Significance
The bacterial cell envelope is the frontline defense against host immune factors and antibiotics. The regulator of capsule synthesis (Rcs) is a complex signaling pathway that maintains the homeostasis of this essential organelle. Several hypotheses for how the sensory component RcsF activates signaling in response to stress were proposed but could not be directly tested because RcsF proper assembly into the complex with outer membrane proteins (OMPs) depends on the essential β-barrel assembly machine (Bam). We used an extensive genetic analysis to tease apart which RcsF interactions are important for the sensing function. We show that RcsF does not monitor the Bam complex function. Instead, the Bam complex is required to assemble the sensory RcsF/OMP complex that monitors membrane integrity.
Keywords: envelope biogenesis, Rcs phosphorelay, surface-exposed lipoproteins, envelope stress response
Abstract
The regulator of capsule synthesis (Rcs) is a complex signaling cascade that monitors gram-negative cell envelope integrity. The outer membrane (OM) lipoprotein RcsF is the sensory component, but how RcsF functions remains elusive. RcsF interacts with the β-barrel assembly machinery (Bam) complex, which assembles RcsF in complex with OM proteins (OMPs), resulting in RcsF’s partial cell surface exposure. Elucidating whether RcsF/Bam or RcsF/OMP interactions are important for its sensing function is challenging because the Bam complex is essential, and partial loss-of-function mutations broadly compromise the OM biogenesis. Our recent discovery that, in the absence of nonessential component BamE, RcsF inhibits function of the central component BamA provided a genetic tool to select mutations that specifically prevent RcsF/BamA interactions. We employed a high-throughput suppressor screen to isolate a collection of such rcsF and bamA mutants and characterized their impact on RcsF/OMP assembly and Rcs signaling. Using these mutants and BamA inhibitors MRL-494 and darobactin, we provide multiple lines of evidence against the model in which RcsF senses Bam complex function. We show that Rcs activation in bam mutants results from secondary OM and lipopolysaccharide defects and that RcsF/OMP assembly is required for this activation, supporting an active role of RcsF/OMP complexes in sensing OM stress.
The bacterial cell envelope is an essential structure, acting as a first line of defense against environmental assault. The gram-negative cell envelope is complex, consisting of an inner (IM) and outer (OM) membrane that encloses the cell wall in an aqueous periplasmic space (1). The OM is asymmetric, with phospholipids and lipopolysaccharides (LPS) in the inner and outer leaflets, respectively. The cation cross-bridged LPS molecules confer extreme resistance to detergents and many antibiotics (2).
The regulator of capsule synthesis (Rcs) signaling cascade is one of several envelope stress responses that monitor envelope integrity and biogenesis (Fig. 1) (3). Rcs involves at least six proteins spanning all cellular compartments from the cell surface to the cytoplasm. At the core of this pathway is the RcsCDB Histidine-Aspartate phosphorelay complex consisting of the IM hybrid histidine protein kinase RcsC, the IM phosphotransferase protein RcsD, and the cytoplasmic DNA-binding response regulator RcsB (4–6). The activity of this Rcs phosphorelay is regulated by interactions with two upstream components, IgaA and RcsF. IgaA is a polytopic IM protein with a large periplasmic domain, and it inhibits the phosphorelay through RcsD (7, 8). The OM lipoprotein RcsF is a sensory component of the Rcs cascade, which activates downstream signaling in response to stress by releasing IgaA inhibition (8–12). However, sensing by RcsF and signal transduction to IgaA are poorly understood at a molecular level, in part because many distinct genetic and chemical stimuli can induce Rcs, including defects in lipoprotein biogenesis (13–15), cell wall biogenesis (12, 16–19), and the defects of LPS at the cell surface (as a result of Polymyxin B [PMB] treatment, for example) (19–22).
Fig. 1.
Proposed mechanistic models for the Rcs stress response. Rcs components (orange) are shown in the context of the envelope structure and biogenesis pathways. The sensory lipoprotein RcsF and the negative regulator IgaA are central to the regulation of RcsCDB phosphorelay. RcsF is exported to the OM by the Lol pathway; the Bam complex assembles RcsF with partner OMPs, leading to a partially surface-exposed topology. Red arrows represent proposed signaling events in response to stress (red stars) that are not yet fully understood. (A) Proposed model for the OM/LPS sensing by RcsF. Cell surface localization of RcsF NTD enables RcsF to monitor the integrity of the outer leaflet. Upon LPS stress (e.g., PMB treatment), the signal is transduced to the periplasmic CTD through the conformational change in the RcsF/OMP complex stimulating downstream signaling. (B) Proposed model for the Bam complex sensing function of RcsF. Envelope stress by an unknown mechanism inhibits the Bam complex function; as a result, RcsF/BamA interaction is prevented, and RcsF is accumulated in the periplasmic-facing orientation stimulating downstream signaling.
At the OM, RcsF forms a complex with β-barrel OM proteins (OMPs) such as OmpA, OmpC, and OmpF, adopting a transmembrane orientation in which RcsF is partially surface exposed (12, 23). The β-barrel assembly machinery (Bam complex) that assembles all OMPs also assembles RcsF/OMP complexes, and RcsF interacts with its central and essential component, BamA (12, 23).
Defective lipoprotein biogenesis results in the retention of RcsF at the IM, promoting physical association with IgaA and the constitutive activation of signaling (12, 13). Two hypotheses have been proposed to explain how RcsF signals from the OM (Fig. 1 A and B): the first suggests that the surface-exposed N-terminal domain (NTD) of RcsF in an RcsF/OMP complex monitors the integrity of LPS at the outer leaflet, transmitting the signal to the periplasmic carboxyl-terminal domain (CTD) to induce downstream signaling (19, 23) (Fig. 1A); the second argues that the RcsF/OMP complex plays no active role in signal transduction, with stress signals altering the RcsF/BamA interaction to retain RcsF in a periplasm-facing orientation, allowing downstream signaling (12) (Fig. 1B). This altering of the RcsF/BamA interaction is thought to allow RcsF to monitor Bam complex activity (12). Testing these hypotheses has proven to be challenging, as the Bam complex is essential, and there was no clear path to identifying point mutations that specifically disrupt RcsF/OMP or RcsF/BamA interactions without compromising OMP biogenesis and OM integrity.
The Bam complex consists of five components, A through E (24): BamA is a β-barrel with five periplasmic Potra domains that scaffold four regulatory lipoproteins, BamB through E. An essential lipoprotein, BamD, recruits OMP substrates to the Bam complex and activates BamA for OMP folding and insertion into the OM (25–29). Coordination of BamA and BamD activities is essential for the OMP assembly and is mediated by their direct interaction at the Potra 5 interface, for which the salt bridge between BamA E373 and BamD R197 is critically important (29, 30). Previously, we reported that the loss of the nonessential Bam component BamE results in a significant decrease in RcsF/OMP assembly (SI Appendix, Fig. S1) (19). The ΔbamE strain and an assembly-defective rcsFA55Y mutant strain (SI Appendix, Table S1) are both significantly deficient in the detection of PMB-induced LPS stress, providing the first evidence to support an active role for RcsF/OMP in signaling under conditions of LPS stress (19). In the absence of BamE, BamA binds RcsF but is unable to engage with BamD to complete RcsF/OMP assembly (31) (SI Appendix, Fig. S1). As a result, RcsF accumulates on BamA, preventing it from functioning in OMP assembly (31, 32). This RcsF-dependent “jamming” of BamA is the reason for the synthetic lethal interaction of ΔbamE and various bam mutants, including a bamB null (SI Appendix, Table S1) (31, 32).
We exploited the lethal interaction between BamA and RcsF in the bamE bamB double mutant to select for mutations that disrupt this RcsF/BamA interaction. Our characterization of the effects of the rcsF and bamA suppressor mutations identified on Rcs signaling and RcsF/OMP assembly demonstrates that assembly of the RcsF/OMP complex is required for Rcs signaling and argues against the model that proposes that RcsF monitors BamA activity. Moreover, our data suggest that the recently published RcsF/BamA structure corresponds to the "jammed" RcsF-BamA complex and not an assembly intermediate, as suggested (33).
Results
BamA Interacts with the RcsF CTD.
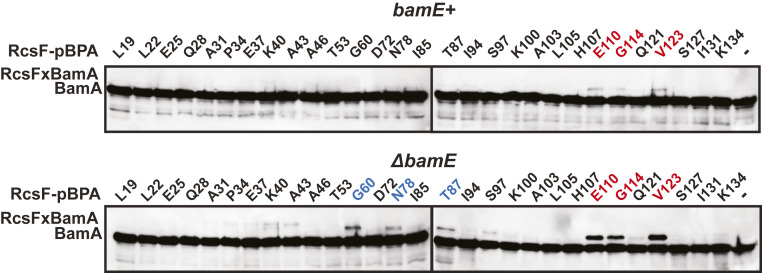
RcsF consists of two structurally distinct domains: the lipidated and largely unstructured NTD (residues 16 to 62), and the folded CTD (residues 63 to 134) (34, 35). The distal end of the NTD (residues 53 to 62) is predicted to span the lumen of partner OMPs (23). To examine the RcsF/BamA interaction interface in bamE+ and ΔbamE backgrounds, we used site-specific cross-linking based on incorporation of photoactivatable p-Benzoylphenylalanine (pBPA) using amber suppression (36). We previously reported that RcsF/BamA cross-linking sites were limited to the distal tip of the RcsF CTD (11). We rebuilt the rcsF amber mutant library into the low-copy number vector pZS21 to avoid overexpression and probed the RcsF/BamA interaction in the wild-type (WT) and ΔbamE strains (Fig. 2). RcsF/BamA cross-linking was prominent at E110, G114, and V123 in both the ΔbamE and WT strain but with increased cross-linking efficiency in the ΔbamE strain. We also detected several additional cross-links at G60, N78, and T87, though it was unclear whether these cross-links are indicative of a distinct RcsF/BamA conformation or are simply detected because of increased RcsF/BamA cross-linking in ΔbamE strain.
Fig. 2.
A pattern of RcsF-pBPA site-specific cross-linking to BamA in bamE+ or ΔbamE backgrounds. Cells expressing RcsFpBPA variants at the indicated codons or an RcsFWT (no pBPA negative control indicated by “−“) were subjected to UV cross-linking, and cell lysates were analyzed by immunoblotting with an α-BamA antibody. Size shifts indicate RcsF/BamA complexes. RcsF sites of BamA cross-link in both bamE+ or ΔbamE backgrounds are highlighted in red. RcsF sites of BamA cross-link specific to ΔbamE are highlighted in blue.
rcsF Suppressors Map to the CTD.
The inhibition of BamA by RcsF is the reason for the bamE bamB synthetic lethality (31, 32). We reasoned that mutations that disrupt the RcsF/BamA interaction in this background would, therefore, suppress this phenotype and restore growth (SI Appendix, Fig. S1). We developed a population-based approach for rcsF mutant selection by combining a codon-saturated rcsF library with next-generation sequencing (NGS; SI Appendix, Fig. S2). When generating the rcsF mutant library, we focused on amino acids 50 to 134, which cover all observed BamA cross-linking sites (Fig. 2), the OMP lumen spanning domain (23), and a residue important for RcsF/OMP assembly (A55) (19). Within this region, we excluded four Cys residues that participate in disulfide bond formation during RcsF folding (34, 35) and several residues with buried sidechains that are more likely to participate in intramolecular folding than in intermolecular interactions. We generated saturated single-codon rcsF libraries using NNK (where N = A/C/G/T, K = G/T) degenerate mutagenesis (SI Appendix, Fig. S2) and transformed our codon-based mutant libraries into strain AK-1261. This strain is a ΔrcsF derivative that contains bamE under the control of an arabinose-inducible promoter and a nonpolar null mutation of bamB, bamB8 (37). In the absence of arabinose, expression of rcsF from the pZS21::rcsF (p-rcsF) construct was toxic to this strain (SI Appendix, Fig. S2). We subjected rcsF mutant pools to growth selection with low starting inoculum (105 cells/mL) to enable outgrowth of approximately 11 generations. This outgrowth depleted BamE and reliably selected for rcsF suppressor mutants. We isolated the plasmids and sequenced the mutations, selecting for further analysis those resulting in an amino acid change detected in three independent replicates. In the absence of selection (+ arabinose), we detected 1,092 amino acid variants, corresponding to 81.25% saturation of our mutant library (Dataset S1). We compared the relative abundance of amino acid variants during selection (− arabinose) with a + arabinose control, plotting fold change (FC) of the variants either per residue or across all residues (Fig. 3 A, Left and Right). Overall, the mutant library had a wide distribution of 8.6 log2[FC] (Fig. 3A). As expected, synonymous mutants were depleted, while nonsense mutants were enriched across all but the two last codons (Fig. 3 A, Left). With a cutoff of 1 log2[FC], we identified 110 missense mutants targeting 20 residues. All residues were located at the distal tips of the CTD in the region identified by site-specific cross-linking.
Fig. 3.
RcsF mutant library performance. (Left) Log2[FC] of individual RcsF variants plotted against the RcsF residue number. Nonsense variants (green squares), synonymous variants (red circles), and RcsFSUP variants characterized in detail (blue diamonds) are highlighted. (Right) Violin plot of log2[FC] distribution within RcsF variant groups. The solid horizontal line represents the median. Statistical analysis was performed using one-way ANOVA (multiple comparison). (A) A total of 11 generations of outgrowth in an rcsB+ background; (B) A total of 18 generations of outgrowth in an rcsB+ background; (C) A total of 18 generations of outgrowth in a ΔrcsB background. The complete data for all detected RcsF variants are presented in Dataset S1. n.s. = P ≥ 0.05, ***P < 0.001, ****P < 0.0001.
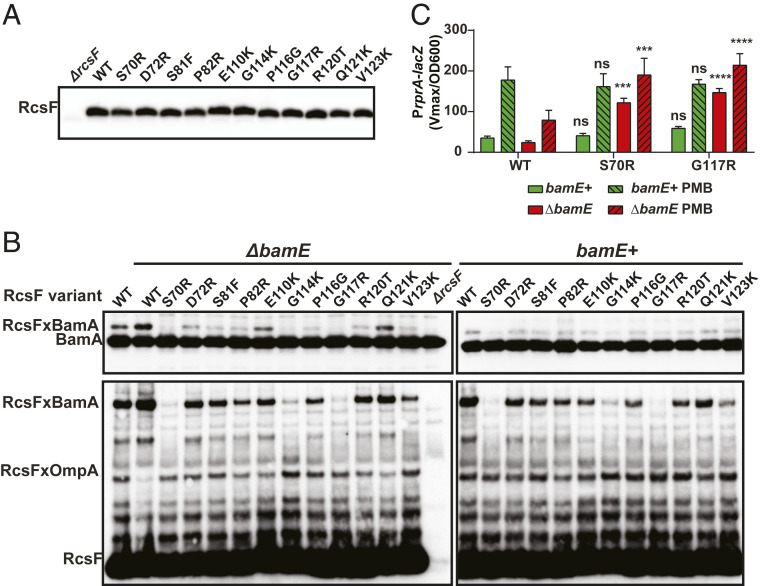
Eleven representative mutants, collectively referred to as rcsFSUP, were characterized (Fig. 3A, blue diamonds). Interestingly, rcsFSUP mutants performed slightly better than nonsense mutants, with several rcsFSUP mutants outperforming nonsense variants at the same codon (SI Appendix, Fig. S3). This observation prompted us to repeat selection with a longer outgrowth of 18 generations. With prolonged selection, mutants at these codons outcompeted all other variants, including nonsense mutations (Fig. 3B and SI Appendix, Fig. S3). We considered two potential explanations for this phenomenon: truncated RcsF protein retained some ability to bind and inhibit BamA, or rcsFSUP conferred a gain-of-function phenotype. Consistent with the latter, rcsFSUP mutant strains constructed de novo grew more rapidly in monocultures than an empty vector control (SI Appendix, Fig. S4).
We next introduced these alleles at the native chromosomal locus to examine the mechanism of suppression (Fig. 4A). All mutants displayed reduced RcsF/BamA cross-linking in both ΔbamE and bamE+ backgrounds to varying degrees, while the functional RcsF/BamA interaction and the assembly of RcsF/OmpA complexes were restored in the absence of bamE (Fig. 4B and SI Appendix, Fig. S5). As our previous data suggested that the inability of ΔbamE mutants to respond to PMB was caused by the lack of RcsF/OMP assembly (19), we next tested whether rcsFSUP had physiological consequences for signaling. All rcsFSUP mutants functioned as WT in a bamE+ background; all responded to PMB and did not display constitutive activation of Rcs (Fig. 4C and SI Appendix, Fig. S6, green bars). When introduced into a ΔbamE background, these rcsFSUP mutants not only restored the ability to respond to PMB but also resulted in highly active Rcs during normal growth (Fig. 4C and SI Appendix, Fig. S6, red bars). This observation suggested that the competitive advantages for rcsFSUP in a bamB8 bamE–depletion background were due to the activation of Rcs, allowing cells to combat OM stress. Indeed, when Rcs signaling was inactivated by the introduction of a ΔrcsB mutation, strains lost this growth advantage both in monocultures and by population-based selection (Fig. 3C and SI Appendix, Fig. S4). Importantly, all rcsFSUP alleles rescued growth in the absence of arabinose in a ΔrcsB background (Fig. 3C and SI Appendix, Figs. S3 and S4). rcsFSUP alleles were recessive, as RcsFWT could still bind to and inhibit BamA (SI Appendix, Fig. S7 A and B). In the rcsFWT/rcsFSUP merodiploid strains, no RcsF/OMP were formed in a ΔbamE background, and RcsFSUP did not increase Rcs signaling (SI Appendix, Fig. S7C). We concluded that RcsFSUP mutants restored growth by alleviating BamA jamming in the absence of BamE but gained a growth advantage due to restored RcsF/OMP assembly and signaling.
Fig. 4.
Phenotype of RcsFSUP variants in different genetic backgrounds. (A) Chromosomal rcsFSUP mutants express RcsF at a WT level. (B) Immunoblot analysis of in vivo formaldehyde cross-linked samples probed with α-BamA (Top) or α-RcsF antibodies (Bottom). Immunoblot quantification can be found in SI Appendix, Fig. S5. (C) Rcs activity as measured by a β-galactosidase assay using a PrprA-lacZ transcriptional reporter. Strains grown in LB and treated with 0.75 μg/mL PMB, where indicated, for 40 min. Graphs represent mean β-galactosidase activity normalized to OD600 ± SEM. Statistical analysis was performed, using the two-way ANOVA (multiple comparison), between RcsFSUP variants and a WT RcsF control under the same conditions. n.s. = P ≥ 0.05, ***P < 0.001, ****P < 0.0001.
bamA Suppressors Map to the β-Barrel Domain.
Mutations in the bamA β-barrel domain suppressing bamE bamB synthetic lethality have been reported (38). Several of these mutations have been characterized, including BamAF494L (a loop [L] 3 variant) and BamAG655A (an L6 variant) (25, 31, 39). The introduction of BamAF494L and BamAG655A into either a WT or ΔbamE strain had no effect on RcsF cross-linking or signaling (SI Appendix, Fig. S8) (31). Therefore, we sought to perform more extensive screening using a bamA plasmid library (40) in a bamA diploid background, as suppressors were expected to be dominant (SI Appendix, Fig. S9). We anticipated two different classes of bamA* mutants: class 1 mutants would prevent RcsF binding to BamA*, allowing BamA* to function together with the rest of the Bam complex in OMP assembly, and class 2 mutants would bypass the requirement for BamE and reduce levels of stalled RcsF/BamA* complex by promoting RcsF/OMP assembly (SI Appendix, Fig. S9).
We isolated 23 unique bamA alleles with missense mutations within the BamA β-barrel domain (SI Appendix, Fig. S10A). We confirmed and characterized five single-codon mutations in further detail (SI Appendix, Fig. S10B): four in extracellular loops (T434I in L1, A499S and L501R in L3, and A654T in L6) and one in a lumen-facing residue of a β14-strand (S715R). All mutations, referred to as BamASUP, are in the same region as previously reported bamE bamB suppressor mutations (SI Appendix, Fig. S10C) (38). These mutants, together with the well-studied bamAF494L mutant, were transformed into a bamA-depletion background with or without bamE. We observed that only one variant, BamAS715R, resulted in decreased RcsF/BamA cross-linking (Fig. 5A and SI Appendix, Fig. S11). Like rcsFSUP mutants, bamAS715R led to the restoration of RcsF/OmpA cross-linking and Rcs activation in a ΔbamE background (class 2 mutant) (Fig. 5B and SI Appendix, Fig. S11). The other variants behaved like bamAF494L, with no significant changes in the pattern of cross-linking in either a bamE+ or ΔbamE background (Fig. 5A and SI Appendix, Fig. S11). We hypothesized that these substitutions destabilized, but did not abolish, the RcsF/BamA interaction, a phenotype that could be masked by cross-linking, which traps even unstable interactions. Therefore, we examined the RcsF/BamA interaction directly by in vivo pull-down using N-terminally Twin-Strep-tagged BamA variants (Fig. 5C). In a bamE+ background, the RcsF was detected only weakly, while RcsF copurified with Strep-BamA in a ΔbamE background (Fig. 5C). This interaction was reduced in all BamASUP variants and was indistinguishable from those in BamAS715R and RcsFSUP variants (Fig. 5C), demonstrating that BamASUP loop mutants and BamAF494L destabilize the RcsF/BamA complex (class 1 mutants).
Fig. 5.
Phenotype of BamASUP variants in different genetic backgrounds. (A) Immunoblot analysis of in vivo formaldehyde cross-linked samples probed with α-BamA (Top) and α-RcsF antibodies. Immunoblot quantification can be found in SI Appendix, Fig. S11. (B) Rcs activity measured as described in Fig. 4. Statistical analysis was performed using two-way ANOVA (multiple comparison) between the BamAS715R variant and a WT BamA control under the same conditions. (C) Strep-Tactin purification of RcsF/BamASUP and RcsFSUP/BamA complexes in the absence of cross-linking. Immunoblots were probed with α-BamA and α-RcsF antibodies. n.s. = P ≥ 0.05, **P < 0.01, ***P < 0.001.
RcsFSUP and BamASUP Disrupt the RcsF Interaction with the BamA β-Barrel Domain Directly.
The location of the rcsFSUP and bamASUP mutations suggests an interaction between the RcsF CTD and BamA β-barrel domain. Because the BamA β-barrel and the periplasmic POTRA domains regulate each other, β-barrel mutations are frequently isolated as suppressors of periplasmic defects (38, 41–43). Therefore, we sought to examine whether RcsF directly interacts with the BamA β-barrel. We purified full-length RcsF-Strep without lipid modification and the detergent-refolded His-tagged full-length BamA (His-BamAFL) and BamAβ-barrel domain (His-BamAβ) using established protocols (27, 34, 44). When incubated at an equimolar ratio, RcsF-Strep coeluted with both His-BamAFL and His-BamAβ, while the RcsFSUP variants RcsFS70R and RcsFG117R failed to interact with either His-BamAFL or His-BamAβ (Fig. 6 A and B). We next introduced four bamASUP mutations into His-BamAβ: T434I, F494L, A654T, and S715R. With the exception of BamAT434I, all mutations disrupted RcsF binding (Fig. 6C). These data suggest a direct interaction between RcsF and the BamA β-barrel.
Fig. 6.
In vitro pull-down of variant RcsF/BamA complexes. Purified RcsF-Strep and His-BamA, and their variants, were incubated in equimolar amounts before being subjected to Ni-NTA purification. Equal volume of the first elution fractions was resolved on an SDS-PAGE gel and visualized using Imperial Protein Stain. All other fractions are shown in SI Appendix, Fig. S12. (A) Interactions between RcsF variants and full-length BamA (BamAFL). (B) Interactions between RcsF variants and the BamA β-barrel domain (BamAβ). (C) Interactions between BamAβ variants and RcsFWT.
BamA Overexpression Phenocopies ΔbamE.
An RcsF/BamA crystal structure was recently solved, showing that the RcsF CTD plugs the lumen of the BamA β-barrel domain (33) (Fig. 7A). This structure was obtained by BamA overexpression. Previously, we have shown that the BamA:BamD ratio was important for the RcsF/OMP assembly and that the bamDL13P strain with a reduced BamD relative to BamA phenocopies the ΔbamE mutant (31). We reasoned that increasing levels of BamA relative to BamD may also result in the same phenotype as observed in the ΔbamE strain. We introduced a plasmid expressing bamA into a bamA+ background to induce moderate overexpression of BamA (Fig. 7B, referred to as a BamA+++ background). BamA+++ led to an increase in RcsF/BamA cross-linking and the titration of RcsF away from its assembly pathway (Fig. 7B and SI Appendix, Fig. S13). An E373K mutation in BamA disrupts a critical salt bridge essential for BamA/BamD binding, an interaction essential for BamA function and cell viability (SI Appendix, Table S1) (30). BamAE373K bound RcsF with WT efficiency (Fig. 7B and SI Appendix, Fig. S13), demonstrating that BamA binds RcsF independently of BamD. The Rcs was not induced by PMB in the BamA+++ strain, consistent with a lack of RcsF/OMP complexes (Fig. 7C). We next examined whether rcsFSUP mutants could suppress the BamA+++ phenotype. In the presence of RcsFS70R and RcsFG117R, we observed a significant reduction in RcsF/BamA cross-linking, a restoration of RcsF/OMP assembly and PMB-induced signaling in the BamA+++ background (Fig. 7 C and D and SI Appendix, Fig. S13). Because our RcsFSUP and BamASUP residues fit perfectly with the RcsF/BamA structure (Fig. 7A) and RcsFSUP also suppressed BamA overexpression phenotype, we concluded that the structure represents a RcsF/BamA complex as observed in the ΔbamE strain.
Fig. 7.
Phenotypes of RcsF and BamA variants in a BamA+++ background. (A) The structure of the RcsF/BamA complex (Protein Data Bank 6T1W); RcsF is shown in orange, and BamA is shown in green (33). The BamA Potra domains are omitted for clarity. RcsFSUP residues are shown as yellow spheres. BamASUP residues described previously (38) and in this study are shown as purple and magenta spheres, respectively (B and D). Immunoblot analysis of in vivo formaldehyde cross-linked samples probed with α-BamA (Top) and α-RcsF antibodies. Immunoblot quantification is shown in SI Appendix, Fig. S13. (C) Rcs activity measured as described in Fig. 4. Statistical analysis was performed, using two-way ANOVA (multiple comparison), in comparison with the untreated WT with EV. n.s. = P ≥ 0.05, **P < 0.01, ****P < 0.0001.
The observation that BamA overexpression inhibits RcsF/OMP assembly despite the presence of the other Bam components suggested that this RcsF/BamA complex is a dead-end product rather than an assembly intermediate. To test this hypothesis directly, we used the AK-1478 strain, in which rcsF and bamE expression is under the tight control of tetracycline- and arabinose-inducible promoters. We first grew this strain in the absence of BamE, allowing RcsF to accumulate on BamA (Fig. 8, lane 1). Cells were washed to remove tetracycline and grown in several cultures with different combinations of inducers for 30 min (Fig. 8). We observed that BamE could not promote RcsF/OMP assembly from the existing RcsF/BamA complex (Fig. 8, lane 2) and that the restoration RcsF/OMP cross-linking required de novo RcsF synthesis (Fig. 8, lane 3). We concluded that the RcsF/BamA complex that forms in the absence of BamE is not an assembly intermediate but an off-pathway, dead-end product.
Fig. 8.
The RcsF/BamA complex that forms in the absence of BamE is a dead-end product. The AK-1478 strain was grown until midlog in the presence of tetracycline to allow expression of RcsF, and the sample was taken (time point 0). Remaining cells were washed and resuspended in fresh media supplemented with tetracycline and arabinose to promote RcsF and BamE expression, as indicated, and grown for 30 min. Immunoblot analysis of total cell pellet (Top) and in vivo formaldehyde cross-linked samples (Middle) probed with α-BamE and α-RcsF antibodies. Quantification of RcsFxOmpA band (Bottom) as a percentage of total RcsF based on the three biological replicates. Statistical analysis was performed using one-way ANOVA in comparison with the time point 0. ***P < 0.0001.
RcsF Monitors OM/LPS Integrity and Not Bam Complex Function.
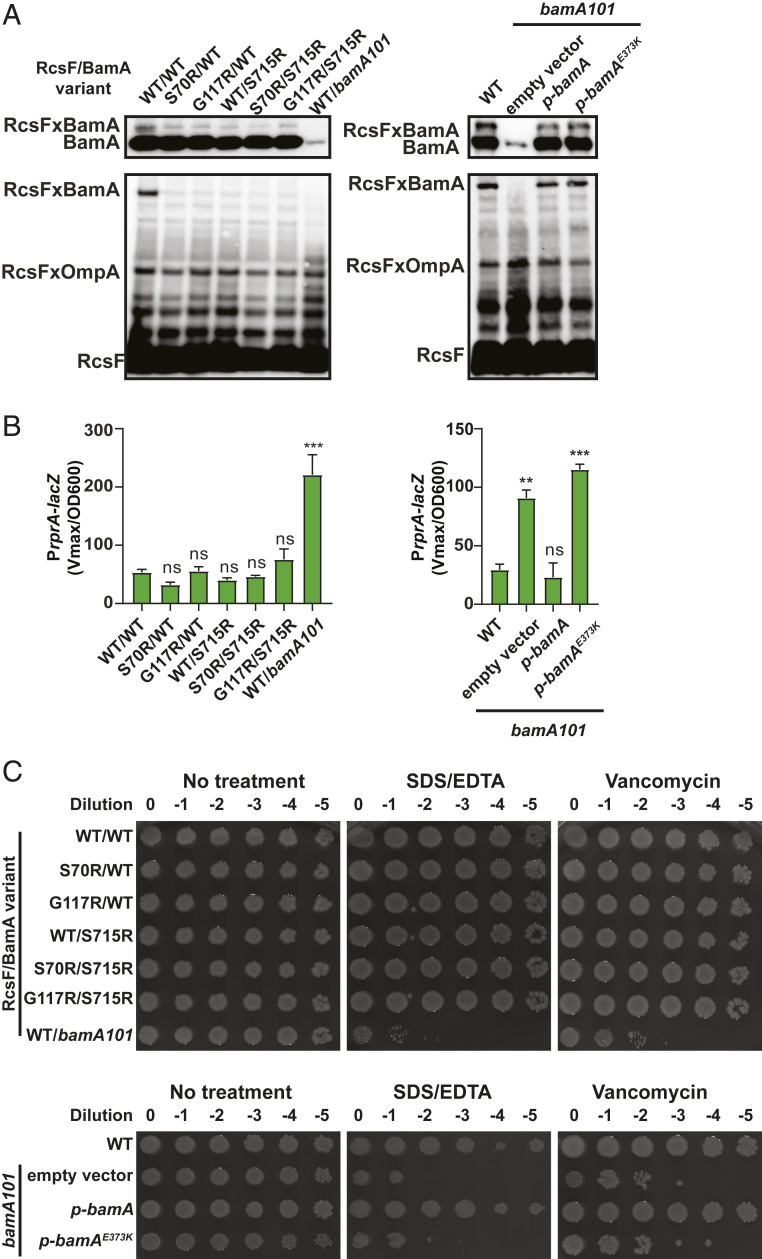
It has previously been suggested that RcsF plays a role in the monitoring of BamA function, failing to bind nonfunctional BamA and leading to an accumulation of RcsF in a periplasmic orientation, stimulating signaling (12) (Fig. 1C). This model is based largely on the phenotype conferred by the bamA101 promoter mutation, which reduces BamA levels about 10-fold (45, 46). RcsF/BamA cross-linking is undetectable in bamA101 cells, which correlated with strong induction of Rcs, as described previously (12, 31) (Fig. 9 A and B). However, bamA101 also affects OM integrity, and bamA101 is extremely sensitive to toxic agents that are selectively excluded by the OM, such as detergent and vancomycin (Fig. 9C). Therefore, the true cause of RcsF induction was unclear.
Fig. 9.
Disruption of the RcsF/BamA complex does not induce Rcs signaling. (A) Immunoblot analysis of in vivo formaldehyde cross-linked samples probed with α-BamA (Top) and α-RcsF antibodies. Immunoblot quantification is shown in SI Appendix, Fig. S14. (B) Rcs activity measured as described in Fig. 4. Statistical analysis was performed, using two-way ANOVA (multiple comparison), in comparison with a WT control. (C) RcsF and BamA variant sensitivity to detergent or vancomycin. Overnight cultures were serially diluted and plated on LB agar supplemented with 0.5% SDS/0.5 mM EDTA or 75 µg/mL vancomycin. n.s. = P ≥ 0.05, ***P < 0.001.
Our screen yielded several rcsF and bamA mutants in which the RcsF/BamA complex was reduced to undetectable levels; however, these mutations alone or in combination did not induce Rcs in an otherwise WT background, demonstrating that a reduction in RcsF/BamA complex alone is insufficient to induce Rcs (Fig. 9 A and B, Left, and SI Appendix, Fig. S14). Unlike bamA101, these mutants are resistant to detergent and antibiotics (Fig. 9C), suggesting that Rcs induction in bamA101 is due to the sensing of OM defects. To test this hypothesis directly, we performed bamA101 complementation experiments with WT BamA and the nonfunctional BamAE373K variant (Fig. 9 A–C and SI Appendix, Fig. S14). While BamAE373K was able to form a complex with RcsF (Fig. 9 A, Right, and SI Appendix, Fig. S14) like the BamAWT, it was unable to restore OM integrity (Fig. 9C). Interestingly, Rcs signaling remained activated in this mutant (Fig. 9 B, Right). We concluded that Rcs activation in bamA101 is due to a loss of OM integrity rather than a loss of RcsF/BamA complex formation.
bamDL13P and bamA101 are analogous mutations that lower the number of functional Bam complexes by decreasing expression of essential BamD and BamA (SI Appendix, Table S1) (46). The bamDL13P and bamA101 mutations are nonadditive and confer similar OMP assembly and OM defects. Unlike bamA101, bamDL13P fails to assemble RcsF/OMP complexes (31, 46). Interestingly, bamDL13P did not activate Rcs (Fig. 10A), suggesting that Rcs sensing of OM integrity requires the RcsF/OMP complex.
Fig. 10.
Rcs activation upon Bam complex inhibition. (A and B) Rcs activity of strains grown in LB or LB supplemented with 10 mM MgSO4 or CaCl2 were measured as described in Fig. 4. Statistical analysis was performed using one-way ANOVA and indicated as follows: P > 0.05 (ns), **P < 0.01. (C) Activity of fluorescent Rcs (PrprA-GFP) and σE (PmicA-GFP) reporters expressed as relative fluorescence units (RFU) normalized to OD600. Strains were grown in LB or LB supplemented with 10 mM MgSO4 or CaCl2, as indicated. After 90 min, cells were treated with 32 μg/mL MRL-494, 1 μg/mL darobactin, or a vehicle control (DMSO or H2O, respectively) for 3 h. Statistical analysis was performed, using one-way ANOVA, in comparison with the vehicle control. n.s. = P ≥ 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
OM mechanical stability, asymmetric organization, and permeability barrier function requires tight cation-mediated packing of LPS in the outer leaflet (2, 47, 48). Disruption of LPS packing by cationic antimicrobial peptides such as PMB, or by genetic alteration in LPS structure, is a strong Rcs-inducing signal (19, 20, 49–52). Addition of divalent cations (such as Mg2+ and Ca2+) to otherwise cation-poor Lysogeny Broth (LB) medium promotes growth in many OM-defective mutants and reduces Rcs signaling upon LPS stress (19, 53, 54). To examine whether activation of Rcs following Bam complex inhibition is related to the sensing of OM/LPS stress, we monitored Rsc activity in the presence of MgSO4 and CaCl2 (Fig. 10 A and B). While both Mg2+ and Ca2+ bind abundantly to LPS in vivo (55), cellular concentration of Ca2+ is very low (56), and it does not play an important role in regulating enzymatic functions. Interestingly, the addition of either 10 mM MgSO4 or 10 mM CaCl2 to the growth media led to a reduction in Rcs activity in ΔbamE strains (Fig. 10B and SI Appendix, Fig. S6) independent of the PhoPQ two-component system (SI Appendix, Fig. S15), suggesting that LPS/OM defects lead to the activation of Rcs in this background.
Finally, we wanted to examine whether RcsF sensing of LPS/OM defects could more broadly explain the Rcs activation seen in response to Bam complex inhibition. Darobactin and MRL-494 are chemical inhibitors of BamA (40, 57). Treatment with these compounds activates the σE stress response, indicative of an accumulation of unfolded OMPs in the periplasm (Fig. 10C) (40, 57). Similarly, these compounds activated Rcs (Fig. 10C). While the addition of cations had little effect on the activation of σE, Rcs activity was significantly reduced (Fig. 10C). To ensure that this phenotype is specific to BamA, not a result of any unappreciated off-targets, we also performed BamA-depletion experiments, in which Rcs, but not σE, showed Mg2+/Ca2+ dependency (SI Appendix, Fig. S16). Importantly, the addition of divalent cations did not broadly inhibit Rcs, as the Rcs response to other antibiotics, such as globomycin, was unaffected by the presence of Mg2+ or Ca2+ (SI Appendix, Fig. S17A). Finally, while PMB activated Rcs, it had no effect on σE signaling, suggesting, as reported previously, that PMB does not inhibit the Bam complex (19) (SI Appendix, Fig. S17B). Together, these data suggest that Rcs activation in response to the Bam complex inhibition is a result of secondary OM/LPS defects.
Discussion
OM biogenesis is complex, and while each component has its own biogenesis pathway, these pathways are interdependent (58). For example, the localization of lipoproteins (Lol) pathway supplies lipoprotein components to the Bam complex and the LPS translocon. Likewise, the Bam complex assembles the LPS translocon and other OMPs required for LPS homeostasis in the OM (59, 60). As a result, perturbation of one pathway can indirectly affect others, leading to pleiotropic phenotypes. Thus, the study of stress responses that monitor envelope homeostasis is fundamentally challenging, and the molecular mechanisms responsible for the activation of these pathways remain elusive. This complexity is particularly evident in the Rcs stress response (3). Rcs monitors LPS and cell wall integrity and is influenced by both the Lol and Bam pathways, which play a role in the localization of the Rsc OM sensory component RcsF. While some mutations in the Bam complex have been shown to induce Rcs, the mechanism of activation was poorly defined. Here, we have isolated a collection of rcsF and bamA mutations that suppress RcsF-dependent “jamming” of BamA, and their characterization has clarified the relationship between Bam complex–mediated RcsF/OMP assembly and Rcs signaling.
BamE Prevents Unproductive Binding of RcsF to BamA.
While BamA alone is responsible for catalyzing OMP folding and insertion into the OM, presentation of proper substrates, overall efficiency and the assembly of certain complex substrates require the Bam lipoproteins (42, 43). BamD plays an essential regulatory function, activating BamA-dependent OMP assembly upon substrate binding (25–29). A direct interaction between BamA and BamD coordinates their activity (29). While BamE is not required for BamA-BamD coordination during normal OMP assembly, this coordination is critical for RcsF/OMP assembly, likely because RcsF binding alters BamA conformation in a manner that prevents BamD engagement (31). In cells lacking BamE, RcsF does not disengage from the RscF/BamA complex and effectively “jams” BamA, preventing its function. This explains the synthetic lethal interaction between ΔbamE and various other bam mutants. In these double mutants, the increased stress on the BAM complex caused by the additional bam mutation results in a lethal defect in global OMP assembly (31, 32).
We isolated a collection of rcsFSUP and bamASUP mutants that suppress all ΔbamE phenotypes. All of these mutations disrupted the stable RcsF/BamA complex. None of these mutants disrupted efficient RcsF/OMP assembly; moreover, they allow efficient assembly regardless of whether BamE is present or not. These results demonstrate that this type of RcsF/BamA interaction is not only not required but also has to be prevented for productive RcsF/OMP assembly. Using experiments-inducible promoters, we showed that BamE cannot act on this stable RcsF/BamA complex to promote RcsF/OMP assembly. Based on our data, we conclude that this RcsF/BamA complex is not an assembly intermediate but rather a dead-end complex that forms in the absence of BamE and compromises the BamA function.
RcsF/BamA Structure Corresponds to the “RcsF-Jammed” BamA.
A recent RcsF/BamA complex crystal structure, in which the CTD of RcsF plugs the BamA lumen, was described by the authors as an RcsF assembly intermediate (33). This structure was purified from cells overexpressing BamA. In this strain, BamA:BamD stoichiometry, and thus BamA-BamD coordination, is prevented as effectively as it is in a ΔbamE mutant. In this strain, the same dead-end RcsF/BamA complex that is observed in the bamE mutants accumulates. Importantly, in neither case is proper RcsF/OMP assembly observed. The rcsF suppressors of ΔbamE also suppressed the BamA overexpression phenotype, providing direct genetic evidence for an identical RcsF/BamA interaction in both backgrounds. Strikingly, the location of RcsFSUP and BamASUP residues is consistent with this structure. Together, these data suggest that the published crystal structure represents not an assembly intermediate but the “jammed” dead-end RcsF/BamA complex that is observed in the ΔbamE strain.
While this structure is not informative about the RcsF/OMP assembly pathway or the final RcsF topology, it does provide an insight into how RcsF may inhibit BamA activity. The BamA β-barrel can adopt various conformations (61–64), with only the inward–open conformation compatible with RcsF binding to the lumen (33). Once bound, RcsF likely stabilizes the BamA β-barrel in this conformation (33). When barrel conformation is similarly restricted by disulfide bonding, it renders BamA nonfunctional in vivo (65). In this conformation, RcsF also occludes the BamA β-barrel lumen and the lateral gate that are critical for folding and membrane insertion of incoming OMPs substrates (66–68). As disrupting this interaction restores BamA function, we suggest that in this complex, RcsF may block OMP access to the BamA lumen, resulting in a dead-end complex.
RcsF Is Not a Sensor of Bam Complex Activity.
A previous study suggested that RcsF monitors BamA activity during OMP assembly (12) (Fig. 1B). According to this model, in the absence of stress, BamA assembles RcsF in a nonfunctional complex with OMPs that titrate RcsF away from signaling to the downstream IM components. In the presence of stress, BamA function is inhibited, decreasing the RcsF/BamA interaction and allowing RcsF to accumulate in the inner leaflet of the OM in a periplasmic-facing orientation, promoting downstream signaling to the IM components (12). The data presented here provide several lines of evidence that challenge this model.
First, mutations that inhibit the RcsF/BamA interaction do not induce signaling. Several RcsFSUP variants, including S70R, G117R, and BamAS715R, reduce the RcsF/BamA interaction dramatically without activating Rcs. Second, RcsF interacts with nonfunctional BamA variants, such as BamAE373K, clearly demonstrating that RcsF cannot tell the difference between BamA that is, or is not, in a functional BAM complex. BamAE373K produced in trans restores RcsF/BamA cross-linking in the bamA101 strain without lowering Rcs signaling, demonstrating that the activation of RcsF signaling was not due to the reduction in the RscF/BamA interaction. Third, when BamA is overexpressed, cells accumulate only RcsF/BamA, and this inactivates the normal Rcs response to the LPS/OM defects caused by PMB, further demonstrating that this dead-end complex is inactive. Finally, while both bamA101 and bamDL13P mutations lead to a similar decrease in Bam complex activity (46), only bamA101, but not bamDL13P, cells induce Rcs, suggesting that a reduction in Bam complex activity is not a direct signal for Rcs. This seeming paradox is explained by the fact that bamA101 cells retain the ability to assemble RcsF/OMP complexes, while bamDL13P cells do not (31). Together, these results show that the levels of this nonfunctional, dead-end RcsF/BamA complex have no role in Rcs signaling. Rcs induction requires the assembly of the RcsF/OMP complex.
An Active Role for RcsF/OMP Complexes in Sensing OM/LPS Stress.
OMPs contribute substantially to the overall OM stability. Indeed, many play direct functions in LPS transport, modifications, and overall OM lipid homeostasis (59, 60). Accordingly, all bam mutants display well-documented secondary OM bilayer/LPS defects evident by sensitivities to detergent and antibiotics, which are typically occluded by the OM (37). It is well documented that direct disruption of LPS structure and/or packing induces Rcs signaling in all enteric bacteria (19, 20, 49–52). We show that Rcs activation upon BamA depletion, genetic or chemical inhibition was ameliorated by the stabilization of LPS crossbridges with Mg2+ or Ca2+. Since these cations do not correct defects in OMP assembly and do not lower activity of the σE stress response, we conclude that Rcs is activated by OM bilayer/LPS defects that are an indirect consequence of compromised Bam complex function.
In strains defective for RcsF/OMP assembly, including ΔbamE, BamA+++, rcsFA55Y, and bamDL13P, PMB-induced signaling is disrupted. BamAS715R and RcsFSUP variants restore RcsF/OMP assembly and signaling in ΔbamE strains. Moreover, this restoration of Rcs signaling is physiologically relevant, as rcsFSUP mutations improve the growth of the bamE bamB double mutant far better than rcsF null mutations. Together, these mutations provide strong supporting evidence demonstrating that the RcsF/OMP complex is not inactive but rather functions directly in signaling.
We have shown that the transmembrane orientation of RcsF in complex with OMP enables it to monitor the integrity of the OM outer leaflet. LPS stress is detected by the surface-exposed RcsF NTD, leading to a conformational change in the RcsF/OMP complex and signal transmission to the periplasmic CTD (Fig. 1A) (19, 23). The RcsF CTD activates the downstream signaling by interacting with the large periplasmic domain of IgaA, releasing the inhibition of phosphorelay (7, 11, 12).
One of the challenges in dissecting the Rcs activation mechanism is the lack of knowledge about the regions/residues of the RcsF CTD that are important for IgaA interaction. Indeed, no rcsF mutants that affect downstream signaling have been identified. A recent biochemical study showed that the tip of the RcsF CTD can interact with the OmpA CTD using cross-linking and NMR (69). The periplasmic IgaA domain competes with OmpA for RcsF in vitro, suggesting that the modulation of these RcsF/OmpA/IgaA interactions may regulate the induction of Rcs. However, our analysis yielded over 110 rcsF mutations targeting the RcsF CTD, including mutations in residues known to cross-link to OmpA in vivo (P116, Q121, and E110) (69). Interestingly, none of the mutations disrupted the RcsF/OmpA interaction or Rcs signaling, suggesting that the RcsF/OmpA interaction in this region is not functionally relevant for Rcs regulation. Specifically, none resulted in the constitutive “ON” phenotype expected from a loss of a proposed inhibitory RcsF/OmpA interaction, and none resulted in a constitutively “OFF” phenotype expected from a loss of the RcsF/IgaA interaction.
To fully understand how Rcs detects and transmits stress signals across the multilayered gram-negative envelope, we first need to understand RcsF interaction with its partners, including BamA, OMPs, and IgaA, using genetics and structural biology approaches. Only then we can dissect the contribution of individual components and distinguish direct from indirect responses and physiological signals from side-effects of RcsF mislocalization.
Materials and Methods
Growth condition and general strain construction are described in SI Appendix, Extended Materials and Methods. All strains and primers used in this study are listed in SI Appendix, Tables S2 and S3.
Construction of the rcsF Mutant Library.
All residues spanning RcsF amino acids 50 to 134 were mutated, with the exception of four cysteine residues and any residue considered to have a buried side chain by solvent accessibility (SA) analysis using GETAREA (70). An SA ration of <20% was considered to indicate a buried side chain (34, 35). The rcsF mutant library was constructed using high-throughput site-directed mutagenesis (SDM), using primers encoding NNK (where n = A/C/G/T, K = G/T) diversified codons and pZS21::rcsF as a template. Purified PCR product (100 ng) was subjected to in vitro phosphorylation/ligation using T4 Polynucleotide Kinase, T4 DNA ligase, and DpnI (New England Biolabs) and transformed into Escherichia coli Mach1 competent cells. Transformation resulted in between 300 and 2,000 colonies per codon, sufficient to achieve full saturation based on NNK diversification, with 0.99 probability (71).
rcsF Suppressor Screen and Data Analysis.
Plasmids isolated from the individual codon libraries were independently transformed into host strains (AK-1261 and AK-1377), and colonies were recovered on selective LB Kan with 0.4% arabinose plates. Resulting individual codon libraries were combined into two pools: pool 1 contained residues 50 to 82, and pool 2 contained residues 84 to 134. For the selection, 11 mL LB Kan Tet media with or without arabinose was inoculated with pooled libraries at 105 cells/mL (for 11 generations selection) or 103 cell/mL (for 18 generations selection). Cultures of 10 mL were grown in flasks at 37 °C with orbital shaking; 1 mL of the cultures was taken to monitor growth alongside the WT and empty vector (EV) controls in the BioTek Synergy H1 plate reader. When the optical density at a wavelength of 600 nm (OD600) of EV control and pooled cultures reached saturation (11 to 13 h depending on the inoculum size), cells were collected, and plasmids were isolated. rcsF was PCR amplified using plasmid-specific primers (AK-387 + AK-842), and amplicons containing partial NGS-adapters were generated with pool-specific primers (AK-852 + AK-853 for pool 1 and AK-851 + AK-850 for pool 2). Purified PCR product (500 ng) was submitted for the Illumina-based Genewiz Amplicon-EZ service. Genewiz performed sequencing and initial variant detection analysis. For downstream analysis, we only considered variants with one to three single-nucleotide changes within one codon with at least three independent reads. Using a custom Python script (https://github.com/annaklab/variant_detector), we converted nucleotide variants into amino acid variants by combining nonidentical nucleotide reads encoding the same amino acid variants and keeping track of synonymous variants. log2[FC] was calculated by comparing the relative abundance of each variant grown in the absence of arabinose compared with an arabinose-containing control culture. If a variant was not detected postselection, we used the relative abundance corresponding to two reads (below the threshold) to facilitate the log2[FC] calculation. The full analysis is presented in Dataset S1. Only variants detected in three replicates were considered for further analysis. GraphPad Prism 9.0 was used for statistical analysis.
bamA Suppressor Screen.
A previously published, error-prone PCR-mutagenized bamA mutant library (40) was transformed into AK-1255 cells, and colonies recovered on LB Kan agar with 0.4% arabinose overnight at 37 °C. Colonies were pooled and grown overnight in LB Kan with 0.4% arabinose. Cultures were diluted to 105 cells/mL in LB Kan Tet and grown for 11 h at 37 °C. A total of 15 µL of the cultures was plated onto LB Kan Tet agar, and plasmids from two colonies were isolated and retransformed into AK-1255 to confirm a plasmid-linked suppression phenotype, after which bamA was sequenced. The alleles of interest were rebuilt by SDM before phenotypic characterization.
In Vivo Pull-Down Assay with bamA Variants.
The tetracycline-inducible pZS21-Twin-Strep-tag-bamA and its mutant derivates were transformed into MT-382 and MT-404, derivates of the bamA-depletion strain JCM320 (72). These strains express TetR repressors from the Tn10 transposon, allowing precise control of BamA expression by aTc. Strains harboring plasmids were grown at 37 °C in LB Kan with 3 ng/mL aTc (Alfa Aesar). Cells from midlog cultures were harvested, washed twice with 1 mL phosphate-buffered saline (PBS; 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, pH 7.6) and resuspended to an OD600 of 10 in membrane solubilization buffer (20 mM Tris⋅HCl pH 8.0, 300 mM NaCl, 1% n-Dodecyl-β-d-maltoside) (DDM, Goldbio) containing 2 μg/mL lysozyme (Goldbio), protease inhibitor mixture (Thermo Fisher Scientific), and 8 U/mL benzonase (Millipore Sigma). After 60 min of incubation at room temperature (RT) with rotation, cellular debris was removed by centrifugation (15,000 × g, 15 min at RT). Soluble supernatant (200 µL, input) was then mixed with 50 µL 50% suspension of Strep-Tactin Sepharose beads (IBA Life Sciences) preequilibrated in membrane solubilization buffer. After incubation for 1 h at RT with rotation, beads were collected by centrifugation, washed 10 times with 200 µL membrane solubilization buffer, and proteins were eluted twice with 200 µL membrane solubilization buffer containing 5mM desthiobiotin (IBA Life Sciences). Fractions were analyzed by immunoblotting.
In Vivo Cross-Linking and Immunoblot Analysis.
For site-specific cross-linking, strains with pZS21::rcsFam-Strep variants and pSup (36) were grown in LB Kan Cam with 10 mM MgSO4 and 0.08 mM pBPA (Bachem) to midlog phase. Cells were concentrated in ice-cold PBS to an OD600 of 20. The cell suspension was subjected to ultraviolet (UV) irradiation at 365 nm for 15 min at 4 °C. An equal volume of 2× sodium dodecyl sulfate (SDS) sample loading buffer was added, and samples were analyzed by immunoblotting. In vivo formaldehyde cross-linking and immunoblot analysis were performed as described (31). Immunoblots were visualized and quantified using the ChemiDoc MP Imaging System (Bio-Rad). All figures are representative images from at least three independent biological replicates.
In Vitro Interaction Assay.
RcsF-Strep expression and purification were adapted from ref. 34. His-BamAFL–devoid signal sequence (residues 22 to 810) and His-BamAβ domain (residues 417 to 810) were purified from inclusion bodies and refolded as previously described (27, 73). Detailed protocols are described in SI Appendix, Extended Materials and Methods. The RcsF-Strep variant and His-BamAFL or His-BamAβ (5 μM each) were mixed in buffer C (25 mM Tris⋅HCl pH 8.0, 150 mM NaCl, 0.05% DDM [Goldbio]) in 300 μL total reaction volume and incubated for 1 h at RT. Ni-NTA His Bind Resin (60 μL, EMD Millipore), preequilibrated with buffer C beads, was added to each tube and collected in Pierce Centrifuge Columns (0.8 mL, Thermo Fisher Scientific). Beads were washed four times with 500 μL buffer C, and proteins were eluted three times with 100 μL buffer C with 250 mM imidazole. Each fraction (20 μL each) was analyzed on a 15% SDS-PAGE (SDS–polyacrylamide gel electrophoresis) and visualized with Imperial Protein Stain (Thermo Fisher Scientific).
Fluorescent Reporter Assays.
Strains containing promotor-less gfp (EV control) and PrprA-gfp and PmicA-gfp reporters were diluted in LB containing 10 mM MgSO4 and 10 mM CaCl2 where applicable, and 200 µL was grown in black 96-well plates with optical bottoms (Thermo Fisher Scientific) at 37 °C with orbital shaking in a BioTek Synergy H1 plate reader. After 1.5 h, cultures were treated with 32 μg/mL MRL-494, 1 μg/mL darobactin, or corresponding vehicle controls (dimethylsulfoxide [DMSO] or H2O, respectively). The OD600 and green fluorescence (excitation, 481 nm; emission, 507 nm) were measured. Nonspecific fluorescence from the EV control was subtracted, and specific GFP fluorescence of PrprA-gfp and PmicA-gfp reporters were normalized to OD600. Experiments were performed in four independent biological replicates. Mean GFP/OD600 SEM at the 3-h time point was plotted. GraphPad Prism 9.0 was used for statistical analysis.
Supplementary Material
Acknowledgments
We thank Dr. Scott S. Walker and Dr. Juliana C. Malinverni (Merck Sharp & Dohme Corp.) for providing MRL-494 and Prof. Kim Lewis (Northeastern University) for providing darobactin. We thank Makayla Wells for technical assistance with Fig. 2 and all colleagues who read the manuscript and provided constructive feedback. The research in the A.K. laboratory is supported by The National Institute of General Medical Sciences Grant 1R01GM133904-01, the Welch Foundation Research Grant AU-1998-20190330, and by the University of Texas System Rising STAR Award.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100369118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
Change History
December 17, 2021: The Abstract and Figures 5 and 6 have been updated.
References
- 1.Silhavy T. J., Kahne D., Walker S., The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikaido H., Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wall E., Majdalani N., Gottesman S., The complex Rcs regulatory cascade. Annu. Rev. Microbiol. 72, 111–139 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Brill J. A., Quinlan-Walshe C., Gottesman S., Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 170, 2599–2611 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout V., Gottesman S., RcsB and RcsC: A two-component regulator of capsule synthesis in Escherichia coli. J. Bacteriol. 172, 659–669 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M. H., et al., Characterization of the RcsC–>YojN–>RcsB phosphorelay signaling pathway involved in capsular synthesis in Escherichia coli. Biosci. Biotechnol. Biochem. 65, 2364–2367 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Wall E. A., Majdalani N., Gottesman S., IgaA negatively regulates the Rcs phosphorelay via contact with the RcsD phosphotransfer protein. PLoS Genet. 16, e1008610 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domínguez-Bernal G., et al., Repression of the RcsC-YojN-RcsB phosphorelay by the IgaA protein is a requisite for Salmonella virulence. Mol. Microbiol. 53, 1437–1449 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Gervais F. G., Drapeau G. R., Identification, cloning, and characterization of rcsF, a new regulator gene for exopolysaccharide synthesis that suppresses the division mutation ftsZ84 in Escherichia coli K-12. J. Bacteriol. 174, 8016–8022 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majdalani N., Heck M., Stout V., Gottesman S., Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 187, 6770–6778 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussein N. A., Cho S. H., Laloux G., Siam R., Collet J. F., Distinct domains of Escherichia coli IgaA connect envelope stress sensing and down-regulation of the Rcs phosphorelay across subcellular compartments. PLoS Genet. 14, e1007398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho S. H., et al., Detecting envelope stress by monitoring β-barrel assembly. Cell 159, 1652–1664 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Shiba Y., et al., Exploring the relationship between lipoprotein mislocalization and activation of the Rcs signal transduction system in Escherichia coli. Microbiology (Reading) 158, 1238–1248 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Shiba Y., et al., Activation of the Rcs signal transduction system is responsible for the thermosensitive growth defect of an Escherichia coli mutant lacking phosphatidylglycerol and cardiolipin. J. Bacteriol. 186, 6526–6535 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao K., Narita S., Tokuda H., Defective lipoprotein sorting induces lolA expression through the Rcs stress response phosphorelay system. J. Bacteriol. 194, 3643–3650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laubacher M. E., Ades S. E., The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 190, 2065–2074 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranjit D. K., Young K. D., The Rcs stress response and accessory envelope proteins are required for de novo generation of cell shape in Escherichia coli. J. Bacteriol. 195, 2452–2462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callewaert L., Vanoirbeek K. G., Lurquin I., Michiels C. W., Aertsen A., The Rcs two-component system regulates expression of lysozyme inhibitors and is induced by exposure to lysozyme. J. Bacteriol. 191, 1979–1981 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konovalova A., Mitchell A. M., Silhavy T. J., A lipoprotein/β-barrel complex monitors lipopolysaccharide integrity transducing information across the outer membrane. eLife 5, e15276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farris C., Sanowar S., Bader M. W., Pfuetzner R., Miller S. I., Antimicrobial peptides activate the Rcs regulon through the outer membrane lipoprotein RcsF. J. Bacteriol. 192, 4894–4903 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steenhuis M., Ten Hagen-Jongman C. M., van Ulsen P., Luirink J., Stress-Based High-Throughput Screening Assays to Identify Inhibitors of Cell Envelope Biogenesis. Antibiotics (Basel) 9, 808 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker C. T., et al., Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J. Bacteriol. 174, 2525–2538 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konovalova A., Perlman D. H., Cowles C. E., Silhavy T. J., Transmembrane domain of surface-exposed outer membrane lipoprotein RcsF is threaded through the lumen of β-barrel proteins. Proc. Natl. Acad. Sci. U.S.A. 111, E4350–E4358 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomasek D., Kahne D., The assembly of β-barrel outer membrane proteins. Curr. Opin. Microbiol. 60, 16–23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., et al., Substrate binding to BamD triggers a conformational change in BamA to control membrane insertion. Proc. Natl. Acad. Sci. U.S.A. 115, 2359–2364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagan C. L., Wzorek J. S., Kahne D., Inhibition of the β-barrel assembly machine by a peptide that binds BamD. Proc. Natl. Acad. Sci. U.S.A. 112, 2011–2016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagan C. L., Westwood D. B., Kahne D., bam lipoproteins assemble BamA in vitro. Biochemistry 52, 6108–6113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlova O., Peterson J. H., Ieva R., Bernstein H. D., Mechanistic link between β barrel assembly and the initiation of autotransporter secretion. Proc. Natl. Acad. Sci. U.S.A. 110, E938–E947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe A. L., Ricci D., Adetunji M., Silhavy T. J., Conformational changes that coordinate the activity of BamA and BamD allowing β-barrel assembly. J. Bacteriol. 199, e00373-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricci D. P., Hagan C. L., Kahne D., Silhavy T. J., Activation of the Escherichia coli β-barrel assembly machine (Bam) is required for essential components to interact properly with substrate. Proc. Natl. Acad. Sci. U.S.A. 109, 3487–3491 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tata M., Konovalova A., Improper coordination of BamA and BamD results in Bam complex jamming by a lipoprotein substrate. mBio 10, e00660-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart E. M., Gupta M., Wühr M., Silhavy T. J., The synthetic phenotype of ΔbamB ΔbamE double mutants results from a lethal jamming of the Bam complex by the lipoprotein RcsF. mBio 10, e00662-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez-Alonso R., et al., Structural insight into the formation of lipoprotein-β-barrel complexes. Nat. Chem. Biol. 16, 1019–1025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogov V. V., Rogova N. Y., Bernhard F., Löhr F., Dötsch V., A disulfide bridge network within the soluble periplasmic domain determines structure and function of the outer membrane protein RCSF. J. Biol. Chem. 286, 18775–18783 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leverrier P., et al., Crystal structure of the outer membrane protein RcsF, a new substrate for the periplasmic protein-disulfide isomerase DsbC. J. Biol. Chem. 286, 16734–16742 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin J. W., Martin A. B., King D. S., Wang L., Schultz P. G., Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 99, 11020–11024 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz N., Falcone B., Kahne D., Silhavy T. J., Chemical conditionality: A genetic strategy to probe organelle assembly. Cell 121, 307–317 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Tellez R. Jr, Misra R., Substitutions in the BamA β-barrel domain overcome the conditional lethal phenotype of a ΔbamB ΔbamE strain of Escherichia coli. J. Bacteriol. 194, 317–324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra R., Stikeleather R., Gabriele R., In vivo roles of BamA, BamB and BamD in the biogenesis of BamA, a core protein of the β-barrel assembly machine of Escherichia coli. J. Mol. Biol. 427, 1061–1074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart E. M., et al., A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier. Proc. Natl. Acad. Sci. U.S.A. 116, 21748–21757 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwyer R. S., Ricci D. P., Colwell L. J., Silhavy T. J., Wingreen N. S., Predicting functionally informative mutations in Escherichia coli BamA using evolutionary covariance analysis. Genetics 195, 443–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart E. M., Silhavy T. J., Functions of the BamBCDE lipoproteins revealed by bypass mutations in BamA. J. Bacteriol. 202, e00401-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart E. M., Gupta M., Wühr M., Silhavy T. J., The gain-of-function allele bamA E470K bypasses the essential requirement for BamD in β-barrel outer membrane protein assembly. Proc. Natl. Acad. Sci. U.S.A. 117, 18737–18743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni D., Yang K., Huang Y., Refolding, crystallization and preliminary X-ray crystallographic studies of the β-barrel domain of BamA, a membrane protein essential for outer membrane protein biogenesis. Acta Crystallogr. F Struct. Biol. Commun. 70, 362–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki S. K., Webb J. S., Braaten B. A., Low D. A., Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J. Bacteriol. 191, 1777–1786 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahoney T. F., Ricci D. P., Silhavy T. J., Classifying β-barrel assembly substrates by manipulating essential Bam complex members. J. Bacteriol. 198, 1984–1992 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rojas E. R., et al., The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 559, 617–621 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clifton L. A., et al., Effect of divalent cation removal on the structure of Gram-negative bacterial outer membrane models. Langmuir 31, 404–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng J., Xu J., Huang C., Chen J., Rcs phosphorelay responses to truncated lipopolysaccharide-induced cell envelope stress in Yersinia enterocolitica. Molecules 25, 5718 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little K., Tipping M. J., Gibbs K. A., Swarmer cell development of the bacterium Proteus mirabilis requires the conserved enterobacterial common antigen biosynthesis gene rffG. J. Bacteriol. 200, e00230-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spöring I., et al., Regulation of flagellum biosynthesis in response to cell envelope stress in Salmonella enterica Serovar Typhimurium. mBio 9, e00736-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llobet E., Campos M. A., Giménez P., Moranta D., Bengoechea J. A., Analysis of the networks controlling the antimicrobial-peptide-dependent induction of Klebsiella pneumoniae virulence factors. Infect. Immun. 79, 3718–3732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatterjee A. K., Ross H., Sanderson K. E., Leakage of periplasmic enzymes from lipopolysaccharide-defective mutants of Salmonella typhimurium. Can. J. Microbiol. 22, 1549–1560 (1976). [DOI] [PubMed] [Google Scholar]
- 54.Sutterlin H. A., et al., Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway. Proc. Natl. Acad. Sci. U.S.A. 113, E1565–E1574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coughlin R. T., Tonsager S., McGroarty E. J., Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry 22, 2002–2007 (1983). [DOI] [PubMed] [Google Scholar]
- 56.Gangola P., Rosen B. P., Maintenance of intracellular calcium in Escherichia coli. J. Biol. Chem. 262, 12570–12574 (1987). [PubMed] [Google Scholar]
- 57.Imai Y., et al., A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha S. Sr, Lach S. R., Konovalova A., Homeostasis of the Gram-negative cell envelope. Curr. Opin. Microbiol. 61, 99–106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okuda S., Sherman D. J., Silhavy T. J., Ruiz N., Kahne D., Lipopolysaccharide transport and assembly at the outer membrane: The PEZ model. Nat. Rev. Microbiol. 14, 337–345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson B. W., Trent M. S., Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 17, 403–416 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Y., et al., Structural basis of outer membrane protein insertion by the BAM complex. Nature 531, 64–69 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Han L., et al., Structure of the BAM complex and its implications for biogenesis of outer-membrane proteins. Nat. Struct. Mol. Biol. 23, 192–196 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Bakelar J., Buchanan S. K., Noinaj N., The structure of the β-barrel assembly machinery complex. Science 351, 180–186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iadanza M. G., et al., Lateral opening in the intact β-barrel assembly machinery captured by cryo-EM. Nat. Commun. 7, 12865 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noinaj N., Kuszak A. J., Balusek C., Gumbart J. C., Buchanan S. K., Lateral opening and exit pore formation are required for BamA function. Structure 22, 1055–1062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J., et al., Formation of a β-barrel membrane protein is catalyzed by the interior surface of the assembly machine protein BamA. eLife 8, e49787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomasek D., et al., Structure of a nascent membrane protein as it folds on the BAM complex. Nature 583, 473–478 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doyle M. T., Bernstein H. D., Bacterial outer membrane proteins assemble via asymmetric interactions with the BamA β-barrel. Nat. Commun. 10, 3358 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dekoninck K., et al., Defining the function of OmpA in the Rcs stress response. eLife 9, e60861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braun F., Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J. Comput. Chem. 19, 319–333 (1998). [Google Scholar]
- 71.Nov Y., When second best is good enough: Another probabilistic look at saturation mutagenesis. Appl. Environ. Microbiol. 78, 258–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu T., et al., Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121, 235–245 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Ni D., et al., Structural and functional analysis of the β-barrel domain of BamA from Escherichia coli. FASEB J. 28, 2677–2685 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.