Abstract
Purpose
Agitation is prevalent among inpatients with schizophrenia. The aim of this study was to investigate whether biochemical parameters are associated with agitation in schizophrenia.
Patients and Methods
Agitation was evaluated by the Positive and Negative Syndrome Scale-Excited Component questionnaire (PANSS-EC). Fasting serum levels of C-reactive protein (CRP), free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), uric acid (UA), creatinine, glucose and lipids were measured.
Results
The analysis included 154 inpatients with schizophrenia (71 with agitation, 83 without agitation) and 75 healthy control subjects. Patients with schizophrenia and agitation had higher serum levels of CRP, FT3, FT4 and UA as well as lower levels of serum TSH and creatinine than patients without agitation (all P < 0.05). Multivariate logistic regression analysis indicated that serum CRP (odds ratio [OR] = 1.470, P = 0.001), FT3 (OR = 13.026, P < 0.001), TSH (OR = 0.758, P = 0.033) and creatinine (OR = 0.965, P = 0.004) were significantly associated with agitation in schizophrenia. CRP, FT3, TSH and creatinine achieved an area under the ROC curve of 0.626, 0.728, 0.620 and 0.663 respectively in discriminating schizophrenia with or without agitation.
Conclusion
Increased serum CRP and FT3 levels and decreased serum TSH and creatinine levels are independent risk factors for agitation in hospitalized patients with schizophrenia. Inflammation, thyroid hormones and renal function may be involved in the pathogenesis of agitation in schizophrenia.
Keywords: schizophrenia, psychomotor agitation, C-reactive protein, triiodothyronine, thyroid-stimulating hormone, creatinine
Introduction
Schizophrenia is a severe psychiatric disorder that affects approximately 1% of people worldwide,1 and is one of the top 10 global causes of disability.2 Schizophrenia has a profound effect on both individuals and society.3 This disorder reduces life expectancy by 10–20 years,4 and places a heavy burden of care on the families and friends of those affected.5 Agitation is common in patients with schizophrenia, especially during an acute exacerbation of the disease.6,7 Indeed, schizophrenia accounts for 47% of all episodes of psychiatric agitation in Europe.8 The prevalence of agitation in newly hospitalized patients with schizophrenia is 47.5% in China.9 Agitation is a complex behavior characterized by excessive motor or verbal activity, irritability, uncooperativeness, yelling and threatening other people.9 When agitation escalates into aggression and violence, immediate intervention should be taken to prevent harm to patients, their care providers and others.10–12
Considerable efforts have been made to identify biomarkers for schizophrenia and other psychotic disorders,13 although objective and specific diagnostic biomarkers for schizophrenia have yet to be identified. Nevertheless, in recent years there has been increasing interest in the identification of biomarkers that could be used to facilitate the diagnosis of schizophrenia, disease monitoring, and evaluation of the response to treatment.14,15 The discovery of biomarkers associated with agitation in schizophrenia might help clinicians to instigate early intervention to reduce the risk of agitation developing into aggression and violence.
Although the pathophysiology of schizophrenia is not fully understood, there is evidence that inflammation may play a role in this disorder.16–18 C-reactive protein (CRP) is widely used in clinical practice as a biomarker of inflammatory status. Elevated levels of serum CRP have been reported to be associated with an increased risk of schizophrenia.19,20 Furthermore, in patients with schizophrenia, both agitation,21,22 and severity of positive symptoms,23,24 are positively correlated with serum CRP levels.
Neuroendocrine dysfunction is one of the mechanisms contributing to the pathogenesis of psychiatric illnesses. Thyroid hormones are necessary for the normal development and function of the brain.25 Studies have suggested that an increased rate of subclinical hypothyroidism in patients with schizophrenia is associated with antipsychotic medication and that subclinical hypothyroidism may be associated with sexual dysfunction.26,27 Nevertheless, there has been inconsistency between studies regarding thyroid hormone changes in patients with schizophrenia. For example, one report concluded that drug-naïve patients with schizophrenia had a higher free triiodothyronine (FT3) level than healthy controls,28 while another investigation described a higher free thyroxine (FT4) level in patients with schizophrenia.29 Furthermore, to our knowledge, the relationship between thyroid hormones and agitation in schizophrenia remains unknown.
There is increasing evidence that oxidative stress may be involved in the pathogenesis of schizophrenia.30–32 Uric acid (UA) is the end-product of purine catabolism and an important antioxidant in the central nervous system.33 The relationship between serum UA levels and schizophrenia remains controversial. For example, one study found that patients with schizophrenia had a lower UA level than healthy controls,34 whereas another study showed that schizophrenia was associated with a higher UA level.35 However, whether UA plays a role in the development of agitation in patients with schizophrenia is unclear.
We hypothesized that some biochemical indices used routinely in clinical practice might be biomarkers of agitation in schizophrenia. Therefore, the aim of this comparative cross-sectional study was to compare biochemical indices between patients with schizophrenia and agitation, patients with schizophrenia who do not have agitation and healthy controls and to investigate whether any of these biochemical indices have potential as blood-based biomarkers of agitation in patients with schizophrenia.
Patients and Methods
Study Design and Participants
This comparative cross-sectional study included patients with schizophrenia who were hospitalized at Shandong Mental Health Center (Jinan, China) between January 2020 and October 2020. The inclusion criteria were: (1) aged 18–60 years; (2) diagnosed with schizophrenia according to the International Classification of Disease-10 (ICD-10) criteria; and (3) First-episode of schizophrenia or had not received any psychotropic medication within four weeks before recruitment. The exclusion criteria were: (1) alcohol and/or substance dependence; (2) previously diagnosed with another psychiatric disorder; (3) physical diseases including thyroid-related diseases, kidney diseases, gout, major organic brain diseases, diabetes, immune diseases or other serious medical conditions; (4) infection in the previous four weeks or currently taking anti-inflammatory drugs, glucocorticoids or antibiotics; (5) pregnant or breastfeeding; and (6) had undergone electroconvulsive therapy within the previous four weeks.
In addition, a control group of gender-matched healthy volunteers was recruited from staff members at Shandong Mental Health Center. The exclusion criteria were: (1) alcohol and/or substance dependence; (2) previously diagnosed with a psychiatric disorder; (3) physical diseases including thyroid-related diseases, kidney diseases, gout, major organic brain disease, diabetes, immune diseases or other serious medical conditions; (4) infection in the previous four weeks or currently taking anti-inflammatory drugs, glucocorticoids or antibiotics; (5) pregnant or breastfeeding; and (6) family history of psychiatric disorder.
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee of Shandong Mental Health Center (2019-R43). All patients with schizophrenia and healthy volunteers provided informed written consent for inclusion in the study.
Assessment of Agitation
Agitation was evaluated using the Positive and Negative Syndrome Scale-Excited Component questionnaire (PANSS-EC). The PANSS-EC is a five-item scale that includes the following items: excitement, hostility, tension, uncooperativeness and poor impulse control. Each item is rated from 1 (absent) to 7 (extreme), hence the PANSS-EC total score can range from 5 to 35. A PANSS-EC total score ≥14 with one or more items scoring ≥4 is considered to indicate the presence of agitation symptoms. In this study, the patients with schizophrenia were classified as having an absence of agitation (PANSS-EC score <14) or the presence of agitation (PANSS-EC score ≥14) based on the methods used in previous studies.11,36
Biochemical Measurements
A 5-mL sample of fasting venous blood was collected from each patient with schizophrenia between 7:00 am and 7:30 am on the day after admission to hospital. Blood samples from participants in the healthy control group were also collected between 7:00 am and 7:30 am. The blood samples were centrifuged at 3000g for 10 minutes at 4°C. CRP concentration was determined using an enhanced immunoturbidimetric method. Peripheral levels of FT4, FT3 and TSH were measured using an Architect i2000sr immunoassay analyzer (Abbott Laboratories; Lake Bluff, IL, USA). Serum levels of triglycerides (TG), cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, fasting plasma glucose, creatinine and UA were determined using a Cobas C702 automatic biochemical analyzer (Roche, Basel, Switzerland). All samples were analyzed by the same analyst, who was blind to the sample sources.
Statistical Analysis
All data were analyzed using SPSS 24.0 software (IBM, Armonk, NY, USA). Continuous data were tested for normality with the Kolmogorov–Smirnov test. Normally-distributed continuous data are expressed as mean ± standard deviation (SD), non-normally-distributed continuous data are expressed as median (interquartile range), and categorical data are expressed as n (%). Group differences in categorical variables were examined using χ2 analyses. Some data were not normally distributed. Thus, differences between groups were calculated by non-parametric Kruskal–Wallis H-tests and Mann–Whitney U-tests. The Bonferroni correction method was used for multiple comparisons. Normally-distributed data were compared between groups using one-way ANOVA and the LSD post-hoc test. In addition, creatinine and UA levels were compared among groups after controlling for the potentially confounding effects of variables that significantly differed among groups in univariate analyses using ANCOVA test. Univariate and multivariate forward logistic regression analyses were used to identify factors associated with agitation. Factors returning P < 0.01 in the univariate analysis were entered into the multivariate analysis. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated. Receiver operating characteristic (ROC) analysis was used to show the use of CRP, FT3, TSH and Creatinine in differentiating between schizophrenia with and without agitation. All statistical tests were two-tailed, and P < 0.05 was considered significant.
Results
Demographic and Clinical Characteristics
The final analysis included 71 patients with schizophrenia and agitation, 83 patients with schizophrenia who did not have agitation, and 75 healthy control subjects. The demographic and clinical characteristics of the patients and healthy controls are presented in Table 1. Patients with schizophrenia and agitation were significantly younger than patients without agitation (P = 0.015) or healthy controls (P < 0.001). The healthy control group had a significantly higher proportion of married subjects than the schizophrenia with agitation group (P < 0.001) or the schizophrenia without agitation group (P < 0.001). The proportion of people employed was also significantly higher in the healthy control group than in the schizophrenia with agitation group (P < 0.001) or schizophrenia without agitation group (P < 0.001). Among the patients with schizophrenia, there were no significant differences in education level, smoking status, body mass index, duration of illness, age at onset, proportion with first-episode schizophrenia or family history of psychiatric disorder between those with agitation and those without agitation. The PANSS-EC score was significantly higher in patients with schizophrenia and agitation than in patients without agitation (P < 0.001).
Table 1.
Demographic and Clinical Characteristics of the Study Participants
| Characteristic | Healthy Controls (n = 75) | Schizophrenia without Agitation (n = 83) | Schizophrenia with Agitation (n = 71) | P | SCZ with Agitation-HCs | SCZ without Agitation-HCs | SCZ with Agitation- SCZ without Agitation |
|---|---|---|---|---|---|---|---|
| Bonferroni | Bonferroni | Bonferroni | |||||
| Demographic Data | |||||||
| Gender | 0.746 | - | - | - | |||
| Male | 35 (46.7%) | 37 (44.6%) | 36 (50.7%) | ||||
| Female | 40 (53.3%) | 46 (55.4%) | 35 (49.3%) | ||||
| Age (years) | 35 (30–46) | 34 (29–47) | 30 (22–39)a, b | <0.001 | <0.001 | 0.814 | 0.015 |
| Married | 69 (92.0%) | 30 (36.1%)a | 29 (40.8%)a | <0.001 | - | - | - |
| Employed | 75 (100%) | 48 (57.8%)a | 45 (63.4%)a | <0.001 | - | - | - |
| Education (years) | - | 12 (9–12) | 12 (9–12) | 0.568 | - | - | - |
| Smoking status | - | 0.878 | - | - | - | ||
| Smokers | 19 (22.9%) | 17 (23.9%) | |||||
| Non-smokers | 64 (77.1%) | 54 (76.1%) | |||||
| Body mass index (kg/m2) | - | 23.3±3.5 | 23.9±4.3 | 0.298 | - | - | - |
| Clinical Data | |||||||
| Illness duration (years) | - | 7 (2–17) | 6 (2–9) | 0.122 | - | - | - |
| First episode | - | 18 (21.7%) | 19 (26.8%) | 0.463 | - | - | - |
| Age at onset (years) | - | 25 (20–31) | 21 (18–29) | 0.114 | - | - | - |
| Family history of psychiatric disorder | - | 21 (25.3%) | 21 (29.6%) | 0.553 | - | - | - |
| Agitation Rating | |||||||
| PANSS-EC score | - | 11 (9–12) | 21 (19–24)b | <0.001 | - | - | - |
Notes: Data are presented as n (%) or median (interquartile range) or mean ± standard deviation. aP < 0.05 vs healthy controls; bP < 0.05 vs patients without agitation.
Abbreviations: SCZ with agitation, schizophrenia with agitation; SCZ without agitation, schizophrenia without agitation; HCs, healthy controls; PANSS-EC, Positive and Negative Syndrome Scale-Excited Component scale.
Comparison of Biochemical Indices Between the Three Groups of Patients
The biochemical indices are compared between groups in Table 2. Serum FT3 level was significantly lower in patients without agitation than in patients with agitation (P < 0.001) or healthy controls (P < 0.001), but there was no significant difference between the latter two groups. Serum FT4 level was significantly higher in patients with agitation than in patients without agitation (P = 0.002) or healthy controls (P = 0.011). Furthermore, serum TSH level was significantly lower in patients with agitation than in patients without agitation (P = 0.022).
Table 2.
Comparison of Serum Biochemical Indices Between the Three Groups
| Parameter | Healthy Controls (n = 75) | Schizophrenia without Agitation (n = 83) | Schizophrenia with Agitation (n = 71) | P | SCZ with Agitation-HCs | SCZ without Agitation-HCs | SCZ with Agitation- SCZ without Agitation |
|---|---|---|---|---|---|---|---|
| Bonferroni | Bonferroni | Bonferroni | |||||
| CRP (mg/L) | 0.58 (0.18–1.41) | 0.87 (0.33–20.60) | 1.73 (0.54–4.30)a, b | <0.001 | <0.001 | 0.114 | 0.038 |
| FT4 (ng/dL) | 0.99 (0.90–1.06) | 0.96 (0.81–1.16) | 1.06 (0.94–1.22)a, b | 0.001 | 0.011 | 1 | 0.002 |
| FT3 (pg/mL) | 2.97 ± 0.35 | 2.62 ± 0.48a | 2.95 ± 0.40b | <0.001 | - | - | - |
| TSH (µIU/mL) | 2.17 (1.65–2.67) | 2.54 (1.67–4.20) | 2.02 (1.39–2.66)b | 0.035 | 0.852 | 0.323 | 0.022 |
| TG (mmol/L) | 1.15 (0.82–1.70) | 1.30 (0.74–2.07) | 1.15 (0.80–1.43) | 0.357 | - | - | - |
| CHO (mmol/L) | 4.54 ± 0.88 | 4.40 ± 0.91 | 4.42 ± 0.98 | 0.635 | - | - | - |
| HDL-C (mmol/L) | 1.35 ± 0.26 | 1.23 ± 0.25a | 1.20 ± 0.24a | 0.001 | - | - | - |
| LDL-C (mmol/L) | 2.61 ± 0.64 | 2.63 ± 0.76 | 2.56 ± 0.62 | 0.834 | - | - | - |
| FPG (mmol/L) | 4.89 (4.63–5.27) | 4.60 (4.40–4.90)a | 4.60 (4.30–5.00)a | <0.001 | <0.001 | 0.001 | 1 |
| Creatinine (µmol/L) | 70.49 ± 14.03 | 83.2 ± 17.14a | 73.65 ± 16.85b | <0.001 | - | - | - |
| UA (µmol/L) | 306.04 ± 80.18 | 327.34 ± 100.76 | 378.10 ± 96.16a, b | <0.001 | - | - | - |
Notes: Data are presented as median (interquartile range) or mean ± standard deviation. aP < 0.05 vs healthy controls; bP < 0.05 vs patients without agitation.
Abbreviations: CHO, total cholesterol; CRP, C-reactive protein; FPG, fasting plasma glucose; FT3, free triiodothyronine; FT4, free thyroxine; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; TG, triglycerides; TSH, thyroid-stimulating hormone; UA, uric acid; SCZ with agitation, schizophrenia with agitation; SCZ without agitation, schizophrenia without agitation; HCs, healthy controls.
Creatinine level was significantly higher in patients without agitation than in patients with agitation (P < 0.001) or healthy controls (P < 0.001), but there was no significant difference between the latter two groups. Serum HDL-C level was significantly lower in patients without agitation (P = 0.002) and patients with agitation (P < 0.001) than in healthy control subjects, but the levels were comparable between the two groups of patients with schizophrenia. Serum creatinine level was significantly higher in patients without agitation than that in patients with agitation (p < 0.001) or healthy controls (p < 0.001). Serum UA level was significantly elevated in patients with agitation as compared to patients without agitation (P = 0.001) or healthy controls (P < 0.001), and there was no significant difference between the latter two groups. Serum CRP level was also significantly higher in patients with agitation than in patients without agitation (P = 0.038) or healthy controls (P < 0.001).
Analysis of covariance showed that serum creatinine and UA level differences among the three groups remained statistically significant after adjusting for age, marriage and employee status (F= 11.467, P < 0.001; F=7.072, P = 0.001, respectively). Further pairwise comparisons indicated that schizophrenia without agitation had higher serum creatinine levels than schizophrenia with agitation (P = 0.001) and healthy controls (P < 0.001), respectively. Further pairwise comparisons indicated that schizophrenia with agitation had higher serum UA levels than schizophrenia without agitation (P = 0.011) and healthy controls (P = 0.003), respectively.
Factors Associated with Agitation in Schizophrenia
The results of the binary logistic regression analyses are detailed in Table 3. Univariate analysis indicated that age, CRP level, FT4 level, FT3 level, TSH level, creatinine level, TG level and UA level were associated with agitation in patients with schizophrenia (Table 3). Multivariate regression analysis revealed that increased levels of CRP (OR = 1.470, 95% CI = 1.180–1.832, P = 0.001) and FT3 (OR = 13.026, 95% CI = 4.226–40.143, P < 0.001) and decreased levels of TSH (OR = 0.758, 95% CI = 0.588–0.977, P = 0.033) and creatinine (OR = 0.965, 95% CI = 0.941–0.988, P = 0.004) were independently associated with agitation in patients with schizophrenia.
Table 3.
Logistic Regression Analysis of Factors Associated with Agitation in Patients with Schizophrenia
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 0.961 | 0.933–0.990 | 0.009 | |||
| Smoking status | 1.060 | 0.502–2.240 | 0.878 | |||
| Body mass index | 1.046 | 0.963–1.135 | 0.288 | |||
| C-reactive protein | 1.298 | 1.104–1.527 | 0.002 | 1.470 | 1.180–1.832 | 0.001 |
| Free thyroxine | 8.066 | 1.652–39.384 | 0.010 | |||
| Free triiodothyronine | 6.172 | 2.573–14.802 | <0.001 | 13.026 | 4.226–40.143 | <0.001 |
| Thyroid-stimulating hormone | 0.733 | 0.586–0.917 | 0.007 | 0.758 | 0.588–0.977 | 0.033 |
| Triglycerides | 0.590 | 0.386–0.902 | 0.015 | |||
| Creatinine | 0.967 | 0.948–0.987 | 0.001 | 0.965 | 0.941–0.988 | 0.004 |
| Uric acid | 1.005 | 1.002–1.009 | 0.003 | |||
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
Receiver Operating Characteristics (ROC) for CRP, FT3, TSH and Creatinine for the Diagnosis of Schizophrenia with Agitation
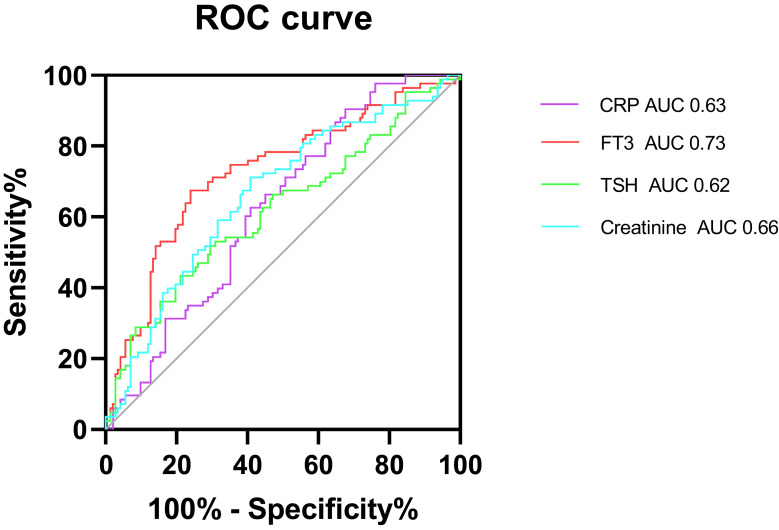
The ROC analysis revealed that the cut-off value of CRP to predict schizophrenia with agitation was 3.470 mg/L with an area under the curve (AUC) of 0.626 (95% CI: 0.536–0.716) and with a sensitivity of 32% and a specificity of 90%, the cut-off value of FT3 to predict schizophrenia with agitation was 2.785 pg/mL with the AUC of 0.728 (95% CI: 0.647–0.808) and with a sensitivity of 75% and a specificity of 68%, the cut-off value of TSH to predict schizophrenia with agitation was 2.720 µIU/mL with the AUC of 0.620 (95% CI: 0.532–0.708) and with a sensitivity of 79% and a specificity of 43%, and the cut-off value of creatinine to predict schizophrenia with agitation was 75.758 µmol/L with the AUC of 0.663 (95% CI: 0.576–0.749) and with a sensitivity of 58% and a specificity of 71% (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curves for the diagnostic ability of CRP, FT3, TSH and Creatinine (Schizophrenia with agitation vs Schizophrenia without agitation). ROC curves for CRP, FT3, TSH and Creatinine values for the diagnosis of Schizophrenia with agitation. CRP: AUC 0.626 (95% CI=0.536 to 0.716), P =0.007; FT3: AUC 0.728 (95% CI=0.647 to 0.808), P <0.001; TSH: AUC 0.620 (95% CI=0.532 to 0.708), P =0.011; Creatinine: AUC 0.663 (95% CI=0.576 to 0.749), P =0.001.
Abbreviation: AUC, area under the ROC curve.
Discussion
The main objective of the present study was to evaluate whether biochemical parameters might be associated with agitation in schizophrenia. A notable finding was that patients with schizophrenia and agitation had higher serum levels of CRP, FT3, FT4 and UA as well as lower serum levels of TSH and creatinine than patients without agitation. Furthermore, logistic regression analysis and ROC analysis revealed that agitation in schizophrenia was associated with higher serum levels of CRP and FT3 and lower serum levels of TSH and creatinine. To the best of our knowledge, this is the first study to investigate differences in thyroid hormones, UA and creatinine between inpatients with schizophrenia who have agitation and those without agitation. We anticipate that our findings will facilitate future research efforts to develop a panel of biomarkers that could be used to screen patients with schizophrenia and identify those at high risk of developing agitation.
The evidence of biomarkers associated with agitation has been found in previous studies. One previous review reported agitation/aggression was the most consistent neuropsychiatric symptom related to core CSF biomarkers (amyloid/tau) in Alzheimer’s disease (AD).37 Another systematic review showed six classes of biomarkers associated with agitation in AD, including neuropathological, neurotransmitter, neuroimaging, apolipoprotein E (APOE) genotype, inflammatory, and clusterin.38 In the study of Mirko and Vassilios, they reported genetic and epigenetic markers had predictive role in aggression of severe mental illness (including schizophrenia, bipolar disorder, autism spectrum disorder, and attention-deficit/hyperactivity disorder), meanwhile they reported plausible connections between gut microbiota and aggressiveness in autism spectrum disorder.39 A meta-analysis including 26 studies showed CRP concentrations in schizophrenia were significantly higher than controls and CRP also appeared to be positively associated with severity of positive symptoms.24 To date, few studies have reported the effects of thyroid hormones, UA and creatinine on agitation in hospitalized patients with schizophrenia.
Our comparative cross-sectional study showed that patients with schizophrenia and agitation had a higher serum CRP concentration than patients without agitation, which is consistent with two previous reports.21,40 Most studies have concluded that the CRP level is higher in patients with schizophrenia than in healthy controls.20,22,24,41 However, we found that patients with schizophrenia and agitation had a higher CRP level than healthy controls, whereas no significant difference was observed between patients without agitation and healthy controls. One possible explanation for this apparent discrepancy is that the patients with schizophrenia enrolled in previous studies included both those with agitation and those without agitation, whereas we analyzed them as two separate groups. Patients with schizophrenia who have agitation are in an acute phase in which inflammatory processes have more potential to be activated. Our logistic regression analysis found that CRP was a risk factor for agitation in schizophrenia. Prior investigations have shown that the CRP level is positively correlated with the severity of positive symptoms in schizophrenia.24,41 Moreover, other studies of patients with schizophrenia have reported that the CRP level is positively correlated with the verbal aggression score of the Modified Overt Aggression Scale (MOAS),22 and with aggressive behavior evaluated by the PANSS-EC.21 CRP is also positively associated with agitation or hostility in patients with other psychiatric disorders.42–44 A recent meta-analysis reported a significant relationship between elevated CRP levels and cognitive impairment in schizophrenia.45 Based on the available data, it is plausible that inflammation is involved in the pathogenesis of schizophrenia and that the serum CRP level might be a candidate biological marker of agitation in patients with schizophrenia. Moreover, A recent study examined C-reactive protein to albumin ratio (CAR), which is considered more sensitive in representing inflammatory status, in both clinically exacerbated and remitted schizophrenia patients.46 Hence, a study is needed to further explore the relationship between CAR and agitation in first-episode and drug-naïve schizophrenia.
We also found that patients with schizophrenia had higher FT3 and FT4 levels and a lower TSH level than those without agitation. By contrast, an animal study reported no differences in the serum levels of FT3, FT4 and TSH between aggressive dogs and non-aggressive dogs,47 while patients with high aggression levels 28 days after alcohol withdrawal had lower serum levels of FT3 and FT4.48 Some studies have described thyroid dysfunction in patients with schizophrenia spectrum disorders.49,50 Moreover, there is evidence that patients with first-episode schizophrenia have higher FT3 and FT4 levels than healthy controls,51,52 as well as higher FT3 and FT4 levels and a lower TSH level than patients with recurrence of schizophrenia.52 However, some investigators have reported that patients with schizophrenia using antipsychotic medications had a lower FT4 level than healthy controls,26,53 and that a lower FT4 level was associated with the use of antipsychotics in regression analyses.53 Notably, our study detected a significantly higher FT4 level and a significantly lower TSH level only in patients with schizophrenia and agitation and not in those without agitation. Numerous factors might contribute to the differing results between these various studies, including differences in sample sizes, cohort characteristics (including ethnicity), disease phase and/or antipsychotic medication use. Notably, our logistic regression analysis identified increased FT3 level and decreased TSH level as risk factors for agitation in schizophrenia. Published data are limited regarding the relationships between thyroid hormones and agitation in schizophrenia. One previous study of patients with schizophrenia suggested a positive correlation between the serum FT4 level and the severity of psychiatric symptomatology as assessed by the Brief Psychiatric Rating Scale. However, the results of studies evaluating patients with other psychiatric diseases are in line with our data: one investigation described a positive association between FT3 level and restless-impulsive ratings in patients with childhood conduct disorder,54 while another reported agitation as an explanatory variable for the reduction in TSH seen in patients with depression.55 There is evidence that thyroid hormones regulate the levels of dopamine receptors,56,57 hence thyroid dysfunction could potentially play a role in the pathogenesis of schizophrenia. Further research is merited to establish whether serum levels of FT3 and TSH might be candidate biomarkers for agitation in patients with schizophrenia.
Another observation made in this study was that serum UA was significantly higher in patients with schizophrenia and agitation than in patients without agitation. There has been debate regarding the relationship between serum UA level and schizophrenia. Some studies have described higher serum levels of UA in patients with schizophrenia than in healthy controls,35,58 whereas a meta-analysis reported lower UA levels in subjects with first-episode psychosis.59 In the present study, only patients with schizophrenia and agitation had higher UA levels than healthy controls. We speculate that the inconsistencies between studies may be due in part to differences in the disease phase and sample sizes. However, our logistic regression analysis did not identify serum UA as a factor associated with agitation in schizophrenia, whereas a decrease in the level of serum creatinine was found to be a risk factor. Previous investigations have reported that patients with schizophrenia exhibit reductions in urinary creatinine concentration and peripheral blood mononuclear cells when compared with healthy controls.60,61 However, our study found that the serum creatinine level was significantly lower in healthy controls and patients with schizophrenia and agitation than in patients without agitation. Possible reasons for the apparent discrepancy include the different sample types used for creatinine analysis and differing cohort sizes. Moreover, our results can be explained from the perspective of energy utilization. Patients with schizophrenia and agitation tend to consume more energy. Creatinine is directly associated with energy metabolism.60 The tricarboxylic acid cycle produces ATP, which can be used to convert creatine to phosphocreatine as a rapidly mobilizable reserve of energy.62 A lower serum creatinine level in patients with agitation implies increased energy production and energy conversion. Hence, serum creatinine might be a candidate biological marker of agitation in patients with schizophrenia. Nevertheless, further research will be required to clarify whether there is a relationship between serum creatinine level and agitation in schizophrenia.
We constructed ROC curves to determine optimum cut-off levels of CRP, FT3, TSH and creatinine for the diagnosis schizophrenia with agitation. The cut-off value of CRP, FT3, FT4 and creatinine found in our study were 3.470, 2.785, 2.720 and 75.758, respectively. To date, few studies have reported cut-off levels of CRP, FT3, TSH and creatinine. Despite there still existing copious room for research to appreciate its diagnostic use, our finding suggests that CRP, FT3, TSH and creatinine may be used to support the diagnosis of schizophrenia with agitation.
Several limitations of the present study should be mentioned. First, the number of individuals in our study was relatively small, and we did not consider the clinical subtype of schizophrenia (including paranoid, catatonic, hebephrenic, undifferentiated and residual types) as a possible confounding factor. Thus, our results will need to be verified by a large-scale study that better addresses possible confounding factors. Second, the cross-sectional design precluded dynamic measurements of biochemical indices and agitation scores over time. A prospective and longitudinal study will be needed to demonstrate a direct causal relationship between CRP, thyroid hormones, creatinine and agitation in schizophrenia. Third, patients with agitation are more likely to have an irregular diet, which may have influenced the routine biochemical indices measured in our study. Fourth, prior investigations have indicated that the hypothalamic–pituitary–adrenal axis and sex hormones may be involved in agitation. However, we did not measure the levels of corticotrophin releasing hormone, adrenocorticotrophic hormone, cortisol or sex hormones in this study. Further research is merited to measure these related hormones and explore their roles in the agitation of schizophrenia. Fifth, although the PANSS-EC is widely used for the assessment of agitation, alternative questionnaires are available such as the MOAS.63 Therefore, our results will need to be verified in a further study using MOAS to assess agitation in schizophrenia.
Conclusion
Increased levels of serum CRP and FT3 and decreased levels of serum TSH and creatinine may be risk factors for agitation in schizophrenia. Our results indicate that inflammation, thyroid hormones and kidney function might be biological markers for agitation in schizophrenia and potentially involved in its pathogenesis.
Acknowledgments
The authors would like to thank all the patients with schizophrenia and healthy volunteers who supported and participated in this study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Abbreviations
CRP, C-reactive protein; FT3, free triiodothyronine; FT4, free thyroxine; UA, Uric acid; ICD-10, International Classification of Disease-10; PANSS-EC, Positive and Negative Syndrome Scale-Excited Component questionnaire; TG, triglycerides; SD, standard deviation; ORs, odds ratios; 95% Cis, 95% confidence intervals.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of Shandong Mental Health Center (2019-R43). All patients with schizophrenia and healthy volunteers provided informed written consent for inclusion in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Borelli CM, Solari H. Schizophrenia. JAMA. 2019;322(13):1322. doi: 10.1001/jama.2019.11073 [DOI] [PubMed] [Google Scholar]
- 2.Gubert C, Kong G, Uzungil V, et al. Microbiome profiling reveals gut dysbiosis in the metabotropic glutamate receptor 5 knockout mouse model of schizophrenia. Front Cell Developmental Biol. 2020;8:582320. doi: 10.3389/fcell.2020.582320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet (London, England). 2016;388(10039):86–97. doi: 10.1016/S0140-6736(15)01121-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657 [DOI] [PubMed] [Google Scholar]
- 5.Fleischhacker WW, Arango C, Arteel P, et al. Schizophrenia–time to commit to policy change. Schizophr Bull. 2014;40 Suppl 3(Suppl3):S165–194. doi: 10.1093/schbul/sbu006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165–172. doi: 10.5811/westjem.2015.12.28763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang SZ, Mu YG, Liu Q, et al. Prescription practices in the treatment of agitation in newly hospitalized Chinese schizophrenia patients: data from a non-interventional naturalistic study. BMC Psychiatry. 2019;19(1):216. doi: 10.1186/s12888-019-2192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.San L, Marksteiner J, Zwanzger P, et al. State of acute agitation at psychiatric emergencies in europe: the STAGE study. CP & EMH. 2016;12:75–86. doi: 10.2174/1745017901612010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mi W, Zhang S, Liu Q, et al. Prevalence and risk factors of agitation in newly hospitalized schizophrenia patients in China: an observational survey. Psychiatry Res. 2017;253:401–406. doi: 10.1016/j.psychres.2017.02.065 [DOI] [PubMed] [Google Scholar]
- 10.Lesem MD, Tran-Johnson TK, Riesenberg RA, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198(1):51–58. doi: 10.1192/bjp.bp.110.081513 [DOI] [PubMed] [Google Scholar]
- 11.Pratts M, Citrome L, Grant W, Leso L, Opler LA. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61–68. doi: 10.1111/acps.12262 [DOI] [PubMed] [Google Scholar]
- 12.Pacciardi B, Calcedo A, Messer T. Inhaled loxapine for the management of acute agitation in bipolar disorder and schizophrenia: expert review and commentary in an era of change. Drugs R D. 2019;19(1):15–25. doi: 10.1007/s40268-019-0262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahn S, Noll R, Barnes A, Schwarz E, Guest PC. Challenges of introducing new biomarker products for neuropsychiatric disorders into the market. Int Rev Neurobiol. 2011;101:299–327. [DOI] [PubMed] [Google Scholar]
- 14.Quintero M, Stanisic D, Cruz G, Pontes JGM, Costa T, Tasic L. Metabolomic biomarkers in mental disorders: bipolar disorder and schizophrenia. Adv Exp Med Biol. 2019;1118:271–293. [DOI] [PubMed] [Google Scholar]
- 15.Lai CY, Scarr E, Udawela M, Everall I, Chen WJ, Dean B. Biomarkers in schizophrenia: a focus on blood based diagnostics and theranostics. World J Psychiatry. 2016;6(1):102–117. doi: 10.5498/wjp.v6.i1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44(5):973–982. doi: 10.1093/schbul/sby024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102–112. doi: 10.1016/j.schres.2014.04.041 [DOI] [PubMed] [Google Scholar]
- 18.Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21(8):1009–1026. doi: 10.1038/mp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsolini L, Sarchione F, Vellante F, et al. Protein-C reactive as biomarker predictor of schizophrenia phases of illness? A systematic review. Curr Neuropharmacol. 2018;16(5):583–606. doi: 10.2174/1570159X16666180119144538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Li P, Chi D, Wu T, Mei Z, Cui G. Association between C-reactive protein and risk of schizophrenia: an updated meta-analysis. Oncotarget. 2017;8(43):75445–75454. doi: 10.18632/oncotarget.17995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barzilay R, Lobel T, Krivoy A, Shlosberg D, Weizman A, Katz N. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8–12. doi: 10.1016/j.eurpsy.2015.09.461 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Hong W, Li H, et al. Increased ratio of high sensitivity C-reactive protein to interleukin-10 as a potential peripheral biomarker of schizophrenia and aggression. Int J Psychophysiol. 2017;114:9–15. doi: 10.1016/j.ijpsycho.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 23.Bolu A, Aydın MS, Akgün A, et al. Serum levels of high sensitivity C-reactive protein in drug-naïve first-episode psychosis and acute exacerbation of schizophrenia. Clin Psychopharmacol Neurosci. 2019;17(2):244–249. doi: 10.9758/cpn.2019.17.2.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes BS, Steiner J, Bernstein HG, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21(4):554–564. doi: 10.1038/mp.2015.87 [DOI] [PubMed] [Google Scholar]
- 25.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. doi: 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telo S, Bilgic S, Karabulut N. Thyroid hormone levels in chronic schizophrenic patients: association with psychopathology. West Indian Med J. 2016;65(2):312–315. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Tang Z, Ruan Y, et al. Prolactin and thyroid stimulating hormone (TSH) levels and sexual dysfunction in patients with schizophrenia treated with conventional antipsychotic medication: a cross-sectional study. Med Sci Monitor. 2018;24:9136–9143. doi: 10.12659/MSM.913759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrikis P, Tigas S, Tzallas AT, Archimandriti DT, Skapinakis P, Mavreas V. Prolactin levels in drug-naïve patients with schizophrenia and other psychotic disorders. Int J Psychiatry Clin Pract. 2016;20(3):165–169. doi: 10.1080/13651501.2016.1197274 [DOI] [PubMed] [Google Scholar]
- 29.Jose J, Nandeesha H, Kattimani S, Meiyappan K, Sarkar S, Sivasankar D. Association between prolactin and thyroid hormones with severity of psychopathology and suicide risk in drug free male schizophrenia. Clin Chim Acta. 2015;444:78–80. doi: 10.1016/j.cca.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Solberg DK, Refsum H, Andreassen OA, Bentsen H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta neuropsychiatrica. 2019;31(4):202–212. doi: 10.1017/neu.2019.14 [DOI] [PubMed] [Google Scholar]
- 31.Wei C, Sun Y, Chen N, Chen S, Xiu M, Zhang X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology. 2020;111:104473. doi: 10.1016/j.psyneuen.2019.104473 [DOI] [PubMed] [Google Scholar]
- 32.Fraguas D, Díaz-Caneja CM, Ayora M, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2019;45(4):742–751. doi: 10.1093/schbul/sby125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. JAD. 2010;19(4):1331–1336. doi: 10.3233/JAD-2010-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao JK, Dougherty GG Jr, Reddy RD, et al. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS One. 2010;5(3):e9508. doi: 10.1371/journal.pone.0009508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Z, Wen T, Wang Y, Kan W, Xun G. Peripheral non-enzymatic antioxidants in patients with schizophrenia: a case-control study. BMC Psychiatry. 2020;20(1):241. doi: 10.1186/s12888-020-02635-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry. 2002;59(5):441–448. doi: 10.1001/archpsyc.59.5.441 [DOI] [PubMed] [Google Scholar]
- 37.Showraki A, Murari G, Ismail Z, et al. Cerebrospinal fluid correlates of neuropsychiatric symptoms in patients with alzheimer’s disease/mild cognitive impairment: a systematic review. JAD. 2019;71(2):477–501. doi: 10.3233/JAD-190365 [DOI] [PubMed] [Google Scholar]
- 38.Ruthirakuhan M, Lanctôt KL, Di Scipio M, Ahmed M, Herrmann N. Biomarkers of agitation and aggression in Alzheimer’s disease: a systematic review. Alzheimer’s Dementia. 2018;14(10):1344–1376. doi: 10.1016/j.jalz.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 39.Manchia M, Fanos V. Targeting aggression in severe mental illness: the predictive role of genetic, epigenetic, and metabolomic markers. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:32–41. doi: 10.1016/j.pnpbp.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 40.Kachouchi A, Sebbani M, Akammar S, et al. C-reactive protein and agitation in patients with schizophrenia: a cohort study with a control group. L’Encephale. 2020;46(4):264–268. doi: 10.1016/j.encep.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 41.Steiner J, Frodl T, Schiltz K, et al. Innate immune cells and C-reactive protein in acute first-episode psychosis and schizophrenia: relationship to psychopathology and treatment. Schizophr Bull. 2020;46(2):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71(2):158–165. doi: 10.1001/jamapsychiatry.2013.3297 [DOI] [PubMed] [Google Scholar]
- 43.Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22(5):753–761. doi: 10.1016/j.bbi.2007.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coccaro EF. Association of C-reactive protein elevation with trait aggression and hostility in personality disordered subjects: a pilot study. J Psychiatr Res. 2006;40(5):460–465. doi: 10.1016/j.jpsychires.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol Med. 2019;49(12):1971–1979. doi: 10.1017/S0033291719001685 [DOI] [PubMed] [Google Scholar]
- 46.Balcioglu YH, Kirlioglu SS. C-reactive protein/albumin and neutrophil/albumin ratios as novel inflammatory markers in patients with schizophrenia. Psychiatry Investig. 2020;17(9):902–910. doi: 10.30773/pi.2020.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radosta LA, Shofer FS, Reisner IR. Comparison of thyroid analytes in dogs aggressive to familiar people and in non-aggressive dogs. Veterinary J. 2012;192(3):472–475. doi: 10.1016/j.tvjl.2011.06.029 [DOI] [PubMed] [Google Scholar]
- 48.Ozsoy S, Esel E, Izgi HB, Sofuoglu S. Thyroid function in early and late alcohol withdrawal: relationship with aggression, family history, and onset age of alcoholism. Alcohol Alcoholism. 2006;41(5):515–521. doi: 10.1093/alcalc/agl056 [DOI] [PubMed] [Google Scholar]
- 49.Radhakrishnan R, Calvin S, Singh JK, Thomas B, Srinivasan K. Thyroid dysfunction in major psychiatric disorders in a hospital based sample. Indian J Med Res. 2013;138(6):888–893. [PMC free article] [PubMed] [Google Scholar]
- 50.Santos NC, Costa P, Ruano D, et al. Revisiting thyroid hormones in schizophrenia. J Thyroid Res. 2012;2012:569147. doi: 10.1155/2012/569147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiibinu MO, Ogundahunsi OA, Ogunyemi EO. Inter-relationship of plasma markers of oxidative stress and thyroid hormones in schizophrenics. BMC Res Notes. 2012;5:169. doi: 10.1186/1756-0500-5-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y, Ji H, Tao L, et al. Functional status of hypothalamic-pituitary-thyroid and hypothalamic-pituitary-adrenal axes in hospitalized schizophrenics in Shanghai. Front Psychiatry. 2020;11:65. doi: 10.3389/fpsyt.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vedal TSJ, Steen NE, Birkeland KI, et al. Free thyroxine and thyroid-stimulating hormone in severe mental disorders: a naturalistic study with focus on antipsychotic medication. J Psychiatr Res. 2018;106:74–81. doi: 10.1016/j.jpsychires.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 54.Dmitrieva TN, Oades RD, Hauffa BP, Eggers C. Dehydroepiandrosterone sulphate and corticotropin levels are high in young male patients with conduct disorder: comparisons for growth factors, thyroid and gonadal hormones. Neuropsychobiology. 2001;43(3):134–140. doi: 10.1159/000054881 [DOI] [PubMed] [Google Scholar]
- 55.Corrigan MH, Gillette GM, Quade D, Garbutt JC. Panic, suicide, and agitation: independent correlates of the TSH response to TRH in depression. Biol Psychiatry. 1992;31(10):984–992. doi: 10.1016/0006-3223(92)90092-E [DOI] [PubMed] [Google Scholar]
- 56.Crocker AD, Overstreet DH, Crocker JM. Hypothyroidism leads to increased dopamine receptor sensitivity and concentration. Pharmacol Biochem Behav. 1986;24(6):1593–1597. doi: 10.1016/0091-3057(86)90491-0 [DOI] [PubMed] [Google Scholar]
- 57.Crocker AD, Overstreet DH. Modification of the behavioural effects of haloperidol and of dopamine receptor regulation by altered thyroid status. Psychopharmacology. 1984;82(1–2):102–106. doi: 10.1007/BF00426390 [DOI] [PubMed] [Google Scholar]
- 58.Wen S, Cheng M, Wang H, et al. Serum uric acid levels and the clinical characteristics of depression. Clin Biochem. 2012;45(1–2):49–53. doi: 10.1016/j.clinbiochem.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 59.He Q, You Y, Yu L, et al. Uric acid levels in subjects with schizophrenia: a systematic review and meta-analysis. Psychiatry Res. 2020;292:113305. doi: 10.1016/j.psychres.2020.113305 [DOI] [PubMed] [Google Scholar]
- 60.Cai HL, Li HD, Yan XZ, et al. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naïve schizophrenia patients after treatment with risperidone. J Proteome Res. 2012;11(8):4338–4350. doi: 10.1021/pr300459d [DOI] [PubMed] [Google Scholar]
- 61.Karoum F, Karson CN, Bigelow LB, Lawson WB, Wyatt RJ. Preliminary evidence of reduced combined output of dopamine and its metabolites in chronic schizophrenia. Arch Gen Psychiatry. 1987;44(7):604–607. doi: 10.1001/archpsyc.1987.01800190020003 [DOI] [PubMed] [Google Scholar]
- 62.Liu ML, Zheng P, Liu Z, et al. GC-MS based metabolomics identification of possible novel biomarkers for schizophrenia in peripheral blood mononuclear cells. Mol Biosyst. 2014;10(9):2398–2406. doi: 10.1039/C4MB00157E [DOI] [PubMed] [Google Scholar]
- 63.Meyer LF, Telles LEB, Mecler K, Soares A, Alves RS, Valença AM. Schizophrenia and violence: study in a general psychiatric hospital with HCR-20 and MOAS. Trends Psychiatry Psychother. 2018;40(4):310–317. doi: 10.1590/2237-6089-2017-0039 [DOI] [PubMed] [Google Scholar]