Abstract
Given the heterogeneity of senescent cells, our knowledge of both the drivers and consequences of cellular senescence in tissues and organs remains limited, as is our understanding of how this process could be harnessed for human health. Here we identified five broad areas that would help propel the field forward.
Background
A variety of stress signals, including telomere dysfunction, oxidative stress, changes in chromatin architecture, mitochondrial dysfunction, mitotic stress, and proteotoxicity induce cellular senescence, a persistent hypo-replicative state characterized by a specialized form of permanent cell-cycle arrest of previously replication-competent cells (He and Sharpless, 2017). Important distinctions between quiescence and senescence are that, while quiescence is a reversible cell-cycle arrest without any cellular damage or characteristic secretory phenotype, senescence is marked by irreversible cell-cycle arrest associated with macromolecular damage and a secretory phenotype (He and Sharpless 2017). Surprisingly, while senescent cells are cell-cycle arrested, they are metabolically active and capable of performing some of the functions of the parent replication-competent cells from which they are derived (Lee and Schmitt, 2019). Thus, hallmarks of cellular senescence include irreversible cell-cycle withdrawal or durable growth arrest via expression of anti-proliferative molecules; macromolecular damage; and activation of damage-sensing signaling pathways, secretory phenotype namely senescence associated secretory proteins (SASPs), and deregulated metabolism (He and Sharpless, 2017; Gorgoulis et al., 2019). Although observed by Hayflick in 1961 as a finite proliferative capacity of cultured normal human fibroblasts (Hayflick and Moorhead, 1961), the phenomenon was ignored for several decades and not widely accepted until recently, because it was generally believed to represent an in vitro artifact. However, biological relevance of senescence has become apparent in many normal physiological states as well as in several pathological conditions (Campisi et al., 2019; Childs et al., 2017; Rhinn et al., 2019; Hou et al., 2019; Khosla et al., 2020). For instance, cellular senescence is required for tissue remodeling during embryogenesis, during wound healing, and to prevent malignant transformation of damaged cells (Lee and Schmitt, 2019; Rhinn et al., 2019). In contrast to their involvement in these normal physiological processes, excessive accumulation and activity of senescent cells is also associated with many of the chronic diseases of aging, such as atherosclerosis, cancer, cardiac and kidney dysfunctions, neurodegeneration, pulmonary fibrosis, and many others (Campisi et al., 2019; Hou et al., 2019; Khosla et al., 2020; Lee and Schmitt, 2019). Moreover, recent studies have identified "senescent-like" signatures in post-mitotic cells such as cardiomyocytes, osteocytes, osteoclasts, adipocytes, and neurons (Pańczyszyn et al., 2020). Interestingly, elimination of these cell types in aged and diseased mice is associated with improvements in health-span (Raffaele et al., 2020). On the other hand, a recent study demonstrated that continuous or acute elimination of p16High senescent cells in murine models disrupted blood-tissue barriers with corresponding health deterioration due to liver and tissue fibrotic pathologies with associated shortening of lifespan (Grosse et al., 2020). Thus, characterizing the post-mitotic senescent signatures along with evaluating the consequences of senolysis of these cell types for tissue integrity and function in either human tissues and/or in murine models will be important. However, it remains to be proven whether elimination of senescent cells, which is more easily achieved and its consequences more easily assessed in mice, would have similar beneficial and/or adverse effects in humans.
While the relevance of senescence for human biology is increasingly becoming evident, a major stumbling block has been the challenge of defining a "universal" marker for identification of senescent cells in vivo. In fact, the emergent view is that there will perhaps be a panel of biomarkers necessary to identify potentially heterogeneous senescent cells in vivo (Campisi et al., 2019; He and Sharpless, 2017; Sharpless and Sherr, 2015). These observations raise some important questions. Are there different senescence programs triggered by different forms of stress and/or insult in different cell types? What is the extent of heterogeneity of senescent cells? To answer this question, should large scale transcriptomics and proteomics studies be undertaken at single-cell level—i.e., mapping and atlasing senescence cells? Could there be a universal senescent program regardless of input and resident tissues? How representative are SASPs as reporters for senescence in vivo? If there is extensive heterogeneity, how to characterize a panel of markers for each type? How representative are in vitro studies as models for in vivo senescence? When should human tissues versus animal models be used as experimental systems? How can senescence be perturbed via senolytics and approaches like immune therapy in order to regulate and validate senescence in model systems, with the goal of benefiting human health? How do other pharmacologic approaches such as senostatics (agents that slow the progression of senescence; Kaur et al., 2020) compare to senolytics? These unanswered questions are a barrier to a our understanding of how to modulate senescence and inform clinical trials of promising senolytics, for the betterment of human health.
It should be noted that in a recent phase II trial of a putative senolytic, intra-articular administration of the study drug (UBX0101) failed to reduce the symptoms of osteoarthritis of the knee (https://www.globenewswire.com/news-release/2020/08/17/2079116/0/en/UNITY-Biotechnology-Announces-12-week-data-from-UBX0101-Phase-2-Clinical-Study-in-Patients-with-Painful-Osteoarthritis-of-the-Knee.html). It is unlcear whether this agent was ineffective because it did not induce efficient senolysis in vivo at the dose and schedule administered or whether senolysis occurred in recipient patients but did not provide therapeutic benefit against the symptoms of osteoarthritis. Ongoing studies of this agent at increased dose intensity, as well as several human trials with other potential senolytics, should help to address the validity of this approach for human disease states. This early experience already makes clear the pressing need for pharmacodynamic markers of senolysis—that is, biomarkers that indicate the degree to which therapeutic agents are able to induce senolysis in vivo in a given tissue after treatment. These types of biomarkers to inform clinical trials design are a key research priority presently for the field.
Identification and Characterization of Senescent Cells: A Comprehensive Approach
There is a growing recognition of the physiological importance of senescent cells in health and disease. However, given the heterogeneity of these cells, the first steps are to identify and characterize senescent cells in tissues and organs. NIH recently conducted workshops to receive input from the broad scientific community to address these questions with the goal of identifying opportunities for a possible future Common Fund (https://commonfund.nih.gov) program focusing on drivers and consequences of cellular senescence, including the development of tools and technologies to identify tissue heterogeneity, tracing and tracking these cells in vivo, and to establish model systems and perturbation agents to validate in vivo observational studies (a list of participants is available in Table S1). In addition to these informational discussions, we present our perspective regarding conceptual and technical opportunities as well as barriers hindering progress. It is our sincere hope that in addition to helping NIH prioritize research activities that are likely to propel this field forward in the next 5–10 years, these discussions will broadly help the community of researchers working in the area of cellular senescence.
Five broad areas were identified through this exercise: (1) generation of a multimodal atlas that characterizes the heterogeneity and spatial distribution of senescent cells across tissues; (2) determination of reliable biomarkers to identify senescent cells in vivo; (3) establishment of tools and model systems amenable to perturbations; (4) improvement of imaging tools to identify and validate senescence, both at cellular and whole-body levels; and (5) regulating senescence through senolytics and immune therapy. Below, we elaborate on each of these aspects.
Senescence Cell Atlas
Given the substantial heterogeneity of senescent cells and the idea that they represent a continuum of states, creation of an Atlas of Senescent Cells is a core need of the field. A deep profiling of these cells in various tissues with spatiotemporal resolution is necessary to distinguish between acute and chronic senescent states across developmental timescales and lifespan. The multimodal mapping efforts should include single-cell transcriptomics, genomics and epigenomics, proteomics, metabolomics, and spatial profiling. As part of this effort, a "taxonomy" of senescence could also be developed and annotated to describe age of the host, tissue/cell of origin, senescence triggers, and other attributes. Artificial intelligence and computational approaches for massive scale data analyses will also be critical for this effort. Although strategic coordination with existing groups (e.g., HCA, HuBMAP, and HTAN) working in this space is advisable, given the rarity and morphology of senescent cells, these cells might not be captured in most cellular atlas datasets currently in existence. However, a common platform for data ingestion, processing, and display could be developed across all atlasing efforts that might benefit the community at large.
Biomarkers
A full understanding of which cells senesce in vivo is lacking. An integral aspect of developing a comprehensive atlas of cellular senescence is the ability to establish a set of "gold standard" biomarkers to identify senescent cells in vivo, based on both molecular and physiologic properties. This includes, but is not limited to, SASPs, metabolic signatures, and cell surface and intracellular markers. These markers would also allow a better definition of senescence and establish a uniform vocabulary for the community. This is a challenging but essential step, which will be facilitated by the development of a comprehensive atlas.
Model Systems
The challenging question remains of whether senescent cells in animal models faithfully recapitulate human states. Nevertheless, an amenable, fast-aging but relevant model system that allows rapid experimental iteration is needed to analyze, benchmark, and computationally model the causal effects of different genetic or pharmacological perturbations. In addition to animal models (e.g., murine, non-human primate), other systems could include human organoid and explant platforms for lineage tracing, characterization of senescent cell accumulation, and their features across different timescales and disease contexts to provide a baseline resource for the community. Such systems will be critical for understanding vulnerabilities of different senescent cell types, that likely vary by tissue of origin, cell type within a given tissue, and health of host. Animal models could also enable longitudinal studies to measure the long-term effects of senescent cells in different tissues, as well as the effects of their removal on the long-term host’s physiology. Identification of these different vulnerabilities is important to harness senolytics and immune therapy approaches to removal of the cells.
Finally, these systems would allow the assessment of immune cells in regulating survival and persistence of senescent cells and immunotherapy as an adjunct therapeutic modality to senolytic agents. Apart from experimental systems, computational and/or predictive efforts to model senescence in vivo is also necessary for future studies. Given that it might take considerable effort and time to generate a comprehensive atlas of all tissue-resident senescent cells, computational modeling could help in the immediate future for predicting existence and identification of such cells in health and in various pathological conditions.
Imaging and Visualization Tools
Tracking and identifying senescent cells in vivo are particularly difficult tasks, yet necessary to validate findings from the atlas and biomarker discovery efforts. Thus, there is a need for improved reagents and tools to visualize individual senescent cells and senescent cell burden, such as whole-body live imaging (e.g., non-invasive imaging like computer tomography or PET) to identify a particular metabolic reaction unique to senescent cells and dynamic changes resulting from therapeutic interventions. Furthermore, there is still uncertainty as to how senescent cells are organized in vivo, with experimental evidence supporting either mosaic or clumped models, such that longitudinal imaging of senescent cells for lineage tracing in vivo is extremely critical and would alter the trajectory of research in this field. Additionally, imaging via cell-surface biomarkers, intracellular metabolic, or other changes is also recommended. One must take into consideration the visualization tools that need to be developed for displaying imaging data for use by the scientific community. Computational biologists along with artificial intelligence experts and modelers need to work together with data generators to achieve such goals.
Regulation and Validation of Senescence
The biomarker panels, model systems, and imaging technologies will need to be validated through demonstration projects in which senescence pathways are perturbed to induce, eliminate, or modulate senescence and then to assess the impact of those perturbations on health and disease. For instance, a combination of senolytic drugs with other geroprotectors or disease-specific drugs could be tested on samples in sliced transplant tissues from healthy and diseased donors. The relationship of senescent cells with the immune system in the context of clearing via immunotherapy or activation of the host immune system (e.g., a combination therapy using senolytics and anti-inflammatories) should be considered. As an example, T cells undergo cellular exhaustion (somewhat analogous to senescence) during CAR-T cell therapy. Exploration in this area could benefit both cancer treatment and senescence-related disorders (Amor et al., 2020). Finally, lineage tracing and real-time monitoring of clearing as a result of perturbing senescent cells by senolytic treatments will also be an important capability to demonstrate and validate any senolytic approach in a relevant experimental model system. Ultimately because different senescent cells might react differently to distinct senolytic approaches, a one size fits all approach might not work in all healthy or diseased conditions.
Outlook
It is our view that the time is right to begin to address causes and consequences of cellular senescence along with exploring approaches to identify, track, trace, and perturb senescent cells in relevant experimental model systems (Figure 1). But central to this quest is the establishment of multimodal maps of senescent cells in various tissues leading to a senescence cell atlas that the community can use to interrogate acute and chronic senescent states across developmental timescales and lifespan in normal physiological and pathophysiological conditions. We acknowledge that the challenges are enormous, but these are first and necessary steps toward catalyzing and transforming this particular field of cross-cutting biomedical research.
Figure 1. Five Broad Objectives to Help Build a Comprehensive Blueprint of Senescent Cells in Human Tissues and Organs.
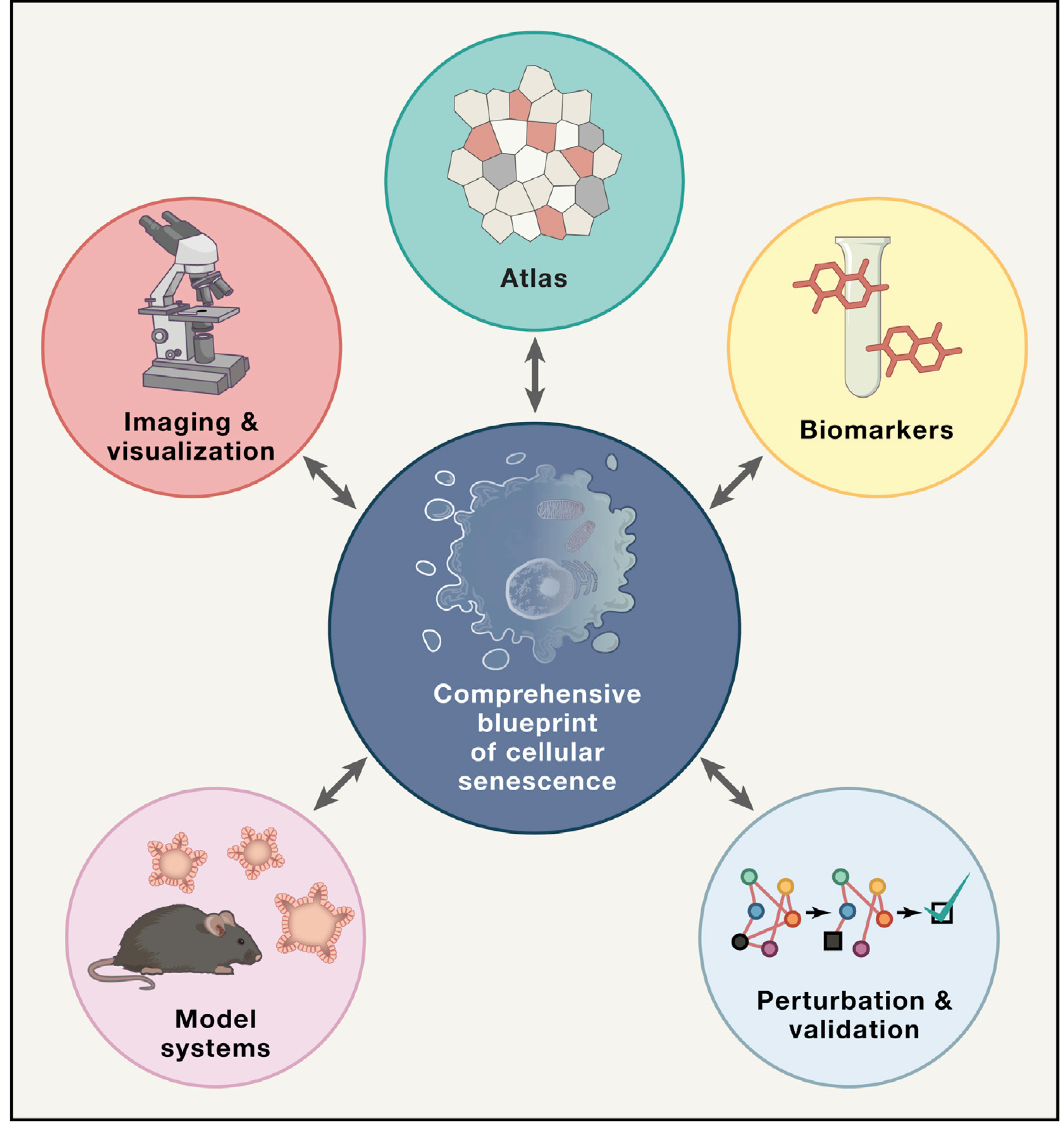
These five objectives are building a multimodal and multidimensional senescent cell atlas (Atlas); identifying a panel of reliable biomarkers (Biomarkers); imaging and visualizing (Imaging & visualization), including artificial intelligence tools to identify senescent cells; establishing experimental model systems (Model systems); and validation experiments (Perturbation & validation) via perturbation to test predictive models.
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank our colleagues at NIH for their help and input with the workshops.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.10.032.
WEB RESOURCES
NIH Common Fund, https://commonfund.nih.gov
UNITY Biotechnology press release, https://www.globenewswire.com/news-release/2020/08/17/2079116/0/en/UNITY-Biotechnology-Announces-12-week-data-from-UBX0101-Phase-2-Clinical-Study-in-Patients-with-Painful-Osteoarthritis-of-the-Knee.html
REFERENCES
- Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, et al. (2020). Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, and Verdin E (2019). From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, and van Deursen JM (2017). Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, et al. (2019). Cellular Senescence: Defining a Path Forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner KD, and Bulavin DV (2020). Defined p16High Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 32, 87–99.e6. [DOI] [PubMed] [Google Scholar]
- Hayflick L, and Moorhead PS (1961). The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. [DOI] [PubMed] [Google Scholar]
- He S, and Sharpless NE (2017). Senescence in Health and Disease. Cell 169, 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, and Bohr VA (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. [DOI] [PubMed] [Google Scholar]
- Kaur A, Macip S, and Stover CM (2020). An Appraisal on the Value of Using Nutraceutical Based Senolytics and Senostatics in Aging. Front. Cell Dev. Biol. 8, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Farr JN, Tchkonia T, and Kirkland JL (2020). The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 16, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, and Schmitt CA (2019). The dynamic nature of senescence in cancer. Nat. Cell Biol. 21, 94–101. [DOI] [PubMed] [Google Scholar]
- Pańczyszyn A, Boniewska-Bernacka E, and Goc A (2020). The role of telomeres and telomerase in the senescence of postmitotic cells. DNA Repair 95, 102956. [DOI] [PubMed] [Google Scholar]
- Raffaele M, Kovacovicova K, Bonomini F, Rezzani R, Frohlich J, and Vinciguerra M (2020). Senescence-like phenotype in post-mitotic cells of mice entering middle age. Aging (Albany N.Y.) 12, 13979–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Ritschka B, and Keyes WM (2019). Cellular senescence in development, regeneration and disease. Development 146, dev151837. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, and Sherr CJ (2015). Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


