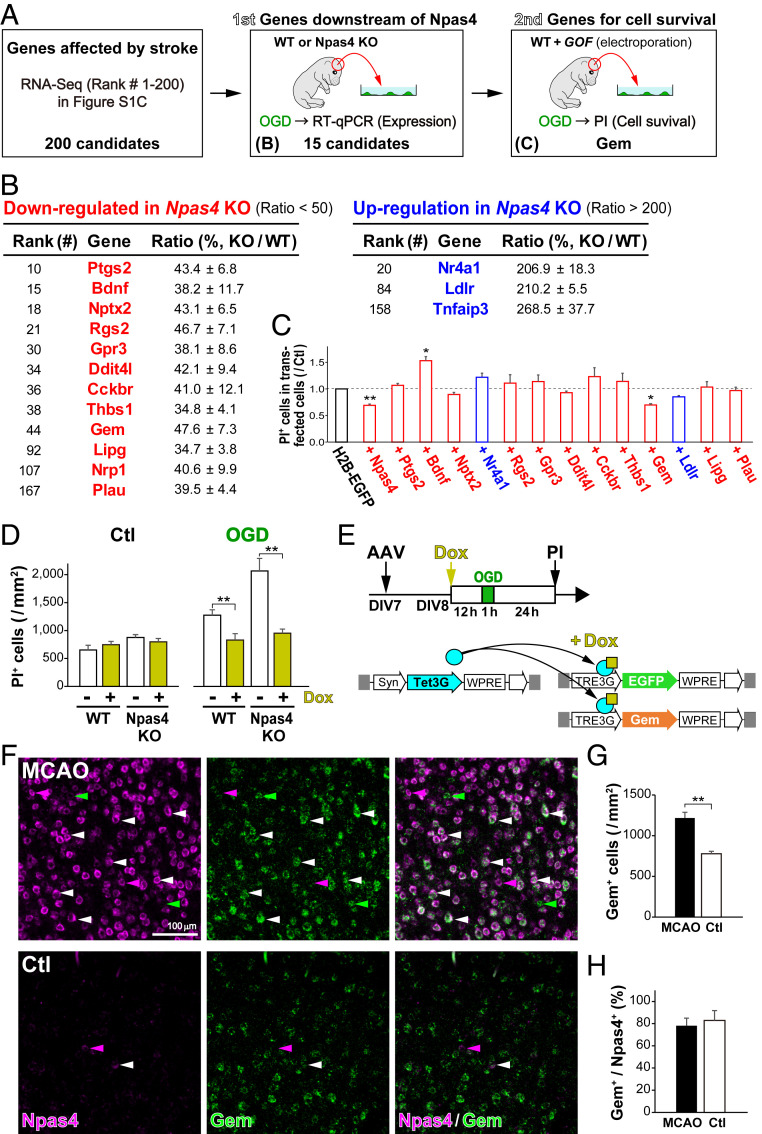
As Npas4 overexpression in primary cortical neurons did not change the expression of L-type VGCC subunits (SI Appendix, Fig. S8), it is possible that the transcription factor Npas4 controls L-type VGCC function via its downstream genes (Fig. 4J). We therefore searched for Npas4 target genes that met the following requirements: 1) expression is regulated by ischemic stroke, 2) expression is altered by Npas4 deficiency, and 3) expression protects neurons from cell death upon infarct. First, ischemia-regulated genes were identified with the RNA-Seq analysis in MCAO mice (SI Appendix, Fig. S2C). Second, qRT-PCR analyses for the top 200 genes from this primary screen were performed to identify 12 genes down-regulated and 3 genes up-regulated after OGD in neurons from Npas4 KO mice compared with expression in neurons from WT mice (Fig. 5 A and B and SI Appendix, Fig. S9). Third, to determine which genes are necessary for cell survival, we overexpressed 13 of the genes as well as Npas4 and an H2B-EGFP control in primary neurons via electroporation. Only two of these reduced the number of PI+ dead cells after OGD: Npas4 and Gem (Fig. 5C). The neuroprotective effect of Gem overexpression against OGD was confirmed via the AAV/Syn–TetOn system in primary neurons from WT and Npas4 KO mice (Fig. 5 D and E), indicating that Gem overexpression is sufficient to prevent the increase in cell death of Npas4-deficient neurons (Fig. 5D). Two-color in situ hybridization (ISH) of cortical neurons after MCAO showed that Gem expression increased significantly mainly in Npas4-expressing neurons (156.4 ± 9.0%; Fig. 5 F–H). Furthermore, the in vivo experiments revealed that Npas4 overexpression with the AAV/Syn–TetOn system increased transcription of Gem and Nptx2 [Npas4-downstream gene (31)] (SI Appendix, Fig. S10). These results suggest that Gem, a newly identified downstream target of Npas4, is sufficient to protect ischemic neurons from cell death.
Fig. 5.
Search for Npas4 target genes conferring protection against ischemic death. (A) Schematic drawing for the search for Npas4 target genes in vitro. Genes whose expression was altered after MCAO were identified by RNA-Seq. (B) The first screen for candidate genes that were regulated in ischemic neurons in an Npas4-dependent manner. Among the top 200 genes listed in SI Appendix, Fig. S2C, expression of the 15 candidate genes was confirmed by qRT-PCR on primary cultured neurons from WT (n = 4) and Npas4 KO (n = 4) pregnant mice subjected to 1 h of OGD. (C) The second screen for the 13 differentially regulated genes listed in B. Primary neurons were cotransfected with each candidate gene and H2B-EGFP, and numbers of dead cells after OGD were counted via PI staining 24 h later. (D) Reduced numbers of dead cells overexpressing Gem in WT and Npas4-KO primary neurons after OGD. (E) Schematic drawings of Gem overexpression with the AAV/Syn–TetOn system in primary neurons and the experimental timeline. Primary neurons from WT and Npas4 KO embryonic cortexes were cotransfected with AAV/Syn–Tet3G and AAV/TRE3G–EGFP or AAV/TRE3G–Gem. After 12 h of Dox administration, primary neurons received 1 h of OGD and were subjected to PI staining 24 h later to count dead cells (D). Note that overexpression of Gem in Npas4-deficient neurons was sufficient to reduce the number of dead cells after ischemia. (F) Gem expression in cortical neurons after stroke. Two-color ISH with Npas4 (magenta) and Gem (green) was performed in the neocortex 1 h after MCAO. (G and H) The numbers of Gem+ cells (G) and the ratio of Gem+ cells to Npas4+ cells (H) in the MCAO side (MCAO) compared with those in the control side (Ctl). **P < 0.01 (Student’s t test in G and H, Student’s t test with Bonferroni correction in C and G, and two-way ANOVA with post hoc Tukey’s test in D).
Gem Expression Protects Ischemic Neurons from Death.
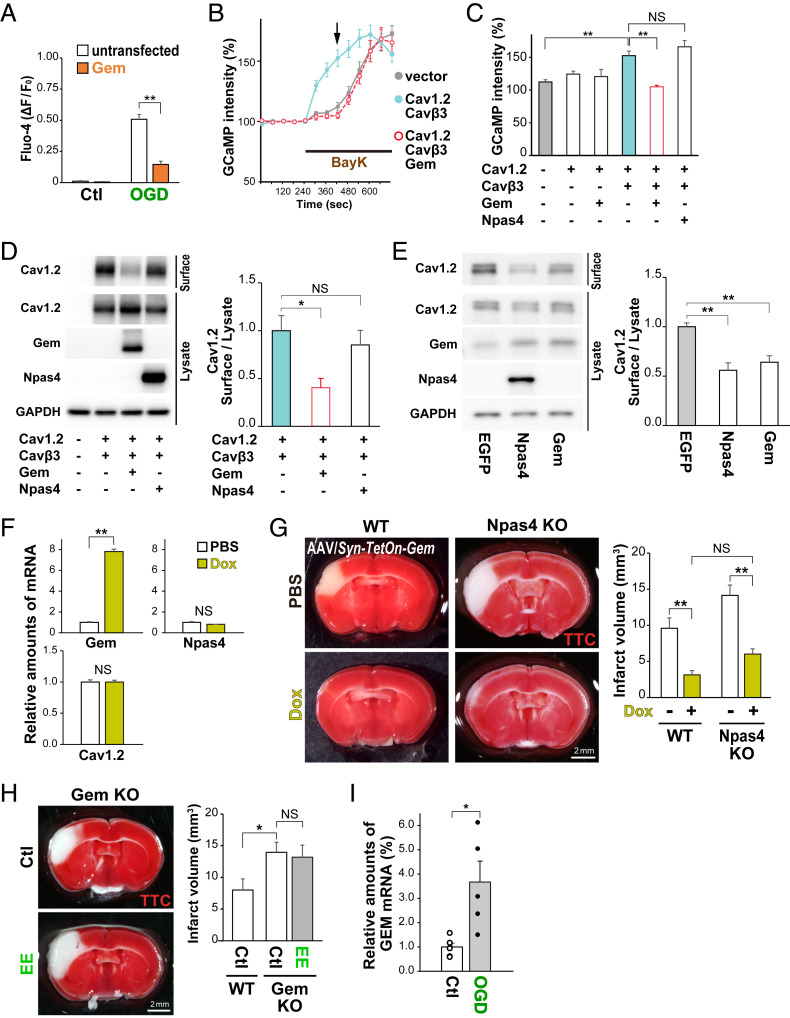
Gem, a member of the Rem, Rad, and Gem/Kir (RGK) family of Ras-related small GTPases, regulates Ca2+ channel function and cytoskeletal rearrangements (32, 33). Gem overexpression before OGD suppressed the OGD-induced increase in Ca2+ influx in primary cortical neurons (75.9 ± 1.8%; Fig. 6A). However, intracellular Ca2+ concentrations were not different in Gem-overexpressing and control primary neurons under normal conditions (105.7 ± 7.5%; SI Appendix, Fig. S11B). To examine the effect of Gem on VGCC function, we performed Ca2+ imaging with GCaMP6f in HEK293T cells cotransfected with the genes encoding L-type VGCC subunits Cav1.2 (Cacna1c; channel) and Cavβ3 (Cacnb3; regulatory). The cells were treated with the L-type VGCC agonist (BayK), leading to a potent increase in Ca2+ influx (Fig. 6B). By contrast, cotransfection of Gem with Cav1.2 and Cavβ3 suppressed the increase in Ca2+ influx induced by BayK treatment (68.8 ± 1.9%; Fig. 6 B and C), while cotransfection of Npas4 along with these did not (Fig. 6C). These observations suggested that Npas4 represses L-type VGCC function via Gem, which regulates the subcellular localization of Cav1.2 (32). In HEK293T cells cotransfected with Gem, Cav1.2, and Cavβ3, the amount of Cav1.2 protein on the cell surface was smaller (41.0 ± 10.0%) than that on HEK293T cells cotransfected with Cav1.2 and Cavβ3 but not Gem (Fig. 6D and SI Appendix, Fig. S11C). Similarly, Gem overexpression with the AAV/Syn–TetOn system in primary neurons also reduced the amount of endogenous Cav1.2 on the cell surface relative to the amount in the cell lysate (55.9 ± 7.6%; Fig. 6E and SI Appendix, Fig. S11E). In the in vivo experiment, Gem overexpression with the AAV/Syn–TetOn system prior to MCAO did not change expression of Npas4 or Cav1.2 (Fig. 6F) but significantly reduced the infarct volume (32.7 ± 6.2%) compared with that with the PBS control (Fig. 6G). Furthermore, Gem overexpression prevented increased cell death in Npas4-KO mice (62.9 ± 7.4%; Fig. 6G). To confirm the effect of Gem deficiency for ischemia, we newly generated Gem KO mice via the CRISPR-Cas9 system (SI Appendix, Fig. S12). Gem KO mice exhibited a larger infarct volume after MCAO than WT mice (174.4 ± 19.9%; Fig. 6H). Interestingly, preexposure to an EE was not neuroprotective against ischemia in Gem KO mice (164.8 ± 23.8%) compared with the effect in control (non-EE) mice (Fig. 6H). Taken together, these results demonstrate that Gem expression is necessary and sufficient to protect neurons from ischemia in vivo. Notably, expression of GEM (the human homolog) was also increased in human cerebral organoids after OGD (370 ± 90%; Fig. 6I), suggesting that Gem also plays a role in neuronal protection after ischemia in the human brain. Moreover, Gem expression was up-regulated transiently in the cortex, hippocampus, and striatum of mice exposed to an EE (SI Appendix, Fig. S13). Therefore, Gem may regulate L-type VGCC function in both the injured and healthy brains in an activity-dependent manner.
Fig. 6.
Gem expression protects ischemic neurons from death. (A) Ca2+ imaging during OGD in primary neurons electroporated with plasmids carrying H2B-mCherry and Gem. Graph shows Fluo-4 intensities of Gem-overexpressing neurons (Gem) relative to those of surrounding untransfected neurons (untransfected) 5 min after the onset of OGD. (B) Ca2+ imaging of GCaMP6f-transfected HEK293T cells during treatment with BayK (VGCC agonist). GCaMP intensities were measured in HEK293T cells expressing GCaMP6f, VGCC subunits (Cav1.2 and Cavβ3), and Gem during BayK treatment. (C) Graph showing relative GCaMP intensities 3 min after BayK treatment (arrow in B). (D) Western blot for Cav1.2 protein on the cell surfaces of HEK293T cells expressing VGCC subunits (Cav1.2 and Cavβ3) and either Gem or Npas4. Graph shows amounts of the cell surface Cav1.2 protein relative to the protein amounts in the cell lysates. (E) Western blot for endogenous Cav1.2 protein in primary neurons in which Npas4 or Gem was overexpressed. Graph shows amounts of the cell surface Cav1.2 protein relative to the amounts in cell lysates. (F) Gem overexpression via the AAV/Syn–TetOn system in the cortex in vivo. AAV/Syn–Tet3G and AAV/TRE3G–Gem were coinjected into the lateral ventricles of postnatal day 0 (P0) pups. qRT-PCR was performed to calculate relative amounts of each messenger RNA (mRNA) after PBS or Dox treatment. (G) Effects of Gem overexpression with the AAV/Syn–TetOn system before MCAO. AAV/Syn–Tet3G and AAV/TRE3G–Gem were coinjected into the lateral ventricles of WT and Npas4 KO pups at P0; 42 d later, the mice received PBS or Dox treatment 24 h before MCAO. Living cells were stained with TTC 24 h after MCAO to calculate the infarct volume. (H) Living cells in WT and Gem KO mice were stained with TTC 24 h after MCAO to calculate the infarct volume. Note that preexposure to an EE is not neuroprotective against ischemia in Gem KO mice. (I) qRT-PCR for relative amounts of GEM mRNA 1 h after OGD in human brain organoid cultures generated from induced pluripotent stem cells. *P < 0.05, **P < 0.01; NS: not significant (one-way or two-way ANOVA with post hoc Tukey’s test in C, D, E, and H or A and G, respectively; Student’s t test in F and I).
Discussion
The main findings of this study are that 1) a short period of neural activation with either natural stimuli (EE) or chemogenetics before stroke is sufficient to facilitate neuronal protection from ischemic death (activity-dependent ischemic tolerance); 2) Npas4 expression, induced just before stroke, is necessary and sufficient to promote activity-dependent ischemic tolerance; 3) Npas4 expression inhibits L-type VGCC function in vitro and in vivo; and 4) expression of Gem, a newly identified downstream target of Npas4, is necessary and sufficient to protect neurons from ischemic death in vitro and in vivo.
Npas4 is neuroprotective in a model of stroke (13–15). Nevertheless, it was not clear how Npas4 promotes survival of ischemic neurons after OGD in vitro and after MCAO in vivo. Synaptotagmin 10 (Syt10) (34) and mitochondrial calcium uniporter (Mcu) (35) are Npas4-downstream factors that affect neuroprotection against excitotoxicity induced by kainic acid and NMDA, respectively, in primary neurons in vitro. However, our RNA-Seq analysis of MCAO-treated mice (SI Appendix, Fig. S2) revealed that expression of Syt10 and Mcu was not altered markedly after stroke. This suggests that in the in vivo model of ischemic stroke, Npas4 might regulate unknown targets to protect ischemic neurons from cell death. In this study, we identified Gem as an Npas4 target, which is necessary and sufficient to protect neurons from cell death after in vitro and in vivo ischemia (Fig. 6).
It is well known that a patient who either had a recent transient ischemic attack or recovered from a mild stroke is at high risk of recurrence (36). Although pretreatment with brief ischemia induces a neuroprotective mechanism (18, 19), it is difficult to apply this to patients. Interestingly, animal experiments reveal that exercise preconditioning (walking on a treadmill) provides significant neuroprotection against stroke (37), although at least 2 or 3 wk of pretraining is necessary to induce ischemic tolerance. By contrast, our mouse ischemic models revealed that a short period of neural activation before stroke is sufficient to acquire ischemic tolerance (Fig. 1). As shown in the results from the DREADD system (Fig. 1H), the method that artificially modulates the brain state may lead to a new therapy for stroke. Furthermore, our results suggest that transient induction of activity-regulated genes in the healthy brain activates a neuroprotective mechanism and facilitates cell survival after stroke (Fig. 1). Consistent with this, our systematic searches for such responsible genes revealed that Npas4 and thus Gem play a central role in activity-dependent ischemic tolerance (Figs. 2 and 5). The number of Npas4+ cells in mice overexpressing Npas4 by the AAV/Syn–TetOn system was larger (1,790 ± 87 cells/mm2; SI Appendix, Fig. S5) than that in mice either activated with chemogenetics or exposed to an EE (824 ± 126 and 643 ± 78 cells/mm2, respectively; SI Appendix, Fig. S4). However, the rate of reduction in infarct volume after MCAO was not significantly different among these conditions (Figs. 1 and 3). Npas4 may facilitate neuroprotection via both cell-autonomous and non–cell-autonomous mechanisms. In the ischemic brain, abnormal depolarization of neurons induces several events that affect surrounding neurons, including uncontrolled glutamate release (3, 6), production of inflammatory cytokines (4), and cell death. It is possible that preinduction of Npas4 in a certain number of neurons before stroke may reduce Ca2+ influx via a cell-autonomous mechanism, leading to propagation to surrounding Npas4-negative neurons.
Although Npas4 and Gem are reported independently as ischemia-induced genes (20, 38, 39), the functional relationship between them is unknown. Our results show that Gem is transcriptionally activated by Npas4, whose expression is induced by excessive Ca2+ influx into the cytoplasm of neurons after stroke (Fig. 6). Gem suppresses localization of the L-type VGCC to the cytoplasmic membrane, leading to inhibition of excess Ca2+ influx and thereby protecting neurons from excitotoxic death. These findings are consistent with the protection conferred by Npas4 against seizure-induced damage in hippocampal neurons (12). Transcription of Gem, as well as the related RGK family member Rem2, is up-regulated by extracellular stimuli (40, 41). Actually, we found that both EE and brief ischemia induce Gem expression (Fig. 2 and SI Appendix, Fig. S2). Rem2 inhibits VGCC currents, promotes development of excitatory and inhibitory synapses, and is involved in dendritic branching (33, 41, 42), whereby it controls neural plasticity in the visual cortex (43). Intriguingly, Gem positively regulates dendritic branching in an activity-dependent manner (40). Npas4 also regulates dendritic spine formation and controls the excitatory–inhibitory balance within neural circuits (21, 31, 44). In the healthy brain, the neural activity–evoked Npas4 increases both inhibitory synapses in excitatory projection neurons and excitatory synapses in inhibitory interneurons (31), enabling neurons to calm down. This raises the possibility that expression of Gem via Npas4 is part of a feedback loop that controls Ca2+ influx into neurons to regulate neuroplasticity in the healthy brain and facilitate neuroprotection in the injured brain. Remarkably, expression of Npas4 and Gem increased not only in mouse brain but also human cerebral organoids after ischemic treatment (Figs. 4C and 6I). Therefore, it is possible that Gem generally functions downstream of Npas4 in various brain areas, nominating it as a good target for drug discovery aimed at neuroprotection from excitotoxicity after stroke as well as seizure.
Materials and Methods
Animal experiments were approved by the animal care committees of Nara Medical University, Kagawa University, and Osaka University in accordance with the policies established in the NIH Guide for the Care and Use of Laboratory Animals (45). Details of materials regarding a list of animals, plasmids, primers for RNA-Seq analysis, and antibodies used for our study can be found in SI Appendix. Furthermore, methods detailing brain surgery techniques, primary neuronal culture techniques, plasmid and adeno-associated viral vector constructions, qRT-PCR analysis, RNA-Seq analysis, immunoblot analysis, immunohistochemistry, ISH, and Ca2+ imaging techniques can also be found in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research on Basic Research-B (A.T.) and -C (H.T.), Innovative Areas (Adaptive circuit shift; A.T. and K.K.), and for Challenging Exploratory Research (A.T.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by a grant for the Drug Discovery Seeds (A) Program (A.T.) in Project of Translational and Clinical Research Core Center in Osaka University from Agency for Medical Research and Development, Japan. A.T. was supported by grants from the Japanese Applied Enzymology Foundation, the Smoking Science Research Foundation, and the Takeda Science Foundation for Collaborative Research Projects, Japan. H.T. was supported by grants from the Takeda Science Foundation, the Terumo Life Science Foundation, the Koyanagi Foundation, and the Naito Foundation, Japan. We thank Drs. Nobuhiko Yamamoto and Makoto Sato for support and Ms. Mayumi Higuchi for technical assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018850118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Feigin V. L., Norrving B., Mensah G. A., Global burden of stroke. Circ. Res. 120, 439–448 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Sekerdag E., Solaroglu I., Gursoy-Ozdemir Y., Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr. Neuropharmacol. 16, 1396–1415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreier J. P., Reiffurth C., The stroke-migraine depolarization continuum. Neuron 86, 902–922 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Lambertsen K. L., Finsen B., Clausen B. H., Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 137, 693–714 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silasi G., Murphy T. H., Stroke and the connectome: How connectivity guides therapeutic intervention. Neuron 83, 1354–1368 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Dreier J. P., The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 17, 439–447 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Kinouchi H., et al., Induction of c-fos, junB, c-jun, and hsp70 mRNA in cortex, thalamus, basal ganglia, and hippocampus following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 14, 808–817 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Mitsios N., et al., Pathophysiology of acute ischaemic stroke: An analysis of common signalling mechanisms and identification of new molecular targets. Pathobiology 73, 159–175 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Volkmar F. R., Greenough W. T., Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science 176, 1445–1447 (1972). [DOI] [PubMed] [Google Scholar]
- 10.Nakahata Y., Yasuda R., Plasticity of spine structure: Local signaling, translation and cytoskeletal reorganization. Front. Synaptic Neurosci. 10, 29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadia S., Stevenson P., Hardingham N. R., Bading H., Hardingham G. E., Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J. Neurosci. 25, 4279–4287 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S. J., et al., Nuclear calcium signaling controls expression of a large gene pool: Identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 5, e1000604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamloo M., et al., Npas4, a novel helix-loop-helix PAS domain protein, is regulated in response to cerebral ischemia. Eur. J. Neurosci. 24, 2705–2720 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Ooe N., Motonaga K., Kobayashi K., Saito K., Kaneko H., Functional characterization of basic helix-loop-helix-PAS type transcription factor NXF in vivo: Putative involvement in an “on demand” neuroprotection system. J. Biol. Chem. 284, 1057–1063 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Leong W. K., Klaric T. S., Lin Y., Lewis M. D., Koblar S. A., Upregulation of the neuronal Per-Arnt-Sim domain protein 4 (Npas4) in the rat corticolimbic system following focal cerebral ischemia. Eur. J. Neurosci. 37, 1875–1884 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Yu K., et al., Neuroprotective effects of prior exposure to enriched environment on cerebral ischemia/reperfusion injury in rats: The possible molecular mechanism. Brain Res. 1538, 93–103 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Sohn J., et al., A single vector platform for high-level gene transduction of central neurons: Adeno-associated virus vector equipped with the tet-off system. PLoS One 12, e0169611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitagawa K., et al., ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 528, 21–24 (1990). [DOI] [PubMed] [Google Scholar]
- 19.Dirnagl U., Simon R. P., Hallenbeck J. M., Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 26, 248–254 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Choy F. C., Klarić T. S., Leong W. K., Koblar S. A., Lewis M. D., Reduction of the neuroprotective transcription factor Npas4 results in increased neuronal necrosis, inflammation and brain lesion size following ischaemia. J. Cereb. Blood Flow Metab. 36, 1449–1463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y., et al., Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455, 1198–1204 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamoorthi K., et al., Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334, 1669–1675 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisani A., Calabresi P., Tozzi A., D’Angelo V., Bernardi G., L-type Ca2+ channel blockers attenuate electrical changes and Ca2+ rise induced by oxygen/glucose deprivation in cortical neurons. Stroke 29, 196–201, discussion 202 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Goldberg M. P., Choi D. W., Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 13, 3510–3524 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano-Abad M. F., Villarroya M., García A. G., Gabilan N. H., López M. G., Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J. Biol. Chem. 276, 39695–39704 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Yamato M., et al., Nifedipine treatment reduces brain damage after transient focal ischemia, possibly through its antioxidative effects. Hypertens. Res. 34, 840–845 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Lai T. W., Zhang S., Wang Y. T., Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 115, 157–188 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Blanke M. L., VanDongen A. M. J., “Activation mechanisms of the NMDA receptor” in Biology of the NMDA Receptor, A. M. VanDongen, Ed. (CRC Press, 2009), pp. 283–312. [PubMed] [Google Scholar]
- 29.Nanou E., Catterall W. A., Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 98, 466–481 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Davis S., Butcher S. P., Morris R. G., The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J. Neurosci. 12, 21–34 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegel I., et al., Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157, 1216–1229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Béguin P., et al., Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 411, 701–706 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Buraei Z., Yang J., Inhibition of voltage-gated calcium channels by RGK proteins. Curr. Mol. Pharmacol. 8, 180–187 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Woitecki A. M., et al., Identification of synaptotagmin 10 as effector of NPAS4-mediated protection from excitotoxic neurodegeneration. J. Neurosci. 36, 2561–2570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu J., et al., Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat. Commun. 4, 2034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf P. A., et al., Preventing ischemic stroke in patients with prior stroke and transient ischemic attack: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke 30, 1991–1994 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Zhang F., Wu Y., Jia J., Exercise preconditioning and brain ischemic tolerance. Neuroscience 177, 170–176 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Buchthal B., Weiss U., Bading H., Post-injury nose-to-brain delivery of Activin A and SerpinB2 reduces brain damage in a mouse stroke model. Mol. Ther. 26, 2357–2365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White R. E., et al., Mice lacking the β2 adrenergic receptor have a unique genetic profile before and after focal brain ischaemia. ASN Neuro 4, e00096 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krey J. F., et al., Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat. Neurosci. 16, 201–209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiretti A. E., et al., Rem2 is an activity-dependent negative regulator of dendritic complexity in vivo. J. Neurosci. 34, 392–407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paradis S., et al., An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron 53, 217–232 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore A. R., et al., Rem2 stabilizes intrinsic excitability and spontaneous firing in visual circuits. eLife 7, e33092 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshihara S., et al., Npas4 regulates Mdm2 and thus Dcx in experience-dependent dendritic spine development of newborn olfactory bulb interneurons. Cell Rep. 8, 843–857 (2014). [DOI] [PubMed] [Google Scholar]
- 45.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.