Abstract
Oxidative stress (OS) is one of the most significant propagators of systemic damage with implications for widespread pathologies such as vascular disease, accelerated aging, degenerative disease, inflammation, and traumatic injury. OS can be induced by numerous factors such as environmental conditions, lifestyle choices, disease states, and genetic susceptibility. It is tied to the accumulation of free radicals, mitochondrial dysfunction, and insufficient antioxidant protection, which leads to cell aging and tissue degeneration over time. Unregulated systemic increase in reactive species, which contain harmful free radicals, can lead to diverse tissue-specific OS responses and disease. Studies of OS in the brain, for example, have demonstrated how this state contributes to neurodegeneration and altered neural plasticity. As the worldwide life expectancy has increased over the last few decades, so has the prevalence of OS-related diseases resulting from age-associated progressive tissue degeneration. Unfortunately, vital translational research studies designed to identify and target disease biomarkers in human patients have been impeded by many factors (e.g. limited access to human brain tissue for research purposes and poor translation of experimental models). In recent years, stem cell-derived three-dimensional tissue cultures known as “brain organoids” have taken the spotlight as a novel model for studying central nervous system diseases. In this review, we discuss the potential of brain organoids to model the responses of human neural cells to OS, noting current and prospective limitations. Overall, brain organoids show promise as an innovative translational model to study CNS susceptibility to OS and elucidate the pathophysiology of the aging brain.
Keywords: Oxidative stress, stem cells, brain organoids, neurodevelopment, aging
Introduction
Oxidative stress and the associated increases in inflammatory markers have long been known to play major roles in both the normal aging process as well as in progressive degenerative disease states including cerebrovascular disease, Alzheimer’s disease (AD), Parkinson’s disease (PD), and neurodevelopmental deficits (Cenini, Lloret, & Cascella, 2019; De Silva & Miller, 2016; Hensley et al., 1996; Ikonomidou & Kaindl, 2011; Metodiewa & Koska, 2000; Sorolla et al., 2008). Indeed, the World Health Organization reports that global efforts are underway to treat aging-related diseases (Tan, Norhaizan, Liew, & Sulaiman Rahman, 2018). Increases in the average human lifespan, thanks to scientific advancements in healthcare, are now at odds with an increased susceptibility to neurocognitive disease (A. Reynolds, Laurie, Mosley, & Gendelman, 2007). Recent studies suggest that as the natural protective mechanisms of the central nervous system (CNS) become less effective with age, oxidative stress and aberrant cell signaling lead to tissue damage, cognitive dysfunction, and behavioral changes (J. K. Andersen, 2004; Berr, Balansard, Arnaud, Roussel, & Alperovitch, 2000; d’Avila et al., 2018; Droge & Schipper, 2007; Vollert et al., 2011) (Figure 1). Despite our current understanding of oxidative stress-induced pathological changes at the tissue level, a lack of knowledge about the etiology of cell-specific changes has presented a major challenge (Markesbery, 1997).
Figure 1.
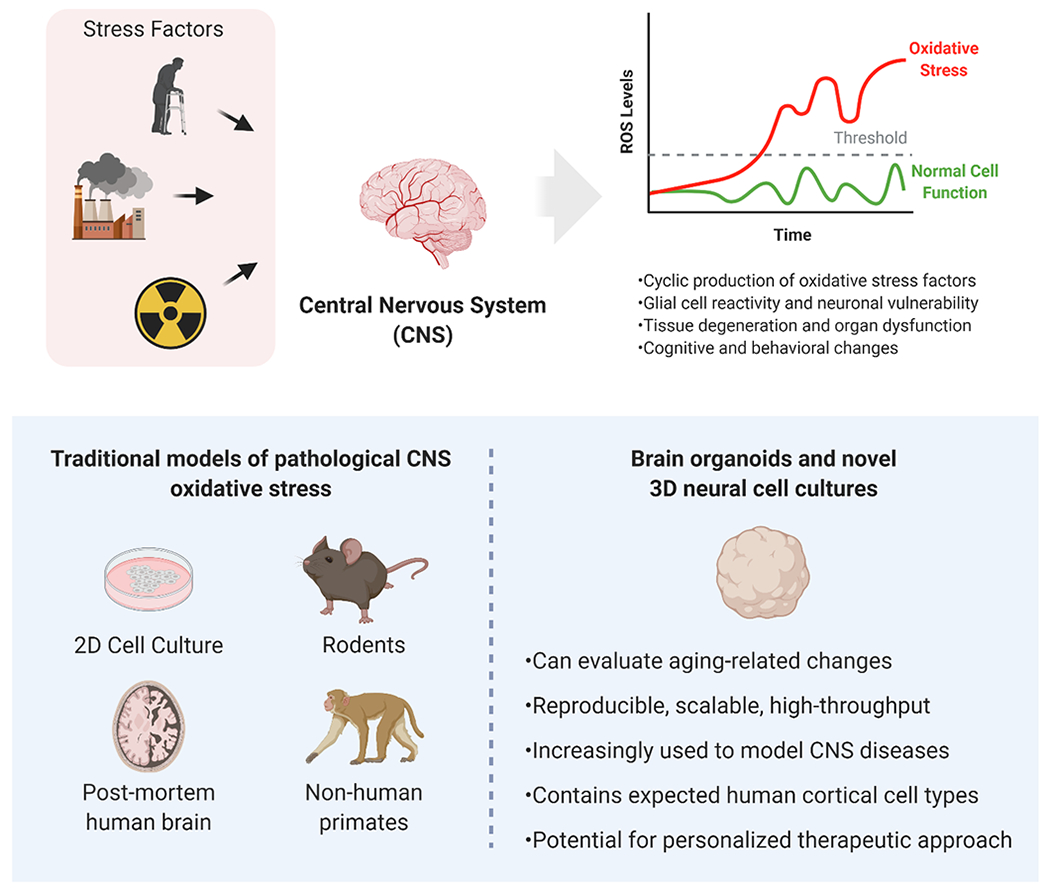
Oxidative stress (OS) within the Central Nervous System (CNS) can be produced by numerous stress factors such as aging, pollution, and exposure to ionizing radiation. Cyclic production of OS factors contributes to damage and disease in a cell-, tissue-, and organ-specific manner. Although traditional models of OS in the CNS are available, three-dimensional cell cultures, notably brain organoids, offer some advantages and novel insights for translational studies. Created with BioRender.com
Oxidative stress has been linked to neural cell stress responses (e.g. altered cell morphology, function, and viability) and progressive endothelial dysfunction (i.e. increasing vascular permeability of the blood brain barrier) and is a critical component of the pathophysiology of CNS diseases (Chong, Li, & Maiese, 2005; Jenner, 2003; Kunsch & Medford, 1999; Taibur Rahman, 2012). Chronically elevated reactive oxygen species (ROS) and cyclical, low level (sometimes sub-clinical) inflammatory responses are increasingly recognized as hallmarks of neurological disease (Halliwell, 1992; Koelink et al., 2012).
Physiological and Pathological roles of Reactive Oxygen Species
ROS are broadly defined as oxygen-containing chemical species with reactive properties (reactive molecules, free radicals, and nonradical species) and include superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) (Schieber & Chandel, 2014; J. Zhang et al., 2016). Under normal physiological conditions, ROS act as cell signaling molecules and are critical in maintaining essential cellular and tissue level processes (Finkel, 2011; Schieber & Chandel, 2014) in addition to maintaining homeostasis (Schieber & Chandel, 2014; J. Zhang et al., 2016). These processes include, but are not limited to, differentiation, proliferation, growth, apoptosis, morphological changes, and migration (Brieger, Schiavone, Miller, & Krause, 2012). For example, energy production in the mitochondria as well as immune defense functions involving peroxisomes and NADPH-dependent enzymes both result in elevated levels of reactive species (Finkel, 2011; Tarafdar & Pula, 2018).
Increases in ROS concentrations can result from tissue damage, disease, and/or dysregulation of normal cellular function (Abdul-Muneer, Chandra, & Haorah, 2015). Additionally, ROS production can be elevated by external factors such as drugs, poor diet, radiation, air pollutants, and environmental chemicals (Gandhi & Abramov, 2012; Joseph, Shukitt-Hale, Casadesus, & Fisher, 2005; Ryter et al., 2007). Given enough time, excessive levels of concentrated ROS can become detrimental to tissue (Ahmadinejad, Geir Moller, Hashemzadeh-Chaleshtori, Bidkhori, & Jami, 2017; Sies, Berndt, & Jones, 2017). To prevent this process of oxidative damage, natural and artificial antioxidants serve as reactive species “scavengers” (Pisoschi & Pop, 2015). The imbalance between prooxidant reactive species and antioxidant scavengers is the primary component of oxidative stress (Dalle-Donne, Rossi, Colombo, Giustarini, & Milzani, 2006; Li, Jia, & Trush, 2016).
During a state of oxidative stress, high ROS levels accelerate cell aging and promote damage to nucleic acids, carbohydrates, proteins, and lipid membranes (Berlett & Stadtman, 1997; Li et al., 2016; Raha & Robinson, 2000; Sohal, 2002). Although cells can protect themselves by employing antioxidants to scavenge ROS, dysfunction and insufficient activity of these agents can result in a chain reaction of oxidative damage that causes DNA strand breaks, increased protein aggregation, and lipid peroxidation (Birben, Sahiner, Sackesen, Erzurum, & Kalayci, 2012; Kalyanaraman, 2013). This damage can lead to cell cycle arrest, signaling pathway dysregulation, and local upregulation of inflammatory factors, consequently causing widespread tissue damage. Accordingly, excessive levels of ROS are reported to play an important role in the development of chronic inflammation (Biswas, 2016). Early in the inflammatory response, oxidative stress induces cells to release proinflammatory cytokines that can contribute to cell activation and tissue remodeling (Zuo et al., 2019). Left unchecked, this response can result in extensive tissue damage (including long-term functional and morphological changes), further release of inflammatory factors, and chronically elevated levels of ROS (D’Ambrosio, Panina-Bordignon, & Sinigaglia, 2003; Federico, Morgillo, Tuccillo, Ciardiello, & Loguercio, 2007; J. M. Zhang & An, 2007). Indeed, this cyclical activity can persist as chronic inflammation for years after initiation (Dinarello, 2007; Schaue, Kachikwu, & McBride, 2012).
Central Nervous System Susceptibility to Oxidative Stress
Specific Neural Cell responses to oxidative stress
ROS have long been known to play an important role in CNS health (Gemma, Vila, Bachstetter, & Bickford, 2007; Salim, 2017). At normal physiological concentrations, ROS are essential to neural cell function (Angelova & Abramov, 2018; Popa-Wagner, Mitran, Sivanesan, Chang, & Buga, 2013). Studies demonstrate that they facilitate cell communication within neural tissue, maintain populations of progenitor cells, and regulate long-term potentiation between neurons (Brieger et al., 2012; Cobley, Fiorello, & Bailey, 2018). However, the brain is particularly susceptible to oxidative stress when antioxidant systems are overwhelmed by high concentrations of ROS (Birben et al., 2012). This increased risk is associated with the abundant polyunsaturated lipids and high metabolic activity of the brain (Hirooka, 2008; Melo et al., 2011; Patel, 2016; Uttara, Singh, Zamboni, & Mahajan, 2009). Furthermore, relatively low physiological levels of antioxidant enzymes, limited regenerative potential, and the presence of neurotransmitters that are easily oxidizable, all contribute to the high sensitivity of the brain to oxidative stress (J. H. Kim, Brown, Jenrow, & Ryu, 2008; Patel, 2016; Uttara et al., 2009). Studies report that prolonged oxidative stress causes region- and cell-specific changes in neural tissue and brain vasculature (Hirooka, 2008; Salim, 2017). The following sections highlight the pervasive influence of oxidative stress on neural cells and call attention to the cell-specific responses which play a role in CNS disease.
Neurons
Neurons are the characteristic cells of the CNS, and they direct a wide range of sensory, motor, and integrative functions for the body. These cells form intricate networks, communicating through means such as neuronal processes, soluble molecules (i.e., neurotransmitters and cytokines), and synaptic or extracellular vesicles (Fainzilber, Budnik, Segal, & Kreutz, 2011; Fruhbeis, Frohlich, Kuo, & Kramer-Albers, 2013). They are generally classified by their overall morphology or function and can be further classified by gene expression profiles or the complexity of their neuronal processes (axons and dendrites) (Chklovskii, 2004; Poulin, Tasic, Hjerling-Leffler, Trimarchi, & Awatramani, 2016; Sharpee, 2014). Within neurons, ROS serve to regulate necessary functions including inflammation, apoptosis, long-term potentiation, and synaptic plasticity (Serrano & Klann, 2004).
Evidence suggests that populations of neurons have selective vulnerability and heightened sensitivity to oxidative stress, particularly within specific brain regions such as the hippocampus CA1 region and frontal cortex (X. K. Wang & Michaelis, 2010). When ROS are unregulated, neurons under oxidative stress conditions can respond by releasing additional ROS and other inflammatory factors. The resultant oxidative damage can lead to neuronal dysfunction or death, triggering apoptotic and inflammatory response pathways in surrounding cells (K. Choi, Kim, Kim, & Choi, 2009; Loh, Huang, De Silva, Tan, & Zhu, 2006; Redza-Dutordoir & Averill-Bates, 2016). This cyclical response is the primary driver of the chronic tissue degeneration associated with many neurological diseases and dysfunctions (Koelink et al., 2012; X. K. Wang & Michaelis, 2010).
Glia (Oligodendrocytes, Astrocytes & Microglia)
Glial cells help to maintain healthy neurons, but during persistent oxidative stress they can become dysfunctional and contribute to neuronal vulnerability (Dringen, Gutterer, & Hirrlinger, 2000; X. K. Wang & Michaelis, 2010). Whereas glial cells are typically more resistant than neurons to oxidative damage, the mechanisms of neuron-glia crosstalk, along with neuron-neuron and glia-glia crosstalk, are key factors in oxidative stress pathology (Benarroch, 2005; L. Huang, Nakamura, Lo, & Hayakawa, 2019; Nutma, van Gent, Amor, & Peferoen, 2020; Peferoen, Kipp, van der Valk, van Noort, & Amor, 2014).
Oligodendrocytes
Oligodendrocytes offer structural and functional support in CNS tissue by ensheathing axons to increase the conduction speed of electrical impulses (Simons & Nave, 2015). These cells help to nourish axons, regulate signal traffic, and maintain the balance of oxidative reactions with anti-oxidative defenses (Beckhauser, Francis-Oliveira, & De Pasquale, 2016; Griot, Vandevelde, Richard, Peterhans, & Stocker, 1990). However, sustained oxidative stress can alter differentiation, compromise production and maintenance of axonal sheaths, and induce apoptosis of oligodendrocyte-lineage cells (French, Reid, Mamontov, Simmons, & Grinspan, 2009; Giacci & Fitzgerald, 2018; Thorburne & Juurlink, 1996). Increased oxidative stress in oligodendrocytes also correlates with increased astrocytic reactivity in vivo (Wellman, Cambi, & Kozai, 2018). Indeed, elevated ROS can cause degeneration of oligodendrocytes and trigger a reactive phenotype in astrocytes (J. W. Choi et al., 2004; Griot et al., 1990).
Astrocytes
Like oligodendrocytes, astrocytes contribute to the structural and functional support of neurons by enveloping synapses, releasing neurotrophic factors, contributing to extracellular ion homeostasis, and regulating the blood-brain barrier (Benarroch, 2005). “Astrogliosis” describes the activation, proliferation, morphological changes, and additional responses of reactive astrocytes associated with pathological conditions in the CNS (Ben Haim, Carrillo-de Sauvage, Ceyzeriat, & Escartin, 2015; Hsieh, Lin, Hsiao, & Yang, 2013). Reactive astrocytes secrete ROS and inflammatory cytokines in an attempt to maintain CNS homeostasis, which can inadvertently promote damage in normal tissues (Ben Haim et al., 2015; Sheng, Hu, Feng, & Rock, 2013). Once activated, reactive astrocytes can cause long-lasting changes to tissue morphology and influence the activity of surrounding cells—particularly within the context of the tripartite synapse (Ben Haim et al., 2015; Liddelow et al., 2017).
Microglia
Microglia are the resident immune cells of the brain and play a major role in maintaining CNS homeostasis (Colonna & Butovsky, 2017; Salter & Stevens, 2017). In response to secreted signaling molecules or inflammatory factors, microglia alter their phenotype and then migrate towards damaged or infected areas of the brain to release additional factors or phagocytose harmful material (Bordt & Polster, 2014; Nakanishi & Wu, 2009). Like astrocytes, microglia release inflammatory cytokines and ROS in response to tissue damage but to a much greater degree (von Bernhardi, Eugenin-von Bernhardi, & Eugenin, 2015). Once these cells arrive at distressed areas, released factors serve as immune cell recruitment factors which lead to additional immune cell migration, ROS activity, and cytokine secretion (Norden, Muccigrosso, & Godbout, 2015).
Due to this active response to damage, microglia play an intimate role in tissue repair and the subsequent changes in tissue morphology. However, as with all cell types involved in the oxidative stress response, if left unchecked, microglia can also contribute to the chronic, cyclical activation of inflammatory factors (Martindale & Holbrook, 2002; A. Reynolds et al., 2007). Indeed, prolonged microglial activation can alter the homeostatic set point and cause long-term dysregulation of signaling pathways in both neural and immune cells (Perry & Teeling, 2013).
Because of the inflammatory nature of these cells, understanding how they respond to oxidative stress is also important for investigating age-related neurodegenerative disease (Patel, 2016; von Bernhardi et al., 2015; Wolf, Boddeke, & Kettenmann, 2017). Studies of such disorders suggest that activated microglia play both neuroprotective (i.e., clearing amyloid plaques) and neurotoxic (i.e., excessive and nonspecific release of inflammatory factors) roles (Nakanishi & Wu, 2009; Salter & Stevens, 2017). Microglia are also essential for synaptic pruning in CNS development and adult neuroplasticity. However, dysregulation of the mechanisms which execute these roles can lead to the aberrant pruning seen in neurodevelopmental and neurodegenerative disorders (Guarente & Kenyon, 2000; Salter & Stevens, 2017).
Endothelial Cells & Cerebral Vasculature
Oxidative stress in neural tissue can significantly increase pathological risk for cells of the cerebral vasculature. The increased migration of activated immune cells through vascular walls, in response to inflammatory signals, damages the neurovascular unit, alters gene expression in endothelial cells, and disrupts tight junctions in blood-tissue barriers such as the Blood-Brain Barrier (BBB) (Carvalho & Moreira, 2018; Faraci, 2005). BBB breakdown is a significant risk factor for neuroinflammation and neurodegeneration (Haorah, Knipe, Leibhart, Ghorpade, & Persidsky, 2005; Haorah et al., 2007).
Similarly, studies also report risks associated with the cerebral lymphatic system. Though research on this subject is limited, lymphatic vessels typically facilitate the “clearing out” of toxic metabolites and immune components in neural tissue, but aging-associated increase in oxidative stress can reduce the contractility of these vessels (Louveau et al., 2015; Sun et al., 2018; Thangaswamy, Bridenbaugh, & Gashev, 2012). Consequently, the meningeal lymphatic drainage routes become blocked and amyloid-β begins to accumulate in the meninges and brain parenchyma, notably within the hippocampus (Da Mesquita et al., 2018; Kruk, Aboul-Enein, Kladna, & Bowser, 2019). Protein aggregates are known risk factors repeatedly identified in patients with neurodegenerative diseases, though the mechanisms by which they contribute to these diseases are not fully understood (Cioffi, Adam, & Broersen, 2019; Olivares, Huang, Branden, Greig, & Rogers, 2009).
Neural Progenitor/Stem Cells (NPSCs)
In mammalian brains, neural progenitor stem cells (NPSCs) are prominent during development and are retained in the adult brain within the dentate gyrus of the hippocampus and subventricular zone of the anterior lateral ventricles. NPSCs are vital for neurogenesis and gliogenesis. At physiological levels, several studies (Cobley et al., 2018; Perez Estrada, Covacu, Sankavaram, Svensson, & Brundin, 2014; Srivastava, Tripathi, & Mishra, 2018) suggest that ROS production, even oxidative stress, is important for the role of NPSC homeostasis, development, repair, regeneration, and neuroplasticity (Chui, Zhang, Dai, & Shi, 2020; T. T. Huang, Zou, & Corniola, 2012; Le Belle et al., 2011; Walton et al., 2012; Yokoyama, Kuroiwa, Yano, & Araki, 2008; Yuan, Gu, Shan, Machado, & Arias-Carrion, 2016). However, persistent oxidative stress induces maladaptive cell responses and disrupts repair mechanisms in these proliferating cells, leading to altered gene expression and protein dysfunction (Musgrove et al., 2019; Perez Estrada et al., 2014; Texel & Mattson, 2011; Vonk et al., 2020; Walton et al., 2012). These pathological conditions can lead to loss of progenitor cells, altered neurogenesis and gliogenesis, and significant morphological changes including reduced brain mass (Walton et al., 2012).
Oxidative Stress Induced Behavioral and Cognitive Changes
Therapeutic strategies aimed at treating neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) have targeted oxidative stress because of its general contribution to the induction and progression of brain disease: increased lipid peroxidation and decreased polyunsaturated fatty acids, accumulation of redox metals, increased protein and DNA oxidation, reduced metabolic activity, decreased cytochrome c oxidase, molecular interactions with amyloid beta (Aβ) peptide, and accumulation of senile plaques and neurofibrillary tangles (W. J. Huang, Zhang, & Chen, 2016; Markesbery, 1997; Mattson, Duan, Pedersen, & Culmsee, 2001; Nunez-Millacura, Tapia, Munoz, Maccioni, & Nunez, 2002; Olivares et al., 2009). For example, in PD patients, disease progression is marked by a loss of dopaminergic neurons of the substantia nigra (Haining & Achat-Mendes, 2017). Dopamine can act as a metal chelator its redox chemistry can promote conditions which generate toxic free radicals, leading to neuronal damage (Uttara et al., 2009). Evidence suggests that oxidative damage in the CNS in PD and other diseases not only leads to localized neuronal degeneration but can also alter emotional well-being and worsen neuropsychiatric disorders (Salim, 2017). Because of significant patient-patient variability in brain network function, characterizing pathogenetic mechanisms at the level of individual neuron and glial cell types provides an incomplete picture of the disease. Accordingly, clinicians have emphasized the importance of using patient-specific models of CNS diseases to identify universally relevant targets for treating the cognitive and behavioral deficits associated with these diseases.
Traditional Oxidative Stress Models of the Human Brain
Our biochemical and physiological knowledge of human neurocognitive disease has predominantly come from studies of post-mortem tissues, cultured human and non-human cells, and non-human organisms, such as, nematodes, fish, rodents, and non-human primates (Lewis, 2002). Despite the clear progress made in the field using traditional techniques, these models are subject to limitations that have hindered the development of effective therapeutic treatments for CNS diseases (Mimetas; Wolf et al., 2017). These limitations include poor sample quality or availability, inconsistent characterization of the disease mechanisms, and ineffective translation from models to patients (Table 1).
Table 1.
Comparative analysis of experimental models for pathological oxidative stress in Central Nervous System tissues
| 2D Cultures | Animal Models | Post-Mortem Human Tissue | Brain Organoids (3D Culture) | |
|---|---|---|---|---|
| Advantages | Abundant culture methods and analytical techniques | Comparable size and anatomical structure | Accurate size and anatomical structure | Recapitulate 3D structural organization and diffusion of biological factors |
| Can be generated from human iPSCs and ESCs | Can monitor the behavior of specific cortical cell types | Specific cortical cell types and precise developmental cues | Can be generated from human iPSCs and ESCs | |
| Widely used to study CNS disease progression | Widely available variants to study disease progression | Visible tissue degeneration in specific brain regions | Increasingly used to model human disease progression | |
| Can model near-physiological morphological changes and responses to oxidative stress | Can obtain useful measures of altered cognitive and behavioral states | Can obtain patient-specific measures of disease states | Capacity for self-directed organization and differentiation | |
| Highly scalable and high-throughput analysis of cell responses to biological factors | Can identify acute and chronic actions of reactive species during disease states | Can identify terminal pathological features of disease states across diverse human populations | Highly scalable and high-throughput analysis of cell responses to biological factors | |
| Can obtain functional cell- and tissue-specific information using simplified and low-cost methods | Can obtain functional whole-body 3D information i.e. systemic responses | Can identify some functional measures with a short “post-mortem interval” | Can obtain functional organ-specific 3D information i.e. electrophysiological network activity patterns | |
| Limitations | Tissue composition and cell state change rapidly and demonstrate limited complexity | Significant metabolic, anatomical, and physiological differences to humans | Rapid biochemical changes during processing | Reliance on growth factors and differentiation protocols |
| Poor representation of the in vivo physiological environment; limited cell-cell interaction | Lifespan of some species unaffected by high levels of oxidative stress; developmental differences | Loss of data on altered cell function and behavior due to tissue degeneration | Current limitations on functional and developmental neural cell maturation | |
| Lack of relevant data on cell-ECM or cell-scaffold interactions | Notable differences in brain mass, cellular organization, and regionalization | Decreasing donor/sample availability | Current methods are expensive, time-consuming, and characteristically provisional | |
| Automatically defined apical-basal polarization of cells | Results from these models often fail to translate to humans due to inter-species differences | Artifacts of neuronal death are rapidly introduced into dissected samples | Studies have reported stressful culture conditions and limited oxygen and nutrient diffusion | |
| Lack of 3D information; morphological constraints of 2D geometry | Greater neuronal density; lesser dendritic branching vs humans | Ethical and practical limitations of interrogating live/dead human brain tissue | Batch-batch or organoid-organoid variability in organization and “discrete” brain regions | |
| Risk of teratoma formation in stem-cell based therapy; limited differentiation capacity | Different patterns of age-related gene expression alterations | Poor study control to determine if observations/results are due to disease or caused by other agents | Lack of consensus for optimal culture conditions and methods to generate brain organoids |
For example, previous work involving tissues collected post-mortem from patients with neurodegenerative diseases has identified signs of oxidative damage including DNA damage and atypical concentrations of GABA, glutamate, and serotonin metabolites (Coppede & Migliore, 2009; Eckman, Dixit, Nackenoff, Schrag, & Harrison, 2018). However, significant biochemical changes can take place during the post-mortem interval before tissue processing and lead to skewed results (Hynd, Lewohl, Scott, & Dodd, 2003). Furthermore, once brain death occurs, we are unable to collect further data on functional processes essential to understanding and targeting cell signaling pathways in humans (Gordon & McKinlay, 2012; Starr, Tadi, & Pfleghaar, 2020).
Besides post-mortem tissues, traditional 2-D cell cultures of human neural cells have provided us with a greater understanding of near-physiological responses to oxidative stress and temporally relevant morphological changes (Walter et al., 2019; Walton et al., 2012). Key markers for neurodegeneration indicative of oxidative stress pathophysiology such as protein misfolding and aggregation, abnormal neural cell reactivity, and neuronal death have all been identified in cell culture models (Wolf et al., 2017; Xu et al., 2002). However, even cultures generated through cell reprogramming technology from patients afflicted with neurodegenerative diseases do not capture the entirety of the in in vivo pathology (Mitchell, Scheibye-Knudsen, Longo, & de Cabo, 2015). Additionally, it can be difficult to maintain these cultures long-term while keeping neural cells in a non-reactive state (Sloan et al., 2017).
Many researchers still consider rodent and primate models to be best suited for evaluating complex associations between environmental factors and biological endpoints, particularly for testing antioxidant and countermeasure interventions for oxidative stress (Lees, Walters, & Cox, 2016; Melov, 2002). Indeed, animal models provide more information about the physiology of integrative systems, age-dependent risks, and the real-time responses of neural cells in fully functional and interconnected brain tissue (Kregel & Zhang, 2007; Schiavone, Jaquet, Trabace, & Krause, 2013; Wilhelm, Vytasek, Uhlik, & Vajner, 2016). For example, the migration of activated microglia through cortical layers in damaged brain regions can be tracked in animal models but not cell-based models (Wolf et al., 2017). Thus, animal models are widely used to interrogate the acute and chronic actions of reactive species in aging and oxidative stress, including genetic and epigenetic modification, regulation of antioxidant defenses, and coordinated tissue responses (Balmus, Ciobica, Antioch, Dobrin, & Timofte, 2016; Lee et al., 2012; Melov, 2002; Pamplona & Costantini, 2011).
Animal studies have also shown how morphological changes in brain tissue are related to higher levels of cognitive or behavioral dysfunction (Butterfield, Howard, & LaFontaine, 2001; Droge & Schipper, 2007; McEwen, 2007; Opii et al., 2008; Picard & McEwen, 2018; Schiavone et al., 2013). In addition to physiology and morphology, numerous studies involving transgenic animals (including some primates) have confirmed the influence of genetic background on responses to oxidative stress (Cioffi et al., 2019; Crowe et al., 2016; Fraser, Khaitovich, Plotkin, Paabo, & Eisen, 2005; J. M. Kim, Kim, & Son, 2018). Indeed, modifications to genetic elements in animal models homologous to human variants have frequently been used to identify oxidative stress-induced cognitive and behavioral changes (Balmus et al., 2016; Cioffi et al., 2019; Schiavone et al., 2013; Sorce & Krause, 2009).
Although considerable progress has been made using animal models, there are significant functional differences between humans and other mammals in such processes as DNA repair, immune response, and multi-system organ integration, which have hampered the translation of experimental results to therapies for degenerative diseases (Mitchell et al., 2015). Additionally, the lifespan of some species appears to be unaffected by high levels of oxidative stress, even if initiated early in life (Buffenstein, Edrey, Yang, & Mele, 2008). There are also significant anatomical differences in brain mass, cellular organization, and regionalization between humans and other mammals which is highly relevant because human brain regions are disproportionately damaged by oxidative stress, and the properties of cerebral vasculature are non-uniform throughout the brain (Coyle & Puttfarcken, 1993; Haces, Montiel, & Massieu, 2010; X. Wang et al., 2005).
Given these differences, it is perhaps not surprising that research conducted with common animal models has often failed to appropriately translate to humans (Hu, Todhunter, LaBarge, & Gartner, 2018; Shi, Buffenstein, Pulliam, & Van Remmen, 2010). Indeed, despite high efficacy animal models, therapeutic strategies often fail in human clinical trials (Carvalho & Moreira, 2018; Floyd, 1999; Kamat et al., 2008; Neal & Richardson, 2018). Extrapolating from these studies has largely failed to slow disease progression in the human CNS (Kamat et al., 2008). Without an understanding of the intricate mechanisms underlying neural cell death and dysfunction in neurodegenerative disorders in human neural tissue, it is difficult to identify targets for therapeutic intervention (Melo et al., 2011). Though attempts at “humanizing” animal models are underway, sophisticated alternative strategies are being developed to model human tissue and organ-level responses (J. K. Andersen, 2004; Kamat et al., 2008).
Novel Complex Models of Oxidative Stress in Human Brain Tissue using Stem-Cell Derived 3D Organoids
Developing stem-cell-derived 3D brain tissue models
Due to the ethical and practical limitations of interrogating live human brain tissue, a major challenge for studying CNS disease progression is the lack of patient tissue samples, particularly for critical developmental periods (Eckman et al., 2018; Sloan et al., 2017). To directly study oxidative stress and neurodegeneration in functional human brain tissue, researchers have developed three dimensional (3-D) human cell cultures derived from induced pluripotent stem cells (iPSCs) (Halliwell & Whiteman, 2004). iPSCs can be generated from human fibroblasts with the help of a few transcription factors, including Oct3/4,Sox2, Klf4, and c-Myc (Takahashi et al., 2007; Yamanaka, 2012). Despite the technical limitations and considerable start-up costs of isolating and culturing iPSCs, they have proven to be a useful biological model due to their physiological relevance, reproducibility, and ability to model patient- and disease-specific mechanisms of interest (Dolmetsch & Geschwind, 2011; Saha & Jaenisch, 2009; Yamanaka, 2012). Though the process of generating these cultures from reprogrammed patient-derived cells can be labor-intensive, once established, iPSC cultures can be used to generate NPSCs. With recent advancements to cell culture methods and analytical tools, these cells are now widely used in models of human CNS diseases (Okano et al., 2013).
Additional approaches can be used to form 3-D aggregates of NPSCs known as “neurospheres” (a.k.a. neural spheroids or neuro-aggregates), free-floating or scaffold-based clusters which retain neural precursor cells but also promote the differentiation of mature cell phenotypes (Campos, 2004; Denham & Dottori, 2011; Hofrichter et al., 2017; Yagi et al., 2012). Neurospheres can generate brain region-specific neurons and astrocytes which model the progression of normal development and even various disease states (Begum et al., 2015; Sloan et al., 2017). For example, they are useful in neurodegenerative disease research to model aspects of familial AD mutations such as the accumulation of amyloid-β and phosphorylated tau (Jorfi, D’Avanzo, Tanzi, Kim, & Irimia, 2018). As a result, the ability of neurospheres to model morphological complexity and multiple levels of pathological changes has provided key insights for both protective and degenerative mechanisms of neural cell sensitivity to oxidative stress (Carletti, Piemonte, & Rossi, 2011; Chui et al., 2020; Fike, Rosi, & Limoli, 2009; Madhavan, Ourednik, & Ourednik, 2006; Puschmann et al., 2013; Tseng et al., 2014). Collectively these studies show that neurospheres, derived from iPSCs, are valuable tools to study CNS development, disease, and tissue repair (Daadi, 2019; B. A. Reynolds & Rietze, 2005; Ring et al., 2012).
These cultures resemble in vivo conditions more closely than traditional 2-D cultures, thus facilitating the investigation of cell-ECM interactions, cell differentiation, cell-cell communication, morphological changes, and functional network activity (Centeno, Cimarosti, & Bithell, 2018; Hofrichter et al., 2017; Pauly et al., 2018). They can be maintained for long periods of time without significant reactive gliosis, allowing researchers to more accurately model disease progression within human brain regions, with cultures demonstrating disease-specific differences in protein/gene expression, cell function and behavior, and coordinated network activity (Matigian et al., 2010; Pasca et al., 2015). Recent advancements in cell-type specific mapping/sequencing techniques and experimental methods will certainly allow researchers to examine these cultures in greater detail throughout the course of development (Giandomenico, Sutcliffe, & Lancaster, 2020; Poli, Magliaro, & Ahluwalia, 2019; Trevino et al., 2020). However, while neurospheres are quite useful to evaluate changes to neural cell structure and function, these models are still limited in their ability to model the complex network activity, spontaneous self-organization, and diverse cell subpopulations found in the human brain.
Brain organoids as models for neurological disease and neurodevelopment
With these concepts in mind, in 2008 the Sasai lab developed a 3-D tissue model of the cerebral cortex (a.k.a. cerebral organoids or cerebroids) (Eiraku et al., 2008). Further methods to generate the structures widely known as “brain organoids” were defined by the work of the Knoblich lab (Lancaster & Knoblich, 2014; Lancaster et al., 2013). Credit is also due to the work of other labs for providing the brain organoid models widely used today for numerous applications, but we will not cover them here as they have been discussed extensively in previous reviews (Poli et al., 2019; Qian, Song, & Ming, 2019; H. Wang, 2018). Due to the pioneering work of these early studies, novel organoid models now assist researchers in recapitulating the complex 3-D organization, spontaneous development of brain-like regions, and functional behavior of differentiating neural cells (Cleber A. Trujillo et al., 2019).
Organoids are generated from embryonic stem cells (ESCs) or iPSCs, typically embedded in Matrigel, and supplemented with factors to promote a certain developmental trajectory or pathological state of the human brain (Clevers, 2016). Once they are of sufficient size and development, organoids can serve as complex functional surrogates with similar mechanics at the molecular, cellular, tissue, and organ level (Budday, Ovaert, Holzapfel, Steinmann, & Kuhl, 2019; Goriely et al., 2015; Poldrack & Farah, 2015). Protocols to generate organoid models of various tissues are now widely available. These cutting-edge methods include guided, unguided, and assembloid strategies to generate brain organoids, which have led to organoid-on-a-chip, xenograft, and chimera models described elsewhere (J. Andersen et al., 2020; Chen et al., 2019; Tambalo & Lodato, 2020). With continued improvements, organoids can be generated in high quantities with little batch-batch variability and thus they may soon be established as thoroughly reproducible, scalable, and high-throughput translational models (Huch, Knoblich, Lutolf, & Martinez-Arias, 2017; C. A. Trujillo & Muotri, 2018; Velasco et al., 2019; Yoon et al., 2019).
Previously, organoids were considered to be best applied as developmental models because research efforts failed to robustly produce endophenotypes of neurodegenerative diseases and cell aging that would allow researchers to transition from other stem cell-based models (Qian et al., 2019). However, more recent approaches demonstrate the potential of iPSC-based 3-D neural cell cultures to model various types of dementia (S. H. Choi et al., 2014; Marotta, Kim, & Krainc, 2020; Zhu et al., 2019). Of particular interest is the etiology of cytoskeletal remodeling, mitochondrial dysfunction, synaptic alterations, protein accumulation, and genetic abnormalities. In light of these approaches, there is an opportunity to apply knowledge from other iPSC-based models to generate 3-D organoids which may provide novel insights about the role of oxidative stress in CNS disease progression.
Brain organoid models of oxidative stress
To date, only a handful of published studies have investigated the oxidative-stress-induced responses in brain organoids and how associated mechanisms may increase susceptibility to CNS diseases. In one such study, researchers generated a multicellular 3-D human neurovascular unit organoid containing endothelial cells, pericytes, astrocytes, microglia, oligodendrocytes and neurons to evaluate the effects of hypoxia and neuroinflammation on BBB function (Nzou et al., 2020). Organoids subjected to hypoxia treatment demonstrated increased BBB permeability, pro-inflammatory cytokine production, and oxidative stress, assessed by binding of reactive oxygen and nitrogen species (RONS)-sensitive dyes and decreased mitochondrial ATP production. The study also reported a reduction in ROS and inflammation upon treatment with the antioxidant and anti-inflammatory molecule secoisolariciresinol diglucoside (SDG), a free radical scavenger, and 2-arachidonoyl glycerol (2-AG), an endocannabinoid.
Additional studies of hypoxia treatment on 3-D cerebral organoids have documented protein disruption, altered differentiation, and cell death in intermediate neural progenitors (Daviaud, Chevalier, Friedel, & Zou, 2019; Pasca et al., 2019). Another study generated human midbrain organoids from iPS cells from patients with LRRK2-associated sporadic PD, and reported increased gene expression of thioredoxin-interacting protein (TXNIP), which is associated with lysosomal dysfunction and may mediate the PD pathophenotype (H. Kim et al., 2019). Together, these studies demonstrate the utility of organoids to evaluate the initial effects of oxidative stress on neural cells in a more complete tissue context, and the secondary roles of the vascular system and of antioxidant treatment.
Organoids are also useful to understand the importance of oxidative stress in the context of radiation medicine and space biology (Schielke, Hartel, Durante, Ritter, & Schroeder, 2020; Vehlow, Deville, & Cordes, 2020). Exposure to ionizing radiation during patient radiotherapy and spaceflight missions is known to alter brain tissue and its vasculature. With the increasing access to radiotherapy treatments and human space travel it is crucial to understand the mechanisms underlying these changes and to develop suitable countermeasures (Xiao W. Mao et al., 2020; Xiao Wen Mao et al., 2016). A consistent phenomenon observed following rodent brain exposure to “low-dose” ionizing radiation is the persistence of oxidative stress and neuroinflammation followed later by cognitive impairment, depending on the type and dose of radiation received (Pariset, Malkani, Cekanaviciute, & Costes, 2020; Tseng et al., 2014). Interestingly, it is precisely because of this property that we see a potential for using ionizing radiation as a tool to reliably produce oxidative stress in brain organoids, which should overcome the challenge of obtaining uniform perfusion when using oxidative stress-inducing agents in cell culture media.
It remains to be investigated how various molecular processes are affected by oxidative stress in brain organoid models, including DNA/RNA damage and repair, lipid peroxidation, protein oxidation, cytokine release, and ROS/RNS dynamics. There is also a need to understand how these effects are regulated by endogenous antioxidants, such as, superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and catalase (Cat) (Mariani, Polidori, Cherubini, & Mecocci, 2005). This will provide important baseline information for assessing disease mechanisms and the actions of potential therapeutics.
Limitations
As with all models, brain organoids are subject to many limitations. The methods used to generate these brain organoids are technically changing and time consuming, which creates challenges for batch-to-batch and study-to-study consistency (Shou, Liang, Xu, & Li, 2020). As the field is still largely exploratory, it has become difficult to define standards for culture methods and to set parameters for different classes of organoids. Reports have also demonstrated that organoid culture conditions are inherently stressful for cells and can impair the differentiation of cellular subtypes (Bhaduri et al., 2020). Furthermore, due to the intrinsic complexity of brain tissue, researchers are currently forced to select for certain features and discriminate against others; no one model features all of the relevant cell types, extracellular matrix components, vasculature, and lymph vessels found in the human brain.
Structurally, the size of brain organoids is limited for reasons not well understood and this creates a challenge for the health and long-term maintenance of cells within the interior of the organoids. This has led to the development of alternative approaches such as air-liquid interface organoid slices (Giandomenico et al., 2019). Though the complexity and self-organization of organoids is of intrinsic interest, it certainly cannot be stated that they fully replicate the developmental trajectory, region specific morphology, molecular patterning, or, disease phenotypes observed in the human brain. Major technical improvements are still required to satisfactorily replicate these characteristics. Fortunately, the pace of research efforts is rapidly increasing, promising to steadily advance state-of-the-art technology for producing and characterizing brain organoids.
Despite the limitations, a number of distinct advantages demonstrate the potential for using brain organoids to model oxidate stress. Pharmacological and genetic tools have made it possible to induce oxidative stress in brain organoids in defined ways for the study of neurodegeneration and adaptive changes in cell function and behavior (Brawner, Xu, Liu, & Jiang, 2017; Faravelli, Costamagna, Tamanini, & Corti, 2020; Hu et al., 2018; Kagias, Nehammer, & Pocock, 2012; Kamat et al., 2008; Setia & Muotri, 2019). The patient-derived iPSCs that can be used to generate organoids have already been demonstrated to exhibit disease-specific effects of oxidative stress (Andrade, Nathanson, Yeo, Menck, & Muotri, 2012). Indeed, a variety of oxidative stress-relevant CNS disorders have already been modeled with brain organoids generated from patient-derived iPSCs including schizophrenia, autism spectrum disorders, Rett syndrome, microcephaly, and ZIKA virus infection (Kathuria et al., 2020; Koh, Tan, & Ng, 2018; Nassor et al., 2020). Although the culture methods vary and the studies did not specifically set out to measure oxidative stress, they capture key functional and anatomical features of development and disease progression that are known to be influenced by ROS and inflammation.
Outlook
Clearly, organoid technology has created powerful tools to facilitate the field of regenerative medicine and the development of personalized therapeutic interventions for diseases where oxidative stress is a major participant. This point is salient as the number of personalized medicines has doubled within four years and yet treatments for neurodegenerative diseases are still largely ineffective (Jeremias, 2020). As culture methods continue to improve, brain organoid models can be expected to provide a fresh perspective on the oxidative theory of aging, identify cell-type specific responses to ROS and enable the evaluation of an assortment of biomolecules as therapeutic targets.
Acknowledgments:
Funding and material support for this research is supported in part by NASA Research Grant NNX13AN34G and the Loma Linda University School of Medicine Department of Basic Sciences.
Footnotes
Conflict of Interest Statements:
Dr. Muotri is a co-founder and has equity interest in TISMOO, a company dedicated to genetic analysis and brain organoids, focusing on therapeutic applications customized for autism spectrum disorder and other neurological disorders with genetic origins. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
References
- Abdul-Muneer PM, Chandra N, & Haorah J (2015). Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol, 51(3), 966–979. doi: 10.1007/s12035-014-8752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadinejad F, Geir Moller S, Hashemzadeh-Chaleshtori M, Bidkhori G, & Jami MS (2017). Molecular Mechanisms behind Free Radical Scavengers Function against Oxidative Stress. Antioxidants (Basel), 6(3). doi: 10.3390/antiox6030051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, … Pasca SP. (2020). Generation of Functional Human 3D Cortico-Motor Assembloids. Cell, 183(7), 1913–1929 e1926. doi: 10.1016/j.cell.2020.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JK (2004). Oxidative stress in neurodegeneration: cause or consequence? Nature Medicine, 10 Suppl, S18–25. doi: 10.1038/nrn1434 [DOI] [PubMed] [Google Scholar]
- Andrade LN, Nathanson JL, Yeo GW, Menck CF, & Muotri AR (2012). Evidence for premature aging due to oxidative stress in iPSCs from Cockayne syndrome. Hum Mol Genet, 21(17), 3825–3834. doi: 10.1093/hmg/dds211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, & Abramov AY (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett, 592(5), 692–702. doi: 10.1002/1873-3468.12964 [DOI] [PubMed] [Google Scholar]
- Balmus IM, Ciobica A, Antioch I, Dobrin R, & Timofte D (2016). Oxidative Stress Implications in the Affective Disorders: Main Biomarkers, Animal Models Relevance, Genetic Perspectives, and Antioxidant Approaches. Oxidative Medicine and Cellular Longevity, 2016, 3975101. doi: 10.1155/2016/3975101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckhauser TF, Francis-Oliveira J, & De Pasquale R (2016). Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J Exp Neurosci, 10(Suppl 1), 23–48. doi: 10.4137/JEN.S39887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum AN, Guoynes C, Cho J, Hao J, Lutfy K, & Hong Y (2015). Rapid generation of sub-type, region-specific neurons and neural networks from human pluripotent stem cell-derived neurospheres. Stem Cell Res, 15(3), 731–741. doi: 10.1016/j.scr.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L, Carrillo-de Sauvage MA, Ceyzeriat K, & Escartin C (2015). Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci, 9, 278. doi: 10.3389/fncel.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2005). Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc, 80(10), 1326–1338. doi: 10.4065/80.10.1326 [DOI] [PubMed] [Google Scholar]
- Berlett BS, & Stadtman ER (1997). Protein oxidation in aging, disease, and oxidative stress. J Biol Chem, 272(33), 20313–20316. doi: 10.1074/jbc.272.33.20313 [DOI] [PubMed] [Google Scholar]
- Berr C, Balansard B, Arnaud J, Roussel AM, & Alperovitch A (2000). Cognitive decline is associated with systemic oxidative stress: the EVA study. Etude du Vieillissement Arteriel. J Am Geriatr Soc, 48(10), 1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x [DOI] [PubMed] [Google Scholar]
- Bhaduri A, Andrews MG, Mancia Leon W, Jung D, Shin D, Allen D, … Kriegstein AR. (2020). Cell stress in cortical organoids impairs molecular subtype specification. Nature, 578(7793), 142–148. doi: 10.1038/s41586-020-1962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, & Kalayci O (2012). Oxidative stress and antioxidant defense. World Allergy Organ J, 5(1), 9–19. doi: 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK (2016). Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Medicine and Cellular Longevity, 2016, 5698931. doi: 10.1155/2016/5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordt EA, & Polster BM (2014). NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radic Biol Med, 76, 34–46. doi: 10.1016/j.freeradbiomed.2014.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner AT, Xu R, Liu D, & Jiang P (2017). Generating CNS organoids from human induced pluripotent stem cells for modeling neurological disorders. Int J Physiol Pathophysiol Pharmacol, 9(3), 101–111. [PMC free article] [PubMed] [Google Scholar]
- Brieger K, Schiavone S, Miller FJ Jr., & Krause KH (2012). Reactive oxygen species: from health to disease. Swiss Med Wkly, 142, w13659. doi: 10.4414/smw.2012.13659 [DOI] [PubMed] [Google Scholar]
- Budday S, Ovaert TC, Holzapfel GA, Steinmann P, & Kuhl E (2019). Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Archives of Computational Methods in Engineering. doi: 10.1007/s11831-019-09352-w [DOI] [Google Scholar]
- Buffenstein R, Edrey YH, Yang T, & Mele J (2008). The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordr), 30(2–3), 99–109. doi: 10.1007/s11357-008-9058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, & LaFontaine MA (2001). Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer’s disease and Huntington’s disease. Curr Med Chem, 8(7), 815–828. doi: 10.2174/0929867013373048 [DOI] [PubMed] [Google Scholar]
- Campos LS (2004). Neurospheres: insights into neural stem cell biology. J Neurosci Res, 78(6), 761–769. doi: 10.1002/jnr.20333 [DOI] [PubMed] [Google Scholar]
- Carletti B, Piemonte F, & Rossi F (2011). Neuroprotection: the emerging concept of restorative neural stem cell biology for the treatment of neurodegenerative diseases. Curr Neuropharmacol, 9(2), 313–317. doi: 10.2174/157015911795596603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C, & Moreira PI (2018). Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front Physiol, 9, 806. doi: 10.3389/fphys.2018.00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenini G, Lloret A, & Cascella R (2019). Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Medicine and Cellular Longevity, 2019, 2105607. doi: 10.1155/2019/2105607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno EGZ, Cimarosti H, & Bithell A (2018). 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol Neurodegener, 13(1), 27. doi: 10.1186/s13024-018-0258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Wolf JA, Blue R, Song MM, Moreno JD, Ming GL, & Song H (2019). Transplantation of Human Brain Organoids: Revisiting the Science and Ethics of Brain Chimeras. Cell Stem Cell, 25(4), 462–472. doi: 10.1016/j.stem.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB (2004). Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron, 43(5), 609–617. doi: 10.1016/j.neuron.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Choi JW, Shin CY, Yoo BK, Choi MS, Lee WJ, Han BH, … Ko KH. (2004). Glucose deprivation increases hydrogen peroxide level in immunostimulated rat primary astrocytes. J Neurosci Res, 75(5), 722–731. doi: 10.1002/jnr.20009 [DOI] [PubMed] [Google Scholar]
- Choi K, Kim J, Kim GW, & Choi C (2009). Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr Neurovasc Res, 6(4), 213–222. doi: 10.2174/156720209789630375 [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, … Kim DY. (2014). A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature, 515(7526), 274–278. doi: 10.1038/nature13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li FQ, & Maiese K (2005). Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Progress in Neurobiology, 75(3), 207–246. doi: 10.1016/j.pneurobio.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Chui A, Zhang Q, Dai Q, & Shi SH (2020). Oxidative stress regulates progenitor behavior and cortical neurogenesis. Development, 147(5). doi: 10.1242/dev.184150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi F, Adam RHI, & Broersen K (2019). Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J Alzheimers Dis, 72(4), 981–1017. doi: 10.3233/JAD-190863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2016). Modeling Development and Disease with Organoids. Cell, 165(7), 1586–1597. doi: 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- Cobley JN, Fiorello ML, & Bailey DM (2018). 13 reasons why the brain is susceptible to oxidative stress. Redox Biol, 15, 490–503. doi: 10.1016/j.redox.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, & Butovsky O (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol, 35, 441–468. doi: 10.1146/annurev-immunol-051116-052358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F, & Migliore L (2009). DNA damage and repair in Alzheimer’s disease. Curr Alzheimer Res, 6(1), 36–47. doi: 10.2174/156720509787313970 [DOI] [PubMed] [Google Scholar]
- Coyle JT, & Puttfarcken P (1993). Oxidative stress, glutamate, and neurodegenerative disorders. Science, 262(5134), 689–695. doi: 10.1126/science.7901908 [DOI] [PubMed] [Google Scholar]
- Crowe EP, Tuzer F, Gregory BD, Donahue G, Gosai SJ, Cohen J, … Torres C. (2016). Changes in the Transcriptome of Human Astrocytes Accompanying Oxidative Stress-Induced Senescence. Frontiers in Aging Neuroscience, 8, 208. doi: 10.3389/fnagi.2016.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio D, Panina-Bordignon P, & Sinigaglia F (2003). Chemokine receptors in inflammation: an overview. J Immunol Methods, 273(1–2), 3–13. doi: 10.1016/s0022-1759(02)00414-3 [DOI] [PubMed] [Google Scholar]
- d’Avila JC, Siqueira LD, Mazeraud A, Azevedo EP, Foguel D, Castro-Faria-Neto HC, … Bozza FA. (2018). Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J Neuroinflammation, 15(1), 28. doi: 10.1186/s12974-018-1059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, … Kipnis J. (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature, 560(7717), 185–191. doi: 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM (2019). Generation of Neural Stem Cells from Induced Pluripotent Stem Cells. Methods Mol Biol, 1919, 1–7. doi: 10.1007/978-1-4939-9007-8_1 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, & Milzani A (2006). Biomarkers of oxidative damage in human disease. Clin Chem, 52(4), 601–623. doi: 10.1373/clinchem.2005.061408 [DOI] [PubMed] [Google Scholar]
- Daviaud N, Chevalier C, Friedel RH, & Zou H (2019). Distinct Vulnerability and Resilience of Human Neuroprogenitor Subtypes in Cerebral Organoid Model of Prenatal Hypoxic Injury. Front Cell Neurosci, 13, 336. doi: 10.3389/fncel.2019.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva TM, & Miller AA (2016). Cerebral Small Vessel Disease: Targeting Oxidative Stress as a Novel Therapeutic Strategy? Front Pharmacol, 7, 61. doi: 10.3389/fphar.2016.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham M, & Dottori M (2011). Neural differentiation of induced pluripotent stem cells. Methods Mol Biol, 793, 99–110. doi: 10.1007/978-1-61779-328-8_7 [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2007). Historical insights into cytokines. Eur J Immunol, 37 Suppl 1, S34–45. doi: 10.1002/eji.200737772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch R, & Geschwind DH (2011). The human brain in a dish: the promise of iPSC-derived neurons. Cell, 145(6), 831–834. doi: 10.1016/j.cell.2011.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, & Hirrlinger J (2000). Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem, 267(16), 4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x [DOI] [PubMed] [Google Scholar]
- Droge W, & Schipper HM (2007). Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell, 6(3), 361–370. doi: 10.1111/j.1474-9726.2007.00294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman J, Dixit S, Nackenoff A, Schrag M, & Harrison FE (2018). Oxidative Stress Levels in the Brain Are Determined by Post-Mortem Interval and Ante-Mortem Vitamin C State but Not Alzheimer’s Disease Status. Nutrients, 10(7). doi: 10.3390/nu10070883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, … Sasai Y (2008). Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell, 3(5), 519–532. doi: 10.1016/j.stem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Fainzilber M, Budnik V, Segal RA, & Kreutz MR (2011). From synapse to nucleus and back again--communication over distance within neurons. J Neurosci, 31(45), 16045–16048. doi: 10.1523/JNEUROSCI.4006-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM (2005). Oxidative stress: the curse that underlies cerebral vascular dysfunction? Stroke, 36(2), 186–188. doi: 10.1161/01.STR.0000153067.27288.8b [DOI] [PubMed] [Google Scholar]
- Faravelli I, Costamagna G, Tamanini S, & Corti S (2020). Back to the origins: Human brain organoids to investigate neurodegeneration. Brain Res, 1727, 146561. doi: 10.1016/j.brainres.2019.146561 [DOI] [PubMed] [Google Scholar]
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, & Loguercio C (2007). Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer, 121(11), 2381–2386. doi: 10.1002/ijc.23192 [DOI] [PubMed] [Google Scholar]
- Fike JR, Rosi S, & Limoli CL (2009). Neural Precursor Cells and Central Nervous System Radiation Sensitivity. Seminars in Radiation Oncology, 19(2), 122–132. doi: 10.1016/j.semradonc.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T (2011). Signal transduction by reactive oxygen species. Journal of Cell Biology, 194(1), 7–15. doi: 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA (1999). Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med, 222(3), 236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x [DOI] [PubMed] [Google Scholar]
- Fraser HB, Khaitovich P, Plotkin JB, Paabo S, & Eisen MB (2005). Aging and gene expression in the primate brain. PLoS Biol, 3(9), e274. doi: 10.1371/journal.pbio.0030274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French HM, Reid M, Mamontov P, Simmons RA, & Grinspan JB (2009). Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res, 87(14), 3076–3087. doi: 10.1002/jnr.22139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, & Kramer-Albers EM (2013). Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci, 7, 182. doi: 10.3389/fncel.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, & Abramov AY (2012). Mechanism of oxidative stress in neurodegeneration. Oxidative Medicine and Cellular Longevity, 2012, 428010. doi: 10.1155/2012/428010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Vila J, Bachstetter A, & Bickford PC (2007). Oxidative Stress and the Aging Brain: From Theory to Prevention. In Riddle DR (Ed.), Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL). [PubMed] [Google Scholar]
- Giacci M, & Fitzgerald M (2018). Oligodendroglia Are Particularly Vulnerable to Oxidative Damage After Neurotrauma In Vivo. J Exp Neurosci, 12, 1179069518810004. doi: 10.1177/1179069518810004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, … Lancaster MA (2019). Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nature Neuroscience, 22(4), 669–679. doi: 10.1038/s41593-019-0350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico SL, Sutcliffe M, & Lancaster MA (2020). Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nature Protocols. doi: 10.1038/s41596-020-00433-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JK, & McKinlay JMA (2012). Physiological changes after brain stem death and management of the heart-beating donor. Continuing Education in Anaesthesia Critical Care & Pain, 12(5), 225–229. doi: 10.1093/bjaceaccp/mks026 [DOI] [Google Scholar]
- Goriely A, Geers MG, Holzapfel GA, Jayamohan J, Jerusalem A, Sivaloganathan S, … Kuhl E (2015). Mechanics of the brain: perspectives, challenges, and opportunities. Biomech Model Mechanobiol, 14(5), 931–965. doi: 10.1007/s10237-015-0662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griot C, Vandevelde M, Richard A, Peterhans E, & Stocker R (1990). Selective degeneration of oligodendrocytes mediated by reactive oxygen species. Free Radic Res Commun, 11(4-5), 181–193. doi: 10.3109/10715769009088915 [DOI] [PubMed] [Google Scholar]
- Guarente L, & Kenyon C (2000). Genetic pathways that regulate ageing in model organisms. Nature, 408(6809), 255–262. doi: 10.1038/35041700 [DOI] [PubMed] [Google Scholar]
- Haces ML, Montiel T, & Massieu L (2010). Selective vulnerability of brain regions to oxidative stress in a non-coma model of insulin-induced hypoglycemia. Neuroscience, 165(1), 28–38. doi: 10.1016/j.neuroscience.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Haining RL, & Achat-Mendes C (2017). Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res, 12(3), 372–375. doi: 10.4103/1673-5374.202928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (1992). Reactive oxygen species and the central nervous system. J Neurochem, 59(5), 1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x [DOI] [PubMed] [Google Scholar]
- Halliwell B, & Whiteman M (2004). Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol, 142(2), 231–255. doi: 10.1038/sj.bjp.0705776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, & Persidsky Y (2005). Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol, 78(6), 1223–1232. doi: 10.1189/jlb.0605340 [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, & Persidsky Y (2007). Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem, 101(2), 566–576. doi: 10.1111/j.1471-4159.2006.04393.x [DOI] [PubMed] [Google Scholar]
- Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, … et al. (1996). Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid beta peptide. Ann N Y Acad Sci, 786, 120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x [DOI] [PubMed] [Google Scholar]
- Hirooka Y (2008). Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Auton Neurosci, 142(1-2), 20–24. doi: 10.1016/j.autneu.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Hofrichter M, Nimtz L, Tigges J, Kabiri Y, Schroter F, Royer-Pokora B, … Fritsche E (2017). Comparative performance analysis of human iPSC-derived and primary neural progenitor cells (NPC) grown as neurospheres in vitro. Stem Cell Res, 25, 72–82. doi: 10.1016/j.scr.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Lin CC, Hsiao LD, & Yang CM (2013). High glucose induces reactive oxygen species-dependent matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Mol Neurobiol, 48(3), 601–614. doi: 10.1007/s12035-013-8442-6 [DOI] [PubMed] [Google Scholar]
- Hu JL, Todhunter ME, LaBarge MA, & Gartner ZJ (2018). Opportunities for organoids as new models of aging. Journal of Cell Biology, 217(1), 39–50. doi: 10.1083/jcb.201709054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Nakamura Y, Lo EH, & Hayakawa K (2019). Astrocyte Signaling in the Neurovascular Unit After Central Nervous System Injury. Int J Mol Sci, 20(2). doi: 10.3390/ijms20020282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Zou Y, & Corniola R (2012). Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol, 23(7), 738–744. doi: 10.1016/j.semcdb.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Zhang X, & Chen WW (2016). Role of oxidative stress in Alzheimer’s disease. Biomed Rep, 4(5), 519–522. doi: 10.3892/br.2016.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Knoblich JA, Lutolf MP, & Martinez-Arias A (2017). The hope and the hype of organoid research. Development, 144(6), 938–941. doi: 10.1242/dev.150201 [DOI] [PubMed] [Google Scholar]
- Hynd MR, Lewohl JM, Scott HL, & Dodd PR (2003). Biochemical and molecular studies using human autopsy brain tissue. J Neurochem, 85(3), 543–562. doi: 10.1046/j.1471-4159.2003.01747.x [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, & Kaindl AM (2011). Neuronal death and oxidative stress in the developing brain. Antioxid Redox Signal, 14(8), 1535–1550. doi: 10.1089/ars.2010.3581 [DOI] [PubMed] [Google Scholar]
- Jenner P (2003). Oxidative stress in Parkinson’s disease. Ann Neurol, 53 Suppl 3, S26–36; discussion S36-28. doi: 10.1002/ana.10483 [DOI] [PubMed] [Google Scholar]
- Jeremias S (2020, December 22). PMC Report Shows Number of Personalized Medicines Doubled in 4 Years. Number of Personalized Medicines Doubled in 4 Years, New Report Says. 2020/12/22. Retrieved from https://www.ajmc.com/view/number-of-personalized-medicines-doubled-in-4-years-new-report-says
- Jorfi M, D’Avanzo C, Tanzi RE, Kim DY, & Irimia D (2018). Human Neurospheroid Arrays for In Vitro Studies of Alzheimer’s Disease. Sci Rep, 8(1), 2450. doi: 10.1038/s41598-018-20436-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G, & Fisher D (2005). Oxidative stress and inflammation in brain aging: nutritional considerations. Neurochem Res, 30(6–7), 927–935. doi: 10.1007/s11064-005-6967-4 [DOI] [PubMed] [Google Scholar]
- Kagias K, Nehammer C, & Pocock R (2012). Neuronal responses to physiological stress. Front Genet, 3, 222. doi: 10.3389/fgene.2012.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B (2013). Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol, 1, 244–257. doi: 10.1016/j.redox.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, & Hensley K (2008). Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis, 15(3), 473–493. doi: 10.3233/jad-2008-15314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM, & Karmacharya R (2020). Transcriptomic Landscape and Functional Characterization of Induced Pluripotent Stem Cell-Derived Cerebral Organoids in Schizophrenia. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Park HJ, Choi H, Chang Y, Park H, Shin J, … Kim J (2019). Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Reports, 12(3), 518–531. doi: 10.1016/j.stemcr.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Brown SL, Jenrow KA, & Ryu S (2008). Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol, 87(3), 279–286. doi: 10.1007/s11060-008-9520-x [DOI] [PubMed] [Google Scholar]
- Kim JM, Kim HG, & Son CG (2018). Tissue-Specific Profiling of Oxidative Stress-Associated Transcriptome in a Healthy Mouse Model. Int J Mol Sci, 19(10). doi: 10.3390/ijms19103174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelink PJ, Overbeek SA, Braber S, de Kruijf P, Folkerts G, Smit MJ, & Kraneveld AD (2012). Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol Ther, 133(1), 1–18. doi: 10.1016/j.pharmthera.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Koh YH, Tan LY, & Ng SY (2018). Patient-Derived Induced Pluripotent Stem Cells and Organoids for Modeling Alpha Synuclein Propagation in Parkinson’s Disease. Front Cell Neurosci, 12, 413. doi: 10.3389/fncel.2018.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, & Zhang HJ (2007). An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol, 292(1), R18–36. doi: 10.1152/ajpregu.00327.2006 [DOI] [PubMed] [Google Scholar]
- Kruk J, Aboul-Enein HY, Kladna A, & Bowser JE (2019). Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res, 53(5), 497–521. doi: 10.1080/10715762.2019.1612059 [DOI] [PubMed] [Google Scholar]
- Kunsch C, & Medford RM (1999). Oxidative stress as a regulator of gene expression in the vasculature. Circ Res, 85(8), 753–766. doi: 10.1161/01.res.85.8.753 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, & Knoblich JA (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science, 345(6194), 1247125. doi: 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, … Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373–379. doi: 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, … Kornblum HI (2011). Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell, 8(1), 59–71. doi: 10.1016/j.stem.2010.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HP, Pancholi N, Esposito L, Previll LA, Wang X, Zhu X, … Lee HG (2012). Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. Plos One, 7(1), e28033. doi: 10.1371/journal.pone.0028033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees H, Walters H, & Cox LS (2016). Animal and human models to understand ageing. Maturitas, 93, 18–27. doi: 10.1016/j.maturitas.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Lewis DA (2002). The human brain revisited: opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology, 26(2), 143–154. doi: 10.1016/S0893-133X(01)00393-1 [DOI] [PubMed] [Google Scholar]
- Li R, Jia Z, & Trush MA (2016). Defining ROS in Biology and Medicine. React Oxyg Species (Apex), 1(1), 9–21. doi: 10.20455/ros.2016.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. doi: 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KP, Huang SH, De Silva R, Tan BK, & Zhu YZ (2006). Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res, 3(4), 327–337. doi: 10.2174/156720506778249515 [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, … Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan L, Ourednik V, & Ourednik J (2006). Increased “vigilance” of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells, 24(9), 2110–2119. doi: 10.1634/stemcells.2006-0018 [DOI] [PubMed] [Google Scholar]
- Mao XW, Nishiyama NC, Byrum SD, Stanbouly S, Jones T, Holley J, … Delp MD (2020). Spaceflight induces oxidative damage to blood-brain barrier integrity in a mouse model. The FASEB Journal, 34(11), 15516–15530. doi: 10.1096/fj.202001754R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao XW, Nishiyama NC, Pecaut MJ, Campbell-Beachler M, Gifford P, Haynes KE, … Gridley DS (2016). Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiation Research, 185(6), 647–657. doi: 10.1667/rr14267.1 [DOI] [PubMed] [Google Scholar]
- Mariani E, Polidori MC, Cherubini A, & Mecocci P (2005). Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci, 827(1), 65–75. doi: 10.1016/j.jchromb.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Markesbery WR (1997). Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med, 23(1), 134–147. doi: 10.1016/s0891-5849(96)00629-6 [DOI] [PubMed] [Google Scholar]
- Marotta N, Kim S, & Krainc D (2020). Organoid and pluripotent stem cells in Parkinson’s disease modeling: an expert view on their value to drug discovery. Expert Opin Drug Discov, 15(4), 427–441. doi: 10.1080/17460441.2020.1703671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale JL, & Holbrook NJ (2002). Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol, 192(1), 1–15. doi: 10.1002/jcp.10119 [DOI] [PubMed] [Google Scholar]
- Matigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, … Mackay-Sim A (2010). Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech, 3(11-12), 785–798. doi: 10.1242/dmm.005447 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Pedersen WA, & Culmsee C (2001). Neurodegenerative disorders and ischemic brain diseases. Apoptosis, 6(1-2), 69–81. doi: 10.1023/a:1009676112184 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev, 87(3), 873–904. doi: 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- Melo A, Monteiro L, Lima RMF, de Oliveira DM, de Cerqueira MD, & El-Bacha RS (2011). Oxidative Stress in Neurodegenerative Diseases: Mechanisms and Therapeutic Perspectives. Oxidative Medicine and Cellular Longevity. doi:Artn 467180 10.1155/2011/467180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S (2002). Animal models of oxidative stress, aging, and therapeutic antioxidant interventions. Int J Biochem Cell Biol, 34(11), 1395–1400. doi: 10.1016/s1357-2725(02)00086-9 [DOI] [PubMed] [Google Scholar]
- Metodiewa D, & Koska C (2000). Reactive oxygen species and reactive nitrogen species: relevance to cyto(neuro)toxic events and neurologic disorders. An overview. Neurotox Res, 1(3), 197–233. doi: 10.1007/bf03033290 [DOI] [PubMed] [Google Scholar]
- Mimetas. 3D Cell Culture vs. Traditional 2D Cell Culture. Retrieved from https://mimetas.com/article/3d-cell-culture-vs-traditional-2d-cell-culture
- Mitchell SJ, Scheibye-Knudsen M, Longo DL, & de Cabo R (2015). Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci, 3, 283–303. doi: 10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- Musgrove RE, Helwig M, Bae EJ, Aboutalebi H, Lee SJ, Ulusoy A, & Di Monte DA (2019). Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular alpha-synuclein transfer. J Clin Invest, 130, 3738–3753. doi: 10.1172/JCI127330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, & Wu Z (2009). Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res, 201(1), 1–7. doi: 10.1016/j.bbr.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Nassor F, Jarray R, Biard DSF, Maiza A, Papy-Garcia D, Pavoni S, … Yates F (2020). Long Term Gene Expression in Human Induced Pluripotent Stem Cells and Cerebral Organoids to Model a Neurodegenerative Disease. Front Cell Neurosci, 14, 14. doi: 10.3389/fncel.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M, & Richardson JR (2018). Time to get Personal: A Framework for Personalized Targeting of Oxidative Stress in Neurotoxicity and Neurodegenerative Disease. Curr Opin Toxicol, 7, 127–132. doi: 10.1016/j.cotox.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, & Godbout JP (2015). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology, 96(Pt A), 29–41. doi: 10.1016/j.neuropharm.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Millacura C, Tapia V, Munoz P, Maccioni RB, & Nunez MT (2002). An oxidative stress-mediated positive-feedback iron uptake loop in neuronal cells. J Neurochem, 82(2), 240–248. doi: 10.1046/j.1471-4159.2002.00971.x [DOI] [PubMed] [Google Scholar]
- Nutma E, van Gent D, Amor S, & Peferoen LAN (2020). Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells, 9(3). doi: 10.3390/cells9030600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzou G, Wicks RT, VanOstrand NR, Mekky GA, Seale SA, El-Taibany A, … Atala AJ (2020). Multicellular 3D Neurovascular Unit Model for Assessing Hypoxia and Neuroinflammation Induced Blood-Brain Barrier Dysfunction. Sci Rep, 10(1), 9766. doi: 10.1038/s41598-020-66487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, … Miura K (2013). Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res, 112(3), 523–533. doi: 10.1161/CIRCRESAHA.111.256149 [DOI] [PubMed] [Google Scholar]
- Olivares D, Huang X, Branden L, Greig NH, & Rogers JT (2009). Physiological and pathological role of alpha-synuclein in Parkinson’s disease through iron mediated oxidative stress; the role of a putative iron-responsive element. Int J Mol Sci, 10(3), 1226–1260. doi: 10.3390/ijms10031226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, … Butterfield DA (2008). Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging, 29(1), 51–70. doi: 10.1016/j.neurobiolaging.2006.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, & Costantini D (2011). Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol, 301(4), R843–863. doi: 10.1152/ajpregu.00034.2011 [DOI] [PubMed] [Google Scholar]
- Pariset E, Malkani S, Cekanaviciute E, & Costes SV (2020). Ionizing radiation-induced risks to the central nervous system and countermeasures in cellular and rodent models. International Journal of Radiation Biology, 1–47. doi: 10.1080/09553002.2020.1820598 [DOI] [PubMed] [Google Scholar]
- Pasca AM, Park JY, Shin HW, Qi Q, Revah O, Krasnoff R, … Pasca SP (2019). Human 3D cellular model of hypoxic brain injury of prematurity. Nature Medicine, 25(5), 784–791. doi: 10.1038/s41591-019-0436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, … Pasca SP (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods, 12(7), 671–678. doi: 10.1038/nmeth.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M (2016). Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol Sci, 37(9), 768–778. doi: 10.1016/j.tips.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly MG, Krajka V, Stengel F, Seibler P, Klein C, & Capetian P (2018). Adherent vs. Free-Floating Neural Induction by Dual SMAD Inhibition for Neurosphere Cultures Derived from Human Induced Pluripotent Stem Cells. Front Cell Dev Biol, 6, 3. doi: 10.3389/fcell.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peferoen L, Kipp M, van der Valk P, van Noort JM, & Amor S (2014). Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology, 141(3), 302–313. doi: 10.1111/imm.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Estrada C, Covacu R, Sankavaram SR, Svensson M, & Brundin L (2014). Oxidative stress increases neurogenesis and oligodendrogenesis in adult neural progenitor cells. Stem Cells Dev, 23(19), 2311–2327. doi: 10.1089/scd.2013.0452 [DOI] [PubMed] [Google Scholar]
- Perry VH, & Teeling J (2013). Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol, 35(5), 601–612. doi: 10.1007/s00281-013-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, & McEwen BS (2018). Psychological Stress and Mitochondria: A Systematic Review. Psychosom Med, 80(2), 141–153. doi: 10.1097/PSY.0000000000000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoschi AM, & Pop A (2015). The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem, 97, 55–74. doi: 10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, & Farah MJ (2015). Progress and challenges in probing the human brain. Nature, 526(7573), 371–379. doi: 10.1038/nature15692 [DOI] [PubMed] [Google Scholar]
- Poli D, Magliaro C, & Ahluwalia A (2019). Experimental and Computational Methods for the Study of Cerebral Organoids: A Review. Front Neurosci, 13, 162. doi: 10.3389/fnins.2019.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Wagner A, Mitran S, Sivanesan S, Chang E, & Buga AM (2013). ROS and brain diseases: the good, the bad, and the ugly. Oxidative Medicine and Cellular Longevity, 2013, 963520. doi: 10.1155/2013/963520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Tasic B, Hjerling-Leffler J, Trimarchi JM, & Awatramani R (2016). Disentangling neural cell diversity using single-cell transcriptomics. Nat Neurosci, 19(9), 1131–1141. doi: 10.1038/nn.4366 [DOI] [PubMed] [Google Scholar]
- Puschmann TB, Zanden C, De Pablo Y, Kirchhoff F, Pekna M, Liu J, & Pekny M (2013). Bioactive 3D cell culture system minimizes cellular stress and maintains the in vivo-like morphological complexity of astroglial cells. Glia, 61(3), 432–440. doi: 10.1002/glia.22446 [DOI] [PubMed] [Google Scholar]
- Qian X, Song H, & Ming GL (2019). Brain organoids: advances, applications and challenges. Development, 146(8). doi: 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha S, & Robinson BH (2000). Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci, 25(10), 502–508. doi: 10.1016/s0968-0004(00)01674-1 [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M, & Averill-Bates DA (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta, 1863(12), 2977–2992. doi: 10.1016/j.bbamcr.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, & Gendelman HE (2007). Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol, 82, 297–325. doi: 10.1016/S0074-7742(07)82016-2 [DOI] [PubMed] [Google Scholar]
- Reynolds BA, & Rietze RL (2005). Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods, 2(5), 333–336. doi: 10.1038/nmeth758 [DOI] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, … Huang Y (2012). Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell, 11(1), 100–109. doi: 10.1016/j.stem.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, & Choi AM (2007). Mechanisms of cell death in oxidative stress. Antioxid Redox Signal, 9(1), 49–89. doi: 10.1089/ars.2007.9.49 [DOI] [PubMed] [Google Scholar]
- Saha K, & Jaenisch R (2009). Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell, 5(6), 584–595. doi: 10.1016/j.stem.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S (2017). Oxidative Stress and the Central Nervous System. J Pharmacol Exp Ther, 360(1), 201–205. doi: 10.1124/jpet.116.237503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, & Stevens B (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018–1027. doi: 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Schaue D, Kachikwu EL, & McBride WH (2012). Cytokines in radiobiological responses: a review. Radiation Research, 178(6), 505–523. doi: 10.1667/RR3031.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S, Jaquet V, Trabace L, & Krause KH (2013). Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal, 18(12), 1475–1490. doi: 10.1089/ars.2012.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, & Chandel NS (2014). ROS function in redox signaling and oxidative stress. Curr Biol, 24(10), R453–462. doi: 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielke C, Hartel C, Durante M, Ritter S, & Schroeder IS (2020). Solving the Issue of Ionizing Radiation Induced Neurotoxicity by Using Novel Cell Models and State of the Art Accelerator Facilities. Frontiers in Physics, 8. doi: 10.3389/fphy.2020.568027 [DOI] [Google Scholar]
- Serrano F, & Klann E (2004). Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev, 3(4), 431–443. doi: 10.1016/j.arr.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Setia H, & Muotri AR (2019). Brain organoids as a model system for human neurodevelopment and disease. Semin Cell Dev Biol, 95, 93–97. doi: 10.1016/j.semcdb.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpee TO (2014). Toward functional classification of neuronal types. Neuron, 83(6), 1329–1334. doi: 10.1016/j.neuron.2014.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Feng A, & Rock RB (2013). Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem Res, 38(10), 2148–2159. doi: 10.1007/s11064-013-1123-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Buffenstein R, Pulliam DA, & Van Remmen H (2010). Comparative studies of oxidative stress and mitochondrial function in aging. Integr Comp Biol, 50(5), 869–879. doi: 10.1093/icb/icq079 [DOI] [PubMed] [Google Scholar]
- Shou Y, Liang F, Xu S, & Li X (2020). The Application of Brain Organoids: From Neuronal Development to Neurological Diseases. Front Cell Dev Biol, 8, 579659. doi: 10.3389/fcell.2020.579659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H, Berndt C, & Jones DP (2017). Oxidative Stress. Annu Rev Biochem, 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]
- Simons M, & Nave KA (2015). Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol, 8(1), a020479. doi: 10.1101/cshperspect.a020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, … Pasca SP (2017). Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron, 95(4), 779–790 e776. doi: 10.1016/j.neuron.2017.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS (2002). Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med, 33(1), 37–44. doi: 10.1016/s0891-5849(02)00856-0 [DOI] [PubMed] [Google Scholar]
- Sorce S, & Krause KH (2009). NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal, 11(10), 2481–2504. doi: 10.1089/ARS.2009.2578 [DOI] [PubMed] [Google Scholar]
- Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, & Cabiscol E (2008). Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med, 45(5), 667–678. doi: 10.1016/j.freeradbiomed.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Srivastava K, Tripathi R, & Mishra R (2018). Age-dependent alterations in expression and co-localization of Pax6 and Ras-GAP in brain of aging mice. J Chem Neuroanat, 92, 25–34. doi: 10.1016/j.jchemneu.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Starr R, Tadi P, & Pfleghaar N (2020). Brain Death. In StatPearls. Treasure Island (FL). [Google Scholar]
- Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, … Yang XY (2018). Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Progress in Neurobiology, 163-164, 118–143. doi: 10.1016/j.pneurobio.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Taibur Rahman IH, Towhidul Islam MM, Uddin Shekhar* Hossain. (2012). Oxidative stress and human health. Scientific Research, 3(7A), 23. doi: 10.4236/abb.2012.327123 [DOI] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, & Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872. doi: 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Tambalo M, & Lodato S (2020). Brain organoids: Human 3D models to investigate neuronal circuits assembly, function and dysfunction. Brain Res, 1746, 147028. doi: 10.1016/j.brainres.2020.147028 [DOI] [PubMed] [Google Scholar]
- Tan BL, Norhaizan ME, Liew WP, & Sulaiman Rahman H (2018). Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front Pharmacol, 9, 1162. doi: 10.3389/fphar.2018.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafdar A, & Pula G (2018). The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int J Mol Sci, 19(12). doi: 10.3390/ijms19123824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texel SJ, & Mattson MP (2011). Impaired adaptive cellular responses to oxidative stress and the pathogenesis of Alzheimer’s disease. Antioxid Redox Signal, 14(8), 1519–1534. doi: 10.1089/ars.2010.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaswamy S, Bridenbaugh EA, & Gashev AA (2012). Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol, 10(2), 53–62. doi: 10.1089/lrb.2011.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburne SK, & Juurlink BH (1996). Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem, 67(3), 1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x [DOI] [PubMed] [Google Scholar]
- Trevino AE, Sinnott-Armstrong N, Andersen J, Yoon SJ, Huber N, Pritchard JK, … Pasca SP (2020). Chromatin accessibility dynamics in a model of human forebrain development. Science, 367(6476). doi: 10.1126/science.aay1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, … Muotri AR (2019). Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell. doi: 10.1016/j.stem.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo CA, & Muotri AR (2018). Brain Organoids and the Study of Neurodevelopment. Trends Mol Med, 24(12), 982–990. doi: 10.1016/j.molmed.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Giedzinski E, Izadi A, Suarez T, Lan ML, Tran KK, … Limoli CL. (2014). Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid Redox Signal, 20(9), 1410–1422. doi: 10.1089/ars.2012.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, & Mahajan RT (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol, 7(1), 65–74. doi: 10.2174/157015909787602823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehlow A, Deville SS, & Cordes N (2020). 3D Radiation Biology for Identifying Radiosensitizers. In Molecular Targeted Radiosensitizers (pp. 115–135). [Google Scholar]
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, … Arlotta P (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature, 570(7762), 523–527. doi: 10.1038/s41586-019-1289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, … Salim S (2011). Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res, 224(2), 233–240. doi: 10.1016/j.bbr.2011.05.010 [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Eugenin-von Bernhardi L, & Eugenin J (2015). Microglial cell dysregulation in brain aging and neurodegeneration. Frontiers in Aging Neuroscience, 7, 124. doi: 10.3389/fnagi.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk WIM, Rainbolt TK, Dolan PT, Webb AE, Brunet A, & Frydman J (2020). Differentiation Drives Widespread Rewiring of the Neural Stem Cell Chaperone Network. Mol Cell, 78(2), 329–345 e329. doi: 10.1016/j.molcel.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Bolognin S, Antony PMA, Nickels SL, Poovathingal SK, Salamanca L, … Schwamborn JC. (2019). Neural Stem Cells of Parkinson’s Disease Patients Exhibit Aberrant Mitochondrial Morphology and Functionality. Stem Cell Reports, 12(5), 878–889. doi: 10.1016/j.stemcr.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Shin R, Tajinda K, Heusner CL, Kogan JH, Miyake S, … Matsumoto M (2012). Adult neurogenesis transiently generates oxidative stress. Plos One, 7(4), e35264. doi: 10.1371/journal.pone.0035264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H (2018). Modeling Neurological Diseases With Human Brain Organoids. Front Synaptic Neurosci, 10, 15. doi: 10.3389/fnsyn.2018.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pal R, Chen XW, Limpeanchob N, Kumar KN, & Michaelis EK (2005). High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res, 140(1-2), 120–126. doi: 10.1016/j.molbrainres.2005.07.018 [DOI] [PubMed] [Google Scholar]
- Wang XK, & Michaelis EK (2010). Selective neuronal vulnerability to oxidative stress in the brain. Frontiers in Aging Neuroscience, 2. doi:ARTN 12 10.3389/fnagi.2010.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman SM, Cambi F, & Kozai TD (2018). The role of oligodendrocytes and their progenitors on neural interface technology: A novel perspective on tissue regeneration and repair. Biomaterials, 183, 200–217. doi: 10.1016/j.biomaterials.2018.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J, Vytasek R, Uhlik J, & Vajner L (2016). Oxidative Stress in the Developing Rat Brain due to Production of Reactive Oxygen and Nitrogen Species. Oxidative Medicine and Cellular Longevity, 2016, 5057610. doi: 10.1155/2016/5057610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HW, & Kettenmann H (2017). Microglia in Physiology and Disease. Annu Rev Physiol, 79, 619–643. doi: 10.1146/annurev-physiol-022516-034406 [DOI] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJ, Song W, Jin LW, & Yankner BA (2002). Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nature Medicine, 8(6), 600–606. doi: 10.1038/nm0602-600 [DOI] [PubMed] [Google Scholar]
- Yagi T, Kosakai A, Ito D, Okada Y, Akamatsu W, Nihei Y, … Suzuki N (2012). Establishment of induced pluripotent stem cells from centenarians for neurodegenerative disease research. Plos One, 7(7), e41572. doi: 10.1371/journal.pone.0041572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S (2012). Induced pluripotent stem cells: past, present, and future. Cell Stem Cell, 10(6), 678–684. doi: 10.1016/j.stem.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Kuroiwa H, Yano R, & Araki T (2008). Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinson’s disease. Neurol Sci, 29(5), 293–301. doi: 10.1007/s10072-008-0986-2 [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Elahi LS, Pasca AM, Marton RM, Gordon A, Revah O, … Pasca SP (2019). Reliability of human cortical organoid generation. Nat Methods, 16(1), 75–78. doi: 10.1038/s41592-018-0255-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TF, Gu S, Shan C, Machado S, & Arias-Carrion O (2016). Erratum to: Oxidative Stress and Adult Neurogenesis. Stem Cell Rev Rep, 12(1), 162. doi: 10.1007/s12015-015-9629-1 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, & Dong W (2016). ROS and ROS-Mediated Cellular Signaling. Oxidative Medicine and Cellular Longevity, 2016, 4350965. doi: 10.1155/2016/4350965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, & An J (2007). Cytokines, inflammation, and pain. Int Anesthesiol Clin, 45(2), 27–37. doi: 10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Sun C, Ren J, Wang G, Ma R, Sun L, … Xu J (2019). Stress-induced precocious aging in PD-patient iPSC-derived NSCs may underlie the pathophysiology of Parkinson’s disease. Cell Death Dis, 10(2), 105. doi: 10.1038/s41419-019-1313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, & Zhou T (2019). Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int J Mol Sci, 20(18). doi: 10.3390/ijms20184472 [DOI] [PMC free article] [PubMed] [Google Scholar]


