Abstract
Microbes inhabit different anatomical sites of the human body including oral cavity, gut, and skin. A growing literature highlights how microbiome variation is associated with human health and disease. There is strong evidence of bidirectional communication between gut and brain mediated by neurotransmitters and microbial metabolites. Here, we review the potential involvement of microbes residing in the gut and in other body sites in the pathogenesis of eight neuropsychiatric disorders, discussing findings from animal and human studies. The data reported provide a comprehensive overview of the current state of the microbiome research in neuropsychiatry, including hypotheses about the mechanisms underlying the associations reported and the translational potential of probiotics and prebiotics.
Keywords: Microbiome, Gut-Brain- Axis, Inflammation, Neuropsychiatric Disorders, Dysbiosis, Probiotics
1. Introduction
Microbes reside in the human body and not all of them are necessarily harmful. Their presence/absence and balance/imbalance are associated with health and disease. Recent estimates revealed that the number of microbial to human cells show a 1:1 proportion. Some microbes are beneficial and their diversity across human body sites contributes to the overall health status of an individual [1; 2]. Fluctuations in the relative diversity and composition of the microbiome across the human body are hypothesized to affect the risk of several diseases, including inflammatory bowel disease (IBD) [3], cancer [4], and immunological disorders [5].
There is considerable literature supporting the association between human microbiome variation and mental health, also including several review articles focused on specific disorders or specific mental health domains, such as depression [6; 7; 8], mood disorders [9; 10], neurodegenerative disorders [11], and neurodevelopment [12]. However, to our knowledge, a review presenting the current state of microbiome research across multiple neuropsychiatric disorders and different body sites is missing. The current article aims to provide a compendium of the current state of human microbiome research across the neuropsychiatric spectrum to help investigators with different expertise to understand the evidence available to date. We include an initial overview of three microbiome domains (gut, mouth, and skin; Figure 1) and then review findings specifically related to eight neuropsychiatric disorders (Alzheimer’s disease, attention deficit hyperactivity disorder, anorexia nervosa, autism spectrum disorder, bipolar disorder, major depressive disorder, schizophrenia, and substance use disorders; Table 1). Due to size constraints, we decided to focus on those psychiatric disorders that were investigated by numerous studies or that were not reviewed previously.
Figure 1:
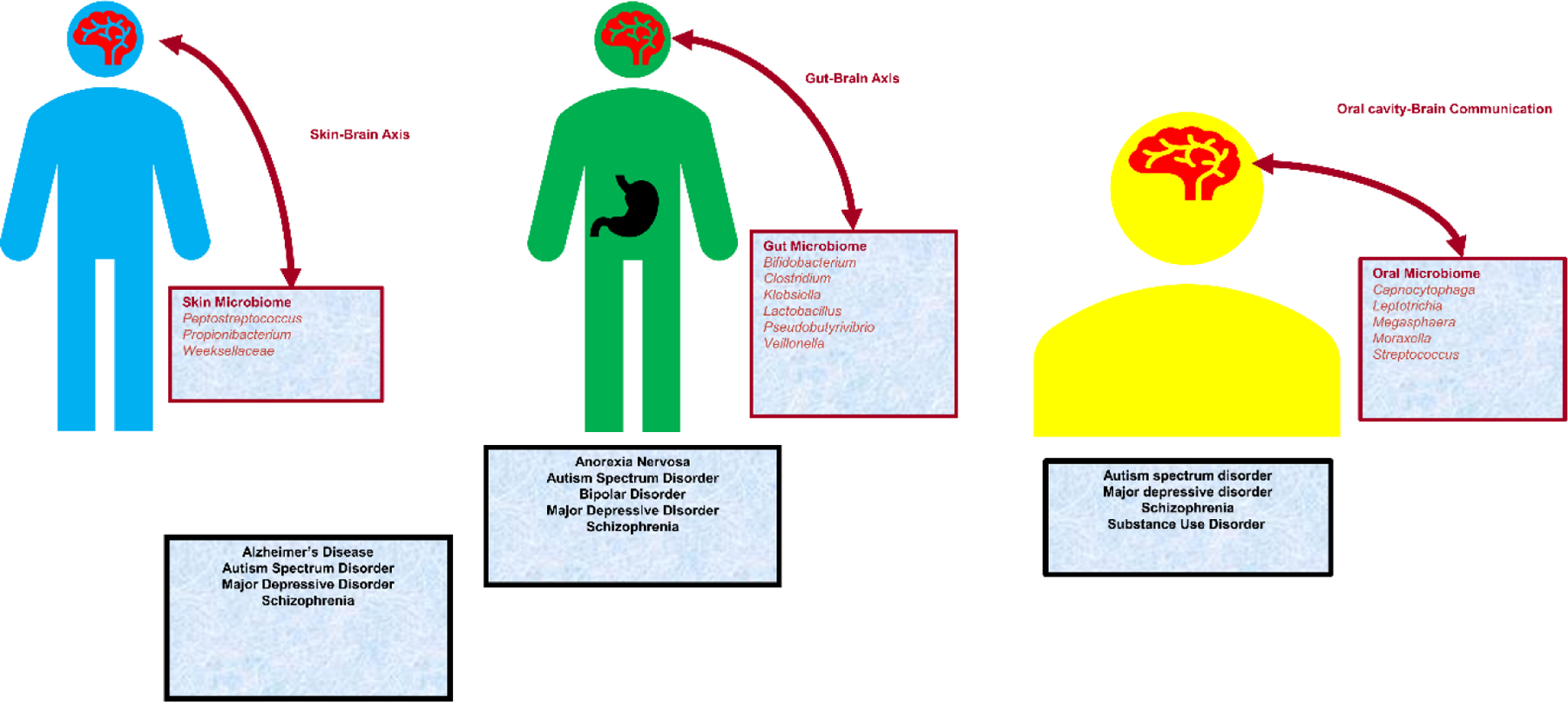
Microbiome across different anatomical sites and associated psychiatric disorders
Table 1:
Microorganisms, anatomical sites, and associated psychiatric disorders.
| Microorganism | Type | Anatomical sites | Psychiatric Disorders |
|---|---|---|---|
| Lactobacillus fermentum | AD | ||
| Lactobacillus acidophilus | |||
| Lactobacilli | |||
| Roseburia inulinivorans | |||
| Roseburia | |||
| Methanobrevibacter smithii | |||
| Ruminococcus | AN, ASD | ||
| B. bifidum | |||
| Bifidobacterium | |||
| Collinsella stercosis | |||
| LactoBacillus bulgaricus | |||
| Lactobacillus fermentum | |||
| Lactobacillus plantarum WCFS | |||
| Veillonella | |||
| Bifidobacterium adolescentis | |||
| Preveotella | |||
| Akkermansia | |||
| Faecalibacterium | |||
| Flavonifractor | |||
| Lachnospiraceae | |||
| Butyrivibrio | |||
| Coprococcus | |||
| Dorea | |||
| Pseudobutyrivibrio | |||
| Lactobacillus phage phiadh | SZ | ||
| Leptotrichia | Gut, Oral Cavity | ||
| Peptostreptococcus | Gut, Oral cavity, Skin, Vagina | ||
| Propionibacterium acnes | Gut, Skin | AD | |
| Corynebacterium | ASD, AD, MDD | ||
| Weeksellaceae | SZ | ||
| Enterobacteria | |||
| Clostridium difficle | |||
| Enterobacter | AD, MDD, ASD | ||
| Clostridium | AN, ASD | ||
| Clostridium bolteae | |||
| Klebsiella | |||
| Clostridium coccoides | |||
| Clostridium histolyticum | |||
| Escherichia coli | |||
| Alistipes | MDD | ||
| Measles morbillivirus | SZ | ||
| Saccharomyces cerevisiae | Gut, Skin | SZ | |
| Streptococcus | Oral cavity | ADHD, ASD, BD, MDD | |
| Neisseria | ASD | ||
| Megasphaera | ASD, SZ | ||
| Moraxella | ASD, SZ, BD | ||
| Capnocytophaga | |||
| Rothia | |||
| Capnocytophaga | |||
| Haemophilus | |||
| Herpes simplex virus | Oral cavity, Skin, Vagina | ||
| Cytomegalovirus | Oral cavity, Urinary tract | ||
| Epstein–Barr virus | Oral cavity, Vagina | ||
| Rubella | Urinary tract | ||
| Candida albicans | Vagina |
Abbreviations- AD: Alzheimer’s disease, ADHD: Attention Deficit Hyperactivity Disorder, AN: Anorexia Nervosa, ASD: Autism Spectrum Disorder, BD: Bipolar Disorder, MDD: Major Depressive Disorder, Schizophrenia: SZ, SUD- Substance Use Disorder.
The studies reviewed were conducted using a wide range of different methods and designs. For example, diversity metrics include several measures of alpha diversity (e.g., the Shannon index assumes the observed abundances reflect random sampling of the microbiome and thus is maximized when abundances increase evenly across all taxonomic units; the Simpson index gives more weight to highly abundant taxanomic groups and is less influenced by very low abundance organisms; the Chao1 index uses a Poisson distribution to estimates the number of taxa in a sample by extrapolating the number of rare organisms that may have been missed due to under-sampling; rarefaction assesses species richness through construction of rarefaction curves). Beta diversity reflects a comparison of abundances between two microbiome samples (e.g., Jaccard distance is measures similarity in the presence or absence of taxonomic groups without regard to abundance; Bray–Curtis dissimilarity measures the differences in abundances of each taxonomic units; Unweighted UniFrac is the distance between two samples by calculating the fraction of the branch length in a phylogenetic tree that leads to descendants) [13]. Because analytic variability undermines comparison between studies and contributes to the lack of reproducibility among microbiome studies, investigators have highlighted the need for establishing standards for microbiome analysis and interpretation [14; 15]. Due to the difficulty of comparing results that used different statistics, we decide to compare the finding of the studies reviewed relying on the interpretation made by the authors.
2. Gut-Brain Axis
The gut microbiome is a reservoir of many microorganisms such as Firmicutes, Bacteroides, Preveotella, and Bifidobacterium associated with the healthy physical and mental state of an individual. [16]. Gut dysbiosis (i.e., altered abundances of gut microbial communities) has been hypothesized to be involved in gastrointestinal disease [16; 17], cardiovascular illnesses [18], metabolic disorders [19]and autoimmune diseases [19; 20]. With respect to neuropsychiatric disorders, the gut-brain axis (GBA) represents the link between the central and the enteric nervous system, linking emotional and cognitive centers of the brain with peripheral intestinal functions. The association between microbiota and GBA appears to be bidirectional with signaling from gut-microbiota to brain and from brain to gut-microbiota mediated neural, endocrine, immune, and humoral mechanisms [21]. The impact of gut microbes on human health also includes potential associations with increased vulnerability to psychiatric disorders. A well-known study on using a germ-free mouse model emphasized an altered hormonal response to stress, suggesting that microbiota influences the neuroendocrine hypothalamic–pituitary–adrenal (HPA) axis [22]. In additional mouse experiments, the lack of normal gut microbiota influences behaviors (e.g., motor activity) and brain transcriptomic profile involved in motor control and behavioral regulations [23]. Another study testing the administration of a mixture of nonabsorbable antimicrobials for 7 days to pathogen-free mice transiently altered the gut microbial composition and was associated with heightened exploratory behavior and hippocampal Brain-Derived Neurotrophic Factor (BDNF) expression [24; 25]. These changes were independent of other inflammatory activities and alterations at the level of gastrointestinal neurotransmitters. Accordingly, the intestinal microbiota was hypothesized to influence brain chemistry and behaviors independently of the autonomic nervous system and gut inflammation [25].
The gut epithelium plays a key role in the GBA regulation because it is the primary target for changes induced by dietary, microbial, and inflammatory components. Enterochromaffin (EC) cells function as chemo- and mechanosensory neuroendocrine cells that release serotonin in order to modulate other gastrointestinal neurons, in order to effect peristalsis and mucus secretion. Serotonin released from EC cells in the mouse colon has been attributed to neuroinflammation [26; 27]. There is evidence of sex-differences in this hormonal response to microbiome perturbations. Female germ-free mice showed greater serotonin concentrations and elevated plasma levels of tryptophan (i.e., the serotonin precursor) than conventionally colonized control animals [28]. In mouse models of stroke and multiple sclerosis, the perturbation of gut microbiota appears to interfere with communicative and sensorimotor behaviors via immunomodulation [29].
The gut consists of a high concentration of immune cells, providing an additional layer of defense from pathogens. Production of pro- and anti-inflammatory cytokines is influenced by gut microbiota which can lead to brain dysfunction through the circulatory system [30]. Inflammation caused by cytokines can result in the release of corticosteroids accelerating stress-induced anxiety and depression [31]. Gut microbiota regulates metabolism of neuroactive compounds (e.g., short-chain fatty acids, indoles, bile acids, choline metabolites, lactate) and their release of these neuroactive compounds can promote additional neuroinflammation [32; 33].
Microbes residing in the gut often metabolize tryptophan, the serotonin precursor, along with a host of other neurotransmitters and neuromodulators [34]. These compounds permeate the gut wall that is innervated by the enteric nervous system (ENS) [35; 36]. This interaction can trigger bidirectional gut-brain communication resulting in inflammation of the gut and brain epithelia and production of stress peptides resulting in anxiety-driven behaviors [21]. Gut-brain module analysis based on human fecal metagenomes identified microbial production of 3,4-dihydroxyphenylacetic acid (a dopamine metabolite), which correlated positively with self-reported quality of life; this study also indicated the role of microbial γ-aminobutyric acid production in psychopathology [33; 37]. Among the pathogenic processes involved in the disruption of gut-brain axis equilibrium, inflammation plays a key role in altering the microbiome homeostasis in response to specific pathogens [38], immune activation [39], and antibiotic supplementation [40]. This altered microbial environment due to gut infection can be restored via beneficial microbes called probiotics [41]. Although this field is still in infancy, it has great potential for the development of future therapeutic applications [42; 43; 44].
3. Oral Microbiome
The human oral cavity is a complex environment presenting a variety of habitats hosting different kinds of microorganisms [45]. Highly prevalent microorganisms in the oral cavity include Staphylococcus [46; 47] and Streptococcus [48]. Data regarding different oral microbiome species are available from the Human Oral Microbiome Database (HOMD) [49]. Compared with the gut microbiome, few studies examined the oral microbiome in the context of neuropsychiatric disorders [50]. The microbiome in the oral cavity has been associated with systemic inflammation linked to altered cognitive functioning [51]. This may be due to indirect factors such as diet, lifestyle, and oral hygiene, but the fact that the association was related to a specific species (i.e., Neisseria subflava) suggests the possibility of a direct link. Unfortunately, to date the underlying mechanisms are still unclear. Due to many confounders that can affect the human variation of oral microbiome composition, most of the current studies should be considered as preliminary evidence that needs confirmation in a larger and more carefully characterized samples [52].
4. Skin Microbiome
Skin serves as a barrier preventing the invasion of external pathogens and also acts as the primary habitat for the commensal microbiota [53]. Sebaceous sites in the skin are reservoirs of specific bacteria like lipophilic Propionibacterium, Staphylococcus, and Corynebacterium species [54]. Skin microbiome diversity is generally conserved at the community level and despite external perturbations like diet, antimicrobial therapy, and long-term environmental interactions, it is considered stable over time in healthy individuals [55]. Alteration of skin microbiota is observed when individuals are affected by wounds, lesions, and/or dermatological disorders. Very few studies investigated the impact of skin microbiome dysbiosis with respect to psychiatric disorders. However, some of them hypothesized the presence of a gut-brain-skin axis related to immune response and inflammatory processes linking these organs [56; 57; 58]. Bidirectional communication between skin and gut microbiota has been reported in the context of immune-related disorders [1; 59; 60]. For example, variation in gut microbiome in patients with skin lacerations has been reported to alter the skin microbiota [57; 61; 62]. Persistent changes in both gut and skin microbiome can lead to neuro-modulatory effects associated with decreased cognitive function via inflammatory cytokines (i.e., TNF-α, IL-1β, IL-6, IFN-α) [56]. These factors have been associated with gut inflammation, increasing the permeation of the gut-brain barrier resulting in the release of neurotransmitters [63; 64; 65]. In patients with psoriasis, alterations in the skin and intestinal microbiome play a role in the pathogenesis of psoriasis, where inflammatory and immune mechanisms are associated with the dysregulation of the hypothalamic-pituitary-adrenal axis [66; 67].
5. Alzheimer’s disease
Alzheimer’s disease (AD) is a leading cause of death worldwide, with an estimated incidence of 1–3% and a prevalence of 10–30% of the population > 65 years of age in the United States [68; 69]. The role of altered gut microbiota and its subsequent impact on the HPA axis has been studied in the context of AD pathophysiology [70]. The leading hypothesis is that the composition of the intestinal microbiome plays a role in the neuroinflammation of the amyloid plaques deposition [71]. Indeed, microbiota-mediated inflammation associated with AD appears to act at the level of the blood-brain barrier (BBB) [72]. Some bacteria are capable of directly crossing the BBB, giving rise to infections of the central nervous systems (CNS) [73]. Certain molecules generated by bacteria (e.g., lipopolysaccharide, LPS) potentially stimulate BBB disruption in patients affected by neurodegenerative disorders. [74]. Consistent with this, the bacterial metabolite propionate, a short-chain fatty acid (SCFA), appears to protect the BBB from damage via inhibiting oxidative stress thereby maintaining its integrity. [75]. LPS is typically associated with pathogenic strains, while commensal or non-pathogenic flora produce SCFAs. In rats, peritoneal LPS administration resulted in increased levels of inflammatory factors, notably IL-1, IL-6, and TNF-α, in the hippocampus, suggesting a role for the microbiome in the initiation of an innate immune response in AD [76]. Zhao and colleagues found immunohistochemical evidence of microbiome-derived LPS within the perinuclear region of human AD brains [77].
The natural biodiversity of the gut microbiome tends to decline with aging, with a relative reduction in commensal species, such as Bacteroides, Bifidobacteria, and Lactobacilli, and a relative increase in opportunists and potentially pathogenic species such as Enterobacteria, C. perfringens, and C. difficile [78]. In the ELDERMET cohort comprised of individuals over 65 years of age, there was a shift in the gut microbiota toward a Bacteroidetes-predominated population in older individuals compared to younger participants[79]. The variation in microbial composition is also dependent on diet and lifestyle [80]. Diet-induced perturbation in the gut microbiome alters the shikimate metabolic pathway (responsible for the de novo synthesis of aromatic compounds in microorganisms [81]), which was associated with elevated levels of the cytotoxic amine tryptamine and increased symptoms among individuals affected by AD [82]. In human brain samples, a large bacterial load of Firmicutes species and P. acnes was observed in the cerebral cortex of AD patients [83].
Ongoing studies are exploring the role of probiotics used in gastrointestinal (GI) diseases for the treatment of AD patients. [84]. Certain probiotic formulations displayed neuroprotective effects in a transgenic mouse model of AD, including attenuation of microglial activation, reduction in Aβ load, and preservation of dendritic spine structure and function [85; 86]. A relatively larger randomized controlled trial (N=60) revealed that intake of milk supplemented with Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum produced significant, albeit small magnitude improvement in Mini-Mental Status Examination scores (widely used to test cognitive function among the elderly [87]) and improvement on various metabolic measures in AD patients [88]. With respect to the oral microbiome, a large scale retrospective case-control study of Alzheimer’s disease (AD) including participants from Taiwan’s national insurance database (N=209,112 cases and 836,448 dementia-free controls) found negative associations with the per-person cost of dental care, number of root canals, and number of tooth extractions, and positive associations with the cost of dental imaging and dental emergencies [89].
6. Attention Deficit Hyperactivity Disorder
Attention-deficit hyperactivity disorder (ADHD) is a heterogeneous neurodevelopmental disorder [90]. Diet potentially plays an important role in ADHD-related behavioral processes via its effect on the composition and functioning of the gut microbiome [91]. Apart from diet, host-microbe interaction with the gut-brain axis could be directly involved in the development of ADHD [92]. The host-microbiome interactions have implicated effects on hormones and neurotransmitter levels thought to be involved in the pathophysiology of ADHD [93; 94]. For instance, GABA production has been associated with different microbial genera including Bifidobacterium, Lactobacillus, and Escherichia coli [95]. Gut dysbiosis in combination with immune dysfunction caused by constant pathogen exposure could contribute to hyperactive behaviors observed in ADHD affected patients [96]. A study administering probiotics to ADHD patients showed that early intervention with Lactobacillus reduced the risk of ADHD development later in childhood [97]. ADHD has been associated with abnormalities in the predicted dopamine and noradrenaline synthesis, whose precursors are provided by the gut bacteria [90]. ADHD is often found to be associated with gut dysbiosis like enrichment or depletion of certain bacteria like Bifidobacterium [94].
7. Anorexia Nervosa
Anorexia nervosa (AN) is a mental illness characterized primarily by feeding restriction, distorted perceptions of and preoccupation with body weight and shape, and obsessive behaviors related to food [98]. Metabolic, immunologic, and weight regulating effects of the microbiome could influence the course and prognosis of the disease. Additionally, the interplay between stress-coping mechanisms and gut microbiome can also have important implications for AN [99].
AN patients have demonstrated altered diversity of bacterial species within the gut [100; 101; 102]. In particular, AN patients showed high gut levels of the archaeon Methanobrevibacter smithii [103]. This methane-producing archaeon is associated with food transformation processes in a very low-calorie diet [104]. AN patients also have significantly low amounts of total bacteria and obligate anaerobes, e.g. Clostridium coccoides group [105]. The reduced microbial diversity is associated with impaired immune response and reduced capacity to absorb calories from the diet [106]. Additionally, the genera Roseburia, Ruminococcus, and Clostridium were reduced in line with the AN depletion of total short-chain fatty acids, butyrate, and propionate [101]. Butyrate concentrations inversely correlated with anxiety levels, whereas propionate was positively associated with insulin levels and with an increased presence of Roseburia inulinivorans [101].
Due to its therapeutic potential, the interaction of the human microbiome with dietary habits is a rapidly expanding research area in AN [107; 108]. For example, Roseburia sp. represents one of the candidates for AN probiotic intervention due to the lower rate of anxiety associated with affected patients [109]. However, more studies are needed to clarify whether the differences observed are a cause or consequence of the disease. Additionally, it will be important to understand how changes in AN microbiota are affected by the interactions among nutritional supply, nutritional supplements, probiotics (i.e., live bacteria), and prebiotics (i.e., fibers supporting the growth of certain types of bacteria) [110].
8. Autism Spectrum Disorder
Autism spectrum disorder (ASD) is characterized by impairment in communication, speech, social interaction, and the presence of restricted interests and repetitive or stereotyped behaviors [111; 112]. Due to the high prevalence of GI symptoms in ASD-affected individuals, considerable attention has been paid to the gut microbiome [113]. Altered age-related patterns have been associated with ASD-affected individuals, including cognitive impairments, difficulty in speech and motor coordination skills [114; 115]. A higher representation of Bacteroidetes, Proteobacteria, and Firmicutes has been reported in ASD patients when compared with healthy controls [116]. Conversely, ASD showed a reduction of Bifidobacterium, Klebsiella, Enterobacter, Prevotella, Coprococcus, and Veillonellacea [117; 118; 119]. Earlier studies also suggested that the imbalance in Bacteroides and Firmicutes gut has been associated with increased autism severity in ASD patients [120]. However, this finding was not replicated in certain cohorts, possibly due to different living conditions and eating habits [121]. Pyrosequencing of fecal microflora of ASD children showed a higher abundance of Desulfovibrio species and Bacteroides vulgatus when compared with healthy controls [122]. Late-onset autism patients presented a high incidence of Clostridium and Ruminococcus species with a particular enrichment for Clostridium cluster groups I and XI and Clostridium bolteae [123]. The accumulation of neurotoxin-producing bacteria such as Clostridia are associated with ASD symptoms [124]. Toxic molecules released by such microbes affect serotonin signalling [125], potentially leading to ASD behavioral patterns such as decreased socialization, decreased response to pain, abnormal language, and self-abusive or repetitive behaviors. ASD-affected individuals show altered levels of other potentially toxic compounds produced by several bacteria (e.g., Bifidobacterium, C. difficile, and C. histolyticum) [126; 127; 128; 129]. Among them, increased abundance of urinary and fecal paracresol (p-cresol) and its conjugated derivative p-cresylsulfate inhibit the enzyme dopamine-beta-hydroxylase [130]. Additionally, increased Clostridia-derived metabolite 3-(3-hydroxyphenyl)-3-hydroxy propionic acid has been detected in ASD-affected individuals, potentially reflecting an altered catecholamine metabolism. Treatment with vancomycin and probiotic Bifidobacterium was associated with normalized metabolite levels, decreased constipation, and a reduction in severity of ASD features [131].
Obesity- or diet-induced changes in the offspring gut microbiome have been examined as a potential mediator of the association of maternal obesity with ASD. In rodent models, offspring of mothers who were fed high fat diets showed altered gut microbial composition and deficits in social behaviors and social reward pathways thought to be relevant to ASDs [132]. Fecal inoculation from offspring whose mothers were fed a typical diet (through either cohabitation or fecal transplant) were associated with normalization of social (but not repetitive) behaviors [132].
The gut microbiome metabolizes three classes of short-chain fatty acids (SCFA): propionic acid (PPA), acetic acid, and butyric acid. PPA and SCFA in general are capable of gaining access to the brain and inducing widespread effects on CNS function, including neurotransmitter synthesis and release, calcium influx, intracellular pH maintenance, lipid metabolism, intercellular gating, immune activation, and gene expression [133]. Perfusion of PPA in rats induced ASD symptoms, supporting the importance of gut-acquired factors [119; 134].
A small open-label clinical trial evaluated the impact of microbiota transfer therapy (MTT; a combination of antibiotics, a bowel cleanse, a stomach-acid suppressant, and fecal microbiota transplant) on gut microbiota composition, GI symptoms, and ASD symptoms in eighteen affected individuals. [135]. The GI symptom rating scale exhibited an approximately 80% decrease of GI symptoms at the end of treatment, including significant improvements in symptoms of constipation, diarrhea, indigestion, and abdominal pain [135]. Additionally, ASD symptoms improved significantly and remained constant 8 weeks after treatment ended [135]. The overall bacterial diversity and the abundance of Bifidobacterium, Prevotella, and Desulfovibrio among other taxa increased following MTT, and these changes persisted after treatment stopped [135]. Parracho and colleagues conducted a double-blind placebo crossover trial in the United Kingdom with 22 children with ASD aged between 3–16 years using Lactobacillus plantarum WCFS as a probiotic [136]. Although no major differences were observed in GI symptoms, a significant increase in Lactobacilli/Enterococci and a decrease in the Clostridium coccoides were reported in the stool samples of children with ASD when compared with the placebo group.
Another interesting area of ASD research is related to maternal immune activation (MIA) triggered by infectious or infection like stimuli [137]. A MIA mouse model showed significant behavioral changes in offspring of potential relevance to ASD [29]. These changes are accompanied by altered gut microbial composition. Oral treatment with human Bacteroides fragilis improved the communicative and stereotyped behaviors, as well as altered intestinal permeability associated with microbial composition [29]. The behavioral deficits appeared to be due to the Clostridium-associated metabolite 4-ethylphenyl sulfate, a molecule like p-cresol (4-methylphenol) [29]. This is a chemically related metabolite reported being a possible urinary ASD biomarker [138; 139]. In another study conducted on ASD-affected children (N=22), a sugar-free diet and probiotic capsules of L. acidophilus were associated with significant improvement in concentration and the ability to follow instructions in affected patients [128; 140]. In an additional clinical trial, an oral liquid dose of vancomycin 500 mg/day and followed by probiotic therapy (a mixture of L. acidophilus, L. bulgaricus, and B. bifidum) was associated with an improvement in the cognitive functioning of ASD patients [141].
Beyond the gut environment, microbiome variation in other body sites was also investigated with respect to ASD. For example, certain components of the oral microbiome (abundance of Rothia, Neisseria, Moraxella, Megasphaera, and Gemella) were associated with autism in children [142].
9. Bipolar Disorder
Bipolar disorder (BD) is a chronic mood disorder characterized by periods of abnormally elevated mood in addition to periods of depression, and it is associated with high morbidity [143]. Several lines of evidence support the presence of chronic low-grade inflammation among BD-affected persons, with increased plasma cytokines, soluble cytokine receptors, chemokines, acute phase reactants, and T-cell activation. It is unclear the extent to which these findings may be linked with dysbiosis.
In a study comparing the gut microbiota of 115 BD patients and 64 healthy controls [144], Faecalibacterium levels were decreased in BD patients and abundance was negatively correlated with self-reported symptoms of depression severity. In a separate study, higher microbial variation was observed in BD-affected patients when compared to healthy controls [145]. Among patients affected by episodes of both mania and depression, the relative increase of Escherichia coli and Bifidobacterium adolescentis was higher in individuals with manic episodes, while Stercoris was higher in individuals with depressive symptoms [145; 146]. Flavonifractor genus was associated with BD patients [147]. However, no difference in gut microbiota were observed between unaffected first-degree relatives of BD patients and healthy controls [147].
Few studies have examined microbiome interventions in BD. An investigation of 20 euthymic BD-affected individuals, who received a probiotic supplement over 3 months, showed significant improvement in cognition and psychomotor processing speed [148]. Additionally, one observational study of atypical antipsychotic treatment showed a decreased alpha diversity of gut microbiota in BD female patients and altered abundance of Lachnospiraceae and Akkermansia in the whole BD group [149].
10. Major Depressive Disorder
Major Depressive disorder (MDD) is the 4th leading course of disability around the world [150]. Multiple studies reported associations between microbiome variation and certain biological changes associated with MDD pathogenesis (e.g., neurotrophic factor alterations neuroanatomical abnormalities, and endocrine and immune system dysfunction) [151; 152]. Depression appears to be generally associated with reduced microbial diversity [153]. The relative abundance of Bacteroidetes, Proteobacteria, and Actinobacteria phyla were increased in MDD patients whereas that Firmicutes abundance was significantly reduced [152]. MDD group also showed elevated levels of Enterobacteriaceae and Alistipes but reduced levels of Fecalibacterium, which were also negatively associated with MDD symptoms [152]. Coprococcus, Pseudobutyrivibrio, Dorea, and Clostridium genera were reported as overrepresented in MDD patients [154]. Conversely, MDD-depleted bacterial genera include butyrate-producing bacteria like Dialister, Fecalibacterium, and Butyvibrio [155; 156]. In female MDD patients, it was observed enrichment for Bacteroidetes, proteobaeteria, and Fusobacteria and depletion for Firmicutes and Actinobacteria phyla [157]. In mouse models, the microbiome profile associated with depressive symptoms has been associated with specific changes in microbial genes and changes in gut metabolism of carbohydrate and amino acids [158; 159]. The composition of gut microbiota is significantly altered in MDD mice versus healthy GF (germ-free) controls [153; 160]. In mouse models, LPS-administration induces neuroinflammatory changes that effect synaptic and non-synaptic plasticity in basolateral amygdala projection neurons associated with anxiety-related behavior [161]. Additionally, the endocrine system communicates bidirectionally with the gut microbiota also via sex hormones, like androgens, estrogens, and others, which can influence MDD-related neuroinflammation [162; 163]. Indeed, androgens seem to exhibit anxiolytic properties whereas estrogens have been found to elevate HPA activity [164]. Fluctuations in levels of oxytocin are correlated positively with changes in the gut bacterial taxa whose abundance was altered in clinical depression [160].
Probiotics consumption for extended periods showed beneficial effects on depression-related behaviors, [165]. One of the other potential therapeutic approaches for depression based on FMT microbial manipulation is the. This therapeutic approach showed positive effects in treating Clostridium difficile mediated infection[166]. GF mice receiving FMT from patients with depression exhibited greater depressive behaviors compared to GF mice receiving FMT from healthy control individuals [158].
11. Schizophrenia
Schizophrenia (SCZ) is a chronic psychiatric disorder characterized by a range of symptoms, including delusions, hallucinations, disorganized thoughts, and cognitive deficits [167]. The role of microbial diversity in contributing to SCZ has been widely discussed. Imbalance of microbes produced either by pathogen invasion, stress, immune gene activation, or endothelial barrier compromise is associated cognitive impairments and has been hypothesized to occur in individuals with SCZ [168]. In 1845, Jean-Étienne Esquiro was the first to suggest that infectious diseases are involved in the vulnerability to psychoses [168]. More recently, SCZ risk has been associated with the exposure to neurotropic viruses (e.g., herpes simplex viruses, cytomegalovirus, Epstein-Barr virus, measles, and rubella) [169; 170; 171; 172]. Nevertheless, viral load in post-mortem brain samples did not show any significant difference between SCZ patients and healthy controls [173]. In addition to the established SCZ-associated pathogens, gut microbes could activate cytokine and complement systems, causing neuroinflammation and increasing the risk of psychotic symptoms [174]. In one study, maternal complement was elevated in peripheral blood at time of birth in mothers whose children went on to develop severe psychoses [175]. This complement activation during pregnancy can affect the development of neuronal networks [176]. Metagenomic studies showed increased fecal abundance of Lactobacillus among individuals with first-episode psychosis, as well as diminished response to treatment in those with the strongest evidence of dysbiosis [177]. Additionally, Lactobacillus phage phiadh showed increased abundance in the oropharyngeal microbiomes of SZ-affected individuals [177; 178].
Candida albicans and Saccharomyces cerevisiae were observed to be elevated in SCZ patients [179]. In a double-blind, placebo-controlled study, C. albicans was elevated in SCZ and was associated with more severe cognitive impairments and psychiatric symptoms [180]. Probiotic treatment reduced C. albicans antibodies over the 14-week study period in males, but not in females.
Another study showed an association of the oropharyngeal microbe Ascomycota, being more abundant in SCZ patients than in non-affected individuals [181]. Significant enrichment of lactic acid-producing microbes like Candida and Eubacterium compared to Neisseria, Haemophilus, and Capnocytophaga, also suggests a specific dysbiosis signature in SCZ patients. Recently, the oropharyngeal microbiome showed an underrepresentation of Neisseria Weeksellaceae, and Prevotella in SCZ and individuals with manic episodes [51].
12. Substance Use Disorders
Gut dysbiosis has been reported among subjects with substance use disorders (SUD) and hypotheses have been made regarding whether these differences are consequences of substance use or they contribute to the psychopathology observed among the patients investigated [182]. As such, this section includes both studies of SUD-affected patients and the effects observed among users of addictive substances. Comparing intestinal microbiota in SUD patients and healthy controls, the species diversity index and the abundance of Thauera, Paracoccus, and Prevotella were increased in SUD individuals with over-representation of microbial pathways related to translation, DNA replication and repair, and cell growth [183]. Changes in microbiome community composition suggest negative impact of alcohol dependence on gut microbiota [184]. Alcohol dependent subjects also show altered gut permeability, which is related to higher scores greater acute depression, anxiety, and alcohol craving after abstinence [185]. Gut permeability appears to induce neuroinflammation associated with changes in mood, cognition, and alcohol abuse [186]. Among the gut-derived bacterial products, the permeation of lipopolysaccharides and peptidoglycans was observed to stimulate certain inflammatory pathways in peripheral blood mononuclear cells associated with alcohol craving [187; 188].
While tobacco smoking generally decreases gut microbiome diversity, it increases the abundance of Proteobacteria, Clostridium, Bacteroides, and Prevotella [189]. Cigarette smoking is also associated with changes in the oral microbiome with differences between active smokers, former smokers, and never smokers. Active smokers show a depletion of Proteobacteria, Capnocytophaga, and Peptostreptococcus and Leptotrichia and an enrichment of Atopobium and Streptococcus [190]. Smoking-associated changes in oral microbiomes were related to changes in microbial genes associated with carbohydrate, energy, and xenobiotic metabolisms [190].
Taking the advantage of the resting-state functional magnetic resonance imaging technique for the analysis of brain functional networks, changes in brain functional connectivity (mainly including connectivity between brain default network and other task-positive networks) were observed to be associated with microbial imbalance caused by nicotine dependence in smokers [191]. A consistent association between gut microbiota and opioid-related traits has been reported across multiple studies [192]. Mu-opioid receptors in neurons within the myenteric ganglia or on nerve terminals innervating smooth muscle cells appear to affect analgesic tolerance to opioids [193]. In patients affected by type-2 diabetes, the abundance of Bifidobacterium and Prevotella genera in the gut microbiome appears to be regulated by the interaction of exogenous opioids with mu-opioid receptors in the gut [194]. In male C57Bl6/J mice treated with intermittent morphine, depletion of the gut microbiota showed increased neuroinflammation, reduced opioid analgesic potency, and impaired cocaine reward [195]. In mice, the bacterial depletion with oral gavage of an antibiotic cocktail reduced gut bacteria and morphine-induced gut permeability, also showing the ability to prevent tolerance while not altering naloxone withdrawal susceptibility [196]. In a self-directed intake model, a diet enriched for omega-3 polyunsaturated fatty acids increases microbial richness, phylogenetic diversity, and evenness, improving oxycodone-seeking behaviors [197].
In cannabis users, the abundance of Prevotella genera in gut microbiome was positively correlated with fluid cognition which is associated with the capacity of an individual to process information, flanker inhibitory control, working memory, and cognitive flexibility [198]. These associations were not present in cannabis non-users.
13. Conclusions
The study of the human microbiome in the context of psychiatric disorders is an emerging and promising field of study. To date, most studies are focused on the gut-brain axis and neuroinflammation, highlighting potential pathogenetic mechanisms in the context of psychiatric traits [63; 70]. Although there are a limited number of investigations, oral and skin microbiome can also affect mental health [56; 89; 199]. The current state of microbiome research in neuropsychiatry presents several major limitations. There is a general lack of statistical power in human studies due to the limited number of individuals tested [153]. Studies conducted examining the association of microbiome variation with psychiatric disorders often fail to account adequately for potential confounding factors, such as diet, age, sex, comorbidities, and their associated medications [200]. Another important limitation that is not limited to neuropsychiatry research in is the lack of established standards for microbiome analysis and interpretation that makes difficult to compare findings generated using different analytic frameworks [14; 15]. Although the number of associations between microbiome variation and psychiatric disorders is rapidly growing, there is a systematic lack of mechanistic studies to understand the underlying processes by which the human microbiome affects mental health. These should include human, in vitro, in vivo, and computational approaches focused on understanding the implications of microbiome function in the context of disease and behavior. A better comprehension of the relationship between the human microbiome and mental health will permit the use of many probiotics and prebiotics, which may augment the effects of current treatment approaches for neuropsychiatric disorders. This could particularly benefit a consistent portion of psychiatric patients not responding to current pharmacological therapies.
Highlights.
The human microbiome in the context of psychiatric disorders is an emerging field of study.
The gut-brain axis has been associated with several neuropsychiatric disorders.
Oral and skin microbiome could affect mental health via inflammatory pathways.
Large samples and appropriate designs are needed to verify the microbiome-brain association.
14. Acknowledgements and Declaration of Interest
The authors acknowledge support from the National Institutes of Health (R21 DA047527, R21 DC018098, and F32 MH122058). Dr. Polimanti is paid for his editorial work on the journal Complex Psychiatry. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lloyd-Price J, Abu-Ali G, and Huttenhower C, The healthy human microbiome. Genome medicine 8 (2016) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, Nelson KE, White O, Methé BA, and Huttenhower C, The Human Microbiome Project: a community resource for the healthy human microbiome. PLoS biology 10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Glassner KL, Abraham BP, and Quigley EM, The microbiome and inflammatory bowel disease. Journal of Allergy and Clinical Immunology 145 (2020) 16–27. [DOI] [PubMed] [Google Scholar]
- [4].Bhatt AP, Redinbo MR, and Bultman SJ, The role of the microbiome in cancer development and therapy. CA: a cancer journal for clinicians 67 (2017) 326–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Belkaid Y, and Hand TW, Role of the microbiota in immunity and inflammation. Cell 157 (2014) 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, Borsini A, Firth J, Pariante CM, Berding K, Cryan JF, Clarke G, Craig JM, Su KP, Mischoulon D, Gomez-Pinilla F, Foster JA, Cani PD, Thuret S, Staudacher HM, Sanchez-Villegas A, Arshad H, Akbaraly T, O’Neil A, Segasby T, and Jacka FN, Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry 26 (2021) 134–150. [DOI] [PubMed] [Google Scholar]
- [7].Bastiaanssen TFS, Cussotto S, Claesson MJ, Clarke G, Dinan TG, and Cryan JF, Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harv Rev Psychiatry 28 (2020) 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cruz-Pereira JS, Rea K, Nolan YM, O’Leary OF, Dinan TG, and Cryan JF, Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu Rev Psychol 71 (2020) 49–78. [DOI] [PubMed] [Google Scholar]
- [9].Margolis KG, Cryan JF, and Mayer EA, The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cruz-Pereira JS, and Cryan JF, In Need of a Quorum: From Microbes to Mood Via the Immune System. Am J Psychiatry 177 (2020) 895–897. [DOI] [PubMed] [Google Scholar]
- [11].Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, and Dinan TG, The gut microbiome in neurological disorders. Lancet Neurol 19 (2020) 179–194. [DOI] [PubMed] [Google Scholar]
- [12].Cowan CSM, Dinan TG, and Cryan JF, Annual Research Review: Critical windows - the microbiota-gut-brain axis in neurocognitive development. J Child Psychol Psychiatry 61 (2020) 353–371. [DOI] [PubMed] [Google Scholar]
- [13].Ashton JJ, Beattie RM, Ennis S, and Cleary DW, Analysis and Interpretation of the Human Microbiome. Inflamm Bowel Dis 22 (2016) 1713–22. [DOI] [PubMed] [Google Scholar]
- [14].Morton JT, Marotz C, Washburne A, Silverman J, Zaramela LS, Edlund A, Zengler K, and Knight R, Establishing microbial composition measurement standards with reference frames. Nat Commun 10 (2019) 2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sczyrba A, Hofmann P, Belmann P, Koslicki D, Janssen S, Droge J, Gregor I, Majda S, Fiedler J, Dahms E, Bremges A, Fritz A, Garrido-Oter R, Jorgensen TS, Shapiro N, Blood PD, Gurevich A, Bai Y, Turaev D, DeMaere MZ, Chikhi R, Nagarajan N, Quince C, Meyer F, Balvociute M, Hansen LH, Sorensen SJ, Chia BKH, Denis B, Froula JL, Wang Z, Egan R, Don Kang D, Cook JJ, Deltel C, Beckstette M, Lemaitre C, Peterlongo P, Rizk G, Lavenier D, Wu YW, Singer SW, Jain C, Strous M, Klingenberg H, Meinicke P, Barton MD, Lingner T, Lin HH, Liao YC, Silva GGZ, Cuevas DA, Edwards RA, Saha S, Piro VC, Renard BY, Pop M, Klenk HP, Goker M, Kyrpides NC, Woyke T, Vorholt JA, Schulze-Lefert P, Rubin EM, Darling AE, Rattei T, and McHardy AC, Critical Assessment of Metagenome Interpretation-a benchmark of metagenomics software. Nat Methods 14 (2017) 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, and Knight R, Diversity, stability and resilience of the human gut microbiota. Nature 489 (2012) 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cummings J, Macfarlane G, and Macfarlane S, Intestinal bacteria and ulcerative colitis. Current issues in intestinal microbiology 4 (2003) 9–20. [PubMed] [Google Scholar]
- [18].Tang WW, and Hazen SL, The contributory role of gut microbiota in cardiovascular disease. The Journal of clinical investigation 124 (2014) 4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Blandino G, Inturri R, Lazzara F, Di Rosa M, and Malaguarnera L, Impact of gut microbiota on diabetes mellitus. Diabetes & metabolism 42 (2016) 303–315. [DOI] [PubMed] [Google Scholar]
- [20].Maeda Y, and Takeda K, Role of gut microbiota in rheumatoid arthritis. Journal of clinical medicine 6 (2017) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carabotti M, Scirocco A, Maselli MA, and Severi C, The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of gastroenterology: quarterly publication of the Hellenic Society of Gastroenterology 28 (2015) 203. [PMC free article] [PubMed] [Google Scholar]
- [22].Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, and Koga Y, Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558 (2004) 263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, and Pettersson S, Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences 108 (2011) 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maqsood R, and Stone TW, The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochemical research 41 (2016) 2819–2835. [DOI] [PubMed] [Google Scholar]
- [25].Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, and McCoy KD, The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141 (2011) 599–609. e3. [DOI] [PubMed] [Google Scholar]
- [26].Del Angel-Meza A, Dávalos-Marín A, Ontiveros-Martinez L, Ortiz G, Beas-Zarate C, Chaparro-Huerta V, Torres-Mendoza B, and Bitzer-Quintero O, Protective effects of tryptophan on neuro-inflammation in rats after administering lipopolysaccharide. Biomedicine & Pharmacotherapy 65 (2011) 215–219. [DOI] [PubMed] [Google Scholar]
- [27].Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, and Julius D, Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170 (2017) 185–198. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney R, Shanahan F, Dinan T, and Cryan J, The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry 18 (2013) 666–673. [DOI] [PubMed] [Google Scholar]
- [29].Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, and Petrosino JF, Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155 (2013) 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, and Cryan JF, Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annual review of pharmacology and toxicology 60 (2020) 477–502. [DOI] [PubMed] [Google Scholar]
- [31].Maydych V, The interplay between stress, inflammation, and emotional attention: relevance for depression. Frontiers in neuroscience 13 (2019) 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, and Mithieux G, Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156 (2014) 84–96. [DOI] [PubMed] [Google Scholar]
- [33].Caspani G, Kennedy S, Foster JA, and Swann J, Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microbial Cell 6 (2019) 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O’Mahony SM, Clarke G, Borre Y, Dinan T, and Cryan J, Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural brain research 277 (2015) 32–48. [DOI] [PubMed] [Google Scholar]
- [35].Dinan TG, Cryan JF, and Stanton C, Gut microbes and brain development have black box connectivity. Biological psychiatry 83 (2018) 97–99. [DOI] [PubMed] [Google Scholar]
- [36].Rea K, Dinan TG, and Cryan JF, The microbiome: a key regulator of stress and neuroinflammation. Neurobiology of stress 4 (2016) 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, and Wijmenga C, The neuroactive potential of the human gut microbiota in quality of life and depression. Nature microbiology 4 (2019) 623–632. [DOI] [PubMed] [Google Scholar]
- [38].Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, and Lukiw WJ, Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD). Frontiers in aging neuroscience 6 (2014) 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zevin AS, McKinnon L, Burgener A, and Klatt NR, Microbial translocation and icrobiome dsybiosis in HIV-associated immune activation. Current Opinion in HIV and AIDS 11 (2016) 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grazul H, Kanda LL, and Gondek D, Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes 7 (2016) 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Petschow B, Doré J, Hibberd P, Dinan T, Reid G, Blaser M, Cani PD, Degnan FH, Foster J, and Gibson G, Probiotics, prebiotics, and the host microbiome: the science of translation. Annals of the New York Academy of Sciences 1306 (2013) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kashyap PC, Chia N, Nelson H, Segal E, and Elinav E, Microbiome at the frontier of personalized medicine, Mayo Clinic Proceedings, Elsevier, 2017, pp. 1855–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zmora N, Zeevi D, Korem T, Segal E, and Elinav E, Taking it personally: personalized utilization of the human microbiome in health and disease. Cell host & microbe 19 (2016) 12–20. [DOI] [PubMed] [Google Scholar]
- [44].Kuntz TM, and Gilbert JA, Introducing the microbiome into precision medicine. Trends in pharmacological sciences 38 (2017) 81–91. [DOI] [PubMed] [Google Scholar]
- [45].Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu W-H, Lakshmanan A, and Wade WG, The human oral microbiome. Journal of bacteriology 192 (2010) 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schulze-Schweifing K, Banerjee A, and Wade WG, Comparison of bacterial culture and 16S rRNA community profiling by clonal analysis and pyrosequencing for the characterization of the dentine caries-associated microbiome. Frontiers in cellular and infection microbiology 4 (2014) 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wade WG, The oral microbiome in health and disease. Pharmacological research 69 (2013) 137–143. [DOI] [PubMed] [Google Scholar]
- [48].Butler RR, Soomer-James JT, Frenette M, and Pombert J-F, Complete genome sequences of two human oral microbiome commensals: Streptococcus salivarius ATCC 25975 and S. salivarius ATCC 27945. Genome Announc. 5 (2017) e00536–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, and Dewhirst FE, The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Logan AC, Jacka FN, Craig JM, and Prescott SL, The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clinical Psychopharmacology and Neuroscience 14 (2016) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yolken R, Prandovszky E, Severance EG, Hatfield G, and Dickerson F, The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophrenia Research (2020). [DOI] [PubMed] [Google Scholar]
- [52].Kong X, Liu J, Cetinbas M, Sadreyev R, Koh M, Huang H, Adeseye A, He P, Zhu J, and Russell H, New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 11 (2019) 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Grice EA, and Segre JA, The skin microbiome. Nature Reviews Microbiology 9 (2011) 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Scharschmidt TC, and Fischbach MA, What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discovery Today: Disease Mechanisms 10 (2013) e83–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Oh J, Byrd AL, Park M, Kong HH, Segre JA, and Program NCS, Temporal stability of the human skin microbiome. Cell 165 (2016) 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hadian Y, Fregoso D, Nguyen C, Bagood MD, Dahle SE, Gareau MG, and Isseroff RR, Microbiome-skin-brain axis: A novel paradigm for cutaneous wounds. Wound Repair and Regeneration 28 (2020) 282–292. [DOI] [PubMed] [Google Scholar]
- [57].Salem I, Ramser A, Isham N, and Ghannoum MA, The gut microbiome as a major regulator of the gut-skin axis. Frontiers in microbiology 9 (2018) 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Arck P, Handjiski B, Hagen E, Pincus M, Bruenahl C, Bienenstock J, and Paus R, Is there a ‘gut-brain-skin axis’? Exp Dermatol 19 (2010) 401–5. [DOI] [PubMed] [Google Scholar]
- [59].Lee S-Y, Lee E, Park YM, and Hong S-J, Microbiome in the gut-skin axis in atopic dermatitis. Allergy, asthma & immunology research 10 (2018) 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Prieto GA, and Cotman CW, Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine & growth factor reviews 34 (2017) 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, and Knight R, Current understanding of the human microbiome. Nature medicine 24 (2018) 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].O’Neill CA, Monteleone G, McLaughlin JT, and Paus R, The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 38 (2016) 1167–1176. [DOI] [PubMed] [Google Scholar]
- [63].Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, and Wakefield S, Gut microbiota’s effect on mental health: the gut-brain axis. Clinics and practice 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, Dayanim G, and Bhatnagar S, The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Molecular psychiatry 25 (2020) 1068–1079. [DOI] [PubMed] [Google Scholar]
- [65].Liu RT, The microbiome as a novel paradigm in studying stress and mental health. American Psychologist 72 (2017) 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang X, Li Y, Wu L, Xiao S, Ji Y, Tan Y, Jiang C, and Zhang G, Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed Pharmacother 137 (2021) 111065. [DOI] [PubMed] [Google Scholar]
- [67].Chen L, Li J, Zhu W, Kuang Y, Liu T, Zhang W, Chen X, and Peng C, Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front Microbiol 11 (2020) 589726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Masters C, Bateman R, Blennow K, and Rowe C, Sperling 559 RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev 560 Dis Primers 1 561. [DOI] [PubMed] [Google Scholar]
- [69].Gaugler J, James B, Johnson T, Marin A, and Weuve J, 2019 Alzheimer’s disease facts and figures. Alzheimers & Dementia 15 (2019) 321–387. [Google Scholar]
- [70].Kim Y-K, and Shin C, The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Current neuropharmacology 16 (2018) 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, and Holtzman DM, Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Scientific reports 6 (2016) 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kowalski K, and Mulak A, Brain-gut-microbiota axis in Alzheimer’s disease. Journal of neurogastroenterology and motility 25 (2019) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Coureuil M, Lecuyer H, Bourdoulous S, and Nassif X, A journey into the brain: insight into how bacterial pathogens cross blood–brain barriers. Nature Reviews Microbiology 15 (2017) 149–159. [DOI] [PubMed] [Google Scholar]
- [74].Xaio H, Banks W, Niehoff ML, and Morley J, Effect of LPS on the permeability of the blood–brain barrier to insulin. Brain research 896 (2001) 36–42. [DOI] [PubMed] [Google Scholar]
- [75].Hoyles L, Snelling T, Umlai U-K, Nicholson JK, Carding SR, Glen RC, and McArthur S, Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome 6 (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhu B, Wang ZG, Ding J, Liu N, Wang DM, Ding LC, and Yang C, Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Experimental and Therapeutic Medicine 7 (2014) 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhao Y, Cong L, Jaber V, and Lukiw WJ, Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Frontiers in Immunology 8 (2017) 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, and Yadav H, Gut microbiome and aging: Physiological and mechanistic insights. Nutrition and healthy aging 4 (2018) 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, and Fitzgerald G, Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proceedings of the National Academy of Sciences 108 (2011) 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nagpal R, Neth BJ, Wang S, Mishra SP, Craft S, and Yadav H, Gut mycobiome and its interaction with diet, gut bacteria and alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 59 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mir R, Jallu S, and Singh TP, The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit Rev Microbiol 41 (2015) 172–89. [DOI] [PubMed] [Google Scholar]
- [82].Paley EL, Diet-related metabolic perturbations of gut microbial shikimate pathway-tryptamine-tRNA aminoacylation-protein synthesis in human health and disease. International Journal of Tryptophan Research 12 (2019) 1178646919834550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Emery DC, Shoemark DK, Batstone TE, Waterfall CM, Coghill JA, Cerajewska TL, Davies M, West NX, and Allen SJ, 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Frontiers in aging neuroscience 9 (2017) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Leblhuber F, Steiner K, Schuetz B, Fuchs D, and Gostner JM, Probiotic supplementation in patients with Alzheimer’s dementia-an explorative intervention study. Current Alzheimer Research 15 (2018) 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kitazawa M, Medeiros R, and M LaFerla F, Transgenic mouse models of Alzheimer disease: developing a better model as a tool for therapeutic interventions. Current pharmaceutical design 18 (2012) 1131–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chunchai T, Thunapong W, Yasom S, Wanchai K, Eaimworawuthikul S, Metzler G, Lungkaphin A, Pongchaidecha A, Sirilun S, and Chaiyasut C, Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. Journal of neuroinflammation 15 (2018) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mitchell AJ, A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 43 (2009) 411–31. [DOI] [PubMed] [Google Scholar]
- [88].Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, and Salami M, Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Frontiers in aging neuroscience 8 (2016) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lin J, Chang C, and Caffrey J, Examining the association between oral health status and dementia: A nationwide nested case-controlled study. Experimental Biology and Medicine 245 (2020) 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Aarts E, Ederveen TH, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, Smeekens SP, Netea MG, Buitelaar JK, and Franke B, Gut microbiome in ADHD and its relation to neural reward anticipation. PloS one 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Szopinska-Tokov J, Dam S, Naaijen J, Konstanti P, Rommelse N, Belzer C, Buitelaar J, Franke B, Aarts E, and Arias Vasquez A, Investigating the gut microbiota composition of individuals with attention-deficit/hyperactivity disorder and association with symptoms. Microorganisms 8 (2020) 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bull-Larsen S, and Mohajeri MH, The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wan L, Ge WR, Zhang S, Sun YL, Wang B, and Yang G, Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children With Attention Deficit Hyperactivity Disorder. Front Neurosci 14 (2020) 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bull-Larsen S, and Mohajeri MH, The potential influence of the bacterial microbiome on the development and progression of ADHD. Nutrients 11 (2019) 2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, and Theoharides TC, Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clinical therapeutics 37 (2015) 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ming X, Chen N, Ray C, Brewer G, Kornitzer J, and Steer RA, A gut feeling: a hypothesis of the role of the microbiome in attention-deficit/hyperactivity disorders. Child neurology open 5 (2018) 2329048X18786799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kumperscak HG, Gricar A, Ülen I, and Micetic-Turk D, A Pilot Randomized Control Trial With the Probiotic Strain Lactobacillus rhamnosus GG (LGG) in ADHD: Children and Adolescents Report Better Health-Related Quality of Life. Frontiers in Psychiatry 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Solomon CG, Mitchell James E., and Peterson Carol B.. N Engl J Med 382 (2020) 1343–51. [DOI] [PubMed] [Google Scholar]
- [99].Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, and Carroll IM, The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom Med 77 (2015) 969–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Seitz J, Trinh S, and Herpertz-Dahlmann B, The microbiome and eating disorders. Psychiatric Clinics 42 (2019) 93–103. [DOI] [PubMed] [Google Scholar]
- [101].Borgo F, Riva A, Benetti A, Casiraghi MC, Bertelli S, Garbossa S, Anselmetti S, Scarone S, Pontiroli AE, Morace G, and Borghi E, Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS One 12 (2017) e0179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gorwood P, Blanchet-Collet C, Chartrel N, Duclos J, Dechelotte P, Hanachi M, Fetissov S, Godart N, Melchior J-C, and Ramoz N, New insights in anorexia nervosa. Frontiers in Neuroscience 10 (2016) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, Miyazaki K, and Sudo N, Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS One 10 (2015) e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Armougom F, Henry M, Vialettes B, Raccah D, and Raoult D, Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PloS one 4 (2009) e7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, and Miyazaki K, Gut dysbiosis in patients with anorexia nervosa. PloS one 10 (2015) e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ruusunen A, Rocks T, Jacka F, and Loughman A, The gut microbiome in anorexia nervosa: relevance for nutritional rehabilitation. Psychopharmacology (2019) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Seitz J, Belheouane M, Schulz N, Dempfle A, Baines JF, and Herpertz-Dahlmann B, The impact of starvation on the microbiome and gut-brain interaction in anorexia nervosa. Frontiers in Endocrinology 10 (2019) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Herpertz-Dahlmann B, Seitz J, and Baines J, Food matters: how the microbiome and gut–brain interaction might impact the development and course of anorexia nervosa. European child & adolescent psychiatry 26 (2017) 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mack I, Penders J, Cook J, Dugmore J, Mazurak N, and Enck P, Is the impact of starvation on the gut microbiota specific or unspecific to anorexia nervosa? A narrative review based on a systematic literature search. Current neuropharmacology 16 (2018) 1131–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Roubalová R, Procházková P, Papežová H, Smitka K, Bilej M, and Tlaskalová-Hogenová H, Anorexia nervosa: Gut microbiota-immune-brain interactions. Clinical Nutrition 39 (2020) 676–684. [DOI] [PubMed] [Google Scholar]
- [111].Matson JL, and Goldin RL, Diagnosing young children with autism. International Journal of Developmental Neuroscience 39 (2014) 44–48. [DOI] [PubMed] [Google Scholar]
- [112].Faras H, Al Ateeqi N, and Tidmarsh L, Autism spectrum disorders. Ann Saudi Med 30 (2010) 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lasheras I, Seral P, Latorre E, Barroso E, Gracia-García P, and Santabárbara J, Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian Journal of Psychiatry 47 (2020) 101874. [DOI] [PubMed] [Google Scholar]
- [114].Schwartz DD, Katzenstein JM, Highley EJ, Stabley DL, Sol‐Church K, Gripp KW, and Axelrad ME, Age‐related differences in prevalence of autism spectrum disorder symptoms in children and adolescents with Costello syndrome. American Journal of Medical Genetics Part A 173 (2017) 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, Chen K, Chen J, Xu R, and Tang J, Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes (2020) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, Borrelli L, Corrado G, De Caro C, and Comegna M, Gut microbiota features in young children with autism spectrum disorders. Frontiers in microbiology 9 (2018) 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, and Tildon JT, Gastrointestinal abnormalities in children with autistic disorder. The Journal of pediatrics 135 (1999) 559–563. [DOI] [PubMed] [Google Scholar]
- [118].Gondalia SV, Palombo EA, Knowles SR, and Austin DW, Faecal microbiota of individuals with autism spectrum disorder. E-journal of applied psychology: clinical and social issues 6 (2010) 24–29. [Google Scholar]
- [119].Adams JB, Johansen LJ, Powell LD, Quig D, and Rubin RA, Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC gastroenterology 11 (2011) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, and Ostatnikova D, Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior 138 (2015) 179–187. [DOI] [PubMed] [Google Scholar]
- [121].Zhang M, Ma W, Zhang J, He Y, and Wang J, Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep 8 (2018) 13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, and Green JA 3rd, Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16 (2010) 444–53. [DOI] [PubMed] [Google Scholar]
- [123].Liu F, Li J, Wu F, Zheng H, Peng Q, and Zhou H, Altered composition and function of intestinal microbiota in autism spectrum disorders: a systematic review. Translational psychiatry 9 (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sivamaruthi BS, Suganthy N, Kesika P, and Chaiyasut C, The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int J Environ Res Public Health 17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Martin CR, Osadchiy V, Kalani A, and Mayer EA, The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol 6 (2018) 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].De Angelis M, Francavilla R, Piccolo M, De Giacomo A, and Gobbetti M, Autism spectrum disorders and intestinal microbiota. Gut Microbes 6 (2015) 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ding HT, Taur Y, and Walkup JT, Gut microbiota and autism: key concepts and findings. Journal of autism and developmental disorders 47 (2017) 480–489. [DOI] [PubMed] [Google Scholar]
- [128].Fattorusso A, Di Genova L, Dell’Isola GB, Mencaroni E, and Esposito S, Autism spectrum disorders and the gut microbiota. Nutrients 11 (2019) 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Mohamadkhani A, Gut microbiota and fecal metabolome perturbation in children with autism spectrum disorder. Middle East journal of digestive diseases 10 (2018) 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Roman P, Rueda-Ruzafa L, Cardona D, and Cortes-Rodríguez A, Gut–brain axis in the executive function of austism spectrum disorder. Behavioural pharmacology 29 (2018) 654–663. [DOI] [PubMed] [Google Scholar]
- [131].Xiong X, Liu D, Wang Y, Zeng T, and Peng Y, Urinary 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid are elevated in children with autism spectrum disorders. BioMed research international 2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, and Costa-Mattioli M, Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165 (2016) 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Silva YP, Bernardi A, and Frozza RL, The role of short-chain fatty acids from gut microbiota in gut-brain communication. Frontiers in Endocrinology 11 (2020) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].MacFabe DF, Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microbial ecology in health and disease 23 (2012) 19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, and Krajmalnik-Brown R, Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5 (2017) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Parracho HM, Gibson GR, Knott F, Bosscher D, Kleerebezem M, and McCartney AL, A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. International Journal of Probiotics & Prebiotics 5 (2010) 69. [Google Scholar]
- [137].Minakova E, and Warner BB, Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res 110 (2018) 1539–1550. [DOI] [PubMed] [Google Scholar]
- [138].Altieri L, Neri C, Sacco R, Curatolo P, Benvenuto A, Muratori F, Santocchi E, Bravaccio C, Lenti C, and Saccani M, Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 16 (2011) 252–260. [DOI] [PubMed] [Google Scholar]
- [139].Gabriele S, Sacco R, Cerullo S, Neri C, Urbani A, Tripi G, Malvy J, Barthelemy C, Bonnet-Brihault F, and Persico AM, Urinary p-cresol is elevated in young French children with autism spectrum disorder: a replication study. Biomarkers 19 (2014) 463–470. [DOI] [PubMed] [Google Scholar]
- [140].Kałużna-Czaplińska J, and Błaszczyk S, The level of arabinitol in autistic children after probiotic therapy. Nutrition 28 (2012) 124–126. [DOI] [PubMed] [Google Scholar]
- [141].Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen M-L, Nelson MN, and Wexler HM, Short-term benefit from oral vancomycin treatment of regressive-onset autism. Journal of child neurology 15 (2000) 429–435. [DOI] [PubMed] [Google Scholar]
- [142].Forsyth A, Raslan K, Lyashenko C, Bona S, Snow M, Khor B, Herrman E, Ortiz S, Choi D, and Maier T, Children with autism spectrum disorder: Pilot studies examining the salivary microbiome and implications for gut metabolism and social behavior. Human Microbiome Journal 15 (2020) 100066. [Google Scholar]
- [143].Müller-Oerlinghausen B, Berghöfer A, and Bauer M, Bipolar disorder. The Lancet 359 (2002) 241–247. [DOI] [PubMed] [Google Scholar]
- [144].Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB, Ellingrod VE, and McInnis MG, The gut microbiome composition associates with bipolar disorder and illness severity. Journal of psychiatric research 87 (2017) 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Guo L, Ji C, Ma Q, Fan Y, Feng J, and Chen C, The diversity and the abundance of gut microbiome in patients with bipolar disorder. Chin. J. Psychiatry 51 (2018) 98–104. [Google Scholar]
- [146].Huang T-T, Lai J-B, Du Y-L, Xu Y, Ruan L-M, and Hu S-H, Current understanding of gut microbiota in mood disorders: an update of human studies. Frontiers in genetics 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Coello K, Hansen TH, Sørensen N, Munkholm K, Kessing LV, Pedersen O, and Vinberg M, Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain, behavior, and immunity 75 (2019) 112–118. [DOI] [PubMed] [Google Scholar]
- [148].Reininghaus EZ, Wetzlmair L-C, Fellendorf FT, Platzer M, Queissner R, Birner A, Pilz R, Hamm C, Maget A, and Koidl C, The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: a pilot study. Neuropsychobiology 79 (2020) 63–70. [DOI] [PubMed] [Google Scholar]
- [149].Flowers SA, Evans SJ, Ward KM, McInnis MG, and Ellingrod VL, Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 37 (2017) 261–267. [DOI] [PubMed] [Google Scholar]
- [150].Friedrich M, Depression is the leading cause of disability around the world. Jama 317 (2017) 1517–1517. [DOI] [PubMed] [Google Scholar]
- [151].Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, and Rudi K, Correlation between the human fecal microbiota and depression. Neurogastroenterology & Motility 26 (2014) 1155–1162. [DOI] [PubMed] [Google Scholar]
- [152].Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, and Shi J, Altered fecal microbiota composition in patients with major depressive disorder. Brain, behavior, and immunity 48 (2015) 186–194. [DOI] [PubMed] [Google Scholar]
- [153].Cheung SG, Goldenthal AR, Uhlemann A-C, Mann JJ, Miller JM, and Sublette ME, Systematic review of gut microbiota and major depression. Frontiers in psychiatry 10 (2019) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, and Bailey MT, Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC microbiology 14 (2014) 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, and Moloney G, Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of psychiatric research 82 (2016) 109–118. [DOI] [PubMed] [Google Scholar]
- [156].Yong SJ, Tong T, Chew J, and Lim WL, Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Frontiers in Neuroscience 13 (2020) 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Chen YH, Xue F, Yu SF, Li XS, Liu L, Jia YY, Yan WJ, Tan QR, Wang HN, and Peng ZW, Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. J Affect Disord 282 (2021) 391–400. [DOI] [PubMed] [Google Scholar]
- [158].Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, and Du X, Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular psychiatry 21 (2016) 786–796. [DOI] [PubMed] [Google Scholar]
- [159].Rogers G, Keating D, Young R, Wong M, Licinio J, and Wesselingh S, From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Molecular psychiatry 21 (2016) 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lach G, Schellekens H, Dinan TG, and Cryan JF, Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15 (2018) 36–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Zheng H-Z, Tu J-L, Li X-H, Hua Q, Liu W-Z, Liu Y, Pan B-X, Hu P, and Zhang W-H, Neuroinflammation induces anxiety-and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain, Behavior, and Immunity (2020). [DOI] [PubMed] [Google Scholar]
- [162].Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, and Flanagan KL, The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility, Seminars in immunopathology, Springer, 2019, pp. 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Peirce JM, and Alviña K, The role of inflammation and the gut microbiome in depression and anxiety. Journal of neuroscience research 97 (2019) 1223–1241. [DOI] [PubMed] [Google Scholar]
- [164].Turner-Cobb JM, Osborn M, da Silva L, Keogh E, and Jessop DS, Sex differences in hypothalamic–pituitary–adrenal axis function in patients with chronic pain syndrome. Stress 13 (2010) 293–301. [DOI] [PubMed] [Google Scholar]
- [165].Wallace CJ, and Milev R, The effects of probiotics on depressive symptoms in humans: a systematic review. Annals of general psychiatry 16 (2017) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Moayyedi P, Yuan Y, Baharith H, and Ford AC, Faecal microbiota transplantation for Clostridium difficile‐associated diarrhoea: a systematic review of randomised controlled trials. Medical Journal of Australia 207 (2017) 166–172. [DOI] [PubMed] [Google Scholar]
- [167].Mattila T, Koeter M, Wohlfarth T, Storosum J, van den Brink W, de Haan L, Derks E, Leufkens H, and Denys D, Impact of DSM-5 changes on the diagnosis and acute treatment of schizophrenia. Schizophrenia bulletin 41 (2015) 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Severance EG, and Yolken RH, From infection to the microbiome: An evolving role of microbes in schizophrenia, Neuroinflammation and Schizophrenia, Springer, 2019, pp. 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Khandaker GM, Stochl J, Zammit S, Lewis G, and Jones PB, Childhood Epstein-Barr virus infection and subsequent risk of psychotic experiences in adolescence: a population-based prospective serological study. Schizophrenia research 158 (2014) 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Alam R, Abdolmaleky HM, and Zhou JR, Microbiome, inflammation, epigenetic alterations, and mental diseases. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 174 (2017) 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Meyer U, Prenatal poly (i: C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry 75 (2014) 307–315. [DOI] [PubMed] [Google Scholar]
- [172].Carter C, Schizophrenia: a pathogenetic autoimmune disease caused by viruses and pathogens and dependent on genes. Journal of pathogens 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Murphy K, Edelstein H, Smith L, Clanon K, Schweitzer B, Reynolds L, and Wheeler P, Treatment of HIV in outpatients with schizophrenia, schizoaffective disorder and bipolar disease at two county clinics. Community mental health journal 47 (2011) 668–671. [DOI] [PubMed] [Google Scholar]
- [174].Allswede DM, Buka SL, Yolken RH, Torrey EF, and Cannon TD, Elevated maternal cytokine levels at birth and risk for psychosis in adult offspring. Schizophrenia research 172 (2016) 41–45. [DOI] [PubMed] [Google Scholar]
- [175].Severance EG, Gressitt KL, Buka SL, Cannon TD, and Yolken RH, Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophrenia research 159 (2014) 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Severance EG, and Yolken RH, Deciphering microbiome and neuroactive immune gene interactions in schizophrenia. Neurobiology of disease 135 (2020) 104331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, Mantere O, Saarela M, Yolken R, and Suvisaari J, Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophrenia research 192 (2018) 398–403. [DOI] [PubMed] [Google Scholar]
- [178].Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, Origoni A, Katsafanas E, Schweinfurth LA, and Savage CL, Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophrenia bulletin 41 (2015) 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Dickerson F, Severance E, and Yolken R, The microbiome, immunity, and schizophrenia and bipolar disorder. Brain, behavior, and immunity 62 (2017) 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, Adamos MB, Sweeney KM, Origoni AE, and Khushalani S, Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain, behavior, and immunity 62 (2017) 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, and Crandall KA, Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 3 (2015) e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Meckel KR, and Kiraly DD, A potential role for the gut microbiome in substance use disorders. Psychopharmacology (Berl) 236 (2019) 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, and Wang K, Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci Rep 7 (2017) 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, and Govorun VM, Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 5 (2017) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, and Delzenne NM, Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 111 (2014) E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Leclercq S, de Timary P, Delzenne NM, and Stärkel P, The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl Psychiatry 7 (2017) e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Temko JE, Bouhlal S, Farokhnia M, Lee MR, Cryan JF, and Leggio L, The microbiota, the gut and the brain in eating and alcohol use disorders: a ‘Ménage à Trois’? Alcohol and Alcoholism 52 (2017) 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Leclercq S, De Saeger C, Delzenne N, de Timary P, and Stärkel P, Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry 76 (2014) 725–33. [DOI] [PubMed] [Google Scholar]
- [189].Savin Z, Kivity S, Yonath H, and Yehuda S, Smoking and the intestinal microbiome. Arch Microbiol 200 (2018) 677–684. [DOI] [PubMed] [Google Scholar]
- [190].Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, Ma Y, Purdue MP, Jacobs EJ, Gapstur SM, Li H, Alekseyenko AV, Hayes RB, and Ahn J, Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 10 (2016) 2435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Lin D, Hutchison KE, Portillo S, Vegara V, Ellingson JM, Liu J, Krauter KS, Carroll-Portillo A, and Calhoun VD, Association between the oral microbiome and brain resting state connectivity in smokers. Neuroimage 200 (2019) 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ren M, and Lotfipour S, The role of the gut microbiome in opioid use. Behav Pharmacol 31 (2020) 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Akbarali HI, and Dewey WL, The gut-brain interaction in opioid tolerance. Curr Opin Pharmacol 37 (2017) 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Barengolts E, Green SJ, Eisenberg Y, Akbar A, Reddivari B, Layden BT, Dugas L, and Chlipala G, Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One 13 (2018) e0194171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, and Taylor AMW, The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43 (2018) 2606–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL, and Akbarali HI, The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep 7 (2017) 42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hakimian JK, Dong TS, Barahona JA, Lagishetty V, Tiwari S, Azani D, Barrera M, Lee S, Severino AL, Mittal N, Cahill CM, Jacobs JP, and Walwyn WM, Dietary Supplementation with Omega-3 Polyunsaturated Fatty Acids Reduces Opioid-Seeking Behaviors and Alters the Gut Microbiome. Nutrients 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Panee J, Gerschenson M, and Chang L, Associations Between Microbiota, Mitochondrial Function, and Cognition in Chronic Marijuana Users. J Neuroimmune Pharmacol 13 (2018) 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Olsen I, and Hicks SD, Oral microbiota and autism spectrum disorder (ASD). Journal of Oral Microbiology 12 (2020) 1702806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Kho ZY, and Lal SK, The human gut microbiome–a potential controller of wellness and disease. Frontiers in microbiology 9 (2018) 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]


