Abstract
Purpose
The use of cerium oxide nanoparticles (CeO2 NPs), a lanthanide element oxide and bivalent compound, has been growing continuously in industry and biomedicine. Due to their wide application, the potential human health problems of CeO2 NPs have attracted attention, but studies on the toxicity of this compound to human eyes are lacking. This study investigated the cytotoxicity and reactive oxygen species (ROS) of CeO2 NPs in human retinal pigment epithelial cells (ARPE-19 cells).
Methods
Using the transmission electron microscope (TEM), the size distribution and shape of CeO2 NPs were characterized. To explore the effect of CeO2 NP size on ophthalmic toxicity in vitro, three sizes (15, 30 and 45 nm) of CeO2 NPs were investigated using ATP content measurement, LDH release measurement and cell proliferation assay in ARPE-19 cells. ROS values and mitochondrial membrane potential depolarization were evaluated by H2DCF-DA staining and JC-1 staining. Morphology changes were detected using a phase-contrast microscope.
Results
The cytotoxicity of 15 nm CeO2 NPs was found to be the highest and hence was further explored. Treatment with 15 nm CeO2 NPs caused the morphology of ARPE-19 cells to change in a dose- and time-dependent manner. Moreover, the treatment induced excessive ROS generation and mitochondrial membrane potential depolarization. In addition, cytotoxicity was attenuated by the application of a ROS scavenger N-acetyl-L- cysteine (NAC).
Conclusion
CeO2 NPs induced cytotoxicity in ARPE-19 cells and excessive production of ROS and decreasing mitochondrial membrane potential. The Overproduction of ROS partially contributes to CeO2 NP-induced cytotoxicity.
Keywords: nanomaterials, ophthalmic toxicity, oxidative stress, mitochondrial membrane potential depolarization
Introduction
Nanomaterials have unique properties, such as small size and enlarged surface area, that enhance regenerative and catalytic enzyme activities and, consequently, their biological effects. Cerium (Ce), a critical rare earth element with a unique f-electron configuration that gives its compounds special properties, has been called a universal new material.1 In cerium oxide nanoparticles (CeO2 NPs), there are two valence states-Ce3+ (reduced state) and Ce4+ (oxidation state)—and these two states can be converted to each other. The transition between Ce4+/Ce3+ on the crystal surface results in catalytic and antioxidant effects.1–3 Pezzini et al reported that CeO2 NPs serve as antioxidants in primary cultured skin fibroblasts.4 As free radical scavengers, CeO2 NPs can treat various diseases induced by oxidative stress.5 Recent studies have found that CeO2 NPs act as ROS scavengers in diabetic nephropathy,6 rheumatoid arthritis,7 and ischemic stroke.8 Furthermore, CeO2 NPs are widely used in single-phase or multiphase drug carriers or delivery devices to solve cancer drug resistance and mistargeting, and to achieve a synergistic anti-tumor activity with drugs.9–11 In addition, some studies have reported that CeO2 NPs are used in the treatment of eye diseases, such as to reduce light-induced retinal degeneration12 and photoreceptor death rate.13 Moreover, CeO2 NPs have been reported to act as antioxidants in the retina and protect against retinal nerve damage induced by high-intensity light exposure.14
Therefore, the widespread use of CeO2 NPs has raised human health concerns. It has been suggested that CeO2 NPs lead to ROS generation, DNA damage, and apoptosis in human lung cells.15,16 Moreover, CeO2 NPs induce cytotoxicity of the human hepatoma cell line SMMC-7721 through oxidative stress and activation of the MAPK signaling pathway.17 In addition, CeO2 NPs induce cytotoxicity and oxidative stress in human skin keratinocytes18 and genotoxicity in human intestinal Caco-2 cells.19 However, despite being an important and sensitive organ, the eyes have been ignored in evaluating the toxicity of CeO2 NPs; whether or not CeO2 NPs exert toxicity to other organs is largely unknown.
ROS are natural byproducts of normal oxidative metabolism and of free radicals such as the highly reactive hydroxyl radical (·OH) or superoxide anion radical (O2·–).20 ROS are unstable and highly reactive compounds that can strip electrons from nearby molecules and induce significant oxidative damage to cellular structures if the amount of ROS exceeds the system’s antioxidant capacity.21,22 The cytotoxicity effect is referred to as “oxidative stress,” which leads to a change in the mitochondrial membrane potential.23 In the whole life cycle of cells, mitochondria use oxidable substrates to produce an electrochemical proton gradient on the mitochondrial membrane, which is used to produce ATP and generate energy for cellular activities.24 The evaluation of the mitochondrial membrane potential (ΔΨ m) of intact cells can provide the necessary information to assess their physiological and pathological status.25,26
This study evaluated the toxicity of CeO2 NPs with different particle sizes in ARPE-19 cells, which are a type of human retinal pigment epithelial cell. We also determined the role of CeO2 NPs in ROS generation. In addition, we explored the change in mitochondrial membrane potential in response to CeO2 NPs treatment.
Materials and Methods
Chemical and Reagents
CeO2 NPs were purchased from Shanghai Xiangtian Nanomaterials Co., Ltd. (Shanghai, China); fetal bovine serum (FBS), DME/F−12 medium, and penicillin/streptomycin were obtained from Life Technologies (Carlsbad, CA, USA); N-acetylcysteine (NAC), and 2′,7′-Dichlorofluorescin diacetate (H2DCF-DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA); and a mitochondrial membrane potential assay kit with JC-1 was purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China).
Characterization of Different Sizes of CeO2 NPs
The size and morphology of CeO2 NPs were examined using a transmission electron microscope (TEM). Briefly, a drop of CeO2 NPs suspension at 50 µg/mL was tested under a TEM (200 kV, Tecnai F20, Philips, The Netherlands).
Cell Culture
The human retinal pigment epithelial cell line (ARPE-19 cells) was from the Fu Heng Cell Center (Shanghai, China). It was cultured with 10% FBS, penicillin (50 U/mL), and streptomycin (50 U/mL at 37°C in a humidified atmosphere with 5% CO2. Next, CeO2 NPs were dispersed in ultrapure water to prepare stock solutions (200 mg/mL). The stock solution was sonicated using a probe sonicator (Ningbo Xinzhi Biotechnology Co., Ltd., China) at 600 W for 40 min and diluted to different concentrations with culture medium and penicillin/streptomycin just before cell exposure. The cells were adjusted to a concentration of 1×105 cells/mL in a volume of 100 μL per well in 96-well plates for toxicity assays.
Cell Morphology
ARPE-19 cells were collected and seeded into 96-well plates at a density of 1×104 cells/well and cultured overnight in a CO2 incubator. Cells were exposed to CeO2 NPs at different concentrations (1–100 µg/mL) for 24 and 48 h. The cell morphological changes were examined using a phase-contrast microscope (Leica DM16000B, Germany).
Cell Viability Assay
The Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI, USA) was employed to examine cell viability. Following 24 or 48 h of exposure to CeO2 NPs at various concentrations, 10 μL of cell titer agents was added to each well. The 96-well plate was incubated in an incubator for 2 h. Absorbance was measured at 490 nm with the Synergy H4 Hybrid microplate reader (Bio Tek Instruments, Inc., Winowinsky, VT, USA).
Measurement of Cellular ATP Levels and Lactate Dehydrogenase (LDH) Release
Cell Titer-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA) was used to assess the ATP levels in CeO2 NPs-treated ARPE-19 cells according to the manufacturer’s instructions. Luminescence was recorded using a Synergy H4 Hybrid microplate reader.
The cytotoxicity of CeO2 NPs was examined using the LDH Release Assay (Beyotime, Beijing, China) as described previously.27
Measurement of Intracellular ROS
Intracellular ROS concentration was measured using the fluorescent dye H2DCF-DA.27 Briefly, ARPE-19 cells were treated with 10 μM H2DCF-DA for 30 min in the cell culture incubator. The cells were washed twice with PBS and then treated with 3.125–100 μg/mL CeO2 NPs in phenol-red-free medium. The cells were continuously incubated, and the fluorescence intensities were measured at 6, 12, 24, and 48 h time points with a Synergy H4 Hybrid microplate reader. Meanwhile, the oxidation of H2DCF-DA was detected using a confocal laser scanning microscope (Leica TCS SP5, Germany) at the 24- and 48-h time points.
Detection of Mitochondrial Membrane Potential
JC-1 Staining Kit (Beyotime, Beijing, China) was used to assess mitochondrial membrane potential changes. Cells were seeded on dishes at a density of 1×105 cell/mL and stored overnight. The cells were treated with different concentrations of CeO2 NPs for 24 and 48 h. At the end of treatment, cells were removed from the medium, washed three times with PBS, and then incubated with JC-1 staining kit (20 μM) for 15 min. The JC-1 staining solution was removed, and the cells were washed three times. PBS was added for imaging by confocal laser scanning microscopy (Leica TCS SP5, Germany).
Statistical Analysis
Results were presented as the mean ± standard deviation (SD). Analyses were performed using Graph Pad Prism 6 (Graph Pad Software; La Jolla, CA, USA). Statistical significance was determined by one-way analysis of variance (ANOVA) followed by the Dunnett’s tests for comparisons between different concentrations to vehicle control or two-way ANOVA followed by the Sidak’s multiple comparisons test for comparisons of two treatment groups in NAC pretreatment experiments. The differences were considered statistically significant when the p value was <0.05.
Results
Characterization of CeO2 NPs
The TEM images of the different sizes of CeO2 NPs are presented in Figure 1. The images showed that the average sizes of CeO2 NPs were about 15 ± 5 nm, 30 ± 5 nm, and 45 ± 5 nm (Figure 1A–C, left panels). The enlarge images showed shapes of CeO2 NPs were mostly irregular spheres (Figure 1A–C, right panels).
Figure 1.
Characterization of CeO2 NPs by transmission electron microscopy (TEM), showing nanoparticles with average diameters of (A) 15 ± 5 nm, (B) 30 ± 5 nm, and (C) 45 ± 5 nm. (Left image scale bar: 50 nm and right image scale bar: 10 nm).
Cytotoxicity of CeO2 NPs in ARPE-19 Cells
A previous study showed that CeO2 NPs induced toxicity in human lung cells.16 In our present study using ARPE-19 cells we compared the cytotoxicity of CeO2 NPs of different diameters, including 15, 30 and 45 nm. The cytotoxicity was determined using three parameters, namely ATP content, LDH release and MTS viability assay (Figure 2). ARPE-19 cells were treated with different sizes of CeO2 NPs at concentrations of 3.125 μg/mL to 100 μg/mL for 24 and 48 h. As shown in Figure 2A and B, CeO2 NPs caused a time-dependent decrease in ATP content (Figure 2A and B). In addition, our results indicate that CeO2 NPs lead to increased LDH release (Figure 2C and D) and growth inhibition (Figure 2E and F), and that ARPE-19 cells have the highest sensitivity to 15 nm CeO2 NPs. Therefore, in the current study, we focused the following studies on 15 nm CeO2 NPs.
Figure 2.
CeO2 NPs induced cytotoxicity in ARPE-19 cells. ARPE-19 cells were exposed to different concentrations (3.125–100 μg/mL) of CeO2 NPs for (A, C and E) 24 h and (B, D and F) 48 h before measurements of (A and B) ATP content, (C and D) LDH release and (E and F) cytotoxicity determined using the MTS assay. Data points represent the mean ± SD from three independent experiments with three samples per concentration in each experiment. *p < 0.05 compared to controls.
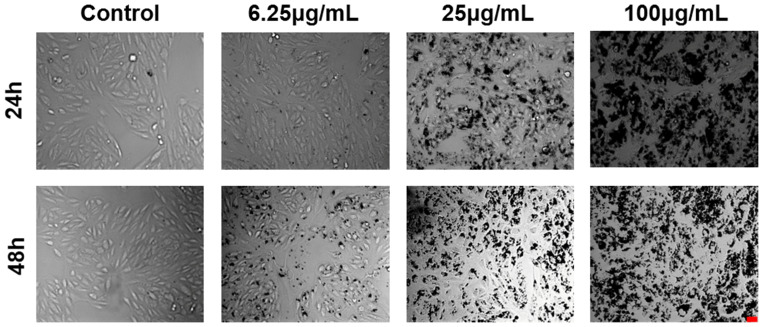
Figure 3.
CeO2 NPs induced morphological changes in cells. Morphological changes of ARPE-19 cells were observed via microscopy following 24 h and 48 h of exposure to CeO2 NPs with indicated concentrations. (Scale bar: 25 μm.).
CeO2 NPs Induce Morphological Changes in Cells
The morphology of ARPE-19 cells changed with the increase of CeO2 NP concentration. Morphological analysis of ARPE-19 cells exposed to CeO2 NPs showed that the morphology of ARPE-19 cells became irregular starting from the concentration of 25 μg/mL after 24 h of exposure (Figure 3). At 48 h, the changes of cell morphology became more prominent with increasing concentration. At 100 μg/mL, most cells detached, and the density was reduced.
CeO2 NPs Induce ROS Generation
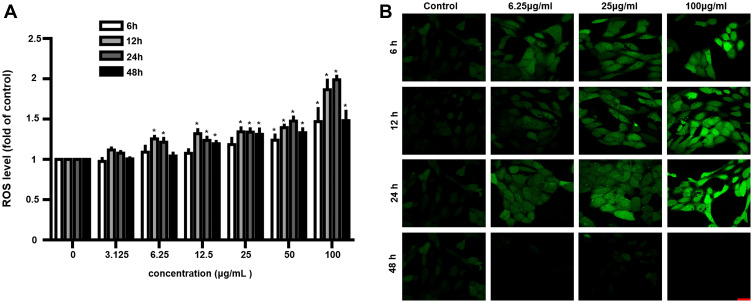
Cytotoxicity can result from ROS accumulation, so it is of interest to investigate whether CeO2 NPs induce ROS generation in human retinal pigment epithelial cells. Therefore, ARPE-19 cells were treated with CeO2 NPs at concentrations ranging between 3.125 μg/mL and 100 μg/mL, and ROS production was monitored at 6, 12, 24 and 48 h (Figure 4A). ROS generation was observed as early as during the 12-h treatment with 6.25 μg/mL. The maximum ROS induction was two-fold that of the control at 24 h and 100 μg/mL CeO2 NPs treatment. At 48 h, ROS levels were less remarkable compared to those at 12 and 24 h even though the production of ROS at 48 h remained significantly elevated compared to the corresponding control. For instance, at the concentration of 12.5 μg/mL, ROS generation was 1.19-fold higher at 48 h for CeO2 NPs, whereas the ROS levels were 1.35-fold higher and 1.23-fold higher at 12 and 24 h, respectively. The reduction in ROS may result from decreased cell viability (Figure 2). To further verify the ROS generation results, we performed ROS fluorescence staining. ARPE-19 cells were treated with CeO2 NPs at concentrations of 6.25, 25 and 100 μg/mL. Confocal laser scanning microscopy (CLSM) images showed that ROS levels increased (Figure 4B) as the incubation time increased. The increased intensity of the ROS indicator suggested that oxidative stress resulted from CeO2 NPs treatment.
Figure 4.
CeO2 NPs induced ROS generation. (A) ROS levels were measured at 6, 12, 24 and 48 h after exposure to various concentrations (3.125–100 μg/mL) of CeO2 NPs by H2DCF-DA staining. (B) ROS levels were monitored under CLSM, which showed that ROS levels increased following 6, 12, 24, and 48 h of exposure to CeO2 NPs with concentrations of 25 and 100 μg/mL. Data points represent the mean ± SD from three independent experiments with three samples per concentration. *p < 0.05 compared to controls. (Scale bar: 25 μm.).
CeO2 NPs Induce Mitochondrial Dysfunction
Mitochondrial dysfunction can lead to cellular energetic depression, which may result in cell death. In addition, mitochondria are the main sites of ATP and ROS generation. Mitochondrial depolarization (the ΔΨm decrease) can lead to ROS accumulation and decreased ATP level. Next, we explored whether CeO2 NPs cause mitochondrial depolarization in ARPE-19 cells. The ΔΨm was accessed by JC-1 dye; the accumulation of JC-1 in organelles leads to the formation of red J-aggregates (emission maximum at 590 nm) at higher mitochondrial concentrations, reflecting higher mitochondrial potential, which, in addition to the typical green fluorescence of J-monomers (emission maximum of 529 nm) at lower mitochondrial concentrations, indicates loss of membrane potential. The ARPE-19 cells were treated with CeO2 NPs at concentrations of 6.25, 25 and 100 μg/mL for 24 and 48 h. The decreased ΔΨm of ARPE-19 cells was observed as early as the 24-h treatment with 6.25 μg/mL(Figure 5A). JC-1 staining images showed that the transition from red fluorescence to green fluorescence became more obvious at 48 h (Figure 5B), which suggested that CeO2 NPs induced a significant time- and concentration-dependent decrease of ΔΨm.
Figure 5.
CeO2 NPs induce mitochondrial dysfunction. ARPE-19 cells were treated with three concentrations (6.25, 25 and 100 μg/mL) of CeO2 NPs for 24 h (A) and 48 h (B). JC-1 staining was performed to assess mitochondrial membrane potential. (Scale bar: 25 μm.).
CeO2 NPs-Induced Cytotoxicity is Attenuated by the ROS Scavenger
To investigate further the role of ROS generation in the cytotoxicity of CeO2 NPs, we used a ROS scavenger, NAC, to suppress intracellular ROS levels. Pretreating ARPE-19 cells with 10 mM NAC for 1 h, prior to exposures of 3.125 −100 μg/mL CeO2 NPs for 12h, significantly attenuated ROS induction and confirmed the effectiveness of NAC pretreatment (Figure 6A). The NAC pretreatment alleviated CeO2 NPs-induced cytotoxicity, as evidenced by reductions in both ATP content (Figure 6B) and LDH release (Figure 6C) in NAC pretreated groups. These results demonstrated that CeO2 NPs cytotoxicity was partially mediated by ROS generation.
Figure 6.
NAC pretreatment alleviates CeO2 NPs-induced cytotoxicity. (A) Intracellular ROS levels were measured after a 12-h CeO2 NPs treatment with and without 1-h pretreatment of 10 mM NAC. (B and C) ATP content and LDH release were evaluated after a 24-h CeO2 NPs treatment with and without 1-h pretreatment of 10 mM NAC. The data points represent the mean ± SD from at least three independent experiments. *,#p < 0.05 compared to the vehicle control without or with NAC pretreatment, respectively. &p < 0.05 between the treatments with and without NAC pretreatment at the same concentration of CeO2 NPs.
Discussion
Given the wide application of CeO2 NPs in the biomedical field raises safety concerns for human health. The toxicity of CeO2 NPs has been studied previously by some investigator.15,28,29 Characteristics of nanomaterials, including synthesis methodologies, size, and coating, affect their toxicity.30 Unfortunately, these previous studies ignored some of these characteristics on CeO2 NPs-induced cytotoxicity. In our present study, we investigated whether the size can affect the cytotoxicity of CeO2 NPs. We assessed the toxicity of CeO2 NPs of different sizes (15 ± 5 nm, 30 ± 5 nm and 45 ± 5 nm) in ARPE-19 cells. Lin et al16 and Mittal et al15 studied the fate of 20 and 177 nm CeO2 NPs in human lung cells. They found that CeO2 NPs can induce oxidative stress, DNA damage and apoptosis in A549 cells. In SMMC-7721 cells, exposure to hexahedral CeO2 NPs with a size of 20–30 nm induced apoptosis and oxidative stress by activation of MAPK signaling pathways.17 Recently, the therapeutic effects of CeO2 NPs in the retinal degenerative process were reported.13,14 With the widespread application of CeO2 NPs to the treatment of ocular diseases, its ocular toxicity requires the attention of scientists and ophthalmologists. Therefore, we used ARPE-19 cells to study the ocular toxicity of CeO2 NPs.
Previous studies indicate that the size of nanoparticles significantly alters their toxicity potential. For example, AgNPs exhibit cytotoxicity and genotoxicity in a size-dependent manner in L5718Y cells.30 Kim et al studied the toxicity of silica nanoparticles with diameters of 20–200 nm in A549 epithelial cells, HepG2 epithelial cells and NIH/3T3 fibroblasts. They found that the cytotoxicity changed in a size-, dose- and cell type-dependent manner.31 Interestingly, among a group of silica nanoparticles ranging in size from 20 to 200 nm, the 60 nm silica nanoparticles exhibited the highest toxicity. However, whether the toxicity of CeO2 NPs is size dependent has not been reported. In this study, we focused on the toxicity of CeO2 NPs of three sizes. First, the particle morphology and average size of CeO2 NPs were examined by TEM (Figure 1). The images showed three dimensions: 15 ± 5 nm, 30 ± 5 nm and 45 ± 5 nm. Next, we compared the cytotoxicity of these three kinds of CeO2 NPs (Figure 2). All three of the tested CeO2 NPs induced different magnitudes of cytotoxicity in ARPE-19 cells. Among them, the 15 nm CeO2 NPs showed the highest cytotoxicity. Thus, in the subsequent toxicity studies, only 15 nm CeO2 NPs were used.
Mittal et al found that 8–20 nm CeO2 NPs accumulated in the cytoplasm of A549 cells, resulting in cell morphology changes.15 We tested whether the cytotoxicity of 15 nm CeO2 NPs affects the morphology of ARPE-19 cells. The density of ARPE-19 cells treated with 15 nm CeO2 NPs became lower in a time- and concentration-dependent manner, and the shape of cells became ambiguous (Figure 3). In the past decade, CeO2 NPs have been reported as one kind of antioxidants for scavenging ROS in many diseases,32 including cancer,33 ocular diseases14 and neurodegenerative diseases.34 However, some studies reported that the small particle size and large reactive surface of nanomaterials can lead to toxicity through the production of ROS and oxidative stress.35,36 It has been reported that ROS accumulation is an upstream event that triggers cytotoxicity.37 Thus, we investigated whether CeO2 NPs induce ROS overproduction in ARPE-19 cells. We found that ROS generation resulted from CeO2 NPs treatment and occurred at a lower concentration and earlier (Figures 4 and 6). Studies have reported ROS overproduction accompanied by mitochondrial membrane potential depolarization.15,38,39 In this study, CeO2 NPs exhibited the ability to induce mitochondrial dysfunction (Figure 5). The result suggested that the excessive production of ROS leads to mitochondrial dysfunction. Our previous study showed it is likely that ROS overproduction is the upstream event triggering cytotoxicity.37 In the present study, we confirmed this phenomenon also existed in the CeO2 NPs-induced cytotoxicity. This assertion was evidenced by the following assays: inhibition of ROS significantly diminished LDH release and cellular ATP depletion caused by CeO2 NPs (Figure 6). It is worth noting that NAC only showed modest protective effect on CeO2 NPs-induced cytotoxicity (Figure 6B and C); therefore, CeO2 NPs-induced ROS may not be the only cause of cytotoxicity.
Conclusion
In summary, the current study suggests that CeO2 NPs induce morphological alteration and cytotoxicity in a time- and dose-dependent manner. Oxidative stress, including ROS overproduction and mitochondrial membrane potential depolarization, may be part of the cause of CeO2 NP-induced toxicity. Our results provide new insights into the toxicity of CeO2 NPs in ophthalmologic research and improve our understanding of potential hazards associated with the application of CeO2 NPs for treating eye diseases. However, this study did not explore molecular pathways related to the CeO2 NP-induced toxicity in ARPE-19 cells. For a better understanding of which signal pathways play a crucial role in CeO2 NP-induced ocular toxicity, additional studies are needed.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81970826 and 81500743), awarded to Zhuhong Zhang, and the Talent Induction Program for Youth Innovation Teams in Colleges and University of Shandong Province to Yanping Zhu.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Celardo I, Pedersen JZ, Traversa E, Ghibelli LJN. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420. doi: 10.1039/c0nr00875c [DOI] [PubMed] [Google Scholar]
- 2.Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Redox-active radical scavenging nanomaterials. Chem Soc Rev. 2010;39(11):4422–4432. doi: 10.1039/b919677n [DOI] [PubMed] [Google Scholar]
- 3.Ponnurangam S, O’Connell GD, Chernyshova IV, Wood K, Hung CT, Somasundaran P. Beneficial effects of cerium oxide nanoparticles in development of chondrocyte-seeded hydrogel constructs and cellular response to interleukin insults. Tissue Eng Part A. 2014;20(21–22):2908–2919. doi: 10.1089/ten.tea.2013.0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pezzini I, Marino A, Del Turco S, et al. Cerium oxide nanoparticles: the regenerative redox machine in bioenergetic imbalance. Nanomed. 2017;12(4):403. doi: 10.2217/nnm-2016-0342 [DOI] [PubMed] [Google Scholar]
- 5.Casals E, Zeng M, Parra-Robert M, et al. Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small. 2020;16(20):e1907322. doi: 10.1002/smll.201907322 [DOI] [PubMed] [Google Scholar]
- 6.Tong Y, Zhang L, Gong R, et al. A ROS-scavenging multifunctional nanoparticle for combinational therapy of diabetic nephropathy. Nanoscale. 2020;12(46):23607–23619. doi: 10.1039/D0NR06098D [DOI] [PubMed] [Google Scholar]
- 7.Kalashnikova I, Chung SJ, Nafiujjaman M, et al. Ceria-based nanotheranostic agent for rheumatoid arthritis. Theranostics. 2020;10(26):11863–11880. doi: 10.7150/thno.49069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CK, Kim T, Choi IY, et al. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl. 2012;51(44):11039–11043. doi: 10.1002/anie.201203780 [DOI] [PubMed] [Google Scholar]
- 9.Gao R, Mitra RN, Zheng M, Wang K, Dahringer JC, Han Z. Developing nanoceria-based ph-dependent cancer-directed drug delivery system for retinoblastoma. Adv Funct Mater. 2018;28(52):1806248. doi: 10.1002/adfm.201806248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhammad F, Wang A, Qi W, Zhang S, Zhu G. Intracellular antioxidants dissolve man-made antioxidant nanoparticles: using redox vulnerability of nanoceria to develop a responsive drug delivery system. ACS Appl Mater Interfaces. 2014;6(21):19424–19433. doi: 10.1021/am5055367 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Wu X, Hou C, et al. Dual-responsive dithio-polydopamine coated porous CeO2 nanorods for targeted and synergistic drug delivery. Int J Nanomed. 2018;13:2161–2173. doi: 10.2147/IJN.S152002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tisi A, Passacantando M, Lozzi L, Maccarone R. Cerium oxide nanoparticles reduce the accumulation of autofluorescent deposits in light-induced retinal degeneration: insights for age-related macular de generation. Exp Eye Res. 2020;199:108169. doi: 10.1016/j.exer.2020.108169 [DOI] [PubMed] [Google Scholar]
- 13.Wong LL, Pye QN, Lijuan C, Sudipta S, Mcginnis JF, Knut S. Defining the catalytic activity of nanoceria in the P23H-1 rat, a photoreceptor degeneration model. PLoS One. 2015;10(3):e0121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tisi A, Passacantando M, Lozzi L, Riccitelli S, Bisti S, Maccarone R. Retinal long term neuroprotection by cerium oxide nanoparticles after an acute damage induced by high intensity light exposure. Exp Eye Res. 2019;182:30–38. doi: 10.1016/j.exer.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Mittal S, Pandey AK. Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. BioMed Res Int. 2014;2014:891934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin W, Huang YW, Zhou XD, Ma YJ. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25(6):451–457. doi: 10.1080/10915810600959543 [DOI] [PubMed] [Google Scholar]
- 17.Cheng G, Guo W, Han L, et al. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways. Toxicol In Vitro. 2013;27(3):1082–1088. doi: 10.1016/j.tiv.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 18.Ngoc LTN, Bui VKH, Moon JY, Lee YC. In-vitro cytotoxicity and oxidative stress induced by cerium aminoclay and cerium oxide nanoparticles in human skin keratinocyte cells. J Nanosci Nanotechnol. 2019;19(10):6369–6375. doi: 10.1166/jnn.2019.17035 [DOI] [PubMed] [Google Scholar]
- 19.Fisichella M, Berenguer F, Steinmetz G, Auffan M, Rose J, Prat O. Toxicity evaluation of manufactured CeO2 nanoparticles before and after alteration: combined physicochemical and whole-genome expression analysis in Caco-2 cells. BMC Genomics. 2014;15:700. doi: 10.1186/1471-2164-15-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halliwell BJ. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2010;97(6):1634–1658. [DOI] [PubMed] [Google Scholar]
- 22.Markovic Z, Trajkovic VJB. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials. 2008;29(26):3561–3573. doi: 10.1016/j.biomaterials.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 23.Caputo F, De Nicola M, Sienkiewicz A, et al. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. J Nanoscale. 2015;7(38):15643–15656. doi: 10.1039/C5NR03767K [DOI] [PubMed] [Google Scholar]
- 24.Logan A, Pell VR, Shaffer KJ, et al. Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry. Cell Metab. 2016;23(2):379–385. doi: 10.1016/j.cmet.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picard M, McEwen BS, Epel ES, Sandi C. An energetic view of stress: focus on mitochondria. Front Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seppet E, Gruno M, Peetsalu A, et al. Mitochondria and energetic depression in cell pathophysiology. Int J Mol Sci. 2009;10(5):2252–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Chen S, Mei H, et al. Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci Rep. 2015;5:14633. doi: 10.1038/srep14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balaji S, Mandal BK, Vinod Kumar Reddy L, Sen D. Biogenic ceria nanoparticles (CeO2 NPs) for effective photocatalytic and cytotoxic activity. Bioengineering. 2020;7(1):26. doi: 10.3390/bioengineering7010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahamed M, Akhtar MJ, Khan MAM, Alaizeri ZM, Alhadlaq HA. Evaluation of the cytotoxicity and oxidative stress response of CeO2-RGO nanocomposites in human lung epithelial A549 Cells. Nanomaterials. 2019;9(12):1709. doi: 10.3390/nano9121709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X, Li Y, Yan J, et al. Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays. Nanotoxicology. 2016;10(9):1373–1384. doi: 10.1080/17435390.2016.1214764 [DOI] [PubMed] [Google Scholar]
- 31.Kim IY, Joachim E, Choi H, Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine. 2015;11(6):1407–1416. doi: 10.1016/j.nano.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 32.Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine. 2013;8(9):1483–1508. doi: 10.2217/nnm.13.133 [DOI] [PubMed] [Google Scholar]
- 33.Wason MS, Zhao J. Cerium oxide nanoparticles: potential applications for cancer and other diseases. Am J Transl Res. 2013;5(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon HJ, Cha MY, Kim D, et al. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano. 2016;10(2):2860–2870. doi: 10.1021/acsnano.5b08045 [DOI] [PubMed] [Google Scholar]
- 35.Xia T, Kovochich M, Liong M, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Ren Z, Chen S, et al. ROS generation and JNK activation contribute to 4-methoxy-TEMPO-induced cytotoxicity, autophagy, and DNA damage in HepG2 cells. Arch Toxicol. 2018;92(2):717–728. doi: 10.1007/s00204-017-2084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Wang Y, Guo R, et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release. 2020;317:259–272. doi: 10.1016/j.jconrel.2019.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X, Chen S, Zhang Z, et al. Reactive oxygen species and c-Jun N-terminal kinases contribute to TEMPO-induced apoptosis in L5178Y cells. Chem Biol Interact. 2015;235:27–36. doi: 10.1016/j.cbi.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]