Abstract
The objective of this study is to assess predictors of genetic beliefs toward cancer risk perceptions among adults, aged 18 years and over, in the United States (US). Data were obtained from the National Cancer Institute’s (NCI) Health Information National Trends Survey 2014 (HINTS) 4 Cycle 4. Bivariate and multivariable logistic regression analyses were conducted to assess factors associated with an individual’s beliefs about genetic and cancer risk perceptions. The results showed that African Americans, Non-White Hispanics, Non-Hispanic Asians, individuals with a high school education or less, and annual household incomes less than $20,000 and do not believe that health behaviors play some role in determining whether a person will develop cancer was significantly less likely to report that genetics plays at least some role in whether a person will develop cancer. Findings of this study provide an opportunity for genetic counselors to address beliefs about genetics and cancer risk perceptions among minority populations and promote health equity.
Keywords: attitudes, beliefs, disparities, diversity, education, genetic counseling, genetic testing, health behavior, health literacy, health promotion, public health, susceptibility testing, underrepresented populations
1 |. INTRODUCTION
Researchers have estimated that inherited genetic variants contribute to 5%–10% of all cancers (National Cancer Institute, 2017). Genetic testing for cancer risk has become an important tool to identify specific inherited or acquired changes or variants in a person’s chromosomes and/or genomic sequence that may convey an increased risk for cancer to an individual. Specifically, genetic testing can be used to determine whether a person has specific variants that increases his/her risk for developing a disease or condition. For example, a person who inherits cancer-predisposing genomic variants may have an increased but not absolute risk of developing cancer (National Cancer Institute, 2017).
Genetic variants can have variable effects ranging from harmful, neutral (no effect), to uncertain or unknown effects on health (National Cancer Institute, 2017). Seeking genetic counseling prior to undergoing testing is important for people considering genetic testing. It allows genetic counselors the opportunity to better address patients’ questions and concerns ranging from medical, psychosocial, social, and psychological issues including individual and familial anxiety, risk and benefits of testing and disease management, and costs (Rink & Kuller, 2018). With the advent of direct-to-consumer genetic testing and the lack of genetic counselors in some clinical settings, onsite genetic counseling may not be an option. Consultations with genetic counselors may be an alternative to providing patients with an understanding of the genetic testing that can help them make informed decisions (Patch & Middleton, 2018). For example, a positive test result indicating an increased cancer risk for a person undiagnosed with cancer may encourage the use of preventive strategies that may reduce his/her risk of developing cancer. Additionally, positive test results that include specific actionable variants for a person diagnosed with cancer may better inform treatment options and familial risks (National Cancer Institute, 2017).
Genetics has been a controversial topic for many people worldwide because of historical and current events where genetics was used to support or justify discrimination, criminalization, and institutionalization of individuals and groups (Green, Lautenbach, & McGuire, 2015; Rothstein & Anderlik, 2001). These historical injustices continue to elicit feelings of hesitation, fear, and misperception of genetics, especially among minority and socially excluded or marginalized individuals and/or communities including those who are homeless, incarcerated, and/or have mental health issues (McDonald et al., 2012; Zimmerman et al., 2006).
Mistrust of medical research, fear of discrimination and abuse, and reduced access to health-related services including genetic counseling, geography (rural/urban), lack of cultural appropriateness of the genetic tests, cost, and an overall lack of trust in the healthcare system are among the reasons disparities in genetic services, genetic testing utilization, and research participation persist. (Salloum et al., 2018). Addressing and overcoming these barriers are becoming increasingly important because of genetic testing’s increased utility and importance in healthcare.
Theory-based approaches have been utilized to better understand why disparities in genetic testing persist between Whites and other racial/ethnic populations. Agurs-Collins et al. (2015) proposed a conceptual framework that was adapted from Karen Glantz’s 1999 Social Cognitive Theory. This theory was designed to explain psychosocial factors that were associated with genetic testing for colorectal cancer (Agurs-Collins et al., 2015). Jones et al. (2016) studied the use of cancer genetic services in African American young breast cancer survivors using the theory of planned behavior. This theory hypothesized links one’s own beliefs to their behavior. It suggests that an intention toward a behavior, subjective norms, and perceived control over one’s own behavior, shapes a person’s intentions and behaviors (Jones et al., 2016). These studies demonstrate the value of incorporating evidence-based theoretical frameworks when assessing the disparities that persist in genetic testing among various populations. Researchers suggests that a combination of knowledge, attitudes, beliefs about genetics, and cancer risk perceptions such as genetics, lifestyle behaviors, and the environment contribute to current disparities (Ashida et al., 2011; Glanz & Bishop, 2010; Hamilton & Waters, 2018; Kendall, Kendall, Catts, Radford, & Dasch, 2007; Smerecnik, Mesters, Vries, & Vries, 2008; Waters, Muff, & Hamilton, 2014).
There are a number of studies that explore the relationship between genetics and personal beliefs (Bustillo et al., 2017; Cohen, Huziak, Gustafson, & Grubs, 2016; Halbert, McDonald, Magwood, & Jefferson, 2017; Hamilton & Waters, 2018; Hann et al., 2017; Huang, Apouey, & Andrews, 2014). For example, Hann et al., 2017 conducted a systematic review of qualitative and quantitative studies published between 2000 and 2015 to understand various groups’ awareness of genetic testing and its acceptability to widening disparities in health care. Hann and colleagues concluded that interventions are needed to increase awareness and knowledge of genetic testing for cancer risk among ethnic minority groups.
Additionally, the perceived stigma and taboo surrounding genetic testing should be addressed and reduced. Because mistrust in the health care has contributed to the disparities in health seen today, research conducted by Rogers et al., 2018 not only explores the relationship between genetics and personal beliefs but also aims to address the disparities seen at the intersection of genetic testing and prostate cancer research. Rogers and colleagues found that PSA testing confusion, healthcare system distrust, and misuse of personal health information were barriers to the participation of most of the African American men who participated in the focus group for this study (Rogers et al., 2018).
Other forms of beliefs can be harmful and may enforce unfounded negative stereotypes. Genetic determinism is one such belief. It is a concept that has many different definitions. For our purposes, genetic determinism is where the gene is believed to be the only, or at least the most relevant contributor for determining individual phenotypic characteristics. It is an attempt to reduce human biology to the physical sciences, with the behavior and personality of individuals being largely shaped by their genes (Gericke et al., 2017). Studies focused on genetic determinism assert that the lay public holds many beliefs, both informed and not informed, about the role genes play on health. Other studies focused on the relationship between beliefs and genetics include genetic relativism, which involves the belief that genes are only partially responsible for human health (Parrott et al., 2004).
In addition, other fields of study, including behavioral and cancer genetics, explore how perceptions about genetics may influence a person’s beliefs about human evolution, personal characteristics, his/her place in the world, and many aspects of everyday life (Jayaratne et al., 2006; Morin-Chassé, 2014; Rogers et al., 2018). Placing focus on beliefs about genetics may be a significant first step in addressing barriers to an individual’s intention to pursue genetic testing for cancer risk.
This study is based on Hochbaum, Rosenstock, and Kegeles’ Health Belief Model that predicts anindividual’s readiness to adopt or engage in a certain health behavior (Baum, 1997). The Health Belief Model can be used to predict a person’s intention to pursue genetic testing, a preventive health behavior. Together, this Health Belief Model’s individual constructs, including personal perception of susceptibility, perceived severity, cues to action, personal demographics (e.g., gender, education, and socioeconomic status), perceived benefits, and perceived barriers have been used to determine whether a person ismotivated to adopt a behavior or take a specific action (Baum, 1997; Cyr, Dunnagan, & Haynes, 2010).
This study also may contribute to a greater understanding of why racial/ethnic disparities exist in genetic testing utilization by assessing to what degree, if any, beliefs toward genetics play a role in a person’s willingness to have a genetic test for cancer risk. Understanding the factors that inform an adult’s belief toward genetics and its ability to predict cancer risk, may proveusefultogenetic counselors by serving to further inform their current practice and position them to play a greater role in eliminating the racial/ethnic disparities seen in cancer prevention and early detection (Underhill, Jones, & Habin, 2016).
Historically, the participation of African Americans in genetic research has been low, and continues to be today (Halbert et al., 2017; Hann et al., 2017; Honda, 2003; Huang et al., 2014; Huo & Olopade, 2007; Long, Thomas, Grubs, Gettig, & Krishnamurti, 2011; McDonald et al., 2012; Pagan, Su, Li, Armstrong, & Asch, 2009; Popejoy & Fullerton, 2016; Salloum et al., 2018; Sayani, 2018). For example, Hann et al. (2017) concluded African Americans were less likely than non-Hispanic Whites to participate in genetic testing because of their suspicions that the government would use their genetic testing results to label them as inferior outweighed their beliefs in the health benefits of genetic testing. In a qualitative study, researchers, Hann et al. (2017), highlighted that while knowledge, awareness, and positive views about genetic testing for cancer risk were observed among a small portion of African American, Hispanic, and Asian ethnic groups, concerns and negative perceptions about genetic testing persisted. These themes can inform the design of educational materials and communications aimed at tailoring dialogue with genetic counselors to address genetic causal beliefs toward perceptions about cancer risk assessments among African Americans and other racial/ethnic minority populations.
The National Institute on Minority Health and Health Disparities (NIMHD) research framework is a model designed to characterize the multilevel and multidomain determinants of health to better understand and address disparities in health (Alvidrez, Castille, Laude-Sharp, Rosario, & Tabor, 2019). Like the Health Belief Model, the NIMHD research framework is an ecological model that can address the racial and ethnic disparities and promote health equity (Alvidrez et al., 2019) by providing insight into the factors that influence causal genetic beliefs.. We propose that the NIMHD research framework and select constructs of the Health Belief Model explain factors that influence an individual’s beliefs about genetics toward cancer risk. This combined framework also serves to guide our understanding of the varying factors associated with beliefs about genetics and how societal, community, interpersonal, and individual level factors can influence adult beliefs and genetic cancer risk (Figure 1).
FIGURE 1.
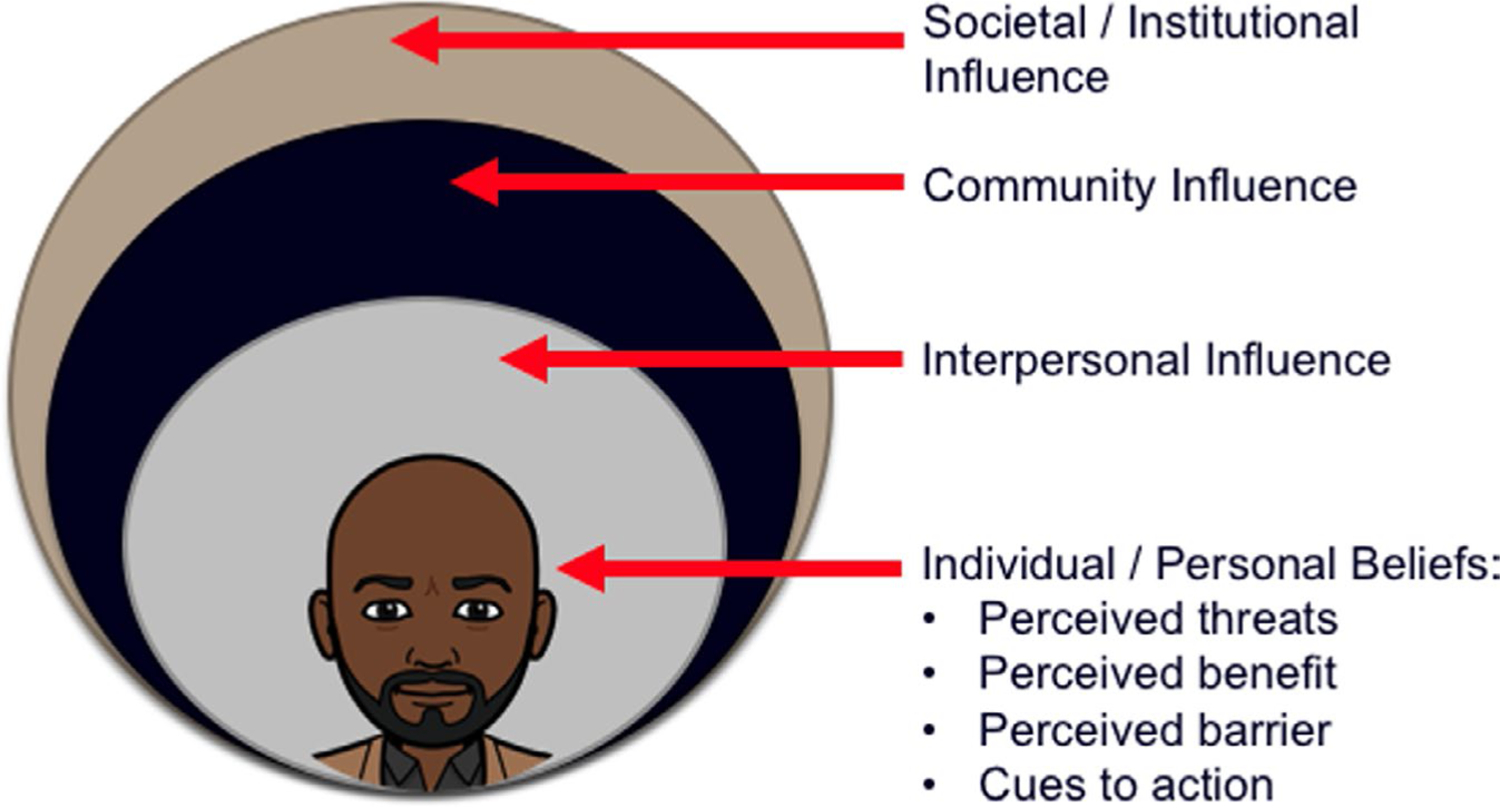
Adapted from the NIMHD Minority Health and Health Disparities Research Network and select constructs from the Health Belief Model. Reflecting the multiple levels of influence relevant to minority health and health disparities
For example, socioeconomic factors, such as low income, may have greater effects on adults’ beliefs about genetics and whether a person will develop cancer than distrust of the medical system due to their inability to gain access to this system (Bonham, Callier, & Royal, 2016). For example, if the financial costs of treatment and genetic testing services outweighs the health benefits an adult may perceive, this may contribute to the justification that genetics does not contribute to his/her developing cancer, thus, influencing his/her decision not to pursue genetic testing (Bonham et al., 2016; Bonham & Knerr, 2008; Joyner & Paneth, 2015; Singer, Antonucci, & Hoewyk, 2004).
Developing interventions or guides for genetic counselors to use when addressing an individual’s causal genetic beliefs may provide a beneficial to many in the field of genetic counseling. These guides may better position genetic counselors who work with individuals from racially and ethnically diverse populations to better understand the impact that lifestyle, family history, and environmental factors can have on their clients and their understanding of their susceptibility to cancer. In addition, this information can contribute to better understanding possible biological effects of housing, racism, discrimination, and other social disadvantages that may negatively impact racial and ethnic minority populations (Fullerton, Knerr, & Burke, 2012; Zimmerman et al., 2006).
Perceived threats, cues to action, perceived benefits and barriers, and the socioeconomic nuances can causal genetic beliefs (Cyr et al., 2010). Research conducted by Allford, Qureshi, Barwell, Lewis, & Kai, 2014 has shown that historical mistrust of the medical system and research, and the stigma about cancer or the inherited risk of cancer among African Americans can influence the perceived threat to genetic testing among African Americans. They and others also showed that perceived benefits that supported genetic causal belief and testing included knowledge of familial disease and risk and satisfying one’s curiosity about knowing one’s own genetic makeup (Allford et al., 2014; Cyr et al., 2010; Dye et al., 2016; Shavers, Lynch, & Burmeister, 2000).
The present study aims to assess factors associated with beliefs about genetics and cancer risk perceptions among adults aged 18 years and older in the United States (US). The results of this study may serve to inform genetic counselors and the broader health community’s understanding of the factors that influence an adult’s genetic causal beliefs when they consider their cancer risk and may provide evidence to support genetic counselors and other health professionals to deliver more culturally responsive care to individuals who are making decisions about their genetic health.
2 |. METHODS
Our research objectives and data analysis were framed through the NIMHD research framework. Our theoretical framework utilized constructs from the Health Belief Model. We assessed the factors associated with an individual’s beliefs toward genetics and whether it may determine their risk for cancer. In addition, we assessed modifying factors associated with those beliefs, which included age, gender, ethnicity, socioeconomic status, perceived threat of disease, knowledge of cancer or genetic testing, and overall education. The research question that guided this study is whether sociodemographic factors, such as age, gender, level of income, level of education, and race/ethnicity, are associated with the belief that one’s genetics determines cancer risk.
2.1 |. Participants
Data from the National Cancer Institute’s 2014 Health Information National Trends Survey (HINTS) 4 Cycle 4 were analyzed. HINTS is a nationally representative, cross-sectional survey that collects statedata from a random sample of non-institutionalized US adults aged 18 years and older concerning their public use of cancer- related information, including, but not limited to, changes in communication trends and practices and cancer risk perceptions. A two-stage design was used. First, a stratified sample of addresses was compiled from a list of residence addresses collected from a database of addresses used by Marketing Systems Group (MSG). The sampling frame of addresses was grouped into three explicit sampling strata: (a) addresses in areas with high concentrations of minority population; (b) addresses in areas with low concentrations of minority population; and (c) addresses located in counties comprising Central Appalachia regardless of minority population. The high- and low-minority strata were formed using the block group level characteristics from the 2010 Decennial Census Summary File. Addresses in census block groups that had a population proportion of Hispanics or African Americans that equaled or exceeded 40% were assigned to the high-minority stratum. All the remaining addresses were assigned to the low-minority stratum. Addresses in counties comprising Central Appalachia were assigned to the Central Appalachia stratum regardless of minority status (Health Information National Trends Survey 4, 2015).
An equal-probability sample of addresses was selected from within each explicit sampling stratum. The total number of addresses selected for Cycle 4 was 14,000:8,855 from the high-minority stratum, 5,025 from the low-minority stratum, and 120 from the Central Appalachia stratum. The high-minority stratum’s proportion of the sampling frame was 25.0% and was oversampled so that its proportion of the sample was 63.3%. Conversely, the low-minority stratum comprised 74.2% of the sampling frame but made up just 35.6% of the sample. The Central Appalachia stratum was sampled proportionally with the stratum comprising about 0.8% of both the sampling frame and sample. To carry out the data collection experiment, the address sample was divided into nine groups with about 70% of the sample addresses assigned to the control treatment group and the remaining 30% of the sample addresses assigned to eight experimental treatment groups (Health Information National Trends Survey 4, 2015).
The second stage consisted of one adult selected from each sample household. To account for and remove duplicate households, a question about how many different ways respondents received mail was included in the survey. In keeping with Cycles 2 and 3, data collection for Cycle 4 implemented the next birthday method to select the one adult in the household. Questions were included on the survey instrument to assist the household in selecting the adult in the household having the next birthday. Data for HINTS 4 cycle 4 were collected from August 20, 2014, through November 14, 2014 (Health Information National Trends Survey 4, 2015).
2.2 |. Procedures
2.2.1 |. Measures
All measures in this study were based on the self-reported data from the 2014 HINTS. The adults sampled who completed a questionnaire in Cycle 4 received a full-sample weight and a set of 50 replicate weights. The full-sample weight was the weight which was used to calculate population and subpopulation estimates from the HINTS data collected in Cycle 4. The replicate weights were then used to compute standard errors for these estimates. The weighting process encompassed the procedures used to create the final full-sample and replicate weights for the survey respondents. The use of sampling weights was done to ensure valid inferences from the responding sample to the population, correcting for nonresponse and noncoverage biases to the extent possible (Health Information National Trends Survey 4, 2015). Only those questions from the HINTS relevant to the present study are described below. The complete survey instrument can be obtained online at https://hints.cancer.gov/docs/Instruments/HINTS_4_Cycle_4_English_Annotated_Form.pdf.
2.2.2 |. Dependent variables
Beliefs toward genetic testing for cancer risk were calculated as the proportions of participants who answered, “a lot”, “somewhat”, “a little”, or “not at all” to the question: “How much do you think genetics, that is characteristics passed from one generation to the next, determine whether or not a person will develop cancer?” Belief that genetic testing does determine whether a person will develop cancer was defined as answering, “a lot”, “somewhat”, or “a little”. Belief that genetic testing does not determine whether a person will develop cancer was defined as answering “not at all” to the same question (Hamilton & Waters, 2018).
2.2.3 |. Independent variables
Knowledge about genetic testing was calculated as the proportion of respondents who answered “yes” to the question: “Genetic tests that analyze your DNA, diet, and lifestyle for potential health risks are currently being marketed by companies directly to consumers. Have you heard or read about these genetic tests?” The response options were “yes” or “no.” Beliefs that health behaviors determine whether a person will develop cancer was defined by a respondent answering, “a lot,” “somewhat,” or “a little” to the question: “How much do you think health behaviors like diet, exercise, and smoking determine whether or not a person will develop cancer?” Response categories were: “a lot,” “a little,” “somewhat,” or “not at all.” Beliefs that health behaviors do not determine whether a person will develop cancer was defined by a respondent answering “not at all” to the same question. The responses were then dichotomized as “yes” if a participant answered, “a lot,” “a little,” or “somewhat” and “no” if a respondent answered, “not at all.”
2.2.4 |. Covariates
Control variables included were sociodemographic characteristics (age, sex, race/ethnicity, education, and income), knowledge about genetic testing, and causal beliefs that health behaviors determine whether a person will develop cancer.
2.3 |. Data analysis
A bivariate analysis was conducted to assess factors independently associated with beliefs toward genetic testing for cancer risk. Variables that achieved p ≤ .05 in the bivariate analysis were included in our final multivariable logistic regression model; odds ratios (AORs) and 95% confidence intervals (95% CIs) were obtained for each of them. A 2-sided p-value of ≤.05 was considered to indicate statistical significance. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3 |. RESULTS
The present study aimed to examine whether sociodemographic factors are associated with beliefs that genetics determines cancer risk. Sixty percent of the survey respondents were female and Non-Hispanic Whites. African Americans (AA) represented 16% of the survey population, whereas Hispanics, non-Hispanic Asians, and those classified as Other represented 16%, 4%, and 4%, respectively (Table 1). Thirty-four percent of the survey respondents were between the ages of 50 and 64. Nearly 42% of the survey respondents had a college degree (41.7%). Ninety-four percent of respondents believed that health behaviors, such as diet, exercise, and smoking, determine whether they will develop cancer. Sixty-three percent of respondents had genetic testing knowledge (had either heard or read about genetic tests), based upon the definition provided by the survey.
TABLE 1.
Number and percentage of respondents who believed that genetics determine whether a person will develop cancer by select characteristics (N = 3,247): 2014 HINTS, United States
| Belief that genetics determine whether a person will develop cancer |
||||
|---|---|---|---|---|
| Total |
No |
Yes |
||
| Select characteristics | n (%) | n (%) | n (%) | p-value |
| Overall | 3,247 | 162 (5) | 3,085 (95) | |
| Gender | <.02 | |||
| Female | 1,935 (100) | 83 (4) | 1,852 (96) | |
| Male | 1,312 (100) | 79 (6) | 1,233 (94) | |
| Race/ethnicity | <.01 | |||
| Non-Hispanic White | 1,898 (100) | 54 (3) | 1,844 (97) | |
| Non-Hispanic Black/African American | 517 (100) | 36 (7) | 481 (93) | |
| Hispanic | 506 (100) | 36 (7) | 470 (93) | |
| Non-Hispanic Asian | 122 (100) | 10 (8) | 112 (92) | |
| Othera | 115 (100) | 8 (7) | 107 (93) | |
| Age group | <.01 | |||
| 18–34 | 461 (100) | 15 (3) | 446 (97) | |
| 35–49 | 718 (100) | 29 (4) | 689 (96) | |
| 50–64 | 1,175 (100) | 50 (4) | 1,125 (96) | |
| 65–74 | 606 (100) | 32 (5) | 574 (95) | |
| 75 and above | 398 (100) | 39 (10) | 359 (90) | |
| Level of education | <.01 | |||
| Less than high school | 287 (100) | 31 (11) | 256 (89) | |
| High school or less | 632 (100) | 62 (10) | 570 (90) | |
| Some college | 1,053 (100) | 47 (4) | 1,006 (96) | |
| College graduate | 1,414 (100) | 32 (2) | 1,382 (98) | |
| Level of income | <.01 | |||
| Less than $20,000 | 727 (100) | 77 (11) | 650 (89) | |
| $20,000 to $49,999 | 933 (100) | 49 (5) | 884 (95) | |
| $50,000 to $99,999 | 898 (100) | 25 (3) | 873 (97) | |
| $100,000 or more | 591 (100) | 8 (1) | 583 (99) | |
| Genetic test knowledge | .02 | |||
| No | 2,182 (100) | 143 (7) | 2,039 (93) | |
| Yes | 1,317 (100) | 38 (3) | 1,279 (97) | |
| Belief that health behaviors (e.g., diet, exercise, smoking) determine whether you will develop cancer | <.01 | |||
| Yes | 3,279 (100) | 101 (3) | 3,178 (97) | |
| No | 198 (100) | 74 (37) | 124 (63) | |
Alaska Native, American Indian, Native Hawaiian or other Pacific Islander, Non-Hispanic multiple race.
As seen in Table 2, the multivariable model included gender, race/ethnicity, age, level of education, level of income, genetic test knowledge, and the belief that health behaviors (i.e., diet, exercise, and smoking) contribute to a person’s beliefs about developing cancer, with females showing the highest odds of reporting that genetics plays at least some role in determining whether a person will develop cancer (AOR = 2.20; 95% CI = 1.46–3.34). The results in Table 2 illustrate factors associated with lower odds of reporting that genetics plays at least some role in determining whether a person will develop cancer, including being African American (AOR = 0.52; 95% CI = 0.30–0.89), Hispanic (AOR = 0.57; 95% CI = 0.32–0.99), non-Hispanic Asian (AOR = 0.33; 95% CI = 0.14–0.79), other races, including Alaska Natives, American Indians, Native Hawaiians or other Pacific Islanders, and non-Hispanic multiple races (AOR = 0.38; 95% CI = 0.15–0.95) compared to whites; attaining a college degree compared to having a high school education (AOR = 0.34, 95% CI = 0.19–0.60); being age 75 and older compared to being less than 35(AOR = 0.40; 95% CI = 0.18–0.88); earning less than $20,000 compared to earning at least $100,000 (AOR = 0.23; 95% CI = 0.09–0.60); and not believing that health behaviors play at least some role in determining whether a person will develop cancer compared to believing it (AOR = 0.08; 95% CI = 0.05–0.13). Knowledge of genetic testing was found to not be statistically significant (AOR = 0.81; 95% CI = 0.50–1.31; p-value = .39).
TABLE 2.
Adjusted odds ratios and 95% confidence intervals for the belief that genetics determine whether a person will develop cancer factors by select characteristics (N = 3,085): 2014 HINTS, United States
| Select characteristics | Adjusted OR (95% CI) for the belief that genetics determine whether a person will develop cancer | p-value |
|---|---|---|
| Gender | ||
| Male | Ref | |
| Female | 2.20 (1.46–3.34) | <.01 |
| Race/ethnicity | ||
| Non-Hispanic White | Ref | |
| Non-Hispanic Black/African American | 0.52 (0.30–0.89) | .01 |
| Hispanic | 0.57 (0.32–0.99) | .04 |
| Non-Hispanic Asian | 0.33 (0.14–0.79) | .01 |
| Othera | 0.38 (0.15–0.95) | .03 |
| Age group | ||
| 18–34 | Ref | |
| 35–49 | 0.87 (0.40–1.84) | .70 |
| 50–64 | 0.83 (0.42–1.66) | .59 |
| 65–74 | 0.71 (0.32–1.57) | .39 |
| 75 and above | 0.40 (0.18–0.88) | .02 |
| Level of education | ||
| College graduate | Ref | |
| Some college | 0.80 (0.44–1.45) | .46 |
| High school | 0.34 (0.19–0.60) | <.01 |
| Less than high school | 0.48 (0.23–0.99) | .04 |
| Level of income | ||
| $100,000 or more | Ref | |
| $50,000 to $99,999 | 0.53 (0.21–1.37) | .19 |
| $20,000 to $49,999 | 0.45 (0.09–0.60) | .09 |
| Less than $20,000 | 0.23 (0.09–0.60) | <.01 |
| Genetic test knowledge | ||
| Yes | Ref | |
| No | 0.81 (0.50–1.31) | .38 |
| Belief that health behaviors (ex. diet, exercise, smoking) determine whether you will develop cancer | ||
| Yes | Ref | |
| No | 0.08 (0.05–0.13) | <.01 |
Alaska Native, American Indian, Native Hawaiian or other Pacific Islander, Non-Hispanic multiple race.
4 |. DISCUSSION
The results of this study show that factors including race/ethnicity, age, gender, level of education, level of income, and not believing that health behaviors (such as diet, exercise, and smoking) play a role in determining whether a person will develop cancer is all associated with beliefs about genetics and cancer risk perceptions among adults aged 18 years and older in the United States.
4.1 |. Minority populations
Our study concurs with Thompson, Valdimarsdottir, Jandorf, and Redd (2003) that found Latinos having greater perceived concerns to genetic testing, including potential negative emotional effects of the testing results on their families, not believing that genetic testing would be beneficial to them, especially if they were healthy at the time of testing, and concerns that a positive test result would lead to shame. Our study results supported findings that even though females were more likely to endorse genetic causal beliefs about cancer risk, self-identified race/ethnicity (i.e., Hispanic descent or Hispanic culture) had more influence than gender in causal genetic beliefs. Overcoming beliefs regarding the perceived disadvantages of genetic testing and identifying to what degree political, community, societal, or interpersonal influences play on personal genetic causal beliefs may be paramount when consulting Latino and other ethnic and racially diverse populations so that the personal barriers to pursuing genetic testing for cancer risk may be removed.
4.2 |. Health behavior beliefs
Culture has an influencing role on the formation of an adult’s beliefs. These beliefs, including religious beliefs, may influence an adult to adopt more fatalistic beliefs. Fatalistic beliefs involve a person believing that there is not much he/she can do to avoid getting cancer, so pursuing genetic testing is pointless.
For example, genetic fatalistic beliefs can involve an adult believing that a diagnosis of cancer is “God’s will” and nothing can be done to mitigate fate. Although religious beliefs may influence an individual’s beliefs about his/her risk for cancer, Leyva et al., (2014) have suggested that they may not outweigh a person’s self-efficacy, or ability to do something about their cancer and may provide an opportunity for a genetic counselor to reaffirm his/her client’s self-efficacy in determining his/her cancer outcome. Kendall et al. (2007) also noted that these religious beliefs may put African Americans at odds with the goals of cancer genetic risk assessment. Our study findings that most of our study respondents believed that, in addition to genetics, health behaviors also play a role in determining whether they will develop cancer replicates other research that reports adults possessing multifactorial beliefs about cancer risk (Hamilton & Waters, 2018).
These multifactorial beliefs about cancer risk suggest there are multiple domains of influence that individuals believe may play a role in their health. In addition to the role of biology, behavioral, physical or build environment, sociocultural environments, and the overarching health system may impact the health of the individual. Hamilton and Waters (2018) suggest that understanding multifactorial nature of cancer “is an important component of genomic health literacy, because this knowledge can help individuals to obtain, process, and utilize rapidly evolving and increasing available genomic information to guide their person health decisions” (pp. 02). Knowing that an individual may possess genetic and health behavior beliefs will provide an opportunity for genetic counselors to help their patients develop a deeper understanding of how genetic and behavioral factors work together to contribute to cancer risk (Hamilton & Waters, 2018). An awareness of the multifactorial causes of cancer also supports the Precision Medicine Initiative’s approach that considers environmental, genetic, and lifestyle factors, such as health behaviors, in its goal for developing prevention measures and treatments for individuals (Ferryman & Picat, 2018; Hamilton & Waters, 2018; Smerecnik et al., 2008; Waters et al., 2014).
Our study demonstrates a need to frame beliefs about genetics and cancer risk perceptions in a social ecological perspective. This perspective allows genetic counselors to gain a more comprehensive understanding of the varying levels of influence that society, community, interpersonal relationships, and personal beliefs have on an adult’s beliefs about genetics (Figure 1). Research conducted using this perspective provides insight into how best to assess and address issues concerning the racial/ethnic disparities seen in the utilization of genetic testing among adults in the United States. In addition, utilizing the NIMHD research framework and the Health Belief Model in the context of a social ecological perspective may prove to be necessary as the patient population and need for genetic counselors grows.
4.3 |. Study strengths
Our study applied a theoretical construct and ecological framework to guide and predict racial/ethnic adult health behavior with regard to the likelihood of pursing genetic testing for cancer risk reduction to a nationally representative sample.
4.4 |. Study limitations
The limitations of our study include low response rates and the inability to infer causation. The reliance on self-reported data from survey respondents may have been subjected to recall and/or reporting bias.
4.5 |. Practice implications
The present study has important implications for genetic counselors interested in cancer prevention and control and risk-reduction behaviors. Utilizing educational outreach strategies, such as community-based capacity building and community-based participatory research (CBPR) that target minority populations, may serve as viable templates for genetic counselors to use when making efforts to address socially driven and health-related needs and also strengthen educational outreach to medically underserved and other underrepresented populations (Rapkin et al., 2017).
This kind of outreach strategy may better inform genetic counselors on factors outside of the individual such as the ability to pay for genetic services and the cultural stigma that may be associated with pursuing genetic testing. Through these collaborative efforts, new skills may be gained and employed in the practice of genetic counseling that may help reduce some of the barriers to genetic testing. In addition, educational outreach through these strategies encourages providers, such as genetic counselors, to work with the community leaders of the populations they serve. Only when providers form collaborative partnerships with the community and not simply provide a service, do barriers to access, resources, and education begin to be removed (Cohn, Husamudeen, Larson, & Williams, 2015). Meeting patients where they are has been one of the strategies used to advance health equity and eliminate barriers to health services. These outreach strategies and community engaged partnerships may prove to be more effective when addressing cancer risk perceptions for those considering or using genetic testing because it may inform genetic counselors on how best to tailor their session that takes into account the varying levels of influence that factor into their patient populations health-related beliefs (Cohen et al., 2016).
Genetic counselors can play a pivotal role in helping to eliminate racial and ethnic disparities in health. Knowledge, compassion, and empathy are all characteristics possessed by the best genetic counselors. These personal qualities may translate well when addressing an individual’s personal beliefs about genetics and its impact on cancer care and overall health (Miranda, Veach, Martyr, & LeRoy, 2015; Witt & Jankowska, 2018). Genetic counselors also are uniquely positioned to aid in removing barriers to benefits of genetic testing for cancer risk by their ability to communicate complicated genetic information to their patients in a way that informs their patients who may be at risk for cancer or inherited syndrome before and after genetic testing consultations. In addition, they are well positioned to address personal and cultural beliefs surrounding genetic testing by conveying the individual and population level effects that genetics may have on health and disease. Particularly for minority populations, addressing the biological effects of health disparities by relating genetic health to the interaction outside of the individual, such as safe housing, racism, discrimination, and other social disadvantages that negatively affect racial/ethnic minority groups, may put beliefs about genetics in a way that may be more palpable and relevant to the individual who may be considering pursing genetic testing for cancer risk.
4.6 |. Research recommendations
As the community of genetic counselors grows and the patient population seen by them continues to be more diverse, it becomes imperative that every practicing genetic counselor be culturally responsive and respectful when counseling all people; more can and should be done (Blair et al., 2013; Smedley, Stith, & Nelson, 2003). Cultural competency training can provide the information that is needed for genetic counselors to deliver more personalized, socially conscious, and empathetic consultations to individuals. Providing cultural competence training that includes increased awareness of unconscious biases in various in-person and electronic formats may allow genetic counselors to become more culturally responsive in ways to deliver quality care to their patient populations.
5 |. CONCLUSION
The present study found that self-identified non-Hispanic White adults over the age of 75 who did not attain more than a high school degree and earned less than $20,000 per year were less likely to have the belief that genetics plays at least some role in determining whether a person will develop cancer. Future studies may provide further understanding of factors that influence causal genetic beliefs toward cancer risk among minority groups in the context of their beliefs about genetics and cancer risk. Additionally, developing culturally competent educational materials that includes understanding socially informed belief systems can be used to inform novel and targeted, community-based cancer risk communication programs aimed at fostering partnerships to educate the public about cancer and genetics in an effort to promote health equity.
Supplementary Material
ACKNOWLEDG MENTS
We would like to thank the Cancer Health Equity Institute and the Master of Public Health program at Morehouse School of Medicine. This manuscript was supported by the U54 Morehouse School of Medicine/Tuskegee University/University of Alabama at Birmingham Comprehensive Cancer Center Partnership Grant #5U54CA118638–13.
Funding information
U54 Morehouse School of Medicine; Tuskegee University; University of Alabama at Birmingham; Comprehensive Cancer Center
Footnotes
COMPLIANCE WITH E THIC AL STANDARDS
Conflict of interest
Lawrence P. McKinney, MPH, Gemechu. B. Gerbi, PhD MSc, Lee S. Caplan, MD PhD MPH, Mechelle D. Claridy, MPH, and Brian M. Rivers, PhD MPH have declared no conflict of interest.
Data source
The data that support the findings of this study are available in HINTS, part of the National Cancer Institute’s Division of Cancer Control and Population Sciences at https://hints.cancer.gov/data/default.aspx.
Human studies and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Secondary data analysis was conducted in this study; informed consent for respondents in this study was not sought after or obtained.
Animal studies
No animal studies were carried out by the authors for this article.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Agurs-Collins T, Ferrer R, Ottenbacher A, Waters EA, O’Connell ME, & Hamilton JG (2015). Public awareness of direct-to-consumer genetic tests: Findings from the 2013 U.S. Health Information National Trends Survey. Journal of Cancer Education, 30(4), 799–807. 10.1007/s13187-014-0784-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allford A, Qureshi N, Barwell J, Lewis C, & Kai J (2014). What hinders minority ethnic access to cancer genetics services and what may help? European Journal of Human Genetics, 10.1038/ejhg.2013.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvidrez J, Castille D, Laude-Sharp M, Rosario A, & Tabor D (2019). The National institute on minority health and health disparities research framework. American Journal of Public Health, 109(S1), S16–S20. 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida S, Goodman M, Pandya C, Koehly LM, Lachance C, Stafford J, & Kaphingst KA (2011). Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics, 14(4–5), 307–316. 10.1159/000316234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A (1997). Cambridge handbook of psychology, health, and medicine Cambridge, UK; New York, NY: Cambridge University Press. [Google Scholar]
- Blair IV, Steiner JF, Fairclough DL, Hanratty R, Price DW, Hirsh HK, … Havranek EP (2013). Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients. The Annals of Family Medicine, 11(1), 43–52. 10.1370/afm.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham VL, Callier SL, & Royal CD (2016). Will precision medicine move us beyond race? New England Journal of Medicine, 374(21), 2003–2005. 10.1056/NEJMp1511294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham VL, & Knerr S (2008). Social and ethical implications of genomics, race, ethnicity, and health inequities. Seminars in Oncology Nursing, 24(4), 254–261. 10.1016/j.soncn.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo NE, McGinty HL, Dahn JR, Yanez B, Antoni MH, Kava BR, & Penedo FJ (2017). Fatalism, medical mistrust, and pretreatment health-related quality of life in ethnically diverse prostate cancer patients. Psycho-oncology, 26(3), 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SA, Huziak RC, Gustafson S, & Grubs RE (2016). Analysis of advantages, limitations, and barriers of genetic counseling service delivery models. Journal of Genetic Counseling, 25(5), 1010–1018. 10.1007/s10897-016-9932-2 [DOI] [PubMed] [Google Scholar]
- Cohn EG, Husamudeen M, Larson EL, & Williams JK (2015). Increasing participation in genomic research and biobanking through community-based capacity building. Journal of Genetic Counseling, 24(3), 491–502. 10.1007/s10897-014-9768-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr A, Dunnagan TA, & Haynes G (2010). Efficacy of the health belief model for predicting intention to pursue genetic testing for colorectal cancer. Journal of Genetic Counseling, 19(2), 174–186. 10.1007/s10897-009-9271-7 [DOI] [PubMed] [Google Scholar]
- Dye T, Li D, Demment M, Groth S, Fernandez D, Dozier A, & Chang J (2016). Sociocultural variation in attitudes toward use of genetic information and participation in genetic research by race in the United States: Implications for precision medicine. Journal of the American Medical Informatics Association, 23(4), 782–786. 10.1093/jamia/ocv214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferryman K, & Picat M (2018). What is precision medicine? Retrieved from Data&Society. [Google Scholar]
- Fullerton SM, Knerr S, & Burke W (2012). Finding a place for genomics in health disparities research. Public Health Genomics, 15(3–4), 156–163. 10.1159/000334717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke N, Carver R, Castéra J, Evangelista NAM, Marre CC, & El-Hani CN (2017). Exploring relationships among belief in genetic determinism, genetics knowledge, and social factors. Science & Education, 26(10), 1223–1259. [Google Scholar]
- Glanz K, & Bishop DB (2010). The role of behavioral science theory in development and implementation of public health interventions. Annual Review of Public Health, 31, 399–418. 10.1146/annurev.publhealth.012809.103604 [DOI] [PubMed] [Google Scholar]
- Green RC, Lautenbach D, & McGuire AL (2015). GINA, genetic discrimination, and genomic medicine. New England Journal of Medicine, 372(5), 397–399. 10.1056/NEJMp1404776 [DOI] [PubMed] [Google Scholar]
- Halbert CH, McDonald JA, Magwood G, & Jefferson M (2017). Beliefs about genetically targeted care in African Americans. Journal of the National Medical Association, 109(2), 98–106. 10.1016/j.jnma.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, & Waters EA (2018). How are multifactorial beliefs about the role of genetics and behavior in cancer causation associated with cancer risk cognitions and emotions in the US population? Psycho-Oncology, 27(2), 640–647. 10.1002/pon.4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann KEJ, Freeman M, Fraser L, Waller JO, Sanderson SC, Rahman B, … Lanceley A (2017). Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: A systematic review. BMC Public Health, 17(1), 503. 10.1186/s12889-017-4375-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Information National Trends Survey 4 (HINTS 4). (2015). Cycle 4 methodology report 68. [Google Scholar]
- Honda K (2003). Who gets the information about genetic testing for cancer risk? The role of race/ethnicity, immigration status, and primary care clinicians. Clinical Genetics, 64(2), 131–136. 10.1034/j.1399-0004.2003.00112.x [DOI] [PubMed] [Google Scholar]
- Huang H, Apouey B, & Andrews J (2014). Racial and ethnic disparities in awareness of cancer genetic testing among online users: Internet use, health knowledge, and socio-demographic correlates. Journal of Consumer Health on the Internet, 18(1), 15–30. 10.1080/15398285.2014.869165 [DOI] [Google Scholar]
- Huo D, & Olopade OI (2007). Genetic testing in diverse populations: Are researchers doing enough to get out the correct message? Genetic Testing in Diverse Populations, 298(24), 2910–2911. 10.1001/jama.298.24.2910 [DOI] [PubMed] [Google Scholar]
- Jayaratne TE, Ybarra O, Sheldon JP, Brown TN, Feldbaum M, Pfeffer C, & Petty EM (2006). White Americans’ genetic lay theories of race differences and sexual orientation: Their relationship with prejudice toward blacks, and Gay men and Lesbians. Group Processes & Intergroup Relations, 9(1), 77–94. 10.1177/1368430206059863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Lockhart JS, Mendelsohn-Victor KE, Duquette D, Northouse LL, Duffy SA, … Katapodi MC (2016). Use of cancer genetics services in African-American young breast cancer survivors. American Journal of Preventive Medicine, 51(4), 427–436. 10.1016/j.amepre.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Joyner MJ, & Paneth N (2015). Seven questions for personalized medicine. JAMA, 314(10), 999–1000. 10.1001/jama.2015.7725 [DOI] [PubMed] [Google Scholar]
- Kendall J, Kendall C, Catts ZA, Radford C, & Dasch K (2007). Using adult learning theory concepts to address barriers to cancer genetic risk assessment in the African American community. Journal of Genetic Counseling, 16(3), 279–288. 10.1007/s10897-006-9070-3 [DOI] [PubMed] [Google Scholar]
- Leyva B, Allen JD, Tom LS, Ospino H, Torres MI, & Abraido-Lanza AF (2014). Religion, fatalism, and cancer control: A qualitative study among Hispanic Catholics. American Journal of Health Behavior, 38(6), 839–849. 10.5993/AJHB.38.6.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KA, Thomas SB, Grubs RE, Gettig EA, & Krishnamurti L (2011). Attitudes and beliefs of African-Americans toward genetics, genetic testing, and sickle cell disease education and awareness. Journal of Genetic Counseling, 20(6), 572–592. 10.1007/s10897-011-9388-3 [DOI] [PubMed] [Google Scholar]
- McDonald JA, Barg FK, Weathers B, Guerra CE, Troxel AB, Domchek S, … Halbert CH (2012). Understanding participation by African Americans in cancer genetics research. Journal of the National Medical Association, 104(7–8), 324–330. 10.1016/s0027-9684(15)30172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C, Veach PM, Martyr MA, & LeRoy BS (2015). Portrait of the master genetic counselor clinician: A qualitative investigation of expertise in genetic counseling. Journal of Genetic Counseling, 25, 767–785. 10.1007/s10897-015-9863-3 [DOI] [PubMed] [Google Scholar]
- Morin-Chassé A (2014). Public (Mis)understanding of news about behavioral genetics research: A survey experiment. BioScience, 64(12), 1170–1177. 10.1093/biosci/biu168 [DOI] [Google Scholar]
- National Cancer Institute. (2017). Genetic testing fact sheet Retrieved from https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet [Google Scholar]
- Pagan JA, Su D, Li L, Armstrong K, & Asch DA (2009). Racial and ethnic disparities in awareness of genetic testing for cancer risk. American Journal of Preventive Medicine, 37(6), 524–530. 10.1016/j.amepre.2009.07.021 [DOI] [PubMed] [Google Scholar]
- Parrott R, Silk K, Weiner J, Condit C, Harris T, & Bernhardt J (2004). Deriving lay models of uncertainty about genes’ role in illness causation to guide communication about human genetics. Journal of Communication, 54(1), 105–122. 10.1111/j.1460-2466.2004.tb02616.x [DOI] [Google Scholar]
- Patch C, & Middleton A (2018). Genetic counselling in the era of genomic medicine. British Medical Bulletin, 126(1), 27–36. 10.1093/bmb/ldy008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popejoy AB, & Fullerton SM (2016). Genomics is failing on diversity. Nature, 538(7624), 161–164. 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin BD, Weiss E, Lounsbury D, Michel T, Gordon A, Erb-Downward J, … Kemeny M (2017). Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. American Journal of Community Psychology, 60(1–2), 145–159. 10.1002/ajcp.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink BD, & Kuller JA (2018). What are the required components of pre- and post-test counseling? Seminars in Perinatology, 42(5), 287–289. 10.1053/j.semperi.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Rogers CR, Rovito MJ, Hussein M, Obidike OJ, Pratt R, Alexander M, … Warlick C (2018). Attitudes toward genomic testing and prostate cancer research among black men. American Journal of Preventive Medicine, 55(5 Suppl 1), S103–S111. 10.1016/j.amepre.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein MA, & Anderlik MR (2001). What is genetic discrimination, and when and how can it be prevented? Genetics in Medicine, 3(5), 354–358. https://doi.org/10.109700125817-200109000-00005. [DOI] [PubMed] [Google Scholar]
- Salloum RG, George TJ, Silver N, Markham M-J, Hall JM, Guo YI, … Shenkman EA (2018). Rural-urban and racial-ethnic differences in awareness of direct-to-consumer genetic testing. BMC Public Health, 18(1), 277. 10.1186/s12889-018-5190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayani A (2018). Inequities in genetic testing for hereditary breast cancer: Implications for public health practice. Journal of Community Genetics, 10(1), 35–39. 10.1007/s12687-018-0370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers VL, Lynch CF, & Burmeister LF (2000). Knowledge of the Tuskegee study and its impact on the willingness to participate in medical research studies. Journal of the National Medical Association, 92(12), 563–572. [PMC free article] [PubMed] [Google Scholar]
- Singer E, Antonucci T, & Hoewyk JV (2004). Racial and ethnic variations in knowledge and attitudes about genetic testing. Genetic Testing, 8(1), 31–43. 10.1089/109065704323016012 [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, & Nelson AR (2003). Institute of medicine, committee on understanding and eliminating racial and ethnic disparities in health care. Unequal treatment: Confronting racial and ethnic disparities in healthcare Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Smerecnik CM, Mesters I, de Vries NK, & de Vries H (2008). Educating the general public about multifactorial genetic disease: Applying a theory-based framework to understand current public knowledge. Genetics in Medicine, 10(4), 251–258. 10.1097/GIM.0b013e31816b4ffd [DOI] [PubMed] [Google Scholar]
- Thompson HS, Valdimarsdottir HB, Jandorf L, & Redd W (2003). Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: Differences across African American, Latina and Caucasian women. Patient Education and Counseling, 51(3), 217–227. 10.1016/s0738-3991(02)00219-7 [DOI] [PubMed] [Google Scholar]
- Underhill ML, Jones T, & Habin K (2016). Disparities in cancer genetic risk assessment and testing. Oncology Nursing Forum, 43(4), 519–523. 10.1188/16.ONF.519-523 [DOI] [PubMed] [Google Scholar]
- Waters EA, Muff J, & Hamilton JG (2014). Multifactorial beliefs about the role of genetics and behavior in common health conditions: Prevalence and associations with participant characteristics and engagement in health behaviors. Genetics in Medicine, 16(12), 913–921. 10.1038/gim.2014.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt MM, & Jankowska KA (2018). Breaking bad news in genetic counseling—problems and communication tools. Journal of applied genetics, 59(4), 449–452. [DOI] [PubMed] [Google Scholar]
- Zimmerman RK, Tabbarah M, Nowalk MP, Raymund M, Jewell IK, Wilson SA, & Ricci EM (2006). Racial differences in beliefs about genetic screening among patients at inner-city neighborhood health centers. Journal of the National Medical Association, 98(3), 370–377. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


