Abstract
Leguminous plants produce nodules for nitrogen fixation; however, nodule production incurs an energy cost. Therefore, as an adaptive strategy, leguminous plants halt root nodule development when sufficient amounts of nitrogen nutrients, such as nitrate, are present in the environment. Although legume NODULE INCEPTION (NIN)-LIKE PROTEIN (NLP) transcription factors have recently been identified, understanding how nodulation is controlled by nitrate, a fundamental question for nitrate-mediated transcriptional regulation of symbiotic genes, remains elusive. Here, we show that two Lotus japonicus NLPs, NITRATE UNRESPONSIVE SYMBIOSIS 1 (NRSYM1)/LjNLP4 and NRSYM2/LjNLP1, have overlapping functions in the nitrate-induced control of nodulation and act as master regulators for nitrate-dependent gene expression. We further identify candidate target genes of LjNLP4 by combining transcriptome analysis with a DNA affinity purification-seq approach. We then demonstrate that LjNLP4 and LjNIN, a key nodulation-specific regulator and paralog of LjNLP4, have different DNA-binding specificities. Moreover, LjNLP4–LjNIN dimerization underlies LjNLP4-mediated bifunctional transcriptional regulation. These data provide a basic principle for how nitrate controls nodulation through positive and negative regulation of symbiotic genes.
Different DNA-binding specificities of LjNLP4 and LjNIN underlie LjNLP4-mediated positive or negative regulation of symbiotic gene expression in nitrate-induced control of nodulation.
Introduction
Nitrogen is an essential macronutrient for the growth of every organism. Plants have developed various strategies for acquiring nitrogen to adapt to a fluctuating nitrogen nutrient environment (Oldroyd and Leyser, 2020). On their roots, leguminous plants form nodules, lateral organs specialized for the acquisition of nitrogen nutrients. Nodules contain nitrogen-fixing bacteria, collectively called rhizobia. Plants have a symbiotic relationship with the rhizobia that reside inside the nodule cells (Suzaki et al., 2015; Ferguson et al., 2019). Root nodule symbiosis makes nitrogen in the atmosphere available as a nutrient and allows legumes to grow in nitrogen-deficient soils. Symbiotic host plants consume photosynthetic products as an energy source for driving nodule development and nitrogen fixation. Unnecessary nodulation can be harmful, as plants lose carbon sources that could be used for their growth. Therefore, a balance between the benefits of obtaining nitrogen sources and the costs of losing carbon sources needs to be maintained during root nodule symbiosis (Nishida and Suzaki, 2018a). In this context, plants have a genetic mechanism to cease nodulation if there are sufficient nitrogen sources, such as nitrate, available in their environment, thereby saving the costs associated with nodulation. In response to nitrate, plants control several key processes required to establish root nodule symbiosis, including rhizobial infection, initiation and growth of nodules, and the nitrogen fixation process (Streeter and Wong, 1988; Carroll and Mathews, 1990; Nishida and Suzaki, 2018b).
Recent genetic studies using two model leguminous plants, Lotus japonicus and Medicago truncatula, identified key transcription factors (TFs) that are required for nitrate-induced control of root nodule symbiosis (Lin et al., 2018; Nishida et al., 2018). In L. japonicus, a forward genetic approach identified NITRATE UNRESPONSIVE SYMBIOSIS 1 (NRSYM1), which encodes a NODULE INCEPTION (NIN)-LIKE PROTEIN (NLP) TF, LjNLP4 (Nishida et al., 2018). LjNLP4 mediates pleiotropic control of root nodule symbiosis by nitrate. The designation of the NLP TF refers to its similarity to the nodulation-specific TF NIN (Schauser et al., 1999, 2005). NLP consists of an N-terminal conserved domain responsible for the nitrate response, an RWP-RK domain and a PB1 domain involved in protein–protein interaction (Schauser et al., 2005; Konishi and Yanagisawa, 2013; Suzuki et al., 2013).
Although the relatively lower conservation in some region of the N-terminal domain of NIN may be relevant to its loss of nitrate-responsiveness (Suzuki et al., 2013), NIN is necessary and sufficient for positively regulating rhizobial infection and nodule organogenesis by inducing the expression of several genes that are required for these processes (Schauser et al., 1999; Soyano et al., 2013, 2014; Kawaharada et al., 2017; Li et al., 2019). In addition to its activating role in nodulation, in some contexts, LjNIN can negatively regulate nodulation through direct activation of CLAVATA3/ESR-related (CLE)-ROOT SIGNAL 1 (CLE-RS1) and CLE-RS2 that function as root-derived signals in a mechanism called autoregulation of nodulation (AON), a systemic long-range signaling pathway between roots and shoots (Okamoto et al., 2013a; Soyano et al., 2014; Ferguson et al., 2019). More recently, the NIN-CLE signaling cascade has been shown to be conserved in M. truncatula (Laffont et al., 2020). DNA-binding sites for LjNLP4 resemble those of NIN (Nishida et al., 2018). In particular, LjNLP4 negatively regulates the nodule initiation process by directly inducing CLE-RS2 expression (Nishida et al., 2018). Furthermore, in nitrate-sufficient conditions, the loss-of-function mutations in the genes involved in AON exhibit tolerance to the nitrate-induced control of nodule number but not to other nitrate-affected processes (Nishida et al., 2018). Hence, the nitrate-induced LjNLP4-AON signaling pathway plays a role predominantly in the control of nodule number. Given that LjNLP4 is involved in the nitrate-induced control of multiple processes involved in root nodule symbiosis, such as rhizobial infection, nodule growth, and nitrogen fixation, it is likely that LjNLP4 uses different downstream target genes from CLE-RS2 to achieve this pleiotropic regulation.
In M. truncatula, the role of another NLP, MtNLP1, was characterized and like LjNLP4, MtNLP1 can control root nodule symbiosis in response to nitrate (Lin et al., 2018). MtNLP1 interacts with MtNIN and negatively regulates MtNIN-mediated induction of CYTOKININ RESPONSE 1 (MtCRE1) encoding a cytokinin receptor required for nodule organogenesis (Vernié et al., 2015). In addition, MtNLP1 binds to the promoter region of MtCRE1, to which MtNIN also binds. Based on these observations, it is proposed that MtNLP1 represses MtCRE1 expression possibly via physical interaction with MtNIN and/or by binding to the same cis-element as MtNIN.
Although the identification of LjNLP4 and MtNLP1 in two legumes has advanced the understanding of how nitrate-mediated control of root nodule symbiosis is achieved, there are only a few nodulation-related genes that have been identified as being regulated by NLPs. In particular, it remains unclear how NLPs can have the bifunctional roles of inducing and repressing gene expression depending on the gene. Here, we identify NRSYM2, a gene that encodes LjNLP1. Phenotypic and transcriptome analyses using Ljnlp4 and Ljnlp1 mutants demonstrate that LjNLP4 and LjNLP1 have overlapping functions and act as master regulators for nitrate-dependent gene expression. We further identify genome-wide LjNLP4-binding sites and their similarities and differences from LjNIN-binding sites. Furthermore, we show that LjNLP4–LjNIN dimerization and different DNA-binding specificities to the cis-elements of LjNLP4 and LjNIN underlie LjNLP4-mediated positive or negative regulation of gene expression. Collectively, these data propose basic principles about the NLP-mediated transcriptional regulation of symbiotic genes that allow nitrate inhibition of nodulation.
Results
NRSYM2 encodes LjNLP1 and mediates the nitrate-induced control of root nodule symbiosis
We identified a new recessive L. japonicus mutant, nrsym2, in a previous screening for ethylmethane sulfonate (EMS) mutants involved in the nitrate response during nodulation (Nishida et al., 2018). Nitrate treatment (10 mM) significantly attenuated nodulation in wild-type (WT) plants, but the nrsym2 plants formed mature nodules in the presence of a high nitrate concentration (Figure 1A). A genome-resequencing approach using nrsym2 identified a point mutation that causes a premature stop codon in the gene, Lj1g3v2295200, which was previously named as LjNLP1 (Figure 1, B and C; Suzuki et al., 2013). A 9.5-kb genomic fragment encompassing the entire locus was introduced into the nrsym2 mutants by Agrobacterium rhizogenes-mediated hairy root transformation. Nodulation on the mutant roots carrying the complementation construct was inhibited by nitrate (Supplemental Figure S1), indicating that the nrsym2 phenotype results from mutation of the gene.
Figure 1.
Identification of the NRSYM2/LjNLP1 gene. A, Nodule phenotypes of WT and the nrsym2/Ljnlp1 mutant grown with 0 or 10 mM KNO3 for 21 dai. Scale bars = 2 mm. B, Structure of the predicted LjNLP1 protein. An N-terminal conserved region is indicated by the gray box. C-terminal regions containing an RWP-RK DNA-binding (RWP-RK) and a conserved oligomerization (PB1) domain are shown as red and blue boxes, respectively. The arrowhead indicates the location of the nrsym2/Ljnlp1 mutation. C, A phylogenetic tree of NLPs from A. thaliana, M. truncatula, and L. japonicus was generated using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates), are shown.
For clarity, hereafter, we unified the nomenclature of NRSYM1 and NRSYM2 as LjNLP4 and LjNLP1, respectively. LjNLP1 encodes a protein with sequence similarity to NLP TFs, consisting of a nitrate-responsive domain, an RWP-RK and a PB1 domain (Figure 1, B and C). Phylogenetic analysis revealed that LjNLP1 is orthologous to MtNLP1 (Lin et al., 2018). The level of LjNLP1 expression was unaffected by nitrate treatment or rhizobial inoculation (Supplemental Figure S2A). One of the features of some NLPs is that they localize within nuclei in response to nitrate (Marchive et al., 2013; Liu et al., 2017; Nishida et al., 2018; Lin et al., 2018). Immunohistochemistry using a functional LjNLP1-myc fusion protein suggested that, unlike these NLPs, LjNLP1 constantly localizes within nuclei with or without nitrate (Supplemental Figure S2, B and C).
We next conducted progressive phenotypic analyses of different stages of nodule development and function. LjNLP4, an NLP TF related to LjNLP1 but distinct, mediates the nitrate-induced control of these processes (Figure 2, A–D; Nishida et al., 2018). We then investigated whether LjNLP1 was implicated in the control of nodulation in response to nitrate and its potential genetic interaction with LjNLP4. In WT, nitrate treatment compromised several symbiotic processes but the effects of nitrate were impaired in the Ljnlp4 and Ljnlp1 mutants (Figure 2, A–D). Collectively, the Ljnlp1 mutant exhibited similar phenotypes to Ljnlp4 in terms of nitrate-mediated reduction in nodule number and growth, and nitrogen fixation. However, unlike Ljnlp4, infection thread formation was reduced by nitrate in Ljnlp1 (Figure 2A). Nitrate-mediated inhibition of these processes was almost completely impaired in the Ljnlp4 Ljnlp1 double mutants. Furthermore, in the presence of extremely high nitrate concentrations (60 mM), the Ljnlp4 Ljnlp1 double mutants formed more normal nodules compared with either of the single mutants (Supplemental Figure S3). These observations indicated that LjNLP4 and LjNLP1 have overlapping roles in nitrate-induced pleiotropic control of root nodule symbiosis. We next investigated the effects of nitrate on plant growth in nonsymbiotic conditions. Whereas each single mutation diminished nitrate-promoted plant growth, the Ljnlp1 mutation had stronger effects than Ljnlp4, and the Ljnlp4 Ljnlp1 double mutation almost abolished nitrate-dependent plant growth (Figure 2, E and F).
Figure 2.
Nitrate effects on nodulation and plant growth in the Ljnlp4 Ljnlp1 double mutants. A–D, Nitrate effects on nodulation in WT, the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants. A, The number of infection threads in plants growing in 0 or 10 mM KNO3 at 7 dai with rhizobia that constitutively express DsRED (n = 11–12 plants). B and C, The number of nodules (B) and maximum nodule diameters (C) in plants grown in the presence of 0 or 10 mM KNO3 for 21 dai (n = 10–12 plants). Scale bars = 1 mm (C). D, Acetylene reduction activity of nodules. Plants grown under nitrate-free conditions for 21 dai were supplied with 0 or 10 mM KNO3, and after 3 d the acetylene reduction activity (μmol/h, g nodules) of nodules from each plant was measured (n = 9–10 plants). E and F, Plant growth (E) and fresh shoot weight (F) of WT, the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants grown in 0 or 10 mM KNO3 in the absence of rhizobia for 16 d (n = 11–12 plants). Scale bars = 5 cm (E). In (A–D) and (F), centerlines in the boxplots show the medians, and upper and lower quartile limits are shown as horizontal bars. Outliers are represented by points, and red crosses indicate the sample means. *P < 0.05 by a two-sided Welch's t test (A, C, and D) or Wilcoxon rank sum test (B). Significant differences were determined with Kruskal–Wallis one-way analysis with a post hoc Steel–Dwass test, indicated by different lower-case letters (P < 0.05; F).
LjNLP4 and LjNLP1 act as master regulators of nitrate-dependent gene expression
We next investigated the effect of Ljnlp4/1 mutations on gene expression. Real-time Reverse transcription polymerase chain reaction (RT-PCR) analysis showed that the transcript abundance of two nitrate-inducible genes, NITRITE REDUCTASE 1 (LjNIR1) and CLE-RS2 (Nishida et al., 2018), were considerably reduced in the Ljnlp4 or Ljnlp1 single mutants (Figure 3). Notably, nitrate-mediated gene expression was completely attenuated in the Ljnlp4 Ljnlp1 double mutants. Therefore, these results indicate that both LjNLPs are required for the nitrate-inducible expression of these genes.
Figure 3.
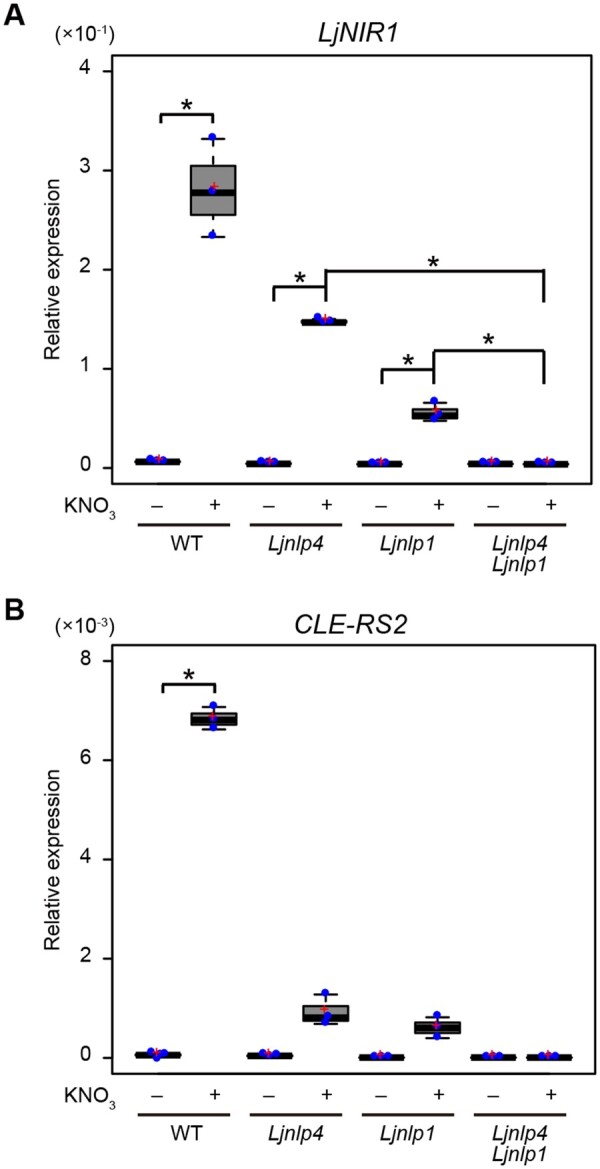
The effect of Ljnlp4 and Ljnlp1 mutations on nitrate-inducible gene expression. A and B, Real-time RT-PCR analysis of (A) LjNIR1 and (B) CLE-RS2 expression in roots of WT, the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants. Each cDNA sample was prepared from total RNA derived from noninoculated roots treated with 0 or 10 mM KNO3 for 24 h (n = 3 independent pools of roots derived from 10 plants). The expression of LjUBQ was used as a normalizer to assess the relative expression of each gene. Centerlines in the boxplots show the medians, and upper and lower quartile limits are shown as horizontal bars. Individual data points are represented by blue points, and red crosses indicate the sample means. *P < 0.05 by a two-sided Welch’s t test. P values were modified by Bonferroni correction.
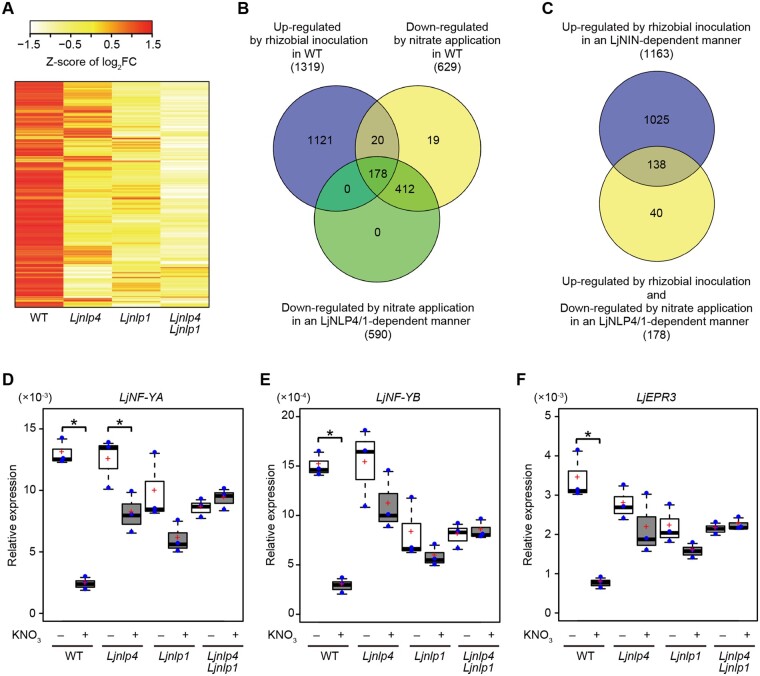
To gain insight into the roles of LjNLP4 and LjNLP1 among genes whose expression is nitrate-inducible, we conducted comparative transcriptome analysis using rhizobia-inoculated roots of WT, Ljnlp4, Ljnlp1, and Ljnlp4 Ljnlp1 mutants grown with or without nitrate. Each plant was pretreated with nitrate a day before rhizobia inoculation and nitrate-treated whole roots of 3 d after inoculation (dai) were collected for RNA-seq. Consequently, we identified 364 nitrate-inducible genes in the WT (Figure 4A;Supplemental Figure S4A; Supplemental Data Set S1A). Of the 364 genes, LjNLP4 and LjNLP1 were, respectively, responsible for the expression of 281 and 347 genes (Supplemental Figure S4B; Supplemental Data Set S1, B and C). Of note, the expression of almost all, namely, 363 genes depended on LjNLP4 and/or LjNLP1 (Figure 4A;Supplemental Figure S4A; Supplemental Data Set S1D). The transcriptome data indicate that LjNLP4 and LjNLP1 have overlapping roles as master regulators of nitrate-inducible gene expression in L. japonicus roots. Although the genes with LjNLP4-dependent expression mostly overlapped with those of LjNLP1, there were a moderate number of LjNLP1-specific downstream genes (Supplemental Figure S4B; Supplemental Data Set S1E).
Figure 4.
Transcriptome analysis of the Ljnlp4 and the Ljnlp1 mutants during root nodule symbiosis. A–F, WT, the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants were grown without nitrate nor rhizobia for 4 d and then treated with 0 or 10 mM KNO3 for 24 h before rhizobia inoculation with continuous treatment of 0 or 10 mM KNO3. WT and the Ljnin-9 mutants were grown at inoculated and noninoculated conditions without nitrate. RNA was extracted for (A–C) RNA-seq and (D–F) real-time RT-PCR analysis from inoculated roots (3 dai) or noninoculated roots (n = 3 independent pools of roots derived from 10 plants). A, A heatmap showing relative transcript abundance (10 mM KNO3/0 mM KNO3) for nitrate-inducible genes (364 genes) in WT, the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants. Nitrate-inducible genes were identified in WT by gene expression levels with at least a 2-fold change (log2 fold changes (log2FC) >1 and FDR <0.05) when grown in 10 mM KNO3 compared with those grown in 0 mM KNO3 (Supplemental Data Set S1A). B, A Venn diagram showing the number of rhizobia-inducible genes in WT (1,319 genes), nitrate-repressible genes in WT (629 genes), and LjNLP4/1-regulated nitrate-repressible genes (590 genes). Genes upregulated between inoculated and noninoculated roots were extracted based on select criteria (log2FC >1 and FDR <0.05). Downregulated genes between 10 and 0 mM KNO3-treated roots were extracted based on similar criteria (log2FC < −1 and FDR < 0.05) (Supplemental Data Set S2, A, B, and E). C, A Venn diagram showing the overlap between LjNIN-dependent rhizobia-inducible genes (1,163 genes) and rhizobia-inducible and LjNLP4/1-dependent nitrate-repressible genes (178 genes) (Supplemental Data Set S2E, S3A and S3D). D–F, Real-time RT-PCR analysis of (D) LjNF-YA, (E) LjNF-YB, and (F) LjEPR3 expression in WT, the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants. The expression of LjUBQ was used as the reference. Centerlines in the boxplots show the medians, and upper and lower quartile limits are shown as horizontal bars. Individual data points are represented by blue points, and red crosses indicate the sample means. *P < 0.05 by a two-sided Welch’s t test.
We previously showed that the LjNLP4-dependent induction of CLE-RS2 expression, a negative regulator for nodulation, is linked to the nitrate-induced control of nodule number (Nishida et al., 2018). Considering nitrate’s pleiotropic effects on root nodule symbiosis, we assumed that expression of other nodulation-related genes also must be controlled by nitrate. We thus focused on the genes whose expression was upregulated by rhizobia inoculation but downregulated by nitrate during early stages of root nodule symbiosis. We identified rhizobia-inducible genes by comparing transcriptome of inoculated (3 dai) WT roots with that of noninoculated, and then sought nitrate-repressible genes using inoculated roots of WT, Ljnlp4, Ljnlp1, and Ljnlp4 Ljnlp1 with or without nitrate. Of the 1,319 rhizobia-inducible genes, 178 genes were downregulated by nitrate in an LjNLP4/1-dependent manner (Figure 4B;Supplemental Figure S5, A and B; Supplemental Data Set S2).
We next incorporated the Ljnin mutants into our transcriptome analyses, as LjNIN is not only an essential regulator for positive regulation of rhizobial infection and nodule organogenesis, but also closely related to NLPs (Schauser et al., 1999, 2005). RNA-seq analysis using inoculated (3 dai) and noninoculated Ljnin roots revealed that LjNIN is required for the expression of 1,163 of 1,319 genes; among them 138 genes were downregulated by nitrate in an LjNLP4/1-dependent manner (Figure 4C;Supplemental Figure S5, C and D; Supplemental Data Set S3). In L. japonicus, some positive regulators for rhizobial infection and nodule organogenesis were identified as direct target genes of LjNIN, including EXOPOLYSACCHARIDE RECEPTOR 3 (LjEPR3), NUCLEAR FACTOR-YA (LjNF-YA), LjNF-YB, and RHIZOBIAL INFECTION RECEPTOR-LIKE KINASE 1 (LjRINRK1) (Soyano et al., 2013; Kawaharada et al., 2015, 2017; Hossain et al., 2016; Li et al., 2019). These LjNIN target genes were included among the 138 genes (Figure 4C;Supplemental Data Set S3D).
Real-time RT-PCR analysis confirmed that the rhizobia-inducible expression of these genes was significantly repressed by nitrate in an LjNLP4/1-dependent manner (Figure 4, D–F). Although either the Ljnlp4 or Ljnlp1 single mutant exhibited sufficient tolerance to the nitrate-mediated downregulation of these genes, there was no nitrate effect on gene expression for the Ljnlp4 Ljnlp1 double mutants. Therefore, our data indicate that the two LjNLPs locate genetically upstream in the nitrate-mediated repression of these LjNIN-target genes.
LjNLP4 and LjNIN bind identical and distinct cis-elements
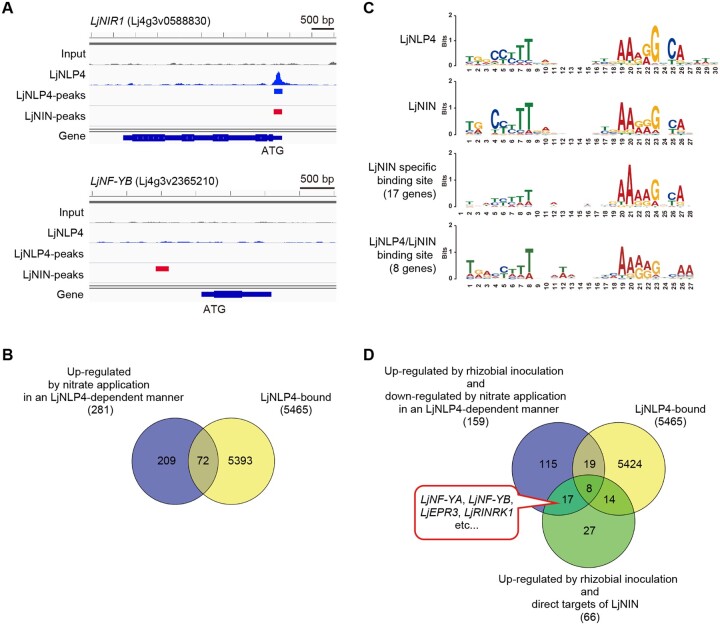
To better understand LjNLP-mediated transcriptional regulation in the nitrate-induced control of root nodule symbiosis, we sought to identify direct target genes of LjNLP4 by combining RNA-seq analysis with DNA affinity purification (DAP)-seq, an in vitro DNA-binding assay to capture genome-wide DNA-binding sites of TFs in their native sequence context (O’Malley et al., 2016). Consequently, we confirmed that LjNLP4 bound to the promoter regions of LjNIR1 and CLE-RS2, known direct target genes of LjNLP4 (Figure 5A;Supplemental Figure S6A); the LjNLP4-binding sites identified by DAP-seq were identical to those reported previously (Nishida et al., 2018). These observations supported the validity of using DAP-seq in combination with RNA-seq to identify LjNLP4-target gene candidates. Of 281 LjNLP4-dependent nitrate-inducible genes, LjNLP4 bound to the gene regulatory regions of 72 genes, including NITRATE-INDUCIBLE GARP-TYPE TRANSCRIPTIONAL REPRESSOR 1 (LjNIGT1) and LATERAL ORGAN BOUNDARY DOMAIN 38 (LjLBD38) (Figure 5B;Supplemental Figure S6A; Supplemental Data Set S4B).
Figure 5.
Identification of genome-wide LjNLP4-binding sites. A, Screenshots of IGV views showing LjNLP4 DAP-seq peaks (blue peaks) within the LjNIR1 locus and LjNF-YB locus. LjNLP4 and LjNIN-binding sites were identified by DAP-seq (blue bars) and ChIP-seq (red bars; Soyano et al., 2014), respectively. B, A Venn diagram showing the overlap between LjNLP4-dependent nitrate-inducible genes (281 genes) and LjNLP4-bound genes (5,465 genes) (Supplemental Data Set S1B and S4A). C, The most highly enriched motifs for the LjNLP4 DAP-seq dataset (top 500 peaks) and the LjNIN ChIP-seq dataset (top 500 peaks). The motifs of LjNIN-specific binding sites and LjNLP4/LjNIN binding sites were predicted using the genes that were directly upregulated by LjNIN in response to rhizobia and downregulated by nitrate in an LjNLP4-dependent manner. LjNLP4 bound to the gene regulatory regions of 8 genes (Figure 5D;Supplemental Data Set S4E) but not 17 genes (Figure 5D;Supplemental Data Set S4D). The sequence logo was generated using WebLogo (http://weblogo.berkeley.edu/logo.cgi). D, A Venn diagram showing the number of rhizobia-inducible genes that were downregulated by nitrate in an LjNLP4-dependent manner (159 genes), LjNLP4-bound genes (5,465 genes), and rhizobia-inducible genes that were directly regulated by LjNIN (66 genes; Supplemental Data Sets S2C; S4A; S4, C and D).
In addition, using the top 500 LjNLP4-binding sites, we predicated their features; they consisted of 30 nucleotides, in which there were two conserved motifs with semi-palindromic structures (Figure 5C). The representative LjNLP4-binding sites had similar nucleotide compositions to a motif originally identified as a nitrate-responsive cis-element (NRE) in Arabidopsis (Konishi and Yanagisawa, 2010).
Next, we analyzed previous chromatin immunoprecipitation (ChIP)-seq data of LjNIN (Soyano et al., 2014) and compared the properties of the LjNLP4- and LjNIN-binding sites. The predicted features of the LjNIN-binding sites based on the top 500 LjNIN-binding sites were very similar to the LjNLP4-binding sites, suggesting that the two TFs shared common target genes (Figure 5C). Indeed, LjNLP4 and LjNIN bound to identical cis-elements within the gene regulatory regions of LjNIR1, CLE-RS2, LjNIGT1, and LjLBD38 (Figure 5A;Supplemental Figure S6A).
Combinational analyses of our RNA-seq with the ChIP-seq data identified 66 nodulation-related LjNIN-target genes; LjNIN bound to the regulatory regions of genes whose expression was upregulated by rhizobial inoculation in an LjNIN-dependent manner (Figure 5D;Supplemental Data Set S4C). Of the 66 genes, we noticed that LjNLP4 did not bind to the gene regulatory regions of some LjNIN-target genes, such as LjNF-YB, LjNF-YA, and LjEPR3, whose expression was upregulated by rhizobia in an LjNIN-dependent manner but downregulated by nitrate in an LjNLP4-dependent manner (Figure 5, A and D; Supplemental Figure S6B). In total 17 genes had similar expression and LjNLP4/LjNIN-binding patterns to LjNF-YB (Figure 5D;Supplemental Data Set S4D). Using these 17 genes, we predicted features of the LjNIN-specific binding sites: they had weaker palindromic structures in contrast to the LjNLP4/LjNIN common binding sites (Figure 5C). We also identified eight genes that were LjNLP4/LjNIN common direct targets, whose expression was activated by rhizobia inoculation but repressed by nitrate (Figure 5, C and D; Supplemental Data Set S4E).
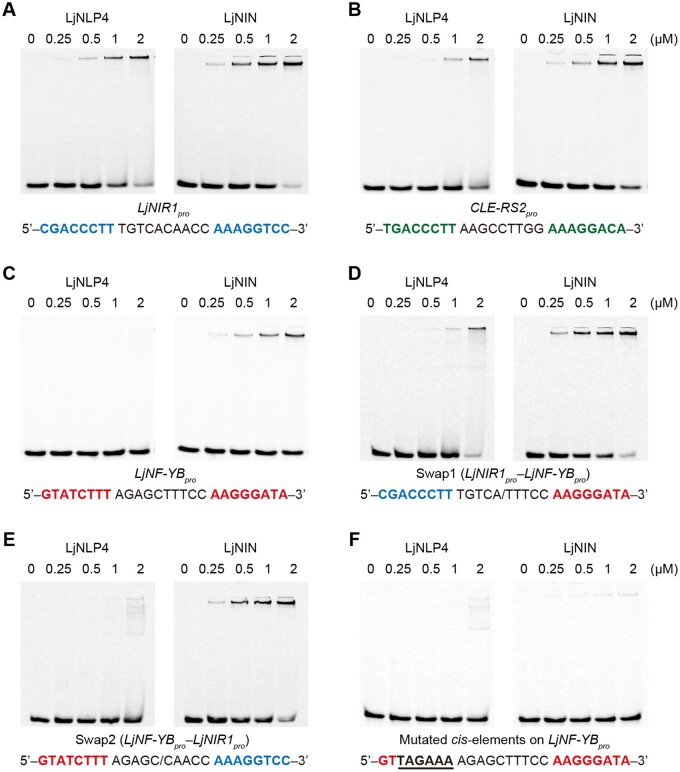
To verify the similarities and differences of LjNLP4/LjNIN-binding to these cis-elements in more detail, we carried out an electrophoretic mobility shift assay (EMSA). Consistent with the DAP-seq and ChIP-seq data described above, the recombinant LjNLP4 and LjNIN proteins, consisting of an RWP-RK and a PB1 domain, bound to cis-elements in the LjNIR1 and CLE-RS2 promoters (Figure 6, A and B), and only LjNIN bound to the cis-elements in the LjNF-YB promoter (Figure 6C). The sequence similarity of one of the two motifs located in the 5ʹ region of the LjNF-YB promoter was lower than those of the LjNIR1 and CLE-RS2 promoters (Figures 5C; 6, A–C). We then used two modified cis-elements of the LjNF-YB promoter, in which one-half of the LjNIN-binding site was replaced with that from a corresponding part of the LjNLP4/LjNIN-binding site in the LjNIR1 promoter. In this case, LjNLP4 bound to one of the modified cis-elements that was more similar to a corresponding region of a typical LjNLP4/LjNIN-binding site than original region in the LjNF-YB promoter (Figures 5C, 6, C–E). Thus, it seems reasonable to conclude that cis-elements bound by LjNLP4 include more rigorous palindromic motifs than those bound by LjNIN. However, a set of at least partially conserved and palindromic motifs seemed to exist to allow LjNIN binding, as mutations in either of the motifs perturbed LjNIN binding (Figure 6, C and F; Soyano et al., 2013).
Figure 6.
Comparison of LjNLP4 and LjNIN-binding sites. A–F, EMSA showing LjNLP4 or LjNIN binding to the cis-elements on the (A) LjNIR1 promoter, (B) CLE-RS2 promoter, (C) LjNF-YB promoter, (D) swap1 (LjNIR1pro–LjNF-YBpro), (E) swap2 (LjNF-YBpro–LjNIR1pro), and (F) mutated cis-elements on the LjNF-YB promoter. MBP-LjNLP4 (564–976) or MBP-LjNIN (551–878) recombinant protein, consisting of an RWP-RK and a PB1 domain, was incubated with the carboxyfluorescein (FAM)-labeled DNA probe (Supplemental Table S1). Swap1 and swap2 were modified cis-elements that combined respective halves of the LjNLP4/LjNIN-binding site of the LjNIR1 promoter and the LjNIN-binding site of the LjNF-YB promoter. Blue and red nucleotides indicate conserved motifs in the LjNLP4 and/or LjNIN-binding sites on the LjNIR1 and LjNF-YB promoters, respectively. The mutated nucleotide sequences in the cis-elements of the LjNF-YB promoter are underlined.
Cis-element-dependent dimerization of LjNLP4/LjNIN and its link to gene expression
Generally, TFs form dimers when they bind to cis-elements with palindromic structures (Schleif, 1988; Siggers and Gordân, 2014). As the LjNLP4/LjNIN-binding sites contained semi-palindromic structures (Figure 5C), we suspected that LjNLP4/LjNIN might dimerize. We performed size-exclusion chromatography coupled to multi-angle scattering (SEC–MALS) analysis to determine the absolute molecular masses of recombinant LjNLP4 and LjNIN, which are composed of an RWP-RK and a PB1 domain, in solution. In this analysis, both proteins showed an elution peak corresponding to a molecular mass of homodimer or homodimer–DNA complex (Figure 7, A and B). A peak derived from aggregation of LjNIN protein was observed in the absence of DNA, and the peak was reduced by the addition of DNA, suggesting LjNIN homodimer can be stable by associating with DNA. These results demonstrate that LjNLP4 and LjNIN exclusively exist as dimers.
Figure 7.
Cis-element-dependent protein–protein interactions. A and B, SEC-MALS analysis using purified (A) MBP-LjNLP4 (564–976) or (B) MBP-LjNIN (551–878) recombinant protein, consisting of an RWP-RK and a PB1 domain, in the presence or absence of the nonlabeled LjNIR1pro DNA fragment. The chromatogram displays the relative intensity of absorbance at 280 nm (left axis) together with the molar mass of the peak calculated by MALS (right axis). The elution volume and the average molar mass of the protein for each peak are indicated. Parentheses indicate molar mass of protein complex excluding DNA (23.9 kDa). Theoretical molar mass, MBP-LjNLP4-monomer, 88.2 kDa; MBP-LjNLP4-dimer, 176.4 kDa; MBP-LjNIN-monomer, 79.3 kDa; MBP-LjNIN-dimer, 158.6 kDa, are indicated. Asterisks and arrowhead represent free DNA and protein aggregation, respectively. C–E, EMSA showing LjNLP4 or LjNIN binding to the (B) LjNIR1, (C) CLE-RS2, and (D) LjNF-YB promoter (Figure 6; Supplemental Table S1). Blue, green, and red arrows respectively indicate the position of shifted bands showing interaction between MBP-LjNLP4 (564–976) homodimer, MBP-LjNLP4 (564–976)–Trx-His-LjNIN (551–878) heterodimer or Trx-His-LjNIN (551–878) homodimer, and the FAM-labeled probe. Black arrows indicate free probes that did not interact with proteins. Top tables show the concentration of MBP-LjNLP4 (564–976) and Trx-His-LjNIN (551–878) recombinant protein used in each assay. Bottom tables indicate the ratio of each band measured using an Amersham Imager 680 system (GE Healthcare).
Consistent with recent findings about protein–protein interactions between NLPs and NIN in several plant species (Lin et al., 2018; Wang et al., 2019; Konishi and Yanagisawa, 2019), our yeast two-hybrid assay showed physical interactions between a PB1 domain of LjNLP4, LjNLP1, and LjNIN (Supplemental Figure S7A). No interaction was observed when we used an RWP-RK domain (Supplemental Figure S7B). Thus, protein–protein interaction can be achieved through a PB1 domain. The physical interaction between NLPs and LjNIN was confirmed by biomolecular fluorescence complementation (BiFC) assay using Nicotiana benthamiana (Supplemental Figure S7C). Taken together, it is reasonable to conclude that LjNLP4 and LjNIN form dimers when they bind to cis-elements of their target genes.
We then investigated how DNA binding by LjNLP4 or LjNIN could be influenced in the presence of the other protein. The difference in molecular size between LjNLP4 and LjNIN recombinant proteins enabled us to distinguish the type of LjNLP4/LjNIN dimerization along with its DNA-binding pattern. In all cases tested, where LjNLP4 and LjNIN coexisted, the ratio of LjNIN homodimer-interacting DNA was substantially reduced followed by the occurrence of the LjNLP4 homodimer or LjNLP4–LjNIN heterodimer-interacting DNA (Figure 7, C–E). Although the interaction between LjNLP4 homodimer and cis-elements on the LjNF-YB promoter was not detected, the LjNLP4–LjNIN heterodimer could bind to the region (Figures 6, C and 7, E). Coexistence of LjNIN, consisting of an RWP-RK and a PB1 domain, and C-terminal part of LjNLP4 lacking a PB1 domain did not cause heterodimer-interacting band shift (Supplemental Figure S7D). Taken together, these results indicate that LjNLP4 can interact with LjNIN through a PB1 domain, and the resulting formation of an LjNLP4–LjNIN heterodimer is likely to reduce the chances of DNA binding by the LjNIN homodimer.
To elucidate how LjNLP4/LjNIN-mediated gene induction or repression is accomplished, we investigated the effects of overexpression of LjNLP4 or LjNIN on gene expression in the presence of nitrate. In transgenic L. japonicus hairy roots, LjNLP4 overexpression had no effect on LjNF-YB expression regardless of the nitrate levels in nonsymbiotic conditions (Figure 8A). This finding is in agreement with the observation that LjNLP4 homodimer did not bind to cis-elements of LjNF-YB (Figures 6, C and 7, E). On the other hand, LjNIN-mediated LjNF-YB induction was inhibited by nitrate treatment (Figure 8A), probably resulting from the presence of nitrate-activated LjNLP4.
Figure 8.
The regulation of gene expression by LjNLP4 and LjNIN. A, The effect of LjNLP4 or LjNIN overexpression on LjNF-YB expression. Real-time RT-PCR analysis of LjNF-YB transcripts in transgenic hairy roots produced from WT containing the LjUBQpro:GUS, LjUBQpro:LjNLP4, or LjUBQpro:LjNIN constructs. Transgenic roots were identified by GFP fluorescence. Each cDNA sample was prepared from total RNA derived from noninoculated roots incubated with 0 or 10 mM KNO3 for 24 h (n = 3 independent pools of roots derived from 10 plants). The expression of LjUBQ was used as the reference. B–D, Transactivation of (B and C) 4xYB1:GUS and (D) LjNIR1pro:GUS in L. japonicus mesophyll protoplasts transformed with respective constructs (n = 3 independent pools of protoplasts). B and C, Full length LjNLP4 (1‒976) and truncated LjNLP4 (LjNLP4ΔPB1; 1‒656) were expressed in the assay. Transformed protoplasts were incubated with (B and D) 0 or 10 or (C) 10 mM KNO3. GUS activity was measured relative to (B and D) LjUBQpro:LUC or (C) 35Spro:LUC activity. E, A schematic diagram of the promoter-GUS constructs used in (F) and (G). Promoter fragments containing the LjNLP4/LjNIN-binding site (blue bar) in the LjNIR1 promoter region were inserted upstream of the GUS gene. In the modified LjNIR1 promoter, LjNIR1(NF-YB)pro, the LjNLP4/LjNIN-binding site was replaced with the LjNIN-binding site (red bar) from the LjNF-YB promoter region. F, Transactivation of LjNIR1pro:GUS or LjNIR1(NF-YB)pro:GUS in L. japonicus mesophyll protoplasts transformed with respective constructs (n = 3 independent pools of protoplasts). Transformed protoplasts were incubated with 10 mM KNO3. G, Real-time RT-PCR analysis of GUS expression in WT transgenic hairy roots expressing each GUS construct. Transgenic roots were identified by GFP fluorescence. Each cDNA sample was prepared from total RNA derived from noninoculated roots incubated with 0 or 10 mM KNO3 for 24 h (n = 3 independent pools of roots derived from five plants). The expression of GFP was used as the reference. Data of transactivation are normalized using the conditions in which GFP is expressed with (C and F) or without (B and D) nitrate. Individual data points are represented by blue points, and red crosses indicate the sample means. *P < 0.05 by a two-sided Student’s t test (A and B) or Welch's t test (D and G). Significant differences were determined with Kruskal–Wallis one-way analysis with Tukey multiple comparisons, indicated by different lower-case letters (P < 0.05; C and F).
We next developed a transactivation assay using mesophyll protoplasts of L. japonicus. Using this system, we found that, although LjNLP4 alone had no ability to induce LjNF-YB expression, LjNLP4 could reduce the LjNIN-mediated LjNF-YB expression (Figure 8B). On the other hand, LjNLP4 that lacks a PB1 domain did not affect LjNF-YB expression induced by LjNIN (Figure 8C). Therefore, these results indicate that LjNLP4 is able to interfere with LjNIN-mediated induction of nodulation-related genes through the PB1 domain. LjNIN had a weaker ability to induce LjNIR1 expression than the LjNLP4 and could reduce the induction level of nitrate-dependent LjNIR1 expression brought by LjNLP4 (Figure 8D).
Lastly, we asked if an apparently subtle difference in nucleotide composition of the cis-elements could be relevant to gene expression. To this end, we replaced an LjNLP4/LjNIN-binding site on the LjNIR1 promoter with an LjNIN-specific binding site on the LjNF-YB promoter and examined GUS reporter gene expression under the control of the modified promoter. In transactivation assay, although an intact LjNIR1 promoter could express GUS by LjNLP4, the modified LjNIR1 promoter, in which an original LjNLP4/LjNIN-binding site was replaced with an LjNIN-specific binding site, significantly reduced the ability to LjNLP4-mediated induction of the reporter gene (Figure 8, E and F). We obtained basically same conclusion when we introduced the modified promoter-GUS construct into L. japonicus hairy roots (Figure 8G). Hence, it is possible that the different specificities of DNA-binding between LjNLP4 and LjNIN affect gene expression.
Discussion
Our understanding of the molecular basis for how legumes cease root nodule symbiosis in the presence of a high nitrate concentration has been incomplete. In this study, we show that L. japonicus LjNLP4 and LjNLP1 have central roles in this regulation; the two NLP TFs have overlapping functions in the nitrate-induced control of root nodule symbiosis and act as master regulators for nitrate-dependent gene expression in L. japonicus roots. In Arabidopsis, AtNLP6 and AtNLP7 are localized within nuclei upon nitrate treatment and regulate the expression of most nitrate-responsive genes (Konishi and Yanagisawa, 2013; Marchive et al., 2013; Liu et al., 2017; Alvarez et al., 2020). In addition to previously identified LjNIR1 and CLE-RS2 as direct target genes of LjNLP4 (Nishida et al., 2018), we identified 72 genes as candidates for LjNLP4 direct targets. LjNIGT1 and LjLBD38, two homologous genes in Arabidopsis that are known to be directly regulated by AtNLP6/7 (Maeda et al., 2018; Alvarez et al., 2020), are included among the LjNLP4-target genes. Thus, it is likely that the nitrate signaling pathway in Arabidopsis is conserved in L. japonicus. In terms of nitrate-affected nodulation and gene expression, Ljnlp1 exhibits a phenotype similar to that of Ljnlp4. During nodulation, although the defect in nitrate responsiveness is clearly observed in the Ljnlp4 or Ljnlp1 single mutants, the phenotypes become more pronounced in the Ljnlp4 Ljnlp1 double mutants, suggesting that the two LjNLPs have overlapping functions. This conclusion is consistent with that reported in M. truncatula, where MtNLP1 and MtNLP4 function redundantly in nitrate inhibition of nodulation (Lin et al., 2018). In contrast, nitrate-dependent plant growth in Ljnlp1 in nonsymbiotic conditions is greatly retarded compared with that of Ljnlp4. This phenotypic difference indicates that LjNLP1 but not LjNLP4 has a predominant role in nitrate utilization.
Revealing how TFs can possess bifunctional roles to positively and negatively regulate gene expression is a fundamental problem for understanding the molecular details of plant transcriptional regulation. WUSCHEL, a crucial regulator of stem cell specification in the shoot apical meristem, possesses the bifunctional role because of comprising both a transcriptional activation and a repressive domain (Ikeda et al., 2009). LEAFY (LFY) can induce the expression of floral meristem identity genes and repress that of immune response genes (Winter et al., 2011). BRASSINAZOLE-RESISTANT 1 (BZR1) also plays dual roles in gene expression for brassinosteroid biosynthesis and downstream growth responses (He et al., 2005; Sun et al., 2010). In the presence of high nitrate levels, LjNLP4 and LjNLP1 can induce and repress the expression of symbiotic genes; the induced genes include CLE-RS2, which has a negative role in nodulation, and the repressed genes include LjNF-YA, LjNF-YB, LjEPR3, and LjRINRK1 that have positive roles in nodulation and are direct targets of LjNIN (Soyano et al., 2013; Kawaharada et al., 2015, 2017; Hossain et al., 2016; Li et al., 2019; Shrestha et al., 2021).
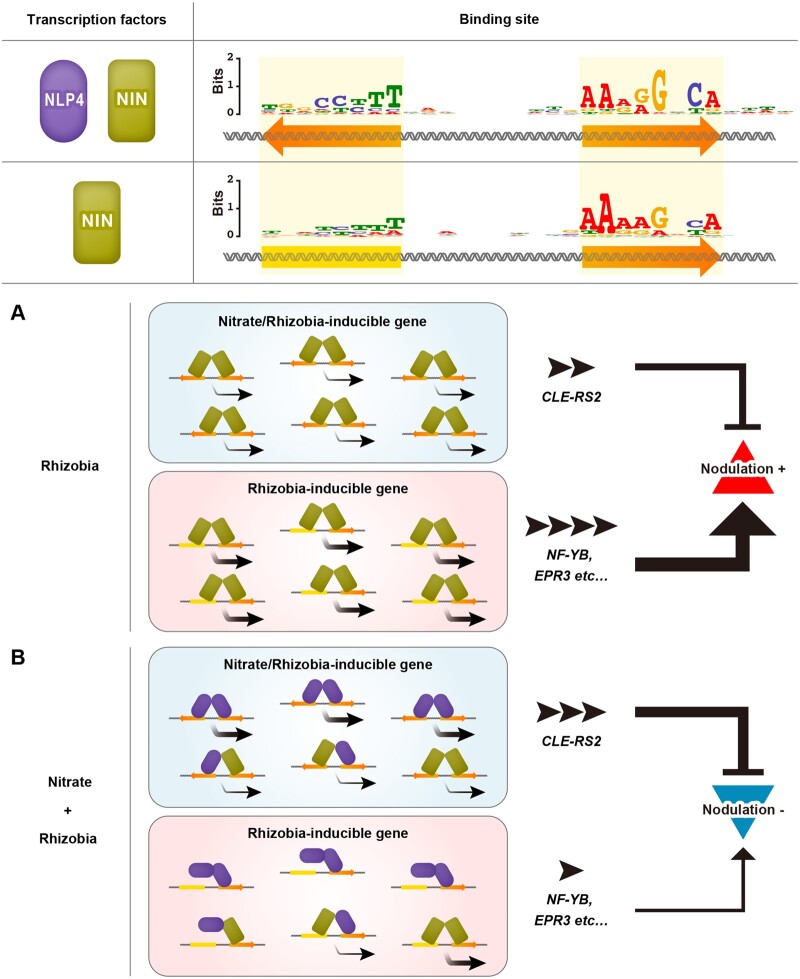
The two conserved motifs in the cis-elements, which we identified as LjNLP4/LjNIN-binding sites, have a semi-palindromic structure with similarity to NRE as shown by a previous study (Konishi and Yanagisawa, 2010). Several types of plant TFs, AUXIN RESPONSE FACTORs, LFY, and BZR1, form either homodimers or heterodimers with their homologs. The dimerization of TFs may be a prerequisite for binding to cis-elements containing a palindromic structure and gene expression (Ulmasov et al., 1999; Hamès et al., 2008; Boer et al., 2014; Nosaki et al., 2018). The LjNLP4 homodimer can bind to promoter regions of LjNIR1 and CLE-RS2, which contain semi-palindromic structures; this protein–DNA interaction induced gene expression. In contrast, the LjNLP4 homodimer did not sufficiently bind to promoter regions of nodulation-related genes, such as LjNF-YB, to which the LjNIN homodimer could bind. These different protein–DNA interaction patterns between LjNLP4 and LjNIN can be attributed to different DNA-binding specificity between the two proteins; unlike LjNLP4, LjNIN bound to cis-elements in which there were weaker palindromic structures compared with LjNLP4-binding sites. Thus, it is probable that the occupation of LjNIN-binding sites by the LjNLP4 homodimer reduces the chances of DNA binding by the LjNIN homodimer, which interferes with LjNIN-mediated gene expression (Figure 9).
Figure 9.
Model for the regulation of nodulation-related gene expression in response to nitrate in L. japonicus. Ellipses and rectangles denote LjNLP4 and LjNIN proteins, respectively. The LjNLP4/LjNIN-binding sites contain two conserved motifs (orange arrows) with semi-palindromic structures. On the other hand, the LjNIN-specific binding sites are less palindromic, as one of the motifs (yellow box) can be variable. A nitrate/rhizobia-inducible gene CLE-RS2 and a rhizobia-inducible gene LjNF-YB are shown as examples of negative and positive regulators for nodulation. CLE-RS2 has an LjNLP4/NIN binding site, whereas LjNF-YB has an LjNIN-specific binding site. A, In the presence of rhizobia only, the LjNIN homodimer binds to cis-elements of LjNF-YB and CLE-RS2 and induces the expression of both genes. The strength of LjNIN-mediated regulation of positive regulators for nodulation exceeds that of negative regulators, thereby nodulation is promoted. B, In the presence of nitrate and rhizobia, the expression of CLE-RS2 is strongly induced by the LjNLP4 homodimer. LjNLP4 homodimer has no ability to induce the expression of LjNF-YB possibly due to incomplete-binding to the LjNIN-specific binding site. LjNIN forms a heterodimer with LjNLP4 rather than forming the LjNIN homodimer. In these cases, LjNLP4 is likely to bind only to the more conserved motif (orange arrow) of the LjNIN-specific binding site. Formations of the LjNLP4 homodimer and LjNLP4–LjNIN heterodimer work together to reduce the chances of DNA binding by the LjNIN homodimer, thereby reducing the LjNIN homodimer-mediated gene induction. Root nodule symbiosis is inhibited by a combination of upregulated expression of negative regulators and downregulated expression of positive regulators for nodulation. The number of black arrowheads represents the levels of induced gene expression.
Moreover, LjNIN formed heterodimers with LjNLP4 rather than homodimers with itself. In terms of LjNF-YB induction, our data suggest that the LjNLP4–LjNIN heterodimer has lower activity than the LjNIN homodimer. In animals, two bZIP TFs, Fos and Jun, act with glucocorticoid receptors and regulate the target gene expression. Whereas the Jun homodimer is able to induce gene expression, transcriptional repression of the gene occurs when Jun forms a heterodimer with Fos (Diamond et al., 1990). This opposite transcriptional regulation is due to differences in DNA bending induced by Jun and Fos (Kerppola and Curran, 1991). Based on this example, it is probable that the direction of gene expression (induction/repression) is determined depending on which TF dimers are bound to the cis-elements.
Taking these observations together, we propose that LjNIN-dependent gene expression can be downregulated by a reduction in transcriptionally active LjNIN homodimers followed by nitrate-induced formation of transcriptionally less active LjNLP4–LjNIN heterodimers (Figure 9). Conversely, the induction level of LjNIR1 by LjNLP4 is reduced by LjNIN, possibly because the ratio of LjNLP4 homodimers is reduced by the formation of LjNLP4–LjNIN heterodimers (Supplemental Figure S8). This hypothesis implies that LjNIN can reduce LjNLP4-mediated gene expression, a proposal that is consistent with a previous observation showing LjNIN overexpression inhibits nitrate-inducible gene expression (Soyano et al., 2015).
Although transcriptome analysis indicated the downstream genes of LjNLP1 overlapped with those of LjNLP4, the detailed role of LjNLP1 in transcriptional regulation remains elusive. MtNLP1 downregulates MtCRE1 expression possibly via physical interaction with MtNIN and/or by binding to the same cis-element as MtNIN (Lin et al., 2018). Thus, it is possible that LjNLP1 controls NIN-target gene expression by a similar mechanism that is observed in M. truncatula. Lastly, unlike most NLPs reported so far but like AtNLP8 (Yan et al., 2016), LjNLP1 is localized within nuclei irrespective of nitrate level. How such constitutively nuclear-localized NLPs can function only when nitrate is present remains unclear. One possibility is that upon nitrate treatment posttranslational modifications (PTMs) may allow the NLPs protein–protein interaction and/or modulate DNA-binding affinities. To date, more than 200 different types of PTMs have been identified, including acetylation, glycosylation and phosphorylation (Deribe et al., 2010). Characterizing the nitrate-induced PTMs should be incorporated into future functional analyses of LjNLP1.
Materials and methods
Plant materials and growth conditions
In this study, the Miyakojima MG-20 ecotype of L. japonicus was used as the WT plant (Kawaguchi, 2000). The nrsym2/Ljnlp1 mutants were isolated in a previous screening for EMS mutants involved in the nitrate response during nodulation (Nishida et al., 2018). The Ljnlp1 mutants were backcrossed with WT once and descendent Ljnlp1 mutants were used for all analyses in this study. A description of Ljnlp4-1, previously named nrsym1-1, and Ljnin-9 plants was published previously (Suzaki et al., 2012; Nishida et al., 2018). Plants were grown with or without Mesorhizobium loti MAFF 303099 in autoclaved vermiculite with Broughton and Dilworth solution under a 16-h light/8-h dark cycle at 24°C (Broughton and Dilworth, 1971). For the nitrate response assay, varying concentrations of KNO3 (0, 10, or 60 mM) were used as supplements.
Genome-resequencing of the nrsym2/Ljnlp1 mutants
The nrsym2/Ljnlp1 mutants were crossed with WT, and F2 progeny displaying nitrate-tolerant phenotype was screened. Genomic DNA was extracted from pools of leaves derived from 20 plants using a DNeasy Plant Mini Kit (Qiagen). Libraries were constructed using a TruSeq DNA Sample Prep Kit (Illumina) and sequenced using a NextSeq 500 (Illumina) instrument with an 86-bp single-end sequencing protocol. Reads were mapped against the L. japonicus genome version 3.0 (http://www.kazusa.or.jp/lotus/) by Bowtie-0.12.9 (Langmead et al., 2009). Single-nucleotide polymorphism candidates were identified using the Mitsucal program (Suzuki et al., 2018).
Phylogenetic analysis
Phylogenetic analysis was conducted in MEGA X (Kumar et al., 2018). The phylogenetic tree was built using the neighbor-joining method. The optimal tree with the sum of branch length = 6.32672472 was shown. One thousand bootstrap replicates were performed for tree reconstruction. The tree was rooted to the outgroup LjNIN and drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and were in the units of the number of amino acid substitutions per site. Sequence alignment and machine-readable tree file are shown in Supplemental Files S1 and S2, respectively.
Acetylene reduction assay
The nitrogenase activity of nodules was indirectly determined by measuring the acetylene reductase activity (Nishida et al., 2018). Nodulated roots detached from intact plants were put into 20 mL vials. Subsequently, acetylene was injected into the vials. After incubation for 30 min at 25°C, the amount of ethylene produced was measured by gas chromatography (Nishida et al., 2018).
Constructs
The primers used for PCR are listed in Supplemental Data Set S5. For the complementation analysis of the nrsym2/Ljnlp1 mutants, a 9.5-kb genomic DNA fragment including the LjNLP1 candidate gene was amplified by PCR from WT genomic DNA. This fragment, including a 4-kb sequence directly upstream of the initiation codon, was cloned into pCAMBIA1300-GFP (Suzaki et al., 2019). For immunohistochemistry, the LjNLP1 coding sequence without a stop codon was amplified by PCR from template cDNA prepared from WT roots and cloned into the pENTR/D-TOPO vector. The insert was transferred into pGWB20 (Nakagawa et al., 2007) by the LR recombination reaction in order to express a C-terminal fusion to a 10xMyc (myc) tag. Using the resulting construct as a template, the LjNLP1-myc fragments were amplified by PCR and cloned into the pENTR/D-TOPO vector. The insert was transferred into pUB-GW-GFP (Maekawa et al., 2008) by the LR recombination reaction. To make the construct for DAP-seq of LjNLP4, a portion of LjNLP4 (531–976) was amplified by PCR from template cDNA prepared from WT roots and cloned into the pENTR/D-TOPO vector. The insert was transferred into the pIX-HALO in vitro expression vector (Bartlett et al., 2017) by the LR recombination reaction. For promoter-GUS analysis using L. japonicus hairy roots, an LjNIN-specific binding site from the LjNF-YB promoter was replaced with the LjNLP4/LjNIN-binding site in the LjNIR1pro:GUS construct (Nishida et al., 2018) by the In-Fusion reaction.
To make the constructs for recombinant protein expression in Escherichia coli, portions of LjNLP4 (564‒976 or 575‒656) and LjNIN (551‒878) were amplified by PCR from template cDNA prepared from WT. The cDNA fragments of LjNLP4 (564‒976 or 575‒656) and LjNIN (551‒878) were cloned into the expression vector pMAL-c2X (New England Biolabs) by the In-Fusion reaction. The latter was also cloned into another expression vector pET-48b (+) (Addgene).
For yeast two-hybrid assays, the cDNA fragments of LjNLP4 (830–976 or 564–656), LjNLP1 (764–903 or 573–662) and LjNIN (730–878 or 551–641) were amplified by PCR from template cDNAs prepared from WT and were cloned into the pGBKT7 or pGADT7 vectors (Clontech) by the In-Fusion reaction. To make the constructs for BiFC, the cDNA fragments of LjNLP4 (564–976), LjNLP1 (573–903), LjNIN (551–878), and LjIAA14 (Lj5g3v1204430) were amplified by PCR from template cDNAs prepared from WT and were cloned into the pSPYNE(R)173 and pSPYCE(MR) vectors (Waadt et al., 2008) by the In-Fusion reaction. For the transactivation assay using protoplasts, two set of fragments, LjUBQpro-NOSter and 35Spro-RBCSter were inserted between XbaI and HindIII sites of pUC19, which made it possible to insert gene of interest downstream of LjUBQpro or 35Spro using newly created SalI site. Each fragment of GFP, LjNLP4 (1‒976 or 1‒656), LjNIN-HA (see below), and LUC was amplified by PCR using original vectors (this study; Soyano et al., 2014; Nishida et al., 2018) as a template and inserted at the SalI site by the In-Fusion reaction. For making LjNIN-HA, the LjNIN coding sequence without a stop codon was amplified by PCR from template cDNA prepared from WT roots and cloned into the pENTR/D-TOPO vector. The insert was transferred into pGWB14 (Nakagawa et al., 2007) by the LR recombination reaction in order to express a C-terminal fusion to a 3xHA (HA) tag. To make reporter constructs, GUS-RBCSter cassette was amplified by PCR using original vector (Nishida et al., 2018) as a template and inserted at the PstI site of pUC19. Each fragment of LjNIR1pro, LjNIR1(NF-YB)pro, and 4xYB1 was amplified by PCR using original vectors (this study; Soyano et al., 2013; Nishida et al., 2018) as a template and inserted upstream of GUS using an appropriate site of restriction enzyme by the In-Fusion reaction. The LjUBQpro:LjNLP4 or LjUBQpro:LjNIN constructs for LjNLP4 or LjNIN overexpression in L. japonicus hairy roots were described previously (Suzaki et al., 2012; Nishida et al., 2018).
Hairy root transformation of L. japonicus
Constructs of interest were introduced into Agrobacterium rhizogenes and were transformed into roots of L. japonicus plants by hairy root transformation (Okamoto et al., 2013b). Agrobacterium rhizogenes AR1193 strains harboring each construct were grown on Yeast extract peptone (YEP) plates with appropriate antibiotics for 2 d at 28°C. Four-day-old seedlings were placed in the A. rhizogenes suspension and then cut at the hypocotyl base. Seedlings with cotyledons were transferred onto hairy root medium (1× Gamborg's B5 salt mixture, 1× Gamborg's vitamin solution, 2% sucrose, 1% agar) and were grown in a growth cabinet (24°C dark for the first day, 24°C 16-h light/8-h dark cycle for next 2 d). Then, plants were transferred onto fresh hairy root medium containing 12.5 μg/mL Meropen and were grown for 7–10 d in a growth cabinet (24°C 16-h light/8-h dark cycle). Plants with transgenic hairy roots were used for further experiments.
Gene expression analysis
The primers used for PCR are shown in Supplemental Data Set S5. Total RNA was isolated from whole roots using the PureLink Plant RNA Reagent (Invitrogen) or the Plant Total RNA Mini Kit (Favorgen Biotech). First-strand cDNA was prepared using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo). Real-time RT-PCR was performed using a 7900HT Real-Time PCR system (Applied Biosystems) with a THUNDERBIRD SYBR qPCR Mix (Toyobo) following the manufacturer’s instructions.
Immunohistochemistry
Transgenic hairy roots carrying the LjUBQpro:LjNLP1-myc construct were fixed in 3% paraformaldehyde in MTSB buffer (50 mM PIPES-KOH (pH 7.0), 5 mM EGTA, 5 mM MgSO4) for 40 min under vacuum. After washing with wash buffer (MTSB buffer with 0.1% Triton X-100), roots were preincubated in 3% BSA in wash buffer at room temperature for 1 h and then incubated with a 1:100 dilution of an anti-myc mouse monoclonal antibody (Santa Cruz Biotechnology Inc.) in 3% BSA in wash buffer at room temperature overnight. After washing with wash buffer, the roots were incubated with a 1:1,000 dilution of anti-mouse IgG-Alexa Fluor Plus 488 (Invitrogen) in 3% BSA in wash buffer at room temperature for 3 h. Before observing the signal, the roots were stained with 5 μg mL−1 4ʹ, 6-diamidino-2-phenylindole (Dojindo) for 15 min. Fluorescent images were obtained using a LSM700 confocal laser-scanning microscope (Carl Zeiss) equipped with ZEN software (Carl Zeiss).
RNA-seq analysis
Total RNA was isolated from roots using the PureLink Plant RNA Reagent (Invitrogen). The RNeasy Plant Mini Kit (Qiagen) was used to remove genomic DNA and clean up RNA. Libraries were generated using a NEBNext Ultra II RNA Library Prep Kit from Illumina (New England Biolabs) following the manufacturer’s instructions and sequenced using a NextSeq 500 (Illumina) instrument with the 86-bp single-end sequencing protocol. RNA-seq data were mapped to the L. japonicus genome version 3.0 using HISAT2-2.2.0 with the default parameters (Kim et al., 2015). The resulting bam files were then assembled using StringTie-1.3.5 (Pertea et al., 2015). Three biological replicates were analyzed, and differentially expressed genes were identified with edgeR-3.30.3 (Robinson et al., 2010) using a 5% false discovery rate (FDR) cut-off. A heatmap was drawn using heatmap.2 from R. Genes that were nitrate-inducible in WT but not in the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants (log2FC ≦1 or FDR ≧0.05), or nitrate-inducible genes (log2FC >1 and FDR <0.05) with higher expression in WT than in each mutant under 10 mM KNO3 conditions were identified as LjNLP4, LjNLP1, or LjNLP4/1-dependent nitrate-inducible genes, respectively. Genes that were nitrate repressible in WT but not in the Ljnlp4-1 mutants, the Ljnlp1 mutants, and the Ljnlp4-1 Ljnlp1 double mutants (log2FC ≧ −1 or FDR ≧0.05), or nitrate-repressible genes (log2FC less than −1 and FDR <0.05) with lower expression in WT than in each mutant under 10 mM KNO3 conditions were identified as LjNLP4, LjNLP1, or LjNLP4/1-dependent nitrate-repressible genes, respectively. Genes that were rhizobia-inducible genes in WT but not in the Ljnin-9 mutants (log2FC ≦1 or FDR ≧0.05), or rhizobia-inducible genes (log2FC >1 and FDR <0.05) with higher expression ) in WT than in the Ljnin-9 mutants under the rhizobia conditions were identified as LjNIN-dependent rhizobia-inducible genes.
DAP-seq analysis
Genomic DNA was extracted from 10 mM KNO3-treated WT roots using a DNeasy Plant Mini Kit (Qiagen). Libraries were generated using NEBNext Ultra II DNA Library Prep Kit from Illumina (New England Biolabs) following the manufacturer’s instructions. DAP-seq was performed according to the method described by Bartlett et al. (2017). Specifically, the HALO-LjNLP4 (531–976) protein was synthesized using the TNT SP6 High-Yield Wheat Germ Protein Expression System (Promega). HALO-LjNLP4 (531–976) was immobilized on Magne HaloTag beads (Promega), washed, and incubated with the DNA library. After bead washing, DNA was eluted and amplified with indexed primers. Sequencing was performed using a NextSeq 500 (Illumina) instrument with an 86-bp single-end sequencing protocol. Reads were mapped against the L. japonicus genome version 3.0 by Bowtie2-2.3.5.1 (Langmead and Salzberg, 2012). To visualize with the Integrative Genome Browser (IGV)-2.8.2 (Robinson et al., 2011), bam files were converted to bigwig files using deepTools-3.4.2 bamCoverage (Ramírez et al., 2016) with a 10-bp bin size and reads per kilobase of exon per million mapped reads normalization. To identify LjNLP4-binding sites, DAP-seq peaks were identified by MACS2-2.2.7.1 (Zhang et al., 2008) using default parameters (q-value <0.05). LjNLP4-bound genes were identified by the closest gene to the LjNLP4-binding site when searched with bedtools-2.29.2 closet (Quinlan and Hall, 2010). Motifs were discovered using MEME Suite-5.0.5 MEME-ChIP (Machanick and Bailey, 2011). DAP-seq peaks and LjNLP4-binding sites were visualized in the IGV.
Identification of LjNIN-binding sites
LjNIN-binding sites in the L. japonicus genome version 3.0 were identified by BLAST searches using the ChIP-seq data of LjNIN that was previously provided using the L. japonicus genome version 2.5 (Soyano et al., 2014). Find individual motif occurrences (Grant et al., 2011; P <0.005) searched the cis-elements for the 8 (LjNLP4 bound) and 17 genes (LjNLP4 did not bind) that were directly upregulated by LjNIN in response to rhizobia and downregulated by nitrate in an LjNLP4-dependent manner (Supplemental Data Set S4). NIN-binding sites were visualized in the IGV.
Recombinant protein expression and purification
Expression vectors were transformed into E. coli strain Rosetta (DE3) (Novagen). The expressed proteins from pMAL-c2X and pET-48b (+) contained a maltose-binding protein (MBP) or a thioredoxin followed by 6× histidine (Trx-His) at the N-terminus, respectively. Isopropyl β-d-1-thiogalactopyranoside-induced overexpression continued for 20 h at 18°C. Cells were harvested by centrifugation at 5,000 rpm for 15 min and stored at –80°C until use. The harvested cells were resuspended in buffer A (20 mM Tris–HCl pH 7.5, 1 M NaCl, 1 mM DTT, 1 mM EDTA, and 10% glycerol) for MBP-fused proteins or in buffer B (20 mM Tris–HCl pH 8.5, 1 M NaCl, 1 mM DTT, 10% glycerol, and 10 mM imidazole) for Trx-His-LjNIN (551–878) or buffer C (20 mM Tris–HCl pH 7.0, 1 M NaCl, 0.25 mM DTT, and 10% glycerol) for Trx-His-LjNLP4ΔPB1 (575–656). After the addition of 0.2 mg/mL lysozyme, 1 mM phenylmethylsulfonyl fluoride and 1× protease inhibitor cocktail (EDTA free) (Nacalai tesque), the resuspended cells were lysed by sonication and separated by centrifugation. The supernatant fractions of MBP-fused LjNLP4 (564–976) and LjNIN (551–878) were then applied to amylose resin (New England Biolabs). After washing with buffer A, the fusion proteins were eluted with buffer containing 30 mM maltose. The supernatant fraction of Trx-His-LjNIN (551–878) was applied to a Ni-NTA Superflow Resin (Qiagen), washed and then eluted with buffer containing 20 mM imidazole followed by 250 mM imidazole. The supernatant fraction of Trx-His-LjNLP4ΔPB1 (575–656) was applied to a TALON metal affinity resin (TAKARA), washed with buffer C and then eluted with buffer containing 200 mM imidazole. The eluates of MBP-LjNLP4 (564–976), MBP-LjNIN (551–878), and Trx-His-LjNIN (551–878) were concentrated with Vivaspin 15R centrifugal concentrators (30,000 MWCO Hydrosart) (Sartorius) and further purified by SEC on a HiLoad 26/600 Superdex 200 prep grade column (GE Healthcare) equilibrated with buffer D (20 mM Tris–HCl pH 7.5, 1 M NaCl, 1 mM DTT, and 10% glycerol) to remove protein impurities and aggregates. The eluate of Trx-His-LjNLP4ΔPB1 (575–656) was concentrated with Vivaspin 20 centrifugal concentrators (10,000 MWCO PES) (Sartorius), followed by SEC purification on a Superdex 200 10/300 GL column (GE Healthcare) equilibrated with buffer D. The purified proteins were concentrated to 40 µM in preparation for EMSA and SEC-MALS.
Electrophoretic mobility shift assay
For preparing the probes, DNA fragments (Supplemental Table S1) were labeled with carboxyfluorescein (FAM). The labeled DNA fragments were purified on the Superdex 200 10/300 GL column (GE Healthcare). The purified DNA fragments (0.25 µM) and poly (dI-dC) (50 ng/µL) were mixed with the purified proteins in buffer D (10 mM Tris–HCl pH 7.5, 100 mM KCl, 100 mM NaCl, 1 mM DTT, 2.5% glycerol, and 5 mM MgCl2), and incubated at 25°C for 30 min. The mixtures were loaded on a 10% polyacrylamide gel, and fluorescence was detected using an Amersham Imager 680 system (GE Healthcare) or LuminoGraph III WSE-6300 (ATTO).
Size exclusion chromatography coupled to multi-angle scattering
The absolute molecular mass determination was carried out with Shodex PROTEIN LW-803 column (8.0 × 300 mm2, Shoko science) connected PROTEIN LW-G 6B guard column (6.0 × 50 mm2, Shoko science). All samples were analyzed in the buffer containing 20 mM Tris–HCl pH 7.5, 250 mM NaCl, 1 mM DTT, 5 mM MgCl2, and 10% glycerol at room temperature. The MBP-LjNLP4 (564–976) and MBP-LjNIN (551–878) proteins (10 µΜ, 150 µL) in the presence or absence of the nonlabeled LjNIR1pro DNA fragment (5 µΜ; Supplemental Table S1) were loaded onto the column and then eluted with a flow rate of 0.5 mL min‒1. Light scattering, refractive index, and absorbance at 280 nm were detected on a DAWN HELEOS II instrument (Wyatt Technology), an Optilab T-rEX (Wyatt Technology) and an SPD-20A UV–VIS detector (Shimadzu), respectively. The detectors were calibrated with BSA before the experiment. Molar masses were calculated with ASTRA software (Wyatt Technology) using the refractive index increment (dn/dc) values of 0.180 mL g‒1 (proteins) and 0.165 mL g‒1 (DNA) corrected with BSA. The extinction coefficients at 280 nm of 1.05 mL mg‒1 cm‒1 (MBP-LjNLP4), 0.82 mL mg‒1 cm‒1 (MBP-LjNIN), and 10.4 mL mg‒1 cm‒1 (LjNIR1pro DNA) were applied in the protein conjugated analysis in the ASTRA software.
Yeast two-hybrid assay
Yeast two-hybrid assays were performed following the instructions for the Matchmaker GAL4 Two-Hybrid System 3 (Clontech). pGBKT7-53 and pGBKT7-Lam contain the Gal4 DNA-binding domain (BD) fused to murine p53 and lamin, respectively. pGADT7-T contains the Gal4 activation domain (AD) fused to the SV40 large T-antigen. To test protein–protein interactions, the Gal4 DNA-BD and Gal4 AD fusion constructs were cotransformed into the Y2HGold Yeast Strain. Transformation was confirmed by the growth of transformants on SD medium lacking leucine and tryptophan (-Leu/-Trp). Protein–protein interactions were assayed by spreading 10 µL of each transformed yeast suspension (OD600 = 1) on SD medium lacking adenine, histidine, leucine, and tryptophan (-Ade/-His/-Leu/-Trp) and growing the cultures for 3–5 d at 30°C.
Biomolecular fluorescence complementation
Infiltration of N. benthamiana for transient expression was performed based on the method previously reported (Betsuyaku et al. 2011). Agrobacterium tumefaciens GV3101 MP90 strains harboring each construct were grown on YEP plates with appropriate antibiotics for 2 d at 28°C. 20-d-old N. benthamiana leaves were observed 2 d after Agrobacterium infiltration. LjIAA14–NLP combination was used as negative control for BiFC as described previously (Lin et al., 2018).
Transactivation using L. japonicus protoplasts
Protoplast transient assays using L. japonicus were based on the method of Yoo et al. (2007). Specifically, plants were grown for 10 d with 5 mM KNO3 and for the next 3 d without KNO3. Mesophyll protoplasts were isolated from the 13-d-old L. japonicus seedlings, including leaves, stems, and cotyledons. The seedlings were chopped by a razor blade and were incubated in an enzyme solution [20 mM MES (pH 5.7), 1.5% Cellulase R10 (Yakult), 2% Macerozyme R10 (Yakult), 0.4 M mannitol, 20 mM KCl, 10 mM CaCl2, and 0.1% BSA] at 24°C in the dark for 5 h. For transformation, 100 µL protoplasts (3 × 105 protoplasts) in MMG solution [4 mM MES (pH 5.7), 0.4 M mannitol, and 15 mM MgCl2] and 30–40 µL DNA mixture solution, including effector, reporter, and internal control plasmids, were mixed with 130–140 µL of PEG solution (40% PEG4000, 0.2 M mannitol, and 100 mM CaCl2). Then, protoplasts were incubated overnight at 24°C in the dark in WI solution [4 mM MES (pH 5.7), 0.5 M mannitol, and 20 mM KCl] with 0 or 10 mM KNO3 and harvested by centrifugation. Harvested protoplasts were resuspended in extraction buffer [100 mM phosphate buffer (pH 7.2), 5 mM DTT, and Complete Protease Inhibitor Cocktail (Roche Diagnostics)] and sonicated for 1 s, 20 times using a Microson Ultrasonic Cell Disruptor (Misonix). For GUS assays, 100 μL cell lysate was mixed with 100 μL GUS assay buffer [100 mM phosphate buffer (pH 7.2), 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1% Triton X-100, 5% glycerol, 1 mM 4-methylumbelliferyl-β-d-glucuronide (4-MUG), and 17% methanol]. The mixture was incubated for 1 h at 37 °C, and then 800 μL 0.2 M Na2CO3 was added. Fluorescence was measured using a DTX 880 Multimode Detector (Beckman) or a Synergy LX (Biotek). Luciferase (LUC) assays were performed using a PicaGene Luminescence Kit (Wako) with the DTX 880 Multimode Detector or the Synergy LX. For transformation of protoplasts, equal amount of DNA (10 µg each) of effector, reporter and internal control plasmids, having pCAMBIA1300GFP or pUC19 as a backbone, were used. 35Spro:GFP, LjUBQpro:GFP, LjUBQpro:LjNLP4 (1–977 or 1–656), LjUBQpro:LjNIN and 35Spro:LjNIN-HA were used as the effector plasmids. The reporter plasmids, 4xYB1:GUS, in which a fragment consisting of four tandem repeats of LjNIN-binding sites on the LjNF-YB promoters and CaMV35S minimal promoter were inserted upstream of GUS, LjNIR1pro:GUS and LjNIR1(NF-YB)pro:GUS were described previously or newly created (see above) (Soyano et al., 2013; Nishida et al., 2018). LjUBQpro:LUC (Soyano et al., 2014) or 35Spro:LUC (see above) was used as the internal control plasmids.
Statistical analysis
Each experiment except RNA-seq and DAP-seq was repeated at least twice. Data statistical analysis was performed using R version 3.6.2 (https://cran.r-project.org/). Normality was checked using the Shapiro–Wilk test and P > 0.05 was considered as normal distribution. The F-test was used to test if the variances of two populations were equal or not. The variance of multiple groups was tested by Bartlett's test (P < 0.05). For comparing data of two groups, the two-tailed Student’s t test, the two-sided Welch's t test or Wilcoxon rank sum test was performed. For multiple comparisons, the Kruskal–Wallis one-way analysis with post hoc Steel–Dwass test, Tukey’s test or Steel’s test were used. The P-value was corrected by the Bonferroni correction in multiple statistical tests. The criterion of P < 0.05 means statistically significant difference in this study. The results of the statistical analyses are shown in Supplemental Data Set S6.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession number: LjNLP1, LC572269. Data from the short reads from genome-resequencing of nrsym2/Ljnlp1, RNA-seq, and DAP-seq analyses were respectively deposited in the DNA Data Bank of Japan Sequence Read Archive under the accession numbers DRA010704, DRA010705, and DRA010712.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Complementation of the nrsym2/Ljnlp1 nodulation phenotype. (Supports Figure 1.)
Supplemental Figure S2. The LjNLP1 expression pattern and subcellular localization of LjNLP1. (Supports Figure 1.)
Supplemental Figure S3. Nodule phenotypes of the Ljnlp4 Ljnlp1 double mutants under extremely high nitrate conditions. (Supports Figure 2.)
Supplemental Figure S4. Comparison of nitrate-inducible genes. (Supports Figure 4.)
Supplemental Figure S5. Comparison between nitrate-repressible and rhizobia-inducible genes. (Supports Figure 4.)
Supplemental Figure S6. Identification of LjNLP4 and NIN-binding sites. (Supports Figure 5.)
Supplemental Figure S7. Interaction between LjNLP4/1 and LjNIN. (Supports Figure 7.)
Supplemental Figure S8. Model for the regulation of nitrate-inducible gene expression in L. japonicus. (Supports Figure 9.)
Supplemental Table S1. EMSA probes.
Supplemental Data Set S1. The list of nitrate-inducible genes.
Supplemental Data Set S2. The list of rhizobia-inducible and nitrate-repressible genes.
Supplemental Data Set S3. The list of LjNIN-dependent rhizobia-inducible and LjNLP4/1-dependent nitrate-repressible genes.
Supplemental Data Set S4. The list of candidates for LjNLP4 and/or LjNIN target genes.
Supplemental Data Set S5. Primers used in this work.
Supplemental Data Set S6. Statistical analysis data.
Supplemental File S1. Sequence alignment of NLPs in fasta format.
Supplemental File S2. Phylogenetic tree in newick format.
Supplementary Material
Acknowledgments
We thank Makoto Hayashi for providing M. loti MAFF303099 expressing DsRED; Takashi Soyano for providing 4xYB1:GUS, LjUBQpro:LUC, pSPYNE(R)173 and pSPYCE(MR) constructs; Mary Galli for providing pIX-HALO vector; Taiji Kawakatsu for technical support and valuable suggestions; Shigeyuki Betsuyaku, Ryo Nishijima, Kazuko Ito for technical support; the customer support staffs of Shoko science for performing the SEC-MALS analysis.
Funding
This work was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI (JP18H04773, JP19H03239 and JP20H05908), by Japan Science Technology Agency (JST) Exploratory Research for Advanced Technology (ERATO) (JPMJER1502), by the Cooperative Research Grant of the Plant Transgenic Design Initiative by Gene Research Center, University of Tsukuba (#2009).
Conflict of interest statement.The authors declare no conflict of interests.
T.S. supervised the research. H.N., S.N., T.M., M.T., M.K., and T.S. designed the experiments. H.N., S.N., T.S., M.I., M.N., Y.T., K.M., and T.S. performed experiments and analyzed the data. H.N., S.N., and T.S. wrote the article, which was discussed and approved by all authors.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Takuya Suzaki (suzaki.takuya.fn@u.tsukuba.ac.jp).
References
- Alvarez JM, Schinke AL, Brooks MD, Pasquino A, Leonelli L, Varala K, Safi A, Krouk G, Krapp A, Coruzzi GM (2020) Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat Commun 11: 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett A, O'Malley RC, Huang S-SC, Galli M, Nery JR, Gallavotti A, Ecker JR (2017) Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat Protoc 12: 1659–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsuyaku S, Takahashi F, Kinoshita A, Miwa H, Shinozaki K, Fukuda H, Sawa S (2011) Mitogen-activated protein kinase regulated by the CLAVATA receptors contributes to shoot apical meristem homeostasis. Plant Cell Physiol 52: 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WAM, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, de Vries S, et al. (2014) Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589 [DOI] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125: 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Mathews A (1990) Nitrate inhibition of nodulation in legumes. InGresshof PM, ed, The Molecular Biology of Symbiotic Nitrogen Fixation. CRC Press, Boca Raton, pp 159–180 [Google Scholar]
- Deribe YL, Pawson T, Dikic I (2010) Post-translational modifications in signal integration. Nat Struct Mol Biol 17: 666–672 [DOI] [PubMed] [Google Scholar]
- Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR (1990) Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 249: 1266–1272 [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2019) Legume nodulation: the host controls the party. Plant Cell Environ 42: 41–51 [DOI] [PubMed] [Google Scholar]
- Grant CE, Bailey TL, Noble WS (2011) FIMO: scanning for occurrences of a given motif. Bioinformatics 27: 1017–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamès C, Ptchelkine D, Grimm C, Thevenon E, Moyroud E, Gérard F, Martiel JL, Benlloch R, Parcy F, Müller CW (2008) Structural basis for LEAFY floral switch function and similarity with helix-turn-helix proteins. EMBO J 27: 2628–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SSL, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Shrestha A, Zhong S, Miri M, Austin RS, Sato S, Ross L, Huebert T, Tromas A, Torres-Jerez I, et al. (2016) Lotus japonicus NF-YA1 plays an essential role during nodule differentiation and targets members of the SHI/STY gene family. Mol Plant-Microbe Interact 29: 950–964 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M (2009) Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21: 3493–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M (2000) Lotus japonicus ‘Miyakojima’ MG-20: an early-flowering accession suitable for indoor handling. J Plant Res 113: 507–509 [Google Scholar]
- Kawaharada Y, Kelly S, Nielsen MW, Hjuler CT, Gysel K, Muszynski A, Carlson RW, Thygesen MB, Sandal N, Asmussen MH, et al. (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523: 308–312 [DOI] [PubMed] [Google Scholar]
- Kawaharada Y, Nielsen MW, Kelly S, James EK, Andersen KR, Rasmussen SR, Füchtbauer W, Madsen LH, Heckmann AB, Radutoiu S, et al. (2017) Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat Commun 8: 14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola TK, Curran T (1991) Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell 66: 317–326 [DOI] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S (2010) Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response. Plant J 63: 269–282 [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S (2013) Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun 4: 1617. [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S (2019) The role of protein-protein interactions mediated by the PB1 domain of NLP transcription factors in nitrate-inducible gene expression. BMC Plant Biol 19: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont C, Ivanovici A, Gautrat P, Brault M, Djordjevic MA, Frugier F (2020) The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat Commun 11: 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zheng Z, Kong X, Xu J, Qiu L, Sun J, Reid D, Jin H, Andersen SU, Oldroyd GED, et al. (2019) Atypical receptor kinase RINRK1 required for rhizobial infection but not nodule development in Lotus japonicus. Plant Physiol 181: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Li X, Luo Z, Mysore KS, Wen J, Xie F (2018) NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat Plants 4: 942–952 [DOI] [PubMed] [Google Scholar]
- Liu KH, Niu Y, Konishi M, Wu Y, Du H, Sun Chung H, Li L, Boudsocq M, McCormack M, Maekawa S, et al. (2017) Discovery of nitrate–CPK–NLP signalling in central nutrient–growth networks. Nature 545: 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P, Bailey TL (2011) MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27: 1696–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Konishi M, Kiba T, Sakuraba Y, Sawaki N, Kurai T, Ueda Y, Sakakibara H, Yanagisawa S (2018) A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat Commun 9: 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Kusakabe M, Shimoda Y, Sato S, Tabata S, Murooka Y, Hayashi M (2008) Polyubiquitin promoter-based binary vectors for overexpression and gene silencing in Lotus japonicus. Mol. Plant-Microbe Interact 21: 375–382 [DOI] [PubMed] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A (2013) Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun 4: 1713. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Nishida H, Tanaka S, Handa Y, Ito M, Sakamoto Y, Matsunaga S, Betsuyaku S, Miura K, Soyano T, Kawaguchi M, et al. (2018). A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat Commun 9: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Suzaki T (2018a) Two negative regulatory systems of root nodule symbiosis: how are symbiotic benefits and costs balanced? Plant Cell Physiol 59: 1733–1738 [DOI] [PubMed] [Google Scholar]
- Nishida H, Suzaki T (2018b) Nitrate-mediated control of root nodule symbiosis. Curr Opin Plant Biol 44: 129–136 [DOI] [PubMed] [Google Scholar]
- Nosaki S, Miyakawa T, Xu Y, Nakamura A, Hirabayashi K, Asami T, Nakano T, Tanokura M (2018) Structural basis for brassinosteroid response by BIL1/BZR1. Nat Plants 4: 771–776 [DOI] [PubMed] [Google Scholar]
- O’Malley RC, Huang SSC, Song L, Lewsey MG, Bartlett A, Nery JR, Galli M, Gallavotti A, Ecker JR (2016) Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 165: 1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Shinohara H, Mori T, Matsubayashi Y, Kawaguchi M (2013a) Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat Commun 4: 2191. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Yoro E, Suzaki T, Kawaguchi M (2013b) Hairy root transformation in Lotus japonicus. Bio-protocol 3: e795 [Google Scholar]
- Oldroyd GED, Leyser O (2020) A plant’s diet, surviving in a variable nutrient environment. Science 368: eaba0196. [DOI] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33: 290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dündar F, Manke T (2016) deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44: W160–W165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat. Biotechnol 29: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195 [DOI] [PubMed] [Google Scholar]
- Schauser L, Wieloch W, Stougaard J (2005) Evolution of NIN-Like proteins in Arabidopsis, rice, and Lotus japonicus. J Mol Evol 60: 229–237 [DOI] [PubMed] [Google Scholar]
- Schleif R (1988) DNA binding by proteins. Science 241: 1182–1187 [DOI] [PubMed] [Google Scholar]
- Shrestha A, Zhong S, Therrien J, Huebert T, Sato S, Mun T, Andersen SU, Stougaard J, Lepage A, Niebel A, et al. (2021) Lotus japonicus Nuclear Factor YA1, a nodule emergence stage-specific regulator of auxin signalling. New Phytol 229: 1535–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggers T, Gordân R (2014) Protein-DNA binding: complexities and multi-protein codes. Nucleic Acids Res 42: 2099–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Kouchi H, Hirota A, Hayashi M (2013) NODULE INCEPTION directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genet 9: e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Hirakawa H, Sato S, Hayashi M, Kawaguchi M (2014) NODULE INCEPTION creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci USA 111: 14607–14612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Shimoda Y, Hayashi M (2015) NODULE INCEPTION antagonistically regulates gene expression with nitrate in Lotus japonicus. Plant Cell Physiol 56: 368–376 [DOI] [PubMed] [Google Scholar]
- Streeter J, Wong PP (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7: 1–23 [Google Scholar]
- Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, et al. (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19: 765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yoro E, Kawaguchi M (2015) Leguminous plants: inventors of root nodules to accommodate symbiotic bacteria. Int Rev Cell Mol Biol 316: 111–158 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Takeda N, Nishida H, Hoshino M, Ito M, Misawa F, Handa Y, Miura K, Kawaguchi M (2019) LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet 15: e1007865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kawai T, Takemura S, Nishiwaki M, Suzuki T, Nakamura K, Ishiguro S, Higashiyama T (2018) Development of the Mitsucal computer system to identify causal mutation with a high-throughput sequencer. Plant Reprod 31: 117–128 [DOI] [PubMed] [Google Scholar]
- Suzuki W, Konishi M, Yanagisawa S (2013) The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signal Behav 8: e25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Dimerization and DNA binding of auxin response factors. Plant J 19: 309–319 [DOI] [PubMed] [Google Scholar]
- Vernié T, Kim J, Frances L, Ding Y, Sun J, Guan D, Niebel A, Gifford ML, de Carvalho-Niebel F, Oldroyd GED (2015) The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 27: 3410–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56: 505–516 [DOI] [PubMed] [Google Scholar]
- Wang L, Sun Z, Su C, Wang Y, Yan Q, Chen J, Ott T, Li X (2019) A GmNINa-miR172c-NNC1 regulatory network coordinates the nodulation and autoregulation of nodulation pathways in soybean. Mol Plant 12: 1211–1226 [DOI] [PubMed] [Google Scholar]
- Winter CM, Austin RS, Blanvillain-Baufumé S, Reback MA, Monniaux M, Wu M-F, Sang Y, Yamaguchi A, Yamaguchi N, Parker JE, et al. (2011) LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Dev Cell 20: 430–443 [DOI] [PubMed] [Google Scholar]
- Yan D, Easwaran V, Chau V, Okamoto M, Ierullo M, Kimura M, Endo A, Yano R, Pasha A, Gong Y, et al. (2016) NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat Commun 7: 13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.