Abstract
Background
Non-communicable diseases (NCD) like hypertension, diabetes, cardiovascular and cerebrovascular diseases are the most common comorbidities among COVID-19 patients. The clinical presentation and mortality pattern of COVID-19 are different for patients with comorbidities and without comorbidities.
Objective
To determine the clinical presentation of COVID-19 and risk factors for COVID-19 mortality among diabetic patients in a tertiary care hospital in South India.
Methods
A record-based cross-sectional study was conducted by reviewing the case records of COVID-19 patients admitted for treatment from June 2020 to September 2020 in a tertiary care centre in South India. Potential risk factors for COVID-19 mortality were analysed using univariate binomial logistic regression, generalized linear models (GLM) with the Poisson distribution. Survival curves were made using the Kaplan–Meier method.
Results
Out of 200 COVID-19 patients with diabetes with a mean (SD) age of 56.1 (11.8) years, 61% were men. The median survival time was slightly lesser in male COVID-19 patients (15 days) as compared to female patients (16 days). The risk of mortality among COVID-19 patients with diabetes is increased for patients who presented with breathlessness (aRR = 4.5 (95% CI: 2.3–8.8)), had positive history of smoking (aRR = 1.9 (95% CI: 1.1–3.8)), who had CKD (aRR = 1.8 (95% CI: 1.1–2.8)) and who had cardiac illness (aRR = 1.6 (95% CI: 0.9–2.7)).
Conclusion
Diabetes patients with COVID-19 need to be given additional care and monitoring especially if they present with breathlessness, positive history of smoking, cardiac illness and, CKD. Public health campaigns and health education activities to control smoking is needed to reduce the COVID-19 mortality in diabetes patients.
Keywords: COVID-19, Diabetes, Non-communicable diseases, SARS CoV-2, Risk factors
1. Introduction
The emerging infection of the SARS CoV-2 virus causing COVID-19 disease resulted in the global pandemic with 119,218,587 cases and 2,642,673 deaths all over the world.1 India has contributed to 10% of the global caseload with 11,646,081 cases and 159,967 deaths.2 The morbidity and mortality pattern of COVID-19 is different for patients with comorbidities and without comorbidities.3 Non-communicable diseases (NCD) like hypertension, diabetes, cardiovascular and cerebrovascular diseases are the most common comorbidities among COVID-19 patients.3 A systematic review and meta-analysis showed that 9% of the COVID-19 patients were comorbid with diabetes.4
India is undergoing the epidemiological transition with a dual burden of diseases, both communicable and non-communicable diseases contributing to morbidity.5 Diabetes is one of the common NCDs contributing to the dual burden of diseases with 65·0 million Indians having diabetes in 2016.6 With the increasing prevalence of diabetes in India among adults aged 20 years or older from 5·5% in 1990 to 7·7% in 20166 and the worse prognosis of COVID-19 among diabetic patients,7 the impact of the COVID-19 pandemic in India will be abominable.
Patients with diabetes who have compromised immunity and a poorly regulated inflammatory cytokine storm might be the possible mechanisms for severe COVID-19 disease among diabetes patients.8 Increased glycation of ACE2 and reduced ACE2 in diabetic patients with poor control of glycemic status explains the increased susceptibility to severe ARDS and lung damage in COVID-19 patients.9 Diabetic- COVID-19 patients with other comorbid health conditions like hypertension, ischemic heart diseases, obesity were more likely to have severe disease, hospitalization, and death.7
A study in Wuhan has shown that diabetic patients with COVID-19 had higher odds of Intensive care unit (ICU) admission and death.10 An Italian study showed that 8.9% of the COVID-19 admitted had diabetes. Another British study has shown that the risk of mortality was more among COVID-19 patients with uncontrolled diabetes with a hazard ratio of 2·36 [95% CI 2·18–2·56].11 The literature related to the clinical presentation of COVID-19 among diabetes patients in India is very limited. With this background, this study was conducted to assess the clinical presentation of COVID-19 and risk factors for COVID-19 mortality among diabetic patients in a tertiary care hospital in South India.
2. Methodology
A record-based cross-sectional study was conducted by reviewing the case sheets of COVID-19 patients admitted for treatment in the tertiary care center in Bangalore, India. Since June 2020, this center was designated as Dedicated COVID Hospital (DCH) by the Government of Karnataka (GoK) and standard care is being free of cost to the COVID-19 patients.
The clinical and socio-demographic details were collected from the case records maintained in the hospital. The data collected were entered in MS Excel. The anonymity and confidentiality of the information collected were maintained throughout the study. During the period from June 2020 till September 2020, 854 records of confirmed COVID-19 cases were analyzed. Out of them, there were 200 COVID-19 patients with diabetes comorbidity which were included in the current study. Patients were followed from the date of admission till the date of discharge/death from the hospital.
The variables included in the study are age, sex, the presence of symptoms like fever, cough, breathlessness, sore throat, loose stools chest pain, headache, myalgia. The details like the history of travel, contact, smoking, alcohol consumption were also included in the study. The presence of other comorbidities like hypertension, chronic kidney disease (CKD), respiratory diseases, malignancies, tuberculosis, and hypothyroidism was also collected from the case records. After the period of stay in the hospital, the outcome of the patients was categorized as discharge and death.
2.1. Operational definitions
2.1.1. History of contact
A person who is involved in any works related to providing direct care without proper personal protective equipment for COVID-19 patients or staying in the same close environment as a COVID-19 patient in the last 14 days before the onset of symptoms.12
2.1.2. History of travel
A person who has travelled to any foreign countries in the last 14 days before the onset of symptoms.
2.1.3. History of smoking
A person who is a current smoker or former smoker is considered as having a positive history of smoking.13
2.1.4. History of alcohol consumption
Use of alcoholic beverages either on individual occasions (binge drinking) or as a regular practice is considered a positive history of alcohol consumption.14
2.2. Statistical analysis
The data collected were entered in MS Excel and analyzed using STATA statistical software version 14 (StataCorp LCC, Lakeway Drive College Station, Texas, USA).15 The continuous variables were summarized using mean (SD) or median (interquartile range (IQR)) based on the distribution of data. Other categorical variables were summarized using frequencies and proportions. Chi-square test and Fischer's exact tests are used to assess the statistical significance of association between the categorical variables. Binomial logistic regression was used to do the univariate analysis to determine the factors associated with mortality among diabetic patients with COVID-19. Relative risk (RR) with a 95% confidence interval (95% CI) was used to express the strength of association. A generalized linear model (GLM) with Poisson distribution was used for multivariate analysis and adjusted relative risk (aRR) with 95% CI was calculated. The variables which were significantly associated with the outcome (p-value <0.05) in the univariate analysis were included in the multivariate analysis. A p-value less than 0.05 was considered statistically significant. Kaplan–Meier method was used to plot the survival curves and the survival distributions between the groups were tested using the Log-rank test.
2.3. Ethical considerations
The ethical approval to conduct the study was taken from the Institutional Ethics Committee and due permissions were taken from the authorities. The anonymity and confidentiality of the data were maintained throughout the study.
3. Results
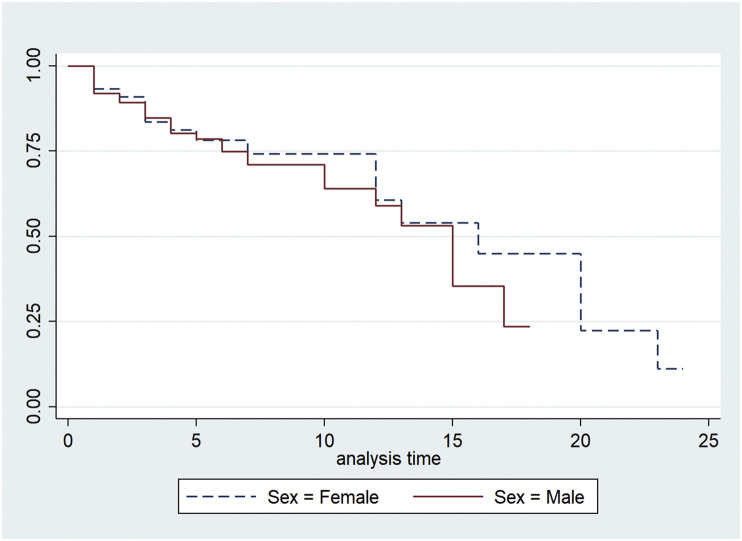
In total 200 COVID-19 patients with diabetes were included in the study whose mean (SD) age was 56.1 (11.8) years. Out of 200 study participants, 78 (39%) were women and 122 (61%) were men. The symptoms with which COVID-19 patients with diabetes presented were fever (59.5%), cough (48%), difficulty in breathing (42%), myalgia (26.5%), sore throat (3%), headache (11%), loose stools (3.5%), loss of taste/smell (5%) and chest pain (5%). The median (IQR) survival time in the hospital among COVID-19 patients with diabetes was 15 (12–20) days. The median survival time slightly lesser for male patients (15 (95% CI: 10–17) days) and female COVID-19 patients (16 (95% CI: 12–20) days) with diabetes but the difference was not statistically significant (p-value 0.490) [Fig. 1 ].
Fig. 1.
Kaplan–Meier survival estimates.
History of contact with a positive case of COVID-19 was present in 38 (19%) and history of travel to a foreign country was present in 23 (11.5%) of the study participants. The comorbidities present in the COVID-19 with diabetes were hypertension (56.5%), ischemic heart disease (9.5%), hypothyroidism (7%), CKD (3.5%), respiratory diseases like chronic obstructive pulmonary disease (COPD), and asthma (3%), tuberculosis (3.0%) and malignancy (1.5%).
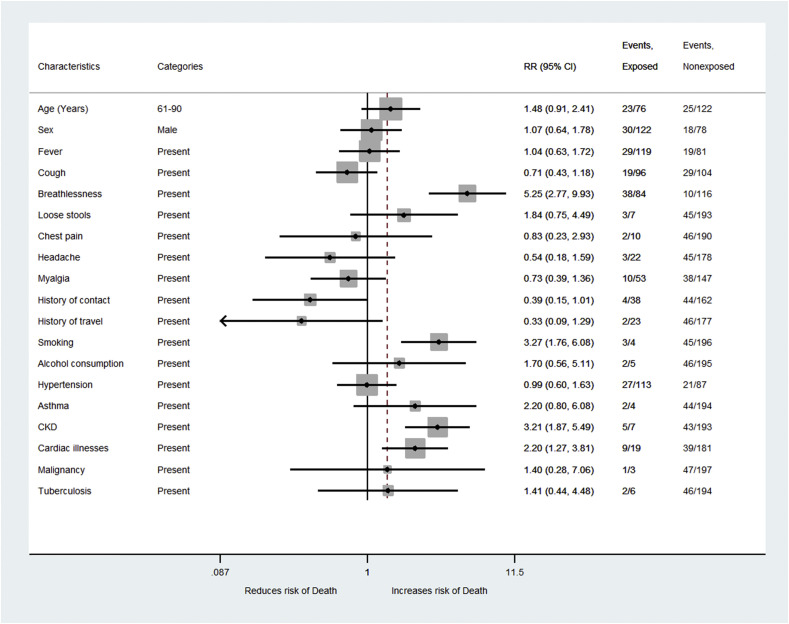
Our results showed that the risk of death due to COVID-19 among diabetes patients increased by RR = 5.2 (95% CI: 2.7–9.9) times among those who presented with breathlessness as compared to those who did not have breathlessness [Table 1 ]. Diabetic COVID-19 patients with a history of smoking had a 3.3 (RR = 3.3, 95% CI: 1.7–6.1) times increased risk of mortality as compared to non-smokers and this association was statistically significant [Fig. 2 ]. COVID-19 Patients with diabetes and CKD had 3.2 times (RR = 3.2, 95% CI: 1.8–5.4) increased risk of death as compared to those without CKD [Table 2 ]. Similarly, COVID-19 patients with diabetes and cardiac illness had 2.1 times (RR = 2.1, 95% CI: 1.2–3.8) increased risk of mortality and the association was statistically significant.
Table 1.
Association of demographic and clinical characteristics with mortality among diabetes patients with COVID-19 admitted to the tertiary care center, N = 200.
| Characteristics | Categories | Discharged n = 152 Frequency (%) |
Died n = 48 Frequency (%) |
RR (95% CI)a | p-value |
|---|---|---|---|---|---|
| Age | 0–30 | 2 (100) | 0 | – | |
| 31–60 | 97 (79.5) | 25 (20.5) | 1 | 0.104 | |
| 61–90 | 53 (69.7) | 23 (30.3) | 1.5 (0.9–2.4) | ||
| Sex | Female | 60 (76.9) | 18 (23.1) | 1 | 0.807 |
| Male | 92 (75.4) | 30 (24.6) | 1.1 (0.6–1.7) | ||
| Fever | No | 62 (76.5) | 19 (23.5) | 1 | 0.882 |
| Yes | 90 (75.6) | 29 (24.4) | 1.0 (0.6–1.7) | ||
| Cough | No | 75 (72.1) | 29 (27.8) | 1 | 0.186 |
| Yes | 77 (80.2) | 19 (19.8) | 0.7 (0.4–1.1) | ||
| Breathlessness | No | 106 (91.4) | 10 (8.6) | 1 | <0.001 |
| Yes | 46 (54.8) | 38 (45.2) | 5.2 (2.7–9.9) | ||
| Loose stools | No | 148 (76.7) | 45 (23.3) | 1 | 0.181 |
| Yes | 4 (57.1) | 3 (42.9) | 1.8 (0.7–4.4) | ||
| Chest pain | No | 144 (75.8) | 46 (24.2) | 1 | 0.767 |
| Yes | 8 (80) | 2 (20) | 0.8 (0.2–2.9) | ||
| Headache | No | 133 (74.7) | 45 (25.3) | 1 | 0.263 |
| Yes | 19 (86.4) | 3 (13.6) | 0.6 (0.2–1.5) | ||
| Myalgia | No | 109 (74.2) | 38 (25.8) | 1 | 0.321 |
| Yes | 43 (81.2) | 10 (18.8) | 0.7 (0.4–1.3) | ||
| Sore throat | No | 146 (75.2) | 48 (24.7) | – | – |
| Yes | 6 (100) | 0 |
The bold values are indicates that the p-values are statistically significant.
RR-relative risk.
Fig. 2.
Forest plot of univariate analysis of risk factors for mortality among COVID-19 patients with diabetes.
Table 2.
Association of demographic and clinical characteristics with mortality among diabetes patients with COVID-19 admitted to the tertiary care center, N = 200.
| Factor | Categories | Discharged n = 152 Frequency (%) |
Died n = 48 Frequency (%) |
RR (95% CI)a | p-value |
|---|---|---|---|---|---|
| History of contact | No | 118 (72.9) | 44 (27.1) | 1 | 0.053 |
| Yes | 34 (89.5) | 4 (10.5) | 0.4 (0.1–1.0) | ||
| History of travel | No | 131 (74) | 46 (26) | 1 | 0.111 |
| Yes | 21 (91.3) | 2 (8.7) | 0.3 (0.1–1.3) | ||
| History of smoking | No | 151 (77) | 45 (23) | 1 | <0.001 |
| Yes | 1 (25) | 3 (75) | 3.3 (1.7–6.1) | ||
| History of alcohol consumption | No | 149 (76.4) | 46 (2.6) | 1 | 0.348 |
| Yes | 3 (60) | 2 (40) | 1.7 (0.5–5.1) | ||
| Pregnancy | No | 149 (75.6) | 48 (24.4) | – | – |
| Yes | 3 (100) | 0 | |||
| Hypertension | No | 66 (75.9) | 21 (24.1) | 1 | 0.968 |
| Yes | 86 (76.1) | 27 (23.9) | 0.9 (0.6–1.6) | ||
| Respiratory disease | No | 150 (77.3) | 44 (22.7) | 1 | 0.126 |
| COPDb | 0 | 2 (100) | – | ||
| Asthma | 2 (50) | 2 (50) | 2.2 (0.8–6.0) | ||
| Tuberculosis | No | 148 (76.3) | 46 (23.7) | 1 | 0.565 |
| Yes | 4 (66.6) | 2 (33.4) | 1.4 (0.4–4.4) | ||
| CKDc | No | 150 (77.7) | 43 (22.3) | 1 | <0.001 |
| Yes | 2 (28.6) | 5 (71.4) | 3.2 (1.8–5.4) | ||
| Cardiac illnesses | No | 142 (78.5) | 39 (21.5) | 1 | 0.005 |
| Yes | 10 (52.6) | 9 (47.4) | 2.1 (1.2–3.8) | ||
| Malignancy | No | 150 (76.2) | 47 (23.8) | 1 | 0.686 |
| Yes | 2 (66.6) | 1 (33.4) | 1.4 (0.3–7.0) | ||
| Hypothyroidism | No | 138 (74.2) | 48 (25.8) | – | – |
| Yes | 14 (100) | 0 |
The bold values are indicates that the p-values are statistically significant.
RR-relative risk.
COPD-Chronic Obstructive Pulmonary Disease.
Chronic Kidney Disease.
In the multivariate analysis, the variables which were significantly associated with mortality in the univariate analysis like presenting with breathlessness, history of smoking, CKD, cardiac illness were included in the model. After adjusting for other variables included in the model, the risk of mortality among COVID-19 patients with diabetes is increased for patients who presented with breathlessness (aRR = 4.5 (95% CI: 2.3–8.8)), had a positive history of smoking (aRR = 1.9 (95% CI: 1.1–3.8)), who had CKD (aRR = 1.8 (95% CI: 1.1–2.8)) and who had a cardiac illness (aRR = 1.6 (95% CI: 0.9–2.7)). These associations were also statistically significant in multivariate analysis [Table 3 ].
Table 3.
Multivariate analysis of clinical characteristics with mortality among diabetes patients with COVID-19 admitted to the tertiary care center, N = 200.
| Characteristics | Categories | RR (95% CI)a | aRR (95% CI)b | p-value |
|---|---|---|---|---|
| Breathlessness | No | 1 | 1 | <0.001 |
| Yes | 5.2 (2.7–9.9) | 4.5 (2.3–8.8) | ||
| History of smoking | No | 1 | 1 | <0.001 |
| Yes | 3.3 (1.7–6.1) | 1.9 (1.1–3.8) | ||
| CKDc | No | 1 | 1 | <0.001 |
| Yes | 3.2 (1.8–5.4) | 1.8 (1.1–2.8) | ||
| Cardiac illnesses | No | 1 | 1 | 0.005 |
| Yes | 2.1 (1.2–3.8) | 1.6 (0.9–2.7) |
The bold values are indicates that the p-values are statistically significant.
RR-relative risk.
aRR-adjusted relative risk.
Chronic Kidney Disease.
4. Discussion
Our analysis has identified that patients presenting with breathlessness, patients with comorbidities CKD and cardiac illness, positive history of smoking were significantly associated with mortality due to COVID among diabetes patients. A systematic review and meta-analysis by Galbadage T et al showed that gender was significantly associated with mortality with the male sex having a high risk of death due to COVID-19.16 But the current study depicted that among COVID-19 patients with diabetes as comorbidity, gender is not significantly associated with mortality [Fig. 1]. This result is similar to another study done in the UK which also showed that gender was not significantly associated with mortality among diabetes patients with COVID-19.17
Our study showed that patients presenting with breathlessness had 4 times increased risk of mortality even after adjusting for other confounding variables which similar to findings among non-diabetic COVID-19 patients.18, 19, 20 Smoking increased the risk of death by 1.9 times among COVID-19 patients with diabetes which is similar to other studies as evinced from a systematic review and meta-analysis by Salah HM et al.17 Smoking increases the expression of Angiotensin-Converting Enzyme-2 (ACE2) which is also linked to the effects of COVID-19.21 Smoking and COVID-19 cause endothelial injury, hypercoagulable state, and disturbed immune system which explain the increased risk of mortality among COVID-19 patients.22 , 23
The current study showed that having the additional co-morbidities like cardiac diseases and CKD along with diabetes increased the risk of mortality among COVID-19 patients by 1.6 times and 1.8 times respectively. ACE2 dependent pathway in the kidney is affected by the SARS-CoV-2 virus leading to acute kidney injury and death which might be the reason for the increased mortality among COVID-19 patients with CKD.24 The effects of the SARS-CoV-2 virus on the cardiovascular system can be explained in many aspects. It anchors on the transmembrane ACE2 to enter the host cells including type 2 pneumocytes, macrophages, endothelial cells, pericytes, and cardiac myocytes.25 The virus can also destabilize atherosclerotic plaques which lead to the development of acute coronary syndromes.25 The above mechanisms lead to inflammation, multi-organ failure, and death.
4.1. Strengths and limitations
Our study has a few strengths. We analyzed the data from a large number of COVID-19 patients with diabetes with appropriate statistical analysis. Our study has few limitations. This is a record-based study from a tertiary hospital, so the generalizability of the study results needs to be done with caution. The duration of symptoms at the time of presenting in the hospital was not studied which describes the health-seeking behavior. The control blood glucose among diabetes patients which might affect mortality was not included in the study. Nevertheless, published literature based on original studies on diabetes and COVID-19 is very limited in India and our study provides valuable evidence on the clinical profile and risk factors for COVID-19 mortality among diabetes patients from India.
5. Conclusion
Almost 1/5th of COVID-19 patients admitted to the hospital had comorbidity diabetes. Among COVID-19 patients with diabetes, those who presented with breathlessness, comorbidities like CKD and cardiac illness, positive history of smoking were significantly associated with mortality. Early identification of these risk factors and their appropriate management is crucial to prevent mortality among COVID-19 patients. Diabetes patients with COVID-19 can be given additional care with prompt monitoring of the symptoms especially breathlessness. Public health campaigns and health education activities to control smoking is needed to reduce the COVID-19 mortality in diabetes patients. The post-COVID-19 sequelae among diabetes patients need to be assessed with cohort studies with a longer follow-up period.
Conflicts of interest
The authors have none to declare.
References
- 1.World Health Organization . World Health Organization; Geneva,Switzerland: 2021 Mar. COVID-19 Weekly Epidemiological Update 22.https://www.who.int/docs/default-source/coronaviruse/situation-reports/weekly_epidemiological_update_22.pdf [Internet] Available from: [Google Scholar]
- 2.Ministry of Health and Family Welfare Government of India . 2021. COVID-19 INDIA.https://www.mohfw.gov.in/ [Internet] [cited 2021 Mar 22]. Available from: [Google Scholar]
- 3.Sanyaolu A., Okorie C., Marinkovic A., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020 Aug;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [Internet] [cited 2021 Mar 22]. Available from: http://pmc/articles/PMC7314621/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paudel S.S. A meta-analysis of 2019 novel corona virus patient clinical characteristics and comorbidities. Res Square. 2020 https://www.researchsquare.com/article/rs-21831/v1 [Internet] Available from: [Google Scholar]
- 5.Yadav S., Arokiasamy P. Understanding epidemiological transition in India. Glob Health Action. 2014;7(suppl 1):23248. doi: 10.3402/gha.v7.23248. http://www.ncbi.nlm.nih.gov/pubmed/24848651 [Internet] [cited 2019 May 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tandon N., Anjana R.M., Mohan V., et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Heal. 2018 Dec 1;6(12):e1352–e1362. doi: 10.1016/S2214-109X(18)30387-5. https://pubmed.ncbi.nlm.nih.gov/30219315/ [Internet] [cited 2021 Mar 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020 Sep 1;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. www.thelancet.com/diabetes-endocrinology [Internet] [cited 2020 Nov 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020 Jun 1;16(6):297–298. doi: 10.1038/s41574-020-0353-9. https://pubmed.ncbi.nlm.nih.gov/32242089/ [Internet] [cited 2021 Mar 30]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020 Apr 1;162:108132. doi: 10.1016/j.diabres.2020.108132. [Internet] [cited 2021 Mar 30]. Available from: http://pmc/articles/PMC7118535/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q., Zhang X., Jiang F., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020 Jul 1;43(7):1382–1391. doi: 10.2337/dc20-0598. https://pubmed.ncbi.nlm.nih.gov/32409504/ [Internet] [cited 2021 Mar 23]. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Williamson E., Walker A.J., Bhaskaran K., et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020 May 7 doi: 10.1101/2020.05.06.20092999. [Internet] [cited 2021 Mar 23]; Available from: [DOI] [Google Scholar]
- 12.National Centre for Disease Control . 2020. The Updated Case Definitions and Contact-Categorisation.https://ncdc.gov.in/WriteReadData/l892s/89568514191583491940.pdf [Internet]. New Delhi. [cited 2020 Nov 24]. Available from: [Google Scholar]
- 13.World Health Organization . 2009 Jul. Global Adult Tobacco Survey (GATS) Indicator Guidelines: Definition and Syntax.https://www.who.int/tobacco/surveillance/en_tfi_gats_indicator_guidelines.pdf [Internet] [cited 2020 Nov 24]. Available from: [Google Scholar]
- 14.Rehm J., Room R., Monteiro M., et al. 1996. Alcohol Use.https://www.who.int/publications/cra/chapters/volume1/0959-1108.pdf [Internet] [cited 2020 Nov 24]. Available from: [Google Scholar]
- 15.StataCorp . StataCorp LP; College Station, TX, USA: 2017. Stata Statistical Software: Release 14. [Google Scholar]
- 16.Galbadage T., Peterson B.M., Awada J., et al. vol. 7. Frontiers Media S.A.; 2020. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes; p. 348. (Frontiers in Medicine). [Internet] [cited 2020 Nov 23]. Available from: http://pmc/articles/PMC7331754/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong Marit, Woodward Mark, Peters Sanne A.E. Diabetes and COVID-19–related mortality in women and men in the UK biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Care. 2020 Dec doi: 10.2337/dc20-2378. https://care.diabetesjournals.org/content/diacare/early/2020/12/07/dc20-2378.full.pdf [Internet] [cited 2021 Apr 3]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abayomi A., Odukoya O., Osibogun A., et al. Presenting symptoms and predictors of poor outcomes among 2,184 patients with COVID-19 in Lagos state, Nigeria. Int J Infect Dis. 2020 Oct 16;102:226–232. doi: 10.1016/j.ijid.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Sun W., Han M., Ying Y., Wang Q. A study on the predictors of disease severity of COVID-19. Med Sci Monit. 2020 Sep 23;26:8. doi: 10.12659/MSM.927167. [Internet] [cited 2020 Nov 23]. Available from: http://pmc/articles/PMC7521067/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain V., Yuan J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020 Jun 25;65(5):533–546. doi: 10.1007/s00038-020-01390-7. http://link.springer.com/10.1007/s00038-020-01390-7 [Internet] [cited 2020 Nov 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salah H.M., Sharma T., Mehta J. Smoking doubles the mortality risk in COVID-19: a meta-analysis of recent reports and potential mechanisms. Cureus. 2020 Oct 7;12(10):e10837. doi: 10.7759/cureus.10837. [Internet] [cited 2021 Apr 3]. Available from: http://pmc/articles/PMC7647838/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallet J., Dubertret C., Le Strat Y. Addictions in the COVID-19 era: current evidence, future perspectives a comprehensive review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020 Aug 12 doi: 10.1016/j.pnpbp.2020.110070. [Internet] [cited 2020 Nov 23]; Available from: http://pmc/articles/PMC7420609/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai G., Bossé Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020 Jun 15;201(12):1557–1559. doi: 10.1164/rccm.202003-0693LE. https://www.atsjournals.org/doi/10.1164/rccm.202003-0693LE [Internet] [cited 2020 Nov 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajaimy M., Melamed M.L. Covid-19 in patients with kidney disease. Clin J Am Soc Nephrol Am Soc Nephrol. Aug 7, 2020:1087–1089. doi: 10.2215/CJN.09730620. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 Aug 1;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. pmc/articles/PMC7197627/ [Internet] [cited 2021 Apr 5]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]