Abstract
Vaccination serves as a cornerstone of global health. Successful prevention of infection or disease by vaccines is achieved through elicitation of pathogen-specific antibodies and long-lived memory T cells. However, a number of microbial threats to human health have proven refractory to past vaccine efforts. These shortcomings have been attributed to either inefficient triggering of memory T- and B-cell responses or to the unfulfilled need to stimulate non-conventional forms of immunological memory. Natural killer (NK) cells have recently emerged as both critical regulators of vaccine-elicited T- and B-cell responses and also as memory cells that contribute to pathogen control. Herein we discuss potential methods to modulate these functions of NK cells to enhance vaccine success.
Keywords: Innate lymphoid cells, immunoregulation, immunization, cytotoxicity, Tfh, adjuvants
Emerging role for NK cells in vaccine success
Licensed vaccines span the spectrum from live, attenuated microbes, to recombinant microbial proteins and nucleic acid-based delivery systems, each designed to educate the immune system by stimulating pathogen-specific T and B cells. The resulting antibodies and cellular immune memory, often bolstered by periodic re-vaccination (i.e. boosting), provide lasting protection against infection. Yet, the dearth of effective vaccines against a number of formidable threats to human health, including human immunodeficiency virus (HIV), highlights the shortcomings of the currently available immunization strategies for triggering adequate immune responses against the gamut of microbial menaces. Two of the prevailing hypotheses guiding strategies to overcome these deficits hold that next generation vaccines must: (1) overcome immunoregulatory roadblocks limiting the magnitude, character, and quality of adaptive immune responses; (2) efficiently engage other arms (e.g. innate immunity) of the immune system necessary for protection.
Natural killer (NK) cells are innate lymphocytes that have emerged as innovative vaccine targets owing to their capacity to stringently regulate adaptive immune responses [1], generate an unconventional form of innate immune memory [2], and functionally cooperate with other vaccine-elicited components (e.g. antibodies) to prevent infection [3]. A variety of germline encoded activating and inhibitory receptors dictate the capacity of NK cells to secrete interferon (See Glossary) gamma (IFN-γ) and mediate cytolytic elimination of tumors and virus-infected cells (Box 1). In addition to these conventional activities, NK-cell expression of IFN-γ and stimulation of antigen-presenting cells can boost vaccine-induced adaptive immunity [4, 5]. In contrast, cytolytic activities of NK cells can both reduce vaccine antigen persistence and eliminate responding T cells [4]. This latter immunoregulatory mechanism implicates NK cells in repression of adaptive immune memory and evolution of antibody responses [6, 7]. Vaccination and infection can also shape the repertoire of NK cells, in some cases favoring hyperfunctional subsets of NK cells more capable of fighting pathogens [2, 8]. In this review, we will discuss the potential to selectively engage these features of NK cells as a means to transform vaccination and achieve higher efficiency.
Box 1. Basic biology of natural killer cells.
NK cells are innate lymphocytes that lack expression of CD3 and are characterized by the expression of markers like NKp46, CD56 in humans, NKG2A in non-human primates, and NK1.1 in some mouse strains. Unlike T and B cells, NK-cell activity is not governed by somatically rearranged antigen-specific receptors, but is instead determined by the net input of activating and inhibitory signals from a variety of germline-encoded receptors. Inhibition is largely achieved via NKG2A, KIR (human/non-human primate), or Ly49 (mouse) engagement of self, class I major histocompatibility complex (MHC) molecules. Downregulation or loss of MHC thus triggers activation of NK cells via missing self. There is strong evidence that NK-cell functional competence is determined by education through these inhibitory receptors. NK cells are also activated via engagement of integrins (e.g. LFA-1), Fc receptors (CD16), natural cytotoxicity receptors (e.g. NKp46), ITAM-associated Ly49s and KIRs, C-type lectin receptors (e.g. NKG2D), and other glycoproteins (e.g. DNAM-1). Other receptors (e.g. SLAMF4) can play both activating and inhibitory roles depending on situational context. Each NK cell exhibits a random repertoire of these receptors that determines per cell responsiveness to environmental cues. Imbalance favoring NK-cell activation results in cytokine secretion and degranulation into synapses formed between the NK-cell and its target.
Promoting NK cells to potentiate vaccine efficacy
Innate functions of NK cells contributing to Immunogenicity
During an immune response, NK cells are a robust, early source of cytokines and chemokines (Figure 1). These include IFN-γ, tumor necrosis factor (TNF), granulocyte-macrophage colony stimulating factor (GM-CSF), macrophage inflammatory protein 1 (MIP-1α, MIP-1β), and RANTES [5]. In this manner, NK cells recruit and activate antigen-presenting cells (APCs) that subsequently stimulate robust adaptive immunity [4]. Moreover, IFN-γ production by NK cells can promote TH1 differentiation of helper T cells and stimulate isotype class switching of B cells [9, 10].
Figure 1. Responses of NK cells that contribute to vaccine efficacy.
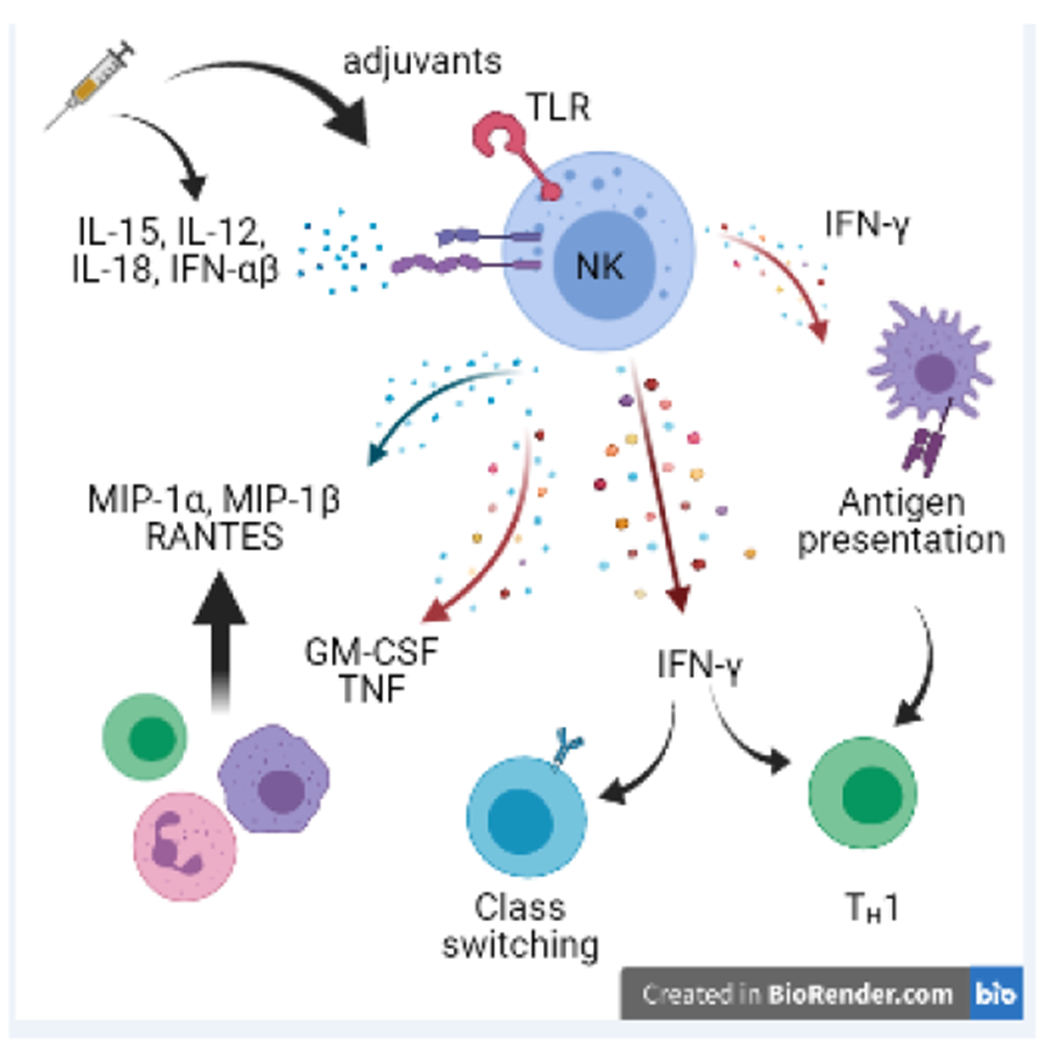
Vaccination can trigger NK cell activation via direct engagement of receptors on NK cells (e.g. adjuvant binding to TLR4) or via triggering of expression of IL-12, IL-18, and type I IFN. Activated NK cells produce IFN-◻, thereby promoting TH1 differentiation of CD4 T cells, IgG2a class switching by B cells, and enhancing antigen presenting functions of myeloid cells. NK cells make cytokines (e.g. GM-CSF) and chemokines (e.g. MIP-1◻) that facilitate recruitment of myeloid cells, granulocytes, and other lymphocytes.
This recruitment and activation mediated by NK cells varies according to the types of cytokines released after various forms of immunization [11]. While comprehensive understanding of the capacity of different vaccine platforms to trigger NK-cell activation is limited, some of the implicated cytokines include type I IFN, IL-12, IL-15, and IL-18. NK-cell activation via IL-15 induction has been seen in rhesus macaques following vaccination with live-attenuated SIV, whereas replication-incompetent Ebola activates human NK cells via IL-18 [12, 13]. There is clear evidence that emerging adjuvants stimulate robust NK-cell activity after vaccination (Box 2). The oil-in-water emulsion-based Adjuvant System 03 (AS03) stimulated early activation and proliferative responses of human NK cells that were associated with robust adaptive immunity at later time points [14]. Improved efficacy of inactivated influenza vaccines in elderly individuals has been attributed to activation of NK cells by MF59 [15], a similar oil-based vaccine adjuvant. Likewise, the liposome-based adjuvant AS01 stimulated robust, early IFN-γ responses by NK cells that promote vaccine immunogenicity in mouse models injected with Hepatitis B surface antigen [16]. DNA vaccine-mediated delivery of IFN-stimulated gene 15 (ISG15) also promotes stronger vaccine-specific T cells responses via adjuvant effects on NK cells in both human cell culture and an in vivo mouse model [17]. Although some adjuvants have been shown to directly influence NK cells, the main goal of adjuvant use is to enhance the costimulatory molecule expression, cytokine production, and antigen-presenting functions of APCs.
Box 2. Adjuvants and NK cells.
Live-attenuated vaccines mimic natural infection resulting in highly efficient induction of long-lasting immunity, albeit not without safety concerns. To achieve the type of robust immunity associated with infection, recombinant proteins and inactivated or killed pathogens frequently require formulation with adjuvants that trigger inflammation and innate responses. Adjuvants approved for human use include aluminum salts (alum), oils (AS03, MF59), Toll-like receptor (TLR) adjuvants (CpG 1018), and combined formulations of these types of compounds (AS04, AS01) (see Table I). There are 25 FDA-approved vaccines containing alum, which was originally included to precipitate antigens during vaccine manufacturing. Alum prolongs vaccine antigen persistence, provokes type 2 cytokines that favor humoral immunity, and modulates membrane lipid content of antigen-presenting cells to enhance soluble antigen delivery. Alum also triggers release of IL-1β and IL-18, which can activate NK cell responses. AS03 and potentially other oil-based adjuvants function by stimulating local inflammation leading to recruitment, activation, and antigen uptake by immune cells. Administration of AS03-adjuvanted vaccine vaccines triggers proliferative responses of NK cells. In contrast, combinatorial adjuvants like AS04 and AS01 trigger substantial IFN-γ expression. In fact, AS01 stimulation of NK-cell IFN-γ is a vital component of the robust immunogenicity of this adjuvant. CpG-ODN (CpG oligodeoxynucleotides) enhance immune responses via TLR9. The resulting type I IFN and IL-12 expression by DCs provides a potent stimulus for NK-cell activation, although NK cells also express TLR9 to respond directly to CpG-ODN [107].
Thus, early innate responses of NK cells may be favorable in certain vaccine responses (Figure 1). Moreover, vaccine platforms and adjuvants can be tailored to optimally trigger NK cell-mediated support for inducing protective adaptive immune memory. A refined understanding of NK-cell contributions to vaccine efficacy is required in order to determine contexts in which acute activation of NK cells would benefit induction of long-lived adaptive immune memory and at which stage of vaccination, prime or boost, this activity would be most beneficial.
Following infection or vaccination, NK cells undergo distinct epigenetic reprogramming that prepares their increased responsiveness to secondary insult [18]. In contrast to classical adaptive memory, this phenomenon called trained innate immunity lasts up to several months and is not antigen specific. For example, vaccination with BCG induced non-specific priming of human and mouse NK cells resulting in increased inflammatory cytokine release against a range of unrelated bacterial and fungal pathogens [19]. Studies in macaques using a prime-boost immunization with a modified vaccinia virus show that the NK cells responding to vaccine boost are functionally distinct from those present at initial priming. Specifically, the priming arm of the vaccine induced NK cells that were more mature and expressed higher levels of cytotoxic, homing, and adhesion molecules compared to those present at baseline [20, 21]. Therefore, an ideal prime-boost vaccine strategy might skew NK cells towards memory differentiation during initial priming (either using an identical or unrelated antigen to their target) to enhance the generation of long-lived memory following vaccine boost.
Functional contributions of NK cells to vaccine recall responses
Whether or not they are functionally modified by vaccination, NK cells that are present in immunized individuals contribute to immunity during recall responses (Figure 2). Studies in mice highlight NK cells as important effectors of protective vaccine responses in response to memory CD4 T cell-derived IL-2 derived during antigen recall [22, 23]. In the setting of Influenza A virus infection in mice, memory CD4 T cell-driven NK-cell responses contribute to pathogenic lung inflammation [24].
Figure 2. Memory NK cells and involvement of NK cells in vaccine recall responses.

Vaccine- or infection-elicited inflammatory cytokines and viral products can promote expansion, differentiation and persistence of memory or adaptive NK cells with heightened effector function relative to their naive counterparts. During recall responses, vaccine-elicited memory T cells promote elevated NK-cell functionality via IL-2 while memory B cell-derived antibodies can promote killing of target cells via engagement of Fc receptor on NK cells (ADCC). Some memory NK cells are highly adept at antibody-dependent effector functions.
NK cells are also key mediators of antibody-dependent cellular cytotoxicity (ADCC). The induction of ADCC-proficient antibodies is a key correlate of vaccine-mediated protection in humans against HIV [25], malaria [26], and influenza [27]. Of note, robust adjuvants of NK-cell activity such as AS01 and 3M-052 (TLR7/8 agonist) also proved more effective at triggering strong ADCC-proficient antibody responses than MF59 and GLA-SE (TLR4) in both infant macaques as well as in a completed randomized double-blind placebo-controlled phase 2 trial (clinical trial number NCT00280033; ClinicalTrials.gov) [28, 29]. These observations suggest that optimization of vaccine-mediated induction of ADCC-inducing antibodies is critical and could potentially be coupled to strategies favoring accumulation of NK-cell subsets with strong ADCC capacity.
Adaptive and memory NK cells in vaccines
Numerous lines of evidence point to adaptive-like functionality of NK cells, including both the capacity of NK-cell subsets to develop and maintain enhanced effector functions and to selectively remember past antigen encounters [2]. While increasing somatic hypermutation may enhance antibody maturation enough to address pathogens with significant antigen variation such as HIV, other limitations to current vaccine efficacy include intracellular pathogens such as Mycobacterium tuberculosis (MTB) for which creation of an antigen-specific cytotoxic population may be possible. Human and mouse NK cells are important in immune responses against MTB, wherein NK cells putatively directly interact with MTB (reviewed in [30] and [31]). Clinical evidence suggests that the creation and persistence of these cells may be possible, as durable activation of the NK-cell compartment following administration of a heterologous prime-boost viral vector-based (Ad26 and modified vaccinia Ankara) Ebola virus vaccine was seen as late as 180 days past the booster dose in a recently completed phase 2 multicenter, randomized, placebo-controlled, observer-blind clinical trial (NCT02416453; clinicaltrials.gov) [32]. However, vaccinology is only just beginning to examine the potential for vaccination to induce a long-lived population of NK cells, so their precise longevity remains to be fully elucidated. While the memory-like functionality of NK cells has not yet been targeted in human vaccine regimens, the potential of such an approach is intriguing (Figure 2).
In the context of cytomegalovirus (CMV) infections of humans and mice, the repertoire of NK cells is skewed and favors subsets with enhanced antiviral or ADCC functionality (Reviewed in [8]). In some strains of mice resistant to murine CMV, NK cells expressing the receptor Ly49H become activated by the viral protein m157 before undergoing clonal expansion and contraction, thereby forming a population of virus-specific memory cells [33]. A similar expansion of highly active NK cells expressing NKG2C is observed in humans during CMV infection, putatively in response to antiviral cytokines (e.g. IL-12) and viral peptides [34, 35]. The other frequently overlapping subset of adaptive NK cells in human CMV infection is characterized by reduced expression of certain signaling mediators (FcRγ, SYK, EAT-2) and transcription factors (PLZF) caused by epigenetic remodeling [36, 37]. This subset is strongly antibody-reactive and is superior at mediating ADCC [38].
Exposure to cytokines, including interleukins such as IL-12, IL-15, and IL-18, elicits similar long-lived functional changes in NK cells that provides strong antitumor functionality [39]. These cytokine-induced memory-like (CIML) NK cells are currently employed in a number of clinical trials targeting various cancers. This cytokine activation is antigen-independent, and these memory NK cells remain effective at least 12 weeks in mice and 3 weeks in humans after first cytokine stimulation [40]. The overlap between cytokines involved in CIML NK-cell differentiation and those triggered by infection or immunization is a compelling hint at the potential utility of this population for enhancing vaccine success.
Virus-specific memory NK cells have been reported in mice [41, 42], non-human primates [43], and humans [44]. In fact, the hepatitis B virus (HBV) vaccine elicits an HBV-reactive subset of memory NK cells in humans [45]. Similarly, hepatitis C Virus (HCV)-, HIV-, and influenza-reactive NK-cell subsets have been observed in humans [46, 47]. The receptors involved in the remarkable specificity of these NK cells for viral antigens remain undefined, leaving intact the possibility that this phenomenon is driven by other mechanisms (i.e. epigenetic imprinting). Indeed, a discrete subset of epigenetically primed human NK cells is implicated in antigen-specific immune responses [48]. Thus, immunization or infection can trigger a long-lived population of NK cells that retains heightened activity specifically recalled by antigens in the original virus or vaccine.
Whether antiviral memory NK cells can be intentionally expanded using vaccines and the extent to which these cells may contribute to protective immunity is unclear. However, development of strategies to concomitantly generate T, B, and NK cell responses against pathogens remains a major vaccine goal. The emergence of robust mRNA vaccine platforms that can be specifically tailored to deliver the necessary antigens and signals may prove transformative in achieving this goal
Restraining NK cells to enhance vaccine efficacy
Cytolytic activity of NK cells suppresses adaptive immunity
A number of publications implicate the cytolytic functionality of NK cells in the suppression of adaptive immune responses after immunization [6, 7, 49] . Perforin-dependent delivery of granzymes via NK-cell cytolytic granules is a major mechanism underlying immunoregulatory killing by NK cells [4]. Yet, death receptors like TRAIL are implicated in this phenomenon as well [50, 51]. In its simplest form, NK-cell cytolytic activity removes infected or antigen-bearing cells, thereby repressing induction of adaptive responses by limiting the availability of antigenic fuel. Yet, NK cells also constrain adaptive immune responses by killing activated CD4 and CD8 T cells [52].
Perforin-dependent elimination of CD4 and CD8 T cells constrains both the number and function of subsequent memory T-cell responses [6, 49]. In a similar fashion, perforin-dependent killing of activated CD4 T cells reduces the magnitude of T follicular helper cells (TFH) responses, thereby inhibiting the quantity and quality of germinal center responses [6, 53]. This mechanism limits antiviral antibody titers and the number of virus-specific plasma cells [6]. Moreover, affinity maturation is enhanced in mice in the absence of NK cells, with antigen-specific germinal center B cells accumulating a greater number and increased quality of somatic mutations [7]. Numerous pathogens and vaccine regimens can trigger NK-cell suppression of T and B cell responses in mice [4, 7], yet this activity is not a ubiquitous feature of immunization. Infections with certain adenoviral vectors or strains of vaccinia virus stimulate very weak to undetectable levels of immune suppression by NK cells [54, 55]. An improved understanding of the distinguishing events in vaccine-induction of immunoregulatory activity is necessary to translate these observations into improved vaccine regimens (Figure 3).
Figure 3. Immunoregulatory functions of NK cells.
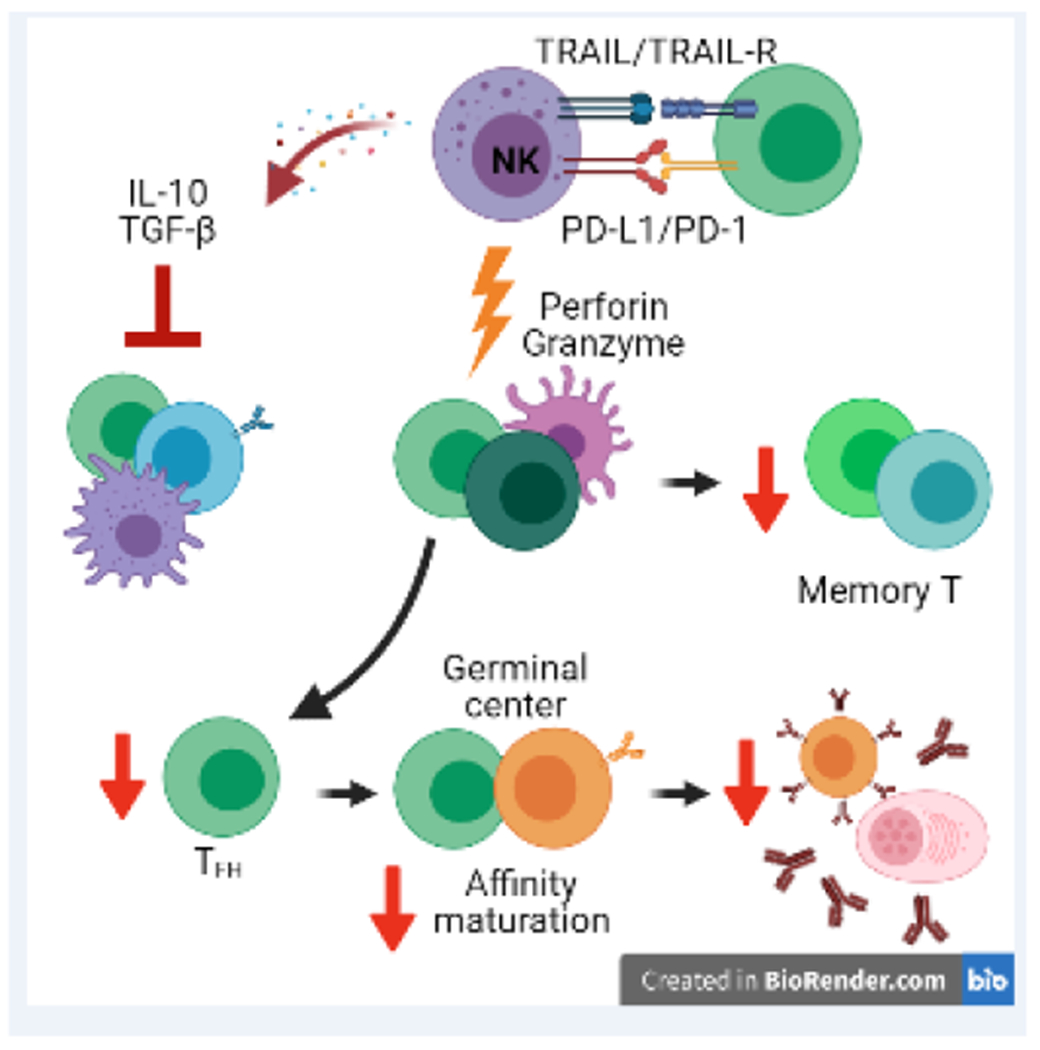
NK cells exert a variety of functions that limit the magnitude and quality of adaptive immune responses. NK-cell production of IL-10 or TGF-◻ can suppress T and B cell responses. TRAIL or PD-L1 on NK cells can suppress T cell responses via TRAIL-R and PD-1 on T cells. NK cells can also use perforin/granzyme-containing cytolytic granules to suppress adaptive immune response. This killing can be targeted against T cells or antigen-presenting cells, resulting in marked deficits in TFH and germinal center responses. Consequently, NK cell regulation limits affinity maturation, elaboration of antibody, and generation of long-lived B-cell memory.
The association between strong NK-cell responses and weak antibody responses is also apparent in humans. High NK-cell activity at the time of yellow fever vaccine administration was linked to weaker antiviral antibody responses in humans [56]. Likewise, gene signatures of NK-cell activation were inversely associated with the strength of antibody responses after both administration of the RTS,S/AS01 malaria vaccine in a completed phase 3 randomized double-blind placebo-controlled trial (Clinicaltrials.gov NCT00866619: NCT00866619) [57] as well as following administration of an adjuvanted HBV vaccine in a completed phase 2 randomized parallel assigned double blind trial (ClinicalTrials.gov NCT00805389: NCT00805389) [58]. Finally, development of broadly neutralizing antibodies in people infected with HIV was associated with NK-cell dysfunction linked to heightened expression of RAB11FIP5 [59]. Collectively, these data suggest that blocking the immunosuppressive functions of NK cells could potentially serve as an innovative path to enhance vaccine efficacy.
Broad functional targeting to alleviate NK-cell suppression
Most studies of immunoregulatory NK-cell function in mice were performed by depleting NK cells with antibodies or using genetically modified mice that lack either NK cells or the cytolytic protein, perforin [6, 7]. Given the vital roles of NK cells in pregnancy [60], immune defense [61], and tumor control [62], whole-sale depletion of these cells is an unlikely translational approach. Instead, transient and local blockade of cytotoxicity at sites of vaccine administration may strike an appropriate balance between maintaining immune defense while circumventing immune suppression (Figure 4). However, long-term suppression of NK cells may upset immunologic balance, perturb tolerance, and increase the risk of off-target complications. Some of these complications are highlighted by the serious herpesvirus infections seen in humans with NK-cell deficiencies [63] and in autoimmune diseases such as systemic lupus erythematous that are associated with defective NK cell function [64, 65].
Figure 4. Strategies to circumvent NK-cell suppression of vaccine responses.
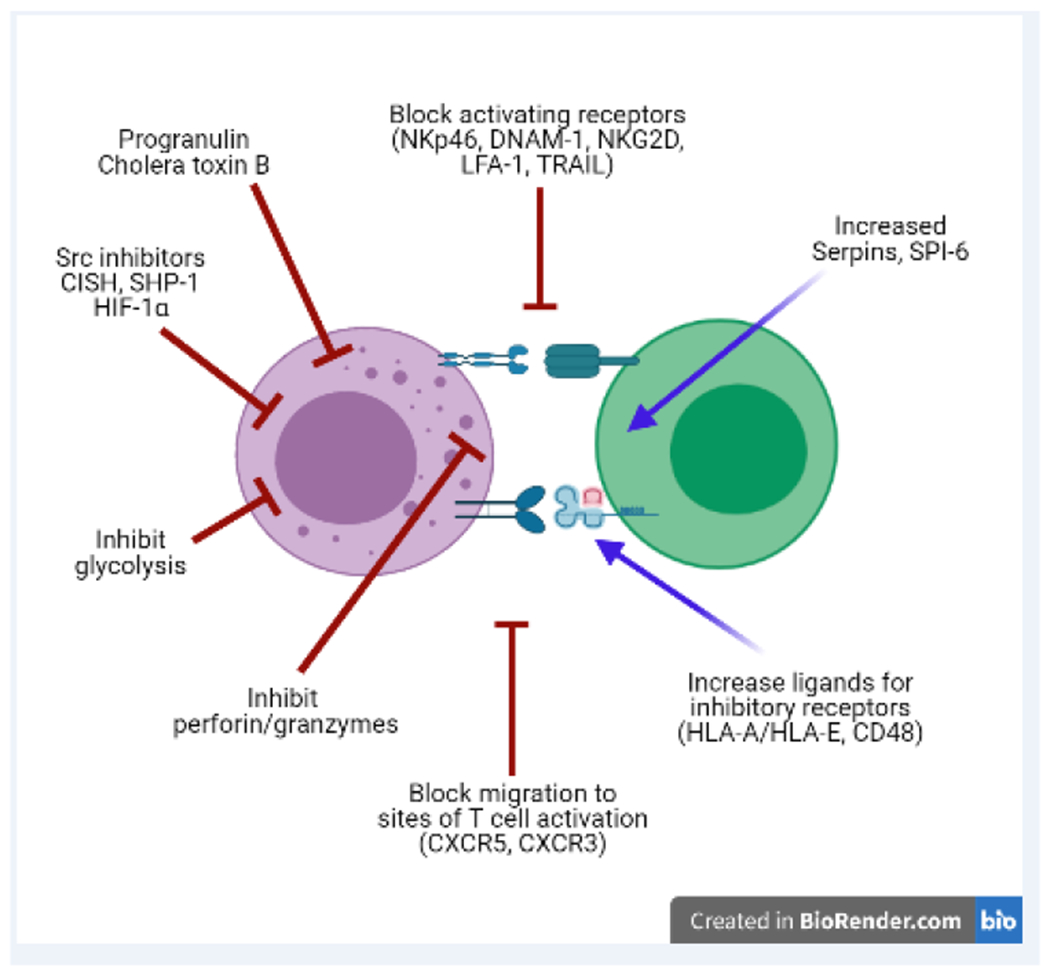
The available evidence accumulated thus far suggests a number of potential strategies to circumvent the immunosuppressive functions of NK cells during vaccination. These include inhibition of NK-cell metabolic processes, activating signals, migration, and cytolytic capacity. Strategies may also focus on enhancing target cell resistance to NK-cell attack or increasing engagement of inhibitory receptors on the NK cells.
Potential strategies to broadly circumvent NK-cell cytolytic suppression of vaccine responses include employment of small-molecule inhibitors targeting key mediators of cytolytic activity. For example, there are selective inhibitors of perforin capable of functioning in vivo [66]. Likewise, inhibitors of granzymes have also been described [67] . Targeting of granzyme K is particularly compelling given its role in NK-cell killing of CD4 T cells during daclizumab (anti-CD25) therapy in patients with multiple sclerosis [68] . An alternative possibility would be provision of granzyme antagonists, including serpinB9, to responding T cells during vaccination in order to protect them from NK-cell attack [69]. Serine protease inhibitors (e.g. SPI-6) also inhibit granzyme B activity and could represent an alternative mechanism for inhibition of NK-cell cytotoxicity.
The perforin-dependent suppression of T and B cell responses by NK cells could also be short-circuited using agents capable of broad inhibition of NK-cell activity (Figure 4). Cholera toxin B is a powerful adjuvant with the capacity to suppress NK-cell cytolytic function [70]. In fact, it is not known to what degree suppression of NK cells may contribute to the adjuvant-like quality of this toxin. Progranulin was shown to suppress NK-cell killing of T cells in mice, resulting in an enhanced magnitude of antiviral T-cell responses [71]. Metabolism is also key for optimal NK-cell cytolytic function. Specifically, glycolysis is essential for NK-cell killing [72]. Yet, glycolysis is dispensable for vaccine-elicited T cells [73] and is minimally involved in germinal center responses [74]. This data supports the hypothesis that inhibition of glycolysis could alleviate NK-cell immune suppression to enhance adaptive immune responses.
Signaling pathways that contribute to NK cell activation may be susceptible to modification in order to block immunosuppressive activity. The NK-cell activating receptors NKp30, NKp46, and NKG2D signal via Src-family kinases [75]. Importantly, inhibition of Src kinases impaired mouse NK-cell activation and reduced cytotoxicity in cell culture models of Ly49D signal transduction [76, 77]. Stimulation of hypoxia pathways also restrained NK-cell cytolytic function via hypoxia inducible factor-1 alpha (HIF-1α) and Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1) as shown in vitro with KHYG-1, NK-92, and primary human NK cells, as well as in vivo in HIF-1α deficient mice [78, 79]. More recently, studies in mice and with human cells revealed cytokine inducible SH2-containing protein (CIS) as a critical checkpoint restraining NK-cell function that can be targeted to enhance target cell killing [80, 81]. Therefore, drugs that modulate kinase and phosphatase function, particularly those with selective function in NK cells versus other cells involved in vaccine responses, may be suitable agents to circumvent regulatory functions of NK cells and potentially enhance vaccine efficacy. Nonetheless, more work needs to be done to dissect the kinetics and vaccine-specific effects of NK-cell inhibition (as opposed to activation in tumor models) in order to fully achieve the predicted translational promise of such therapies.
Blocking activating receptor recognition of NK-cell targets
Studies in mice and in vitro human cells implicate a handful of receptors in promoting or facilitating NK-cell immunoregulatory killing. Therefore, blockade of these receptor/ligand interactions represents a putatively selective means to circumvent immune suppression by NK cells without broadly inhibiting the other functions of these cells. Unfortunately, contradictory evidence casts doubt on the necessity and sufficiency of targeting any of the receptors identified thus far.
Early evidence indicated that ligands for the activating receptor NKG2D are upregulated on activated human and mouse T cells, and that NKG2D is important for NK cells to kill activated T cells [49, 82, 83]. However, in vivo studies of LCMV-infected mice provide evidence that NK-cell killing of T cells is NKG2D-independent [52, 84]. Other activating receptors, including DNAM-1, NKp44, NKp46, and LFA-1, are also implicated in this phenomenon in either mice or human NK cells [83–86]. Intriguingly, reagents that block NKp46 interactions with its ligand in acute LCMV infected mice can enhance T-cell responses in an NK-cell dependent fashion [87]. This strategy may be complicated in the context of influenza, where NKp46 is also implicated in NK-cell recognition of hemagglutinin in both 129/Sv and C57BL/6 mice [88]. TRAIL is also implicated in NK-cell suppression of T cells, though reports vary on whether this involves apoptotic or non-apoptotic functions of this death receptor in human in vitro experiments with HBV [50] or mice infected with LCMV [89]. Likewise, liver resident NK cells inhibit antiviral T cells through upregulation of PD-L1 and engagement with PD-1 on the target T cells [90]. In summary, several candidate receptors have been described that would represent potential targets for selective interference in NK-cell regulatory killing of T cells (Figure 4), but no receptor has emerged as necessary and sufficient to account for the totality of NK-cell mediated immune suppression.
Blocking NK-cell access to adaptive immune cells
Since perforin-dependent lysis depends on cell-contact, prevention of NK cell colocalization with target cells (i.e. T cells) in lymphoid tissues is promising strategy.
Recruitment of mouse NK cells into draining lymph nodes is essential for IFN-γ-dependent support of T cell responses [9, 91]. This migration is dependent upon CXCR3 [91] and may additionally involve CXCR5 [92]. In a similar fashion, interferon-dependent induction of CXCR3 ligands and CXCR3-dependent migration of mouse NK cells into T-cell rich regions of the spleen is important for NK-cell cytolytic suppression of T cells [93]. Thus, blocking access of NK cells to sites of T-cell priming during vaccination via selective inhibition of chemokine receptors, integrins, or other trafficking receptors is another method for potentially enhancing vaccine responses.
Amplification of inhibitory receptor signals to restrain immunoregulatory NK cells
NK cell cytotoxicity can also be curtailed through the increase of inhibitory signals. Major histocompatibility class I (MHC-I) molecules represent a class of ligands which bind inhibitory receptors on NK cells [94]. Strategies that broadly increase expression of classical MHC-I molecules could restrain NK cells while bolstering T-cell responses. In fact, positive regulators of class I MHC expression protects human T cells from NK cell attack, which likely modulates pathogenesis of viral infections [95, 96]. However, the highly polymorphic nature of class I MHC molecules and their corresponding killer immunoglobulin-like receptors (KIR) on NK cells might complicate such strategies on a population level [94].
Instead, less polymorphic receptors like human leukocyte antigen E (HLA-E) or HLA-G may suppress NK cells across a broader swath of the population. The NKG2A/CD94 heterodimeric receptor is an important check on NK-cell activity through interactions with HLA-E in humans or Qa-1b in mice [96, 97]. NKG2A-dervied inhibitory signals restrain NK-cell killing of activated CD4 T cells in mice [98]. In fact, blockade of NKG2A can restore mouse NK-cell killing of T cells as a potential therapeutic in autoimmune disease [99]. Consistent with these findings, LCMV-induced up-regulation of Qa-1b expression on murine B cells restrains immunoregulatory functions [100], while virus-induced downregulation of NKG2A alleviates this roadblock in the same infectious context [55]. Other studies have detailed a similar phenomenon involving HLA-G-mediated suppression of NK cell function via inhibitory KIR or leukocyte Ig-like receptors B (LILRB1, LILRB2) [101]. Methods to increase this inhibitory signal may have utility in boosting vaccine responses (Figure 4).
Several HLA-independent receptor systems are also implicated in tuning NK-cell effector functions. T cell immunoreceptor with Ig and ITIM domains (TIGIT) and PD-1 can restrain NK-cell activity within tumors [102, 103]. The signaling lymphocytic activation molecule (SLAM) receptor 2B4 (CD244, SLAMF4) represents an additional check on NK cell killing, most notably of other murine hematopoietic cells in vivo [104]. In mice, 2B4 expression restrains immunoregulatory killing of T cells by NK cells [85]. In a similar fashion, crosslinking of T cell immunoglobulin mucin 3 (Tim-3) on NK cells and primary human NK cells by glycoprotein ligands significantly reduces NK-cell cytolytic capacity [105]. Sialic acid decorating of Jurkat target cells also provokes inhibitory signals via Siglec-7 that inhibit in vitro cytolytic activity of NK cells [106]. Thus, development of strategies to incorporate or up-regulate expression of inhibitory receptor ligands during vaccination represents a possible strategy to decrease NK-cell function and boost vaccine responses.
The complex role of NK cells in vaccine responses
While NK cells may be stimulated to generate an unconventional form of innate immune memory and cooperate functionally with other vaccine-elicited components to generate an adaptive immune response and ultimately prevent infection, they can also negatively regulate adaptive immune responses. NK cells can secrete a range of cytokines to trigger innate immune responses and stimulate APCs to boost the adaptive immune response, while also reducing the available vaccine antigen and eliminating responding T cells, thereby stunting the memory immune response. This dichotomy increases the complexities inherent to targeting NK cells during vaccination. This is due to the conflicting roles of triggering innate responses and priming adaptive response, and the ways in which NK cells limit the extent to which those responses can develop. This is further complicated by the contrasting roles of NK cell-derived cytokines and cytolytic granules in shaping adaptive immune responses.
Concluding remarks and future perspectives
Vaccines have saved millions of lives through the global eradication of smallpox, widespread elimination of polio, and reduced incidence or severity of countless other infectious diseases. Yet, efficacious vaccines remain unavailable for a number of major threats to human health, including HIV, the causative agent of AIDS. Despite this shortcoming, decades of effort to develop innovative vaccine platforms for the prevention of HIV provided a wealth of options for successfully combating the worldwide COVID-19 pandemic. The full potential of vaccines to prevent pandemics, eradicate cancer, and reduce the burden of chronic diseases is likely to remain unfulfilled until methodologies for engaging unconventional arms of the immune response and overcoming inherent immunoregulatory mechanisms are developed. This review describes newly appreciated activities of NK cells likely to enhance or constrain vaccine efficacy, thereby illuminating innovative strategies for modulating NK-cell responses in next-generation immunization regimens. However, some limitations remain (see Outstanding Questions) before intentional manipulation of NK-cell functionality can be safely, effectively, and reproducibly achieved at a population level.
Outstanding Questions.
Which subsets of NK cells and their associated combinations of receptors or molecular mediators are responsible for the dueling contributions of NK cells to vaccine success?
Can NK cell immunoregulatory functions be blocked to enhance vaccines while important antiviral and antitumor functions of NK cells are left intact?
Which adjuvants and types of vaccines trigger optimal responses of NK cells in the context of vaccination?
Would circumvention of NK-cell regulation of adaptive immune responses during vaccination result in increased autoimmune responses or other adverse events?
Is it possible to trigger sustained accumulation of pathogen-specific memory NK cells via vaccination, and will these cells meaningfully contribute to vaccine efficacy upon pathogen challenge?
The refinement of vaccine strategies to achieve optimal, targeted induction of desirable NK cells functions (i.e. antiviral memory) while minimizing suppressive restraint of T and B cell responses holds promise for the prevention of disease. Detailed understanding of how different adjuvants and vaccine platforms stimulate NK-cell activity is required. Likewise, discovery of mechanisms involved in the development of NK-cell memory and suppression of adaptive immunity will provide targets for translational interventions to surgically guide their contributions to vaccine efficacy. The precise role of NK-cell memory in the broader context of immune responses remains poorly understood, impeding efforts to harness this phenomenon in immunization. There is an equally compelling need to discern the evolutionary value of NK-cell suppression of T- and B- cell responses, perhaps related to prevention of autoimmunity or reduction of immunopathology. These insights will provide crucial guidance in ongoing efforts to manipulate NK cells through vaccination.
Continued development of mRNA and nanoparticle vaccines strategies will facilitate layered, targeted, and coordinated activation of various arms of the immune response. Design of clinical trials to include time points and measurements focused on NK cells will provide a wealth of new information regarding the contributions of these cells to human vaccine responses. We envisage the emergence of methods to selectively amplify or restrain disparate functions of NK cells as means to realize the full potential of vaccines to transform human health.
Table I.
(associated with Box 1). Vaccine adjuvants
| Adjuvant | Ingredients | Vaccines | Mechanism |
|---|---|---|---|
| Alum | Aluminum hydroxide; Aluminum phosphate; Amorphous aluminum hydroxyphosphate sulfate | Many (Hepatitis virus A & B; Diphtheria & tetanus; haemophilus influenza; human papillomavirus) | Type 2 cytokine response and inflammasome activation; antigen depot effect |
| MF59 | Squalene, Span 85, and Tween 80 in 10 mM sodium citrate buffer at pH 6.5 | Adjuvanted influenza vaccine (FLUAD) | Promote type 1 cytokines, recruit antigen-presenting cells and enhance antigen uptake |
| AS01 | Monophosphoryl lipid A (MPL) and the saponin QS-21 | Zoster Vaccine Recombinant, Adjuvanted (SHINGRIX) | Enhance IFN-γ (NK cells) to promote TH1 and T cell cytotoxicity |
| AS03 | Squalene , α-tocopherol, and Tween80 in phosphate buffered saline | Influenza A (H5N1) Virus Monovalent Vaccine, Adjuvanted (N/A) | Increase NK-cell proliferation, enhance antigen uptake, immune cell recruitment, local inflammation |
| AS04 | Aluminum hydroxide or aluminum phosphate with monophosphoryl lipid A (MPL) | Hepatitis B (Fendrix), Human Papillomavirus Bivalent (Types 16, 18) Vaccine, Recombinant (Cervarix) | Bind TLR4, recruit and activate immune cells, promote IFN-γ (NK cells) |
| CpG-ODN | CpG-1018 : CpG-B class oligonucleotide with a phosphorothioate-backbone and the sequence 5’-TGACTGTGAA CG TT CG AGATGA-3’ | Hepatitis B Vaccine (Recombinant), Adjuvanted (HEPLISAV-B) | Bind TLR9 (B cells and DC), activate type I IFN, Promote IFN-γ (NK cell) and TH1 |
| 3M-052 | Synthetic TLR7/8 agonist | Experimental influenza, HIV, malaria, and SARS-CoV-2 vaccines | Stimulate TLR7 and TLR8 to promote inflammation |
| GLA-SE | Synthetic TLR4 agonist | Experimental influenza, MTB, and HIV vaccines | Stimulate innate inflammation via TLR4 |
Highlights.
Natural killer cells exhibit pathogen-specific innate memory and adaptive functions
Innate cytokines from NK cells influence quality of vaccine responses
Antibody-dependent cytolytic activity of NK cells is key to the success of some vaccines
Magnitude and quality of long-lived T- and B-cell memory is determined by NK cells
NK cells can restrain affinity maturation of neutralizing antibodies
Acknowledgements
The authors were supported by NIH grants DA038017, AI148080, AR073228, AI145304 to S.N.W., GM063483 (N.L., H.F.), the L.B. Research and Education Foundation (N.L.), and K12HD000850-34 to A.C. as a Fellow of the Pediatric Scientist Development Program (PSDP).
Glossary
- Adenovirus (Ad)
viral vaccine vectors based on human (Ad5) or chimpanzee (Ad26) species of adenovirus
- Adjuvant systems (AS01, AS03, AS04)
these are combinations of immune stimulants intended to enhance responses to vaccine antigens. The review also covers the various adjuvants designated as alum, 3M-052, MF59, and GLA-SE
- Antibody-dependent cellular cytotoxicity (ADCC)
antibody-mediated recognition of specific antigens on target cells followed by binding of these antibodies by Fc receptors on NK cells, triggering cytolytic elimination of the antibody-bound target cells by NK cells
- Cytokine-inducible SH2-containing protein (CIS, CISH):
an intracellular protein and key negative regulator of cytokine receptor signaling in NK cells
- Chemokine receptors
CXCR5 and CXCR3 are mentioned as examples of receptor that direct immune cell trafficking to various tissues and sites of the body via interaction with soluble chemokine ligands
- Cytokine-induced memory-like (CIML)
a type of memory NK cell imbued with heightened effector and antitumor functionality following priming with IL-12, IL-15, and IL-18
- Cytomegalovirus (CMV)
a betaherpersvirus that causes disease
- DNAX Accessory Molecule-1 (DNAM-1/CD226)
a glycoprotein expressed on NK cells that provides activating signals following engagement with ligands CD122 and CD155
- Granulocyte-macrophage colony-stimulating factor (GM-CSF)
a cytokine made by NK cells that promotes activation and migration of myeloid cells and granulocytes
- Granzymes
serine protease released by activated NK cells and cytotoxic T cells to induce target cell apoptosis
- Human leukocyte antigen (HLA)
a locus of genes in the human major histocompatibility complex (MHC) gene complex involved in presenting peptides to T cells and engaging receptors on other leukocytes, including KIR on NK cells
- Hypoxia inducible factor (HIF)
transcription factor involved in cellular responses to low oxygen levels
- Interferon (IFN)
Cytokines involved in antiviral responses (e.g. IFN-γ)
- Interferon stimulated genes (ISG)
set of genes induced by IFNs and involved in the cellular response to these cytokines (e.g. ISG15)
- Interleukins (IL)
cytokines that promote activation, proliferation, differentiation, and survival of immune cells (e.g. IL-18)
- Killer-cell immunoglobulin-like receptor (KIR)
family of glycoproteins expressed by human NK cells and some T cells that provide activating or inhibitory signals following engagement of class I HLA
- Ly49
family of receptors on mouse NK cells, analogous to KIR on human cells, which trigger activating or inhibitory signaling
- Macrophage inflammatory protein (MIP)
MIP-1◻ (CCL3) and MIP-1◻ (CCL4) are chemokines involved in cellular trafficking
- Major histocompatibility complex (MHC)
a highly polymorphic set of genes involved in immune functions (see HLA)
- NKp46/ Natural cytotoxicity triggering receptor 1 (NCR1)
surface receptor on NK cells, which mediates IFN-γ release and NK killing
- NKG2A
receptor present on NK cells that dimerizes with CD94 and together engage the nonclassical MHCI HLA-E to provide inhibitory signaling
- NKG2C
receptor present on NK cells which also dimerizes with CD94 to engage MHCI HLA-E but produce activating signals to increase cytoxicity
- NKG2D
receptor present on NK cells which forms homodimers and can induce killing of both tumors and virus infected cells through interaction with ligands on these target cells
- T follicular helper (TFH) cells
subset of CD4+ T-cells which play an essential role in the formation of germinal centers via engagement of costimulatory molecule CD40 with CD40L on B cells
- Perforin
cytolytic protein secreted by activated NK cells and cytotoxic T cells which induces cell death by forming a pore in the target cell membrane
- Programmed cell death protein 1 (PD-1)
T-cell surface checkpoint protein which binds to PDL-1 on target cells to promote apoptosis of antigen specific T cells and reduce killing of regulatory T cells
- Rab11 family-interacting protein 5 (RAB11FIP5)
is a protein involves in vesicle trafficking that is highly expressed in dysfunction NK cells in individuals infected with HIV
- Serine protease inhibitors (SPI)
a family of proteins that inhibits activity of serine proteases involved in induction of apoptosis
- Signaling lymphocytic activation molecule (SLAM)
a family of receptors involved in regulation of lymphocyte function
- Src homology region 2 domain-containing phosphatase-1 (SHP-1)
a phosphatase involved in negative regulation of lymphocyte function by countering activating signaling via kinases
- T-cell immunoglobulin and mucin-domain containing-3 (TIM-3)
a receptor on NK and T cells that interacts with glycoproteins to regulate lymphocyte function
- T cell immunoreceptor with Ig and ITIM domains (TIGIT)
an immune receptor on T cells and NK cells that interacts with CD112 and CD155 to regulate lymphocyte function
- TNF-related apoptosis-inducing ligand (TRAIL)
cytokine which binds to death receptors to trigger apoptosis
- Tumor necrosis factor (TNF)
a cytokine that can be soluble or membrane bound that can trigger cell death via death receptor signaling
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Twitter: @AndypedsI; @labwaggoner
References
- 1.Zwirner NW, et al. (2021) Regulatory functions of NK cells during infections and cancer. J Leukoc Biol 109 (1), 185–194. [DOI] [PubMed] [Google Scholar]
- 2.Cerwenka A, and Lanier LL, (2016) Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol 16 (2), 112–23. [DOI] [PubMed] [Google Scholar]
- 3.Ochoa MC, et al. (2017) Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol 95 (4), 347–355. [DOI] [PubMed] [Google Scholar]
- 4.Rydyznski CE, and Waggoner SN, (2015) Boosting vaccine efficacy the natural (killer) way. Trends Immunol 36 (9), 536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodier MR, and Riley EM, (2021) Regulation of the human NK cell compartment by pathogens and vaccines. Clin Transl Immunology 10 (1), e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rydyznski C, et al. (2015) Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nat Commun 6, 6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rydyznski CE, et al. (2018) Affinity Maturation Is Impaired by Natural Killer Cell Suppression of Germinal Centers. Cell Rep 24 (13), 3367–3373 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest C, et al. (2020) NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development. Vaccines (Basel) 8 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Fontecha A, et al. (2004) Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 5 (12), 1260–5. [DOI] [PubMed] [Google Scholar]
- 10.Farsakoglu Y, et al. (2019) Influenza Vaccination Induces NK-Cell-Mediated Type-II IFN Response that Regulates Humoral Immunity in an IL-6-Dependent Manner. Cell Rep 26 (9), 2307–2315 e5. [DOI] [PubMed] [Google Scholar]
- 11.Wagstaffe HR, et al. (2018) Vaccinating for natural killer cell effector functions. Clin Transl Immunology 7 (1), e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagstaffe HR, et al. (2020) Ebola virus glycoprotein stimulates IL-18-dependent natural killer cell responses. J Clin Invest 130 (7), 3936–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, et al. (2020) Protective Immune Responses Elicited by Deglycosylated Live-Attenuated Simian Immunodeficiency Virus Vaccine Are Associated with IL-15 Effector Functions. J Immunol 205 (5), 1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard LM, et al. (2017) Cell-Based Systems Biology Analysis of Human AS03-Adjuvanted H5N1 Avian Influenza Vaccine Responses: A Phase I Randomized Controlled Trial. PLoS One 12 (1), e0167488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li APY, et al. (2021) Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines 6 (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coccia M, et al. (2017) Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNgamma response promoting vaccine immunogenicity. NPJ Vaccines 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iglesias-Guimarais V, et al. (2020) IFN-Stimulated Gene 15 Is an Alarmin that Boosts the CTL Response via an Innate, NK Cell-Dependent Route. J Immunol 204 (8), 2110–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netea MG, et al. (2016) Trained immunity: A program of innate immune memory in health and disease. Science 352 (6284), aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinnijenhuis J, et al. (2014) BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol 155 (2), 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palgen JL, et al. (2019) NK cell immune responses differ after prime and boost vaccination. J Leukoc Biol 105 (5), 1055–1073. [DOI] [PubMed] [Google Scholar]
- 21.Palgen JL, et al. (2021) Optimize Prime/Boost Vaccine Strategies: Trained Immunity as a New Player in the Game. Front Immunol 12, 612747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mooney JP, et al. (2020) Natural Killer Cells Dampen the Pathogenic Features of Recall Responses to Influenza Infection. Front Immunol 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horowitz A, et al. (2010) NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol 185 (5), 2808–18. [DOI] [PubMed] [Google Scholar]
- 24.McKinstry KK, et al. (2019) Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. PLoS Pathog 15 (8), e1007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes BF, et al. (2012) Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366 (14), 1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora G, et al. (2018) NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavian N, et al. (2020) Vaccination with ADCC activating HA peptide epitopes provides partial protection from influenza infection. Vaccine 38 (37), 5885–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau CM, et al. (2020) Selective induction of antibody effector functional responses using MF59-adjuvanted vaccination. J Clin Invest 130 (2), 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips B, et al. (2018) Adjuvant-Dependent Enhancement of HIV Env-Specific Antibody Responses in Infant Rhesus Macaques. J Virol 92 (20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Martino M, et al. (2019) Immune Response to Mycobacterium tuberculosis: A Narrative Review. Frontiers in Pediatrics 7 (350). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esin S, and Batoni G, (2015) Natural Killer Cells: A Coherent Model for Their Functional Role in Mycobacterium tuberculosis Infection. Journal of Innate Immunity 7 (1), 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagstaffe HR, et al. (2021) Durable natural killer cell responses after heterologous two-dose Ebola vaccination. NPJ Vaccines 6 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun JC, et al. (2009) Adaptive immune features of natural killer cells. Nature 457 (7229), 557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolle A, et al. (2014) IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest 124 (12), 5305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammer Q, et al. (2018) Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol 19 (5), 453–463. [DOI] [PubMed] [Google Scholar]
- 36.Schlums H, et al. (2015) Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42 (3), 443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, et al. (2015) Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42 (3), 431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, et al. (2013) Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 190 (4), 1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romee R, et al. (2016) Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 8 (357), 357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gang M, et al. (2020) Memory-like natural killer cells for cancer immunotherapy. Semin Hematol 57 (4), 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paust S, et al. (2010) Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11 (12), 1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillard GO, et al. (2011) Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog 7 (8), e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeves RK, et al. (2015) Antigen-specific NK cell memory in rhesus macaques. Nat Immunol 16 (9), 927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikzad R, et al. (2019) Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol 4 (35). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijaya RS, et al. (2021) HBV vaccination and HBV infection induces HBV-specific natural killer cell memory. Gut 70 (2), 357–369. [DOI] [PubMed] [Google Scholar]
- 46.Jost S, et al. (2020) Human antigen-specific memory natural killer cell responses develop against HIV-1 and influenza virus and are dependent on MHC-E restriction. bioRxiv. [Google Scholar]
- 47.Wijaya RS, et al. (2020) Hepatitis C virus eradication with interferon free, DAA-based therapy results in KLRG1+, hepatitis C virus-specific memory natural killer cells. J Infect Dis. [DOI] [PubMed] [Google Scholar]
- 48.Stary V, et al. (2020) A discrete subset of epigenetically primed human NK cells mediates antigen-specific immune responses. Sci Immunol 5 (52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soderquest K, et al. (2011) Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol 186 (6), 3304–8. [DOI] [PubMed] [Google Scholar]
- 50.Peppa D, et al. (2013) Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 210 (1), 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardoso Alves L, et al. (2020) Non-apoptotic TRAIL function modulates NK cell activity during viral infection. EMBO Rep 21 (1), e48789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waggoner SN, et al. (2011) Natural killer cells act as rheostats modulating antiviral T cells. Nature 481 (7381), 394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook KD, et al. (2015) NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol 98 (2), 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blass E, et al. (2018) Adenovirus Vector Vaccination Impacts NK Cell Rheostat Function following Lymphocytic Choriomeningitis Virus Infection. J Virol 92 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatfield SD, et al. (2018) Weak vaccinia virus-induced NK cell regulation of CD4 T cells is associated with reduced NK cell differentiation and cytolytic activity. Virology 519, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muyanja E, et al. (2014) Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest 124 (7), 3147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazmin D, et al. (2017) Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A 114 (9), 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Mot L, et al. (2020) Transcriptional profiles of adjuvanted hepatitis B vaccines display variable interindividual homogeneity but a shared core signature. Sci Transl Med 12 (569). [DOI] [PubMed] [Google Scholar]
- 59.Bradley T, et al. (2018) RAB11FIP5 Expression and Altered Natural Killer Cell Function Are Associated with Induction of HIV Broadly Neutralizing Antibody Responses. Cell 175 (2), 387–399 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huhn O, et al. (2020) Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat Commun 11 (1), 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali A, et al. (2019) Mutually assured destruction: the cold war between viruses and natural killer cells. Curr Opin Virol 34, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huntington ND, et al. (2020) The cancer-natural killer cell immunity cycle. Nat Rev Cancer 20 (8), 437–454. [DOI] [PubMed] [Google Scholar]
- 63.Mace EM, and Orange JS, (2019) Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol Rev 287 (1), 202–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda K, and Dennert G, (1993) The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med 177 (1), 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kucuksezer UC, et al. (2021) The Role of Natural Killer Cells in Autoimmune Diseases. Front Immunol 12, 622306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spicer JA, et al. (2020) Inhibition of the Cytolytic Protein Perforin Prevents Rejection of Transplanted Bone Marrow Stem Cells in Vivo. J Med Chem 63 (5), 2229–2239. [DOI] [PubMed] [Google Scholar]
- 67.Hiroyasu S, et al. (2021) Granzyme B inhibition reduces disease severity in autoimmune blistering diseases. Nat Commun 12 (1), 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang W, et al. (2011) Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol 187 (2), 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellici TF, et al. (2021) Small-molecule modulators of serine protease inhibitor proteins (serpins). Drug Discov Today 26 (2), 442–454. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe M, et al. (1993) Cholera toxin inhibits lethal hit stage of natural killer cell-mediated cytotoxicity. Microbiol Immunol 37 (4), 317–23. [DOI] [PubMed] [Google Scholar]
- 71.Huang A, et al. (2019) Progranulin prevents regulatory NK cell cytotoxicity against antiviral T cells. JCI Insight 4 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poznanski SM, and Ashkar AA, (2019) What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front Immunol 10, 1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klarquist J, et al. (2018) Clonal expansion of vaccine-elicited T cells is independent of aerobic glycolysis. Sci Immunol 3 (27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weisel FJ, et al. (2020) Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat Immunol 21 (3), 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meza Guzman LG, et al. (2020) Natural Killer Cells: Tumor Surveillance and Signaling. Cancers (Basel) 12 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mason LH, et al. (2006) Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol 176 (11), 6615–23. [DOI] [PubMed] [Google Scholar]
- 77.Oykhman P, et al. (2013) Requirement and redundancy of the Src family kinases Fyn and Lyn in perforin-dependent killing of Cryptococcus neoformans by NK cells. Infect Immun 81 (10), 3912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teng R, et al. (2020) Hypoxia Impairs NK Cell Cytotoxicity through SHP-1-Mediated Attenuation of STAT3 and ERK Signaling Pathways. Journal of Immunology Research 2020, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ni J, et al. (2020) Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1alpha Unleashes NK Cell Activity. Immunity 52 (6), 1075–1087 e8. [DOI] [PubMed] [Google Scholar]
- 80.Delconte RB, et al. (2016) CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 17 (7), 816–24. [DOI] [PubMed] [Google Scholar]
- 81.Daher M, et al. (2021) Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 137 (5), 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lang PA, et al. (2012) Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A 109 (4), 1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nielsen N, et al. (2012) Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS One 7 (2), e31959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crouse J, et al. (2014) Type I Interferons Protect T Cells against NK Cell Attack Mediated by the Activating Receptor NCR1. Immunity 40 (6), 961–73. [DOI] [PubMed] [Google Scholar]
- 85.Guo H, et al. (2016) Deletion of Slam locus in mice reveals inhibitory role of SLAM family in NK cell responses regulated by cytokines and LFA-1. J Exp Med 213 (10), 2187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McQuaid SL, et al. (2020) Low-dose IL-2 induces CD56(bright) NK regulation of T cells via NKp44 and NKp46. Clin Exp Immunol 200 (3), 228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pallmer K, et al. (2019) NK cells negatively regulate CD8 T cells via natural cytotoxicity receptor (NCR) 1 during LCMV infection. PLoS Pathog 15 (4), e1007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gazit R, et al. (2006) Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol 7 (5), 517–23. [DOI] [PubMed] [Google Scholar]
- 89.Cardoso Alves L, et al. (2020) Non-apoptotic TRAIL function modulates NK cell activity during viral infection. EMBO reports 21 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou J, et al. (2019) Liver-Resident NK Cells Control Antiviral Activity of Hepatic T Cells via the PD-1-PD-L1 Axis. Immunity 50 (2), 403–417 e4. [DOI] [PubMed] [Google Scholar]
- 91.Wong E, et al. (2018) Migratory Dendritic Cells, Group 1 Innate Lymphoid Cells, and Inflammatory Monocytes Collaborate to Recruit NK Cells to the Virus-Infected Lymph Node. Cell Rep 24 (1), 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huot N, et al. (2017) Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med 23 (11), 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ali A, et al. (2021) Natural killer cell immunosuppressive function requires CXCR3-dependent redistribution within lymphoid tissues. bioRxiv, 2021.05.11.443590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parham P, and Guethlein LA, (2018) Genetics of Natural Killer Cells in Human Health, Disease, and Survival. Annu Rev Immunol 36, 519–548. [DOI] [PubMed] [Google Scholar]
- 95.Ludigs K, et al. (2016) NLRC5 shields T lymphocytes from NK-cell-mediated elimination under inflammatory conditions. Nat Commun 7, 10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramsuran V, et al. (2018) Elevated HLA-A expression impairs HIV control through inhibition of NKG2A-expressing cells. Science 359 (6371), 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sivori S, et al. (2019) Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol 16 (5), 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C, and Liu Y, (2020) Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy. Front Immunol 11, 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leavenworth JW, et al. (2011) Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc Natl Acad Sci U S A 108 (35), 14584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu HC, et al. (2017) Lymphocytes Negatively Regulate NK Cell Activity via Qa-1b following Viral Infection. Cell Rep 21 (9), 2528–2540. [DOI] [PubMed] [Google Scholar]
- 101.Morandi F, et al. (2011) Soluble HLA-G dampens CD94/NKG2A expression and function and differentially modulates chemotaxis and cytokine and chemokine secretion in CD56bright and CD56dim NK cells. Blood 118 (22), 5840–50. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Q, et al. (2018) Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 19 (7), 723–732. [DOI] [PubMed] [Google Scholar]
- 103.Hsu J, et al. (2018) Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang X, and Kumar V, (2019) Advances in the Study of CD8+ Regulatory T Cells. Crit Rev Immunol 39 (6), 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ndhlovu LC, et al. (2012) Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119 (16), 3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hudak JE, et al. (2014) Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol 10 (1), 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hornung V, et al. (2002) Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168 (9), 4531–7. [DOI] [PubMed] [Google Scholar]


