Abstract
The lactate dehydrogenase isoform A (LDHA) is a key metabolic enzyme that preferentially catalyzes the conversion of pyruvate to lactate. Whereas LDHA is highly expressed in many tissues, its expression is turned off in the differentiated adult β-cell within the pancreatic islets. The repression of LDHA under normal physiological condition and its inappropriate upregulation under a diabetogenic environment is well-documented in rodent islets/β-cells but little is known about LDHA expression in human islet cells and whether its abundance is altered under diabetic conditions. Analysis of public single-cell RNA-seq (sc-RNA seq) data as well as cell type-specific immunolabeling of human pancreatic islets showed that LDHA was mainly localized in human α-cells while it is expressed at a very low level in β-cells. Furthermore, LDHA, both at mRNA and protein, as well as lactate production is upregulated in human pancreatic islets exposed to chronic high glucose treatment. Microscopic analysis of stressed human islets and autopsy pancreases from individuals with type 2 diabetes (T2D) showed LDHA upregulation mainly in human α-cells. Pharmacological inhibition of LDHA in isolated human islets enhanced insulin secretion under physiological conditions but did not significantly correct the deregulated secretion of insulin or glucagon under diabetic conditions.
Keywords: LDH, LDHA, lactate, islets, beta-cell, diabetes
Graphical Abstract
Introduction
Type 2 diabetes (T2D) is a metabolic disorder closely linked to multiple genetic and environmental factors which together evoke the development of multiple pathophysiological metabolic disturbances. T2D is a bi-hormonal disorder manifested by a relative hypoinsulinaemia and hyperglucagonaemia leading eventually to hyperglycemia and diabetes and its complications [1]. Deregulated secretion of both hormones insulin and glucagon produced by pancreatic β- and α- cells respectively is a characteristic feature of T2D [1–5]. The interplay between these two hormones and their respective receptors located in the liver, muscle and adipose tissue enables the maintenance of glucose homeostasis, which is achieved via several mechanisms participating in the fine-tuning of insulin secretion [1]. Insulin secretory function of β-cells is defective in T2D with a higher basal release of insulin in fasting periods and insufficient insulin release after a meal [6]; the secretory defect of β-cells is caused by multiple factors, including chronically elevated glucose (“glucotoxicity”) [7].
A key aspect of β-cell biology is the tight coupling between cellular metabolism and insulin secretion in order to maintain systemic energy homeostasis. To achieve this, islet cells and specifically β-cells show selective repression of some key metabolic genes that otherwise would compromise its functionality, for example by including alternative metabolic pathways. Over 60 of such “disallowed” genes that are specifically repressed in β-cells in a systematic way have been identified through genome-wide mRNA expression analyses [8–13]. De-repression of such disallowed genes could be connected to the impairment of β-cell function in diabetes [10,11,13–15].
Lactate dehydrogenase (LDH) is a metabolic enzyme that catalyzes the inter-conversion of pyruvate and lactate. Its isoform LDHA, which preferentially turns pyruvate into lactate, is such disallowed gene, highly repressed in pancreatic islets/ β-cells. In addition to LDHA, the monocarboxylate transporter 1 (MCT1), a membrane protein that facilitates the transport of lactate and pyruvate across the plasma membrane, is also repressed in α- as well as β-cells [16]. The reason for the low expression of LDHA and MCT1 -as protective mechanism- is to prevent an inappropriate insulin release, triggered by circulating pyruvate or lactate, for example during exercise, which could generate hyperinsulinemia and result in hypoglycemia [11]. Importantly, previous studies have reported upregulation of LDHA in islets/ β-cells from several rodent models of diabetes, either induced by partial pancreatectomy [15,17], in the Goto-Kakizaki (GK) [18–20], Zucker diabetic fatty (ZDF) [21] and human IAPP (HIP) transgenic rats [22] as well as in obese diabetic mice and in chronically high glucose exposed rat INS-1 β-cells [23]. Consistently, mouse senescent β-cells, which have been associated with impaired glucose tolerance and diabetes, also presented elevated LDHA expression levels [24]. Also, forced overexpression of LDHA has been associated with impaired glucose-stimulated insulin secretion (GSIS), a decrease in glucose oxidation rate or unusual insulin secretion stimulated by pyruvate and lactate in pancreatic rodent β-cell lines Min6 or INS-1 [25–28]. Altogether, these studies suggest that the LDHA expression level plays a critical role in the correct channeling of pyruvate into mitochondrial metabolism and overall, a proper insulin secretion, specifically stimulated by glucose.
Although repression of LDHA has been described as key β-cell signature in rodent islets/β-cells, little is known in human islet cells and whether they share a similar LDHA de-repression response documented in mouse models of diabetes. In the present study, we performed multi-approach analyses of LDHA expression in human islet α- and β-cells under normal physiological conditions and in metabolically stressed human islets and in individuals with T2D. We also addressed the impact of pharmacological inhibition of LDHA on insulin and glucagon secretion in human islets.
Materials and methods
Islet isolation, culture, and treatment
Human pancreatic islets isolated from pancreases of nondiabetic donors were provided by the UW Health Transplant Center (University of Wisconsin), the Translational Research Laboratory for Diabetes (University of Lille), the Alberta IsletCore (University of Alberta), the San Raffaele Diabetes Research Institute (ECIT, Milan), the Laboratory for Diabetes Cell Therapy (Plateforme de Recherche Ilots Montpellier Sud (PRIMS)) and the Endocrine surgery, kidney and pancreatic transplantation unit (CHEX, UCLouvain). Islets were cultured in non-coated petri dishes (#628161, Greiner Bio One) in RPMI 1640 (#11879-020, Gibco) at 5mM glucose (UCLouvain) or on Biocoat Collagen I coated dishes (#356400, Corning, ME, USA) in full CMRL medium (Invitrogen) at 5.5 mM glucose (Uni Bremen) during 1–2 days for recovery and then exposed to increased glucose (22.2 mM) or physiological glucose (5.5 mM; control) for 24–72 hours at 5% CO2 and 37°C. For glucose-stimulated insulin secretion (GSIS) or glucose inhibited glucagon secretion assays, human islets were additionally cultured with or without (control) 10 or 20 μM LDHA-selective inhibitor GSK 2837808A (LDHAi; Tocris Bioscence, Bristol, UK) for 48 hours on Collagen I coated plates. The inhibitor was added to the medium one hour before glucose supplement. Ethical approval for the use of human islets had been granted by the Ethics Committee of the University of Bremen. The study complied with all relevant ethical regulations for work with human cells for research purposes and were performed in agreement with the local ethic committees and the institutional ethical committee of the French Agence de la Biomédecine (DC Nos. 2014–2473 and 2016–2716). Informed consent was obtained from all human islet donors’ relatives. Organ donors are not identifiable and anonymous, such approved experiments using human islet cells for research is covered by the NIH Exemption 4 (Regulation PHS 398). Human islets were distributed by the two JDRF and NIH supported approved coordination programs in Europe (Islet for Basic Research program; European Consortium for Islet Transplantation ECIT) and in the US (Integrated Islet Distribution Program IIDP) [29].
Fluorescence-activated cell sorting for gene expression measurements
Islets were trypsinized for dissociation into single cells. Dispersed cells were washed, filtered, and resuspended in a staining buffer (PBS, 1% BSA, 1 mM EDTA). Cells were stained in the dark using a combination of previously described antibodies [30] and sorted with a FACSAriaIII. The gating strategy involved exclusion of leukocytes (CD45+), acinar/ductal cells (CD44+), hematopoietic stem cells/endothelial cells (CD34+), and acinar/ductal cells (CD24+) from endocrine pan-islets cell (HPi2+), and a selective sorting of α- (TM4SF4+) and β-cells (CD9+).
Immunoblot analysis
After medium removal, human islets were washed twice with PBS and lysed with RIPA lysis buffer containing Protease and Phosphatase Inhibitors (Pierce, Rockford, IL, USA). Following the freeze-thaw cycles, the samples were incubated on ice for 30 minutes with intermittent vortexing. The lysate was centrifuged at 16000 × g for 20 minutes at 4°C and the clear supernatant containing the extracted protein was kept at −80°C until needed. The protein concentrations were determined using the BCA Protein Assay Kit (Pierce). Protein samples were fractionated by NuPAGE 4–12% Bis-Tris gel (Invitrogen) and electrically transferred into PVDF membranes. Membranes were then blocked in 2.5% non-fat dry milk (Cell signaling technology; CST) and 2.5% BSA (Sigma) for 1h at room temperature and incubated overnight at 4 °C with the following antibodies: rabbit anti-LDHA (#2012) and rabbit anti-tubulin (#2146) from CST. Primary antibodies were followed by horseradish-peroxidase-linked anti-rabbit IgG secondary antibody (Jackson). All primary antibodies were used at 1:1000 dilution in Tris-buffered saline plus Tween-20 (TBS-T) containing 5% BSA. Membrane was developed using chemiluminescence assay system (Immobilon®, Millipore) and analyzed using the VisionWorksLS image acquisition and analysis software (UVP BioImaging Systems, Upland, CA, USA).
RNA extraction, cDNA synthesis and RT-PCR analysis
Total RNA was isolated from cultured human islets using TriFast™ (peqGOLD; Peqlab) or TriPure (Roche) for FAC-sorted cells according to the manufacturer’s instructions. 500ng to 1μg of RNA were reverse transcribed using the RevertAid RT Reverse Transcription Kit (ThermoFisher) according to the manufacturer’s protocol, including removal of genomic DNA with DNase I prior to reverse transcription. Quantitative RT-PCR was performed as previously described [31]. StepOne Real-Time PCR system (Applied Biosystems, CA, USA) or CFX96 Real Time System (BioRad, CA, USA) with TaqMan® Fast Universal PCR Master Mix for TaqMan assays (Applied Biosystems) were used for analysis. TaqMan® Gene Expression Assays were used for human LDH-A (#Hs01378790_g1), PPiA (#Hs99999904_m1), ACTB (#Hs01060665_g1), GCG (#Hs01031536_m1), and INS (#Hs02741908_m1). qPCR was performed and analyzed by the Applied Biosystems StepOne or the BioRad CFX96 Real-Time Systems. The ΔΔCT or ΔCT methods were used to analyze the relative changes in gene expression.
Lactate assay
At the end of the incubation periods, culture media collected from human islets left untreated or treated with high glucose (22.2 mM) and their corresponding control culture media were subjected to deproteinization using 10kD spin columns (#ab93349 Abcam, Cambridge, UK), in order to minimize the interference of islet-released LDH into the media which could degrade lactate during the lactate assay and kept at −80° C before analysis. Lactate concentration was determined by using a coupled enzymatic assay containing lactate dehydrogenase (LDH) and glutamate pyruvate transaminase (GPT; both Roche Diagnostics, Mannheim, Germany) as previously described in detail [32]. Briefly, aliquote volumes (10 μL or 20 μL) of the media were diluted with pure water to 180 μL in a well of a microtiter plate. To each well, 180 μL of a freshly prepared lactate reaction mixture (5.6 mM NAD+, 3.89 U/mL GPT and 39.7 U/mL LDH in 0.5 M glutamate/NaOH buffer, pH 8.9) was added and the microtiter plate was incubated in a humidified atmosphere of an incubator at 37°C for 90 min before the absorbance of the generated NADH at 340 nm was determined. Due to the 1:1 stoichiometry between lactate oxidation and NADH formation in this assay, the lactate concentration can be calculated by the Lambert-Beer law from the absorbance of NADH using the extinction coefficient of 6.2 mM−1cm−1. The basal lactate content of media that had no contact with islets (1.5 mM) was substracted from the media that had been harvested from the islets to determine the concentration of lactate released from the cells during the 72 h of incubation.
Immunostaining
Human pancreatic sections from nondiabetic and T2D human donors were provided from the National Disease Research Interchange (NDRI). PFA-fixed paraffin-embedded pancreatic sections and bouin-fixed human islets exposed to physiological (5.5 mM) or increased glucose (22.2 mM) were deparaffinized, rehydrated as described before [31] and incubated overnight at 4°C with LDHA rabbit polyclonal antibody (#ab125683; Abcam) or for 2 h at room temperature with guinea pig anti-insulin (#A0546; Dako) or mouse anti-glucagon (#ab10988; Abcam), followed by fluorescein isothiocyanate (FITC)- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, PA, USA). The slides were mounted using mounting medium with DAPI (Vectashield®, Vector Labs). Immunostaining was analyzed using a Nikon MEA53200 (Nikon, Dusseldorf, Germany) microscope, and images were obtained using NIS-Elements imaging software version 3.22.11 (Nikon) and overlays created using ImageJ.
Insulin and glucagon secretion
Human islets (20–30 islets/dish) cultured on collagen-coated plates and treated with the LDHA-selective inhibitor were used for a glucose-stimulated insulin secretion assay performed as described before [31] or glucose inhibition of glucagon secretion. Islets were washed with PBS and preincubated with Krebs-Ringer bicarbonate buffer (KRB) containing 2.8 mM (insulin) or 1 mM glucose (glucagon) at 37° C for 30 minutes followed by fresh KRB containing 2.8 mM (insulin; basal) or 1 mM glucose (glucagon) for 1h and additional 1h in KRB containing 16.7 mM (insulin; stimulated) or 20 mM glucose (glucagon). Islets were washed with PBS and lysed with RIPA buffer for measuring total insulin or glucagon content. Insulin levels were detected by human insulin ELISA (ALPCO, Diagnostics). Glucagon was determined by the MSD Metabolic Assays/ Human Glucagon Kit (#K151HCC-1; Meso Scale, Gaithersburg, MD, USA). Secreted insulin or glucagon was normalized to insulin or glucagon content, respectively.
Statistical analysis
The data are given as means ± SEM. To identify statistically significant differences, two-tailed student’s t-test was conducted when data of at least 3 independent experiments were available. p value < 0.05 was considered statistically significant.
Results and discussion
LDHA is enriched in human pancreatic alpha cells
Due to the multi cell-type composition as well as cellular heterogeneity of human pancreatic islets, targeted cell-based analysis of the transcriptome using RNA-seq provides a valuable resource for the islet research community [33]. Bramswig et al. [34] previously established RNA-seq analysis to determine the transcriptional profiles of sorted α- and β-cell populations. Based on their cluster analysis across different cell types, LDHA is expressed nearly six times more in mature human α-cells compared to β-cells (Fig. 1A). To assess the developmental changes in LDHA expression in human islets, we analyzed LDHA expression in the RNA-seq dataset derived from purified fetal as well as adult α- and β-cells [35]. We found that fetal human α-cells expressed 1.5-fold higher LDHA levels compared to β-cells. However, while stable in α-cells at the adult stage, the expression levels of LDHA were dramatically reduced in human β-cells, LDHA was 15-fold higher expressed in α-cells in comparison to β-cells (Fig. 1B). Thus, the repression of LDHA in β-cells seems to occur from fetal-to adult development, and it is inversely correlated with β-cell maturation and regulated insulin secretion. To further support the α-cell enriched LDHA expression levels, we used several independent recent single-cell RNA-seq (scRNA-seq) datasets of human islets [36–39]. Analysis of such data across different studies confirmed that LDHA is enriched in human α-cells, compared to β-cells (Fig. 1C). Thus, an overall low expression of LDHA in islets can be explained by the lower percentage of α-cells present in islets, compared to β-cells and the strong repression of LDHA within β-cells.
Figure 1. LDHA is predominantly expressed in islet human alpha cells.
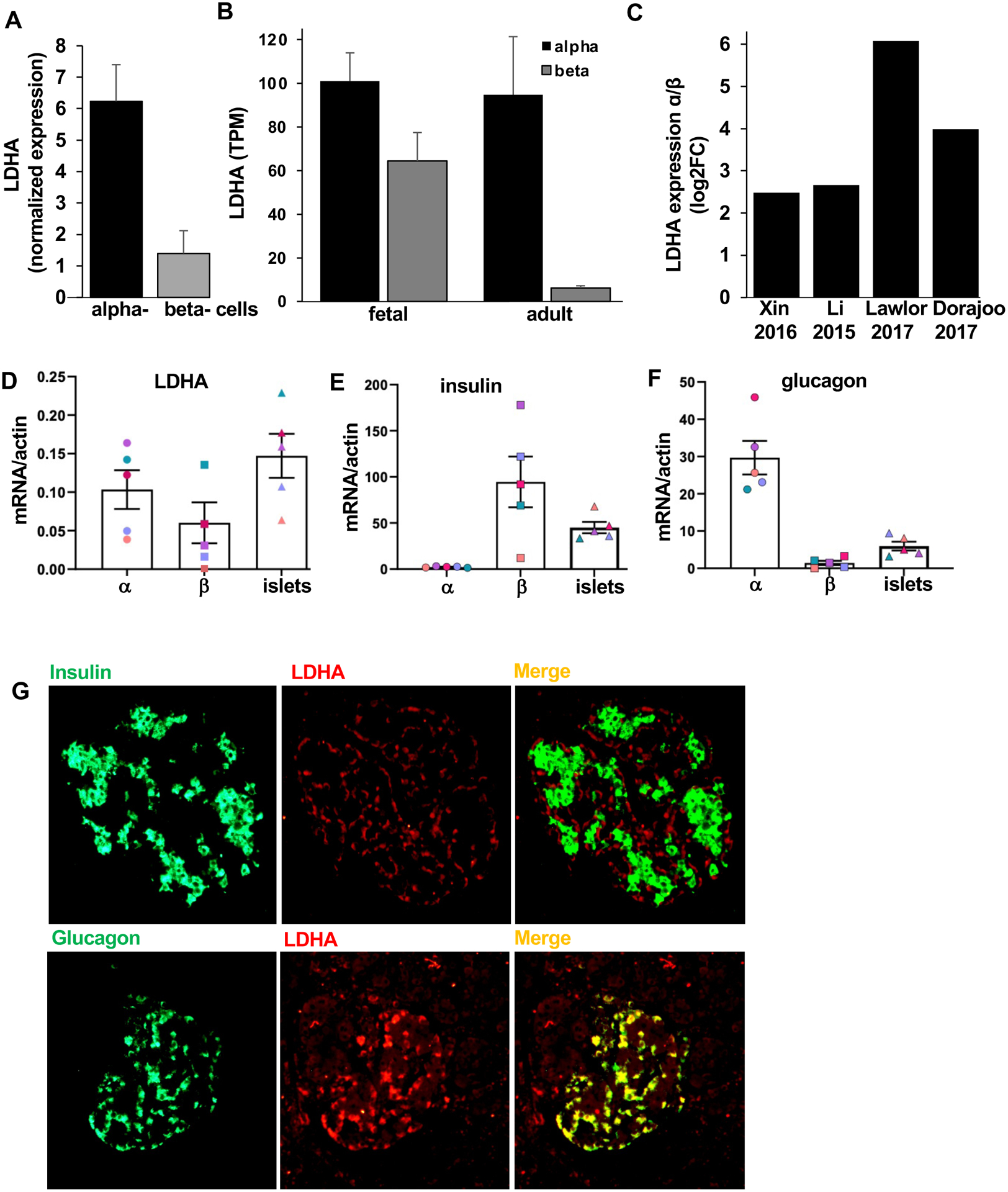
LDHA transcript expression obtained from public available RNA-seq data sets (A) of sorted human α- or β-cells obtained from the analysis from Bramswig et al. , (B) of sorted human fetal as well as adult α- or β-cells from Blodget et al. and (C) of human α- and β-cells expressed as log2FC α/β ratio from Xin et al, Li et al, Lawlor et al and Dorajoo et al. (D-F) LDHA, insulin, glucagon and gene expression in human FAC-sorted α- and β-cells and pancreatic islets normalized to actin (n=5 different human islet isolations from different donors). (E,F) Validation of the population purity through insulin and glucagon expression had been previously performed in 4 out of 5 isolations and thus, results from Fig. 5J,K of our previous publication [30] are included in these panels.
(G) Representative double-stainings for insulin (green; upper panel), or glucagon (green; lower panel), and LDHA (red) are shown from human pancreatic sections from autopsy from patients with T2D (n=3). Data are expressed as means ± SEM.
To corroborate the results from the scRNA-seq data, we performed qPCR analysis on sorted α- and β-cells from five different human islet preparations. LDHA mRNA expression was higher in α-cells than β-cells in 4 out 5 analyzed human islet isolations (Fig. 1D). The two endocrine populations were validated by insulin and glucagon mRNA, which were almost exclusively expressed in β-cell and α-cell fractions, respectively, as shown before [30]; (Fig. 1E,F). Additionally, the cellular source of LDHA protein expression in human pancreatic islets was investigated through double-immunostaining of pancreatic autopsy samples from patients with T2D. In line with gene expression data, LDHA immunodetection revealed a strong colocalization of LDHA with glucagon-positive α-cells but not insulin-positive β-cells. Altogether, these data show that the human α-cell, but not the β-cell is the major source of LDHA expression in the human islets.
LDHA is upregulated in human islets in T2D
To investigate whether LDHA is upregulated in metabolically stressed human islets, they were exposed to high glucose concentrations for different periods of time. Notably, upregulation of LDHA mRNA was evident under high glucose at 24 or 72 hours compared to control (Fig. 2A). Also, human islets exposed to high glucose for 72 hours exhibited significantly increased LDHA protein levels (Fig. 2B,C). LDHA is the isoform that has been shown to preferentially catalyze the conversion of pyruvate into lactate [40]. Consistently, the levels of lactate released by human islets cultured under high glucose were significantly higher than the levels from the control, confirming an increased activity of LDHA in human islets under glucotoxic condition (Fig. 2D). Such marked upregulation of both LDHA mRNA and protein observed under hyperglycemia in human islets supports the existing evidence of LDHA “derepression” as possible consequence of hyperglycemia in pancreatic islets [11,15,17].
Figure 2. LDHA is upregulated in human alpha cells.
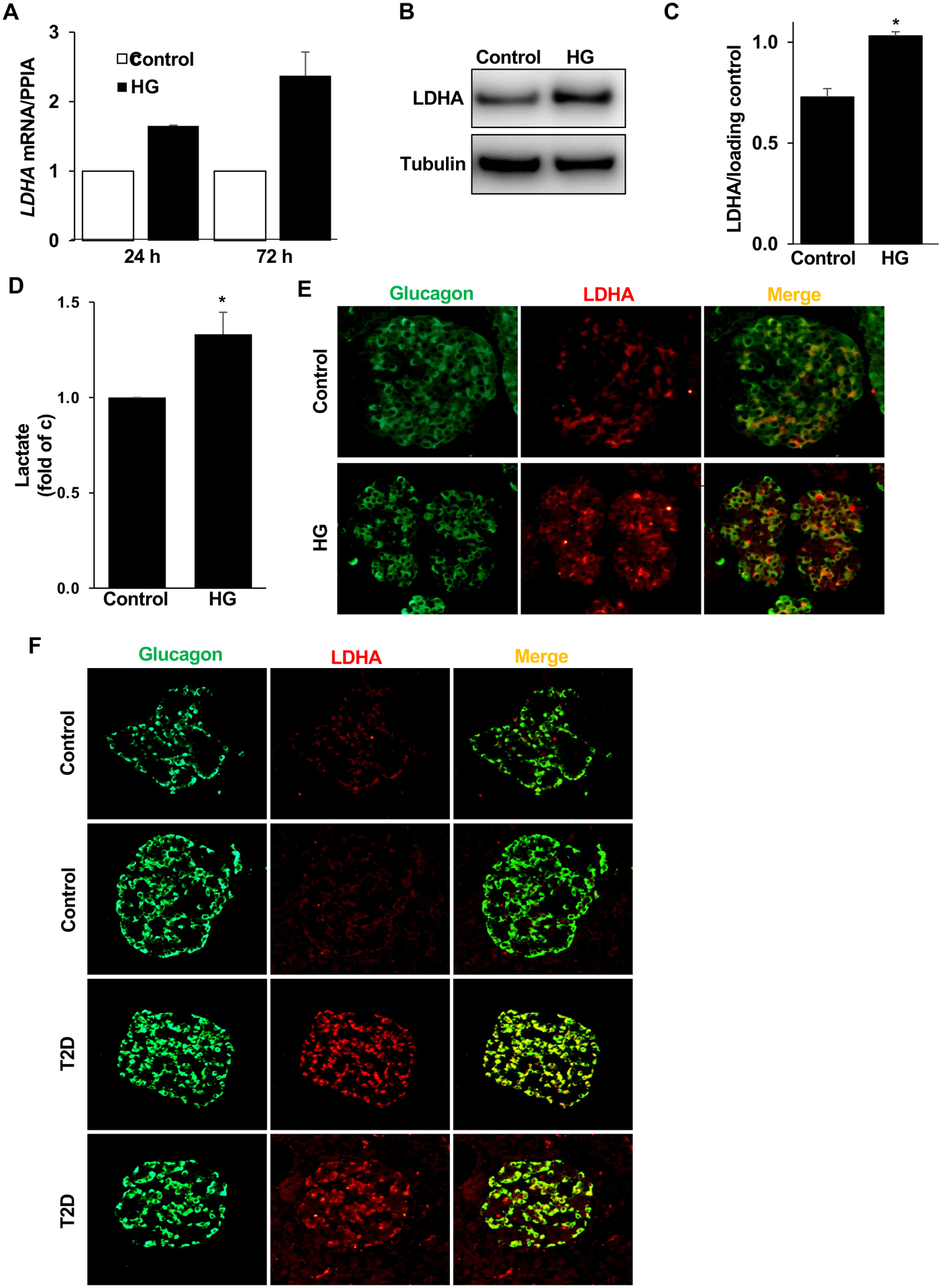
(A) qPCR for LDHA mRNA expression in isolated human islets treated with high glucose (HG; 22 mM) for 1 or 3 days normalized to PPIA (n=2 different human islet isolations). (B,C) Representative Western blots (B) and quantitative densitometry analysis (C) of isolated human islets treated with high glucose (HG; 22 mM) for 3 days (n=3 different human islet isolations). (D) Lactate levels in culture media collected from isolated human islets treated with high glucose (HG; 22 mM) for 3 days normalized to control conditions (n=4 different human islet isolations from 4 different donors). (E) Representative double-stainings for LDHA (red) and glucagon (green) shown from isolated human islets left untreated (cont) or treated with high glucose (HG; 22 mM) for 3 days. (F) Two sets of representative double-stainings for LDHA (red) and glucagon (green) are shown from human pancreatic sections from autopsy from nondiabetic controls (n=3) or from donors with type 2 diabetes (T2D) (n=3). Data are expressed as means ± SEM. *p<0.05 compared to untreated control.
Considering the heterogeneous composition of the islets in the pancreas, it is important to determine a cellular source of LDHA upregulation and its correlation to T2D. Bouin-fixed isolated human islets sections exposed to physiological (5.5 mM; control) or increased glucose (22.2 mM) were double immunostained for LDHA and glucagon. Again, LDHA colocalized with glucagon-expressing α-cells and was upregulated under glucotoxic conditions (Fig. 2E) confirming our expression data from bulk human islets. This suggests that basal as well as glucose-induced LDHA expression mainly occurred in α-cells. Pancreatic autopsy samples from T2D patients and controls were also double-stained to check whether α-cells from donors with established T2D also show LDHA upregulation. Also here, LDHA was found highly colocalized with glucagon-expressing α-cells and was markedly upregulated in the T2D pancreatic sections as compared to nondiabetic-control pancreases (Fig. 2F). Consistent with our finding, a study carried out by Lawlor et al. [37] has recently shown an upregulation of LDHA mRNA levels in human α-cells in T2D compared to control. Also, transcriptome profiling of a β-cell enriched fraction obtained by laser capture microdissection from individuals with T2D showed an increase of LDHA expression levels [41]; however, a purified α-cell fraction was not investigated in this work.
Similar to α-cell dysfunction in T2D [1,5], glucagon secretion is noticeably impaired in type 1 diabetes (T1D), which contributes to the susceptibility of patients to hypoglycemia [42,43]. Importantly, single-cell transcriptomes from cryo-preserved human islets isolated from T1D patients showed an elevated expression of LDHA transcript in α-cells compared to controls matched for BMI, age, sex, and storage time [44] suggesting a similar pathologic response might exist in the human islet α-cells under hyperglycemic T1D conditions.
Altogether, these findings indicate that upregulation of LDHA in stressed human islets ex vivo as well as in human T2D pancreatic islets occurred mainly in α-cells rather than in β-cells.
Impact of LDHA inhibition on the human islet hormones secretion
It has been proposed that impaired insulin secretion observed under hyperglycemic conditions is correlated with a metabolic shift involving upregulation of LDHA [19,20]. Having confirmed the upregulation of LDHA in diabetic human islets, the impact of chemical inhibition of LDHA on islet function was investigated in human islets exposed to chronically elevated glucose. The selective LDHA inhibitor GSK2837808A (LDHAi) has previously successfully been used to target upregulated glycolysis as a metabolic hallmark of cancer cells [45–48]. LDHAi clearly decreases lactate release and increases glucose consumption in cancer cells [47]. Isolated human islets were exposed to 10 or 20 μM of LDHAi and then cultured with increased glucose concentrations for 48 hours. Chronic high glucose exposure strongly abolished glucose-induced insulin secretion (Fig. 3A,B). Notably, the high glucose treated human islets presented a clear and robust increase in the basal insulin secretion phenocopying basal hyperinsulinemia triggered by exhausted dysregulated β-cells as a well-accepted feature of obese prediabetic as well as obese T2D patients [49,50].
Figure 3. Impact of LDHA inhibition on insulin and glucagon secretion.
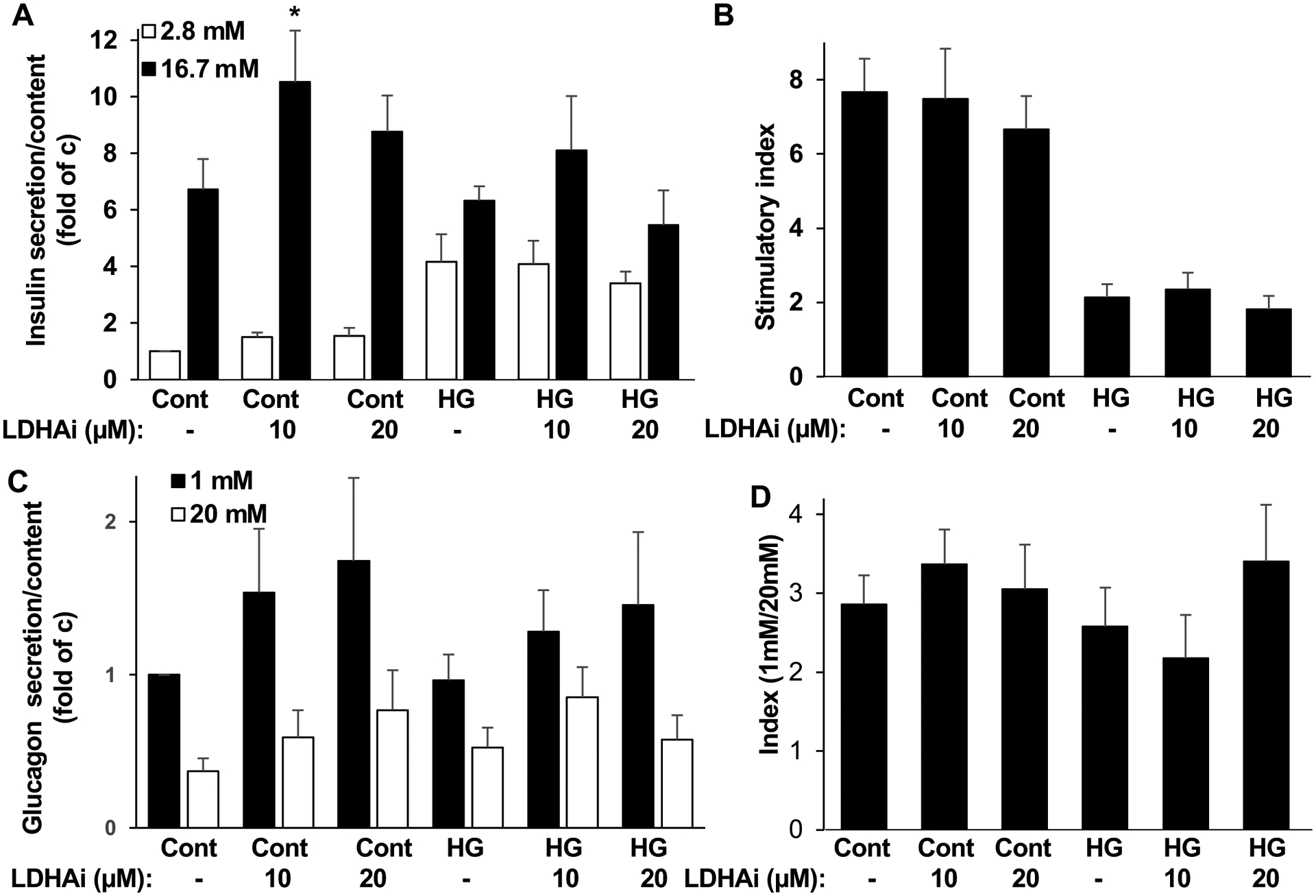
Isolated human islets treated with or without (control: cont) 10 or 20 μM LDHA inhibitor GSK2837808A (LDHAi) 1 hour before and during the 48 hour exposure to physiological glucose (5.5 mM; cont) or to high glucose (22 mM; HG). Thereafter, (A) Insulin secretion was analyzed during 1-h incubation with 2.8 mM (basal) and 16.7 mM (stimulated) glucose normalized to insulin content, (B) the insulin stimulatory index denotes the ratio of secreted insulin during 1-h incubation with 16.7 mM to secreted insulin at 2.8 mM glucose. (C) In a parallel set of islets, glucagon secretion was analysed during 1-h incubation with 1 mM and 20 mM glucose normalized to glucagon content, (D) the glucagon secretory index denotes the ratio of secreted glucagon during 1-h incubation with 1 mM to secreted glucagon at 20 mM glucose. A-D (n=8–9; from 3 different human islet isolations). Data are expressed as means ± SEM. *p<0.05 compared to untreated stimulated control.
LDHA inhibition by LDHAi, at 10 μM, increased glucose stimulated insulin secretion under physiological glucose concentrations and also revealed a tendency of increased glucose stimulated insulin secretion in islets under glucotoxic condition (Fig. 3A), but it did otherwise not show any significant impact on insulin secretion and had no effect on the stimulatory index on human islets exposed to high glucose (Fig. 3B).
The regulation of glucagon secretion is complex, multifactorial and includes cell intrinsic and paracrine elements [1,5,51]. As LDHA is enriched and upregulated in α-cells in T2D and glucagon physiologically released from α-cells potently participates in the regulation of glycemia, LDHAi was used to investigate its impact on glucagon release in human islets exposed to hyperglycemia. LDHAi showed a tendency of increased glucagon secretion at 1mM low glucose, both in islets chronically exposed to physiological as well as elevated glucose, however, glucagon secretion showed a high variability and neither of the effects could reach significance in our setting suggesting that changes in LDHA might be dispensable for glucagon secretion in human islets.
In summary we show that LDHA belongs to the group of “disallowed genes” in human β-cells; it is predominantly expressed in α-cells and its level is highly elevated in islets/ α-cells in metabolically stressed human islets as well as in pancreases from donors with T2D.
Previous work has suggested a link between de-repressed LDHA and β-cell dysfunction in T2D [11,15,17]. Although the mechanisms underlying this connection were not fully elucidated, a number of attempts to block the activity of LDHA in islets/β-cells have been carried out. Initially, Sasaki et al. showed that neutralizing diabetes-associated elevation of reactive oxygen species (ROS) in rat diabetic GK islets by antioxidants counteracts the upregulation of the major metabolic transcription factor HIF1α and its downstream target genes including LDHA. Consequently, lactate production decreased, whereas insulin secretion improved [19]. Also, in vivo treatment of obese diabetic db/db mice with the LDHA inhibitor oxamate, an analog of pyruvate showed a significant restoration in metabolic parameters such as fasted blood glucose, insulin sensitivity and insulin secretion as well as of pancreatic islets morphology [52]. The elevated glucose level has not only been associated with differential expression of LDHA but also with an overall alteration of the coordinated expression of genes involved in glycolytic and mitochondrial metabolism, generating a metabolic shift observed at an early stage of T2D [20]. In contrast and under physiological conditions, current data show that LDHB, the other LDH isoform, is highly expressed in human β-cells and has been acknowledged as a “β-cell signature gene” [37,39] that favors the internal lactate to pyruvate flux in order to preserve and maximize, when needed, mitochondrial transport, oxidative phosphorylation and subsequent insulin secretion. Thus, these studies suggest that elevated LDHA and/or potentially declined LDHB and subsequent lactate overproduction could potentially associate with T2D as it might compromise β-cell mitochondrial oxidative phosphorylation and subsequent insulin secretion by lowering the level of pyruvate and reducing equivalents in mitochondria. It is important to note that the aforementioned studies tested LDHA inhibition in the rodent diabetic models but not in human islets. Our data show that LDHA inhibition moderately enhanced stimulated insulin secretion under physiological conditions but overall did not restore β-cell function or corrected glucagon secretion under high glucose conditions. However, in depth studies including a greater number of human islet preparations -as we were only able to perform the LDHA inhibition experiment in three independent isolations- as well as dynamic perfusion analysis of insulin and glucagon release ex vivo or in vivo is required for a further detailed evaluation of a possible causative role of LDHA in the islet secretory dysfunction and diabetes progression.
Supplementary Material
Highlights.
LDHA is localized in α-cells and only minimally in β-cells in human islets.
LDHA expression and lactate production are upregulated by glucotoxicity.
LDHA is upregulated in α-cells in T2D.
LDHA inhibition stimulates insulin secretion.
Acknowledgments
We thank Julie Kerr-Conte and Francois Pattou (European Genomic Institute for Diabetes, Lille) and Christophe Broca (Laboratory for Diabetes Cell Therapy (Plateforme de Recherche Ilots Montpellier Sud (PRIMS)) for high quality human islet isolations, Yvonne Koehler (University of Bremen) for competently determining the lactate concentrations in the incubation media, Katja Thode (Boehringer Ingelheim) and Katrischa Hennekens (University of Bremen) for excellent technical assistance and Petra Schilling (University of Bremen) for pancreas sectioning. Human pancreatic sections were provided from the National Disease Research Interchange (NDRI), supported by the NIH.
Funding
This work was supported by JDRF and the German Research Foundation (DFG). Human pancreatic islets were kindly provided by the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope, NIH Grant # 2UC4DK098085, the JDRF-funded IIDP Islet Award Initiative and through the ECIT Islet for Basic Research program supported by JDRF (JDRF award 31-2008-413 and 31-2008-416) and the Leona M. & Harry B. Helmsley Charitable Trust. P.G. is Research Director and E.G. is Postdoctoral Researcher of the Fonds National de la Recherche Scientifique, Brussels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no conflict of interests.
Data availability
Raw Western Blots are included in this submission. All data are included in the manuscript and will be further made available upon request.
References
- [1].Gromada J, Chabosseau P, Rutter GA, The alpha-cell in diabetes mellitus, Nat Rev Endocrinol 14 (2018) 694–704. 10.1038/s41574-018-0097-y. [DOI] [PubMed] [Google Scholar]
- [2].Briant L, Salehi A, Vergari E, Zhang Q, Rorsman P, Glucagon secretion from pancreatic alpha-cells, Ups J Med Sci 121 (2016) 113–119. 10.3109/03009734.2016.1156789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ashcroft FM, Rorsman P, Diabetes mellitus and the beta cell: the last ten years, Cell 148 (2012) 1160–1171. 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Holman RR, Clark A, Rorsman P, beta-cell secretory dysfunction: a key cause of type 2 diabetes, Lancet Diabetes Endocrinol 8 (2020) 370. 10.1016/S2213-8587(20)30119-4. [DOI] [PubMed] [Google Scholar]
- [5].Gilon P, The Role of alpha-Cells in Islet Function and Glucose Homeostasis in Health and Type 2 Diabetes, J Mol Biol 432 (2020) 1367–1394. 10.1016/j.jmb.2020.01.004. [DOI] [PubMed] [Google Scholar]
- [6].Nagaraj V, Kazim AS, Helgeson J, Lewold C, Barik S, Buda P, Reinbothe TM, Wennmalm S, Zhang E, Renstrom E, Elevated Basal Insulin Secretion in Type 2 Diabetes Caused by Reduced Plasma Membrane Cholesterol, Mol Endocrinol 30 (2016) 1059–1069. 10.1210/me.2016-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weir GC, Bonner-Weir S, Five stages of evolving beta-cell dysfunction during progression to diabetes, Diabetes 53Suppl 3 (2004) S16–21. [DOI] [PubMed] [Google Scholar]
- [8].Quintens R, Hendrickx N, Lemaire K, Schuit F, Why expression of some genes is disallowed in beta-cells, Biochem Soc Trans 36 (2008) 300–305. 10.1042/BST0360300. [DOI] [PubMed] [Google Scholar]
- [9].Pullen TJ, Khan AM, Barton G, Butcher SA, Sun G, Rutter GA, Identification of genes selectively disallowed in the pancreatic islet, Islets 2 (2010) 89–95. 10.4161/isl.2.2.11025. [DOI] [PubMed] [Google Scholar]
- [10].Thorrez L, Laudadio I, Van Deun K, Quintens R, Hendrickx N, Granvik M, Lemaire K, Schraenen A, Van Lommel L, Lehnert S, Aguayo-Mazzucato C, Cheng-Xue R, Gilon P, Van Mechelen I, Bonner-Weir S, Lemaigre F, Schuit F, Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation, Genome Res 21 (2011) 95–105. 10.1101/gr.109173.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schuit F, Van Lommel L, Granvik M, Goyvaerts L, de Faudeur G, Schraenen A, Lemaire K, beta-cell-specific gene repression: a mechanism to protect against inappropriate or maladjusted insulin secretion?, Diabetes 61 (2012) 969–975. 10.2337/db11-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Martinez-Sanchez A, Nguyen-Tu MS, Rutter GA, DICER Inactivation Identifies Pancreatic beta-Cell “Disallowed” Genes Targeted by MicroRNAs, Mol Endocrinol 29 (2015) 1067–1079. 10.1210/me.2015-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lemaire K, Granvik M, Schraenen A, Goyvaerts L, Van Lommel L, Gomez-Ruiz A, In ‘t Veld P, Gilon P, Schuit F, How stable is repression of disallowed genes in pancreatic islets in response to metabolic stress?, PLoS One 12 (2017) e0181651. 10.1371/journal.pone.0181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, Girotti M, Marie S, MacDonald MJ, Wollheim CB, et al. , Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing, J Biol Chem 269 (1994) 4895–4902. [PubMed] [Google Scholar]
- [15].Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC, Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia, J Biol Chem 277 (2002) 10912–10921. 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- [16].Zhao C, Wilson MC, Schuit F, Halestrap AP, Rutter GA, Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas, Diabetes 50 (2001) 361–366. 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- [17].Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC, Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes, J Biol Chem 274 (1999) 14112–14121. 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- [18].Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, Halban P, Portha B, Serradas P, Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat, Diabetes 55 (2006) 1625–1633. [DOI] [PubMed] [Google Scholar]
- [19].Sasaki M, Fujimoto S, Sato Y, Nishi Y, Mukai E, Yamano G, Sato H, Tahara Y, Ogura K, Nagashima K, Inagaki N, Reduction of reactive oxygen species ameliorates metabolism-secretion coupling in islets of diabetic GK rats by suppressing lactate overproduction, Diabetes 62 (2013) 1996–2003. 10.2337/db12-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hou J, Li Z, Zhong W, Hao Q, Lei L, Wang L, Zhao D, Xu P, Zhou Y, Wang Y, Xu T, Temporal Transcriptomic and Proteomic Landscapes of Deteriorating Pancreatic Islets in Type 2 Diabetic Rats, Diabetes 66 (2017) 2188–2200. 10.2337/db16-1305. [DOI] [PubMed] [Google Scholar]
- [21].Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF, Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes, Diabetes 55 (2006) 2965–2973. 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- [22].Montemurro C, Nomoto H, Pei L, Parekh VS, Vongbunyong KE, Vadrevu S, Gurlo T, Butler AE, Subramaniam R, Ritou E, Shirihai OS, Satin LS, Butler PC, Tudzarova S, IAPP toxicity activates HIF1alpha/PFKFB3 signaling delaying beta-cell loss at the expense of beta-cell function, Nat Commun 10 (2019) 2679. 10.1038/s41467-019-10444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sacco F, Seelig A, Humphrey SJ, Krahmer N, Volta F, Reggio A, Marchetti P, Gerdes J, Mann M, Phosphoproteomics Reveals the GSK3-PDX1 Axis as a Key Pathogenic Signaling Node in Diabetic Islets, Cell Metab 29 (2019) 1422–1432 e1423. 10.1016/j.cmet.2019.02.012. [DOI] [PubMed] [Google Scholar]
- [24].Aguayo-Mazzucato C, Andle J, Lee TB Jr., Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S, Acceleration of beta Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes, Cell Metab 30 (2019) 129–142 e124. 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao C, Rutter GA, Overexpression of lactate dehydrogenase A attenuates glucose-induced insulin secretion in stable MIN-6 beta-cell lines, FEBS Lett 430 (1998) 213–216. 10.1016/s0014-5793(98)00600-0. [DOI] [PubMed] [Google Scholar]
- [26].Ishihara H, Wang H, Drewes LR, Wollheim CB, Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells, J Clin Invest 104 (1999) 1621–1629. 10.1172/JCI7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ainscow EK, Zhao C, Rutter GA, Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion, Diabetes 49 (2000) 1149–1155. 10.2337/diabetes.49.7.1149. [DOI] [PubMed] [Google Scholar]
- [28].Alcazar O, Tiedge M, Lenzen S, Importance of lactate dehydrogenase for the regulation of glycolytic flux and insulin secretion in insulin-producing cells, Biochem J 352Pt 2 (2000) 373–380. [PMC free article] [PubMed] [Google Scholar]
- [29].Hart NJ, Powers AC, Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions, Diabetologia 62 (2019) 212–222. 10.1007/s00125-018-4772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chae H, Augustin R, Gatineau E, Mayoux E, Bensellam M, Antoine N, Khattab F, Lai BK, Brusa D, Stierstorfer B, Klein H, Singh B, Ruiz L, Pieper M, Mark M, Herrera PL, Gribble FM, Reimann F, Wojtusciszyn A, Broca C, Rita N, Piemonti L, Gilon P, SGLT2 is not expressed in pancreatic alpha- and beta-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans, Mol Metab 42 (2020) 101071. 10.1016/j.molmet.2020.101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ardestani A, Paroni F, Azizi Z, Kaur S, Khobragade V, Yuan T, Frogne T, Tao W, Oberholzer J, Pattou F, Conte JK, Maedler K, MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes, Nat Med 20 (2014) 385–397. 10.1038/nm.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R, Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. , Neuromethods 90: Brain Energy Metabolism (2014) 45–72. [Google Scholar]
- [33].Wang YJ, Kaestner KH, Single-Cell RNA-Seq of the Pancreatic Islets--a Promise Not yet Fulfilled?, Cell Metab 29 (2019) 539–544. 10.1016/j.cmet.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH, Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming, J Clin Invest 123 (2013) 1275–1284. 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ, Kim S, Kucukural A, Davis RJ, Kent SC, Greiner DL, Garber MG, Harlan DM, diIorio P, Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets, Diabetes 64 (2015) 3172–3181. 10.2337/db15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dorajoo R, Ali Y, Tay VSY, Kang J, Samydurai S, Liu J, Boehm BO, Single-cell transcriptomics of East-Asian pancreatic islets cells, Sci Rep 7 (2017) 5024. 10.1038/s41598-017-05266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lawlor N, George J, Bolisetty M, Kursawe R, Sun L, Sivakamasundari V, Kycia I, Robson P, Stitzel ML, Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes, Genome Res 27 (2017) 208–222. 10.1101/gr.212720.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li J, Klughammer J, Farlik M, Penz T, Spittler A, Barbieux C, Berishvili E, Bock C, Kubicek S, Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types, EMBO Rep 17 (2016) 178–187. 10.15252/embr.201540946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Lin C, Gromada J, RNA Sequencing of Single Human Islet Cells Reveals Type 2 Diabetes Genes, Cell Metab 24 (2016) 608–615. 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- [40].Doherty JR, Cleveland JL, Targeting lactate metabolism for cancer therapeutics, J Clin Invest 123 (2013) 3685–3692. 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Marchetti P, Weir GC, Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes, PLoS One 5 (2010) e11499. 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ, Cheng P, Beck RW, Diabetes N Research in Children, Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis, Diabetes Care 37 (2014) 1741–1744. 10.2337/dc13-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, Bottino R, Campbell-Thompson M, Aramandla R, Poffenberger G, Lindner J, Pan FC, von Herrath MG, Greiner DL, Shultz LD, Sanyoura M, Philipson LH, Atkinson M, Harlan DM, Levy SE, Prasad N, Stein R, Powers AC, alpha Cell Function and Gene Expression Are Compromised in Type 1 Diabetes, Cell Rep 22 (2018) 2667–2676. 10.1016/j.celrep.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Camunas-Soler J, Dai XQ, Hang Y, Bautista A, Lyon J, Suzuki K, Kim SK, Quake SR, MacDonald PE, Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes, Cell Metab 31 (2020) 1017–1031 e1014. 10.1016/j.cmet.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Thongon N, Zucal C, D’Agostino VG, Tebaldi T, Ravera S, Zamporlini F, Piacente F, Moschoi R, Raffaelli N, Quattrone A, Nencioni A, Peyron JF, Provenzani A, Cancer cell metabolic plasticity allows resistance to NAMPT inhibition but invariably induces dependence on LDHA, Cancer Metab 6 (2018) 1. 10.1186/s40170-018-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Massey AJ, Modification of tumour cell metabolism modulates sensitivity to Chk1 inhibitor-induced DNA damage, Sci Rep 7 (2017) 40778. 10.1038/srep40778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hanse EA, Ruan C, Kachman M, Wang D, Lowman XH, Kelekar A, Cytosolic malate dehydrogenase activity helps support glycolysis in actively proliferating cells and cancer, Oncogene 36 (2017) 3915–3924. 10.1038/onc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lin S, Sun L, Lyu X, Ai X, Du D, Su N, Li H, Zhang L, Yu J, Yuan S, Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5–CCR5 axis: a positive metabolic feedback loop, Oncotarget 8 (2017) 110426–110443. 10.18632/oncotarget.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Corkey BE, Banting lecture 2011: hyperinsulinemia: cause or consequence?, Diabetes 61 (2012) 4–13. 10.2337/db11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Erion K, Corkey BE, beta-Cell Failure or beta-Cell Abuse?, Front Endocrinol (Lausanne) 9 (2018) 532. 10.3389/fendo.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, Asterholm IW, Benrick A, Briant LJB, Chibalina MV, Gribble FM, Hamilton A, Hastoy B, Reimann F, Rorsman NJG, Spiliotis II, Tarasov A, Wu Y, Ashcroft FM, Rorsman P, Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion, Nat Commun 10 (2019) 139. 10.1038/s41467-018-08193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ye W, Zheng Y, Zhang S, Yan L, Cheng H, Wu M, Oxamate Improves Glycemic Control and Insulin Sensitivity via Inhibition of Tissue Lactate Production in db/db Mice, PLoS One 11 (2016) e0150303. 10.1371/journal.pone.0150303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


