Abstract
Objective
Our goal was to define trigeminal nerve ending quantities and patterns in rat molar dentine, their responses to attrition (tooth wear), and their associated odontoblasts and connections with pulpal plexuses.
Design
Trigeminal ganglia were labeled for axonal transport of 3H-proteins to dentinal nerve endings in male rats (3–13 months old). Autoradiography detected radio-labeled dentinal tubules as indicators of nerve ending locations. Quantitative morphometry (ANOVA, t-tests) was done, and littermates were compared for attrition and innervation.
Results
There were six dentinal patterns, only two of which had an associated neural plexus of Raschkow and cell-free zone (Den-1, Den-2). Others entered dentin from bush-like endings near elongated odontoblasts (Den-B), as single fibers (Den-X), as networks in predentine (PdN), or as single fibers in tertiary dentine at cusp tips (Den-S). There were at least 186,600 innervated dentinal tubules within the set of three right maxillary molars of the best-labeled rat, and similar densities were found in other rats. Attrition levels differed among cusps and in littermates (t-test p< 0.02–0.0001), but the matched right/left cusps per rat were similar. Innervations of tertiary and enamel-free dentine (Den-S, Den-X) were preserved in all rats. Den-B and Den-2 coronal patterns were unchanged unless displaced by dentinogenesis. Den-1 losses occurred in older cusps, while Den-2 patterns increased near cervical and intercuspal odontoblasts.
Conclusions
The extensive molar dentinal innervation had unique distributions per rat per cusp that depended on region (buccal, middle, palatal) and attrition, but only two of six patterns connected to a plexus of Raschkow.
Keywords: Dental innervation, odontoblasts, pulp-dentine complex, axonal transport, plexus of Raschkow
1. Introduction
Extensive sensory nerve patterns in crown dentine are found in animal and human teeth (for reviews: Gunji, 1982; Byers, 1984; Hildebrand et al., 1995; Fried & Gibbs, 2014; Sole-Magdalena et al., 2018; Emrick et al., 2020) and depend on interactions with the cells of peripheral pulp, especially odontoblasts (Magloire et al., 2009, 2010; Sato et al., 2018; Shibukawa et al., 2015; Bernal et al., 2021). Odontoblasts are complex cells originating from neural crest mesoderm. They form an epithelioid barrier layer in the crown that is a highly-specialized, electrically-coupled syncytium (Ikeda & Suda, 2013) linked by junctional complexes that separate the pulpal and dentinal compartments, and that help regulate dentinal interstitial fluid (Bishop & Yoshida, 1992; Bleicher, 2014; Couve et al., 2013; Heyeraas & Berggreen, 2000; Okiji, 2012; Orchardson & Cadden, 2001; Pashley, 1996; Turner et al., 1989; Vongsavan & Matthews, 2007). Odontoblasts not only support neuronal extensions into dentinal tubules, they also make and repair dentine, and they have barrier functions which include immune responses. In addition, both the nerves and odontoblasts interact with immune cells, autonomic nerves, neuroglia, vasculature, and each other (Bletsa et al., 2009; Byers & Närhi, 1999; Couve et al., 2013, 2018; Diogenes, 2020; El Karim et al., 2008; Farahani et al., 2011; Fried & Gibbs, 2014; Fristad et al., 2006; Haug & Heyeraas, 2006; Hildebrand et al., 1995; Horst et al., 2011; Komiya et al., 2018; Lundy & Linden, 2004; Lundy et al., 2020; Magloire et al., 2010; Olgart, 1996; Pashley, 1996; Shibukawa et al., 2015; Veerayutthwilai et al., 2007). Other important considerations for dentinal innervation are that the odontoblasts in crown and root have very different innervation (dense in crown, sparse in roots), and they also have different shapes and ion channels (Byers & Sugaya, 1996; Byers and Westenbroek, 2011; Westenbroek, et al., 2004), Our present study of neural patterns in the crown dentine of rat molars is, therefore, one part of the much larger story of the pulp-dentine complex, its innervation, and its integument functions that are related to antimicrobial defense, immunity, inflammation, wound healing, and regeneration, as well as nociception and mechanosensation.
Neurohistologic silver stains initially showed extensive branching of nerves in crown pulp, but could not demonstrate many nerve fibers in dentine, leading to the idea that the few nerves in dentine had been entrapped during dentinogenesis and might not be functional (Bernick, 1962, 1964, 1967). Refinements of silver and methylene blue stains (Fearnhead 1957, 1961; Hattyasy, 1961; Gunji, 1982) were able to prove that nerve fibers occur in dentin with morphologies that are consistent with active, living innervation. The development of dental axonal transport tracing (Fink et al., 1975; Rogers, 1979) revealed both the vitality of dentinal innervation and its extensive distribution in crown dentine (Berger et al., 1983; Byers, 1984; Byers & Dong, 1983; Byers & Kish, 1976; Byers & Matthews, 1981; Marfurt & Turner, 1983). The patterns for those labeled dentinal endings were asymmetric and concentrated in the occlusal regions of the cusps, and they agree with (1) nerve locations demonstrated by electron microscopy of human or animal dentine (Arwill et al., 1973; Frank et al., 1972; Gunji, 1982; Holland, 1994; Lilja, 1979; Maeda, 1996); (2) trigeminal nerve transection and regeneration studies (Arwill et al., 1973; Berger et al., 1983; Holland et al., 1987); (3) retrograde axonal transport from teeth to trigeminal ganglion (Emrick et al., 2020; Taddese et al., 1995; Yang et al., 2006); (4) immunohistochemistry of specific types of trigeminal dental innervation (Alavi et al., 2001; Byers & Cornel, 2018; Byers & Närhi, 1999; Couve et al., 2013, 2018; Egbuniwi at al., 2014; Farahani et al., 2011; Fristad et al., 2006; Fried & Gibbs, 2014; Henry et al., 2012; Maeda et al., 1986, 1987; Magloire et al., 2010; Silverman & Kruger, 1987; Sole-Magdelena et al., 2018; Swift & Byers, 1992; Taddese et al., 1995); and (5) currently evolving imaging and molecular genetic labeling technologies (Bernal et al., 2021; Chung et al., 2012; Emrick et al., 2020; Sato et al., 2018; Shibukawa et al., 2015).
Neural mapping methods show that coronal dentinal tubules are well-innervated and contain terminal branches from a variety of axonal sizes in dental nerves (large: greater than 6μm diameter; medium: 2–6μm; and small: less than 2μm). Those sizes match electrophysiological data for A-beta, A-delta and C-fiber conduction rates from teeth to trigeminal ganglia (Cadden et al., 1983; Dong et al., 1985) all of which may play roles in tooth pain (Fried, et al., 2011). The extensive research on functions of dental nerves has made great strides over many decades (Azerad & Woda, 1977; Berger et al., 1983; Brännström, 1981; Byers, 2019; Cadden et al., 1983; Dong et al., 1993; Fried, 1992; Fried et al., 2011; Henry et al., 2012; Kubo et al., 2008; Lavigne & Sessle, 2016; Naftel et al., 1999; Närhi, 1985; Närhi et al., 1992), with recent research increasingly focused on mechanisms and treatments for dental pain and for pulpal sensory regeneration (Bernal et al., 2021; Chrepa et al., 2020; Fried et al., 2011; Diogenes, 2020; Holland & Botero, 2014).
Prior analyses of rat molar innervation have shown that dentinal axons usually come from the nearby neural plexus of Raschkow which is separated from the odontoblast layer by a cell-free zone. Here, we present morphometic studies of the innervation of dentine in molars of young adult rats at 3–3.5 mo (months old) compared with older rats at 5 mo and at 10–13 mo, each at different stages of attrition (molar wear). This study was feasible because our samples had strong, clearly-defined neural label via axonal transport for the entire trigeminal innervation of dentine in serially-sectioned rat jaws, with additional radio-labeling of dentine matrix as sites of active dentinogenesis.
Our goal was to define trigeminal nerve ending quantities and patterns in rat molar dentine, their responses to attrition (tooth wear), their associated odontoblasts, and their connections with pulpal plexuses. We wanted this study to improve knowledge of (1) the variety, asymmetry, and total numbers of trigeminal nerve endings in rat molar dentine, (2) the association of the plexus of Rashkow with some but not all dentinal innervation, and (3) the adjustments of the different dentinal patterns to molar attrition. We initially quantified all cuspal innervation in the three right maxillary molars of the young adult rat with the strongest axonal transport label. Earlier work (Lovschall et al. 2002) demonstrated that experimental studies of rat molars require comparisons of the same cusp(s) across all groups, to differentiate endogeous variations from those that may be induced during tooth use or experiments. We therefore focused the rest of our quantitative analyses on the posterior cusp of the maxillary second molar because it is large and has the most uniform shape. Our quantitative data were then compared with qualitative studies of the innervation of all right molars in all ten rats.
2. Materials and Methods
2.1. Experimental design and animal groups
This work was authorized by the University of Washington Animal Care Committee and followed the NIH guidelines for animal research (Nat. Res. Council, 1978, 2011). Trigeminal innervation patterns in rat molar dentine were labeled in 10 male Sprague-Dawley rats at four ages [3–3.5 mo (n = 5), 5 mo (n = 2), 10 mo (n = 1), and 13 mo (n = 2)] via overnight axonal transport of 3H-labeled proteins. The rats were housed in standard cages with freely available water and food (Purina rat chow pellets, #5350). The four ages came from two groups of male rats that were selected for this study because the serial sections of their molar teeth had strong, clearly-defined autoradiographic labeling of nerves. This type of labeling always has interanimal variations that depend on the success of ganglion injections and on the duration of exposure of the autoradiographic photo emulsion that coats the radioactive slides (Fig. 1). The initial autoradiographic exposures were evaluated after 3–6 weeks, when every fourth slide was developed. Subsequent developments were scheduled for a total of 9–12 weeks exposure, and/or for 18–32 weeks, depending on the label intensity of the first sets of slides. Autoradiographic label intensity builds up linearly, and so the calibrated full exposures times allowed equivalent label intensities to occur in the later groups of slides. Initial work with axonal transport labeling for dental nerves was validated by electron microscopy in which labeling above background was only found over nerve axons and endings (Byers & Kish, 1976; Byers, 1984; Marfurt & Turner, 1983).
Figure 1: Axonal transport labeling and autoradiography.
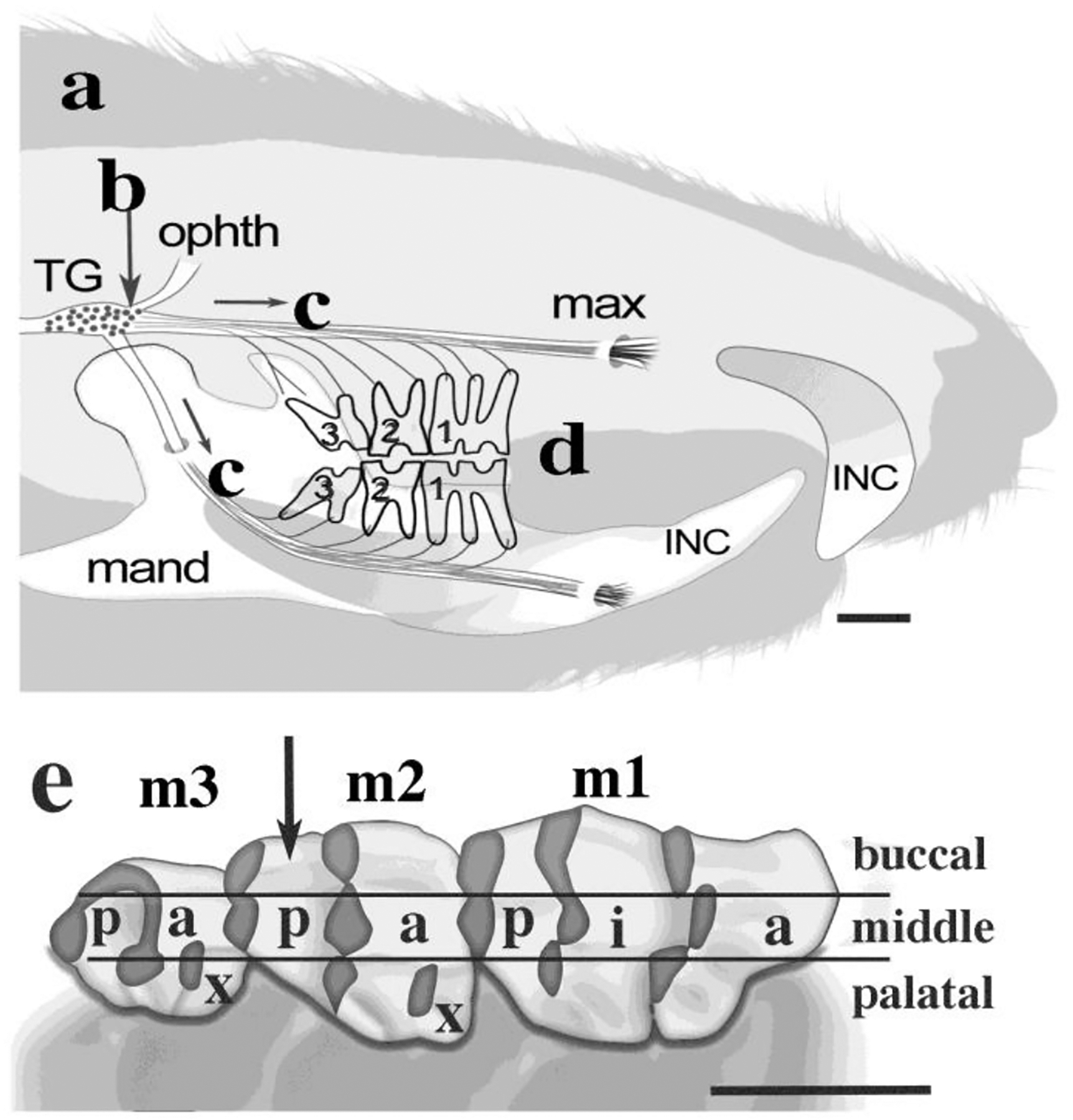
(a): Growth lines: Intravascular 3H-proline was injected to label new dentine matrix at 15 and 21 days of age (n = 4). (b) Ganglion Injection. Rats (n = 10, ages 3 – 13 mo) were anesthetized for injections of the right trigeminal ganglion (TG, arrow) with 3H-proline to label dental nerves and to show dentinogenesis that was in progress during axonal labeling. (c) Axonal transport. Radioactive protein traveled from nerve cell bodies to their endings in teeth for 18 – 25 hours. (d) Tissue Fixation. Each rat was re-anesthetized after axonal transport for the tissue fixation, followed by jaw & nerve excisions, tissue processing, serial sections at 5μm thickness, and autoradiography for all maxillary molars (m1, m2, m3) and some mandibular molars (e) Maxillary molars cusps. The nine cusps were anterior (a), intermediate (i), posterior (p) or extra (x), and the innervation patterns differed for buccal, middle, and palatal regions per cusp. (anterior (a) -posterior (p) = mesial-distal). Arrow: cusp M2p (quantified). Scales: 2.0 mm.
The labeled jaw tissues came from two different groups of male rats: (1) Four rats at ages 3–3.5 mo (n = 3) or 10 mo (n = 1) also had had intravascular injections of 3H-proline at 15 and 21 days of age, using a method that was developed in order to mark locations of primary dentine synthesis (‘growth lines’) related to subsequent neural ingrowth into dentine (Byers, 1980). (2) The other six rats (n = 6, with 2 each at 3 mo, 5 mo, and 13 mo) did not have growth lines. Their information about maturation of jaw opening reflex has been reported (Byers et al., 1982), but this is the first quantitation of their innervation.
2.2. Injections to label the trigeminal endings in molar dentine
Each rat was deeply anesthestized (sodium pentobarbital, 36 mg/kg) and the right trigeminal ganglion was injected slowly with 3–10 μL 3H-proline (Amersham, concentrated to 10 μC/μL) at each of 3–5 sites (TG, Fig. 1), as described in detail (Berger et al., 1983; Byers & Kish, 1976; Byers, 1980; Fink et al., 1975). After injection, rats received a subcutaneous nutritional supplement (Ambex diluted 1:3 with 5.0% dextrose in saline, 1–2 mL/kg) and were kept warm (30°C) with water and Purina rat chow freely available. They were awake within 2–4 hours after surgery, had intermittent sleep during the day, and were awake and moving well by late afternoon.
2.3. Tissue fixation, paraffin embedment, and serial sectioning
Each rat was re-anesthetized for transcardiac perfusion fixation (4% formaldehyde in 0.1M phosphate buffer, pH 7.4) after 18–25 hr axonal transport. The jaws were dissected out and then post-fixed another 1–2 days. The radioactivity of the axonally-transported protein was monitored by scintillation counting (Fink et al., 1975). For this study, nerve labeling per 3 mm segment of right alveolar nerves varied between 20,900–54,700 dpm, while background radioactivity in left control nerves was well below the resolution for neural autoradiography (0.9–4.0% of the right nerve totals). The excised jaws were fixed another 1–2 days, decalcified, dehydrated, and embedded in paraffin. Serial sections (5 μm thickness) were cut in the longitudinal anterior-posterior (i.e. mesio-distal) plane all the way through right maxillary molars from buccal to palatal sides, as well as for some sets of right mandibular molars. Left maxillary and mandibular molars were also sectioned as controls. Successive ribbons of 3–6 serial sections were mounted on slides and stored until ready for autoradiography.
2.4. Autoradiography and slide storage
The autoradiography procedures (Berger et al., 1983; Byers, 1980; Fink et al., 1975; Rogers, 1979) had the following key steps: (1) The paraffin sections that had been mounted on slides were hydrated and then were coated with melted emulsion (Ilford L2 or Kodak NTB2, diluted 1:1 with water). (2) The slides were dried in a humidified box to avoid drying artefacts. (3) The dried slides were stored in light-tight boxes at 2–4°C for periods of 3–32 weeks. Exposure times were planned for each experiment to achieve similar labeling intensities for all animals. (5) Tissues were counterstained with Richardson’s stain (methylene blue plus Azure II) prior to coverslip attachment with Permount. (6) Labeling patterns in any section (5 μm thickness) were compared with adjacent serial sections to add three-dimensonal information and to confirm validity. The groups of slides that were examined here were unchanged during storage in standard histology slide boxes for four decades, and therefore were suitable for this quantitative morphometry.
2.5. Imaging and quantitation
2.5.1. Resolution.
The resolution of autoradiography is less than the dimensions of dentinal tubules (Salpeter et al., 1969; Byers and Kish, 1976; Rogers, 1979), so that labeled tubules were reliably identified. Each labeled tubule was counted as one, even though individual dentinal tubules often contain multiple nerve fibers (Byers & Kish, 1976; Holland, 1994). We compared the NIH Image (Fuji) program with morphometry at the microscope (300–400x magnification), and chose the latter method because of its greater reliability and resolution.
2.5.2. Quantitative Morphometry.
All right maxillary molars from the 10 rats were analysed qualitatively, as were some of the right mandibular molars. Attrition levels were measured in left (control) molars where possible as well as for the right molars. In addition the following quantitative studies were done: (a) Specific Counts: The nine maxillary molar cusps (Fig. 1, 3 cusps/molar, 3 molars) were counted in the 3.5 mo rat with the strongest nerve label. Sections were counted at 300–400x magnification with a Nikon microscope. A hand counter was used to register all labeled dentinal tubules along the predentine-dentine border per section, moving from the crown-root border on one side around the cusp to the intercusp dentine. During the counting process per section, the microscopist did not know the totals. One section in every third slide (moving from buccal to palatal sides of the cusp) was counted, and multiplied by the number of sections for that set of slides to obtain the total number of labeled tubules for that cusp. (b) Regional Estimations*: Evaluation of the posterior cusp of the maxillary second molar at all ages involved a simpler system in which an average was obtained (at 300–400x) for 2–3 sections on the slide that was located in the middle of each of the seven main regions. Each of these averages was multiplied by the number of sections for its region, and then the seven regional values were totaled to obtain an estimate of the number of innervated dentinal tubules throughout each m2p cusp for that rat. Edge pieces were counted so long as there were dentinal tubules connecting with pulp. *REGIONS: buccal edge, buccal zone, buccal near middle, middle zone, palatal near middle, palatal zone, and palatal edge).
2.5.3. Endogenous variations in cusp shape.
There was a three-fold variation in the volume of different cusps, with the narrow cusps (m1a, m2x, m3x, m3p) having section totals that ranged from 59–76 for the best labeled 3.5 mo rat (e.g., cusps in Fig. 1E), while the much wider cusps (m1i, m1p, m2a and m2p) had 139–189 sections per cusp. Every rat had these variations in cuspal volume and shape, but the seven regions could always be identified, despite a 3-fold volume difference.
2.6. Statistical tests
The data for the number of labeled dentinal tubules per section had 90.3% reproducibility for counting that was repeated after a gap of several days. The values for cusp and age group comparisons were tested for significance with one-way ANOVA (Prism 8.3.1, p < 0.05 level for statistical significance). Paired comparisons utilized t-tests (Prism 8.3.1, significance at p < 0.05). The Brown-Forsythe and Bartlett tests were the post hoc assessments for significance.
3. Results
3.1. Trigeminal nerve patterns in rat molar dentine.
Six different patterns of dentinal innervation were found (Fig. 2), based on the strong label over nerves of pulp and dentinal tubules in serial sections of right maxillary and mandibular molars after 18–25 hours of axonal transport in adult rats (n = 10). The right and left molars of four of the rats also had growth lines in dentine matrix via previous intravascular injections of 3H-proline that were helpful for seeing the extent of tooth wear in each cusp (Fig. 3AB). Dental nerves in rats have large, medium and small axons (3A-inset), as well as thin nerve fibers ending in apical pulp (Fig. 3A inset, Fig. 4D). Only two of the six dentinal patterns (Den-1, Den-2) had an associated plexus of Raschkow (pxR) that also had a cell-free zone in between the plexus and the odontoblast layer (Figs. 3ABC), with Den-1 having a thicker plexus than Den-2. Pattern Den-B (Fig. 3AB, 3E, 4A) had a large bush-like neural array that extended into peripheral pulp and the odontoblast layer, thereby filling what would have been a cell-free zone and extending in buccal-palatal directions through dozens of sections. Also, most of the odontoblasts at that site were elongated. Pattern Den-X (Figs. 3B, 3D) was formed by axons that traveled near the odontoblast layer or that came directly from pulp to enter the dentin close to the tertiary tip, without a pxR in both cases. The Den-X axons also were found in the enamel-free zone along the occlusal surface of each cusp. Special tertiary dentin at the tip of the cusp (Tip*, Fig. 3F) had asymmetric sparse dentinal tubules as well as varying odontoblast shapes, layered matrix. and innervation by scattered single trigeminal axons (Den-S). The tubular dentine next to the tertiary tip region had Den-X patterns plus some of the Den-B endings (Fig. 3D, 4A). Networks of beaded endings in predentine (PdN) were found in the cervical regions including intercusps (Fig. 4BC) for the 3, 5, and 10 mo rats, but were sparse in the two 13 mo rats. They occurred on the dentine side of the odontoblast layer along with some extensions into calcified dentine. Finally, the strong, clearly-defined neural label in these rat molars also revealed thin axons that were either free within the root pulp or directed towards the root odontoblasts (Fig. 4D), but almost never found in root dentine.
Figure 2. Six dentine patterns.

Two patterns had an associated plexus of Raschkow in pulp (Den-1, large pxR; Den-2, small pxR) each with a cell-free zone (cfz) separating the nerves from the odontoblasts (ob). Axons, associated cells, and elongated odontoblasts fill, and thereby cancel, the cell-free-zone for the third pattern (Den-B). Other nerves reach dentine either by traveling near the odotoblast layer or directly from pulp (Den-X). In cervical regions a network was found in predentine (PdN) that was oriented perpendicular to dentinal tubules. Special tertiary dentine at each cusp tip had asymmetric reduced tubules, and reduced innervation by thin single (Den-S) axons. Odontoblast shapes were variable for tip dentine. Color codes: Nerves (black), odontoblasts (blue), pulp cells (green), immune or other cells (yellow/tan), extracellular space for predentine and dentine (orange), plexus of Raschkow (pxR) black nerves and green associated cells.
Figure 3. Labeled nerves in right maxillary molar dentine (10 mo rat).
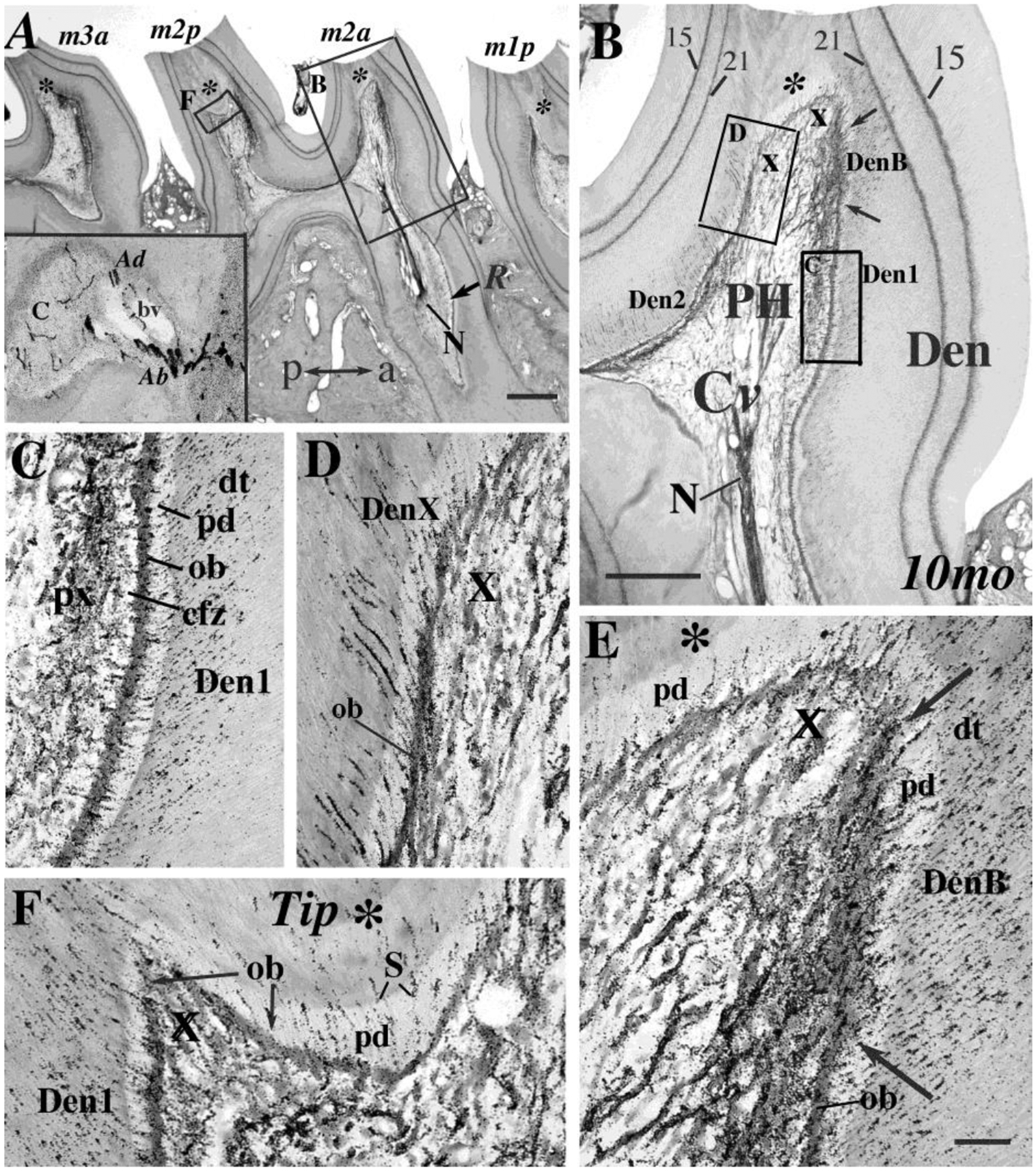
A. Four right maxillary molar cusps (m1p, m2a, m2p, m3a) are shown. These cusps have dentine growth lines (made at ages 15d and 21d) plus nerve label for 25 hours. Boxes show sites for figures 3B & 3F. Inset: labeled Abeta large, Adelta medium, and C,thin axons at root foramen (bv: blood vessel). a, anterior; p, posterior; Tip dentine (*) for each cusp. Scale: 250μm. B. This section has a labeled dental nerve (N) in the root (R) with asymmetric preterminal branches in cervical pulp (Cv) and branches in the pulp horn (PH) with many extending into dentine (Den). The four dentine patterns (Den-1, Den-2, Den-B, Den-X) are also shown in Figs. 3C–F. Scale: 100 μm. C. Pattern Den-1. This pattern from Fig. 3B has labeled nerve fibers in a plexus (px) with many branches crossing a relatively cell-free zone (cfz) to enter the odontoblast layer (ob), then into pale predentine (pd), and then dentinal tubules (dt). D. Pattern Den-X. In some regions the pulpal plexus is absent (X). Dentinal axons arrive from preterminal axons travelling in or near the odontoblast layer (ob) or directly from pulp. E. Pattern Den-B Widely branching endings (between arrows in Figs. 3B, 3E) invade the cell free zone, as do the elongated odontoblasts (ob), with many axons entering predentine (pd) and dentinal tubules (see also Fig. 4A). F. Cusp Tip. This field from cusp m2p (Fig. 3A, F*) shows tertiary dentine (*) and scattered Den-X single endings (S). Scale for C–F in Fig. 3E: 20 μm.
Figure 4. High magnifications of Den-B, PdN, and Root pulp.

A. This Den-B pattern from a m2a cusp shows the elongated odontoblasts (OB), increased vascularity (BV, arrows) on the anterior side of the tertiary tip (*). The Den-B pattern shifts to Den-1 where the odontoblast layer becomes cuboidal and separated by a cell-free zone from the pxR (ob, arrow). Scale bar: 100 μm. BC. Two different views of cervical regions (Cv) and intercusp (IC) show PdN networks parallel to the odontoblasts in the narrow predentine region with some axons possibly branching into overlying tubules of calcified dentin (arrows). D. Root pulp below the m2p cusp of the same rat as Figs. 4BC had detectable thin axons (arrows) in pulp between the main root nerve (N) and the odontoblast layer, with some extensions partway into predentine. Scales in C, D: 20 μm.
3.2. Reactions of dentinal innervation to altered odontoblast functions and attrition
Injections of trigeminal ganglion also release some tritiated proline into the vasculature, and it is then taken up into new matrix by odontoblasts if they are actively engaged in dentinogenesis. Reparative dentine that is no longer making matrix is shown for a completed repair site compared with the nearby tertiary tip (Fig. 5A), and with intense incorporation of label into matrix at a rare site of infection (Fig. 5B). Labeled matrix is also shown at sites of normal repair that blocked neural entry into dentin (Figs. 5C) in a right maxillary molar, compared to a similar site from a left mandibular molar without nerve label (Fig. 5 DE). Those sites differ from a typical cusp tip in a 13 mo rat (Fig. 5F) in which the tertiary tip dentine is well-formed and includes predentine and a patch of innervation (Den-S) in a cusp that also has so much attrition that there is only a narrow band of dentine between pulp and oral cavity. Typical relocation of trigeminal innervation to intercuspal dentine was in regions with a narrow odontoblast layer, a thin or missing plexus of Raschkow, but often some PdN fibers (Fig. 5G).
Figure 5. Odontoblast Varieties.

Different shapes and functional activity for odontoblasts are shown at sites of repair, tertiary dentin, ongoing dentinogenesis, and intercusps. A. Sites with completed reparative dentine (double arrows, RD) no longer had odontoblasts, compared with the nearby special tertiary tip dentine that has predentine (pd), S-type innervation and tip matrix (*). B. Active repair at a rare site of inflammation has cuboidal odontoblasts, no dentinal tubules in the reparative dentine (RD), and no innervation where an intense silver grain layer (small arrows) shows that dentinogenesis was in progress at the time of ganglion injection. C. Ongoing dentinogenesis was found for some cusp tips in which the region of new RD dentine (black silver grains over matrix) has blocked innervation by trigeminal axons. DE. This left mandibular molar has growth lines from matrix that were made at 15 and 21 days of age, but it has no labeled nerves because its ganglion was not injected. Box in Fig. 5D shows location for Fig. 5E where there is ongoing dentinogenesis on one side (thick arrows), with incorporation of 3H-proline into matrix by low cuboidal odontoblasts (ob, short arrow) compared with elongated odontoblasts (OB) on the opposite side (long arrow). F. This cusp tip of a 13 mo rat had normal tertiary tip dentin with Den-S innervation and predentine (pd), plus Den-X on either side of the tip, and a healthy pulp even though the dentin is less than 50 μm thick. Labeled nerves come within 30μm (arrow) of the occusal surface. G. The innervation shown here had moved into the intercusp region of a 10 mo rat, with many endings in dentin, low cuboidal odontoblasts (ob), some PdN (arrows), and Den-2 patterns. Scales: 50μm.
3.3. Quantitative morphometry of dentine innervation at different stages of attrition
Labeled dentinal tubules were counted for the seven regions in each of the nine right maxillary cusps for a 3.5 mo rat that had strong, clearly-defined axonal transport (e.g. Fig. 6A–D). The average incidence of labeled dentinal tubules per section was similar for cusps of first and second molars despite a three-fold difference in cusp volumes (Fig. 6E), while the third molar cusps were both greater (m3a) and less (m3p) than m1 and m2 cusps (p < 0.02–0.05). The values for the three right maxillary molars totaled 186,600 (Fig. 6E). That high number may be undercount, because the autoradiographic emulsion may not have detected the entire 5 μm section since the wave length of tritium is only 1–2 microns, as discussed below (Section 4.3).
Figure 6. Neural quantitative morphometry in maxillary molars (young adult) ABCD.
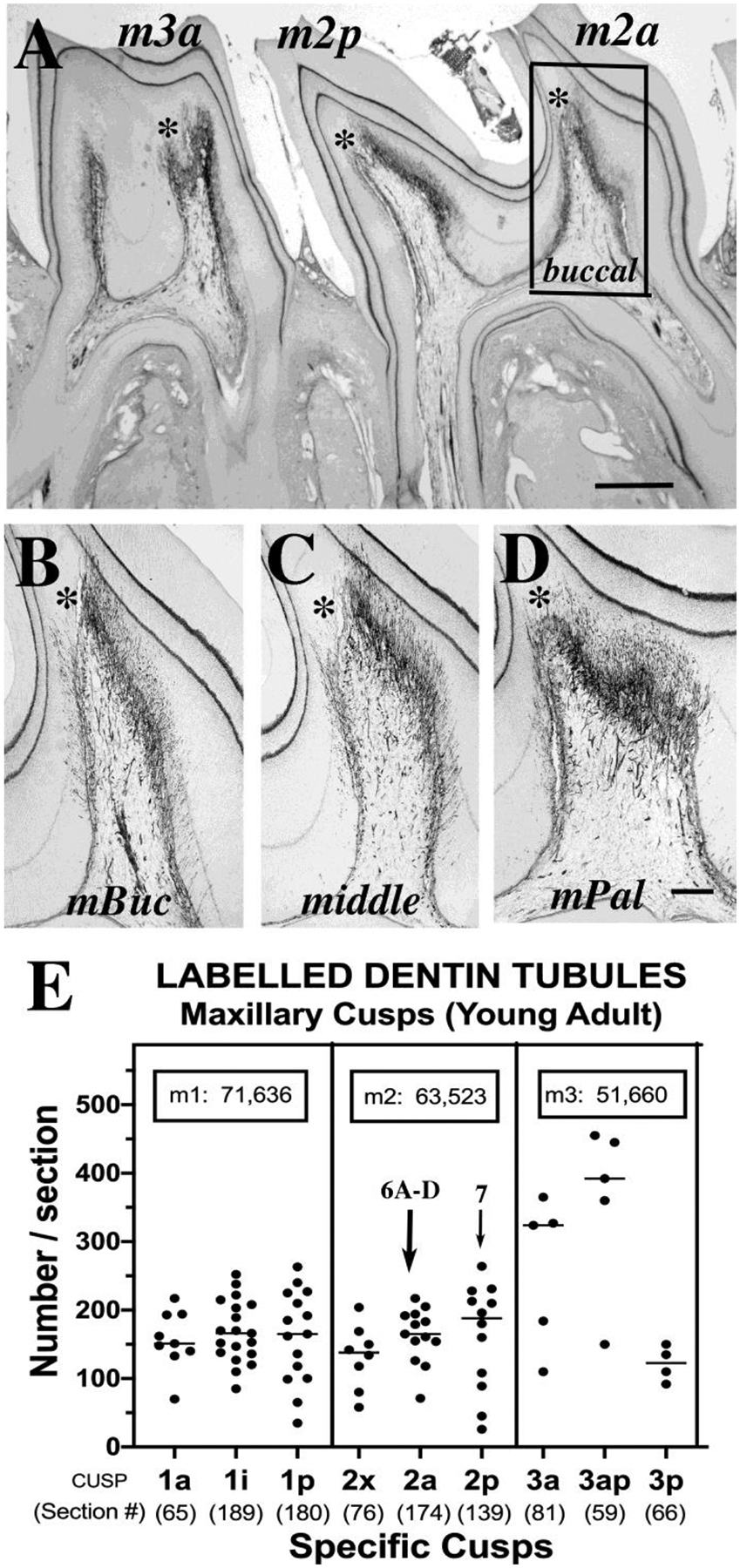
This low magnification overview shows the anterior and posterior cusps of the second (m2a, m2p) and third (m3a) maxillary molars of the 3.5 mo rat that had its innervation counted (Fig. 6E). Note the distinctive shapes of each cusp and the asymmetric innervation of dentine near the tip tertiary dentine (*). The boxed area in 6A shows the m2a cusp near its buccal edge, for comparison with other regions of that cusp in Figs. 6 (B: m2a at buccal near middle, C: middle, and D: palatal near middle). The sections in Figs. 6B–D illustrate shifting pulp horn shapes accompanied by shifting nerve patterns, compared to the buccal zone (Fig 6A). E. The data for all maxillary cusps for the 3.5 mo rat in this scatter plot show mean number of labeled dentinal tubules +/−SD per section, per cusp. The number of sections that are included per cusp (shown in parentheses below each cusp code) reveal volume differences among the cusps. The boxes give calculated totals per molar (m1, m2, m3) that when added, give a total of at least 186,600 trigeminal endings per set of 3 maxillary molars. Images 6A–D are all from m2a (large arrow). Data for the m2p cusp (thin arrow) were compared with other rats in three age groups (Fig.7K). Scales: A, 200μm, B–D, 50μm.
Regional estimations for the posterior (distal) m2p cusp of all age groups (Fig. 7) were compared to the specific counts for cusp m2p (Fig. 6E, thin arrow), and were found to be as accurate for determining the total number of innervated dentinal tubules. Images of the middle of each of 5 zones (buccal edge and palatal edge not shown) at 3 mo (Figs. 7A–E) were compared with similar regions of a 10 mo rat (Fig. 7F–J). The pulp horn height is reduced in the old rat, but in the 3 middle regions of each cusp (Figs. 7BCD vs 7GHI)) the extent of innervation is similar because of its shifted location to the cervical (Cv) and intercuspal (IC) regions in the 10 mo rat (see also Figs. 7LM). Comparison of the nerve totals for the 3 mo, 5 mo, and 10–13 mo groups did not show significant differences (Fig. 7K, p = 0.85, ANOVA). At the sites of increased innervation in intercusp dentin in the older rats (Fig. 7M), the odontoblast shape retained the flat form that occurs in the 3 mo rat (Fig. 7L), and the pulpal plexuses in that region are thin.
Figure 7. Maxillary second molar posterior cusp.

A–E. Young Adult. The posterior m2p cusp of young adult rat (3.5 mo) had different shapes in 5 main regions (buccal, buccal near middle, center of middle, palatal near middle, and palatal) as well as at buccal and palatal edges (not shown). F–I. Old Adult. These regions of the 10 mo rat have reduced pulp horn height plus neural shifts into cervical (Cv) and intercusp (IC) zones compared to the 3 mo rat in Figs. 7A–E. Scale:100μm. K. Age Group Comparison. The posterior cusp of the second molar was compared for eight rats in 3 different age groups, that were not significantly different (p = 0.85, ANOVA). Dark bars, are for rats 3c and 10a, (shown in Figs. 7A–J and Fig. 8D). LM. Neural migration to intercusp and cervical dentin. Dentine innervation for IC and Cv regions is greater at 10 mo than 3.5 mo. However, odontoblast shapes stayed flat and the furcation dentine beneath IC remained unlabeled. Odontoblasts (ob), special tertiary tip dentine (*). Scales: 100μm.
3.4. Comparisons of the extent of dentinal attrition and dentine patterns in littermates
Each cusp of each molar in the right maxillary jaws had unique wear patterns. This was found for four pairs of littermates with significantly different attrition levels despite their identical age, housing, and food (Figs. 8A–D). Measurements of remaining thickness of dentine over the tip of the pulp horn for the maxillary second molar posterior cusp were made on ten adjacent sections where attrition was greatest for each jaw (Fig. 8D). Each matched littermate pair (I-IV) had significant differences (t-test, p < 0.02 to p < 0.0001), with the largest difference found for the two 5 mo rats (pair III, Figs. 8AB). The lengths along the anterior side of the cusp (e.g. Figs. 3B, 4A) for pattern Den-B in littermate pairs were also measured. There was a significant decrease in one rat at 5 mo and one at 13 mo, but their partners (pairs III and IV) were not different from the amount found for Den-B at 3 months (Fig. 8E). There were six rats with well-matched right and left m2p cusps in which no differences in attrition were found per rat (R/L ratio: 0.99 +/− 0.18 SD, n = 6), compared to variations for the shortest remaining dentine for the six right m2p cusps (212.2 +/− 93.3 μm) and six left cusps (208.3 +/− 73.6 μm).
Figure 8. Differing littermate responses to attrition.

AB. There were different depths of wear (8A, 8B) for the right m2p cusps of two littermate rats (pair III) but there were similar shapes for their tertiary tip dentine (*). Despite the differences in attrition, both had labeled nerve endings in the Den-X, and Den-B dentinal tubules that lacked an enamel cover (enamel-free zone at bars above occlusal surface). The posterior side of each cusp (left of the double-headed arrows) had dentinal endings mostly in tubules that were aimed towards enamel-covered dentine. BC. The right cusp (Fig. 8B) had a similar depth of wear and dentine shape as its left m2p cusp (Fig. 8C). Scales: 100 μm. D. Deepest Dentin Wear: Data for the least dentine thickness for four littermate pairs (I, II, III, IV) are shown. The two rats within each pair differed from each other (p < 0.02 – 0.0001). The mean +/− standard deviation for each value represents the range of surviving dentine thickness for 10 serial sections at the narrowest dentine. E. Extent of Pattern Den-B: Violin plot shows data for the length of the Den-B pattern along the anterior side of sections of m2p for each pair. Rat 3d had the least dentin wear and the longest extent of pattern Den-B (short arrows, 8DE), and rats 5b and 13a had the shortest Den-B patterns and deepest wear (longer thin arrows, 8DE). Rat 13b (13 mo) was the same as rats 3abc (3 mo) for their extent of pattern Den-B (*, asterisks, at cusps 3abc and 13b) (ANOVA p = 0.3341).
Dentinal nerve pattern proportions and locations for Den-B, Den-1, and Den-2 were measured in the m2p cusps for all ten rats. Den-B and Den-1 were mostly on the anterior (mesial) side of the cusps (anterio/posterior mean differences of 326 +/− 75 vs. 63 +/− 48, and 543 +/− 84 vs. 189 +/−158, respectively). Den-2 by contrast was almost entirely on the posterior (distal) side. Den-B patterns were close to the occlusal tip and did not change significantly from young rats (n = 5 at 3–3.5 mo) to old (n=5 at 5–13 mo) (t-test, p = 0.2521). Den-2 patterns also did not change from young to old rats. However, the extent of Den-1 decreased from a mean +/− SD of 597 +/− 77 in 3–3.5 mo rats (n = 5) to 489 +/− 54 in old rats (n = 5) (t-test p < 0.05). Neither Den-B nor Den-1 were found in cervical or intercusp dentine of old rats, but Den-2 was found there.
3.5. Innervation of dentinal tubules that lack enamel cover
In all rats, the innervated tubular dentine that was adjacent to tertiary tip dentine had asymmetric innervation where the dentine was not covered by enamel (Fig. 8AB). Most dentinal nerve endings in each cusp enter the anterior (mesial) side for maxillary molars, or the posterior (distal) side for mandibular cusps, where the enamel-free dentine occurs. Innervation of the enamel-free dentine was by Den-X as well as Den-B axons. This tip asymmetry was found for all maxillary and mandibular cusps studied here (total: 153 cusps for all right jaws of 10 rats, and 30 left maxillary cusps for 6 rats). The special tertiary tip dentin was resilient, since it was only breached at the buccal half of one m3a cusp (out of 183 total cusps) where there were an ongoing infection and replacement of tip dentine by reparative matrix lacking dentinal tubules (Fig. 5B).
4. Discussion
4.1. Advantages and Limitations of Autoradiographic Quantitation
This new information about the variety of dentine innervation patterns adds to our fundamental knowledge of the innervation of teeth. It was possible to do this because these particular rats had clearly-defined, strong radio-labeling of their innervated dentinal tubules, and their sections of jaws were unchanged during lengthy storage. Advantages of this method are that the entire trigeminal sensory innervation and its endings can be revealed when injections fill the ganglion with tritiated amino acids, of which 3H-proline is especially appropriate because it is a prominent amino acid in the transported proteins (Rogers, 1979). Posterior molar teeth in rat, cat, or human mandibles can also receive innervation from lingual or myohyoid branches of trigeminal nerve (Kim et al., 2016; Naftel et al., 1999; Robinson, 1980), and those, if present, would also be labeled in addition to the inferior alveolar nerve after trigeminal ganglion injections. Here, we focused only on molar teeth because prior work has shown that the rat incisor teeth have sparse innervation of the odontoblast layer, no dentinal innervation, and no associated pulpal plexuses (Byers & Kish, 1976; Byers, 1984). Furthermore, labeled proteins accumulate in nerve endings and their preterminal axons, thereby revealing the vitality of the sensory innervation. Also, the release of tritiated proline into the blood during the injection of the trigeminal ganglion, is taken up by odontoblasts thereby providing a label for sites of ongoing dentinogenesis and the status of odontoblast activity.
A key limitation for autoradiography is that it is an indirect label that requires close attachment of the photo emulsion to the sections in order to localize the radioactivity within a few microns of its source. That works well when tritium is the signal and the slides are coated with liquid photo emulsion, because the beta radiation of tritium only extends 1–2 μm, and a liquid photo emulsion can come close enough to the sections. However, the short wave length also means that the photo emulsion may only be reading the top part of the 5 μm sections. Another problem is that autoradiographic labels need excessive exposure times, as shown by the samples for this study, many of which needed 18–32 weeks of exposure to have strong, clearly-defined label of the dentinal tubules.
There were also challenges in this study because quantitation of labeled dentinal tubules could not be done automatically, and so a hand-counting technique was used (see Methods Section 2.5.2). In addition, it was not feasible to do the entire collection of data with coded slides, because each jaw’s features (autoradiographic label intensity, counterstain, block size, sequence of tooth arrivals in the section series), in most cases were unique and easily recognized. To obviate this situation, we did repeated counts (Fig. 6E, 7K) on different days with comparable data. Finally, this study only concerns male rats, and it will be important to determine in future work whether similar neural reponses occur in molars of female rats during their attrition.
4.2. Different responses to attrition
Our results are consistent with the idea that different types of sensory innervation respond differently to changes in pulp horn shape during attrition. Some nerve patterns were maintained during attrition in this study, while others were increased, decreased, or no longer present in the oldest rats. For example, patterns Den-X and Den-S were maintained in all rats where tertiary and enamel-free dentine occurred, and Den-B was preserved, even though it was displaced by foci of reparative dentine. Den-1 decreased in m2p cusps, while pattern Den-2 was increased in cervical regions where the nerves had relocated (Fig. 5G). Den-B and Den-1 were cusp-specific and were not found in cervical or intercuspal sites. However, Den-X remained in the dentine adjacent to the tertiary tip dentine, and the latter was preserved in 182 of 183 cusps examined here. We do not know how displaced nerve endings set up their own new dentine territory, but they may require odontoblast interactions and/or altered expression of the neurotrophic factors that are important for development (Fried et al., 2000), normal tooth functions (Kvinnsland et al., 2004; Pan et al., 2000; Sarram et al., 1997; Yang et al., 2006), and injury reactions (Byers et al., 1988; Byers et al., 1992; Fried et al., 1991; Kimberly & Byers, 1988; Taylor et al., 1988; Swift & Byers, 1992). However, we do know that following inferior alveolar nerve transection, the dentine of denervated mandibular rat molars was reinnervated within a few weeks (Berger et al., 1983; Holland et al., 1987), as were many denervated cat teeth, with that reinnervation coming from lingual as well as alveolar trigeminal nerves (Robinson et al., 1980). Clearly, the innervation of dentine possesses ongoing mechanisms to maintain position and to shift locations if the pulp-dentine habitat changes. It is worth noting that the odontoblasts in innervated, intercuspal zones maintained a cuboidal or flat shape, unlike the more columnar odontoblasts near the cusp tip. In addition, the relocated innervation completely avoided the furcation dentine on the root side of the intercuspal zone. It will be important to link sensory functions to the patterns described here, since differential reactions to attrition might create altered proporotions of neuro-pulpal interactions or affect somatosensitivity.
4.3. Density of Trigeminal Innervation of Coronal Dentine of Rat Molars
We found that an intensely labeled set of 3 maxillary molars had at least 186,600 labeled dentinal tubules. The qualitative evaluation of mandibular molars found similar densities, and so, if the maxillary total is doubled to include the right mandibular molars, the right six molars could have at least 370,000 labeled tubules. If that is doubled to include the left teeth, the total per rat might be at least 740,000. That is an impressive number, but two issues with the quantitation suggest that this total may be low, and that full innervation of an adult rat’s molar dentition may be well above one million nerve endings. First, autoradiography of tritium signals may have missed the lower half of each section because its beta-radiation wave length is 1–2μm (Rogers, 1979), Second, electron microscopy shows multiple endings within dentinal tubules of many species (Arwill et al., 1973; Gunji, 1982; Byers 1984; Holland 1994; Holland et al., 1987). An important challenge will be to determine why such massive innervation of coronal dentin occurs, what the functions are, how those sensory systems adapt to being in a tissue that wears down, and whether dental attrition in elderly patients affects their clinical treatment.
4.4. Cusp differences and similarities
Each cusp in the rat molars had a characteristic volume, such as the narrow cusps at maxillary m1a, m2x, m3x, and m3p, compared with the 3-fold larger cusps: m1i, m1p, m2a, m2p and m3a (Figs. 3A, 6E). Despite those differences, all three cusps of first molars and those of second molars each maintained roughly the same number of innervated dentinal tubules per section (Fig. 6E), and each cusp had seven regions from the buccal side to the palatal side. This would be consistent with ongoing local neural adjustments to cusp shape and pulpal features in order to maintain the same set of sensory capabilities in every cusp. Furthermore, each cusp had different attrition challenges, but each appears to try to retain their dentinal nerve patterns. Ongoing cusp-specific neuroplasticity is supported by the unusual amount of growth associated protein-43 (GAP-43) in the innervation of adult rat molars (Maeda, 1996). Finally, discoveries about somatosensory mechanisms that are obtained from rodent incisors would not include mechanisms related to dentinal innervation, since that is missing from the rodent incisors.
4.5. Species differences
The dentine innervation in human teeth and rat molars share many features (Gunji, 1982; Henry et al., 2012; Kim et al., 2017; Maeda et al., 1986, 1987). The human teeth have many millions more dentinal tubules (Pashley, 1996) and may have far more nerve endings in their molar dentin than the rats, although axonal transport label in large animal teeth (monkeys, cats) found the innervation concentrated in the 0.5 mm near the cusp tip regardless of cusp size (Byers & Dong, 1983; Byers & Matthews, 1981). Most studies of human teeth involve unerupted third molars or young teeth removed for orthodontia, which are too immature to have reactions to attrition (Bernick 1964; Hattyasy, 1961; Gunji, 1982). However, some studies of clinical sensitivity found extensive attrition and pulpal reductions in extracted teeth from elderly patients, whose responses to electric stimulation were normal (Brännström, 1981, pp. 27–40), even though important neural and glial changes might have occurred in their teeth (Couve et al., 2018). In rat molars a high density of nerves in the tubular dentine adjacent to each molar cusp tip was seen, especially where there was no enamel covering the dentine (Figs. 5F, 7A–E), and this density was maintained in the worn-down older molars (Fig. 7F–J). We do not know whether uncovered (enamel-free) dentine offers different sensory or efferent neuropeptide possibilities compared with regions covered by enamel, nor whether all the dentinal patterns in rat molars occur in human teeth. Attrition leads to individually distinct cusps that in humans enable specific forensic identifications that are unique for each person (Dyke, 2018). This suggests that each cusp in every kind of dentition may be a highly specialized sensory organ that adjusts itself as needed for its species, in relation to its own local attrition dynamics.
4.6. Plexus of Raschkow and its cell-free zone next to the odontoblast layer
Our results have focused on variations of the plexus of Raschkow (pxR). Similar features have also been described in detail for young human teeth in pioneering studies (Gunji, 1982, p.47): “The density of the nerve plexus varied. Occasionally it was very clearly observed; on other occasions it could hardly be defined as a nerve plexus. The plexus appeared differently even between teeth from children of the same age. Regional differences were also marked within one tooth.” It is possible that the presence or absence of a cell-free zone (Cfz) next to the odontoblast layer is a key feature for the functions of the pxR, especially when it vanishes at pattern Den-B where there are elongated odontoblasts and other cells filling that site. Further examination of the pxR and its Cfz in older human teeth would be of interest, along with determining whether they are similar in all teeth, or perhaps more prominent in molars (with grinding, chewing functions) than in incisors and canines that are specialized for delicate touch as well as cutting, biting, and fighting.
4.7. Predentine network (PdN) of nerve-odontoblast associations
The PdN occurs on the dentinal side of the tight junctions of the odontoblast layer (Figs. 2, 4BC) in a predentine compartment separated from the pulp by the intercellular junctions of the odontoblasts (Bishop & Yoshida, 1992; Turner et al., 1989; Okiji, 2012). Many ultrastructural studies noted close association of odontoblast processes in predentine with the enlarged neural beads that contain organelles typical of sensory receptor cytoplasm (e.g., Arwill et al., 1973; Frank et al.,1972) as well as smaller neural processes (Holland et al., 1987). That network and its associations with odontoblast processes were well defined for young human teeth (Gunji, 1982) using silver stains and electron microscopy, with evidence also for some dentinal extensions from PdN nerve fibers. PdN beaded fibers were found here in cervical regions of all molars (Fig. 4BC) for rats in the 3 mo, 5 mo, and 10 mo age groups. Unexpectedly, their occurrence in the two 13 mo rats was rare in the serial sections of their maxillary and mandibular molars. Our autoradiograms also suggested possible extensions into dentine from the PdN, and newer labeling technologies (e.g. Chung et al., 2012) likely will expand our understanding of those possible special dentinal endings. Similar nerve endings have been confirmed in human and rat molars by immunocytochemistry (Maeda et al., 1986; Maeda et al., 1987). Here, we have designated these endings the ‘predentinal network’ (PdN). Prior names have included ‘marginal plexus’ (Fearnhead, 1961) or ‘complex endings’ (Gunji, 1982), but we wanted to emphasize that the location within predentine may be a defining feature.
5. Conclusions
The quantitative morphometry presented here reveals a surprisingly high number of innervated dentinal tubules in rat molars, showing that the entire molar dentition in an adult rat may have well over one million dentinal tubules containing trigeminal sensory endings. There were six different dentinal patterns, only two of which had an associated plexus of Raschkow plus cell-free zone in the pulp. Some Den-1 innervation was lost from the cuspal dentine during attrition, but Den-B was unchanged unless displaced by ongoing dentinogenesis, and there were increased Den-2 patterns in cervical and intercuspal dentine. The special tertiary dentine at the cusp tip was maintained during the first year of atttition in rat molars along with its asymmetric neighboring innervation of enamel-free dentinal tubules. The differences between pairs of littermate rats show the unique shapes and adjustments to attrition that characterize each molar cusp in each rat. A parallel situation underlies the unique signature of an individual’s forensic data, and it means that future experiments with rodents should compare similar molar cusps across all treatment groups, as demonstrated here and earlier (Lovschall et al., 2002), to distinguish between endogenous variations and those induced during attrition or by experiments. The pulp-dentine complex of rodent teeth is becoming a better understood barrier tissue, and it will be a model site for defining somatosensory mechanisms in general, including neuropeptide functions in integument, as well as defining the specifics of dental pain and its treatment.
Highlights.
Trigeminal innervation of rat molar dentine had at least six different patterns.
Only two of the dentinal patterns had a nearby pulpal plexus of Raschkow
Cervical dentine gained innervation during attrition making up for cuspal loss.
Innervation of special tertiary dentine at cusp tips persisted during attrition.
Each rat molar cusp had a unique shape, innervation, and patterns of attrition.
Acknowledgments:
We thank Marie Doman for superb serial section preparations. The diagrams by Seattle illustrator, Kate Sweeney, are much appreciated, as are the helpful suggestions by our colleague, Dr. Eric H. Chudler. This paper is in honor of the leaders of dental research at the University of Washington, Dr. Leo M. Sreebny (1922-2020) and Dr. Roy C. Page (1932-2020) whose local block grants for their Dental and Oral Biology Research Centers (NIH) were essential for many new investigations, including the initial studies of trigeminal axonal transport. In addition, we are grateful for the superb work by Dr. Rebecca L. Berger when she pioneered many of the procedures used here, during her training and faculty work in the Department of Endodontics, University of Washington.
FUNDING:
NIH funding during this work: Career award DE00099 (MRB, 1979-85) and research grant R01-05159 (MRB, 1979-2006). Continuing facilities support since 2007 by the Dept. of Anesthesiology and Pain Medicine, University of Washington, is gratefully acknowledged. The sources of funding did not influence the manuscript in any way.
Main Abbreviations:
- a-p
anterior-posterior (synonymous with ‘mesio-distal’)
- Cfz
cell-free zone
- Den-1
dentinal nerve pattern with full plexus of Raschkow (pxR)
- Den-2
pattern with a thin pxR
- Den-B
bush-like dentinal pattern without Cfz or standard pxR
- Den-X
single dentinal endings near cusp tip with no pxR
- Den-S
single axons in cuspal tertiary dentine
- Dt
dentinal tubules
- IC
intercuspal dentine
- m1, m2, m3
first, second and third molars
- mo
months old
- Ob
odontoblasts
- px, pxR
plexus of Raschkow
- Pd
predentine
- PdN
predentine network
- RD
reparative dentine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTERESTS: We do not have any competing interests
References:
- Alavi AM, Dubyak GR & Burnstock G (2001) Immunohistochemical evidence for ATP receptors in human dental pulp. Journal of Dental Research, 80(2), 476–483. 10.1177/00220345010800021501. [DOI] [PubMed] [Google Scholar]
- Arwill T, Edwall L, Lilja J, Olgart L, & Svensson S-E (1973). Ultrastructure of nerves in the dentinal pulp border zone after sensory and autonomic nerve transection in the cat. Acta Odontologica Scandinavia, 31:273–281. 10.3109/00016357309002514. [DOI] [PubMed] [Google Scholar]
- Azerad J & Woda A (1977). Sensation evoked by bipolar intrapulpal stimulation in man. Pain, 4(2):145–152. 10.1016/0304-3959(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Berger RL, Byers MR, & Calkins DF (1983). Dental nerve regeneration in rats. Pain 15:345–370. 10.1016/0304-3959(83)90071-4. [DOI] [PubMed] [Google Scholar]
- Bernal L, Sotelo-Hitschfeld P, König C, Sinica V, Wyatt A, Winger A, et al. (2021). Odontoblast TRPC5 channels signal cold pain in teeth. Science Advances 7:eabf5567 (https://advances.sciencemag.org). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernick S (1962). Age changes in the nerves of molar teeth of rats. The Anatomical Record, 143:121–126. 10.1002/ar.1091430205. [DOI] [PubMed] [Google Scholar]
- Bernick S (1964). Differences in nerve distribution between erupted and non-erupted human teeth. Journal of Dental Research 43:406–411. 10.1177/00220345640430031201. [DOI] [PubMed] [Google Scholar]
- Bernick S (1967). Effect of aging on the nerve supply to human teeth. Journal of Dental Research 46(4):694–699. 10.1177/00220345670460031501. [DOI] [PubMed] [Google Scholar]
- Bleicher F (2014). Odontoblast physiology. Experimental Cell Research 325(6):65–71. 10.1016/yescr2013.12.012. [DOI] [PubMed] [Google Scholar]
- Bletsa A, Fristad I, & Berggreen E (2009). Sensory pulpal nerve fibres and trigeminal ganglion neurons express IL-1R1: A potential mechanism for development of inflammatory hyperalgesia. International Journal of Endodontics, 42(11): 978–986. 10.1111/j.1365-2591.2009.01605.x [DOI] [PubMed] [Google Scholar]
- Bishop MA & Yoshida S (1992). A permeability barrier to lanthanum and the presence of collagen between odontoblasts in pig molar. Journal of Anatomy, 181:29–38. [PMC free article] [PubMed] [Google Scholar]
- Brännström M (1981). Dentine and Pulp in Restorative Dentistry. Wolfe Med. Publ. Ltd. London, pp. 21–41. [Google Scholar]
- Byers MR (1980). Development of sensory innervation in dentine. Journal of Comparative Neurology, 191(3):413–27. 10.1002/cne.901910307. [DOI] [PubMed] [Google Scholar]
- Byers MR (1984). Dental sensory receptors. International Review of Neurobiology, 25:39–94. 10.1016/s0074-7742(08)60677-7. [DOI] [PubMed] [Google Scholar]
- Byers MR (2019). Chewing causes rapid changes in immunoreactive nerve patterns in rat molar teeth: Implications for dental proprioception and pain. Archives of Oral Biology, 107:1–12 10.1016/j.archoralbio.2019.104511 [DOI] [PubMed] [Google Scholar]
- Byers MR & Cornel LM (2018). Multiple complex somatosensory systems in mature rat molars defined by immunohistochemistry. Archives of Oral Biology, 85:84–97. 10.10.1016/j.archoralbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Byers MR & Dong WK (1983). Autoradiographic location of sensory nerve endings in dentine of monkey teeth. The Anatomical Record, 205:441–454. 10.1002/ar.1092050409. [DOI] [PubMed] [Google Scholar]
- Byers MR & Kish SJ (1976). Delineation of somatic nerve endings in rat teeth by radioautography of axon-transported protein. Journal of Dental Research, 55(3):419–425. 10.1177/00220345760550032001. [DOI] [PubMed] [Google Scholar]
- Byers MR & Matthews B (1981). Autoradiographic demonstration of ipsilateral and contralateral sensory nerve endings in cat dentine, pulp, and periodontium. The Anatomical Record, 201(2):249–60. 10.1002/ar.1092010205 [DOI] [PubMed] [Google Scholar]
- Byers MR & Närhi MVO (1999). Dental Injury Models: Experimental tools for understanding neuro-inflammatory interactions and polymodal nociceptor functions. Critical Reviews of Oral Biology & Medicine, 10(1):4–39. 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- Byers MR & Sugaya A (1996). Odontoblast processes in dentine revealed by fluorescent Di-I. Journal of Histochemistry Cytochemistry, 43(2):159–168. 10.1177/43.2.7529786 [DOI] [PubMed] [Google Scholar]
- Byers MR & Westenbroek RE (2011). Odontoblasts in developing, mature and aging rat teeth have multiple phenotypes that variably express all nine voltage-gated sodium channels. Archives of Oral Biology, 56:1199–1220. 10.1016/j.archoralbio.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MR, Gerlach RG, & Berger RL (1982). Development of sensory receptor structure and function in rat molars. In: Matthews B, Hill RG (Eds.) Anatomial, Physiological, and Pharmacological Aspects of Trigeminal Pain (pp. 41–52). Exerpta Medica, Amsterdam. [Google Scholar]
- Byers MR, Mecifi KB & Närhi MVO (1988). Acute and chronic reactions of dental sensory nerve fibers to cavities and desiccation in rat molars. Anatomical Record, 221:872–883. 10.1002/ar.1092210412. [DOI] [PubMed] [Google Scholar]
- Byers MR, Wheeler E,F & Bothwell M (1992). Altered expression of NGF and p75NGF-receptor by fibroblasts of injured teeth precedes sensory nerve sprouting. Growth Factors, 6(1):41–52. 10.3109/08977199209008870 [DOI] [PubMed] [Google Scholar]
- Cadden SW, Lisney SJ, & Matthews B (1983). Cat canine tooth-pulp with A-beta, A-delta and C-fibre conduction velocities. Brain Research, 261:31–41. 10.1016/0006-8993(83)91280-5. [DOI] [PubMed] [Google Scholar]
- Chrepa V, Joon R, Austah O, Diogenes A, Hargreaves KM, Ezeldeen M & Ruparel NB (2020). Clinical outcomes of immature teeth treated with regenerative endodontics procedures. San Antonio study. Journal of Endodontics, 2020 August;46(8):1074–1084. 10.1016/j.joen.2020.04.008. [DOI] [PubMed] [Google Scholar]
- Chung M-K, Jue SS & Dong X (2012). Projection of non-peptidergic afferents to mouse tooth pulp. Journal of Dental Research,, 91:777–781. 10.1177/0022034512450298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve E, Osorio R, & Schmachtenberg O, (2013). The amazing odontoblast: Activity, autophagy, and aging. Journal of Dental Research, 92(9):765–772. 10.1177/0022034513495874. [DOI] [PubMed] [Google Scholar]
- Couve E, Lovera M, Suzuki K & Schmachtenberg O (2018). Schwann cell phenotype changes in aging human dental pulp. Journal of Dental Research, 97(3):347–355. 10.1177/0022034517733967. [DOI] [PubMed] [Google Scholar]
- Diogenes A (2020). Trigeminal sensory neurons and pulp regeneration. Journal of Endodontics, Sep46(9S):S71–S80 10.1016/j.joen.06.038. [DOI] [PubMed] [Google Scholar]
- Dong WK, Chudler EH, & Martin RF (1985). Physiological properties of intradental mechanoreceptors. Brain Research, 334:389–395. [DOI] [PubMed] [Google Scholar]
- Dong WK, Shiwaku T, Kawakami Y, & Chudler EH (1993). Static and dynamic responses of periodontal ligament mechanoreceptors and intradental mechanoreceptors. Journal of Neurophysiology, 69:1567–1582. 10.1152/jn.1993.69.5.1567. [DOI] [PubMed] [Google Scholar]
- Dyke AEC, Cunningham S, Hunt N, & Ruff C (2018). A comparative study to investigate the effects of orthodontic treatment on the uniqueness of the human anterior dentition. Forensic Science International 289:368–373. 10.1016/j.forsciint.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Egbuniwi O,, Grover S, Duggal AK, Mavroudis A, Yazdi M, Renton T, et al. , (2014). TRPA1 and TRPV4 activation in human odontoblasts stimulated ATP release. Journal of Dental Research, 93(9):911–17. 10.1177/0022034514544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Orr DF, & Lundy T (2008). Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. Journal of Neuroimmunology August 30;200(1–2):11–6. 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Emrick JJ, von Buchholtz LJ, & Ryba NJP (2020). Transcriptomic classification of neurons innervating teeth. Journal of Dental Research, 99(13) 1478–1485. 10.1177/0022034520941837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani RM, Somonian M, & Hunter N, (2011). Blueprint of an ancestral neurosensory organ revealed in glial networks in human dental pulp. Journal of Comparative Neurology, 519(16):3306–26. 10.1002/cne.22701. [DOI] [PubMed] [Google Scholar]
- Fearnhead RW (1957). Histological evidence for the innervation of human dentine. Journal of Anatomy, 91:267–77,. [PMC free article] [PubMed] [Google Scholar]
- Fearnhead RW (1961). The neurohistology of human dentine. Proceedings of the Royal Society of Medicine, 54:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BR, Kish SJ, & Byers MR, (1975). Rapid axonal transport in trigeminal nerve of rat. Brain Research, 90:85–95. 10.1016/0006-8993(75)90684-8 [DOI] [PubMed] [Google Scholar]
- Frank RM, Sauvage C, & Frank P, (1972). Morphological basis of dental sensitivity. International Dentistry Journal March;22(1):1–19. [PubMed] [Google Scholar]
- Fried K, (1992). Changes in pulpal nerves with aging. Proceedings of the Finnish Dental Society 88(suppl 1), 517–528. [PubMed] [Google Scholar]
- Fried K & Gibbs JL (2014). Dental pulp innervation. In: Goldberg M (Ed) The Dental Pulp (pp. 75–95). Springer, Berlin. 10.1007/978-3-642-55160-4_6 [DOI] [Google Scholar]
- Fried K & Hildebrand C, 1981. Pulpal axons in developing, mature, and aging feline permanent incisors. A study by electron microscopy. Journal of Comparative Neurology, 203(1), 23–36. 10.1002/cne.902030104 [DOI] [PubMed] [Google Scholar]
- Fried K, Arvidsson J, Robertson B, & Pfaller K (1991). Anterograde horseradish peroxidase tracing and immunohistochemistry of trigeminal ganglion tooth pulp neurons after dental nerve lesions in the rat. Neuroscience, 43(1):269–78. 10.1016/0306-4522(91)90434-p. [DOI] [PubMed] [Google Scholar]
- Fried K, Nosrat C, Lillesaar C, & Hildebrand C, (2000). Molecular signaling and pulpal nerve development. Critical Reviews of Oral Biology and Medicine, 11(2) :318–332. 10.1177/10454411000110030301. [DOI] [PubMed] [Google Scholar]
- Fried K, Sessle BJ, & Devor M, (2011). The paradox of pain from tooth pulp: Low-threshold “algoneurons”. Pain 152:2685–2689. 10.1016/j.pain.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad I, Berggreen E, Haug SR (2006). Delta (delta) opioid receptors in small and medium-sized trigeminal neurons supporting the dental pulp of rats. Archives of Oral Biology, 51(4):273–81. 10.1016/j.archoralbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Gunji T (1982). Morphological work on the sensitivity of dentine. Arch Histology Jpn, 45:45–67. [DOI] [PubMed] [Google Scholar]
- Hattyasy D (1961). Continuous regeneration of the dentinal nerve-endings. Nature, 189;72–74 10.1038/189072a0. [DOI] [PubMed] [Google Scholar]
- Haug SR, & Heyeraas KJ, (2006). Modulations of dental inflammation by the sympathetic nervous system. Journal of Dental Research, 85(6):488–495. 10.1177/154405910608500602. [DOI] [PubMed] [Google Scholar]
- Henry MA, Luo S, & Levinson SR (2012). Unmyelinated nerve fibers in the human dental pulp express markers for myelinated fibers and show sodium channel accumulations. Neuroscience, 13:29–40. 10.1186/1471-2202-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyeraas KJ, & Berggreen E, (2000). Interstitial fluid pressure in normal and inflamed pulp. Critical Reviews of Oral Biology and Medicine 10(3):328–336 [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Fried K, Tuisku F, & Johansson CS, (1995). Teeth and tooth nerves. Progress in Neurobiology, 45(3):165–222. 10.1016/0301-0082(94)00045. [DOI] [PubMed] [Google Scholar]
- Holland GR, (1994). Morphological features of dentine and pulp related to dentine sensitivity. Archives in Oral Biology, 39(suppl):3S–11S. 10.1016/0003-9969(94)90182-1 [DOI] [PubMed] [Google Scholar]
- Holland GR, & Botero TM, (2014). Pulp Biology: 30 years of progress. Endodontic Topics 31, (1) 19–35. 10.1111/etp.12064. [DOI] [Google Scholar]
- Holland GR, Matthews B, Robinson PP (1987). An electrophysiological and morphological study of the innervation and reinnervation of cat dentine. Journal of Physiology 386:31–43. DOI: 10.1113/jphysiol.1987.sp016520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst OV, Horst JA, Samudrala R, Dale BA (2011). Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol, 24;12:9 10.1186/1471-2172-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H & Suda H (2013) Odontoblastic syncytium through electrical coupling in the human dental pulp. Journal of Dental Research, 92(4):371–375. 10.1177/0022034513478430 [DOI] [PubMed] [Google Scholar]
- Kim S, Uzbelger-Feldman D, & Yang J (2016) A Systematic review of the cervical plexus accessory innervation and its role in dental anesthesia. Journal of Anesthesia History 2:79–84 10.1016/janh.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Kim TH, Park SK, Choi SY, Lee JS, & Bae YC (2017). Morphologic change of parvalbumin-positive myelinated axons in the human dental pulp. Journal of Endodontics, 43:977–982. 10.1016/j.joen.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Kimberly CL, & Byers MR (1988). Inflammation of rat molar pulp and periodontium causes increased calcitonin gene-related peptide and axonal sprouting. (1988). The Anatomical Record, 222:289–300. 10.1002/ar.1092220310. [DOI] [PubMed] [Google Scholar]
- Komiya H, Shimisu K, Noma N, Tsuboi Y, Honda K, Kanno K, Ohara K, Shinoda M, Osiso B, & Iwata K (2018). Role of neuron-glial interaction mediated by Il-1b in ectopic tooth pain. Journal of Dental Research 97(4):467–475. 10.1177/0022034517741253. [DOI] [PubMed] [Google Scholar]
- Kubo K, Shibukawa Y, Shintani M, Suzuki T, Ichinohe T, Kaneko Y (2008). Cortical representation area of human dental pulp. Journal of Dental Research, 87:358–62. 10.1177/154405910808700409. [DOI] [PubMed] [Google Scholar]
- Kvinnsland IH, Luukko K, Fristad I, Kettunen P, Jackson DL, Fjeld K, von Bartheld CS, & Byers MR (2004). Glial cell line-derived neurotrophic factor (GDNF) from adult rat tooth serves a distinct population of large-sized trigeminal neurons. European Journal of Neuroscience, 19:2089–2098. 10.1111/j.0953-816X.2004.03291.x [DOI] [PubMed] [Google Scholar]
- Lavigne GJ & Sessle BJ (2016). The neurobiology of orofacial pain and sleep and their interactions. Journal of Dental Research. 95(10):1109–16. 10.1177/0022034516648264. [DOI] [PubMed] [Google Scholar]
- Lilja J (1979). Innervation of different parts of the predentine and dentine in young human premolars. Acta Odontological Scandanavia, 37:339–346. 10.3109/00016357909004706 [DOI] [PubMed] [Google Scholar]
- Lovschall H, Fejerskov O, & Josephsen K (2002). Age-related and site-specific changes in the pulpodentinal morphology of rat molars. Archives of Oral Biology 47:361–367. 10.1016/s0003-9969(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Lundy FT & Linden GJ (2004). Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Critical Reviews of Oral Biology and Medicine, 15(2):82–90 10.1177/154411130401500203 [DOI] [PubMed] [Google Scholar]
- Lundy FT, Irwin CR, McLean DF, Linden GJ, & El Karim IA (2020). Natural antimicrobials in the dental pulp. Journal of Endodontics 46:S2–S9. 10.1016/joen.2020.06.021. [DOI] [PubMed] [Google Scholar]
- Luukko K, & Kettunen P (2014). Coordination of tooth morphogenesis and neuronal development through tissue interactions: lessons from mouse models. Experimental Cell Research. July 15;325(2):72–7. 10.1016/j.yexcr.2014.02.029.11. [DOI] [PubMed] [Google Scholar]
- Maeda T (1996). Expression of growth asociated protein (GAP-43) in mature and developing rat dental pulp. In: (Shimono M, Maeda T, Suda H, Takahashi H,K, Eds), Dentine/Pulp Complex (pp. 130–135). Quintessence, Tokyo. [Google Scholar]
- Maeda T, Iwanaga T, Fujita T, & Kobayashi S (1986). Immunohistochemical demonstration of nerves in the predentine and dentine of human third molars with the use of an antiserum against neurofilament protein (NFP). Cell and Tissue Research 243:469–475. [DOI] [PubMed] [Google Scholar]
- Maeda T, Iwanaga T, Fujita T, Takahashi Y, & Kobayashi S (1987). Distribution of nerve fibers immunoreactive to neurofilament protein in rat molars and periodontium. Cell and Tissue Research 249:13–23. [DOI] [PubMed] [Google Scholar]
- Magloire H, Couble M-L, Thivichon-Prince B, Maurin J-C, & Bleicher F (2009). Odontoblast: A mechano-sensory cell. Journal of Experimental Zoology (Mol Dev Evol), 312B:416–424. [DOI] [PubMed] [Google Scholar]
- Magloire H, Maurin JC, Couble ML, Shibukawa Y, Tsumura M, Thivichon-Prince B, & Bleicher F (2010). Topical review. Dental pain and odontoblasts: Facts and hypotheses. Journal of Orofacial Pain, 24(4):335–349. [PubMed] [Google Scholar]
- Marfurt CF & Turner DF (1983). Sensory nerve endings in the rat oro-facial region labeled by the anterograde and transganglionic transport of horseradish peroxidase: a new method for tracing perioheral nerve fibers. Brain Research 1983 February 14;261(1):1–12. 10.1016/0006-8993(83)91277-5. [DOI] [PubMed] [Google Scholar]
- Naftel JP, Richards LP, Pan M, & Bernanke JM (1999). Course and composition of the nerves that supply the mandibular teeth of the rat. The Anatomical Record, 256(4):433–447 [DOI] [PubMed] [Google Scholar]
- Närhi MV, (1985). The characteristics of intradental sensory units and their responses to stimulation. Journal of Dental Research, April;64 Spec No:564–71. 10.1177/002203458506400411. [DOI] [PubMed] [Google Scholar]
- Närhi M, Kontturi-Närhi V, Hirvonen T, Ngassapa D (1992). Neurophysiological mechanisms fo dentine hypersensitivity, Proceedings of the Finnish Dental Society 88:Suppl 1., 15–22. [PubMed] [Google Scholar]
- National Research Council (NIH, USA) (2011). Guide for the Care and Use of Laboratory Animals (8th ed.). Washington (DC): National Academies Press (US); SBN-13: 978-0-309-15400-0ISBN-10: 0-309-15400-6. 10.17226/5140. [DOI] [Google Scholar]
- Okiji T (2012) Pulp as a connective tissue. In: Hargreaves KM, Goodis HE, Tay FR (Eds.), Seltzer and Bender’s Dental Pulp (pp. 67–89) (2nd ed.). Quintessence, Chicago. [Google Scholar]
- Olgart L (1996). Neural control of pulpal blood flow. Critical Reviews of Oral Biology and Medicine 7(2):159–71. 10.1177/10454411960070020401. [DOI] [PubMed] [Google Scholar]
- Orchardson R, & Cadden SW (2001). An update on the physiology of the dentine-pulp complex. Dental Updates, 28:200–209. 10.12968/denu.2001.28.4.200. [DOI] [PubMed] [Google Scholar]
- Pan M, Naftel JP, & Wheeler EF (2000). Effects of deprivation of neonatal nerve growth factor on the expression of neurotrophin receptors and brain-derived neurotrophic factor by dental pulp afferents of the adult rat. Archives of Oral Biology, 45(5):382–99. 10.1016/s0003-9969(00)00002-9 [DOI] [PubMed] [Google Scholar]
- Pashley DH (1996) Dynamics of the pulpo-dentine complex. Critical Reviews of Oral Biology and Medicine 7:104–133. doi: 10.1177/10454411960070020101. [DOI] [PubMed] [Google Scholar]
- Robinson PP (1980). An electrophysiology study of the pathways of pulpal nerves from mandibular teeth in the cat. (1980). Archives of Oral Biology, 25:825–29. 10.1016/0003-9969(80)90141-7. [DOI] [PubMed] [Google Scholar]
- Rogers AW (1979). The Techniques of Autoradiography (3rd. Ed). Elsevier/N Holland Biomedical Press, Amsterdam, The Netherlands. ISBN 0–444-80063–8 [Google Scholar]
- Salpeter MM, Bachmann L, & Salpeter EE (1969) Resolution in electron microscopic radioautography. Journal of Cell Biology, 41(1):1–32. 10.1083/jcb.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarram S, Lee K-F, & Byers MR (1997). Dental Innervation and CGRP in adult p75-deficient mice. Journal of Comparative Neurology 385:297–308. [DOI] [PubMed] [Google Scholar]
- Sato M, Ogura K, Kimura M, Nishi K, Ando M, Tazaki M, & Shibukawa Y (2018). Activation of mechanosensitive transient receptor potential/piezo channels in odontoblasts generates action potentials in cocultured isolectinB4-negative medium sized trigeminal ganglion neurons. Journal of Endodontics, 44:984–991. 10.1016/joen.2018.02.020 [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Sato M, Kimura M, Sobhan U, Shimada M, Nishiyama A, Kawaguchi A, Soya M,, Kuroda H, Katakura A,, Ichinohe T, & Tazaki M (2015). Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast neuron signal transductiion. Pflugers Archives, 467(4):843–63. 10.1007/s00424-014-1551-x. [DOI] [PubMed] [Google Scholar]
- Silverman JE, & Kruger L (1987). An interpretation of dental innervation based on the pattern of calcitonin-related peptide (CGRP) immunoreactive thin sensory axons. Somatosensory Research 5:157–175. [DOI] [PubMed] [Google Scholar]
- Solé-Magdelena A, Marinez-Alonso M, Coronado CA, Junquera LM, Cobb J, Vega JA (2018). Molecular basis of dental sensitivity: The odontoblasts are multisensory cells and express multifunctional ion channels. Annals of Anatomy, 215:20–29. 10.1016/j.aanat.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Swift ML & Byers MR (1992). Effect of aging on responses of nerve fibers to pulpal inflammation in rat molars analyzed by quantitative immunocytochemistry. Archives of Oral Biology 37:901–912. 10.1016/0003-9969(92)90061-c. [DOI] [PubMed] [Google Scholar]
- Taddese A, Nah SY & McCleskey EW (1995). Selective opioid inhibition of small nociceptive neurons. Science 270(5240):1366–9. 10.1126/science.270.5240.1366. [DOI] [PubMed] [Google Scholar]
- Taylor PE, Byers MR, & Redd PE (1988). Sprouting of CGRP nerve fibers in response to dentine injury in rat molars. Brain Research October 4;461(2):371–6. 10.1016/0006-8993(88)90270-3 [DOI] [PubMed] [Google Scholar]
- Turner DF, Marfurt CF, & Sattelberg C (1989). Demonstration of physiological barrier between pulpal odontoblasts and its perturbation following routine restorative procedures: a horseradish peroxidase tracing study in the rat. Journal of Dental Research 68(8)1262–8, 10.1177/00220345890680081001. [DOI] [PubMed] [Google Scholar]
- Veerayutthwilai O, Byers MR, Pham T-T, T., Darveau RP, & Dale BA (2007). Differential regulation of immune responses by odontoblasts. Oral Microbiology and Immunology. February;22(1):5–13. 10.1111/j.1399-302X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Vongsavan N & Matthews B (2007). The relationship between the discharge of intradental nerves and the rate of fluid flow through dentine in the cat. Archives of Oral Biology July;52(7):640–7. 10.1016/j.archoralbio.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Anderson NL, & Byers MR (2004) Altered localization of Cav1.2(L-type) calcium channels in nerve fibers, Schwann cellse, odontoblasts, and fibroblasts of tooth pulp after injury. Journal of Neuroscience Research. 75:371–83. 10.1002/jnr.10863. [DOI] [PubMed] [Google Scholar]
- Yang H, Bernanke JM, & Naftel JP (2006). Immunocytochemical evidence that most sensory neurons of the rat molar pulp express receptors for both glial cell-line-derived neurotrophic factor and nerve growth factor. Archives of Oral Biology, 51(1):69–78. 10.1016/j.archoralbio.2005.05.002. [DOI] [PubMed] [Google Scholar]


