Abstract
The world has been suffering from COVID-19 disease for more than a year, and it still has a high mortality rate. In addition to the need to minimize transmission of the virus through non-pharmacological measures such as the use of masks and social distance, many efforts are being made to develop a variety of vaccines to prevent the disease worldwide. So far, several vaccines have reached the final stages of safety and efficacy in various phases of clinical trials, and some, such as Moderna/NIAID and BioNTech/Pfizer, have reported very high safety and protection. The important point is that comparing different vaccines is not easy because there is no set standard for measuring neutralization. In this study, we have reviewed the common platforms of COVID-19 vaccines and tried to present the latest reports on the effectiveness of these vaccines.
Keywords: Covid-19, Sars-Cov-2, Vaccine, Infection, Adenovirus, Inactivated, Effectiveness
1. Introduction
Discovery of several cases of pneumonia in late 2019 in Wuhan, China, was the beginning of a major global catastrophe and a widespread pandemic [1]. On February 11 of the following year, the cause of this pandemic, which was a novel coronavirus, was named by the international virus classification commission as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the resulting disease was named by the World Health Organization (WHO) as Coronavirus Disease 2019 (COVID-19) [2], [3]. Coronaviruses are a great family of viruses that cause various diseases in animals and humans. Seven viruses of the genus Alpha and beta coronavirus can cause disease in humans. Among these, three viruses that cause Middle East Respiratory Syndrome (MERS-CoV), Severe Acute Respiratory Syndrome (SARS-CoV), and SARS-CoV-2 are the most common coronavirus-related deaths in the world [4], [5]. COVID-19 disease has a wide range of clinical manifestations, from asymptomatic, mild symptoms to acute respiratory distress syndrome (ARDS) and death [6], [7], [8]. Mortality rate of this disease was reported to be 1–3%, and this rate was higher in elderly patients, especially in men [9]. It was also found that the mortality rate varies in different geographical areas, which may be due to different immune responses in people in different regions [10], [11]. SARS and MERS were also found to have higher mortality rates in parts of the United States and Europe than in Southeast Asian countries [12]. So far, no definitive and completely treatment is available to treat this disease, and most of the treatment strategies used in the world are to reduce the symptoms of the disease and prevent the progression of the disease in the infected person [13]. One way to design effective drugs is to understand the virus cycle and its pathogenicity, which helps scientists achieve a specific drug [14].
Still, using a mask and observing the social season and washing your hands are the most common ways to prevent getting the virus. One way to prevent and control the disease was to use herd immunity, which is possible by obtaining natural immunity through infection, but experience has shown that the consequences of this method can be devastating [6]. In Sweden, where officials thought that by infecting 60% of the population, they could create a herd safety to protect the people of their country, this failed, and the death rate per million of the population infected with COVID-19 was at least 5 times higher than in Germany [15]. So, production of effective vaccines for providing long-term immunity is considered as the only principled way to create herd immunity. In this regard, many companies in different countries have made great progress in making new vaccines. The use of new methods and advanced technologies to produce a variety of vaccines, including RNA, DNA, virus-like particles, and subunit vaccines, has been widely tested [16]. In addition, in order to produce a safe and effective vaccine, it is critical to perform pre-clinical and clinical trials to thoroughly investigate and determine any side effects of these vaccines [17]. The purpose of this study is to review the different types of vaccines that are currently being produced and tested around the world, as well as the mechanism of action of these vaccines and the extent of progress and efficacy and safety of these vaccines.
2. Specific features of coronavirus for vaccine development
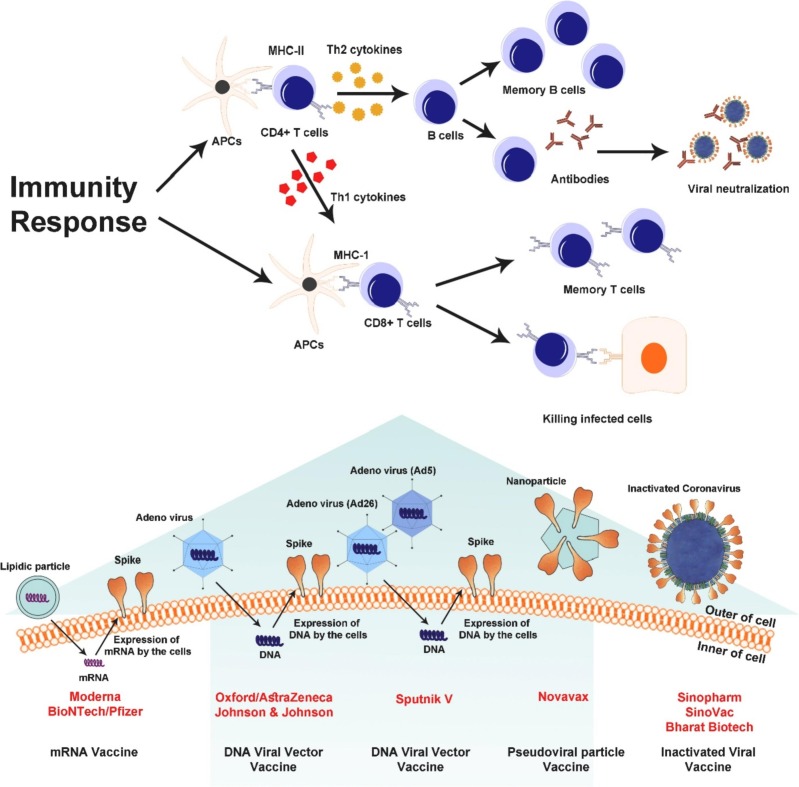
SARS-CoV-2 is a spherical and positive-sense single-stranded RNA virus with a helical nucleocapsid. Size of SARS-CoV-2 genome is 30 kb. One third of this genome is responsible for encoding the structural proteins of the virus, and the rest of the genome shows expression and replication [18]. Structure of this virus consists of membrane glycoproteins (M), spike proteins (S), hemagglutinin ester dimer proteins (HE), nucleocapsid proteins (N), and envelope proteins (E). The major coat glycoprotein expressed at the virus surface (S-protein) is the main target of vaccines [19]. S-protein consists of two subunits: S1 subunit is responsible for binding to the receptor and S2 subunit is responsible for fusion with the cell membrane [20]. The main receptor for SARS-CoV-2 entry into human cells is angiotensin-converting enzyme 2 (ACE2) identified by S-protein [21]. ACE2, an attached carboxypeptidase to the plasma membrane of kidney, intestines, testis, heart, lung and gallbladder cells, is consisting of a 17-amino-acid signal peptide at the amino terminal, a single metalloproteinase active site containing a zinc ion binding motif, a transmembrane domain, and a 22-amino acid small cytoplasmic domain at the carboxyl terminus [22]. The main activity of ACE2 is to lower blood pressure by converting angiotensin II to angiotensin I-VII [23], [24]. It has also been shown that the epithelial expression of ACE2 in the small intestine and lung provides a pathway for SARS-CoV entry [25]. During SARS-CoV-2 outbreak, high expression of ACE2 was detected in type II alveolar cells of lung [26], [27], [28]. The binding affinity of ACE2 and SARS-CoV-2 is approximately 10- to 20-fold higher than that affinity between ACE2 and SARS-CoV [29], [30]. Genomic sequence of S-protein is really diverse among coronaviruses. This variation in S-protein is related to S1 subunit, which consists of two parts: a receptor-binding domain (RBD) and N-terminal domain (NTD) [20]. S-protein has a polybasic cleavage site between S1 and S2 parts which leads to the acquisition of three O-linked glycans around the site. The adjacent predicted O-linked glycans and polybasic cleavage sites have not been found in any of the previous beta-coronaviruses and are unique to the SARS-CoV-2 [19], [31]. Binding of the S-protein to the ACE2 receptor and its proteolytic cleavage and integration with the membrane led to release viral RNA into the cytosol [31], [32]. Unlike the S-protein, E and M proteins of SARS- CoV-2 have weak immune humoral responses, possibly due to their small molecular size and small ectodomines, which make them indistinguishable from immune cells [33]. One study found that transmitting serum from donors immunized with a viral vector expressing E and M protein did not provide any protection against coronary infection [34]. Because the neutralizing antibodies produced in COVID-19 patients are also more likely to be produced against S-protein epitopes, S-protein is a promising target for vaccination against the SARS-CoV-2 [35]. One of the important points in designing vaccines is knowing the isoelectric point. An isoelectric point (IEP or pI) is a pH value at which the net charge of a molecule or biomolecules is zero. Because the electrical repulsion and solubility are at a point lower than the pI, there is a greater tendency for precipitation and aggregation [36]. There is currently no accurate experimental data for isoelectric point of SARS-CoV-2, or its structural and nonstructural proteins. Using bioinformatics tools such as Ex-PASy can give us an isoelectric point prediction [37]. These predictions are based on the amino acid sequence, and it should be noted that bioinformatics tools cannot take into account the post-translational changes that occur in the amino acid sequence of proteins [38]. The pI values of the three SARS-CoV-2 proteins (S, E, and M) that are important in vaccine design are 6.24, 8.57, and 9.51, respectively [39], [40]. The pH changes directly affect the isoelectric point and cause precipitation or insolubility. In one study, the stability of SARS-CoV-2 in different environmental conditions, in the exposure of different disinfectants, and at different pH was evaluated. This study reported that SARS-CoV-2 is stable in a wide range of pH, i.e., pH 3-10 [41]. Another item that is important in inactivating SARS-CoV-2 is the lipophilic or hydrophilic nature of the virus. SARS-CoV-2 has lipophilic properties due to its lipid envelope, so it allows lipophilic disinfectants such as aldehydes, proteases, phenolics, peroxides, alcohols, and detergents to penetrate [42]. However, it should be noted that the penetration of a substance into the virus does not necessarily cause the loss of the mechanism of nucleic acid replication and virus replication [43]. Properly inactivating SARS-CoV-2 is very important in making vaccines. For example, if SARS-CoV-2 epitopes are destroyed during inactivation, we may have a very weak antibody response and therefore the vaccine may not be very protective. Also, if the virus is not completely deactivated, the viral prevalence may happen after vaccination [44]. In general, different designs for SARS-CoV-2 vaccines are currently being developed by large companies and are undergoing various stages of preclinical and clinical trials [45], [46], [47], [48], [49], [50]. Some of these vaccines are based on inactivated viruses or subunits of the virus, and others are based on nucleic acids, each of which has advantages and limitations over the others. One classification that can be considered for these vaccines is based on whether the vaccines exert their immunogenicity as soon as they enter the host body or whether processes such as protein translation are required. Accordingly, inactivated vaccines and nanoparticles containing S-protein stimulate the immune system upon arrival, but vaccines based on nucleic acids and viral vectors must undergo the translation of nucleic acid into immunogenic protein within the host cell (Fig. 1 ).
Fig. 1.
Classification of common vaccines based on the translation process requirement for immunization.
3. Different designs of SARS-CoV-2 vaccines
3.1. Inactivated vaccines
Inactivated vaccines are the fastest option for antiviral vaccines, which are traditionally obtained from virus-infected cells. This method was first invented in 1940 using embryonated eggs to produce the flu vaccine [51]. To date, various chemical and physical methods, including the use of formalin, formaldehyde, β-propiolactone and UV alone or a combination of these methods, have been used to inactivate coronaviruses [52], [53]. Subunits of these inactivated viruses are commonly used to produce antibodies because the use of whole viruses increases the risk of reactogenicity. Tetanus and diphtheria vaccines are examples of subunit vaccines, and polio vaccine is a classic example of a whole killed virus vaccine [54]. Because these vaccines provide weaker immunity than live vaccines, the use of adjuvants is required to achieve an effective and robust immune response [55]. Several inactive SARS-CoV-2 vaccines are currently being developed rapidly ( Table 1 ). A major problem in inactivating vaccines is the selection of the appropriate viral strain. In one study, it was reported that the CoronaVac inactivated vaccine with alum adjuvant had very acceptable neutralizing effects against the SARS-CoV-2 [56]. BBIBP-CorV is one of the inactivated vaccines that is made in Wuhan Institute of Biological Products (Sinopharm) and Beijing Institute of Biological Products, has passed the phase 3 clinical trial. The results of one research have shown that this type of vaccine has been safe and tolerated in all tested doses and in two age groups. Humoral immune responses against SARS-CoV-2 were induced in all recipients of vaccine on day 42. Also, this study reported that two-dose immunization with 4 μg vaccine on days 0 and 21 or days 0 and 28 achieved higher neutralizing antibodies titres than the single 8 μg dose or 4 μg dose on days 0 and 14 [57]. Although using of inactivated vaccines is one of the commonly immunization methods in worldwide, they also have drawbacks, for example, the use of aluminum hydroxide as an adjuvant has previously been linked to vaccine-associated enhanced respiratory disease (VAERD) [58]. However, so far no signs of VAERD have been noted in the performed SARS-CoV-2 clinical trials to date. Another concern with inactivated vaccines is their short immunity period. Previously inactivated vaccines against SARS-CoV showed that antiviral IgG levels dropped rapidly 16 months after inoculation [59].
Table 1.
Different types of SARS-CoV-2 vaccine candidates and their effectiveness.
| Vaccine type | Vaccine type and immunogenic | Vaccine name | Developers | Dose schedule | Cold chain required for storage | Efficacy | Registry index | Ref |
|---|---|---|---|---|---|---|---|---|
| Inactivated vaccine | – | CoronaVac | Sinovac Research and Development Co. | 3 μg of CoronaVac in 0.5 ml of diluent (two doses - day 0, day 14) | 2-8 °C | Brazil study: where the prevalence of p.1 variant was more than 75%: efficacy was 50.7%, 83.7% and 100% against mild, moderate and severe infection, respectively. Turkey study: 83.5% in 18-59 years old volunteers Chile study: 65.9%, 87.5%, 90.3% and 86.3% against prevention of Covid-19, prevention of hospitalization, ICU admission and prevention of Covid-19 related death, respectively |
NCT04456595, NCT04582344, NCT04617483, INA-WXFM0YX | [48], [126], [127], [128] |
| – | BBIBP-CorV | Beijing Institute of Biotechnology / China National Pharmaceutical Group Co. (Sinopharm) | 4 μg (two doses - day 0, day 21) | 2-8 °C | 79% | ChiCTR2000034780, NCT04560881. NCT04510207 | [57], [129] | |
| – | Not Reported | Wuhan Institute of Biological Products / China National Pharmaceutical Group Co. (Sinopharm) | Undisclosed quantity of dosage (two doses - day 0, day 21) | 2-8 °C | – | ChiCTR2000034780, ChiCTR2000039000, NCT04612972, NCT04510207 | [47] | |
| – | BBV152 (Covaxin) | Bharat Biotech | 6 μg (two doses - day 0, day 28) | 2-8 °C | 81% | CTRI/2020/11/028976 |
[130], [131] |
|
| Non-Replicating Viral vector vaccine | Non-Replicating Ad5 vectored vaccine expressing the SARS-CoV-2 full length spike protein | Ad5-nCoV | CanSino Biological / Beijing institute of biotechnology / Academy of Military Medical Sciences | 0.5 × 1011 vp (one dose) | 2-8 °C | – | NCT04526990, NCT04540419 | [69], [132] |
| Non-Replicating Chimpanzee adenovirus-vectored vaccine expressing the SARS-CoV-2 full length spike protein | AZD1222 (ChAdOx1 nCoV-19) | AstraZeneca / Oxford University | 3.5-6.5 × 1010 vp (two doses - day 0, day28) | 2-8 °C | Half dose + standard dose: 90% Two standard dose: 62.1% Overall: 70.4%, Recent studies results: 81.5% for non-B.1.1.7 variants, 70.4% for B.1.1.7 variant and 10.4% for B.1.351 variant, Single dose effectiveness against B.1.617.2 and B.1.1.7 variants: 33.5% and 51.1%, respectively. Two dose effectiveness against B.1.617.2 and B.1.1.7 variants: 59.8% and 66.1%, respectively. |
ISRCTN89951424, NCT04516746, NCT04540393, CTRI/2020/08/ 027170 |
[45], [46], [66], [126], [133], [134], [135] | |
| Non-Replicating rAd6 and rAd5 both of them carry the SARS-CoV-2 full length spike protein gene | Gam-COVID-Vac (Sputnik V) | Gamaleya Research Institute of Epidemiology and Microbiology/ Russian Ministry of Health | 1011 vp (two doses - day 0, day21) | Two explanations: Frozen at -18 °C Lyophilized at 2-8 °C |
91.6% |
NCT04530396, NCT04564716, NCT04642339 |
[136], [137] | |
| Non-Replicating Ad26 vectored vaccine carry the full-length spike protein gene with two proline substitutions and two mutations at furin cleavage site | JNJ-78436735(Ad26.COV2. S) | Janssen Pharmaceutical Companies | 1011 vp (one dose) | 2-8 °C | 66% against infection and 85% against severe-critical covid-19, against b.1.351 variant: 64% against moderate disease and 82% against severe-critical disease |
NCT04505722, NCT04614948, ISRCTN14722499 |
[138], [139], [140], [141], [142] | |
| DNA vaccine | SARS-CoV-2 spike protein gene was cloned into the expression vector pGX0001 | INO-4800 | Coalition for Epidemic Preparedness Innovation (CEPI), Inovio Pharmace ticals | – | can be stored for 4.5 years at 2–8 °C, room temperature for 1 year and 1 month at 37 °C | – | NCT04336410, NCT04642638, NCT04447781 | [79] |
| SARS-CoV-2 spike protein gene was cloned into the pVAX-1 plasmid | ZyCoV-D | Cadila pharmaceuticals limited | – | – | – | CTRI/2020/07/026352 | [143] | |
| mRNA vaccine | mRNA encoding the SARS CoV-2 full length spike protein (modified by two proline substitutions) | BNT162b2 | Pfizer / BioNTech / Fosun pharma | 30 μg (two doses - day 0, day 21) | Stable at: -70 °C for up to 6-month, 2-8 °C for 5 days | 95% overall, 94.7% (>65 years old), Israel vaccination results: 94%, Recent studies results: Single dose 80% Two doses 90%. In 12-15 years old adolescents: 100% Single dose effectiveness against B.1.617.2 and B.1.1.7 variants: 33.5% and 51.1%, respectively. Two dose effectiveness against B.1.617.2 and B.1.1.7 variants: 87.9% and 93.4%, respectively/ |
NCT04368728 | [49], [92], [135], [144], [145], [146], [147] |
| mRNA encoding the SARS CoV-2 full length spike protein (modified by two proline substitutions) | mRNA-1273 | Moderna / NIAID | 100 μg of mRNA-1273 in a volume of 0.5 ml (two doses - day 0, day 28) | Stable at: -20 °C for up to 6-month, 2-8 °C for up to 30 days, room temperature(8-25 °C) for up to 12 h | 94.1%, Recent studies results: Single dose 80% Two doses 90% |
NCT04470427 | [91], [94], [144], [146], [148] | |
| Protein subunit vaccine | Nanoparticle vaccine constructed from the Full-length spike protein with two proline substitutions and three mutations at furin cleavage site | NVX-CoV2373 | Novavax | 5 μg of SARS-CoV-2 rs + 50 μg of Matrix-M1 adjuvant (two doses - day 0, day 21) | 2-8 °C | 96.4% against non-B.1.1.7 variants, 86.3% against B.1.1.7 variant, 60% against B.1.351 variant | NCT04611802, 2020-004123-16 | [50], [149], [150], [151], [152] |
| Virus like particle vaccine (VLP) | Plant derived VLP displays multiple copies of SARS-CoV-2 spike protein on their surface + adjuvant | CoVLP | Medicago/Glaxo Smith Kline (GSK) | 3.75 μg of CoVLP Vaccine adjuvanted with AS03 adjuvant (two doses - day 0, day 21) each arm will be injected once | – | – | NCT04636697 | [153] |
3.2. Non-replicating viral vector vaccine
The use of viral vectors first began in 1972 with recombinant DNA from the SV40 virus. Subsequently, the vaccinia virus was introduced as a transient gene expression vector in 1982 [60], [61]. Among the types of virally vectored candidates for non-replicating SARS-CoV-2 vaccines, most of them are based on adenoviruses. Adenoviruses are two-stranded DNA viruses that are inactivated by deleting their E1A and E1B gene region [62]. These vaccines induce strong immune responses and enhance both humoral and cellular immunity. Enteric adenovirus type 41 and adenovirus type 5 previously had been used against spike proteins of MERS-CoV, and acceptable immune responses, including both B-cell and T-cell responses, have been observed in vaccine recipients [63]. Adenovirus vectors are characterized by the easy growth of these viruses at high titers in cell lines, high expression of transgene, and great transduction effect, as well as a wide range of viral tropism [64], [65]. Numerous clinical trials have been performed to evaluate the efficacy of adenoviral-vectored vaccines, some of which are listed in Table 1. The results of clinical trials showed that nonreplicating vector vaccines are in good immunogenicity and safety. Also, the induction of antibodies against S-protein was confirmed by enzyme-linked immunosorbent assays (ELISA) in these studies [66]. One of the limitations of adenoviral-vectored vaccines is that most people have previous immunity to different strains of adenovirus (pathogens of upper respiratory infection) or they become immunized quickly after the first dose of vaccine [67]. For example, the results of a clinical trial based on Ad5vector-based HIV1 vaccine candidate failed due to patients’ prior immunity against the Ad5 vector [68]. In the first randomised controlled trial for assessment of the immunogenicity of a candidate non-replicating adenovirus type-5 (Ad5)-vectored COVID-19 vaccine, Zhu et al., demonstrated that is effective and safe, but they also reported that previous immunity to Ad5 and aging resulted in a slight decrease in vaccine efficacy in certain groups, especially in the case of hemorrhagic immune responses [69]. One way to overcome this problem is to use alternative adenoviral vectors such as Ad35, Ad26, and non-human adenovirus-derived vectors that are less common in humans [70], [71]. AstraZeneca, Johnson & Johnson, and sputnik V are among the non-replicating SARS-CoV-2 vaccines that are being mass-produced by relevant companies and they are currently injecting in many countries ( Fig. 2 ).
Fig. 2.
Different types of SARS-CoV-2 vaccine candidates and immune response to vaccines.
3.3. DNA vaccines
One of the reasons for the worsening of the infection in COVID-19 disease is the imbalance in the production of different immune cells, so that the increase of CD3+ CD8+ T cells is associated with the decrease of CD14+ HLA-DR+ monocytes [72]. Therefore, the point that is considered in the design of vaccines is to produce an effective immune response without creating such an imbalance. On the other hand, these designed vaccines are able to not only activate cellular immunity, but also activate humoral immune responses by producing immunogenic substances and releasing them from the cell and detected by B-cell receptors [73]. The first use of nucleic acids as a vaccine was about three decades ago, where DNA and RNA molecules expressing luciferase, chloramphenicol acetyltransferase, and beta-galactosidase genes were used to create immunogenicity in mouse. It was reported that the expression of these genes continued until two months after injection [74]. In general, DNA-based vaccines insert genes encoding an antigen into the host cells especially the antigen-presenting cells (APCs), using DNA plasmids as a vector. The mechanism of these vaccines is based on the principle that the genetic material delivered by them is located inside the cell nucleus. The mammalian promoter in the vector is then activated and transcription of the target genes is performed by the host cellular mechanism [75], [76]. The method used to inject plasmid DNA into the cells is electroporation, which uses short electrical pulses to create temporarily and reversibly penetration into the cell membrane. This disruption of membrane allows large molecules such as plasmids to enter the cells. Using electroporation for plasmid injection can greatly increase the expression of the target protein [77]. The advantages of these vaccines are non-infectious, easy production in a short time, cost-effectiveness and stability. In addition, one of the benefits of DNA vaccines is greater thermal stability, so these vaccines have less refrigeration requirements than mRNA vaccines [78]. In COVID-19 disease, injection of a DNA vaccine (INO-4800) consisting of a plasmid containing a N-terminal Ig E leader sequence that encoded the S-protein of SARS-CoV-2 in guinea pigs and BALB/c mice produced neutralizing antibodies against SARS-CoV-2 [79]. Genexine Company, which has previously published the results of its Phase1/2 clinical trials using of electroporation for Human papillomavirus (HPV) vaccination, has conducted phase 1 and 2 clinical trials against SARS-CoV-2. Recent research by this company has shown that vaccines containing DNA encoding whole S-protein (pGX27-S) or S-protein without S2 fragment (pGX27-S ΔTM), both induce the production of neutralizing antibodies, but pGX27-S ΔTM vaccine induces a higher antibody titer [80]. Several DNA vaccines against COVID-19 are currently in various clinical trials stages and no complete information of the efficacy percent of these vaccines has been published so far ( Table 1 ).
3.4. mRNA vaccines
The mRNA-based vaccines contain mRNA molecules that encode protein antigens. New vaccine designs with the great technological innovation are constantly evolving to improve the stability of mRNA molecules and efficiency of protein translation, which improved immune response [81]. One way to overcome the instability of the mRNA is to place mRNA molecules inside lipid nanoparticles, which these nanoparticle delivery carriers also act as adjuvants and induce B-cell and T follicular helper immune response [82], [83]. Codon optimization also reduces the degradation of mRNA molecules and increases the expression of coding antigens. In addition to, mRNA cap modification with synthesized anti-reverse cap analogs (ARCAs) increases translation efficiency and also leads to long-term protein expression and mRNA half-life in cells [84], [85], [86]. In this type of vaccine, the mRNA molecule is injected directly into the host cell and translated into the target protein in the cytoplasm. The overall design of mRNA vaccines contains an open reading frame (ORF) with a 3′ poly-adenylated tail that can induce both cellular and humoral immune responses [87]. The mRNA-based vaccines have advantages over other vaccines: a) There is no risk of infection during vaccine production. b) Examination of mouse models has shown that repeated immunizations with mRNA-vaccines are associated with Long-term immunity in them. c) Since there is no require to enter the cell nucleus to the antigen expression, there is no possibility of insertional mutations creation in them [88]. So far, mRNA-based vaccine technology has been used to produce vaccines against various infectious diseases, including Ebola, respiratory syncytial virus, influenza virus, and HIV [81], [89], [90]. One of the main candidates for the SARS CoV-2 vaccine (mRNA-1273-based vaccine) have been developed with collaboration the National Institute of Allergy and Infectious Diseases (NIAID) and Moderna. The mRNA-1273 molecule encodes the S2-protein antigen, which is made up of SARS-CoV-2 glycoprotein with the transmembrane anchor and an intact S1-S2 cleavage site [91]. This vaccine has gone through various approval stages and is now being used in most parts of the world. Pfizer and BioNTech have also developed several RNA-based vaccines that have been successful in clinical trials and are now being used in many countries [49], [92]. These vaccines, like the Moderna vaccine, are embedded lipid nanoparticles (LNPs) and encode the membrane-anchored full-length S-protein (BNT162b2) and secreted receptor-binding-domain (BNT162b1) of SARS-CoV-2 [93]. BioNTech and Moderna vaccines also require booster doses to ensure high antibody titration and long-term safety. However, the response of the antibody to the receptor-binding domain in both vaccines showed a higher titer of antibody than in patients recovering from COVID-19 [91], [94]. One of the points about these vaccines is that the injection of these vaccines in the first dose caused a mild headache, chills, fatigue, and myalgia and in the booster dose caused moderate to severe systemic or local reactions in some patients [91], [92], [95]. A major advantage of nucleic acid-based vaccines is the short time required from the design of these vaccines to clinical trials. Therefore, testing these vaccines against various likely mutations that occur in the SARS virus is easily and quickly possible [96].
3.5. Protein subunit-based vaccines
Another of the safe vaccines to fight SARS-CoV-2 are protein-based subunit vaccines. These vaccines are highly dependent on adjuvants to increase their immunogenicity. Peptides, like RNAs, are usually unstable and are therefore located within nanoparticles adsorbed onto adjuvants. Different S-protein subunits including RBD and RBD-Fc, and N-terminal domain of S-protein have shown different degrees of protection and immune responses in several animal models [97], [98], [99], [100]. Process of preparing these vaccines is that the genes encoding the predominant antigenic components of the virus are cloned and expressed in various expression systems such as bacterial cells or mammalian cells, and then purified. These recombinant products are used to make stable vaccines [54]. In previous researches, S1 subunit vaccines for SARS-CoV produced from baculovirus Spodoptera frugiperda (Sf9) insect cell with saponin adjuvant showed a reduction in lung viral titer in young mice and using protollin adjuvant showed a reduction in lung viral titer in aged mice after injection [101], [102]. Type of adjuvant can alter the effects of the vaccine, and sometimes the use of several adjuvants in combination can have synergistic effects on the level of immune response. For example, Lan et al. showed that the use of RBD subunit vaccine with alum and cysteine-phosphate-guanine (CpG) oligodeoxynucleotides (ODN) as the adjuvant against MERS-CoV induced much stronger humoral and cellular immune responses [100]. Several SARS-CoV-2 vaccine candidates, including a recombinant S-protein expressed in different cell lines, are currently being developed and used [103]. Among these candidates for the SARS-CoV-2 subunit vaccine, their leader is NVX-CoV2373, which is produced by Novavax and has gone through various stages of clinical trial (Table 1). Production of this protein is done in the sf9 expression system of and the adjuvant used for it is Novavax's Matrix-M1 [50], [104]. This vaccine contains a recombinant full-length spike glycoprotein that has been engineered to resist proteolytic degradation and has a high binding to ACE2 receptors [50]. Among the advantages of these vaccines are their safety and cost-effectiveness, and their limitations include the need for adjuvants in order to uptake of more protein into host antigen presenting cells and producing a long-term immune response [99].
4. SARS-CoV-2 vaccines challenges and considerations
Despite the efforts of scientists and researchers around the world to advance the production of effective vaccines against SARS, there is still much to be discovered that can be used to improve the quality and effectiveness of vaccines and to overcome the limitations of current vaccines. One of the important points that impair the efficiency of COVID-19 vaccines is the occurrence of mutations in the genome sequence of SARS CoV-2 virus. Although the variety of mutations that have occurred so far is not very large, but the high transmission power and global spread of the virus can cause the phenomenon of natural selection to act on certain mutations [105], [106], [107], [108]. Recognition of mutations in coding and non-coding sequences and genetic diversity of the virus is essential for the development of vaccines with long-term efficacy [21], [109]. Another challenge for SARS CoV-2 vaccines is immunization programs and how to use the vaccines. Almost all available vaccine candidates have been delivered to recipients in clinical trials via intradermal or intramuscular route. The question is whether the antibodies produced in the blood reach the lungs and can work there. One method of vaccination that has been considered is mucosal vaccination, which may be effective in inducing an immune response in the mucosa and preventing the transmission of the virus to the respiratory tract and lungs [110]. In addition, each of these vaccine candidates has their own challenges and limitations, for example, previous immunity to adenoviruses is a concern for vaccines that use the virus as a carrier, as it may reduce the immune response [69]. Therefore, in the design of some of these vaccines using genetic engineering, an attempt has been made to use a modified chimpanzee-derived adenovirus [66]. In the case of mRNA-based vaccines, there is the problem of temperature-sensitive lipid nanoparticles containing mRNA and their limitations for production in large scale [92]. In DNA vaccines, the use of electroporation to deliver the vaccine to recipients increases the immune response, but this technology has made the use of these vaccines difficult and complicated [111]. There is also risk of vaccine-enhanced disease for inactivated vaccine candidates, specially vaccine-associated enhanced respiratory disease (VAERD), that needs to be considered [112], [113]. Since antibody dependent enhancement (ADE) has always been a risk factor for vaccines of other coronaviruses, this should also be considered for the SARS CoV-2 vaccine candidates [114]. Although ADE phenomenon is more common in the case of Favivirus and some feline coronaviruses, if it occurs in the case of the SARS-CoV-2 vaccines, nucleic acid-based vaccines can quickly adopt solutions such as engineering or removing the motifs responsible for generating ADE from the vaccine antigen [115], [116]. Given the re-infection observed in patients who had recovered from the COVID-19, this uncertainty about long-term protection for vaccines is also raised. Ideally, vaccines should provide long-term immunity, but based on experience with influenza vaccines, vaccination against SARS CoV-2 can be repeated annually [94], [117]. Despite all the efforts and hopes for the vaccine, attention should not be diminished from trying to find effective drugs that reduce COVID-19 infection [114]. Another common concern about vaccines is that because vaccine candidates are tested on the certain numbers of a population in country of manufacture of the vaccine, it may have unknown effects when used globally that have not been seen in previous experiments. In addition, if not all population groups, including the elderly, young, special patients, and immunocompromised individuals, are not considered in the initial vaccine research, unwarranted side effects may occur during general and universal use of the vaccine in these groups [118]. The issue of political pressures to speed up the production process and approval of a vaccine may reduce the accuracy of the various stages of vaccine production, and ultimately make a vaccine available to the public with low efficacy or unexamined side effects [119]. In addition, if a vaccine receives the necessary approvals and reports a high percentage of efficacy in preclinical and clinical trials, but when used publicly in the population, it does not have the desired effect as expected, it erodes confidence in the vaccine. As a result, fewer people accept the vaccine, which will make the pandemic worse. Therefore, it is very important to build trust and establish effective communication between the people and the public health system and to provide transparent reports on vaccines [120]. One of the challenges of vaccination is the issue of inequality in the distribution and injection of vaccines. As this challenge has affected many people life around the world, especially in poor and less developed countries. In this regard, bollyky and bown chose the term “vaccine nationalism” to describe this tragedy [121]. Factors that have led some countries to turn to vaccine nationalism, stockpiling vaccines to prioritize rapid access to their citizenry, include intense public and political pressure and fears of declining immunity in their country [122]. Indeed, the bitter fact that developed countries continue to sign agreements with the world's largest pharmaceutical companies to purchase vaccines at prices that are not possible for most countries, and to buy and store vaccines more than their requirements, is a confirmation of the issue of vaccine nationalism. As a result, developing countries face acute problems in obtaining vaccines [123], [124], [125]. Raising the issue of vaccine nationalism once again exposed the bitter reality of the lives of the world's poor, who are constantly deprived of basic human rights, and justice in general. While the existence of these inequalities has raised concerns about the continuation of the COVID-19 pandemic, a series of short- and long-term reforms are underway to overcome this inequality. Due to this unfortunate fact, one of the concerns of some organizations such as the People's Vaccine Alliance, was that almost 70 lower-income countries would only be able to vaccinate 10% of their population, so COVAX, a part of a set of initiatives by the International Public Health Organization to accelerate the development and equitable distribution of health products, provided millions of doses of the vaccine for over 92 registered countries. However, these doses cannot fully cover the international need [121].
5. Conclusion
COVID-19 disease has been a serious threat to global health for more than sixteen months, and there is no definitive cure for it, and it is still associated with a high mortality rate worldwide. Researchers around the world have decided to develop effective vaccines to prevent the virus. To date, various vaccines have been produced with very high efficacy and are being used worldwide. Pfizer, BioNTech, Novavax, BBIBP-CorV, AstraZeneca, and Sputnik V vaccines with acceptable efficacy are including the most common vaccines against SARS CoV-2. Despite all this, there are still many ambiguities about vaccines. How long the immunity created by the vaccine lasts, whether the immunity developed after the infection is different from the immunity resulting from the vaccination, whether the general vaccination reduces the spread of the virus, do vaccines work the same in different populations and many other questions are still unanswered. In general, more studies are needed to determine the efficacy and safety of each vaccine, as well as the duration of immunization. While vaccines are now being given rapidly around the world, and many people hope to be able to return to normal life with these vaccines, even vaccine recipients are advised to follow the principles of preventing the transmission of the SARS CoV-2, including social distance and wearing a mask. With current evidences, all provided vaccines will help us to conquer this pandemic but developing general vaccines again all corona viruses and changes in study protocols to ease vaccine development in future pandemics are needed.
Declaration of competing interest
None to declare.
Acknowledgment
This study was supported by TUMS with Grant number 67235 and was approved by local ethic committee with registration number IR.TBZMED.REC.1399.1071. We thank all authors who provided their data to enrich this study and we hope this study help termination of Covid-19 pandemic soon.
References
- 1.Ozma M.A., Maroufi P., Khodadadi E., Köse S., Esposito I., Ganbarov K., Dao S., Esposito S., Dal T., Zeinalzadeh E. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez. Med. 2020;28(2):153–165. [PubMed] [Google Scholar]
- 2.Khodadadi E., Maroufi P., Khodadadi E., Esposito I., Ganbarov K., Espsoito S., Yousefi M., Zeinalzadeh E., Kafil H.S. Study of combining virtual screening and antiviral treatments of the Sars-CoV-2 (Covid-19) Microb. Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Najafi K., Maroufi P., Khodadadi E., Zeinalzadeh E., Ganbarov K., Asgharzadeh M., Kafil H.S. SARS-CoV-2 receptor ACE2 and molecular pathway to enter target cells during infection, reviews in medical. Microbiology. 2020 [Google Scholar]
- 4.Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathizadeh H., Taghizadeh S., Safari R., Khiabani S.S., Babak B., Hamzavi F., Ganbarov K., Esposito S., Zeinalzadeh E., Dao S., Köse S., Kafil H.S. Study presence of COVID-19 (SARS-CoV-2) in the sweat of patients infected with Covid-19. Microb. Pathog. 2020;149:104556. doi: 10.1016/j.micpath.2020.104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathizadeh H., Maroufi P., Momen-Heravi M., Dao S., Köse S., Ganbarov K., Espsoito S., Kafil H.S. Protection and disinfection policies against SARS-CoV-2 (COVID-19) Infez. Med. 2020;28(2):185–191. [PubMed] [Google Scholar]
- 8.Gholizadeh P., Safari R., Marofi P., Zeinalzadeh E., Pagliano P., Ganbarov K., Esposito S., Khodadadi E., Yousefi M., Kafil H.S. Alteration of liver biomarkers in patients with SARS-CoV-2 (COVID-19) J. Inflamm. Res. 2020;13 doi: 10.2147/JIR.S257078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spychalski P., Blazynska-Spychalska A., Kobiela J. Estimating case fatality rates of COVID-19. Lancet Infect. Dis. 2020;20(7):774–775. doi: 10.1016/S1473-3099(20)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gholizadeh P., Sanogo M., Oumarou A., Mohamed M.N., Cissoko Y., Sow M.S., Pagliano P., Akouda P., Soufiane S.A., Iknane A.A., Oury M., Diallo S., Köse S., Dao S., Kafil H.S. Fighting COVID-19 in West Africa after experiencing the Ebola epidemic. Health Promot. Perspect. 2021;11(1) doi: 10.34172/hpp.2021.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadian S., Fathizadeh H., Khiabani S.Shabestari, Asgharzadeh M., Kafil H.S. COVID-19 reinfection in a healthcare worker after exposure with high dose of virus: a case report. Clin. Case Rep. 2021;9(6) doi: 10.1002/ccr3. Jun 23. 4257 eCollection 2021 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narenji H., Gholizadeh P., Aghazadeh M., Rezaee M.A., Asgharzadeh M., Kafil H.S. Peptide nucleic acids (PNAs): currently potential bactericidal agents. Biomed. Pharmacother. 2017;93:580–588. doi: 10.1016/j.biopha.2017.06.092. [DOI] [PubMed] [Google Scholar]
- 13.Alihosseini S., Leylabadlo H.E., Parsaei M., Sarafraz N., Ghanbarov K., Esposito S., Kafil H.S. Current drugs with potential for COVID-19 therapy: a literature review. Rev. Med. Microbiol. 2021 [Google Scholar]
- 14.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 15.Jung F., Krieger V., Hufert F., Küpper J.-H. Herd immunity or suppression strategy to combat COVID-19. Clin. Hemorheol. Microcirc. 2020:1–5. doi: 10.3233/CH-209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calina D., Docea A.O., Petrakis D., Egorov A.M., Ishmukhametov A.A., Gabibov A.G., Shtilman M.I., Kostoff R., Carvalho F., Vinceti M. Towards effective COVID-19 vaccines: updates, perspectives and challenges. Int. J. Mol. Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sempowski G.D., Saunders K.O., Acharya P., Wiehe K.J., Haynes B.F. Pandemic preparedness: developing vaccines and therapeutic antibodies for COVID-19. Cell. 2020;181:1458–1463. doi: 10.1016/j.cell.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tse L.V., Meganck R.M., Graham R.L., Baric R.S. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front. Microbiol. 2020;11:658. doi: 10.3389/fmicb.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020;1866(10) doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Deng W., Li S., Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell. Mol. Life Sci. 2021;78(2):531–544. doi: 10.1007/s00018-020-03611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehm M., Nabel E.G. Angiotensin-converting enzyme 2—a new cardiac regulator. N. Engl. J. Med. 2002;347(22):1795–1797. doi: 10.1056/NEJMcibr022472. [DOI] [PubMed] [Google Scholar]
- 24.Fu J., Zhou B., Zhang L., Balaji K.S., Wei C., Liu X., Chen H., Peng J., Fu J. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol. Biol. Rep. 2020;1 doi: 10.1007/s11033-020-05478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamming I., Timens W., Bulthuis M., Lely A., G.v. Navis H.van Goor. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., Cui X., Xiao J., Meng T., Zhou W. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv. 2020 [Google Scholar]
- 28.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020;16(6):305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou X., Zheng W., Shan Y., Mu Z., Dominguez S.R., Holmes K.V., Qian Z. Identification of the fusion peptide-containing region in betacoronavirus spike glycoproteins. J. Virol. 2016;90(12):5586–5600. doi: 10.1128/JVI.00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du L., He Y., Jiang S., Zheng B.-J. Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today. 2008;44(1):63–74. doi: 10.1358/dot.2008.44.1.1131830. [DOI] [PubMed] [Google Scholar]
- 34.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4 T cells are important in control of SARS-CoV infection. J. Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2020;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michen B., Graule T. Isoelectric points of viruses. J. Appl. Microbiol. 2010;109(2):388–397. doi: 10.1111/j.1365-2672.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- 37.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R., Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nucleic Acids Res. 2021;49(D1):D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahid F., Ashfaq U.A., Aslam S., Fatima I., Fareed M.M., Zohaib A., Chen L.-L., Qamar M.T.u. 2020. Structural Modeling and Conserved Epitopes Prediction Against SARS-COV-2 Structural Proteins for Vaccine Development. [Google Scholar]
- 40.Rehman A., Ashfaq U., Awan M., Fatima I., Shahid F., Chen L. 2020. Designing of a Next Generation Multiepitope Based Vaccine (MEV) Against SARS-COV-2: Immunoinformatics and in Silicoapproaches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin A., Chu J., Perera M. Correspondence. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(10) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapil S., Oberst R., Bieker J.M., Tucker M.D., Souza C.A., Williams C.V. Sandia National Laboratories; 2004. Rapid Inactivation of SARS-like Coronaviruses. [Google Scholar]
- 43.Block S.S. Lippincott Williams & Wilkins; 2001. Disinfection, Sterilization, and Preservation. [Google Scholar]
- 44.Delrue I., Verzele D., Madder A., Nauwynck H.J. Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges. Expert Rev. Vaccines. 2012;11(6):695–719. doi: 10.1586/erv.12.38. [DOI] [PubMed] [Google Scholar]
- 45.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barberis I., Myles P., Ault S., Bragazzi N., Martini M. History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J. Prev. Med. Hyg. 2016;57(3):E115. [PMC free article] [PubMed] [Google Scholar]
- 52.Moghaddam A., Olszewska W., Wang B., Tregoning J.S., Helson R., Sattentau Q.J., Openshaw P.J. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006;12(8):905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 53.Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome,SARS-CoV. J. Virol. Methods. 2004;121(1):85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nascimento I., Leite L. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012;45(12):1102–1111. doi: 10.1590/S0100-879X2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A., Meldgaard T.S., Bertholet S. Novel platforms for the development of a universal influenza vaccine. Front. Immunol. 2018;9:600. doi: 10.3389/fimmu.2018.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fulginiti V.A., Eller J.J., Downie A.W., Kempe C.H. Altered reactivity to measles virus: atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202(12):1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- 59.Cao W.-C., Liu W., Zhang P.-H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 60.Jackson D.A., Symons R.H., Berg P. Biochemical method for inserting new genetic information into DNA of simian virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl. Acad. Sci. 1972;69(10):2904–2909. doi: 10.1073/pnas.69.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackett M., Smith G.L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc. Natl. Acad. Sci. 1982;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benihoud K., Yeh P., Perricaudet M. Adenovirus vectors for gene delivery. Curr. Opin. Biotechnol. 1999;10(5):440–447. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 63.Guo X., Deng Y., Chen H., Lan J., Wang W., Zou X., Hung T., Lu Z., Tan W. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015;145(4):476–484. doi: 10.1111/imm.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ura T., Yoshida A., Xin K.Q., Yoshizaki S., Yashima S., Abe S., Mizuguchi H., Okuda K. Designed recombinant adenovirus type 5 vector induced envelope-specific CD8 cytotoxic T lymphocytes and cross-reactive neutralizing antibodies against human immunodeficiency virus type 1. J. Gene Med. 2009;11(2):139–149. doi: 10.1002/jgm.1277. [DOI] [PubMed] [Google Scholar]
- 65.Abe S., Okuda K., Ura T., Kondo A., Yoshida A., Yoshizaki S., Mizuguchi H., Klinman D., Shimada M. Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5. J. Gene Med. 2009;11(7):570–579. doi: 10.1002/jgm.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang Z., Gao G., Reyes-Sandoval A., Cohen C.J., Li Y., Bergelson J.M., Wilson J.M., Ertl H.C. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 2002;76(6):2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekaly R.-P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2008;205(1):7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geisbert T.W., Bailey M., Hensley L., Asiedu C., Geisbert J., Stanley D., Honko A., Johnson J., Mulangu S., Pau M.G. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J. Virol. 2011;85(9):4222–4233. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dicks M.D., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C., Hill A.V., Cottingham M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PloS one. 2012;7(7) doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duerr G., Heine A., Hamiko M., Zimmer S., Luetkens J., Nattermann J., Rieke G., Isaak A., Jehle J., Held S. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batty C.J., Heise M.T., Bachelder E.M., Ainslie K.M. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Adv. Drug Deliv. Rev. 2020;169:168–189. doi: 10.1016/j.addr.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 75.Lee L.Y.Y., Izzard L., Hurt A.C. A review of DNA vaccines against influenza. Front. Immunol. 2018;9:1568. doi: 10.3389/fimmu.2018.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L., Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines. 2016;15(3):313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupuis M., Denis-Mize K., Woo C., Goldbeck C., Selby M.J., Chen M., Otten G.R., Ulmer J.B., Donnelly J.J., Ott G. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 2000;165(5):2850–2858. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 78.Kim J.H., Jacob J. DNA vaccines against influenza viruses. Vaccines Pandemic Influenza. 2009:197–210. doi: 10.1007/978-3-540-92165-3_10. [DOI] [PubMed] [Google Scholar]
- 79.Smith T.R., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seo Y.B., Suh Y.S., Ryu J.I., Jang H., Oh H., Koo B.-S., Seo S.-H., Hong J.J., Song M., Kim S.-J. Soluble spike DNA vaccine provides long-term protective immunity against SARS-CoV-2 in mice and nonhuman primates. Vaccines. 2021;9(4):307. doi: 10.3390/vaccines9040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Presnyak V., Alhusaini N., Chen Y.-H., Martin S., Morris N., Kline N., Olson S., Weinberg D., Baker K.E., Graveley B.R. Codon optimality is a major determinant of mRNA stability. Cell. 2015;160(6):1111–1124. doi: 10.1016/j.cell.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furuichi Y., LaFiandra A., Shatkin A.J. 5'-terminal structure and mRNA stability. Nature. 1977;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- 86.Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R.E. Synthesis and properties of mRNAs containing the novel "anti-reverse" cap analogs 7-methyl (3'-O-methyl) GpppG and 7-methyl (3'-deoxy) GpppG. RNA. 2001;7(10):1486–1495. [PMC free article] [PubMed] [Google Scholar]
- 87.Schlake T., Thess A., Fotin-Mleczek M., Kallen K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.An D., Frassetto A., Jacquinet E., Eybye M., Milano J., DeAntonis C., Nguyen V., Laureano R., Milton J., Sabnis S. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine. 2019;45:519–528. doi: 10.1016/j.ebiom.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Espeseth A.S., Cejas P.J., Citron M.P., Wang D., DiStefano D.J., Callahan C., O’Donnell G., Galli J.D., Swoyer R., Touch S. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines. 2020;5(1):1–14. doi: 10.1038/s41541-020-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldman R.A., Fuhr R., Smolenov I., Ribeiro A.M., Panther L., Watson M., Senn J.J., Smith M., Almarsson ?., Pujar H.S. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37(25):3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 91.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 93.Walsh E.E., Frenck R., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R. Medrxiv; 2020. RNA-based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020;383(16):1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silveira M.M., Moreira G.M.S.G., Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2020;118919 doi: 10.1016/j.lfs.2020.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T., Tao X., Tasneem S., Lu L., Tseng C.-T.K. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell. Mol. Immunol. 2016;13(2):180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adney D.R., Wang L., Van Doremalen N., Shi W., Zhang Y., Kong W.-P., Miller M.R., Bushmaker T., Scott D., de Wit E. Efficacy of an adjuvanted Middle East respiratory syndrome coronavirus spike protein vaccine in dromedary camels and alpacas. Viruses. 2019;11(3):212. doi: 10.3390/v11030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiaming L., Yanfeng Y., Yao D., Yawei H., Linlin B., Baoying H., Jinghua Y., Gao G.F., Chuan Q., Wenjie T. The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Vaccine. 2017;35(1):10–18. doi: 10.1016/j.vaccine.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lan J., Deng Y., Chen H., Lu G., Wang W., Guo X., Lu Z., Gao G.F., Tan W. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bisht H., Roberts A., Vogel L., Subbarao K., Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334(2):160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu M.C., Jones T., Kenney R.T., Barnard D.L., Burt D.S., Lowell G.H. Intranasal protollin-formulated recombinant SARS S-protein elicits respiratory and serum neutralizing antibodies and protection in mice. Vaccine. 2007;25(34):6334–6340. doi: 10.1016/j.vaccine.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.WHO https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 104.Magnusson S.E., Altenburg A.F., Bengtsson K.L., Bosman F., de Vries R.D., Rimmelzwaan G.F., Stertman L. Matrix-M™ adjuvant enhances immunogenicity of both protein-and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol. Res. 2018;66(2):224–233. doi: 10.1007/s12026-018-8991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Korber B., Fischer W., Gnanakaran S.G., Yoon H., Theiler J., Abfalterer W., Foley B., Giorgi E.E., Bhattacharya T., Parker M.D. BioRxiv; 2020. Spike Mutation Pipeline Reveals the Emergence of a More Transmissible Form of SARS-CoV-2. [Google Scholar]
- 106.Makhoul M., Ayoub H.H., Chemaitelly H., Seedat S., Mumtaz G.R., Al-Omari S., Abu-Raddad L.J. Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. Vaccines. 2020;8(4):668. doi: 10.3390/vaccines8040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malik Y.S., Kumar N., Sircar S., Kaushik R., Bhat S., Dhama K., Gupta P., Goyal K., Singh M.P., Ghoshal U. Coronavirus disease pandemic (COVID-19): challenges and a global perspective. Pathogens. 2020;9(7):519. doi: 10.3390/pathogens9070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Asadi Faezi N., Gholizadeh P., Sanogo M., Oumarou A., Mohamed M.N., Cissoko Y., Saliou Sow M., Keita B.S., Baye Y.A.M., Pagliano P., Akouda P., Soufiane S., Iknane A.A., Safiatou Diallo M.O., Gansane Z., Ali Khan B., Köse S., Allahverdipour H., Ganvarov K., Soumaré M., Asgharzadeh M., Dao S., Samadi Kafil H. Peoples' attitude toward COVID-19 vaccine, acceptance, and social trust among african and Middle East countries. Health Promot. Perspect. 2021;11(2):171–178. doi: 10.34172/hpp.2021.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gothelf A., Gehl J. What you always needed to know about electroporation based DNA vaccines. Hum. Vaccines Immunother. 2012;8(11):1694–1702. doi: 10.4161/hv.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharpe H.R., Gilbride C., Allen E., Belij-Rammerstorfer S., Bissett C., Ewer K., Lambe T. The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world. Immunology. 2020;160(3):223–232. doi: 10.1111/imm.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morris K.V. The improbability of the rapid development of a vaccine for SARS-CoV-2. Mol. Ther. 2020;28(7):1548–1549. doi: 10.1016/j.ymthe.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quinlan B.D., Mou H., Zhang L., Guo Y., He W., Ojha A., Parcells M.S., Luo G., Li W., Zhong G. 2020. The SARS-CoV-2 Receptor-binding Domain Elicits a Potent Neutralizing Response Without Antibody-dependent Enhancement. [Google Scholar]
- 116.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5(10):1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 118.Zenuz A.T., Eslami H., Kafil H.S., Safari E M.A. The application of antimicrobial photodynamic therapy on Pseudomonas aeuroginosa and Enterococcus fecalis using hypericin and methylene blue photosensitizers. Biomed. Pharmacol. J. 2016;9(2):443–450. [Google Scholar]
- 119.Jiang S. Don't rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020:321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 120.Barach P., Fisher S.D., Adams M.J., Burstein G.R., Brophy P.D., Kuo D.Z., Lipshultz S.E. Disruption of healthcare: will the COVID pandemic worsen non-COVID outcomes and disease outbreaks? Prog. Pediatr. Cardiol. 2020;59:101254. doi: 10.1016/j.ppedcard.2020.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chohan U.W. 2021. Coronavirus & Vaccine Nationalism. [Google Scholar]
- 122.Fidler D.P. American Association for the Advancement of Science; 2020. Vaccine Nationalism's Politics. [Google Scholar]
- 123.Saeed U., Sherdil K., Ashraf U., Younas I., Butt H., Ahmad S. Identification of potential lockdown areas during COVID-19 transmission in Punjab,Pakistan. Public Health. 2021;190:42–51. doi: 10.1016/j.puhe.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salamzadeh A., Dana L.P. The coronavirus (COVID-19) pandemic: challenges among Iranian startups. J. Small Bus. Entrep. 2020:1–24. [Google Scholar]
- 125.Hussain Y., Muhammad K., Umer M.F., Omarkhail A., Khan S., Kamran M., Rashid H., Khan Z. Coronavirus disease 2019 in 5 neighboring limited-resource countries: a financial and health threat. Value. Health Reg. Issues. 2021;24:114–116. doi: 10.1016/j.vhri.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hitchings M., Ranzani O.T., Torres M.S., de Oliveira S.B., Almiron M., Said R., Borg R., Schulz W.L., de Oliveira R.D., da Silva P.V. medRxiv; 2021. Effectiveness of CoronaVac in the Setting of High SARS-CoV-2 P. 1 Variant Transmission in Brazil: A Test-negative Case-control Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanriover M.D., Doganay H.L., Akova M., Güner H.R., Azap A., Akhan S., Köse S., Erdinç F.S., Akalin E.H., Tabak Ö.F. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., Pizarro A., Acevedo J., Leo K., Leon F. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quagliariello A., Aloisio I., Bozzi Cionci N., Luiselli D., D'Auria G., Martinez-Priego L., Perez-Villarroya D., Langerholc T., Primec M., Micetic-Turk D., Di Gioia D. Effect of Bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: a pilot study. Nutrients. 2016;8(10) doi: 10.3390/nu8100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ella R., Reddy S., Jogdand H., Sarangi V., Ganneru B., Prasad S., Das D., Raju D., Praturi U., Sapkal G., Yadav P., Reddy P., Verma S., Singh C., Redkar S.V., Gillurkar C.S., Kushwaha J.S., Mohapatra S., Bhate A., Rai S., Panda S., Abraham P., Gupta N., Ella K., Bhargava B., Vadrevu K.M. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Memar M.Y., Varshochi M., Shokouhi B., Asgharzadeh M., Kafil H.S. Procalcitonin: the marker of pediatric bacterial infection. Biomed. Pharmacother. 2017;96:936–943. doi: 10.1016/j.biopha.2017.11.149. [DOI] [PubMed] [Google Scholar]
- 132.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., Wu S.-P., Wang B.-S., Wang Z., Wang L. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil,South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Emary K.R., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., Blane B., Bonsall D., Cicconi P., Charlton S. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B. 1.1. 7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Tessier E., Groves N., Dabrera G., Myers R. medRxiv; 2021. Effectiveness of COVID-19 Vaccines Against the B. 1.617. 2 Variant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I. Interim results of a phase 1–2a trial of Ad26. COV2. S Covid-19 vaccine. N. Engl. J. Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oliver S.E., Gargano J.W., Scobie H., Wallace M., Hadler S.C., Leung J., Blain A.E., McClung N., Campos-Outcalt D., Morgan R.L. The advisory committee on immunization practices’ interim recommendation for use of janssen COVID-19 Vaccine—United States, february 2021. Morb. Mortal. Wkly Rep. 2021;70(9):329. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keijser B.J., Zaura E., Huse S.M., van der Vossen J.M., Schuren F.H., Montijn R.C., ten Cate J.M., Crielaard W. Pyrosequencing analysis of the oral microflora of healthy adults. J. Dent. Res. 2008;87(11):1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 143.Dey A., Rajanathan C., Chandra H., Pericherla H.P., Kumar S., Choonia H.S., Bajpai M., Singh A.K., Sinha A., Saini G. bioRxiv; 2021. Immunogenic Potential of DNA Vaccine Candidate, ZyCoV-D Against SARS-CoV-2 in Animal Models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fan X., Alekseyenko A.V., Wu J., Peters B.A., Jacobs E.J., Gapstur S.M., Purdue M.P., Abnet C.C., Stolzenberg-Solomon R., Miller G., Ravel J., Hayes R.B., Ahn J. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67(1):120–127. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E., Caban-Martinez A.J., Fowlkes A., Lutrick K. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb. Mortal. Wkly Rep. 2021;70(13):495. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frenck R.W., Jr., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., Walter E.B., Senders S., Bailey R. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N. Engl. J. Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Castillo G.D.V., Blanc S.L., Sotomayor C.E., Azcurra A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018;91:35–41. doi: 10.1016/j.archoralbio.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 150.Giuliani M., Troiano G., Cordaro M., Corsalini M., Gioco G., Lo Muzio L., Pignatelli P., Lajolo C. Rate of malignant transformation of oral Lichen planus: a systematic review. Oral Dis. 2018;8(10):12885. doi: 10.1111/odi.12885. [DOI] [PubMed] [Google Scholar]
- 151.Mehrabani M.G., Karimian R., Rakhshaei R., Pakdel F., Eslami H., Fakhrzadeh V., Rahimi M., Salehi R., Kafil H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018;116:966–976. doi: 10.1016/j.ijbiomac.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 152.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., Chadwick D.R., Clark R., Cosgrove C., Galloway J. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ward B.J., Gobeil P., Seguin A., Atkins J., Boulay I., Charbonneau P.-Y., Couture M., D'Aoust M.-A., Dhaliwall J., Finkle C. medRxiv; 2020. Phase 1 Trial of a Candidate Recombinant Virus-like Particle Vaccine for Covid-19 Disease Produced in Plants. [Google Scholar]