Abstract
The pandemic of the 2019 novel coronavirus disease (COVID-19) has brought viruses into the public horizon. Since viruses can pose a threat to human health in a low concentration range, seeking efficient virus removal methods has been the research hotspots in the past few years. Herein, a total of 1060 research papers were collected from the Web of Science database to identify technological trends as well as the research status. Based on the analysis results, this review elaborates on the state-of-the-art of membrane filtration and disinfection technologies for the treatment of virus-containing wastewater and drinking water. The results evince that membrane and disinfection methods achieve a broad range of virus removal efficiency (0.5–7 log reduction values (LRVs) and 0.09–8 LRVs, respectively) that is attributable to the various interactions between membranes or disinfectants and viruses having different susceptibility in viral capsid protein and nucleic acid. Moreover, this review discusses the related challenges and potential of membrane and disinfection technologies for customized virus removal in order to prevent the dissemination of the waterborne diseases.
Keywords: Virus removal, Drinking water treatment, Wastewater treatment, Membrane, Disinfection
Graphical abstract
Abbreviations of viruses
- AdV
Adenovirus
- HAdV
Human adenovirus
- AiV
Aichi virus
- AsV
Astrovirus
- CV
Coxsackievirus
- CVB
coxsackievirus B
- EV
Enterovirus
- ECHO
Echovirus
- HA(E)V
Hepatitis A(E) virus
- NoV
Norovirus
- hNoV
Human norovirus
- MNV
Murine norovirus
- NV GI/II/IV
Norovirus genotype (I/II/IV)
- PMMoV
Pepper mild mottle virus
- PyV
Polyomavirus
- JC/BK PyV
JC/BK polyomavirus
- RV
Rotavirus
- HRV
Human rotavirus
- SaV
Sapovirus
1. Introduction
Global outbreaks of infectious diseases induced by viruses are escalating and seriously threatening human health. Viruses are noncellular biological entities with a simple structure composed of proteins and nucleic acids. Compared with bacteria and fungi, viruses have the characteristics of a small size, special structure, wide distribution, low infectious dose and strong pathogenicity (Xiao et al., 2013). It is well-documented that the global number of phages is estimated to be 4.80 × 1031 (Guemes et al., 2016), which is much higher than the number of organisms with a cellular structure.
According to epidemiological studies, human and animal excreta often contain numerous virus particles, which can enter the water environment via sewage discharge, septic-tank system filtrate, and runoff from agricultural areas (Rzezutka and Cook, 2004). For instance, human enteroviruses can be excreted at high concentrations (1011 viruses/g-feces) from infected individuals and disseminated through the fecal-oral route (Haramoto et al., 2018). It is estimated that 2 to 12 million people die from waterborne diseases every year (US EPA, 2006a), and waterborne diseases can pose a substantial threat to the world public health security. The novel coronavirus (SARS-CoV-2) causing symptoms called COVID-19, causes respiratory infection, thus the major route of transmission is believed to be the inhalation of the virus. Still, the SARS-CoV-2 is also typified by fecal-oral transmission (Arslan et al., 2020), with important pollution sources from urban sewage and sewer overflow (Zhu et al., 2020). Wastewater treatment plants (WWTPs) with conventional procedures such as conventional activated sludge process serving as a significant barrier can essentially impede the spread of waterborne viruses to a certain degree (Sano et al., 2016), but more enhanced methods should be conducted to fulfill more rigorous standards.
At present, there is extensive research on pathogenic bacteria in wastewater treatment processes but relatively fewer studies on viruses. The occurrence, states of existence and decay of viruses are distinct from those of pathogenic bacteria. Hence, in this paper, we analyzed the research hotspots and development about virus removal in virus-containing wastewater/drinking water treatment in recent years. Subsequently, the membrane and disinfection technologies were reviewed along with a comprehensive evaluation and comparative analysis. Finally, we proposed some detailed conclusions regarding the removal effects and mechanisms of several common viruses by membrane and disinfection processes, along with the related challenges and comprehensive perspectives, to provide guidance for the further efficient elimination of waterborne viruses in future practical applications.
2. Research development of virus removal in water bodies
2.1. Overview of virus removal in water from Web of Science
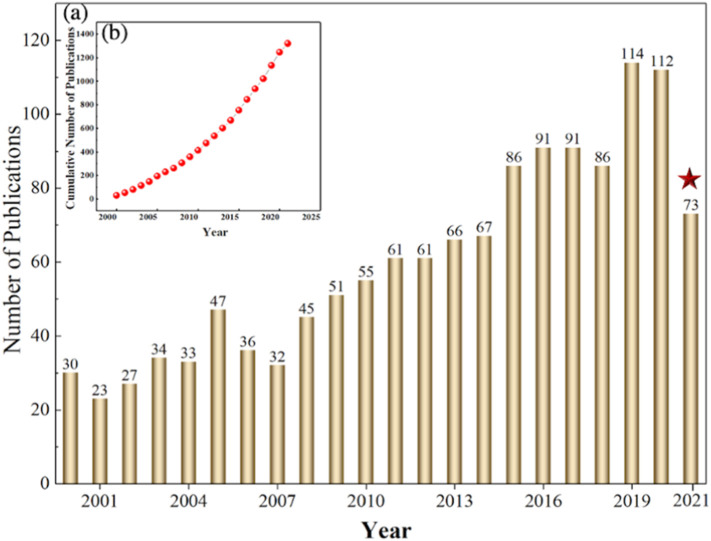
To track the latest research progresses and hotspots, publications about virus removal in water were analyzed. A total of 1321 results were selected between 1st of Jan. 2000 and 16th of July 2021 from the core collection in web of science database with the topic “virus”, and the search terms were designed as (TS = (virus removal AND “water”)). Fig. 1(b) portrays that the field has received increasing attention over the past two decades, as the number of scientific articles has nearly tripled from 2007 to 2016 (Fig. 1(a)).
Fig. 1.
(a) Annual distribution and (b) cumulative quantities of the studies on virus removal from water from the Web of Science. (All the documents were collected until July 16, 2021).
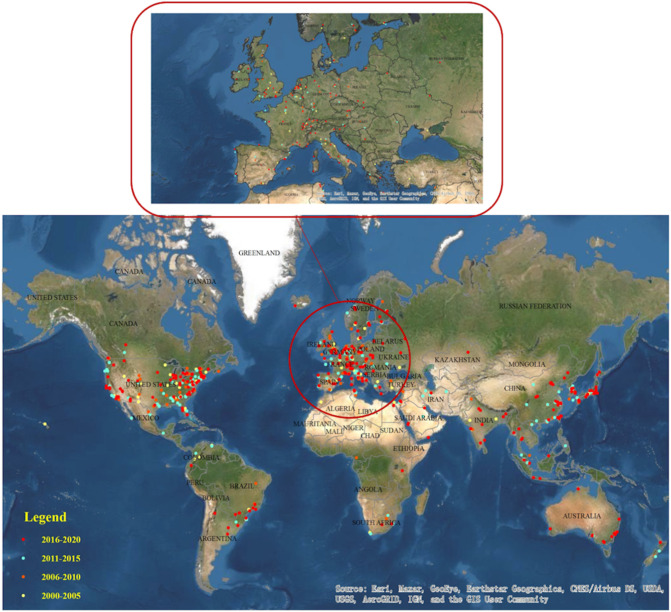
The global geographic distribution map of research production is visualized using ArcGis (as shown in Fig. 2 ) to identify the distribution of countries and institutions performing virus removal research in water, and the specific publication number in some countries as a function of year is displayed in Fig. S1. The research units are mainly distributed in North America (the United States and Canada), Europe (the Netherlands, France, Spain, etc.) and Asia (China, Japan, Korea, etc.). Typically, the USA has played a leading role during this period, generating 29.8% of the total publication number, followed by China and Japan, accounting for 7.2% and 6.02%, respectively, and more detailed data are shown in Table S1. In general, this field has been appealing to increasing attention.
Fig. 2.
Global geographic distribution of research on virus removal from water (TS = (virus removal AND water)). The dots indicate the location of these 1060 literature research institutions, which are mapped by ArcGis according to the longitude and latitude of each research institution. The attribution is Source: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community.
2.2. Categories and elimination of viruses in water treatment
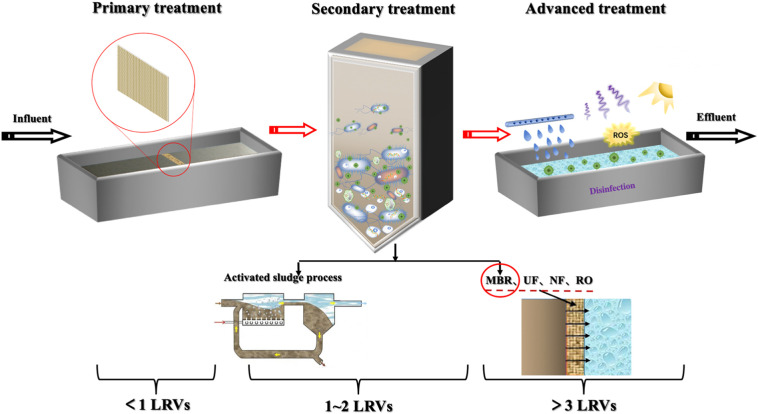
Viruses are tiny (10–100 nm) noncellular particles with nucleic acids (DNA and RNA) wrapped in a protein shell (Hata et al., 2011). Compared with other pathogens (i.e., bacteria and protozoa), viruses survive in the host for its lifetime in their natural state and retain their infectivity, making them difficult to be eliminated (Brooks et al., 2020). For example, AdVs, which have double-stranded DNA and are the abundant human viruses in WWTPs (Hata et al., 2013), are resistant to UV disinfection. Additionally, EVs, RVs, HAV and NoVs, etc., can survive in natural water with strong infectivity for more than one month (Brooks et al., 2020; WHO, 2011). Wastewater treatment is one of effective approaches to remove viruses, and the systematic diagram of virus control in water treatment is exhibited in Fig. 3 . In the influent of WWTPs, most viruses are negatively charged because their isoelectric point (IEP) is lower than the pH of the influent (Michen and Graule, 2010), and the seasonal differences can also indirectly affect the virus distribution and their treatment effects (Pang et al., 2019; Nordgren et al., 2009), notably, viruses in untreated wastewater will contaminate surface water in periods of heavy rain and melting snow. Viruses usually exist in two states: one is exposed to various physical, chemical, and biological environments and the other is parasitic in host cells and coexists with microorganisms (Templeton et al., 2008). Some common viruses in water bodies and their elimination in WWTPs are tabulated in Table 1 . Virus removal efficiency is usually expressed as the log reduction values (LRVs).
Fig. 3.
Schematic diagram and relative removal efficiency of the system containing primary treatment, secondary treatment and advanced treatment used for virus control in wastewater treatment.
Table 1.
Categories and the corresponding diseases caused by waterborne viruses as well as quantitative virus reduction in WWTPs.
| Viruses | Genome | Dimension (nm) | Major diseases | Influent | Effluent | Virus reduction (log10) | Technologies | Detection methods | References |
|---|---|---|---|---|---|---|---|---|---|
| Enteroviruses | ssRNA | 20–100 (Guo and Hu, 2011) | Minor febrile illness, gastroenteritis, aseptic meningitis, paralysis, myocarditis (Iaconelli et al., 2017) | 3.3 × 107 GC/mL | 7.6 × 106 GC/mL | 0.63 | Italy; grid separation, primary sedimentation, secondary bio-logical treatment and disinfection | RT-PCR, Real-time qPCR | (Rosa et al., 2010) |
| Coxsackieviruses | 3.24 × 105 copies/L | 1.54 × 103 copies/L | 2.32 | Arizona, United States; activated sludge and trickling filter | RT-PCR | (Kitajima et al., 2015) | |||
| Astroviruses | ssRNA | 25–35 (Jacukowicz and Domanska-Blicharz, 2017) | Gastroenteritis (Vu et al., 2019) | NG | 2.69 × 103 copies/L | – | France; primary decantation and biological secondary treatment From May 2013 to May 2014 | RT-qPCR | (Prevost et al., 2015) |
| Pepper mild mottle virus | ssRNA | – | Infections to solanaceous plants, mottled or yellow and green floral leaves on plants, malformation or bump spots on fruits | 3.7–4.4 × 106/3.2–9.4 × 106 copies/L | 4.6–6.3 × 105/copies/L | 0.76–0.99/1.8 ± 0.2 | Southern Arizona, USA; Plant A (conventional activated sludge process); Plant B (biological trickling filter process) | TaqMan-based qPCR | (Kitajima et al., 2014) |
| Norovirus genotypes GI/GII | ssRNA | 25–40 (Cheetham et al., 2006) | Acute gastroenteritis (evacuation, vomiting, fever, abdominal pain) (Teixeira et al., 2016) | 8.8 × 104 GC/L | 3 × 104 GC/L | 0.47 | North Wales, UK; WWTP with filter beds for secondary treatment and serves ca. 4000 inhabitants | RT-qPCR | (Farkas et al., 2018) |
| Sapoviruses | ssRNA | 25–40 (Cheetham et al., 2006) | 7.8 × 106 GC/L | NG | – | New Caledonia; sample collected from April 2012 to March 2013 | RT-qPCR | (Kaas et al., 2016) | |
| Rotaviruses | dsRNA | 55 (double-capsid) 70 (single-capsid) (Saif et al., 1980) |
Gastroenteritis, diarrhea (especially for young children) (Banyai et al., 2018) | 1.2 × 105 GC/L | 2.6 × 104 GC/L | 0.66 | Eastern Cape, South Africa; activated sludge system with 40,000 m3/day flow rate | Quantitative TaqMan real-time PCR | (Osuolale and Okoh, 2017) |
| Adenoviruses | dsDNA | 75–90 (Needle et al., 2019; San Martin and Burnett, 2003) | Respiratory disease, gastroenteritis, pneumonia, urinary disease, conjunctivitis, hepatitis, myocarditis, encephalitis (Iaconelli et al., 2017) | 4.3 × 105–8.7 × 106 GC/mL | 1.22 × 104–3.7 × 106 GC/mL | – | Egypt; 330,000 m3/day capacity | Real time PCR | (Elmahdy et al., 2019) |
| Aichi viruses | ssRNA | 30 (Burutaran et al., 2015) | Acute gastroenteritis | 9.7 × 104/2.0 × 106 copies/L | 1.1 × 104/2.0 × 105 copies/L | 0.94–0.99 | Southern Arizona, USA; conventional activated sludge process/biological trickling filter process | TaqMan-based qPCR | (Cheetham et al., 2006) |
| Hepatitis A virus | ssRNA | 27–30 (Feinstone et al., 1973) | Sporadic hepatitis (Iaconelli et al., 2017) | 2.01 × 103–8.39 × 103 copies/L | 1.93 × 103–8.70 × 103 copies/L | – | Kampala, Uganda; conventional activated sludge method, in summer 2016 | qPCR and quantitative RT-PCR | (O'Brien et al., 2017) |
| Polyomaviruses | dsDNA | 40 (Wen et al., 2004) | Malignancies, cancer (skin, prostate, colorectal) (Ugo, 2018) | 3.9 × 105 GC/L | 4.51 × 103 GC/L | 1.93 | Greater Cairo, Egypt; activated sludge as secondary treatment process with 600,000 m3/day | Real time PCR | (Hamza and Hamza, 2018) |
| SARS-CoV | ssRNA | 80–120 | Respiratory disease, lung/liver/kidney injury, multiorgan dysfunction, shock, metabolic acidosis (Li et al., 2020) | NG | 2.4 × 103 copies/L | – | Japan; Conventional activated sludge process | RT-qPCR | (Haramoto et al., 2020) |
Notes for abbreviations: NG: not given, GC/L: genome copies/L, ssRNA: single-stranded RNA, ds RNA: double-stranded RNA, ssDNA: single-stranded DNA, dsDNA: double-stranded DNA, qPCR: quantitative polymerase chain reaction, RT-(q)PCR: reverse transcriptase-(quantitative) polymerase chain reaction.
From Table 1, we can observe that the removal efficiency of WWTPs for viruses exists discrepancies, and the overall removal (0.47–2.32 LRVs) is still incomplete when deploying conventional activated sludge or trickling filter as the treatment approach. Therefore, technical improvement of these processes or the introduction of some advanced treatment processes may be the effective measures to ensure the effluent quality in WWTPs as well as the safety of water quality, especially during the period of epidemic outbreak.
2.3. Keyword analysis of virus removal from water
Keyword analysis plays an important role in clarification of current hot topics in virus removal. The co-occurrence of the collected keywords in the integrated cluster view was analyzed using CiteSpace 5.6.R5 from the 1060 research articles (from 2000 to 2020) listed in Web of Science. After executing threshold configuration, the term in the Node Types function panel was selected, and Top 1.0% of most cited or occurred items from each slice as well as 100 of the maximum number of selected items per slice were designed. CiteSpace 5.6.R5's automatic clustering function was then utilized to picture cluster and timeline view figures after screening to remove duplicates. Fig. 4 depicts a co-occurring analysis of keywords and the trend of the evolution of keywords recommended by authors is illustrated in Fig. S2. For virus removal technologies and evaluation, the cluster titles are “microfiltration” and “quantitative microbial risk assessment”, manifesting that membrane filtration and the prevention of waterborne diseases have attracted more attention recently. Moreover, the title “inactivation” denotes that disinfection technology is a good choice for virus removal. According to the size of the nodes and the number of interleaving points, the keywords “virus removal”, “drinking water”, “wastewater treatment”, “membrane filtration”, “removal efficiency” and “disinfection” appear more frequently than others, with numbers of 171, 102, 73, 59, 44 and 39, respectively, and the relevant values are shown in Table S2. Additionally, the timeline shown in Fig. S2 embodies that research has been covering an increasing number of keywords over time and mainly rest with membrane filtration and disinfection technology, therefore, we picked these two methods as the review highlights.
Fig. 4.
Cluster view of co-occurring analysis of keywords from the scientific literature on virus removal from water with the minimized overlap. The schematic representation of the keyword timeline and its corresponding elaboration are provided in Fig. S2 (the time threshold is set from 2000 to 2020 on CiteSpace 5.6.R5).
3. Membrane and disinfection technologies for virus elimination
3.1. MBR process for virus removal
Secondary treatment covers microbial metabolism, dissolution of organic matter, and precipitation and separation of biological metabolites. Virus removal in the conventional activated sludge (CAS) process is at 0.3–3.1 LRVs (Taboada-Santos et al., 2020), which is 1 LRVs more removal than in primary treatment. Membrane bioreactors (MBRs), which integrate CAS with membrane filtration, have a relatively high virus removal capacity, reaching 1.4 to 6.8 LRVs (Simmons and Xagoraraki, 2011; Sano et al., 2016) so that they are widely used as an advanced treatment technology.
In general, the removal efficacy of the MBR mainly relies on the following four mechanisms (Chaudhry et al., 2015b; Hai et al., 2014): i) the adsorption of viruses to sludge particles. Viruses existing as aggregates can attach to sludge particles which consist of many bacteria and organic compounds that are larger than the pore size of membranes, rending smaller probability of viruses' passing; ii) membrane interception. During membrane separation process, viruses that are larger than membrane pores or have the same electric charge as membrane can be validly intercepted; iii) adsorption and retention of the cake layer and gel layer on the membrane surface. Membrane fouling, including reversible and irreversible fouling, plays an important role in virus removal (Marti et al., 2011). The fouling caused by activated sludge on the membrane surface forms a dynamic filter layer composed of a cake layer and a gel layer that changes the separation performance of the membrane (Marti et al., 2011); iv) decay and inactivation. Proteases in enzymatic catalysis will break the viral capsid protein to inhibit viability of viruses. Besides, the virus decay by the predation by other microorganisms will also increase the virus removal. Normally, the membrane interception together with the adsorption and retention caused by the cake/gel layer accounts for over 50% of the total removal when the 0.04 μm nominal pore size of membrane module was used in MBR, otherwise the virus removal was mainly caused by cake layer and biomass considering the membrane module is 0.4 μm nominal pore size (Chaudhry et al., 2015b; Shang et al., 2005; Wu et al., 2010). The scheme of the MBR removal mechanisms is revealed in Fig. S3.
Membrane modules of MBRs with different pore sizes show no obvious variation in retention of the same virus (Hirani et al., 2010), while the removal efficiency of MBR systems varies for different viruses (Purnell et al., 2016). Presumably, besides mechanical sieving action, the biofilm and sludge particles attached to the membrane surface also play a certain role in virus removal in MBRs (O'Brien and Xagoraraki, 2020). These mechanisms are meanwhile influenced by the surface properties of viruses, such as the electrostatic charge and hydrophobicity of the viral capsid protein (Armanious et al., 2016). Miura et al. (2015) evaluated the removal performance of a MBR system and found that the contents of NV GII and parvovirus in solid phase were equal to or higher than in liquid phase, while EVs could not be detected in the solid phase, indicating that the adhesion between EVs and sludge was not strong. A similar conclusion was also drawn in the research of Sima et al. (2011), they discovered that NoVs were more efficiently removed than SaVs in full- and pilot-scale MBR plants, although they have a similar structure, morphology and size.
In addition, water quality parameters, including pH, temperature, suspended solids concentration, particle size distribution, dissolved organic concentration, viscosity, etc., can impact viruses' behavior is decided by viral protein (such as hydrophobic and charged regions) in aqueous medium (Prado et al., 2019a). Operational changes in MBRs have affected virus elimination owing to changing the composition of microbial community and membrane surfaces. For example, a longer hydraulic retention time (HRT) can enhance the adsorption of viruses by sludge particles, thereby improving the virus removal efficiency (Prado et al., 2019a). High sludge retention times (SRTs) can endow MBR facilities with high mixed liquor suspended solids (MLSS) concentration and abundant microbial community to produce nitrified effluents so that increasing removal performance (Hirani et al., 2014). The addition of powdered activated carbon or polymer flocculant has been verified to effectively alleviate the blockage of MBR membrane pores and improve the removal of bacteria, antibiotic resistance genes and viruses (Ravindran et al., 2009; Nnadozie et al., 2017). Besides, Delanka-Pedige et al. (2020) proposed a low-energy algae wastewater treatment system to substitute for MBRs that can be translated to lower disinfection by-products (DBPs) formation while reducing pathogens via virus inactivation by algae. Therefore, it is more reasonable to optimize the design of MBR for further applications.
The pore size of membrane module used in MBRs normally ranges from 0.1 to 0.4 μm which is far beyond the size of most viruses (usually at nanometer), so the virus removal presented in MBRs predominantly rests on adsorption and aggregation processes by biofilm and cake layer instead of size exclusion. Herein, it would be prudent to elucidate the law of virus diffusion and adsorption and explore the interaction between viruses and different membrane foulant layers (Zhu et al., 2021).
3.2. Virus separation by membrane technology
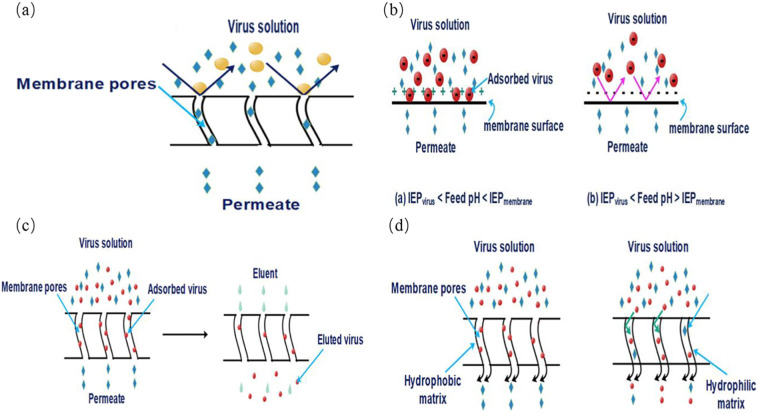
Membrane technology, including mainly microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO), is often used as an advanced treatment. The membrane virus retention mechanisms (illuminated pictorially in Fig. 5 ) are usually divided into four types (Duek et al., 2012; Gentile et al., 2018; Goswami and Pugazhenthi, 2020): mechanical screening, electrostatic interactions, adsorption retention, and hydrophilic and hydrophobic interactions. Mechanical sieving, i.e., size exclusion, basically occurs on the membrane surface; electrostatic interactions are associated with the charge of viruses and the membrane; adsorption retention as well as hydrophilic-hydrophobic interactions are ascribed to physicochemical properties of the viruses and membranes themselves, which allow viruses to penetrate the membrane surface to be deposited on the membrane internal matrix. The size of most viruses is normally smaller than the pore size of MF membranes but larger than that of NF membranes and RO membranes, in this condition, size exclusion plays a decisive role in virus removal. While the pore size of UF membranes is comparable to virus particle size, the virus removal efficiency depends on both the surface properties of viruses and membrane. Specifically, the removal effect of MF for viruses is approximately 0.3–2.2 LRVs (Qiu et al., 2015). In UF, removal of NoV, AdV and RV is greater than 3 LRVs (Qiu et al., 2015), while NF and RO can normally achieve greater than 5 or 6 LRVs, respectively (Cetlin et al., 2018). Furthermore, the external surface of most viruses is primarily composed of proteins that endow them with physicochemical properties similar to colloids and proteins, so the virus removal mechanisms of the membrane can be elucidated by Derjaguin-Landau-Verwey-Overbeek (DLVO) and extended Derjaguin-Landau-Verwey-Overbeek (XDLVO) theories to some extent (Gentile et al., 2018). For those reasons, several aspects related to the materials, modification, fouling and hybrid processes of the membrane are further explained below.
Fig. 5.
Schematic illustration of virus removal in membrane separation: (a) size exclusion, performing a dominant removal efficiency when the size of virus particles is bigger than the nominal pore size of membranes, (b) electrostatic interactions that is more prone to be affected by the PH of feed water, (c) adsorption and elution, and (d) hydrophilic and hydrophobic interactions that are highly influenced by the properties of membrane material and virus particles (Goswami and Pugazhenthi, 2020).
3.2.1. Membrane modification
Membranes have different removal effects on different viruses. Fig. S4 shows the discrepancy in virus removal between MF and UF. This distinction depends on the pore size of the membrane, size and structure of the virus and electrostatic interactions (Gentile et al., 2018). The pore size of the membrane displays a distribution range. Different membrane modules with similar average pore sizes may have different rejection rates for the same virus, and the more uneven the pore size distribution of the membrane, the lower the retention rate of viruses (Fallahianbijan et al., 2017). Madaeni (1999) used a 0.2 μm MF membrane with a retention efficiency of 2 LRVs for poliovirus. However, Herath et al. (1999) used a hydrophilic membrane with a smaller pore size of 0.05 μm but found only a 0.2 to 0.7 LRVs removal of the bacteriophage Qβ, which is almost same size as poliovirus.
3.2.1.1. Reinforcing interaction
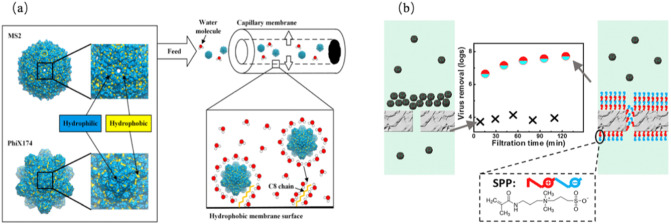
Viruses can easily be adsorbed on the membrane surface or within its pores (Michen and Graule, 2010), and the adsorption behavior varies according to membrane material (Goswami and Pugazhenthi, 2020). Meanwhile, electrostatic and hydrophobic interactions between viruses and membrane surfaces can increase the removal efficiency, which often happens in modified membranes by introducing functionalized materials, such as the functionalization of the yttria-stabilized zirconia capillary membranes with n-hexyltriethoxysilane and n-octyltriethoxysilane, for virus removal based on hydrophobic and electrostatic adsorption (Larson et al., 2018; Bartels et al., 2019) (as shown in Fig. 6(a)). The structure and removal effects of the modified composite membranes used in existing research for virus separation are tabulated in Table 2 . UF membrane grafted with a zwitterionic polymer hydrogel enhances the electrostatic repulsion between viruses and the membrane surface to remarkably facilitate the removal of viruses, which can increase 4 LRV in HAdV and 3 LRV in MS2 higher removal than the unmodified membrane (Lu et al., 2017) (as portrayed in Fig. 6(b)).
Fig. 6.
Schematic diagram pertaining to modified membranes. (a) Hydrophobic yttria-stabilized zirconia capillary membranes are hydrophobized with n-hexyltriethoxysilane and n-octyltriethoxysilane, which can be beneficially utilized for virus retention as a result of hydrophobic interaction (Bartels et al., 2019); (b) graft-polymerization functionalization of 150 kDa ultrafiltration polyether-sulfone membrane with zwitterionic ([3-(methacryloylamino) propyl] dimethyl (3-sulfopropyl) ammonium hydroxide), achieving a higher virus removal on account of the weakened virus accumulation upon the modified membrane surface (Lu et al., 2017).
Table 2.
Separation of viruses from wastewater using a polymer-modified membrane.
| Membrane specification/charge (pH = 7.4) | Average pore size | Viruses | Operating conditions | Removal efficiency (LRVs) | References |
|---|---|---|---|---|---|
| Microfiltration/0.1–1 μm | |||||
| Polyethersulfone membrane coated with PEI/positive | 0.45 μm | Bacteriophage MS2 | 0.2 bars (4 ± 0.9) × 109 PFU/mL |
>3 | Sinclair et al., 2018 |
| TiO2 tubular ceramic microfilters | 0.8 μm | Bacteriophage P22 | 5 × 109 PFU/mL | 5 | Guo et al., 2015 |
| Nano-composite electrospun nanofiber membrane (PAN-ATTM, PAN-TEOS) | 0.8 μm | Semliki Forest virus | 106 PFU/mL | 1.96 | Al-Attabi et al., 2019 |
| Chitosan membranes modified by pyromellitic dianhydride | – | Bacteriophage MS2 | 109 PFU/mL | 3 | Majiya et al., 2019 |
| PAN/PET-cellulose nanofibers/positive | 0.73 μm | Bacteriophage MS2 | 106 PFU/mL | 4 | Wang et al., 2013 |
| Microporous ceramics with ZrO2 and Y2O3 coatings/positive | 0.2–2 μm | Bacteriophage MS2 | 3 bars 107 PFU/mL |
4, 7 |
Wegmann et al., 2008,Wegmann et al., 2009 |
| SiO2-Y2O3 composite nanofiber membrane/positive | 0.1 μm | Bacteriophage MS2 | – | 4 | Liu et al., 2019 |
| PEI-TA-PES membrane (LBL); PEI-Ag/CuNPs-PES membrane | 0.45 μm | Bacteriophage MS2 | 4 × 108 PFU/mL | 4.5–5 | Sinclair et al., 2019 |
| Coating of the ceramic membrane with HTS and OTS | 0.15 μm | Bacteriophage MS2 Bacteriophage PhiX174 |
2.5 bars 109 PFU/mL |
0.3 ± 0.1 3.4 ± 0.2 |
Bartels et al., 2019 |
| Nano-TiO2-PVDF flat membrane | 0.2 μm | Phage F2 | 1.35 × 107 PFU/mL | 3.88 | Zheng et al., 2013 |
| Coating of fiber filter with MWCNTs-copper hydroxide precipitate | 0.4 μm | Bacteriophage MS2 | 108 PFU/mL pH = 5 |
>5 | Domagala et al., 2020 |
| Spray-dried alumina granules modified with copper (oxide) nanoparticles on ceramic filter | 1–2 μm | Bacteriophage MS2 | 104 PFU/mL | 3.1; 3.2 |
Mazurkow et al., 2020. |
| Ultrafiltration/2–100 nm | |||||
| Polysulfone (capillary) | 20 nm | Bacteriophage MS2 | 106–107 PFU/mL | 2.5–6 | Kreißel et al., 2012 |
| Graft-polymerized zwitterionic SPP on polyethersulfone membrane | 50 nm | Bacteriophage MS2, Human adenovirus | 0.69 bar 2.3–3.0 × 109 PFU/mL |
>6, 6.6–7.8 | Lu et al., 2017 |
| Polysulfone membrane coated with Columnar LC-PET film | 3.5 nm | Bacteriophage Qβ, Bacteriophage MS2, Aichi virus |
0.3 MPa 107–108 PFU/mL |
>6.7, 6.3, 7.6 |
Kuo et al., 2020 |
| Coating of the polysulfone ultrafiltration membrane with nAg/negative | – | Bacteriophage MS2 | 5 ± 0.2 × 105 PFU/mL | 4 | Zodrow et al., 2009 |
| Nano-TiO2-PAN flat membrane | 50 nm | Phage F2 | 1.35 × 107 PFU/mL | 6.4 | Zheng et al., 2013 |
| Nanofiltration/1–2 nm | |||||
| Polysulfone membrane coated with Two-Component Columnar LC-PET film | 1.6 nm | Bacteriophage Qβ | 0.3 MPa 108 PFU/mL |
4.4 ± 0.3 | Kreißel et al., 2012 |
| Polysulfone membrane coated with Columnar LC/PET film | 1.8 nm | Bacteriophage Qβ | 0.3 MPa 108 PFU/mL |
4.7 ± 0.3 | Gupta et al., 2019 |
| Reverse osmosis/<1 nm | |||||
| Polysulfone membrane coated with Cubbi LC | 0.6 nm | Bacteriophage Qβ | 0.8 MPa NaCl 500 mg/L 108 PFU/mL |
>6.3 | Marets et al., 2017 |
Notes: PEI: polyethyleneimine; PAN: polyacrylonitrile; ATTM: ammonium tetrathiomolybdate; TEOS: tetraethyl orthosilicate; PET: poly(ethylene terephthalate); TA: terephthalaldehyde; PES: polyethersulfone; LBL: layer-by-layer; HTS: n-hexyltriethoxysilane; OTS: n-octyltriethoxysilane; PVDF: polyvinylidene fluoride; SPP: ([3-(methacryloylamino) propyl]dimethyl (3-sulfopropyl) ammonium hydroxide); LC: liquid-crystalline; Cubbi: bicontinuous cubic. MWCNTs: multi-walled carbon nanotubes.
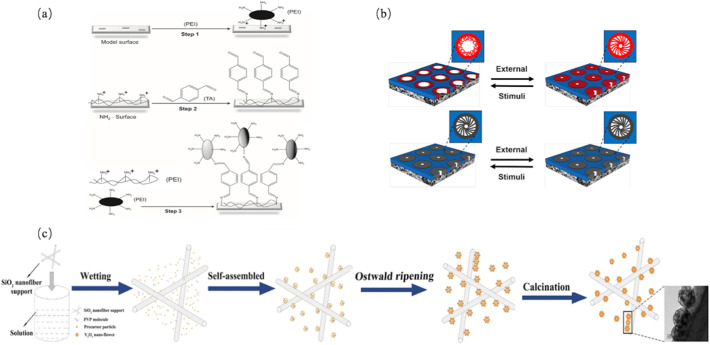
Using terephthalaldehyde (TA) as a cross-linking agent, polyethyleneimine (PEI) was assembled layer by layer on the surface of a polyethersulfone (PES) MF membrane to form a positively charged membrane that can trap and inactivate viruses and eventually achieved 4.5–5 LRVs of virus reduction (Sinclair et al., 2019) (as shown in Fig. 7(a)). What's more, self-assembled block polymer membranes functionalized with macromolecular template capacitate highly selective separations (as shown in Fig. 7(b)). Gu et al. (2018) fabricated a highly porous and permeable functionalized monoliths with well-defined pore structures for the separation of large molecular virus particles, and a self-standing composite membrane with regular mesoporous has been designed, which realized highly size-selective permeation of biomolecules at the nanometer-level (Yamauchi and Kimura, 2013).
Fig. 7.
Schematic diagram pertaining to modified membranes. (a) A stable covalent layer-by-layer strategy used to fabricate ultrathin polyelectrolyte/polyethyleneimine (PEI) multilayers by chemical-crosslinking with terephthalaldehyde (TA) (Sinclair et al., 2019); (b) self-assembled block polymer membrane with tailor-made functionality designed by the macromolecular template, which possessed a precise Molecular Weight Cut Off (MWCO) with 8 Å selective separation (Zhang et al., 2017); (c) self-assembly of the positively charged SiO2-Y2O3 composite nanofiber membrane with a plum-flower-like structure, rendering an excellent virus retention as a result of high adsorption capacity (Liu et al., 2019).
The current materials used in fabrication methods for membranes are primarily based on empirical approaches and lack molecular-level design (Werber et al., 2016). The introduction of nanoparticles has been shown to reinforce the structural integrity and hydrophilicity of the membrane as well as the electrostatic interaction between the membrane and viruses to increase the water flux while improving the virus removal efficiency (Khin et al., 2012). Introducing metal nanoparticles (Liu et al., 2019), carbon nanotubes (Németh et al., 2019) and liquid crystals (Kuo et al., 2020) into the membrane or onto the membrane surface is currently an effective strategy. Y2O3 nanoparticles have the merits of a high isoelectric point (Zhao et al., 2018), large specific surface area (Zhang et al., 2019b), and multiple active surface sites (Meng et al., 2016). The positively charged plum-shaped SiO2-Y2O3 composite nanofiber membrane shown in Fig. 7(c) has a high adsorption capacity of 87.96 mg/cm3 for viruses and can reach 5 LRVs (Liu et al., 2019). Loading AgNPs onto a polysulfone membrane also leads to a significant improvement in virus removal (Zodrow et al., 2009). In addition, a novel microporous nano-MgO-diatomite ceramic membrane has been prepared, which may provide a feasible method based on the strongly positive charge for virus removal (Meng et al., 2016). Moreover, a new type of composite film, in which nanoparticles of copper oxide, titanium oxide, and iron oxide are coated on a polytetrafluoroethylene membrane with an inorganic composite-based multiwalled carbon nanotube (MWCNT) carrier, has been introduced to remove viruses efficiently by increasing the electrostatic interaction between the membrane and viruses (Németh et al., 2019).
3.2.1.2. Enhancing precise screening
Meanwhile, a nanostructured liquid-crystalline (LC) membrane has received widespread attention due to its ability to form ordered nanostructures via a self-organization process (Marets et al., 2017). As portrayed in Fig. S5, the nanostructures can be classified as cubic, columnar and layered shapes (Marets et al., 2017; Hamaguchi et al., 2019; Kuo et al., 2020). Marets et al. (2017) demonstrated for the first time the possibility of using an LC-structured membrane with precise nanochannels for virus removal in water purification. These channels can supply pathways larger enough for water molecules to permeate but small for virus to pass through. Here, a nanostructured bicontinuous cubic LC thin film was photopolymerized onto a polysulfone support layer to make a regular and controllable pore structure, and its removal of bacteriophage Qβ reached 6 LRVs. To further increase the water flux while efficiently rejecting viruses, a smectic A liquid-crystalline (SmA-LC) material with layered nanochannels and two-component columnar LC membranes was prepared, providing prewetted channels with hydrophilic conditions, which were more conducive to water molecules to penetration. The removal of these two membranes on viruses reached 4.4 ± 0.3 and 7.3 LRVs, and the water fluxes were 20 ± 6 and 15 L m−2 h−1, respectively, 40 times and 30 times the flux of the cubic LC membrane (0.54 ± 0.05 L m−2 h−1) (Hamaguchi et al., 2019; Kuo et al., 2020). In addition, Lee et al. (2018) employed a bioconjugated method to load feline calicivirus (FCV), a model virus with a structure similar to NoVs, onto the membrane surface, creating a dual functional membrane capable of both easily detecting and blocking NoVs. This concept of using 2D materials could likely endow the increase of the regular size and the transport nanochannels of pores, and the minimum pinholes for the fabrication of membranes to achieve precise sieving.
3.2.2. Membrane fouling
The presence of membrane fouling improves the retention of the membrane by either clogging breach or forming a cake layer on the membrane surface, which can impact the composition of membrane surfaces to remove viruses as a secondary barrier. As shown in Fig. S6, one study used four membranes to investigate their separation performance and found that the LRVs for MS2 ranked as follows: fouled membrane > backwashed membrane > chemically cleaned membrane ≈ pristine membrane (Lu et al., 2013), which implies that membrane fouling plays a positive role in virus retention. An analogous conclusion was also reflected in another investigation in which the LRVs gradually increased when the time after the membrane wash increased from 0 to 24 h (Chaudhry et al., 2015b), hence grasping the influence of fouling on virus reduction is a pivotal consideration.
Organic fouling can alter the nature and location of nanoparticle capture by competitive adsorption to change the capacity of the membrane for virus retention, as suggested in the studies of Fallahianbijan et al. and Nazem-Bokaee et al. (Fallahianbijan et al., 2019; Nazem-Bokaee et al., 2020). One study has shown that different membrane fouling layer can either improve or attenuate the removal of adenovirus relying on layer properties, as an illustration, humic acid can increase the removal since humic acid containing carboxylic and functional groups can adsorb onto the membrane surface, thereby altering the electrostatic properties and surface hydrophobicity of the membrane and enhancing the interaction between adenovirus and membrane surface, while SiO2 fouling can decrease the removal as the fouling layer caused by larger SiO2 particles is too porous to effectively reject adenovirus yet may lead to the accumulation of virus near the cake-membrane interface (Yin et al., 2015). The increase in virus removal owing to irreversible fouling is higher than reversible fouling (ElHadidy et al., 2014). Membrane biofouling, generally regarded as an irreversible fouling (Nagaraj et al., 2018), will help to achieve more than 7 LRVs of virus removal during long-term filtration in various membrane processes, including the coagulation-MF system, MBR, UF and coagulation-ceramic MF, etc. (Shirasaki et al., 2008; Chaudhry et al., 2015a; Alansari et al., 2015; Michalsky et al., 2009). During these processes, the effect on virus removal by different mechanisms, such as physical sieving, adsorption, cake and gel layer formation that could be expounded by biofouling surface characteristics and structural properties.
Meanwhile, some studies have demonstrated that irreversible fouling is one of the main factors that causes membrane aging (Antony et al., 2016), and the effect of aged RO membranes on virus removal shows certain improvements (Chesters et al., 2013; Pype et al., 2016). Intermittent operation can retain some microbes and organics in the interval of the RO element and increase their propagation during shutdown, leading to irreversible fouling, which can partially enhance virus retention (Torii et al., 2019b). However, intermittent operation can also cause deterioration of the surface of the membrane, which would lead to an increase in permeability but with compromised virus retention (Wang et al., 2017). A study has shown that virus retention is reduced to 2 LRVs after repeated pressurization for 10,000 times (Torii et al., 2019a).
Although membrane fouling is one of major drawbacks of the membrane treatment procedure, fouling will increase virus separation to some degree. By performing an in-depth study of the different types and degrees of membrane fouling and of the mechanism of virus retention to find the best equilibrium point, the operation cost of the membrane treatment can be greatly reduced to provide a theoretical proposal for membrane cleaning and strengthened virus separation during long-term operation.
3.2.3. Hybrid processes
The application of membrane-based hybrid processes can also further enhance inactivation and removal of viruses (Matsushita et al., 2011). A graph of the integration of coagulation and membrane filtration as well as of practical treatment effects is displayed in Fig. S7.
Adding an iron electrocoagulants to an MF system or the addition of glycine before NF can aggregate viruses into larger particles, making virus removal up to 6.5 LRVs (pH = 6.4) (Tanneru and Chellam, 2012). By adding polyaluminum chloride (PACL) as pretreatment coagulant for ceramic membrane filtration, the floc size was increased to form a looser and more porous filter cake layer so as to render more MS2 transferring to the floc phase and reduce the number of small particles that can lead to pore plugging, so the virus removal and membrane anti-fouling performance were all promoted (Im et al., 2019). One study that utilized UF process combined with coagulation and sedimentation to treat virus-containing wastewater showed removal of 1.8–4.2 LRVs more than UF alone (Lee et al., 2017), the introduction of sedimentation could limit the load of coagulation floc on membrane, which achieve a continuous operation in practical use.
Furthermore, the incorporation of two membrane systems can provide a double guarantee of effluent quality. UF integrated with RO can achieve 7.7 LRVs for high norovirus concentrations (Yasui et al., 2017). The MBR combined with the RO process, which takes advantage of size exclusion, electrostatic repulsion, hydrophilic and hydrophobic interaction, sorption of the membrane, and the adsorption of attached and suspended biomass, demonstrated excellent performance for the removal of viruses, emerging pathogens and micropollutants and was suitable for reclaimed water production (Prado et al., 2019b). In short, both the addition of coagulants prior to membrane filtration and the combination of different membrane process systems are capable of increasing virus retention to ensure favorable effluent conditions.
As for membranes, owing to the limitations of membrane materials and membrane fabrication methods, traditional separation membranes have an uneven pore size distribution and thick selective separation layers, which restrict improvements of water flux and separation selectivity (Werber et al., 2016). Therefore, overcoming the “trade-off” between flux and retention by designing and preparing high-performance membranes with a uniform pore size and nanometer thickness is an advisable direction for progress. It is important to effectively control virus transmission in water by understanding how virus removal is affected by interactions between viruses and membranes instead of only relying on membrane retention.
3.3. Disinfection for virus inactivation
To date, the major disinfection methods include chlorine (including free chlorine, chlorine dioxide, monochloramine), ozone and ultraviolet radiation. Various strains of the same virus usually have different susceptibilities to these disinfectants. For example, some mutant NoV strains were less sensitive to disinfectants than others and can still be alive even after wastewater treatment (Rachmadi et al., 2018). Meister et al. (2018) also found that the inactivation kinetics of EVs with diverse serotypes isolated in the laboratory or outdoors vary considerably. Therefore, it is necessary to select an appropriate disinfection strategy under specific conditions.
3.3.1. Chlorination
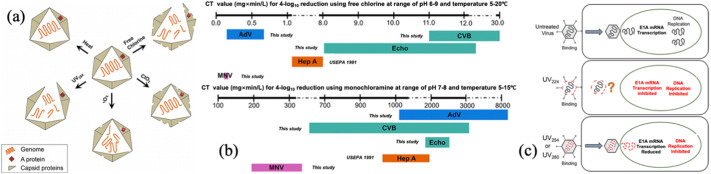
At present, the dominant disinfection is still chlorination. Free chlorine commonly damages the proteins and nucleic acids of viruses, while chlorine dioxide (ClO2) and monochloramine (NH2Cl) mainly damage the viral capsid (Wigginton et al., 2012). The corresponding inactivation mechanism is shown in Fig. 8(a). During the disinfection process, the CT value, which is obtained by multiplying the disinfectant concentration by the contact time and is affected by temperature and pH, can be adjusted to achieve satisfying disinfection outcomes. The US EPA notes that CT value needs to be 3-, 4-, and 6-mg × min/L to attain 2, 3, and 4 LRVs, respectively, at a temperature of 10 °C and pH of 6.0-9.0 when using chlorination disinfection (US EPA, 1991).
Fig. 8.
(a) Principle of different disinfections for damaging the virus structure. UV irradiation and free chlorine caused inactivation primarily by damaging both viral genome and protein, 1O2 caused inactivation by impairing genome replication and ClO2 by the degradation of proteins (Wigginton et al., 2012); (b) CT values needed to achieve 4 LRVs of the removal of AdV, CVB, ECHO and MNV using free chlorine at pH 6–9 and temperature 5–20 °C (above) and using monochloramine at pH 7–8 and temperature 5–15 °C (below) (Rachmadi et al., 2020); (c) distinctive inactivation mechanism of UV radiation at different wavelengths. UV224 mainly affected the integrity of viral capsid to inhibit the delivery of viral genome into the host cell, while UV254 and UV280 mainly restrained the DNA replication (Bravo et al., 2018).
Generally, the disinfectant type and its initial concentration as well as the virus category have a certain impact on the disinfection efficiency. Rachmadi et al. (2020) utilized Tobit and simple linear regression analysis to calculate the CT values required to reach 4 LRVs of virus removal in chlorine and NH2Cl disinfections (as shown in Fig. 8(b)). It shows that the CT values in free chlorine disinfection are much lower than those in NH2Cl disinfection for the same virus, which probably means that the subcellular components (e.g., viral DNA, RNA, capsid) are more susceptible to free chlorine than NH2Cl and the activity of free chlorine still remains outstanding. A study showed that HRV inactivation reached up to 5 LRVs by increasing the initial concentration of the disinfectant, whereas the inactivation could not be further increased by only prolonging the contact time (Xue et al., 2013), this phenomenon demonstrates that the initial concentration of the disinfectant is a necessary factor for disinfection. In addition, the CT values for CVB and ECHO were higher than those for AdVs and MNV with free chlorine and the AdVs demanded higher CT values than others with monochloramine, which may be due to the different chemical reactivities of the viral proteins and genomes to these disinfectants or because of the enhanced resistance of the viruses to the disinfectants from the high rate of mutation in the evolutionary process (Rachmadi et al., 2020).
However, chlorination disinfectants can often react with organics or nitrogen in wastewater, producing toxic and harmful DBPs, such as trihalomethanes (THMs) or haloacetic acid (HAA) carcinogens, haloacetonitrile (HANs) and N-nitrosodimethylamine (NDMA) (Zhang et al., 2020b). Therefore, we can take full advantage of some natural or synthetic compounds to replace chlorination disinfectants. Park et al. (2018) and Dunkin et al. (2017) used a micrometer-sized silica hybrid composite decorated with silver nanoparticles (AgNP-SiO2) and peroxyacetic acid (PAA) to successfully remove hNoV, MNV and bacteriophage MS2 from different water media. In addition, ferrate, as a newly emerging disinfectant, has a better disinfection efficiency than other materials due to its higher redox potential. Some research results adopting pseudo-second-order kinetics and Chick-Watson inactivation dynamic models expounded that H2FeO4 and HFeO4 − had far higher MS2 MNV inactivation than FeO4 2− and the increase of pH decreased the amount of H2FeO4 to reduce the inactivation rate constant (kd) (Wu et al., 2019; Manoli et al., 2020). On the other side, the intracellular algal organic matter (IAOM) that exhibited a stronger reaction with ferrate has a stronger inhibitory effect on MS2 inactivation than extracellular algal organic matter (EAOM). Therefore, it is significant to explore the influence of disinfectant types and their initial concentration, virus category and sensitivity, DBP generation, pH and temperature, coexisting substances, etc. in detail when using chlorination disinfection.
3.3.2. Ultraviolet radiation
The radiation in the wavelength range of ultraviolet (UV) has relatively high energy, and microorganisms absorb protons in high absorption coefficient between 200 and 300 nm. UV radiation disinfection does not produce detrimental byproducts that appear in chlorination disinfection. Employing UV to irradiate viruses can destroy the viral genome (including the phosphate bond between DNA/RNA) and the cross-link between capsid proteins (Wigginton et al., 2012). Different wavelengths of UV have distinguishable inactivation mechanisms for viruses. Bravo et al. (2018) found that UV254 and UV280 can cause mutations in the viral genome and thus inhibit DNA replication, while UV224 irradiation barely affects the integrity of the HAdV-2 genome, but the changes it causes in the capsid structure may restrain the translocation of the viral genome into the nucleus in host cells (As depicted in Fig. 8(c)).
However, traditional UV mercury vapor lamps are fragile and pose a risk associated with mercury. Therefore, some believe that UV light-emitting diodes (UV-LEDs) are expected to substitute for traditional UV lamps for virus disinfection (Li et al., 2019; Nguyen et al., 2019). The development of UV-LEDs will evidently reduce the need of energy and the price of electricity ascribed to the higher use of wind power and sun power. Song et al. (2019) used UV-LEDs to irradiate E. coli and bacteriophage MS2 and found that compared with E. coli removal, the damaged RNA of MS2 could not be repaired owing to the lack of repair enzyme. The application of UV-LEDs to virus disinfection has only attracted attention in recent years (Keshavarzfathy et al., 2021; Rattanakul and Oguma, 2018), so deeper studies need to be performed.
To further improve the disinfection efficiency, altering work pattern or adding other substances including sodium hypochlorite, chlorine dioxide, ozone or Fenton's reagent can be employed. For some insensitive viruses, Zyara et al. (2016) combined UV with chlorine to successfully reduce the chlorine-resistant strains by 3–5 LRVs with reduced DBP production. Additionally, Schijven et al. (2019) conducted a microbiological quantitative risk assessment (QMRA) and used somatic coliphages and bacteriophage MS2 as indicators of AdVs to explore the inactivation efficiency of UV and ClO2, proving that the predetermined target could be realized if low-concentration ClO2 (0.05–0.1 mg/L) was added during UV (40 mJ/cm2 or 73 mJ/cm2) disinfection.
The adsorption and aggregation of viruses must be considered in UV disinfection. Feng et al. (2016) discovered that MS2 formed aggregates and was less inactivated if cations such as sodium (at pH 3) or calcium (at pH 7) were introduced, as the virions situated inside the aggregates were prevented from the UV irradiation, but MS2 was more likely to adsorb to particles and inactivated in the presence of organic particles such as microcystis aeruginosa. As for WWTPs that adopt UV for disinfection, ultraviolet wavelengths and intensity should be considered, and 100, 143, and 186 mJ/cm2 are theoretically required for 2-, 3-, and 4-log virus inactivation, respectively (US EPA, 2006b). In the dynamic process of wastewater treatment, it is inevitable to encounter matrix objects that have complicated interactions with viruses, so researchers should use qualitative or quantitative methods to explore the relationships in depth.
3.3.3. Ozonation
Ozone is a strong oxidant and can interact with water to generate free radicals to destroy the protein and nucleic acids of viruses (Wigginton and Kohn, 2012; Zhang et al., 2016). Ozonation can reduce viruses beyond conventional treatments or even make viruses undetectable, so it may be used to reduce the spread of human viruses (Wang et al., 2018). Cai et al. (2014) determined that with a pH increase and temperature decrease and in the presence of particles, organics and coexisting ions, inactivation would be reduced. Concretely, first, pH affects the oxidation ability and attenuation rate of ozone, which were respectively weaker and rose in alkaline conditions; second, a temperature increase would augment the energy of ozone molecules to facilitate the movement of ozone; third, particles would bind to the virus to a certain extent, thereby shielding the virus and influencing inactivation; lastly, the presence of dissolved organic matters would consume a large amount of ozone, thus compromising the ozone inactivation efficiency.
Meanwhile, integrating ozone with biologically activated carbon, free chlorine or other catalysts may lead to better virus inactivation. Im et al. (2018) combined ozone, coagulation and ceramic membrane filtration to remove viruses for water reclamation and found that the reduction of MS2 could be increased from 2.1 to 6.8 LRVs as the amount of ozone increases since this increase can support more MS2 removal by subsequent membranes. Moreover, ozone can act with catalysts to form hydroxyl radicals that can accelerate the oxidization and decomposition of viruses. For example, when utilizing volcanic rocks as catalysts, ozone could almost completely remove NV GI, GII and JC PyV, but if only ozone was used, JC PyV was still not removed after 150 min (Gomes et al., 2019). However, it is relatively difficult to detect virus inactivation in ozone disinfection because of the lack of real-time tracking methods and the high reactivity of ozone. Some investigators have established Bayesian power models and an experimental batch system to overcome this issue and use the second-order rate constant (kO3-virus) to quantify ozone exposure as well as to evaluate the results of ozonation for the inactivation of EVs and bacteriophages, finally, they received a good disinfection efficiency (Wolf et al., 2019; Wolf et al., 2018). What's more, arising from the extraordinary instability of ozone, the bromide ion in water is easily oxidated to bromate that is mentioned as a kind of DBPs in the drinking water standard in many countries (US EPA, 2009; China, Standards for drinking water quality (GB5749-2006), 2006; Japan, Water Quality Standard, 2015), which is a remarkable barrier for its extensive application. As such, we should optimize treatment process to minimize the formation potential of DBP precursors and reasonably control the disinfection amount and time.
3.3.4. Catalytic oxidation
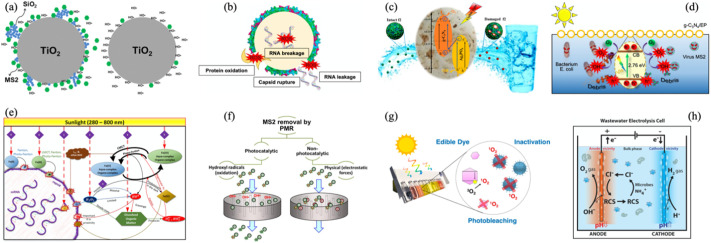
Light irradiation excites electrons in the valence band (VB) of semiconductors to the conduction band (CB), and the holes formed in the VB and the excited electrons can both participate in redox reactions. Photocatalysts include natural photocatalysts (García-Gil et al., 2020;Ryberg et al., 2018), semiconductor oxides (such as titanium dioxide) (Reddy et al., 2017; Liga et al., 2011; Zheng et al., 2018), plasma (Guo et al., 2018), metal oxides, and graphene-based photocatalysts (such as g-C3N4) (Zhang et al., 2019a; Cheng et al., 2018).
Semiconductor oxide-based photocatalysis has recently been one of the hottest research topics. Under light irradiation at ambient temperature, semiconductors will yield many kinds of reactive species (RS), including hydroxyl radical (•OH), superoxide radical (•O2 −), singlet oxygen (1O2), hydrogen peroxide (H2O2), etc. (Li et al., 2008; Lee et al., 2011; Wu et al., 1998), to destruct viral genome and protein for virus inactivation. Among these photocatalysts, titanium dioxide (TiO2) is widely applied in the field of drinking water and wastewater treatment since it can generate reactive oxygen species (ROS), including a surface-produced 1O2 and free metal ions to damage the capsid protein (Reddy et al., 2017). To overcome inherent limitation of the slow reaction kinetics and reduce the electron-hole recombination rate in TiO2 catalyze, Liga et al. (2011) and Liga et al. (2013) studied the ability of photocatalytic silver-doped and SiO2-doped titanium dioxide nanoparticles (nAg-TiO2 and SiO2-TiO2) to inactivate bacteriophage MS2 and found that compared to TiO2 alone, the MS2 inactivation rates induced by nAg-TiO2 and SiO2-TiO2 increased by more than 5 and 2.7 times, respectively, mainly attributed to the increased generation of free •OH from silver and the larger adsorption surface area of the catalyst with the addition of silica (as shown in Fig. 9(a)). Zheng et al. (2018) mentioned that the addition of Cu-TiO2 can provide a large active surface of catalysts and produce more free •OH, but with its continuous increment, the increase in solution turbidity and the decrease of photon penetration ability as well as enhanced catalyst agglomeration led to a decline in catalytic performance. When the visible light intensity and temperature were increased, the catalysts were fully excited to generate more holes and electrons, intensifying the oxidation effect of the catalyst. In addition to TiO2, Hu et al. (2010) also used Ag-AgI/Al2O3 as a plasma to achieve a 3.2 LRVs for RV removal under visible light, which mainly because the generated inorganic anion radicals caused by plasmon resonance of Ag nanoparticles not only boost the electron transfer but have high bactericidal activity. Sarkar et al. (2018) prepared nanopores by electrospray deposition of silver ions on a single-layer molybdenum disulfide (MoS2) nanosheet, the caused molybdenum-rich defects can efficaciously generate ROS under visible light to remove 7 LRVs of bacteriophage MS2 in water.
Fig. 9.
(a) Photocatalytic inactivation of TiO2 toward bacteriophage MS2 with (left) and without (right) the addition of SiO2 nanoparticles (Liga et al., 2013); (b) response of viral death to g-C3N4-based photocatalytic disinfection including protein oxidation, capsid rupture, RNA breakage and RNA leakage (Zhang et al., 2019a); (c) a possible Z-scheme inactivation mechanism of bacteriophage f2 by Ag3PO4-g-C3N4 photocatalytic material. The f2 was oxidized by photogenerated holes (h+) under visible light irradiation, together with hydroxyl radicals (•OH) formed by reaction between h+ and H2O or OH− near the surface of Ag3PO4 and superoxide radical (•O2−) caused by trapping electrons by dissolved oxygen near the surface of g-C3N4 (Cheng et al., 2018); (d) proposed mechanism of bacteriophage MS2 inactivation by g-C3N4-EP520 under visible-light irradiation (Zhang et al., 2018); (e) proposed MS2 inactivation route during the photo-Fenton process through (1) direct sunlight, (2) oxidative stress exerted by H2O2, (3) irradiation of the DOM to generate H2O2, O2−, 1O2 and other ROS, (4) enhancement of the •OH production under solar light in Fenton reaction, (5) aquo-complexes by hydrolysis and organo-complexes in the presence of DOM for Fe(III) in the wastewater and (6) organo-complexes from the interaction of Fe(II) and Fe(III) with amino acids in MS2 capsid (Giannakis et al., 2017); (f) process of bacteriophage MS2 removal by PMR (including the oxidation of hydroxyl radicals in photocatalytic and electrostatic force in non-photocatalytic) (Horovitz et al., 2018); (g) enhanced solar disinfection method using an edible dye as a photosensitizer to generate 1O2 for virus inactivation and signify the finish of solar disinfection by photobleaching (Ryberg et al., 2018); (h) a electrolysis cell for toilet wastewater disinfection in which the free reactive chlorine produced in situ instead of •OH and other reactive oxygen species was the main disinfection ingredient (Huang et al., 2016).
For some nonmetallic photocatalysts, besides common graphene oxide-based catalysts (Akhavan et al., 2012), graphitic carbon nitride (g-C3N4), a visible-light-response semiconductor with a two-dimensional conjugated structure characterized by robust physicochemical stability, low cytotoxicity, facile synthesis and suitable electronic band structures (~2.7 eV), has been studied extensively (Zhang et al., 2019a; Lin et al., 2014). The mechanism of g-C3N4 for virus inactivation under visible-light irradiation is shown in Fig. 9(b) (Zhang et al., 2019a). Cheng et al. (2018) prepared the Ag3PO4-g-C3N4 (AgCN) photocatalytic composite and observed that this composite could completely inactivate 3 × 106 PFU/mL bacteriophage f2 within 80 min and exhibited superior stability because of the production of photogenerated holes (h+), •OH and •O2 − (Fig. 9(c)). Others incorporated g-C3N4 with a low-density porous expanded perlite (EP) mineral (Fig. 9(d)) to achieve 8 LRVs of MS2 inactivation in 240 min under visible-light irradiation, in which the increments of dissolved oxygen (DO), proton concentration, salinity (Na+) and hardness (Ca2+) decreased the electrostatic repulsion between MS2 and the photocatalyst to facilitate MS2 inactivation (Zhang et al., 2018).
As a green and sustainable technology, advanced oxidation processes (AOPs), especially the photo-Fenton technique, have been increasingly utilized to achieve conspicuous treatment of chemical pollutants but have been less applied to microbes. Therefore, Giannakis et al. (2017) introduced H2O2 or Fenton's reagent along with light to inactivate viruses (Fig. 9(e)) and discovered that higher iron concentrations were able to improve virus inactivation and that Fe(II) could interact with key constituents in the viral capsid, generating intermediate ROS near viruses to inactivate them. Furthermore, the ROS that formed from the complexation reaction of iron and dissolved organic matter (DOM) also played a role in photocatalytic disinfection. Sun et al. (2016) also combined UV with peroxy chemicals, including H2O2 and peroxydisulfate (PDS), to achieve disinfection in which three reactive species, •OH, sulfate radical (SO4 • −) and carbonate radical (CO3 • −), were responsible for MS2 inactivation.
Photocatalysis has also been emerging in another promising technology, the Photocatalytic Membrane Reactor (PMR), to inhibit viruses and other microorganisms. The PMR is a hybrid reactor that combines photocatalysis and membranes. Zhang et al. (2020a) employed a PMR driven by visible light-emitting diodes (Vis-LEDs) and used a self-made metal-free heterojunction with the merits of efficient virucidal effects and easy recovery via microfiltration as a catalyst to totally inactivate HAdV under optimal conditions. Other researchers proposed a N-doped TiO2-coated Al2O3 photocatalytic membrane reactor to remove MS2 (as shown in Fig. 9(f)), and the results demonstrated that the removal of MS2 absorbed to the coated membrane in the PMR throughout photocatalysis due to the •OH was 4.9 ± 0.1 LRVs. Meanwhile, natural organic matter (NOM) has obviously been shown to be a negatively charged photocatalytic inhibitor (Horovitz et al., 2018).
Solar water disinfection (SODIS) is inherent clean, simple, economical and space-saving and has therefore been implemented in developing countries. The water is placed into polyethylene terephthalate (PET) or polypropylene (PP) bottles which are later exposed to the direct sunlight for 6 h in clear days or for 48 h in cloudy weather in order to ensure the safety of drinking water (Luzi et al., 2016; Oates et al., 2003; García-Gil et al., 2020). The optical and thermal effects act synergistically for the inactivation of organisms, in the meantime, the aluminum foil reflectors, container volume and the turbidity of water can retard the radiation efficiency (Kehoe et al., 2001), so some avenues like filter prior to solar exposure are deployed (Reed, 1997). Viruses are generally inactivated through indirect sunlight-mediated inactivation induced by ROS, notably 1O2, produced by exogenous photosensitizers (e.g., humic acids) in waters (Kohn and Nelson, 2007). SODIS is able to inactivate some ssRNA viruses but requires extra auxiliary measures when effectively reducing DNA viruses resistant to oxidation (Carratalà et al., 2016). Walker et al. (2004) reduced the F-specific RNA bacteriophage MS2 by 3.5 LRVs after 6 h of natural sunlight via applying a solar disinfection pouch. To improve the efficacy of solar disinfection, Ryberg et al. (2018) proposed an enhanced SODIS method using a food dye—erythrosine—as a photosensitizer and disinfection indicator, finally accomplishing more than 4 LRVs of bacteriophage MS2 inactivation within 5 min (as shown in Fig. 9(g)). It must be noticed that the working life span of plastic bottles is limited. On the other hand, SODIS might be inefficient in places with weak sunlight radiation and inadequate heat, such as places far from the equator or lacking sunshine.
For systems in which it is difficult to remove viruses with ordinary disinfectants, we can consider electrochemical treatment. Electrocatalytic oxidation adopts an electrolysis cell where the electrodes under redox reactions with an applied voltage to remove microbial pathogens. When using chlorine as the disinfectant, the generated reactive chlorine species (RCS, such as (Cl2), (HOCl), (ClO−)) and chlorine free radicals (such as (•Cl), (•Cl2)) are the main disinfection agents, while water or other saline solutions are used as the electrolyte and the formed •OH, H2O2, ozone (O3) and·O2 − serve as the main roles in disinfection, as evinced in Fig. 9 (h) (Huang et al., 2016). Heffron et al. (2019) first applied sequential electrocoagulation-electrooxidation (using boron-doped diamond electrodes) to better mitigate bacteriophages MS2, FX174 and human echovirus, which resulted principally from the positive stimulation effect in the physical reduction of coagulation-filtration, ferrous iron-based disinfection and electrooxidation disinfection. To date, there has been little research on (photo-) electrocatalytic oxidation for virus treatment, but as a developing technology, this kind of oxidation is worthy of more public attention.
On the whole, catalytic oxidation technology possesses the strengths of easy operation, mild reaction condition, toxicity-free, high permanent oxidative stability and environmental friendliness. Different catalyst loadings, light intensities and wavelengths, as well as different doped elements including metal ions, non-metal, sulfide, etc., can influence the disinfection effect by changing the type and quantity of reactive species. Generally, there is electrostatic repulsion between viruses and catalysts, which can be weakened by increasing DO, proton concentrations and cation amount as well as by reducing NOM to improve the catalytic effect. It should be noted that lower synthesis cost, superior catalytic efficiency and the large-scale availability ought to be the imperative goal for the following research.
3.4. Comparison of virus elimination processes
Numerous research studies are dedicated to finding a way to yield highly productive and high-quality water from wastewater. The removal efficiency varies for different technologies and viruses. The comparison shown in Fig. 10 indicates that NF/RO have relatively stable removal values of 4.1–7 LRVs, while the removal efficiency of MBR, MF and UF is more affected by the heterogenous infectivity of viruses and shows a removal range of 1.4–7.1, 0.7–4.7 and 0.5–5.9 LRVs, respectively. Among the disinfections, the deviation of chlorination is relatively small, which may be one of the reasons for its widespread application, and by adjusting the CT values can we achieve sufficient virus removal. As for UV radiation, ozonation and catalytic disinfection, different levels of virus inactivation can be achieved (with 0.09–5, 0.6–7.7 and 1–8 LRVs, respectively) through various disinfectant concentration, light intensity, photocatalyst type (for generating validly reactive substances) and the contact probability between viruses and disinfectants. On the whole, all the methods have acceptable effects for virus removal, and we can opt for an appropriate method to increase selectively targeted virus elimination as much as possible.
Fig. 10.
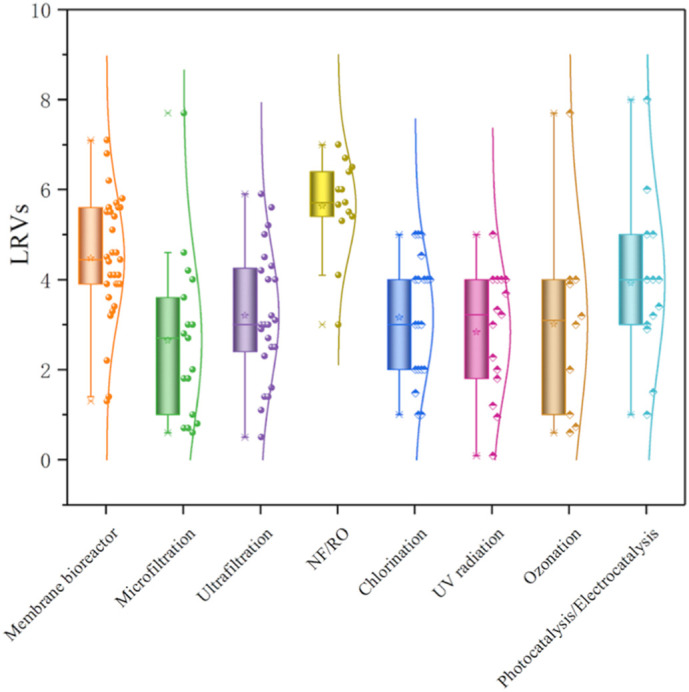
Comparison of the removal efficiencies of viruses by each water/wastewater treatment process. The detailed quantitative data and the relevant operation settings are described in Table S3.
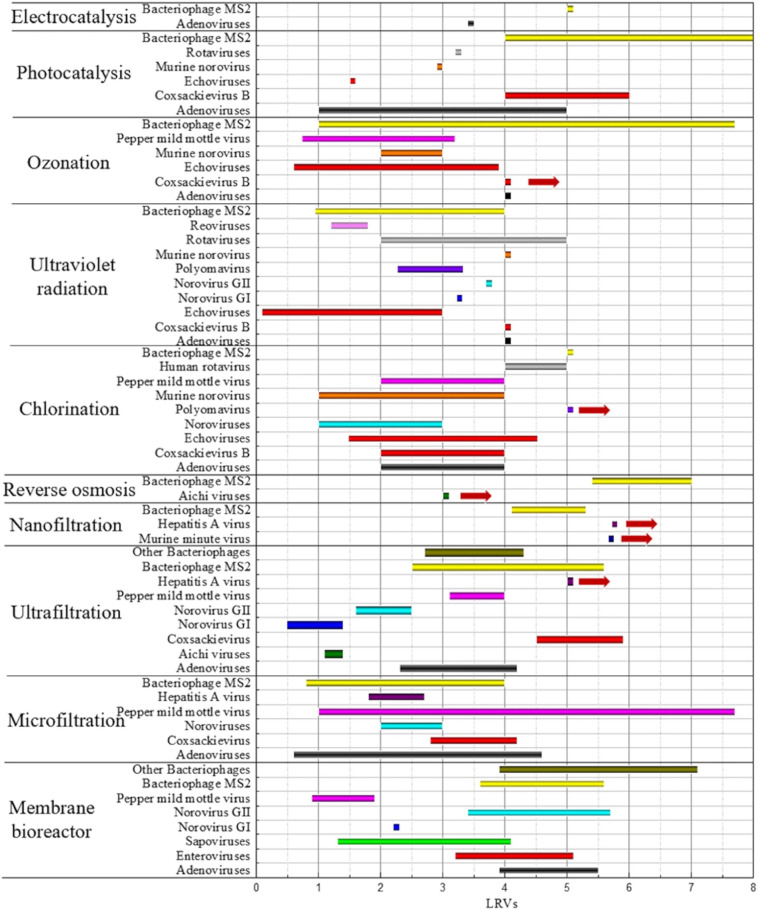
Recent studies have revealed that various technologies have already been used for virus-containing wastewater/drinking water treatment. Fig. 11 summarizes the range of the various types of virus removal in each process, and all processes are compared in Table 3 . Overall, virus elimination by means of membrane filtration and disinfection technology was within the range of 0.5–7 LRVs and 0.09–8 LRVs, respectively.
Fig. 11.
Ranges of the removal efficiency of viruses by various water/wastewater processes originating form information in Table S3.
Table 3.
Virus removal range and the strength and weakness of each technology.
| Process | Removal (LRVs) | Major function mechanism | Strength | Weakness |
|---|---|---|---|---|
| MBR | 1.4–7.1 | Attachment of virus to mixed liquor solids; retention by membrane; retention by membrane cake layer; inactivation of viruses by enzyme | High removal efficiency; high flux and less space demand | Incomplete removal of dissolved organic matters (<500 kDa); high cost for operation and maintenance |
| Microfiltration | 0.7–4.6 | Adsorption largely onto membrane surface or within its pores; follow by size exclusion | High permeability; low pressure-driven process | Low removal effect; health risk potential for humans |
| Ultrafiltration | 0.5–5.9 | Retention by membrane and attachment of virus onto membrane surface or sorption within its pores | High flux and permeability; low energy cost and effective removal of high molecular weight matter | High capital investment and operation; removal efficiency is unstable |
| Nanofiltration/reverse osmosis | 4.1–7 | Size exclusion; Electrostatic interactions | High performance, security and reliability, dedicated removal of enveloped and nonenveloped viruses based only on size-exclusion | High requirements for influent quality |
| Chlorination disinfection | 1–>5 | Damage in protein, nucleic acid and viral capsid | Easy to handle, economical, long residual | DBP formation, corrosive, residual toxicity |
| UV radiation disinfection | 0.09–5 | Formation of lesions in viral genome and destruction of the cross-link between genome and protein | No DBP formation, short contact time, less operating process and space, no extra chemicals, less susceptible to temperature and pH | No residual disinfection efficiency, relatively high level of energy consumption with a certain compromise in UV-LEDs |
| Ozonation disinfection | 0.6–7.7 | Free radical formation from reaction between ozone and water | Short contact time, inactivation of viruses | No residual disinfection efficiency, high energy consumption, relatively hard to detect |
| Photocatalysis disinfection | 1–8 | Redox reaction of some reactive species (h+, e−, •OH, •O2−, 1O2, H2O2, etc.) with visible or UV light | Facile preparation, favorable catalytic performance, low operation cost, good stability | low quantum yield for a few materials |
| Electrocatalysis disinfection | 3.4–5 | Redox reaction of some reactive species (HOCl, •Cl, •OH, •O2−, O3, H2O2, etc.) in electrolysis cell | Applicable to some hard-removing viruses | Electricity consumption |
In the case of enteric viruses (such as Coxsackievirus and HAV), UF has a relatively higher retention efficiency for HAV than even for AdVs with a large size, which indicates that electrostatic and hydrophobic interactions may plays crucial roles in determining virus retention. As for NoVs, more removal occurs in the MBR than in MF and UF, which may suggest stronger reactivity between NoVs and activated sludge or that NoVs may be prone to adsorb on sludge particles with subsequent separation by a membrane, and NoVs GI shows more resistant to remove than NoVs G II. The smaller removal values of PMMoV obtained in MBR denote that this virus is significantly resistant to biological treatment. Separation of viruses using a membrane cannot be regarded as a simple screening process, and the generalization of the behaviors of these viruses has not been unified. The influence of the characteristics of the viruses themselves must also be considered.
The inactivation of all the disinfection methods for the same virus is mostly between 1 and 4 LRVs, and the inactivation is sometimes over 5 LRVs. The data collected so far have indicated that chlorine or chlorine dioxide is sufficient for SARS-CoV reduction (Li et al., 2020); chlorination and ozonation show slightly better inactivation for PMMoV; ozonation and photocatalysis are better for AdVs (highly resistant to monochloramine and UV irradiation (Hu et al., 2010)) and CVB elimination; chlorination is better for RVs, JC PyV and ECHO removal; and UV radiation is better for NoVs reduction, and all the methods are acceptable for bacteriophage MS2 reduction. Some assumptions can be made: (1) since the phage MS2 is a non-pathogenic bacterial virus, its special nature may be more facilely sensitive to external disinfection conditions, so its inactivation has adequate effects overall; (2) judging from the available results, UV relatively exhibits less preponderance for most virus inactivation, probably because of its non-durable disinfection efficiency and the adverse consequences from coexisting ions, or obtaining better inactivation would be costly and the different wavelengths of UV light needs to be optimized to promote virus inactivation; (3) during photocatalysis and other processes involving free radicals for oxidation, viruses have different resistances, and the activity of free radicals under different conditions is also an important factor.
4. Conclusions and future perspectives
Viruses are distinct from other pathogens and show varying reactions in treatment processes, leading to discrepancies in their fate and behavior in water. Better understanding the characteristics, behavior and migration laws of viruses in water treatment and improving the comprehensive assessment of virus contamination along will help guide future research and provide theoretical guidance for the control of waterborne diseases.
The removal efficiencies for AdVs, NoVs GII and Bacteriophage MS2 are comparable in the MBR process, which demonstrates that the MS2 can be regarded as a good indicator or surrogate for AdVs and NoVs GII in MBR. Nevertheless, it has not yet been clearly determined whether virus reduction is attributable to decomposition by activated sludge or retention by membranes. In the former case, determining which one of the microbial communities plays a leading role will be the focus, and the coexistence relationship and interactive mechanisms (like adsorption) between some component (such as particulate matter, other microorganisms, chemical substances and protozoa) in activated sludge and viruses need to be intentionally explored. While in the latter condition, the decisive function from membrane ought to come into people's sight.
Even though the inherent nature of viruses, such as difference in nucleic acid, has a few influences over their removal from membrane, the removal effect is more closely dependent on the membrane properties like the pore size distribution and the interaction between the virus and membrane. In most of cases, the pore sizes in MF and UF membranes are often bigger than the diameter of viruses, thus the acquired propensities of virus removal are susceptibly affected by electrostatic and hydrophobic interactions. Introducing nanoparticles or nano-sheets including liquid crystals, carbon nanotubes, and block polymers (Kuo et al., 2020) that have uniform-sized nano-channels, large specific surface area and high reactivity can be treated as a measure to obtain filtration membrane with high pore interconnectivity and long-term antiviral performance, where the extra removal attributed by the fouling layer is certainly worth to be concerned. A single antiviral strategy can only weaken typical interactions between viruses and the membrane surface, limiting the scope of pollutants that can be treated. However, the synergistic effect achieved by multiple antiviral methods is still vague, which makes it hard to be controlled with high accuracy.
Moreover, it is expected to develop integrated membrane processes to promote virus elimination such as coagulation, adsorption, precipitation and disinfection. Yet different combinations may provide more or less effective removal of the viruses, and place one technique in pretreatment or posttreatment would also produce discrepant results (Im et al., 2019). Furthermore, there is a need to judge the type of the treated water. Compared with domestic sewage and industrial wastewater, the medical wastewater contains more pathogens like viruses, as such, it deserves more exceptional cautions. On the other hand, maintaining good property stability during the long-term operation in large-scale application is of great importance, and it is also apparent that there is a large deviation between the laboratory simulation experiment which is more inclined to mechanism investigation and the large-scale application to verify actual treatment effects, so we must seek strategies to close the gap between them.
Major disinfection treatments include chlorination, UV radiation, ozonation and catalytic oxidation. Some alternatives or combined techniques, such as ferrate and novel g-C3N4 and TiO2-based photocatalytic composites, can be considered as beneficial measures to increase virus inactivation. Many factors should be considered for disinfection, such as the type and initial concentration of the disinfectant and virus and the pH, temperature and matrices (such as particles, DOM, coexisting ions, dissolved oxygen) of the treated water and others. If some coexisting substances cause flocculation to wrap viruses or compete with viruses to react with disinfectants, the disinfection efficiency will be compromised. Otherwise, the efficiency will increase if the adsorption between particles and viruses dominates.
Viruses have different disinfectant-resistance due to their unique genome structure and morphology (i.e., icosahedral or helical symmetry, whether containing envelope and spike). The disinfection mechanism for virus reduction and inactivation primarily lies in the interaction between the disinfectants and viruses to destroy the viral capsid protein and nucleic acid, thereby irreversibly inhibiting the transmission of viruses to host cells as well as their reproduction. Other biological factors such as bacterial compounds will influence the disinfection effect, for example, in drinking water production, adding lipopolysaccharide or peptidoglycan of bacterial origin to enterovirus defended the viral capsid from thermal breakage when the capsid was the prime disinfection target (Waldman et al., 2017), and mixture with E. coli 285 obviously affected the removal of bacteriophage f2 (Zheng et al., 2018). Till now, enteric viruses with ssDNA genome are few. Albeit chlorination probably gives rise to secondary pollution, it displays relatively better inactivation effects for JC PyV (dsDNA), PMMoV (ssRNA), and RVs (dsRNA) than other disinfections. UV radiation, less susceptible to temperature and pH than other disinfectants, is relatively effective when eliminating ssRNA (e.g., NoVs) through mutating viral genome or changing capsid protein under different wavelengths. Ozonation with short operation time has over 4 LRVs of reduction for dsDNA (e.g., AdVs) and ssRNA (e.g., CVB, bacteriophage MS2 and PMMoV) but less for dsRNA by generating hydroxyl radicals to oxide and damage nucleic acid. Catalytic oxidation has less satisfactory removal for some dsRNA viruses such as RVs and ssRNA viruses including NoVs and ECHO but exhibits good results for other dsRNA viruses (e.g., AdVs) and ssRNA viruses like CVB by means of producing reactive species (including free radicals) for the purpose of protein oxidation, capsid rupture and genome breakage. In the near future, new emerging disinfection technology especially photocatalysis or photo-electrocatalysis with fantastic disinfection performance has great potential in virus inactivation in water. Of course, it is worth noting that during the disinfection process, the damaged protein may self-repair and regenerate under appropriate conditions (Nelson et al., 2018), so we are supposed to pay our attention to destroying viral genome so as to inactivate viruses thoroughly.
With regard to the quantitative virus removal, the evidence from this study suggests that membrane filtration and disinfection technologies can treat virus-containing wastewater/drinking water over a wide range (0.5–7 LRVs and 0.09–8 LRVs, respectively). From another perspective, the combination of membrane-based separation and other technologies may harness the strengths and circumvent their respective shortcomings. Tertiary treatment including membrane filtration and disinfection technology will be indispensable in the near future. The presence of organic matters and inorganic ions will impact the inactivation by disinfectant toward viruses (Cai et al., 2014), so membranes separation can be necessary prior to disinfection in some cases. UF and MF membranes are generally an effective barrier for protozoa and bacteria but have limited effects to remove viruses due to their small size. Studies have suggested that a hybrid MF-UV process with a photocatalytic membrane for the removal and inactivation of bacteriophage P22 was more effective (LRV = 5.0 ± 0.7) than stand-alone MF/UV disinfection or MF-UV with a non-photocatalytic membrane (Guo et al., 2015), and the integrated coagulation-MF/UF filtration-UV obtained the highest removal of fluorescent-conjugated MS2 at 5 mg Al3+/L (Guo and Hu, 2014). Specifically, some inspiring results in our work can be proposed: for AiVs, it is suggested to use a combined UF-disinfection process; for AdVs, adopting an MBR system alone or using UF/MF with ozonation/photocatalysis (also suitable for CVB) can be considered; for NoVs, linking MF/UF and UV disinfection together may lead to superior virus removal outcomes; and for PMMoV reduction, simultaneously supplementing the UF/MBR unit with chlorination/ozonation would be a nice choice.
In all processes for eliminating viruses, we should avoid the secondary pollution that may be caused by building extra components such as disinfection by-products and removing viruses may encounter the low-come and specialization issues in small-scale water supply plants. In the context of COVID-19 pandemic, it may be a smart strategy to combine disinfection and membrane separation with molecular imprinting technology to enhance the selective targeted removal of viruses, at the same time, some traditional and emerging monitoring equipment can be deployed to online real-time water quality monitoring of viruses to reduce the higher risk that may be caused by the leakage of the pipe network.
Finally, since there is a large number of viral strains in water, some indicators or surrogates that have behaviors similar to viral pathogens and are easy to detect and quantify should be used to assess water pollution, virus attenuation and virus migration in water. Importantly, bacteriophage MS2 can be adopted as a substitute for NoVs and RVs to explore the disinfection efficiency of UV, but MS2 is often detected with higher LRVs and usually does not have the strong correlation with other indigenous viruses regarding removal efficiency in chlorine and photocatalysis disinfection, which may lead to an overestimation of the effectiveness of water treatment. Thus, it is recommended to look for adequate potential indicators of indigenous viruses.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant No. 52070018 and No. 51920105012) and Beijing Municipal Natural Science Foundation (Grant No. L182030).
Editor: Baoliang Chen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.149678.
Appendix A. Supplementary data
Supplementary material
References
- Akhavan O., Choobtashani M., Ghaderi E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene-tungsten oxide composite under visible light irradiation. J. Phys. Chem. C. 2012;116(17):9653–9659. [Google Scholar]
- Alansari A., Selbes M., Karanfil T., Amburgey J. Optimization of coagulation pretreatment conditions in a ceramic membrane system. J. Am, Water Works Ass. 2015;107(12):E693–E701. [Google Scholar]
- Al-Attabi R., Rodriguez-Andres J., Schütz J.A., Bechelany M., Chen X., Kong L.X., Morsi Y.S., Dumée L.F., des Ligneris E. Catalytic electrospun nano-composite membranes for virus capture and remediation. Sep. Purif. Technol. 2019;229:115806. [Google Scholar]
- Antony A., Branch A., Leslie G., Le-Clech P. Impact of membrane ageing on reverse osmosis performance-implications on validation protocol. J. Membrane Sci. 2016;520:37–44. [Google Scholar]
- Armanious A., Aeppli M., Jacak R., Refardt D., Sigstam T., Kohn T., Sander M. Viruses at solid-water interfaces: a systematic assessment of interactions driving adsorption. Environ. Sci. Technol. 2016;50(2):732–743. doi: 10.1021/acs.est.5b04644. [DOI] [PubMed] [Google Scholar]
- Arslan M., Xu B., El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai K., Estes M.K., Martella V., Parashar U.D. Viral gastroenteritis. Lancet. 2018;392(10142):175–178. doi: 10.1016/S0140-6736(18)31128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels J., Batista A.G., Kroll S., Maas M., Rezwan K. Hydrophobic ceramic capillary membranes for versatile virus filtration. J. Membrane Sci. 2019;570:85–92. [Google Scholar]
- Bravo B.V., Gonca¸lves K., Shisler J.L., Mariñas B.J. Adenovirus replication cycle disruption from exposure to polychromatic ultraviolet irradiation. Environ. Sci. Technol. 2018;52(6):3652–3659. doi: 10.1021/acs.est.7b06082. [DOI] [PubMed] [Google Scholar]
- Brooks Y.M., Spirito C.M., Bae J.S., Hong A., Mosier E.M., Sausele D.J., Fernandez-Baca C.P., Epstein J.L., Shapley D.J., Goodman L.B., Anderson R.R., Glaser A.L., Richardson R.E. Fecal indicator bacteria, fecal source tracking markers, and pathogens detected in two Hudson River tributaries. Water Res. 2020;171 doi: 10.1016/j.watres.2019.115342. [DOI] [PubMed] [Google Scholar]
- Burutaran L., Lizasoain A., Victoria M., Garcia M., Colina R., Tort L.F.L. Detection and molecular characterization of aichivirus 1 in wastewater samples from Uruguay. Food Environ. Virol. 2015;8:13–17. doi: 10.1007/s12560-015-9217-1. [DOI] [PubMed] [Google Scholar]
- Cai X., Han J.C., Li R., Zhang Y., Liu Y., Liu X., Dai R.H. Phage MS2 inactivation in pure and filtered water: effect of pseudo-kinetics and other factors. Ozone Sci. Eng. 2014;36(1):86–93. [Google Scholar]
- Carratalà A., Calado A.D., Mattle M.J., Meierhofer R., Luzi S., Kohn T. Solar disinfection of viruses in polyethylene terephthalate bottles. Appl. Environ. Microbiol. 2016;82(1):279–288. doi: 10.1128/AEM.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetlin D., Pallansch M., Fulton C., Vyas E., Shah A., Sohka T., Dhar A., Pallansch L., Strauss D. Use of a noninfectious surrogate to predict minute virus of mice removal during nanofiltration. Biotechnol. Prog. 2018;34(5):1213–1220. doi: 10.1002/btpr.2694. [DOI] [PubMed] [Google Scholar]
- Chaudhry R.M., Holloway R.W., Cath T.Y., Nelson K.L. Impact of virus surface characteristics on removal mechanisms within membrane bioreactors. Water Res. 2015;84(1):144–152. doi: 10.1016/j.watres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Chaudhry R.M., Nelson K.L., Drewes J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015;49(5):2815–2822. doi: 10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- Cheetham S., Souza M., Meulia T., Grimes S., Han M.G., Saif L.J. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 2006;80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R., Shen L.J., Yu J.H., Xiang S.Y., Zheng X. Photocatalytic inactivation of bacteriophage f2 with Ag3PO4/g-C3N4 composite under visible light irradiation: performance and mechanism. Catalysts. 2018;8(10):406. [Google Scholar]
- Chesters S.P., Pena N., Gallego S., Fazela M., Armstrong M.W., Vigo F. Results from 99 seawater RO membrane autopsies. IDA J. Desalin. Water Reuse. 2013;5(1):40–47. [Google Scholar]
- Delanka-Pedige H.M.K., Munasinghe-Arachchige S.P., Zhang Y.Y., Nirmalakhandan N. Bacteria and virus reduction in secondary treatment: potential for minimizing post disinfectant demand. Water Res. 2020;117 doi: 10.1016/j.watres.2020.115802. [DOI] [PubMed] [Google Scholar]
- Domagala K., Jacquin C., Borlaf M., Sinnet B., Julian T., Kata D., Graule T. Efficiency and stability evaluation of Cu2O/MWCNTs filters for virus removal from water. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115879. [DOI] [PubMed] [Google Scholar]
- Duek A., Arkhangelsky E., Krush R., Brenner A., Gitis V. New and conventional pore size tests in virus-removing membranes. Water Res. 2012;46(8):2505–2514. doi: 10.1016/j.watres.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Dunkin N., Weng S.C., Coulter C.G., Jacangelo J.G., Schwab K.J. Reduction of human norovirus GI, GII, and surrogates by peracetic acid and monochloramine in municipal secondary wastewater effluent. Environ. Sci. Technol. 2017;51(20):11918–11927. doi: 10.1021/acs.est.7b02954. [DOI] [PubMed] [Google Scholar]
- ElHadidy A.M., Peldszus S., Van Dyke M.I. Effect of hydraulically reversible and hydraulically irreversible fouling on the removal of MS2 and fX174 bacteriophage by an ultrafiltration membrane. Water Res. 2014;61:297–307. doi: 10.1016/j.watres.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Elmahdy E.M., Ahmed N.I., Shaheen M.N.F., Mohamed E.C.B., Loutfy S.A. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J. Water Health. 2019;17:287–294. doi: 10.2166/wh.2019.303. [DOI] [PubMed] [Google Scholar]
- Fallahianbijan F., Giglia S., Carbrello C., Zydney A.L. Use of fluorescently-labeled nanoparticles to study pore morphology and virus capture in virus filtration membranes. J. Membrane Sci. 2017;536:52–58. [Google Scholar]
- Fallahianbijan F., Giglia S., Carbrello C., Bell D., Zydney A.L. Impact of protein fouling on nanoparticle capture within the viresolve pro and viresolve NFP virus removal membranes. Biotechnol. Bioeng. 2019;116(9):2285–2291. doi: 10.1002/bit.27017. [DOI] [PubMed] [Google Scholar]
- Farkas K., Cooper D.M., McDonald J.E., Malham S.K., de Rougemont A., Jones D.L. Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and estuarine receiving waters. Sci. Total Environ. 2018;634:1174–1183. doi: 10.1016/j.scitotenv.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Feinstone S.M., Kapikian A.Z., Purceli R.H. Hepatitis a: detection by immune electron microscopy of a virus like antigen associated with acute illness. Science. 1973;182:1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Feng Z., Lu R.Q., Yuan B.L., Zhou Z.M., Wu Q.Q., Nguyen T.H. Influence of solution chemistry on the inactivation of particle-associated viruses by UV irradiation. Colloids Surf. B: Biointerfaces. 2016;148:622–628. doi: 10.1016/j.colsurfb.2016.09.025. [DOI] [PubMed] [Google Scholar]
- García-Gil Á., Pablos C., García-Muñoz R.A., McGuigan K.G., MarugÁn J. Material selection and prediction of solar irradiance in plastic devices for application of solar water disinfection (SODIS) to inactivate viruses, bacteria and protozoa. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.139126. [DOI] [PubMed] [Google Scholar]
- Gentile G.J., Cruz M.C., Rajal V.B., de Cortalezzi M.M.F. Electrostatic interactions in virus removal by ultrafiltration membranes. J. Environ. Chem. Eng. 2018;6(1):1314–1321. [Google Scholar]
- Giannakis S., Liu S.T., Carratal A., Rtimi S., Bensimon M., Pulgarin C. Effect of Fe(II)/Fe(III) species, pH, irradiance and bacterial presence on viral inactivation in wastewater by the photo-Fenton process: kinetic modeling and mechanistic interpretation. Appl. Catal. B Environ. 2017;204:156–166. [Google Scholar]
- Gomes J., Frasson D., Ferreira R.M.Q., Matos A., Martins R.C. Removal of enteric pathogens from real wastewater using single and catalytic ozonation. Water. 2019;11(1):127. [Google Scholar]
- Goswami K.P., Pugazhenthi G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: a review. J. Environ. Manag. 2020;268 doi: 10.1016/j.jenvman.2020.110583. [DOI] [PubMed] [Google Scholar]
- Gu H.M., Liu Y.B., Yin D.Z., Cai L.K., Zhang B.L., Zhang Q.Y. Heparin-immobilized polymeric monolithic column with submicron skeletons and well-defined macropores for highly efficient purification of enterovirus 71. Macromol. Mater. Engi. 2018;303(12):1800411. [Google Scholar]
- Guemes A.G.C., Youle M., Cantu V.A., Felts B., Nulton J., Rohwer F. Viruses as winners in the game of life. Annu. Rev. Virol. 2016;3:197–214. doi: 10.1146/annurev-virology-100114-054952. [DOI] [PubMed] [Google Scholar]
- Guo H., Hu J.Y. Optimization study of a hybrid alum coagulation-membrane filtration system for virus removal. Water Sci. Technol. 2011;64:1843–1850. doi: 10.2166/wst.2011.147. [DOI] [PubMed] [Google Scholar]
- Guo H., Hu J.Y. Fluorescent-conjugated MS2 as surrogates for viruses during drinking water treatment. Water Sci. Technol. Water Supply. 2014;14(6):991–1000. [Google Scholar]
- Guo B., Pasco E.V., Xagoraraki I., Tarabara V.V. Virus removal and inactivation in a hybrid microfiltration-UV process with a photocatalytic membrane. Sep. Purif. Technol. 2015;149:245–254. [Google Scholar]
- Guo L., Xu R.B., Gou L., Liu Z.C., Zhao Y.M., Liu D.X., Zhang L., Chen H.L., Kong M.G. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl. Enviro. Microbiol. 2018;84(17) doi: 10.1128/AEM.00726-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., Suzuki Y., Sakamoto T., Yoshio M., Torii S., Katayama H., Kato T. Polymerizable photocleavable columnar liquid crystals for nanoporous water treatment membranes. ACS Macro Lett. 2019;8(10):1303–1308. doi: 10.1021/acsmacrolett.9b00513. [DOI] [PubMed] [Google Scholar]
- Hai F.I., Riley T., Shawkat S., Magram S.F., Yamamoto K. Removal of pathogens by membrane bioreactors: a review of the mechanisms, influencing factors and reduction in chemical disinfectant dosing. Water. 2014;6(12):3603–3630. [Google Scholar]
- Hamaguchi K., Kuo D., Liu M., Sakamoto T., Yoshio M., Katayama H., Kato T. Nanostructured virus filtration membranes based on two-component columnar liquid crystals. ACS Macro Lett. 2019;8(1):24–30. doi: 10.1021/acsmacrolett.8b00821. [DOI] [PubMed] [Google Scholar]
- Hamza H., Hamza I.A. Oncogenic papillomavirus and polyomavirus in urban sewage in Egypt. Sci. Total Environ. 2018;610–611:1413–1420. doi: 10.1016/j.scitotenv.2017.08.218. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakail O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Katayama H., Kitajima M., Visvanathan C., Nol C., Furumai H. Validation of internal controls for extraction and amplification of nucleic acids from enteric viruses in water samples. Appl. Environ. Microb. 2011;77:4336–4343. doi: 10.1128/AEM.00077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Kitajima M., Katayama H. Occurrence and reduction of human viruses, F-specific RNA coliphage genogroups and microbial indicators at a full-scale wastewater treatment plant in Japan. J. Appl. Microbiol. 2013;114(2):545–554. doi: 10.1111/jam.12051. [DOI] [PubMed] [Google Scholar]
- Heffron J., Ryan D.R., Mayer B.K. Sequential electrocoagulation-electrooxidation for virus mitigation in drinking water. Water Res. 2019;160:435–444. doi: 10.1016/j.watres.2019.05.078. [DOI] [PubMed] [Google Scholar]
- Herath G., Yamamoto K., Urase T. Removal of viruses by microfiltration membranes at different solution environments. Water Sci. Technol. 1999;40(4–5):331–338. [Google Scholar]
- Hirani Z.M., DeCarolis J.F., Adham S.S., Jacangelo J.G. Peak flux performance and microbial removal by selected membrane bioreactor systems. Water Res. 2010;44(8):2431–2440. doi: 10.1016/j.watres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Hirani Z.M., Bukhari Z., Oppenheimer J., Jjemba P., Lechevallier M.W., Jacangelo J.G. Impact of MBR cleaning and breaching on passage of selected microorganisms and subsequent inactivation by free chlorine. Water Res. 2014;57:313–324. doi: 10.1016/j.watres.2014.03.038. [DOI] [PubMed] [Google Scholar]
- Horovitz I., Avisar D., Luster E., Lozzi L., Luxbacher T., Mamane H. MS2 bacteriophage inactivation using a N-doped TiO2-coated photocatalytic T membrane reactor: influence of water-quality parameters. Chem. Eng. J. 2018;354:995–1006. [Google Scholar]
- Hu X.X., Hu C., Peng T.W., Zhou X.F., Qu J.H. Plasmon-induced inactivation of enteric pathogenic microorganisms with Ag-AgI/Al2O3 under visible-light irradiation. Environ. Sci. Technol. 2010;44(18):7058–7062. doi: 10.1021/es1012577. [DOI] [PubMed] [Google Scholar]
- Huang X., Qu Y., Cid C.A., Frinke C., Hoffmann M.R., Lim K., Jiang S.C. Electrochemical disinfection of toilet wastewater using wastewater electrolysis cell. Water Res. 2016;92:164–172. doi: 10.1016/j.watres.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli M., Muscillo M., Della Libera S., Fratini M., Meucci L., De Ceglia M., Giacosa D., La Rosa G. One-year surveillance of human enteric viruses in raw and treated wastewaters, Downstream River waters, and drinking waters. Food. Environ. Virol. 2017;9(1):79–88. doi: 10.1007/s12560-016-9263-3. [DOI] [PubMed] [Google Scholar]
- Im D., Nakada N., Fukuma Y., Kato Y., Tanaka H. Performance of combined ozonation, coagulation and ceramic membrane process for water reclamation: effects and mechanism of ozonation on virus coagulation. Sep. Purif. Technol. 2018;192:429–434. [Google Scholar]
- Im D., Nakada N., Kato Y., Aoki M., Tanaka H. Pretreatment of ceramic membrane microfiltration in wastewater reuse: a comparison between ozonation and coagulation. J. Environ. Manag. 2019;251 doi: 10.1016/j.jenvman.2019.109555. [DOI] [PubMed] [Google Scholar]
- Jacukowicz A., Domanska-Blicharz K. Astroviruses in poultry. Med. Weter. 2017;73:329–333. [Google Scholar]
- Japanese Ministry of Health, Labour, and Welfare . 2015. Water Quality Standard. [Google Scholar]
- Kaas L., Gourinat A.C., Urbès F., Langlet J. A 1-year study on the detection of human enteric viruses in New Caledonia. Food Environ. Virol. 2016;8:46–56. doi: 10.1007/s12560-015-9224-2. [DOI] [PubMed] [Google Scholar]
- Kehoe S.C., Joyce T.M., Ibrahim P., Gillespie J.B., Shahar R.A., McGuigan K.G. Effect of agitation, turbidity, aluminium foil reflectors and container volume on the inactivation efficiency of batch-process solar disinfectors. Water Res. 2001;35(4):1061–1065. doi: 10.1016/s0043-1354(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Keshavarzfathy M., Hosoi Y., Oguma K., Taghipour F. Experimental andcomputational evaluation of a flow through UV-LED reactor for MS2 and adenovirus inactivation. Chem. Eng. J. 2021;407 [Google Scholar]
- Khin M.M., Nair A.S., Babu V.J., Murugan R., Ramakrishna S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012;5(8):8075–8109. [Google Scholar]
- Kitajima M., Iker B.C., Pepper I.L., Gerba C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes - identification of potential viral indicators. Sci. Total Environ. 2014;488:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Rachmadi A.T., Iker B.C., Haramoto E., Pepper I.L., Gerba C.P. Occurrence and genetic diversity of human cosavirus in influent and effluent of wastewater treatment plants in Arizona, United States. Arch. Virol. 2015;160(7):1775–1779. doi: 10.1007/s00705-015-2435-x. [DOI] [PubMed] [Google Scholar]
- Kohn T., Nelson K.L. Sunlight-mediated inactivation of MS2 coliphage via exogenous singlet oxygen produced by sensitizers in natural waters. Environ. Sci. Technol. 2007;41:192–197. doi: 10.1021/es061716i. [DOI] [PubMed] [Google Scholar]
- Kreißel K., Bösl M., Lipp P., Franzreb M., Hambsch B. Study on the removal efficiency of UF membranes using bacteriophages in bench-scale and semi-technical scale. Water Sci. Technol. 2012;66(6):1195–1202. doi: 10.2166/wst.2012.299. [DOI] [PubMed] [Google Scholar]
- Kuo D., Liu M.M., Kumar K.R.S., Hamaguchi K., Gan K.P., Sakamoto T., Ogawa T., Kato R., Miyamoto N., Nada H., Kimura M., Henmi M., Katayama H., Kato T. High virus removal by self-organized nanostructured 2D liquid-crystalline smectic membranes for water treatment. Small. 2020;16(23):2001721. doi: 10.1002/smll.202001721. [DOI] [PubMed] [Google Scholar]
- Larson A.M., Hsu B.B., Rautaray D., Haldar J., Chen J., Klibanov A.M. Hydrophobic polycationic coatings disinfect poliovirus and rotavirus solutions. Biotechnol. Bioeng. 2018;108(3):720–723. doi: 10.1002/bit.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hong S., Mackeyev Y., Lee C., Chung E., Wilson L.J., Kim J.H., Alvare P.J.J. Photosensitized oxidation of emerging organic pollutants by tetrakis C60 aminofullerene-derivatized silica under visible light irradiation. Environ. Sci. Technol. 2011;45:10598–10604. doi: 10.1021/es2029944. [DOI] [PubMed] [Google Scholar]
- Lee S., Ihara M., Yamashita N., Tanaka H. Improvement of virus removal by pilot-scale coagulation-ultrafiltration process for wastewater reclamation: effect of optimization of pH in secondary effluent. Water Res. 2017;114:23–30. doi: 10.1016/j.watres.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Lee B.Y., Kim J., Kim W.J., Kim J.K. Dual functional membrane capable of both visual sensing and blocking of waterborne virus. J. Membrane Sci. 2018;549:680–685. [Google Scholar]
- Li Q., Page M.A., Mariñas B.J., Shang J.K. Treatment of coliphage MS2 with palladium-modified nitrogen-doped titanium oxide photocatalyst illuminated by visible light environ. Sci. Technol. 2008;42:6148–6153. doi: 10.1021/es7026086. [DOI] [PubMed] [Google Scholar]
- Li X.L., Cai M., Wang L., Niu F.F., Yang D.G., Zhang G.Q. Evaluation survey of microbial disinfection methods in UV-LED water treatment systems. Sci. Total Environ. 2019;659:1415–1427. doi: 10.1016/j.scitotenv.2018.12.344. [DOI] [PubMed] [Google Scholar]
- Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liga M.V., Bryant E.L., Colvin V.L., Li Qilin. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011;45:535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Liga M.V., Maguire-Boyle S.J., Jafry H.R., Barron A.R., Li Q.L. Silica decorated TiO2 for virus inactivation in drinking water - simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013;47(12):6463–6470. doi: 10.1021/es400196p. [DOI] [PubMed] [Google Scholar]
- Lin L.S., Cong Z.X., Li J., Ke K.M., Guo S.S., Yang H.H., Chen G.N. Graphitic-phase C3N4 nanosheets as efficient photosensitizers and pH-responsive drug nanocarriers for cancer imaging and therapy. J. Mater. Chem. B. 2014;2(8):1031–1037. doi: 10.1039/c3tb21479f. [DOI] [PubMed] [Google Scholar]
- Liu Z., Tang Y., Zhao K., Chen W. Self-assembly of positively charged SiO2/Y2O3 composite nanofiber membranes with plum-flower-like structures for the removal of water contaminants. Appl. Surf. Sci. 2019;489:717–724. [Google Scholar]
- Lu R.Q., Mosiman D., Nguyen T.H. Mechanisms of MS2 bacteriophage removal by fouled ultrafiltration membrane subjected to different cleaning methods. Environ. Sci. Technol. 2013;47(23):13422–13429. doi: 10.1021/es403426t. [DOI] [PubMed] [Google Scholar]
- Lu R.Q., Zhang C., Piatkovsky M., Ulbricht M., Herzberg M., Nguyen T.H. Improvement of virus removal using ultrafiltration membranes modified with grafted zwitterionic polymer hydrogels. Water Res. 2017;116:86–94. doi: 10.1016/j.watres.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Luzi S., Tobler M., Suter F., Meierhofer R. EAWAG; Switzerland: 2016. SODIS manual: guidance on solar water disinfection. [Google Scholar]
- Madaeni S.S. The application of membrane technology for water disinfection. Water Res. 1999;33(2):301–308. [Google Scholar]
- Majiya H., Chowdhury K.F., Stonehouse N.J., Millner P. TMPyP functionalised chitosan membrane for efficient sunlight driven water disinfection. J. Water Process Eng. 2019;30 UNSP 100475. [Google Scholar]
- Manoli K., Maffettone R., Sharma V.K., Santoro D., Ray A.K., Passalacqua K.D., Carnahan K.E., Wobus C.E., Sarathy S. Inactivation of murine norovirus and fecal coliforms by ferrate (VI) in secondary effluent wastewater. Environ. Sci. Technol. 2020;54(3):1878–1888. doi: 10.1021/acs.est.9b05489. [DOI] [PubMed] [Google Scholar]
- Marets N., Kuo D., Torrey J.R., Sakamoto T., Henmi M., Katayama H., Kato T. Highly efficient virus rejection with self-organized membranes based on a crosslinked bicontinuous cubic liquid crystal. Adv. Healthc. Materi. 2017;6(14):1700252. doi: 10.1002/adhm.201700252. [DOI] [PubMed] [Google Scholar]
- Marti E., Moncls H., Jofre J., Rodriguez-Roda I., Comas J., Balczar J.L. Removal of microbial indicators from municipal wastewater by a membrane bioreactor (MBR) Bioresour. Technol. 2011;102(8):5004–5009. doi: 10.1016/j.biortech.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Matsushita T., Shirasaki N., Matsui Y., Ohno K. Virus inactivation during coagulation with aluminum coagulants. Chemosphere. 2011;85(4):571–576. doi: 10.1016/j.chemosphere.2011.06.083. [DOI] [PubMed] [Google Scholar]
- Mazurkow J.M., Yuzbasi N.S., Domagala K.W., Pfeiffer S., Kata D., Graule T. Nano-sized copper (Oxide) on alumina granules for water filtration: effect of copper oxidation state on virus removal performance. Environ. Sci. Technol. 2020;54(2):1214–1222. doi: 10.1021/acs.est.9b05211. [DOI] [PubMed] [Google Scholar]
- Meister S., Verbyla M.E., Klinger M., Kohn T. Variability in disinfection resistance between currently circulating enterovirus B serotypes and strains. Environ. Sci. Technol. 2018;52(6):3696–3705. doi: 10.1021/acs.est.8b00851. [DOI] [PubMed] [Google Scholar]
- Meng X., Liu Z., Deng C., Zhu M., Wang D., Li K., Deng Y., Jiang M. Microporous nano-MgO/diatomite ceramic membrane with high positive surface charge for tetracycline removal. J. Hazard. Mater. 2016;320:495–503. doi: 10.1016/j.jhazmat.2016.08.068. [DOI] [PubMed] [Google Scholar]
- Michalsky R., Passarelli A.L., Pfromm P.H., Czermak P. Purification of the baculovirus Autographa californica M nucleopolyhedrovirus by tangential flow ultrafiltration. Desalination. 2009;245(1–3):694–700. [Google Scholar]
- Michen B., Graule T. Isoelectric points of viruses. J. Appl. Microbiol. 2010;109(2):388–397. doi: 10.1111/j.1365-2672.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- Miura T., Okabe S., Nakahara Y., Sano D. Removal properties of human enteric viruses in a pilot-scale membrane bioreactor (MBR) process. Water Res. 2015;75:282–291. doi: 10.1016/j.watres.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Nagaraj V., Skillman L., Li D., Ho G. Review-bacteria and their extracellular polymeric substances causing biofouling on seawater reverse osmosis desalination membranes. J. Environ. Manag. 2018;122:586–599. doi: 10.1016/j.jenvman.2018.05.088. [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China . 2006. Standards for Drinking Water Quality (GB5749-2006) [Google Scholar]
- Nazem-Bokaee H., Chen D.Y., O'Donnell S.M., Zydney A.L. Visualizing effects of protein fouling on capture profiles in the planova BioEX and 20N virus filters. J. Membrane Sci. 2020;610 [Google Scholar]
- Needle D.B., Selig M.K., Jackson K.A., Delwart E., Tighe E., Leib S.L., Seuberlich T., Pesavento P.A. Fatal bronchopneumonia caused by skunk adenovirus 1 in an african pygmy hedgehog. J. Vet. Diagn. Investig. 2019;31:103–106. doi: 10.1177/1040638718812123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K.L., Boehm A.B., Davies-Colley R.J., Dodd M.C., Kohn T., Linden K.G., Liu Y.Y., Maraccini P.A., McNeill K., Mitch W.A., Nguyen T.H., Parker K.M., Rodriguez R.A., Sassoubre L.M., Silverman A.I., Wigginton K.R., Zepp R.G. Sunlight-mediated inactivation of health-relevant microorganisms in water: a review of mechanisms and modeling approaches. Environ Sci Process Impacts. 2018;20:1089–1122. doi: 10.1039/c8em00047f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh Z., Szekeres G.P., Schabikowski M., Schrantz K., Traber J., Pronk W., Hernádi K., Graule T. Enhanced virus filtration in hybrid membranes with MWCNT nanocomposite. R. Soc. Open Sci. 2019;6(1) doi: 10.1098/rsos.181294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.M.H., Suwan P., Koottatep T., Beck S.E. Application of a novel, continuous-feeding ultraviolet light emitting diode (UV-LED) system to disinfect domestic wastewater for discharge or agricultural reuse. Water Res. 2019;153:53–62. doi: 10.1016/j.watres.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnadozie C.F., Kumari S., Bux F. Status of pathogens, antibiotic resistance genes and antibiotic residues in wastewater treatment systems. Rev. Environ. Sci. Bio. 2017;16(3):491–515. [Google Scholar]
- Nordgren J., Matussek A., Mattsson A., Svensson L., Lindgren P.E. Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res. 2009;43(4):1117–1125. doi: 10.1016/j.watres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Oates P.M., Shanahan P., Polz M.F. Solar disinfection (SODIS): simulation of solar radiation for global assessment and application for point-of-use water treatment in Haiti. Water Res. 2003;37(1):47–54. doi: 10.1016/s0043-1354(02)00241-5. [DOI] [PubMed] [Google Scholar]
- O'Brien E., Xagoraraki I. Removal of viruses in membrane bioreactors. J. Environ. Engi. 2020;146(7) [Google Scholar]
- O'Brien E., Nakyazze J., Wu H.Y., Kiwanuka N., Cunningham W., Kaneene J.B., Xagoraraki I. Viral diversity and abundance in polluted waters in Kampala, Uganda. Water Res. 2017;127:41–49. doi: 10.1016/j.watres.2017.09.063. [DOI] [PubMed] [Google Scholar]
- Osuolale O., Okoh A. J. Infect. Public Health. 2017;10:541–547. doi: 10.1016/j.jiph.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Pang X.L., Qiu Y.Y., Gao T.J., Zurawell R., Neumann N.F., Craik S., Lee B.E. Prevalence, levels and seasonal variations of human enteric viruses in six major rivers in Alberta, Canada. Water Res. 2019;153:349–356. doi: 10.1016/j.watres.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Park S.J., Ko Y.S., Jung H., Lee C., Woo K., Ko G.P. Disinfection of waterborne viruses using silver nanoparticle-decorated silica hybrid composites in water environments. Sci. Total Environ. 2018;625:477–485. doi: 10.1016/j.scitotenv.2017.12.318. [DOI] [PubMed] [Google Scholar]
- Prado T., Bruni A.D., Barbosa M.R.F., Garcia S.C., Melo A.M.D., Sato M.I.Z. Performance of wastewater reclamation systems in enteric virus removal. Sci. Total Environ. 2019;678:33–42. doi: 10.1016/j.scitotenv.2019.04.435. [DOI] [PubMed] [Google Scholar]
- Prado T., Bruni A.D., Barbosa M.R.F., Garcia S.C., Moreno L.Z., Sato M.I.Z. Noroviruses in raw sewage, secondary effluents and reclaimed water produced by sand-anthracite filters and membrane bioreactor/reverse osmosis system. Sci. Total Environ. 2019;646:427–437. doi: 10.1016/j.scitotenv.2018.07.301. [DOI] [PubMed] [Google Scholar]
- Prevost B., Lucas F.S., Moulin L., Ambert-Balay K., Pothier P., Moulin L., Wurtzer S. Deciphering the diversities of astroviruses and noroviruses in wastewater treatment plant effluents by a high-throughput sequencing method. Appl. Environ. Microbiol. 2015;81(20):7215–7222. doi: 10.1128/AEM.02076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell S., Ebdon J., Buck A., Tupper M., Taylor H. Removal of phages and viral pathogens in a full-scale MBR: implications for wastewater reuse and potable water. Water Res. 2016;100:20–27. doi: 10.1016/j.watres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Pype M.L., Donose B.C., Martí L., Patureau D., Wery N., Gernjak W. Virus removal and integrity in aged RO membranes. Water Res. 2016;90:167–175. doi: 10.1016/j.watres.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Lee B.E., Neumann N., Ashbolt N., Craik S., Maal-Bared R., Pang X.L. Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. J. Appl. Microbiol. 2015;119(6):1729–1739. doi: 10.1111/jam.12971. [DOI] [PubMed] [Google Scholar]
- Rachmadi A.T., Kitajim M., Watanabe K., Yaegashi S., Serrana J., Nakamura A., Nakagomi T., Nakagomi O., Katayama K., Okabe S., Sano D. Free-chlorine disinfection as a selection pressure on norovirus. Appl. Environ. Microbiol. 2018;84(13) doi: 10.1128/AEM.00244-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmadi A.T., Kitajima M., Kato T., Kato H., Okabe S., Sano D. Required chlorination doses to fulfill the credit value for disinfection of enteric viruses in water: a critical review. Environ. Sci. Technol. 2020;54(4):2068–2077. doi: 10.1021/acs.est.9b01685. [DOI] [PubMed] [Google Scholar]
- Rattanakul S., Oguma K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, legionella pneumophila, and surrogate microorganisms. Water Res. 2018;130:31–37. doi: 10.1016/j.watres.2017.11.047. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Tsai H.H., Williams M.D., Pirbazari M. Hybrid membrane bioreactor technology for small water treatment utilities: process evaluation and primordial considerations. J. Membrane Sci. 2009;344(1–2):39–54. [Google Scholar]
- Reddy P.V.L., Kavitha B., Reddy P.A.K., Kim K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: a review. Environ. Res. 2017;154:296–303. doi: 10.1016/j.envres.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Reed R.H. Solar inactivation of faecal bacteria in water: the critical role of oxygen. Lett. Appl. Microbiol. 1997;24:276–280. doi: 10.1046/j.1472-765x.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- Rosa G.L., Pourshaban M., Iaconelli M., Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann. Ist. Super. Sanita. 2010;46:266–273. doi: 10.4415/ANN_10_03_07. [DOI] [PubMed] [Google Scholar]
- Ryberg E.C., Chu C., Kim J.H. Edible dye-enhanced solar disinfection with safety indication. Environ. Sci. Technol. 2018;52(22):13361–13369. doi: 10.1021/acs.est.8b03866. [DOI] [PubMed] [Google Scholar]
- Rzezutka A., Cook N. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 2004;28(4):441–453. doi: 10.1016/j.femsre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Saif L.J., Bohl E.H., Theil K.W., Cross R.F., House J.A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J. Clin. Microbiol. 1980;12:105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin C., Burnett R.M. Book Series: Current Topics in Microbiology and Immunology. Vol. 272. 2003. Structural studies on adenoviruses. adenoviruses: model and vectors in virus-host interactions; pp. 57–94. [DOI] [PubMed] [Google Scholar]
- Sano D., Amarasiri M., Hata A., Watanabe T., Katayama H. Risk management of viral infectious diseases in wastewater reclamation and reuse: review. Environ. Int. 2016;91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D., Mondal B., Som A., Ravindran S.J., Jana S.K., Manju C.K., Pradeep T. Holey MoS2 nanosheets with photocatalytic metal rich edges by ambient electrospray deposition for solar water disinfection. Glob. Chall. 2018;2:1800052. doi: 10.1002/gch2.201800052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijven J., Teunis P., Suylen T., Ketelaars H., Hornstra L., Rutjes S. QMRA of adenovirus in drinking water at a drinking water treatment plant using UV and chlorine dioxide disinfection. Water Res. 2019;158:34–45. doi: 10.1016/j.watres.2019.03.090. [DOI] [PubMed] [Google Scholar]
- Shang C., Wong H.M., Chen G.H. Bacteriophage MS-2 removal by submerged membrane bioreactor. Water Res. 2005;39(17):4211–4219. doi: 10.1016/j.watres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Ohno K. Effects of reversible and irreversible membrane fouling on virus removal by a coagulation –microfiltration system. J. Water Supply Res Technol. 2008;57(7):501–506. [Google Scholar]
- Sima L.C., Schaeffer J., Le Saux J.C., Parnaudeau S., Elimelech M., Le Guyader F.S. Calicivirus removal in a membrane bioreactor wastewater treatment plant. Appl. Environ. Microbiol. 2011;77(15):5170–5177. doi: 10.1128/AEM.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45(12):3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sinclair T.R., Robles D., Raza B., van den Hengel S., Rutjes S.A., Husman A.M.D., de Grooth J., de Vos W.M., Roesink H.D.W. Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids Surf. A Physicochem. Eng. Asp. 2018;551:33–41. [Google Scholar]
- Sinclair T.R., Patil A., Raza B.G., Reurink D., van den Hengel S.K., Rutjes S.A., Husman A.M.D., Roesink H.D.W., de Vos W.M. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membrane Sci. 2019;570:494–503. [Google Scholar]
- Song K., Mohseni M., Taghipour F. Mechanisms investigation on bacterial inactivation through combinations of UV wavelengths. Water Res. 2019;163 doi: 10.1016/j.watres.2019.114875. [DOI] [PubMed] [Google Scholar]
- Sun P.Z., Tyree C., Huang C.H. Inactivation of E. coli, bacteriophage MS2 and bacillus spores under UV/H2O2 and UV/peroxydisulfate advanced disinfection conditions. Environ. Sci. Technol. 2016;50(8):4448–4458. doi: 10.1021/acs.est.5b06097. [DOI] [PubMed] [Google Scholar]
- Taboada-Santos A., Rivadulla E., Paredes L., Carballa M., Romalde J., Lema J.M. Comprehensive comparison of chemically enhanced primary treatment and high-rate activated sludge in novel wastewater treatment plant configurations. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115258. [DOI] [PubMed] [Google Scholar]
- Tanneru C.T., Chellam S. Mechanisms of virus control during iron electrocoagulation - microfiltration of surface water. Water Res. 2012;46(7):2111–2120. doi: 10.1016/j.watres.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Teixeira D.M., Hernandez J.M., Sliva L.D., Oliveira D.D., Spada P.K.D., Gurjão T.C.M., Mascarenhas J.D.P., Linhares A.C., Morais L.L.C.D., Gabbay Y.B. Occurrence of norovirus GIV in environmental water samples from Belem City, Amazon Region, Brazil. Food. Environ. Virol. 2016;8(1):101–104. doi: 10.1007/s12560-015-9220-6. [DOI] [PubMed] [Google Scholar]
- Templeton M.R., Andrews R.C., Hofmann R. Particle-associated viruses in water: impacts on disinfection processes. Crit. Rev. Env. Sci. Tec. 2008;38(3):137–164. [Google Scholar]
- Torii S., Hashimoto T., Do A.T., Furumai H., Katayama H. Impact of repeated pressurization on virus removal by reverse osmosis membranes for household water treatment. Environ. Sci.: Water Res. Technol. 2019;5(5):910–919. doi: 10.1016/j.scitotenv.2019.133814. [DOI] [PubMed] [Google Scholar]
- Torii S., Hashimoto T., Do A.T., Furumai H., Katayama H. Repeated pressurization as a potential cause of deterioration in virus removal by aged reverse osmosis membrane used in households. Sci. Total Environ. 2019;695 doi: 10.1016/j.scitotenv.2019.133814. [DOI] [PubMed] [Google Scholar]
- Ugo M. Human polyomaviruses and papillomaviruses. Int. J. Mol. Sci. 2018;19(8):2360. doi: 10.3390/ijms19082360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA . Guidance Manual for Compliance With the Filtration and Disinfection Requirements for Public Water Systems Using Surface Water Sources. EPA; 1991. pp. 1–580. 68-01-6989. [Google Scholar]
- US EPA . 2006. Prepublication of the Ground Water Rule Federal Register Notice. [Google Scholar]
- US EPA . 2006. Long Term 2 Enhanced Surface Water Treatment Rule: Toolbox Guidance Manual. [Google Scholar]
- US EPA . 2009. National Primary Drinking Water Regulations (EPA 816-F-09-004) [Google Scholar]
- Vu D.L., Sabrià A., Aregall N., Michl K., Garrido V.R., Goterris L., Bosch A., Pintó R.M., Guix S. Novel human astroviruses: prevalence and association with common enteric viruses in undiagnosed gastroenteritis cases in Spain. Viruses-Basel. 2019;11(7):585. doi: 10.3390/v11070585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman P., Meseguer A., Lucas F., Moulin L., Wurtzer S. Interaction of human enteric viruses with microbial compounds: implication for virus persistence and disinfection treatments. Environ. Sci. Technol. 2017;51(23):13633–13640. doi: 10.1021/acs.est.7b03875. [DOI] [PubMed] [Google Scholar]
- Walker D.C., Len S.V., Sheehan B. Development and evaluation of a reflective solar disinfection pouch for treatment of drinking water. Appl. Environ. Microbiol. 2004;70(4):2545–2550. doi: 10.1128/AEM.70.4.2545-2550.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Guan S.H., Sato A.N., Wang X., Wang Z., Yang R., Hsiao B.S., Chu B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membrane Sci. 2013;446(11):376–382. [Google Scholar]
- Wang K., Abdalla A.A., Khaleel M.A., Hilal N., Khraisheh M.K. Mechanical properties of water desalination and wastewater treatment membranes. Desalination. 2017;401:190–205. [Google Scholar]
- Wang H., Sikora P., Rutgersson C. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg. Envir. Heal. 2018;221(3):479–488. doi: 10.1016/j.ijheh.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann M., Michen B., Luxbacher T., Fritsch J., Graule T. Modification of ceramic microfilters with colloidal zirconia to promote the adsorption of viruses from water. Water Res. 2008;42(6):1726–1734. doi: 10.1016/j.watres.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Wegmann M., Michen B., Graule T. Nanostructured surface modification of microporous ceramics for efficient virus filtration. J. Eur. Ceram. Soc. 2009;28(8):1603–1612. [Google Scholar]
- Wen M.C., Wang C.L., Wang M.L., Cheng C.H., Wu M.J., Chen C.H., Shu K.H. Deching Chang. Association of JC virus with tubulointerstitial nephritis in a renal allograft recipient. J. Med. Virol. 2004;72:675–678. doi: 10.1002/jmv.20037. [DOI] [PubMed] [Google Scholar]
- Werber J.R., Osuji C.O., Elimelech M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016;1(5):16018. [Google Scholar]
- Wigginton K.R., Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr. Opin. Virol. 2012;2:84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton K.R., Pecson B.M., Sigstam T., Bosshard F., Kohn T. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012;46(21):12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- Wolf C., Gunten U., Kohn T. Kinetics of inactivation of waterborne enteric viruses by ozone. Environ. Sci. Technol. 2018;52(4):2170–2177. doi: 10.1021/acs.est.7b05111. [DOI] [PubMed] [Google Scholar]
- Wolf C., Pavese A., Gunten U., Kohn T. Proxies to monitor the inactivation of viruses by ozone in surface water and wastewater effluent. Water Res. 2019;166 doi: 10.1016/j.watres.2019.115088. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Guidelines for drinking-water quality. 2011. http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf
- Wu T.X., Liu G.M., Zhao J.C., Hidaka H., Serpone N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B. 1998;102:5845–5851. [Google Scholar]
- Wu J.L., Li H.T., Huang X. Indigenous somatic coliphage removal from a real municipal wastewater by a submerged membrane bioreactor. Water Res. 2010;44(6):1853–1862. doi: 10.1016/j.watres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Wu X.Y., Tang A.X., Bi X.C., Nguyen T.H., Yuan B.L. Influence of algal organic matter of Microcystis aeruginosa on ferrate decay and MS2 bacteriophage inactivation. Chemosphere. 2019;236 doi: 10.1016/j.chemosphere.2019.124727. [DOI] [PubMed] [Google Scholar]
- Xiao H., Guan D., Chen R., Chen P., Monagin C., Li W., Su J., Ma C., Zhang W., Ke C. Molecular characterization of echovirus 30-associated outbreak of aseptic meningitis in Guangdong in 2012. Virol. J. 2013;10:263. doi: 10.1186/1743-422X-10-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Jin M., Yang D., Guo X., Chen Z.L., Shen Z.Q., Wang X.W., Qiu Z.G., Wang J.F., Zhang B., Li J.W. Effects of chlorine and chlorine dioxide on human rotavirus infectivity and genome stability. Water Res. 2013;47(10):3329–3338. doi: 10.1016/j.watres.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y., Kimura T. Self-standing mesoporous membranes toward highly selective molecular transportation. Chem. Commun. 2013;49(97):11424–11426. doi: 10.1039/c3cc45766d. [DOI] [PubMed] [Google Scholar]
- Yasui N., Suwa M., Minamiyama M. Infectious risk assessment of reclaimed water by UF membrane treatment process focusing attention on norovirus. Water Sci. Technol. 2017;18(1):270–278. [Google Scholar]
- Yin Z.Q., Tarabara V.V., Xagoraraki I. Human adenovirus removal by hollow fiber membranes: effect of membrane fouling by suspended and dissolved matter. J. Membrane Sci. 2015;482:120–127. [Google Scholar]
- Zhang C.M., Xu L.M., Xu P.C., Wang X.C. Elimination of viruses from domestic wastewater: requirements and technologies. World J. Microbiol. Biotechnol. 2016;32(4):69. doi: 10.1007/s11274-016-2018-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Z., Mulvenna R.A., Qu S.Y., Boudouris B.W., Phillip W.A. Block polymer membranes functionalized with nanoconfined polyelectrolyte brushes achieve sub-nanometer selectivity. ACS Macro Lett. 2017;6(7):726–732. doi: 10.1021/acsmacrolett.7b00278. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li Y., Shuai D.M., Zhang W.L., Niu L.H., Wang L.F., Zhang H.J. Visible-light-driven, water-surface-floating antimicrobials developed from graphitic carbon nitride and expanded perlite for water disinfection. Chemosphere. 2018;208:84–92. doi: 10.1016/j.chemosphere.2018.05.163. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li Y., Shuai D.M., Shen Y., Xiong W., Wang L.Q. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: a review. Chemosphere. 2019;214:462–479. doi: 10.1016/j.chemosphere.2018.09.137. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Song X., Xu Y., Shen H., Kong X., Xu H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019;210:366–375. [Google Scholar]
- Zhang C., Li Y., Li J. Improved disinfection performance towards human adenoviruses using an efficient metal-free heterojunction in a Vis-LED photocatalytic membrane reactor: operation analysis and optimization. Chem. Eng. J. 2020;392 [Google Scholar]
- Zhang H., Tang W.Z., Chen Y.S., Yin W. Disinfection threatens aquatic ecosystems. Science. 2020;368(8487):146–147. doi: 10.1126/science.abb8905. [DOI] [PubMed] [Google Scholar]
- Zhao S.S., Ba C.Y., Yao Y.X., Zheng W.H., Economy J., Wang P. Removal of antibiotics using polyethylenimine cross-linked nanofiltration membranes: relating membrane performance to surface charge characteristics. Chem. Eng. J. 2018;335:101–109. [Google Scholar]
- Zheng X., Chen D., Wang Z., Lei Y., Cheng R. Nano-TiO2 membrane adsorption reactor (MAR) for virus removal in drinking water. Chem. Eng. J. 2013;230:180–187. [Google Scholar]
- Zheng X., Shen Z.P., Cheng C., Shi L., Cheng R., Yuan D.H. Photocatalytic disinfection performance in virus and virus/bacteria system by cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018;237:452–459. doi: 10.1016/j.envpol.2018.02.074. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D., Zhao X., Huang B.Y., Shi W.F., Lu R.J., Niu P.H., Zhan F.X., Ma X.J., Wang D.Y., Xu W.B., Wu G.Z., Gao G.G.F., Tan W.J. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.F., Chen R., Li Y.Y., Sano D. Virus removal by membrane bioreactors: a review of mechanism investigation and modeling efforts. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116522. [DOI] [PubMed] [Google Scholar]
- Zodrow K., Brunet L., Mahendra S., Li D., Zhang A., Li Q., Alvarez P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009;43(3):715–723. doi: 10.1016/j.watres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Zyara A.M., Torvinen E., Veijalainen A.M., Heinonen-Tanski H. The effect of chlorine and combined chlorine/UV treatment on coliphages in drinking water disinfection. J. Water Health. 2016;14(4):640–649. doi: 10.2166/wh.2016.144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material