Abstract
Palladium has proven to be effective in catalyzing the (hetero)annulation of C=C bonds with ambiphilic organo(pseudo)halides. Through the employment of appropriate ambiphilic coupling partners, efficient annulation of a variety of allenes, 1,3-dienes, strained alkenes, styrenes, and other C=C bond variants can be achieved to provide direct access to numerous useful hetero- and carbocyclic scaffolds. In this Feature Article, we summarize palladium-catalyzed (hetero)annulation methods reported since 2005 (spanning just over 15 years) and discuss outstanding challenges in this area of study.
1. Introduction
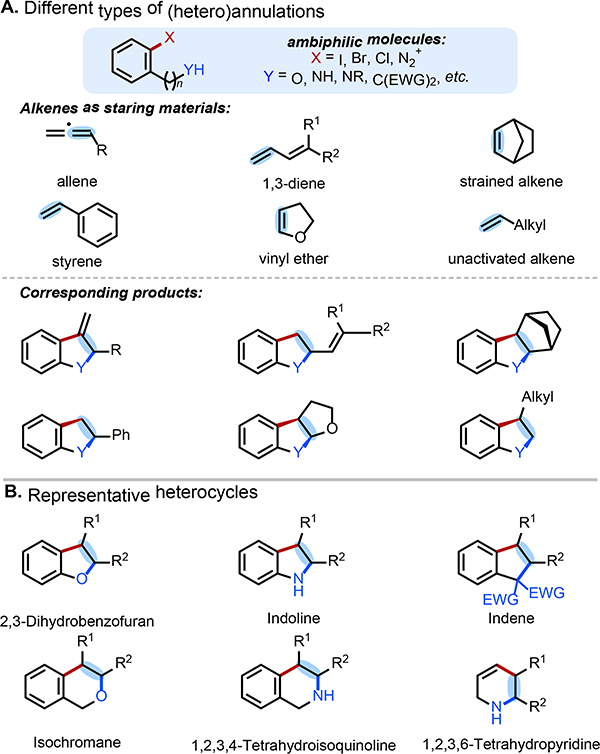
Heterocycles and carbocycles, such as indolines, piperidines, 2,3-dihydrobenzofurans and indanes, are common substructures in pharmaceuticals and natural products. Over the past few decades many new synthetic approaches to access these core structures have emerged,1 among which palladium-catalyzed (hetero)annulation of C=C bonds with ambiphilic aryl (pseudo)halides has proven to be particularly useful (Figure 1A).
Figure 1:
Representative C=C bond starting materials and heterocycle products.
Prior to 1990, few examples of Pd-catalyzed heteroannulation of C=C bonds existed. Additionally, the few protocols that were reported generally suffered from major drawbacks, including the use of stoichiometric palladium and toxic organomercury substrates.2 The first example of catalytic annulation between 2-iodoanilines and 1,3-dienes was reported by Dieck and co-workers in 1983; however, only two dienes, isoprene and 1,3-hexadiene were examined.3 Later, the Larock group successfully expanded this protocol to the heteroannulation of cyclic and acyclic 1,3-dienes with 2-iodo-phenols, -anilines, -benzylic amines and carbonyl ambiphilic coupling partners.4 Following these results, Pd-catalyzed heteroannulation of alkynes emerged, and this field initially progressed faster than that of C=C bond heteroannulation due to the increased reactivity of alkynes and the lack of competitive β-H elimination processes.5
Over the last thirty years, Pd-catalyzed C=C bond heteroannulation has sustained significant interest, and many advancements have been made by research groups worldwide. Currently, a broad array of alkene-based coupling-partners can be reliably annulated under Pd-catalyzed conditions. The ambiphilic molecules in such reactions are usually functionalized aryl or alkenyl halides, among which 2-halophenols and 2-haloanilines are most commonly employed to generate 2,3-dihydrobenzofurans and indolines respectively (Figure 1B). The heteroannulated products can be generated via various mechanistic pathways, dependent upon the coupling-partner, precatalyst, and conditions employed.
The Larock group has performed pioneering research in Pd-catalyzed heteroannulation over the past three decades and provided an insightful and comprehensive review of this field up to 2006.6,7 The aim of this review is to summarize more recent contributions (2005–2021), during which time more classes of C=C bonds have been integrated into this type of transformation and efforts to better understand the mechanisms and elucidate origins of chemo-, regio-, and stereoselectivity have been made. Additionally, important advances in developing enantioselective Pd-catalyzed annulation reactions have been described. This review is organized based on the type of C=C coupling partner employed: (2) allenes, (3) 1,3-dienes (4) strained alkenes, (5) styrenes, and (6) other C=C bond variants. We discuss the reaction mechanism that is operative with the various substrate and coupling partners involved and highlight the diversity of (hetero)annulated products that can be obtained. Overall, this review serves to showcase the exciting recent developments in this area and to discuss current challenges and opportunities to inspire future work.
2. Allenes
Allenes have been extensively investigated as π-components in (hetero)annulations with ambiphilic organo(pseudo)halides due to their increased reactivity compared to their alkene counterparts and their ability to undergo highly regioselective carbopalladation to form π-allyl–Pd(II) species.8 The Pd(0)-catalyzed heteroannulation of allenes is widely believed to proceed via oxidative addition of the Pd(0) catalyst (which is sometimes generated in situ from a Pd(II) precatalyst) into the Ar–X bond (X=Br, I, etc.) of the ambiphilic molecule to give 1, followed by coordination of the allene (2) and migratory insertion to give π-allyl–Pd(II) species 3 (Scheme 1).6 The π-allyl-Pd(II) species 3 can be formed in either a syn- or an anti-fashion. Deprotonation of the nucleophilic moiety (YH) in the presence of base (4) and attack onto π-allyl–Pd(II) intermediate 2 generates the annulated product.
Scheme 1:
The mechanism for Pd(0)-catalyzed heteroannulation of allenes.
Larock was a pioneer in this area, and since his first publication in the 1990s,9 many reports from other groups followed. For example, in 2006, Swamy and Chakravarty reported the coupling of phosphono-functionalized allenes with 2-iodophenols to generate phosphono-benzofurans/dihydrobenzofurans (Scheme 2).10 Due to the more electropositive nature of phosphorus compared to carbon, the products were found to have different structures than their non-phosphorylated counterparts. The desired heteroannulated products were isolated in moderate to good yields; however, in some cases a mixture of regioisomers was observed, reflecting competitive migratory insertion of the allene at either the α/β or β/γ position. Additionally, the heteroannulation of allenes with 2-iodobenzoic acids to form phosphono-isocoumarins in 61–80% yield was disclosed, and the phosphono moiety could be further functionalized via a Horner–Wadsworth–Emmons reaction to give alkene products. Building upon this, Swamy and co-workers published an additional report, in which they further expanded the scope to include 2-iodobenzyl alcohols, which formed benzopyrans in moderate yields under modified reaction conditions.11
Scheme 2:
Pd(0)-catalyzed reactions of allenylphosphonates and related allenes.
Heteroannulations of allenes bearing a heteroatom typically result in mixtures of regioisomers, with the product distribution dependent on the electronic effects of the heteroatom.12 To address this, Sakamoto and co-workers reported that Pd(OAc)2, in combination with P(o-Tol)3, catalyzes the heteroannulation of allenes bearing oxygen- or nitrogen-based substituents using a range of aryl iodides and -bromides via the pathway shown in Scheme 3.13 Both 5- and 6-membered heterocycles were generated in moderate to excellent yields. The catalytic cycle is proposed to proceed in a similar manner to that previously reported by Larock (see Scheme 1). In this case, the nucleophilic attack of the heteroatom onto π-allyl–Pd(II) intermediate 5a can occur at either the α- or γ-position of the allene relative to the heteroatom. However, under the reported conditions, only α-attack was observed, and the product was generated as a single regioisomer. This is most likely due to the α-position being more electropositive, owing to the proximal electronegative heteroatom. Alternatively, the heteroatom could assist in the elimination of Pd(0) as shown in intermediate 5b.
Scheme 3:
Highly regioselective Pd(0)-catalyzed annulation of allenes bearing heteroatom substituents.
In 2009, Li and Shi reported the annulation of diarylvinylidenecyclopropanes with 2-iodophenols to produce a range of heterocycles bearing cyclopropane moieties, which are prevalent in natural products (Scheme 4).14 The combination of PdCl2 and dppp (1,3-bis(diphenylphosphino)propane) was found to give 2,3-dihydrobenzofuran structures in moderate to good yields after 3–5 days. Different aryl groups at the terminal position of the allene were tolerated, and methyl- and chloro-substituents at the C4 position of 2-iodophenol were demonstrated. The reaction was expanded to heteroannulation with N-tosyl-protected 2-iodoanilines, to form 2,3-dihydro-1H-indole derivatives in moderate yields (30–64%) through the addition of Ag2CO3 and [Et3NH][BF4] additives. Ag2CO3 is proposed to abstract the iodide from the initial PdII(Ar)(I) oxidative addition intermediate. The resultant cationic PdII(Ar)+ species is stabilized by the BF4– anion from [Et3NH][BF4], resulting in a Pd(II) intermediate that is more reactive in migratory insertion (see Scheme 1).
Scheme 4:
Annulation of diarylvinylidenecyclopropanes.
Deagostino and co-workers were able to access a range of heteroannulated products in good yields from protected 3-alkyl-1,2-dienols and 2-iodophenols or protected 2-iodoanilines via development of a phosphine-free Pd(0)-catalyzed annulation reaction (Scheme 5).15 Here, the presence of DMSO/TBAB, was crucial to achieve the desired levels of stereoselectivity for the exocyclic C=C bond (60:40–99:1). Interestingly, 2-iodophenols and 2-iodoanilines bearing an electron-withdrawing N-protecting group generated 2-alkoxy-3-alkylidene-2,3-dihydrobenzofuranes and -indolines, respectively. Alternatively, N-methyl or N-benzyliodoanilines generated alkenyl indoles, which is most likely due to the more electron-rich N-atoms promoting elimination of the alkoxy group.
Scheme 5:
Phosphine-free Pd(0)-catalyzed heteroannulation of 2-alkyl-1,2-dienols.
In 2009, Ma, Yu and Shu reported a protocol for the Pd(0)-catalyzed enantioselective cyclization of allenes with 2-aminoiodobenzenes (Scheme 6).16 Key to their success was the development of novel chiral spiro-bisoxazoline ligand L1. Enantioenriched 3-alkylideneindolines were generated in good yields with excellent enantiomeric excess (94–98%). The reaction tolerates halogen-substituted 2-iodoanilines and a range of alkyl substituted allenes, giving the products with complete E-stereoselectivity.
Scheme 6:
Enantioselective annulation of allenes with 2-iodoanilines.
More recently, Zhang et al. reported that Pd(0)/PC-Phos catalyzes the enantioselective intramolecular denitrogenative annulation of allenes and N-allenamides with benzotriazoles (Scheme 7).17 Here, benzotriazoles undergo ring-opening to diazonium species 6 in situ via a Dimroth-type equilibrium.18 Enantioenriched 3-methyleneindolines were produced regioselectively in excellent yields (88–97%) and enantioselectivities (89–98% ee) for a range of diverse substrates. The reaction was successfully carried out on gram scale, and under modified conditions the protocol was expanded to N-allenamides (Pd(dba)2 (5 mol%), PC-Phos (7.5 mol%), NaBF4 (50 mol%), toluene, 40 °C; 19 examples, 88–93% yield, 80–95% ee). Notably, the reaction occurs under mild conditions, at a temperature of 40 °C, and in the absence of a base. The authors suggest this protocol proceeds via oxidative addition of the Pd(0) catalyst into the C–N2 bond followed by expulsion of N2. Subsequent allene migratory insertion forms the π-allyl–Pd(II) intermediate, which forms the annulated products upon reductive elimination (see Scheme 1).
Scheme 7:
Pd(0)/PC-Phos-catalyzed enantioselective inter- molecular denitrogenative of benzotriazoles and annulation of allenes.
In 2019, Liu and co-workers reported a chemo- and regioselective intermolecular [4+2] reaction of (Z)-3-iodo allylic nucleophiles and allenamides (Scheme 8).19 In this example, the ring formation takes pace through an SN2’ pathway in preference to alternative SN2 or Heck-type pathways. An array of 2-amino-dihydropyrans and 2-amino-tetrahydropiperidines with electronically diverse substituents at the C3 position were afforded in 38–97% yield. The allylic group on the allenamide proved crucial for achieving higher yield, presumably because it coordinates to the Pd(II) intermediate, increasing its stability. The products were then derivatized to 2,6,7,7a-tetrahydropyrano[2,3-b]pyrroles and 2,6,7,7a-tetrahydro-1H-pyrrolo[2,3-b]pyridines via ring-closing metathesis (RCM) with Grubbs II catalyst.
Scheme 8:
Pd(0)-catalyzed intermolecular [4+2] annulation of allenamides with (Z)-3-iodo allylic nucleophiles.
3. 1,3-dienes
An alternative strategy to access 2-substituted heterocycles, such as 2,3-dihydrobenzofuran and indoline structures, is the Pd(0)-catalyzed [3+2] heteroannulation of 1,3-dienes with 2-iodophenols or 2-iodoanilines.4b This transformation proceeds via a similar mechanism to that discussed previously (see Scheme 1), whereby the PdII(Ar)(X) oxidative addition intermediate 7 adds to the 1,3-diene to form π-allyl–Pd(II) species 9.6 Here, the exocyclic C=C bond forms in the more thermodynamically stable trans configuration following annulation, regardless of the geometry of the starting alkene. Subsequent deprotonation of the nucleophile (10) and intramolecular nucleophilic displacement of Pd(II) via either an inner- or outer-sphere mechanism then affords the heteroannulated product (Scheme 9).
Scheme 9:
The mechanism for Pd(0)-catalyzed heteroannulation of 1,3-dienes.
More recent studies by Larock and Rozhkov demonstrated that the Pd(0)-catalyzed annulation of 1,3-dienes with 2-iodoaryl acetates could be employed to form dihydrobenzofurans (Scheme 10).20 Here, the use of an acetyl protecting group, compared to a free phenol,4b resulted in a significant improvement in yield, allowing the scope to be expanded to electron-rich aryl iodides. The annulation is suggested to proceed via oxidative addition of the Pd(0) catalyst into the Ar–I bond, syn-addition of the resulting PdII(Ar)(X) species across the 1,3-diene, and intramolecular coordination of the phenolic oxygen to the Pd(II) centre to form cationic species 11. Cationic aryl–Pd(II) species are more reactive towards C=C bonds than neutral aryl–Pd(II) species, which may explain the higher reactivity of the acetyl derivative compared to a free phenol. Hydrolysis of the acetyl group in the presence of base then gives intermediate 12, which can undergo reductive elimination to form the desired products in good to excellent yields. This methodology tolerates terminal, cyclic, and internal 1,3-dienes and electron-rich and -poor 2-iodoaryl acetate coupling partners. However, electron-rich acetates gave lower yields and in the presence of internal or aryl-substituted 1,3-dienes, undesired Heck-type products were often formed, whereby the desired cyclization step failed to proceed. The authors propose that this is due to slower hydrolysis of the acetyl group in the presence of electron-donating substituents, leading to β-hydride elimination from the π-allyl–Pd(II) intermediate and inhibition of the desired cyclization pathway.
Scheme 10:
Pd(0)-catalyzed annulation of 1,3-dienes with 2-iodoaryl acetates.
In 2013, a complimentary approach was reported by Deagostino et al. in which alkoxy-1,3-dienes and 2-iodophenols and -aniline derivatives underwent Pd(0)-catalyzed annulation to generate 2,3-dihydrobenzofurans and indolines respectively (Scheme 11).21 The products were formed in moderate to good yields, with excellent stereoselectivity. However, in the case of N-methyl-2-iodoaniline, only moderate levels of stereoselectivity were achieved; this is presumably due to the smaller size of the methyl group compared to the bulkier tosyl group used in the other examples. The less hindered methyl group would allow rotation of the substituents and isomerization of the C=C bond through re-insertion of a Pd(II)–hydride species. In this case, steric factors dominate over electronic factors in determining the regioselectivity; namely, nucleophilic attack of the heteroatom occurs at the less sterically hindered γ-position rather than the more electrophilic α-position. Under mildly acidic conditions, the enol ether on the products can be hydrolysed to the corresponding carbonyl group, which is primed for further functionalization. Interestingly, use of DMSO and TBAB were necessary for reactivity, with little to no product observed in their absence.
Scheme 11:
Pd(0)-catalyzed heteroannulation of functional 1-alkoxy-1,3-butadienes with 2-iodophenols and 2-iodoanilines.
The first enantioselective variant of a Pd(0)-catalyzed heteroannulation was reported by Han et al. in 2016 (Scheme 12A).22 The Pd(0)-catalyzed heteroannulation of 1,3-dienes with 2-iodoanilines to form enantioenriched indolines was disclosed. Key to their success was use of phosphoramidite ligand L2, which is comprised of a BINOL backbone bearing electron-withdrawing substituents. Both aryl- and cycloalkyl-substituted 1,3-dienes worked well, alongside a range of 2-iodoanilines with both electron-donating and -withdrawing substituents, giving the desired products in 30–83% yield with 58–87% ee. The indoline products were converted to the more stable N-Ac protected moieties by treating the product with AcCl/Et3N. Furthermore, the scope was expanded to 2-iodobenzyl alcohols to afford isochromans in moderate yields with good enantioselectivities.
Scheme 12:
Pd(0)-catalyzed enantioselective heteroannulation of 1,3-dienes.
Later, Gong and co-workers employed the same BINOL-phosphoramidite ligand L2 to achieve the carboannulation of 1,3-dienes, giving access to enantioenriched chiral indanes (Scheme 12B).23 This protocol also proceeds through a similar annulation mechanism (see Scheme 9), in which the required anionic carbon nucleophile was generated in the presence of base. The desired products bearing a variety of substituents were generated in moderate to excellent yields with up to >99% ee. The protocol was only applicable to 1,3-butadienes that bear aryl or heteroaryl substituents, such as 2-furyl-butadiene. The geometry of the C=C bond in the 1,3-diene was found to have a significant impact on both the yield and enantioselectivity of the carboannulated product. For example, (Z)-1-naphthylbutadiene gave the desired product in 51% yield and 47% ee, while (E)-1-naphthylbutadiene gave the annulated product in 98% yield with 93% ee. Analysis of the reaction mixture via HRMS revealed that one molecule of ligand L2 was present on the Pd(0) catalyst during the stereodetermining allylic substitution step. DFT studies supported the proposed annulation mechanism and suggest that the carbon nucleophile is generated in the presence of base via hydrogen-bonding between the two carbonyl functionalities and the base, rather than by a discrete deprotonation event (see Scheme 9).
In 2017, Tang disclosed a method for the Pd(0)-catalyzed functionalization of benzotriazoles with alkenes and 1,3-dienes (Scheme 13).24 Here, the benzonitrile underwent ring-opening to form the isomeric ortho-amino-arenediazonium species 13 in situ in the presence of AgBF4 via a Dimroth-type equilibrium (see Scheme 7).18 A range of electronically diverse N-Tf-protected 2-vinylindolines were generated in good to excellent yields. DFT studies showed that after alkene insertion, [3+2] cyclization was more favorable than β-hydride elimination for 1,3-dienes and vice versa for simple alkenes. Thus, the ortho-amino-arenediazonium species 13 served as an aza-[3C]-synthon for the denitrogenative [3+2] cycloaddition with 1,3-dienes to form 2-vinylindolines. Alternatively, 13 served as an aza-[1C]-synthon for denitrogenative cross-couplings with alkenes to form ortho-amino styrenes via a Heck-type mechanism. The authors attributed this difference to the highly electrophilic nature of the π-allyl–Pd(II) species formed from 1,3-dienes compared to the analogous alkyl–Pd(II) species formed from alkenes, resulting in a higher barrier to β-hydride elimination. Additionally, a significant agostic interaction results in lengthening of the C–H bond for styrenes. This results in more favorable β-hydride elimination; however, this effect is not present in 1,3-dienes.
Scheme 13:
Pd(0)-catalyzed denitrogenative annulation of 1,3-dienes via a Dimroth-type equilibrium.
Many of the examples discussed so far are limited to 1,3-dienes with substitution only at the terminal position (see Larock e.g., Scheme 10),20 which is due in part to a lack of robust methods for the synthesis of highly functionalized 1,3-dienes.25,26 To address this, Wang and co-workers expanded the Pd(0)-catalyzed heteroannulation approach to allenoates, which act as precursors to highly functionalized 1,3-dienes (14) (Scheme 14A).27 2,3-Dihydrobenzofurans bearing a range of ester substituents at the alkenyl carbon atom were produced in 70–88% yield. It was noted that an aryl substituent at the β’-position of the allenoate coupling partner was necessary, with an ethyl substituent resulting in an inseparable mixture. The reaction was successfully conducted on a 5 mmol scale, facilitating derivatizations of the product. Later, this protocol was expanded to aryl allyl ethers as 1,3-butadiene synthons to provide indolines in good to excellent yields (Scheme 14B).28
Scheme 14:
Pd(0)-catalyzed heteroannulation of 1,3-diene synthons.
Takasu and co-workers disclosed an alternative approach towards the generation of 1,3-dienes in situ (Scheme 15).29 Under Pd(0) catalysis, strained fused-cyclobutenes were used to generate short-lived cis,trans-cycloalkadienones 15. These intermediates were then functionalized via a Heck-type arylation or reacted with N-tosyl-protected 2-iodoanilines to form indolines as a single diastereomer. Varied substitution on the fused-cyclobutenes generated medium-ring-fused trans-indolines with yields of 45–80%; however, no substituted 2-iodoanilines were investigated. When optically pure cyclobutanes were employed, chirality transfer was observed to the indoline products, which provides evidence for the formation of the 1,3-diene intermediate. The extent of the chirality transfer was dependent upon the ring size of the cis,trans-cycloalkadienones, with increased ring size facilitating racemization by a ring flip.
Scheme 15:
Pd(0)-catalyzed ring expansion/annulation cascade of cyclobutanes.
4. Strained alkenes
Utilizing strained alkenes in heteroannulation reactions has resulted in the rapid generation of interesting heterocyclic moieties. For example, in 2007, Lautens and Hulcoop demonstrated that a range of polycyclic heterocycles could be generated through the annulation of strained norbornene (NBE) and norbornadiene (NBD) derivatives with aryl halides that bear a pyrrole moiety (Scheme 16).30 Here, the pyrrole moiety takes the place of the phenol/aniline functionality discussed in previous examples, to generate the desired annulated products in excellent yields. The catalytic cycle was proposed to proceed through the same initial sequence of steps outlined above: oxidative addition of the Pd(0) catalyst into the Ar–Br bond, followed by carbopalladation of the alkene. In the absence of the pyrrole, prior literature had shown that reaction of the Pd(II) intermediate, results in a cyclobutene product;31 however, reaction at the C2 position of the pyrrole gave the desired annulated products. The authors did not investigate whether this step occurred via a Heck-type process, electrophilic aromatic substitution, or direct C–H activation. The reaction tolerates electronically diverse groups at the C9 position of the benzene ring; however, a 1:1 mixture of the annulated product to the cyclobutene byproduct was obtained in the absence of substitution. Substituents at C5 of the pyrrole were well tolerated, although substitution at the C3 position of the pyrrole resulted in annulation at both C2 and C5. Furthermore, less strained alkenes such as bicyclo[2.2.2]oct-2-ene were not suitable. Interestingly, when norbornadiene was used, the expected annulated products were obtained at 80 °C; however, when increasing the reaction temperature to 120 °C, pyrroloquinoline derivatives formed selectively, via a retro-Diels–Alder mechanism, with concomitant loss of cyclopentadiene.
Scheme 16:
Pd(0)-catalyzed annulation of strained alkenes with aryl heterocycles.
Two years later, Lautens, Hulcoop and co-workers expanded this protocol to the annulation of norbornadienes with N-Boc-protected haloanilines, to give indolines and isoquinolinones in moderate to excellent yields (Scheme 17).32 Under the previously developed conditions,30 the subsequent retro-Diels–Alder reaction was unsuccessful, leading them to develop new conditions (treatment with silica gel in xylenes at 170 °C or heating in ethylene glycol at 170 °C) to give the corresponding indoles with simultaneous deprotection of nitrogen. The protocol was expanded to halobenzamides, but in this case, cyclobutene moieties were observed alongside the desired product. Despite this, the desired products could still be isolated in 30–86% yields.
Scheme 17:
Pd(0)-catalyzed annulation of norbornadiene with haloanilines and halobenzamides.
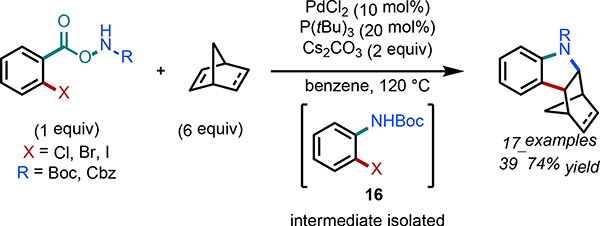
In 2019, Hu and Dai et al. demonstrated that N-Boc-protected haloanilines 16 could be synthesized in situ via decarboxylation/amination of 2-halo-aroyloxycarbamates under Pd(0)-catalyzed conditions (Scheme 18).33 Subsequent Heck-type migratory insertion/heteroannulation with norbornene and norbornadiene resulted in a range of indolines in moderate to good yields. Substitution was tolerated at all positions around the 2-halo-aroyloxycarbamate, with electron-donating groups resulting in higher yields. 1,3-Dienes gave the desired products in moderate yields, and simple styrenes and 1,2-disubstituted alkenes failed to react.
Scheme 18:
Pd(0)-catalyzed tandem decarboxylative/ amination/Heck-type/annulation reaction.
In 2019, Gansäuer et al. reported an asymmetric protocol to generate enantioenriched indolines through the use of Josiphos ligand L3 (Scheme 19A).34 The desired indolines were synthesized regioselectively in good yields and with up to 92% ee from the annulation of norbornadienes with 2-iodoanilines. The products were then subjected to a second annulation event to functionalize the remaining C=C bond. Using regioconvergent catalysis and selecting the absolute configuration of L3, pseudo-C2-symmetrical bis-indolines or pseudo-meso bis-indoline scaffolds could be accessed selectively. In this example, the initial carbopalladation event of norbornadiene to forge the C(sp3)–Ar bond is proposed to be how the absolute stereochemistry is established. Furthermore, the authors found norbornene to be a competent substrate.
Scheme 19:
Pd(0)-catalyzed enantioselective annulation of NBE/NBD.
Following this report, Zhang, Li and Tao employed a Pd(0) catalyst bearing L4 to promote the asymmetric annulation of norbornene with 2-bromoanilines and generate chiral norbornane-fused dihydropyrroles (Scheme 19B).35 The desired products were produced in good to excellent yields with 84–99% ee for 2-bromoanilines with electronically diverse substituents and a range of nitrogen protecting groups. Additionally, this protocol was carried out on gram scale with a minor reduction in yield and enantioselectivity. Through fine-tuning of the solvent and base, the protocol was expanded to become the first example of asymmetric carboetherification to generate norbornane-fused dihydrobenzofurans with good to excellent enantioselectivities; however, lower yields were achieved. Both the carboetherification and carboamination reactions were also compatible with norbornadiene.
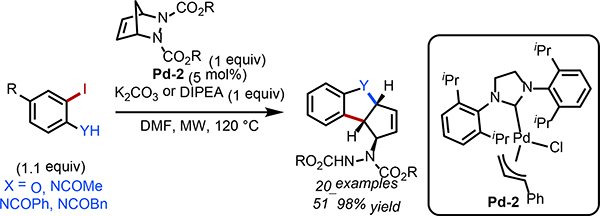
In 2020, Joo, Lim, and co-workers reported a complimentary approach in which they utilized the innate reactivity of 5-memebered heteroaromatic halides as the nucleophilic component (Scheme 20).36 Under the optimized conditions, formation of the 5-memebered palladacycle required for a Catellini-type reaction was slow and, therefore C–H functionalization was preferable over a Catellini-type reaction. Through placement of the halide on the heteroaromatic and judicious choice of the ligand and base, both heteroaryl iodides and -bromides yielded 1,2-annulated products in moderate to excellent yields. In this case, migratory insertion of NBD occurs to generate 17, which is then followed by a second migratory insertion of an additional NBD to afford 18. In this step NBD added in with exo-selectivity, which resulted in the two bicycles in the products being in trans relationship to one another. This methodology was applicable to the annulation of a variety of decorated norbornadienes with a range of 5-membered heterocycles.
Scheme 20:
Pd(0)-catalyzed C–H annulation of NBE/NBD with 5-membered heteroaryl halides.
Radharkrishnan et al. explored the heteroannulation of diazabicyclic alkenes to form cyclopentene-fused dihydrobenzofurans and indolines via a tandem ring opening/annulation reaction (Scheme 21A).37,38 The catalytic cycle is proposed to proceed by oxidative addition of the Pd(0) catalyst into the Ar–I bond (19), followed by coordination of the C=C bond on its exo face and carbopalladation of the alkene (20). Oxypalladation then occurs (20 to 21), with concomitant ring-opening of the azabicycle through the endo face, giving trans intermediate 21. The trans stereochemistry is a result of both attack of the PdII(Ar)(I) intermediate at the exo face and ring opening by cleavage of the C–N bond along the endo face. Consistent with the notion that 21 is an intermediate in the catalytic cycle, the authors found that an independently prepared trans-3,4-disubstituted cyclopentene could be converted to the desired product under the standard reaction conditions. Finally, oxy-/aminopalladation across the C=C bond gives 22, which undergoes β-hydride elimination to furnish the desired product. Like previous examples, Bu4NCl is required as an additive, with only the trans-3,4-disubstituted cyclopentene intermediate 21 observed in its absence. Beyond facilitating synthesis of a range of cyclopentene-fused dihydrobenzofurans and dihydrobenzopyrroles in good to excellent yields, the methodology was also expanded to the annulation of fulvene-derived bicyclic hydrazines with 2-iodophenols. However, in this case, the reaction proceeded at ambient temperature, and no ring-opening C–N bond cleavage was observed (Scheme 21B).38
Scheme 21:
Pd(0)-catalyzed annulation of azabicyclic olefins with 2-iodophenol/anilines.
In 2010, Gilberton reported a Pd0(NHC)-catalyzed version of this reaction (Scheme 22).39 In this case the addition of quaternary ammonium salts inhibited the reaction, which the authors suggest is due to the strongly σ-donating nature of the NHC, which makes the metal stable in its reduced state, even in the absence of quaternary ammonium salt additives. By using microwave irradiation, both the reaction time and the palladium loading could be reduced, with the optimized conditions being 5 mol% Pd and a reaction time of 30 minutes. Additionally, the scope of the reaction was expanded to more substituted and electronically diverse 2-iodophenols. The desired annulated products were obtained in moderate to excellent yields as single diastereomers. However, when N-acetyl protected 2-iodoaniline was utilized, only the corresponding ring-opened Heck-type byproduct was observed.
Scheme 22:
Pd(0)-catalyzed annulation of azabicyclic olefins with 2-iodophenols and -anilines under microwave irradiation.
Radhakrishman and co-workers published two additional reports on this topic (Scheme 23).40,41 In these examples, Sc(OTf)3 was employed as a Lewis acid (LA) to promote the C–N bond cleavage and ring-opening of pentafulvene derived diazabicyclic olefins to give desymmetrization products in up to 93% yield. Under Pd(0) catalysis, these structures then underwent intramolecular heteroannulation with 2-iodoanilines, to from substituted spiropentacyclic cores fused to both indoline and pyrazolidine moieties.40 When combined into a one-pot process, the desired products were prepared in 40–75% yield; however, only substitution at the C4 position of 2-iodoaniline was demonstrated. The proposed mechanism involves coordination of the LA to the carbonyl group of the diazobicyclic olefin (23), which promotes cleavage of the C–N bond and subsequent formation of allylic cation 24. Nucleophilic attack of the aniline from the opposite face then generates trans-1,2-disubstituted alkylidenecyclopentene 25. In the LA cycle, the stereoselective nature of the reaction can be attributed to the large steric substituents on the exocyclic C=C bond. The Pd cycle then begins with oxidative addition of the Pd(0) catalyst into the Ar–I bond of 25, followed by coordination to the C=C bond to give intermediate 26 and migratory insertion to form indoline ring 27. Generation of π-allyl–Pd(II) intermediate 28 and base-promoted intramolecular nucleophilic attack, then gives final product 29. The LA-catalyzed process was later expanded to 2-iodophenols and -thiophenols, but the analogous Pd(0)-mediated heteroannulations have not been disclosed to date.41
Scheme 23:
Pd(0)/LA-mediated domino reaction of pentafulvene derived diazabicyclic olefins.
5. Styrenes
The π-benzyl stabilization present in styrenes has enabled them to be utilized in Pd(0)-catalyzed heteroannulation reactions to prepare biologically active molecules, such as pterocarpans. Pterocarpans contain a cis-fused benzofurano-benzopyran skeleton and are naturally occurring in plants (Scheme 24A).42,43,44
Scheme 24:
(A) Introduction of pterocarpan; (B) Pd(II)-mediated Heck-1,2-oxyarylation.
A direct approach to generate the heterocyclic core of these compounds is the Heck-1,2-oxyarylation reaction of 2H-chromenes with 2-chloromercuriphenols. This method was first established in 1976 by Horino and Inoue who employed stochiometric amounts of Li2[PdCl4] to promote the reaction.45 However, later studies by Antus et al. found that this protocol actually generated a mixture of regioisomers (Scheme 24B).46,47
In 2010, Costa and Eberlin et al. reported a catalytic variant and utilized electrospray ionization mass spectrometry to probe the mechanism (Scheme 25).48 Here, Pd(OAc)2 was the precatalyst, and Ag2CO3 was employed as the base, and they avoided the use of toxic 2-mercury phenols. Under these conditions, they propose that a cationic mechanistic pathway is operative whereby oxidative addition of the Pd(0) catalyst into the Ar–X bond forms cationic PdII(Ar)+ intermediate 30. Regioselective migratory insertion of the styrene then generates intermediate 31. Finally, reductive elimination delivers the annulated product and regenerates the Pd(0) catalyst.
Scheme 25:
Costa and Eberlin’s reported mechanism for Pd(0)-catalyzed heteroannulation of styrenes.
In 2011, Costa and Nájera reported that when they employed Pd(OAc)2 or an oxime-based palladacycle to catalyze the Heck-1,2-oxyarylation reaction, microwave irradiation greatly improved the conversion (Figure 2A).49 Here, the annulation of electron-rich dihydronaphthalenes and chromenes with 2-iodo-phenols and -aryl acetates occurred to give ptercarpans and their derivatives in moderate to good yields and with high selectivity. The Heck-1,2-oxyarylation of electron-poor olefin, 2H-benzo[g]chromene-5,10-dione was also studied giving the products in moderate yields (18–43%).
Figure 2:
Pd(0)-catalyzed [3+2] synthesis of pterocarpan derivatives.
A few years later, Costa and co-workers employed the same Pd(II) pre-catalysts under ligand-free conditions for the oxyarylation of dihydronaphthalenes and chromenequinone with 2-iodophenols and 3-iodolawsone for the synthesis of 5-carbapterocarpans, which are isosters of naturally occurring pterocarpan, and pterocarpanquinones (Figure 2B).50 PEG-400 was employed as the solvent and was proposed to increase the catalytic reactivity of Pd through promoting the reduction of Pd(II) to Pd(0). The products were obtained in moderate to excellent yields and the protocol was successfully carried out on gram-scale.
In the examples discussed so far, only cis-substituted dihydrobenzofurans were generated (see Scheme 15 for trans-substituted indolines).29 In 2010, Sefkow and co-workers developed a one-pot diastereoselective Pd(0)-catalyzed oxyarylation Heck-type reaction to generate trans-2,3-disubstituted dihydrobenzofurans, which are biologically active (Scheme 26).51 Here, diazonium salt electrophile 32 was formed in situ, from an amine and NOPF6. Echoing the findings by Eberlin,52 it was noted that the reaction only proceeded in nitrile solvents, whereby the solvent participates in ligand exchange with the catalyst to form a stable cationic Pd(II) species. Under the optimized conditions, the desired trans-products were generated in moderate to good yields, and a range of substituents with different electronic properties were tolerated on the 2-amino-phenol and phenylpropene moieties. However, unsubstituted aryl rings on the phenylpropene resulted in no reaction, and substitution at the ortho position of the styrene gave drastically reduced yields. The authors disclosed that 2-halophenols were not suitable substrates to generate trans-dihydrobenzofurans; however, they did not comment on how the trans-selectivity arises when using diazonium salts.
Scheme 26:
Pd(0)-catalyzed one-pot synthesis of trans-dihydrobenzofurans from 2-aminophenols.
Building upon Sefkow’s initial contribution to this field50 and previous studies on the enantioselective Heck–Matsuda reaction,53 Correia et al. expanded this protocol to synthesize chiral trans-dihydrobenzofurans via an enantioselective oxy-Heck–Matsuda reaction (Scheme 27).54 Here, a pre-formed arenediazonium salt acted as the electrophile, and chiral N,N-ligands L5 and L6 provided stereoinduction. The tetrafluoroborate counterion on the arenediazonium salt proved to be crucial for reactivity, with the corresponding tosylate and hexafluorophosphate salts resulting in diminished yields. The trans-substituted dihydrobenzofurans were afforded in yields of 28–76% and up to 80% ee and >20:1 d.r.; however, only electron-rich styrenes were demonstrated. Notably, the trans product is favored when starting from either the E- or Z-styrene coupling partner. The trans selectivity most likely arises from the formation of more stable oxypalladium species 33, which upon reductive elimination affords the trans-heteroannulated products. The authors demonstrated the utility of this protocol in the total synthesis of the neolignan (+)-conocarpan, reporting 4- and 5-step routes that both utilize the developed methodology.
Scheme 27:
Pd(0)-catalyzed enantioselective synthesis of dihydrobenzofurans.
6. Other C=C bond variants
Several useful synthetic processes that employ more unique C=C bonds have also been reported. For example, in 2009 Zhu and Wang expanded the Pd(0)-catalyzed heteroannulation of [60]fullerene with 2-iodoanilines, to give unusual [60]fullereneindoline structures (Scheme 28).55 The desired [60]fullereneindolines were afforded in moderate yields for a range of 2-iodoanilines, including N-acetyl protected as well as free aniline. N-Acetyl-protected aniline gave higher yields than free aniline, presumably due to the formation of a more reactive cationic PdII(Ar)+ species, as observed previously in Larock’s chemistry (see Scheme 10).20
Scheme 28:
Pd(0)-catalyzed annulation of C60 with a variety of 2-iodoanilines.
Over the last five years, significant interest has arisen in employing 2,3-dihydrofurans as C=C coupling partners, due to the potential bioactivity of the corresponding fused tetrahydrofurobenzofurans and furoindolines (Scheme 29). The first example of enantioselective intermolecular Pd(0)-catalyzed syn-carboetherification of 2,3-dihydrofurans was reported by the Mazet group in 2016.56,57 Zhang, Liu and co-workers further optimized the reaction conditions and employed newly modified ligands L4 and L9. By doing so, the scope was further expanded, and the transformation was able to proceed more efficiently under more mild conditions.58
Scheme 29:
Pd(0)-catalyzed intermolecular carbohetero-functionalization of 2,3-dihydrofurans.
The mechanism of carboetherification and carboamination of 2,3-dihydrofurans was investigated by the Mazet group in 2017 via crystallographic, spectroscopic, and spectrometric methods.59 They propose that the reaction is initiated by oxidative addition (34), followed by ligand exchange (35), deprotonation (36), and coordination of the alkene (37) (Scheme 30). In contrast to the mechanisms discussed thus far involving C–C-bond forming migratory insertion, in this case C–Y bond forming syn-nucleopalladation occurs (37 to 38). This method grants access to benzo-fused heterocycles with high regio- and enantioselectivity. Mazet et al. observed formation of the competing Heck product 40, via β-hydride elimination from 39 when electron-withdrawing substituents were present on the aryl ring of the 2-bromophenol coupling partner or when 2-bromoanilines with electron-donating substituents on the N-protecting group were utilized. This can be explained by the increased nucleophilicity of the Y group.
Scheme 30:
The mechanism of Pd(0)-catalyzed carboetherification and carboamination of 2,3-dihydrofurans.
The hetero(annulation) of unactivated (i.e., unstrained and non-conjugated) alkenes is extremely rare, but a few examples have been described. In 2018, the Buchwald group reported a two-step, one-pot synthesis of indolines from β,γ-unsaturated ketones and 2-bromoanilines (Scheme 31).60 The authors proposed that the reaction proceeds through α,β-unsaturated ketone intermediate 41, which then generates the indoline product via a conjugate addition event. Quaternary carbon centers were formed in good yields, and advances towards an asymmetric version were made.
Scheme 31:
Pd(0)-catalyzed γ-arylation of β, γ-unsaturated ketones.
More recently, the Yang group disclosed an intramolecular aminoalkylation reaction, where the electrophilic site of the ambiphilic molecule is a C(sp3)–halogen, which is a rare substrate for this class of reactions (Scheme 32).61 The reaction tolerated both alkyl bromides and chlorides, to provide the fused-heterocyclic products in good yields and diastereoselectivity. Mechanistic studies revealed that the reaction likely proceeds via four-membered PdII(alkyl)(amido) intermediate 42.
Scheme 32:
Pd(0)-catalyzed intramolecular aminoalkylation to access polycyclic lactams.
Through employment of a strongly coordinating bidentate directing group, 8-aminoquinoline (AQ), our lab in collaboration with the Liu group, and Pfizer have achieved the (hetero)annulation of non-conjugated alkenes (Scheme 33).62 Here, 2-iodophenols, -anilines, and carbon-based coupling partners were all effective to generate a broad range of heterocycles in excellent yields and regioselectivity. X-ray crystallography revealed that the products were formed in an anti-selective fashion.
Scheme 33:
Pd(II)-catalyzed anti-selective [3+2] annulation of non-conjugated alkenes.
In contrast to the catalytic cycles discussed so far, experimental and computational studies suggest that this transformation proceeds via a Pd(II)/Pd(IV) redox manifold (Scheme 34).62 The cycle is initiated by alkene coordination to Pd(II) as in 43, followed by Wacker-type anti-nucleopalladation to generate intermediate 44. Intramolecular oxidative addition into the Ar–X bond then gives species 45 and subsequent reductive elimination generates the heteroannulated product.
Scheme 34:
The mechanism of anti-selective Pd(II)-catalyzed [3+2] annulation of non-conjugated alkenes.
7. Challenges and opportunities
Although major advances have been made in this field, undoubtedly this family of methods still has its limitations. For example, the synthesis of aryl or alkenyl halides can be challenging due to selectivity issues. Additionally, the heteroannulated products are often formed with poor atom economy.63 As a result, a C–H activation strategy has recently been employed by many groups, which avoids the use of halogenated substrates and the generation of salt waste. Representative examples of C–H activation-based coupling partners are shown in Scheme 35.64 This protocol is an attractive alternative to heteroannulation with aryl halides, but it is typically limited to functionalization of the most reactive C-H bond and often requires a substituent that can act as a directing group.
Scheme 35:
Selective examples of C-H activation-based coupling partners
Additionally, heteroannulation of unactivated alkenes beyond the specific types described above remains challenging. Here, different metal catalyst and catalytic strategies may play an important role. In particular, an elegant method to access indolines from unactivated alkenes was reported by the Jamison group in 2015, in which they took advantage of Nickel/photoredox dual catalysis (scheme 36A).65 This method is not without its limitations; the reaction required use of specific N-acetyl protecting groups which was invoked as a chelating group following migratory insertion, and the scope of the reaction was limited to terminal alkenes.
Scheme 36:
Other existing methodologies involving ambiphilic molecules and alkenes.
Furthermore, coupling partners containing C(sp3)–X electrophilic moieties would grant access to an even broader collection of attractive target compounds. However, these have remained difficult to employ owing to the comparatively slow nature of C(sp3)–X oxidative addition and the presence of competitive processes such as base-mediated elimination. Recently, α-haloacetamides, which are not commonly used in Pd catalysis, have been shown to function as versatile and efficient reagents for the synthesis of aza-heterocycles via copper catalysis or simple basic media (Scheme 36B).66
Lastly, the ability to integrate a third reaction component into established (hetero)annulation processes holds great promise. In recent examples, under carefully tuned reaction conditions, CO has been shown to function in this role (Scheme 36C).67
Conclusions
This feature article summarizes the Pd-catalyzed (hetero)annulation reactions of C=C bonds with ambiphilic aryl (pseudo)halides developed in the past sixteen years (2005–2021). This chemistry employs simple procedures, all-in-one starting materials, mild reaction conditions, and demonstrates good compatibility for various ambiphilic coupling partners. Important advances include the ability to use more varied alkenes, especially in the annulation of non-conjugated alkenes developed by our group and others.
With the help of modern experimental and computational studies, the mechanism, regioselectivity, and diastereoselectivity issues have become better understood. The enantioselective reactions, although still early in the investigation with only a few limited examples, provide a basis for future studies. It is reasonable to believe that annulation methodology will continue to flourish and result in more applications both in academia and industry.
Acknowledgements
We thank the NIH for financial support (NIH grant no. 5R35GM125052–04). We also thank the Lindemann Trust and the English-Speaking Union for the provision of a fellowship to P.C.
Footnotes
Conflicts of interest
In accordance with our policy on Conflicts of interest please ensure that a conflicts of interest statement is included in your manuscript here. Please note that this statement is required for all submitted manuscripts. If no conflicts exist, please state that “There are no conflicts to declare”.
Footnotes relating to the main text should appear here. These might include comments relevant not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Notes and references
- 1.For selected reviews, see: Liu D, Zhao Gand Xiang L, Eur. J. Org. Chem, 2010, 2010, 3975;Silva TS, Rodrigues MT Jr., Santos H, Zeoly LA, Almeida WP, Barcelos RC, Gomes RC, Fernandes FS and Coelho F, Tetrahedron, 2019, 75, 2063;Bertolini F and Pineschi M, Org. Prep. Proc. Int, 2009, 41, 385;Garlets ZJ, White DR and Wolfe JP, Asian J. Org. Chem, 2017, 6, 636.
- 2.(a) Larock RC, Harrison LW and Hsu MH, J. Org. Chem, 1984, 49, 3662; [Google Scholar]; (b) Larock RC and Song H, Synth. Commun, 1989, 19, 1463; [Google Scholar]; (c) Larock RC, Varaprath S, Lau HH and Fellows CA, J. Am. Chem. Soc, 1984, 106, 5274; [Google Scholar]; (d) Larock RC, Liu C-L, Lau HH and Varaprath S, Tetrahedron Lett, 1984, 25, 4459. [Google Scholar]
- 3.O’Connor JM, Stallman BJ, Clark WG, Shu AYL, Spada RE, Stevenson TM and Dieck HA, J. Org. Chem, 1983, 48, 807. [Google Scholar]
- 4.(a) Larock RC and Fried CA, J. Am. Chem. Soc, 1990, 112, 5882; [Google Scholar]; (b) Larock RC, Berrios-Peña N and Narayanan K, J. Org. Chem, 1990, 55, 3447. [Google Scholar]
- 5.Herraiz-Cobo J, Albericio F and Álvarez M, in Advances in Heterocyclic Chemistry, ed. Scriven EFV and Ramsden CA, Academic Press, USA, 1st edn, 2015, vol. 116, ch. 1, pp. 1–35. [Google Scholar]
- 6.For a review, see: Larock RC, J. Organomet. Chem, 1999, 576, 111.
- 7.For a review, see: Zeni G and Lsarock RC, Chem. Rev, 2006, 106, 4644.
- 8.Cazes B, Pure Appl. Chem, 1990, 62, 1867. [Google Scholar]
- 9.Larock RC, Berrios-Peña NG and A Fried C, J. Org. Chem, 1991, 56, 2615. [Google Scholar]
- 10.Chakravarty M and Kumara Swamy KC, J. Org. Chem, 2006, 71, 9128. [DOI] [PubMed] [Google Scholar]
- 11.Pavan MP; Chakravarty M and Kumara Swamy KC, Eur. J. Org. Chem, 2009, 2009, 5927. [Google Scholar]
- 12.Desarbre E and Mérour J-Y, Tetrahedron Lett, 1996, 37, 43. [Google Scholar]
- 13.Inamoto K, Yamamoto A, Ohsawa K, Hiroya K and Sakamoto T, Chem. Pharm. Bull, 2005, 53, 1502. [DOI] [PubMed] [Google Scholar]
- 14.Li W and Shi M, Eur. J. Org. Chem, 2009, 2009, 270. [Google Scholar]
- 15.Boi T, Deagostino A, Prandi C, Tabasso S, Toppino A and Venturello P, Org. Biomol. Chem, 2010, 8, 2020. [DOI] [PubMed] [Google Scholar]
- 16.Shu W, Yu Q and Ma S, Adv. Synth. Catal, 2009, 351, 2807. [Google Scholar]
- 17.Zhang P-C, Han J and Zhang J, Angew. Chem. Int. Ed, 2019, 58, 11444. [DOI] [PubMed] [Google Scholar]
- 18.Habraken CL, Erkelens C, Mellema JR and Cohen-Fernandes P, J. Org. Chem, 1984, 49, 2197. [Google Scholar]
- 19.Yan F, Liang H, Ai B, Liang W, Jiao L, Yao S, Zhao P, Liu Q, Dong Y and Liu H, Org. Biomol. Chem, 2019, 17, 2651. [DOI] [PubMed] [Google Scholar]
- 20.Rozhkov RV and Larock RC, J. Org. Chem, 2010, 75, 4131. [DOI] [PubMed] [Google Scholar]
- 21.Toppino A, Arru P, Bianco N, Prandi C, Venturello P and Deagostino A, Eur. J. Org. Chem, 2013, 2013, 6990. [Google Scholar]
- 22.Chen S-S, Meng J, Li Y-H and Han Z-Y, J. Org. Chem, 2016, 81, 9402. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Chen S-S, Zhang L, Wang H-J and Gong L-Z, Chem. Commun, 2018, 54, 9595. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li Y, Fan Y, Wang Z and Tang Y, Chem. Commun, 2017, 53, 11873. [DOI] [PubMed] [Google Scholar]
- 25.Mąkosza M and Judka M, Synthesis, 2003, 820. [Google Scholar]
- 26.Zoeller JR, J. Org. Chem, 1988, 53, 4716. [Google Scholar]
- 27.Yang J, Mo H, Wu H, Cao D, Pan C and Wang Z, Chem. Commun, 2018, 54, 1213. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Mo H, Jin X, Cao D, Wu H, Chen D and Wang Z, J. Org. Chem, 2018, 83, 2592. [DOI] [PubMed] [Google Scholar]
- 29.Arichi N, Yamada K.-i., Yamaoka Y and Takasu K, J. Am. Chem. Soc, 2015, 137, 9579. [DOI] [PubMed] [Google Scholar]
- 30.Hulcoop DG and Lautens M, Org. Lett, 2007, 9, 1761. [DOI] [PubMed] [Google Scholar]
- 31.Catellani M and Cugini F, Tetrahedron, 1999, 55, 6595. [Google Scholar]
- 32.Thansandote P, Hulcoop DG, Langer M and Lautens M, J. Org. Chem, 2009, 74, 1673. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Li P, Fu H, Dai Q and Hu C, Adv. Synth. Catal, 2019, 361, 192. [Google Scholar]
- 34.Dolja E, Funken N, Slak D, Schnakenburg G and Gansäuer A, ChemCatChem, 2019, 11, 5421. [Google Scholar]
- 35.Tao M, Li W and Zhang J, Chem. Commun, 2020, 56, 13125. [DOI] [PubMed] [Google Scholar]
- 36.Padhi B, Kang G, Kim E, Ha J, Kim HT, Lim J and Joo JM, ACS Catal, 2020, 10, 1792. [Google Scholar]
- 37.John J, Indu U, Suresh E and Radhakrishnan KV, J. Am. Chem. Soc. 2009, 131, 5042. [DOI] [PubMed] [Google Scholar]
- 38.John J, Rajan R, Chand SS, Prakash P, Joseph N, Suresh E and Radhakrishnan KV, Tetrahedron, 2013, 69, 152. [Google Scholar]
- 39.Prasad BAB, Buechele AE and Gilbertson SR, Org. Lett, 2010, 12, 5422. [DOI] [PubMed] [Google Scholar]
- 40.Chand SS, Jijy E, Prakash P, Szymoniak J, Preethanuj P, Dhanya BP and Radhakrishnan KV, Org. Lett, 2013, 15, 3338. [DOI] [PubMed] [Google Scholar]
- 41.Chand SS, Saranya S, Preethanuj P, Dhanya BP, Jijy E, Prakash P, Sasidhar BS, Szymoniak J, Santhini PV and Radhakrishnan KV, Org. Biomol. Chem, 2014, 12, 3045. [DOI] [PubMed] [Google Scholar]
- 42.Szappanos Á, Mándi A, Gulácsi K, Lisztes E, Tóth BI, Bíró T, Antus S and Kurtán T, Org. Biomol. Chem, 2020, 18, 2148. [DOI] [PubMed] [Google Scholar]
- 43.Buarque CD, Militão GCG, Lima DJB, Costa-Lotufo LV, Pessoa C, de Moraes MO, Cunha-Junior EF, Torres-Santos EC, Netto CD and Costa PRR, Bioorg. Med. Chem, 2011, 19, 6885. [DOI] [PubMed] [Google Scholar]
- 44.Kakuda S, Ninomiya M, Tanaka K and Koketsu M, ChemistrySelect, 2016, 1, 4203. [Google Scholar]
- 45.Horino H and Inoue N, J. Chem. Soc., Chem. Commun, 1976, 500. [Google Scholar]
- 46.Tőkés AL, Litkei G, Gulácsi K, Antus S, Baitz-Gács E, Szántay C and Darkó L, Tetrahedron, 1999, 55, 9283. [Google Scholar]
- 47.Gulácsi K, Németh I, Szappanos Á, Csillag K, Illyés TZ, Kurtán T and Antusa S, Croat. Chem. Acta, 2013, 86, 137. [Google Scholar]
- 48.Buarque CD, Pinho VD, Vaz BG, Eberlin MN, da Silva AJM and Costa PRR, J. Organomet. Chem. 2010, 695, 2062. [Google Scholar]
- 49.Leão RAC, Pinho VD, Coelho AS, Buarque CD, Moraes PF, Alonso DA, Nájera C and Costa PRR, Eur. J. Org. Chem, 2011, 2011, 3313. [Google Scholar]
- 50.de Moraes P. d. F., Gaspar FV, Borges RHF, Netto CD, Leão RAC, Nájera Cand Costa PRR, Synthesis, 2015, 47, 3505. [Google Scholar]
- 51.Coy B ED, Jovanovic L and Sefkow M, Org. Lett, 2010, 12, 1976. [DOI] [PubMed] [Google Scholar]
- 52.Sabino AA, Machado AHL, Correia CRD and Eberlin MN, Angew. Chem. Int. Ed, 2004, 43, 2514. [DOI] [PubMed] [Google Scholar]
- 53.Correia CRD, Oliveira CC, Salles AG Jr. and Santos EAF, Tetrahedron Lett. 2012, 53, 3325. [Google Scholar]
- 54.Silva AR, Polo EC, Martins NC and Correia CRD, Adv. Synth. Catal, 2018, 360, 346. [Google Scholar]
- 55.Zhu B and Wang G-W, J. Org. Chem, 2009, 74, 4426. [DOI] [PubMed] [Google Scholar]
- 56.Borrajo-Calleja GM, Bizet V and Mazet C, J. Am. Chem. Soc, 2016, 138, 4014. [DOI] [PubMed] [Google Scholar]
- 57.Bizet V, Borrajo-Calleja GM, Besnard C and Mazet C, ACS Catal, 2016, 6, 7183. [Google Scholar]
- 58.Tao M, Tu Y, Liu Y, Wu H, Liu L and Zhang J, Chem. Sci, 2020, 11, 6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borrajo-Calleja GM, Bizet V, Besnard C and Mazet C, Organometallics, 2017, 36, 3553. [Google Scholar]
- 60.Hyde AM and Buchwald SL, Angew. Chem. Int. Ed, 2008, 47, 177. [DOI] [PubMed] [Google Scholar]
- 61.Ye L, Lo K-Y, Gu Q and Yang D, Org. Lett, 2017, 19, 308. [DOI] [PubMed] [Google Scholar]
- 62.Ni H-Q, Kevlishvili I, Bedekar PG, Barber JS, Yang S, Tran-Dubé M, Romine AM, Lu H-X, McAlpine IJ, Liu P and Engle KM, Nat. Commun, 2020, 11, 6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.(a) Adimurthy S, Ramachandraiah G, Ghosh PK and Bedekar AV, Tetrahedron Lett, 2003, 44, 5099; [Google Scholar]; (b) Ganguly NC, Barik SK and Dutta S, Synthesis, 2010, 1467; [Google Scholar]; (c) Mei T-S, Wang D-H and Yu J-Q, Org. Lett, 2010, 12, 3140. [DOI] [PubMed] [Google Scholar]
- 64.For representative examples, see: Houlden CE, Bailey CD, Ford JG, Gagneé MR, Lloyd-Jones GC and Booker-Milburn KI, J. Am. Chem. Soc, 2008, 130, 10066;Khan I, Chidipudi SR and Lam HW, Chem. Commun, 2015, 51, 2613;Cendón B, Casanova N, Comanescu C, García-Fandiño R, Seoane A, Gulías M and Mascareñas JL, Org. Lett, 2017, 19, 1674;Bai L, Wang Y, Ge Y, Liu J and Luan X, Org. Lett, 2017, 19, 1734;Son J-Y, Kim H, Jeon WH, Baek Y, Seo B, Um K, Lee K and Lee PH, Adv. Synth. Catal, 2017, 359, 3194;Chen S-S, Wu M-S and Han Z-Y, Angew. Chem. Int. Ed, 2017, 56, 6641;Sun Y and Zhang G, Chin. J. Chem, 2018, 36, 708;Zhang T, Shen H-C, Xu J-C, Fan T, Han Z-Y and Gong L-Z, Org. Lett, 2019, 21, 2048;Velasco-Rubio Á, Varela JA and Saá C, Org. Lett, 2020, 22, 3591;González JM, Cendón B, Mascareñas JL and Gulías M, J. Am. Chem. Soc, 2021, 143, 3747.
- 65.Tasker SZ and Jamison TF, J. Am. Chem. Soc, 2015, 137, 9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.For a review, see: Bouakher AE, Martel A and Comesse S, Org. Biomol. Chem, 2019, 17, 8467.
- 67.(a) Ye F and Alper H, J. Org. Chem, 2007, 72, 3218; [DOI] [PubMed] [Google Scholar]; (b) Ying J, Wang J-S, Yao L, Lu W, and Wu X-F, Chem. Eur. J, 2020, 26, 14565; [DOI] [PubMed] [Google Scholar]; (c) Wang J-S, Na Y, Ying J and Wu X-F, Org. Chem. Front, 2021, Advance Article. DOI: 10.1039/D1QO00290B [DOI] [Google Scholar]