Cell volume recovers from hypertonicity-induced shrinkage via the process of regulatory volume increase (RVI). The sodium-potassium-2-chloride cotransporter NKCC1 was the first molecule identified as important in RVI (1) and is considered central to the process (2). NKCC1 regulation has been studied in the contexts of RVI and transepithelial transport (3–5). Two distinct signals, hypertonicity (low volume) and low intracellular chloride, control NKCC1 in these contexts. Both regulatory mechanisms were found to be phosphorylation dependent, leading to the hypothesis of a volume- and chloride-regulated protein kinase (6). Lytle and McManus (3) concluded that the volume and chloride signals are “mutually interdependent” and “converge on a single protein kinase.” The with-no-lysine (WNKs) [K] kinases, discovered by Cobb and colleagues (7), are proving to be the long sought-after kinases activated dually by hypertonicity and low intracellular chloride. Serra et al. (8) report the chloride channel leucine rich repeat containing 8 subunit A (LRRC8A) as an gene essential for RVI and show that it is essential for WNK activation.
WNKs are activated by osmotic stressors (7, 9), and roles for WNKs in volume recovery have been demonstrated in human embryonic kidney 293 (HEK293) cells and in worms (10–12). WNKs are also activated by experimental maneuvers that lower intracellular chloride (9, 13). The discovery of LRRC8A coincides with an improved molecular-level understanding of how WNKs are regulated by chloride and osmotic stresses. Chloride binds and stabilizes an auto-inhibited dimeric configuration (14). Osmotic stress, mimicked in vitro with crowding agents (15), induces WNK1 and WNK3 to autophosphorylate (16). Crowding agents induce monomerization as measured by gel filtration and low-angle X-ray scattering. Overall, WNKs undergo a solvent- and chloride-driven conformational equilibrium between a Cl−-bound inactive state and a Cl−-unbound activation-competent state (16). Osmotic pressure exerts a demand on solvent to shift the equilibrium to the latter state (16, 17) (Fig. 1A). In support of these in vitro studies, mice carrying amino acid mutation of the chloride binding sites of WNK4 exhibit constitutive activation of the kinase (18). The data provide compelling support that WNKs are the volume-regulated kinases that sense intracellular chloride. An apparent conundrum remains, however; osmotic stress activates WNKs and NKCCs, but the intracellular chloride concentration rises as a result of shrinkage-induced water loss and from NKCC1-mediated chloride entry during RVI, which is expected to inhibit WNKs.
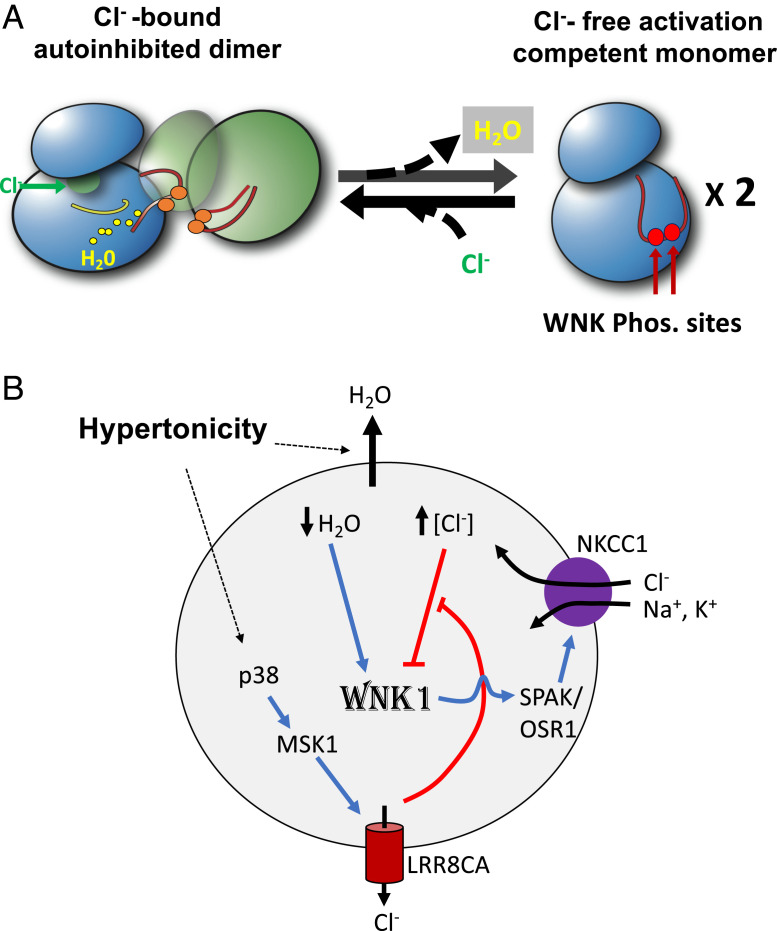
Fig. 1.
Regulation of WNK by chloride and hypertonicity. (A) At a molecular level, WNKs exist in conformational equilibrium between chloride-bound autoinhibited dimer and chloride free activation-competent monomer. Osmotic pressure puts a demand on solvent to induce autophosphorylation-competent monomers (Protein Data Bank [PDB] ID code 6CN9). (Left) The inactive dimer (based on PDB ID code 6CN9). The two subunits are blue and green, activation loops and unphosphorylated phosphorylation sites are orange, stabilizing chloride is green, and water molecules and catalytic loop are yellow. (Right) A monomer with an exposed activation loop and phosphorylation sites (red). (B) At a cellular level, extracellular hypertonicity extracts water from cells, which activates WNK. Water extraction and cell shrinkage raise the intracellular chloride, leading to WNK inhibition. Hypertonicity also activates chloride LRR8CA via p38-MSK1 cascade. Chloride exiting through LRR8CA blunts cell shrinkage–induced intracellular chloride increases to allow WNK to be activated. WNK activation stimulates NKCC1 through the intermediate SPAK/OSR1 (Ste20-related proline/alanine-rich kinase/oxidative stress responsive-1) and results in electrolyte uptake followed by water entry and cell volume recovery. Blue and red lines indicate stimulation and inhibition, respectively.
In PNAS, Serra et al. (8) report a gene essential for hypertonic stress responses and thereby, may have resolved this long-standing question in cell volume regulation. The authors used the Toronto KO library, a large CRISPR-Cas9 guide RNA library (19), to identify genes essential for survival after hypertonic stress. The screen, conducted in HeLa cells (immortal cervical cancer cell line from Henrietta Lacks), identified the chloride channel LRRC8A (Swell1) as the most significant osmo-protective gene among nonlethal genes. Apparently, a chloride channel is involved in regulating intracellular chloride levels in the context of RVI. By reducing intracellular chloride, LRRC8A permits the osmotically induced activation. This occurs in symmetrical cells in the context of RVI but reflects the mechanism of chloride reduction in chloride transepithelial transport (20). It is interesting that many of the genes essential to cell volume recovery, NKCC1, WNKs, and p38 mitogen activated protein (MAP) kinase, did not appear in the Serra et al. (8) screen, apparently an indication of their essentiality.
The LRRC8A hit was validated in cell survival and volume recovery assays in HeLa-LRRC8A-KO (knockout) cells. An LRRC8A inhibitor also reduced cell survival and volume recovery after hypertonic stress. The MAP kinase p38 is activated by both hypertonic and hypotonic stresses in cells (reviewed in ref. 21). Here, the Posas group (8) further showed that cell volume recovery was reduced by knockout or inhibition of p38 to the same extent as in LRRC8A knockout cells. Then, they show that inhibition of the p38 substrate mitogen- and stress-activated protein kinase-1 (MSK1), an MAP kinase-activated protein kinase (MAPKAP) homolog, reduced volume recovery similarly. They went on to identify activating phosphorylation sites in LRRC8A. Like WNKs, LRRC8A is known to be regulated by large conformational changes, but how phosphorylation feeds these equilibria is currently unknown (22). In the end, they postulate that hypertonicity activates LRRC8A through MSK1, which leads to cellular chloride exit. The result is such that the cell shrinkage–induced rise in the intracellular chloride is tempered, allowing WNK1 to be activated by hypertonicity. WNK1 activation leads to stimulation of NKCC1, RVI, and cell survival. The overall dual action of hypertonicity on NKCC1 and LRRC8A through WNKs and p38 MAP kinase is shown in Fig. 1B.
Footnotes
The authors declare no competing interest.
See companion article, “LRRC8A-containing chloride channel is crucial for cell volume recovery and survival under hypertonic conditions,” 10.1073/pnas.2025013118.
References
- 1.Haas M., Forbush B. 3rd, [3H]bumetanide binding to duck red cells. Correlation with inhibition of (Na + K + 2Cl) co-transport. J. Biol. Chem. 261, 8434–8441 (1986). [PubMed] [Google Scholar]
- 2.Parker J. C., In defense of cell volume? Am. J. Physiol. 265, C1191–C1200 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Lytle C., McManus T., Coordinate modulation of Na-K-2Cl cotransport and K-Cl cotransport by cell volume and chloride. Am. J. Physiol. Cell Physiol. 283, C1422–C1431 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Breitwieser G. E., Altamirano A. A., Russell J. M., Osmotic stimulation of Na(+)-K(+)-Cl- cotransport in squid giant axon is [Cl-]i dependent. Am. J. Physiol. 258, C749–C753 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Haas M., McBrayer D., Lytle C., [Cl-]i-dependent phosphorylation of the Na-K-Cl cotransport protein of dog tracheal epithelial cells. J. Biol. Chem. 270, 28955–28961 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Lytle C., Forbush B. 3rd, The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J. Biol. Chem. 267, 25438–25443 (1992). [PubMed] [Google Scholar]
- 7.Xu B., et al., WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 275, 16795–16801 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Serra S. A., et al., LRRC8A-containing chloride channel is crucial for cell volume recovery and survival under hypertonic conditions. Proc. Natl. Acad. Sci. U.S.A. 118, e2025013118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenertz L. Y., et al., Properties of WNK1 and implications for other family members. J. Biol. Chem. 280, 26653–26658 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Rangel S., Gamba G., Ramos-Mandujano G., Pasantes-Morales H., Influence of WNK3 on intracellular chloride concentration and volume regulation in HEK293 cells. Pflugers Arch. 464, 317–330 (2012). [DOI] [PubMed] [Google Scholar]
- 11.de Los Heros P., Pacheco-Alvarez D., Gamba G., Role of WNK kinases in the modulation of cell volume. Curr. Top. Membr. 81, 207–235 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Choe K. P., Strange K., Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am. J. Physiol. Cell Physiol. 293, C915–C927 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Moriguchi T., et al., WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 280, 42685–42693 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Piala A. T., et al., Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci. Signal. 7, ra41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuznetsova I. M., Turoverov K. K., Uversky V. N., What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 15, 23090–23140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akella R., et al., Osmosensing by WNK kinases. Mol. Biol. Cell, 10.1091/mbc.E20-01-0089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akella R., et al., A phosphorylated intermediate in the activation of WNK kinases. Biochemistry 59, 1747–1755 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J. C., et al., WNK4 kinase is a physiological intracellular chloride sensor. Proc. Natl. Acad. Sci. U.S.A. 116, 4502–4507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart T., et al., High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell 163, 1515–1526 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Lytle C., Forbush B. 3rd, Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: Modulation by cytoplasmic Cl. Am. J. Physiol. 270, C437–C448 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Zhou X., Naguro I., Ichijo H., Watanabe K., Mitogen-activated protein kinases as key players in osmotic stress signaling. Biochim. Biophys. Acta 1860, 2037–2052 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Kern D. M., Oh S., Hite R. K., Brohawn S. G., Cryo-EM structures of the DCPIB-inhibited volume-regulated anion channel LRRC8A in lipid nanodiscs. eLife 8, e42636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]