Abstract
Objective:
To review published oral microbiome studies and create a comprehensive list of bacterial species found in saliva and dental plaque among healthy children and adults associated with presence of carious lesions and caries-free state (oral health).
Design:
This review followed PRISMA-ScR guidelines. We searched published studies querying PUBMED and EMBASE using the following keywords: (plaque OR saliva) AND caries AND (next generation sequencing OR checkerboard OR 16s rRNA or qPCR). Studies were limited to human studies published in English between January 1, 2010 and June 24, 2020 that included ≥ 10 caries active and ≥ 10 caries-free participants, and assessed the entire bacterial community.
Results:
Our search strategy identified 298 articles. After exclusion criteria, 22 articles remained; we considered 2 studies that examined saliva and plaque as separate studies, for a total of 24 studies. Species associated with caries or oral health varied widely among studies reviewed, with notable differences by age and biologic sample type. No bacterial species was associated with caries in all studies. Streptococcus mutans was found more frequently among those with caries (14/24 (58.3%)) and Fusobacterium periodonticum was found more frequently among those that were caries-free (5/24 (20.8%)).
Conclusion:
No bacterial species was associated with caries or oral health across all studies supporting multiple pathways to cariogenesis. However, the variation may be due to sampling at different time points during caries development, varying methods of specimen sampling, storage, sequencing or analysis or differences in host factors such as age.
Keywords: oral microbiome, dental caries, 16S rRNA
Introduction
Dental caries are the most prevalent oral disease in both children and adults (NIDCR, 2018), and can greatly compromise quality of life (World Health Organization, 2020). Caries result from an interaction between cariogenic species in the mouth, poor oral hygiene and a high sugar diet (Baker & Edlund, 2019), but can be prevented or limited by regular cleaning to remove cariogenic microbes within the oral cavity, increasing the acid-resistance of the teeth (via sealants), and controlling the carbohydrate composition of the diet (Balakrishnan et al., 2000). Improved understanding of which taxa are cariogenic, and which are protective may lead to additional strategies for oral disease prevention and treatment.
Although dental caries is associated with presence of acid producing bacterial species, particularly Streptococcus mutans, these species appear to be neither necessary nor sufficient. Therefore, the field has embraced an ecological hypothesis of cariogenesis, that complex interactions among multiple microbes found in the oral cavity are required for caries development (Hurley et al., 2019; Marsh, 2018). Studies using methods that do not require microbial culture have identified - in saliva and dental plaque - multiple bacterial species as associated with dental caries (Chen & Jiang, 2014; Jiang et al., 2011). However, these differences have yet to be comprehensively reviewed. At the time of writing, we found only one review summarizing results of studies identifying cariogenic species and species associated with caries absence (oral health), and it focused on children (Fakhruddin et al., 2019). Our scoping review fills this gap, by creating a comprehensive list of bacterial species associated with caries and oral health found in saliva and dental plaque among children and adults. We focused our review on results from oral microbiome studies that identified species associated with caries-active and caries-free states among populations free of underlying illnesses.
Materials and Methods
Search Strategy
We followed the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta Analyses Extension for Scoping Reviews) guidelines and registered the review protocol with Open Science Framework. We searched published studies querying the two most relevant databases, PUBMED and EMBASE (Wong et al., 2006), using the following keywords: (plaque OR saliva) AND caries AND (next generation sequencing OR checkerboard OR 16s rRNA or qPCR). Studies were limited to human studies published in English between January 1, 2010 and June 24, 2020. Initially, the PUBMED database was searched on June 24, 2020 followed by a search on the EMBASE database on August 20, 2020. D.B and B.F reviewed each full text article for inclusion (Figure 1). Studies were included if they met the following criteria: (1) a human study; (2) comparison of a healthy (free from any diagnosed disease or illness) caries-free population to a caries-active population; (3) sample size greater than 10 for each population; (4) assessment of the entire bacterial community (sequencing, DGGE); and (5) had taxa resolved to the species level.
Figure 1.
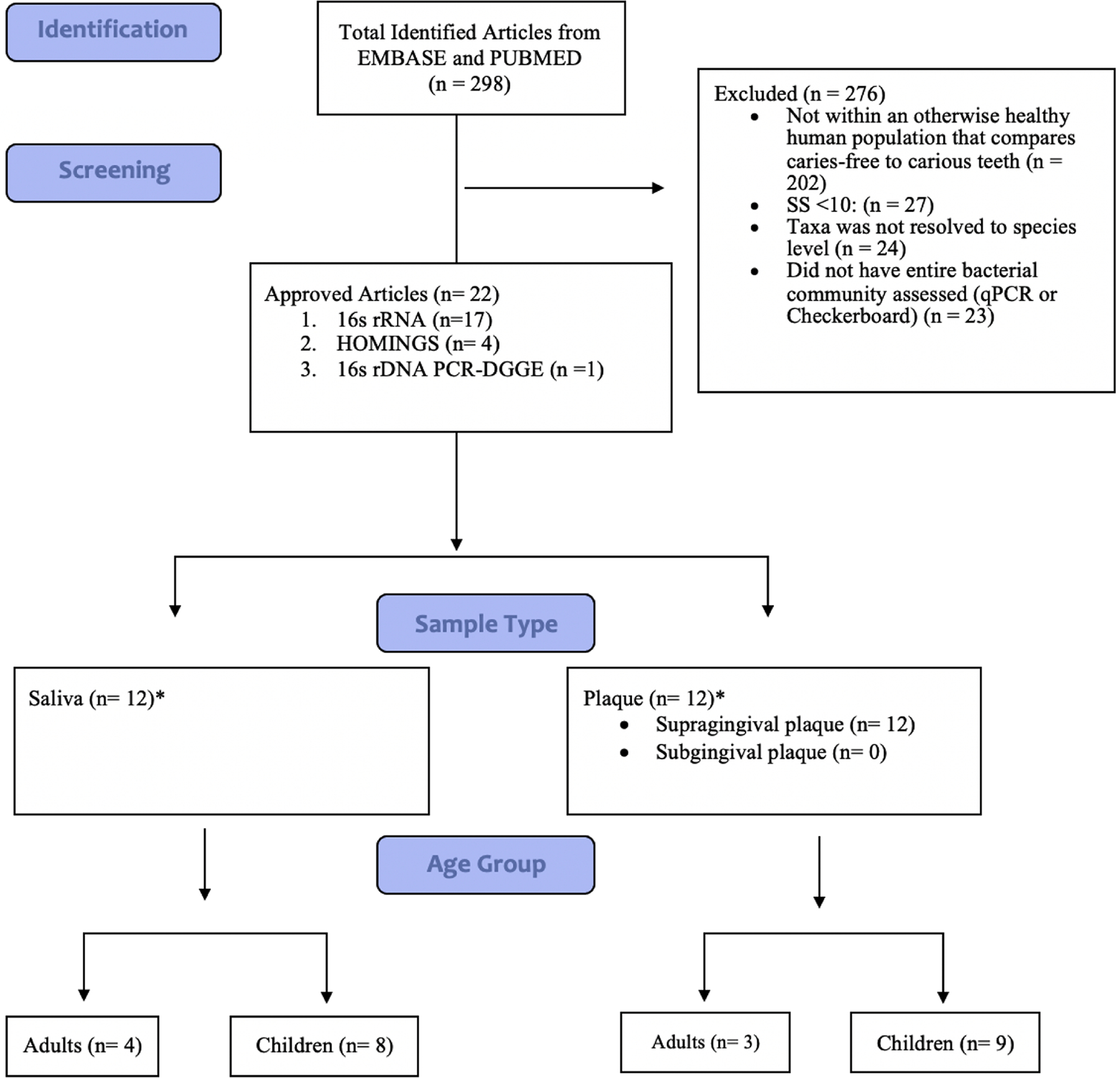
Schematic diagram depicting literature search process for oral microbiome studies conducted in the past 10 years from EMBASE and PUBMED.
*Two studies that included saliva and plaque sample were treated as two separate studies each
Data Synthesis
From each study, we included all taxa occurring statistically significantly more frequently among the caries-active group than caries-free group (cariogenic) or vice versa (oral health) and noted if the comparisons were corrected for multiple testing. We calculated the frequency of each taxa across all included studies, further stratifying by age group (children versus adults) and sample type (saliva versus plaque).
Results
Study Selection
Our search strategy identified 298 identified articles (Additional File 1). After applying the exclusion criteria, 22 articles remained (Figure 1, Tables 1 and 2). Two studies were longitudinal; the remainder compared caries active to caries-free individuals at a single point in time. Six studies were conducted in adults and 16 conducted in children. Seventeen articles used 16s rRNA amplicon sequencing, 4 used HOMINGS, and one used 16s rDNA PCR-DGGE. 10 analyzed saliva (see Table 1 for stimulated vs unstimulated), 10 analyzed supragingival plaque and 2 analyzed both saliva and plaque. For presentation, we included each of the 2 studies analyzing plaque and saliva as separate studies of plaque and saliva, giving us a denominator of 24 studies. Only 8 studies corrected for multiple comparisons.
Table 1.
Characteristics of included supragingival plaque studies (n = 12)
| First Author, Year | Sample Type | Storage of Sample | Study Population | Caries Definition | Sample Size | Method of Analysis | Caries-Active Taxa | Caries-Free Taxa |
|---|---|---|---|---|---|---|---|---|
| Agnello et al., 2017 | Plaque samples were collected from each subject by swabbing a sterile interdental brush on all available tooth surfaces | Samples were immediately frozen at −80 °C in 15% glycerol until used for analysis. | Children; Age <72 months | S-ECC: had severe tooth decay involving multiple primary teeth and were recruited from the Misericordia Health Centre in Winnipeg, Canada, on the day of their scheduled dental rehabilitative surgery under general anesthesia. Caries-free: children were assessed to ensure that there was no evidence of caries (dmft = 0, no cavitations or white spot lesions) | n = 50; S-ECC: n = 30; Caries-free children: n = 20 | 16S rRNA ; V3–V4; Illumina; Statistical Significance: Benjamini Hochberg; p < 0.05 |
Streptococcus mutans, Veillonella sp. HOT-780, Porphyromonas HOT 284 |
Streptococcus gordonii, Streptococcus sanguinis |
| de Jesus et al., 2020 | Supragingival plaque | Samples were dislodged into 1 mL of RNAprotect Bacteria Reagent (Qiagen) and immediately frozen at −80 °C until further analysis. | Children; Ages <72 months | All children with S-ECC were recruited at the Misericordia Health Centre on the day of their dental rehabilitative surgery; Caries-free: dmft index equal to 0; no decayed, missing, or filled primary tooth surface | n=80; Severe Early Childhood Caries: n = 40; Caries-Free: n = 40 | 16S rRNA and ITS1 rRNA gene amplicon sequencing; V4; Illumina MiSeq; Statistical Significance: p < 0.05 |
Streptococcus mutans, Veillonella dispar, Veillonella sp. oral taxon 780 |
Candida dubliniensis, Corynebacterium durum, Lautropia mirabilis |
| Eriksson et al., 2018 | Supragingival biofilm collected from all accessible tooth surfaces; whole stimulated saliva | Supragingival biofilm was collected from all accessible tooth surfaces with sterile wooden toothpicks and pooled by subject into 100 μL of TE buffer (10mM Tris, 1mM ethylenediaminetetraacetic acid [EDTA], pH 7.6). Whole saliva collected for 3 min into ice-chilled sterile test tubes while subjects chewed paraffin wax. All samples were stored at −80 °C. | Adolescents; Age 17 | Numbers of tooth surfaces that had caries in the enamel or into the dentine, had a filling, or were missing were recorded from visual and radiographic examinations. Caries in the enamel was scored according to a visual color change or demineralization within the enamel on bitewing X-rays. Caries in the dentine was scored according to local breakdown in the enamel or a cavity or when demineralization extended into the dentine on bitewing X-rays. | n=154; 71 present caries (46%); 82 no caries. | Multiplex 16S rDNA amplicon sequencing with HOMINGS protocol ; V3–V4; Illumina Miseq Sequencing; Statistical Significance: P ≤ 0.005 | Only S. mutans was significantly (p<=0.005) more abundant in saliva and tooth biofilm samples from subjects with caries than those from caries-free adolescents | NONE FOUND |
| Ihara et al., 2019 | In Vivo Dental Plaque formed on hydroxyapatite disks | The disks were retrieved from the oral cavity 6 h later and placed in a microcentrifuge tube after rinsing with phosphate-buffered saline. | Adults; Ages 20–32 | Subjects with no caries-experienced teeth; Subjects with a moderate number of caries-experienced teeth (1 to 7); Subjects with a high number of caries-experienced teeth (>=8) | n=74; No caries-experienced teeth: n = 20; Moderate number of caries-experienced teeth n = 36; High number of caries-experienced teeth: n = 18 | Full length16S rRNA gene sequences of plaque, V2–V2 from saliva and plaque, and quantitative PCR to determine total bacterial amounts; V1–V9 (full length) and sequencing of V1–V2; PacBio Sequel for full length sequencing, Ion PGM for V1–V2 region of 16S rRNA; Statistical Significance: ---- |
NONE FOUND | NONE FOUND |
| Jiang et al., 2011 | Healthy subjects: pooled Supragingival Plaque from buccal surfaces and accessible proximal surfaces of primary molars. Caries subject: pooled Supragingival Plaque from buccal and accessible proximal surfaces of intact enamel adjacent to carious lesions in primary molars. | The collected plaque samples were wiped onto endodontic paper, was immediately transported on ice to the microbiology laboratory and stored at −20 C before extraction of the genomic DNA. | Children; Ages 3–5 | Caries-Susceptible: dmfs >10; Caries Moderate: 4 <dmfs < 6; Caries-Free: dmfs = 0 | n = 45; 15 Caries-Susceptible; 15 Caries Moderate; 15 Caries-Free | 16s rDNA PCR-DGGE combined with primers specific for oral streptococci; V3–V5; Statistical Significance: p<0.05 |
NONE FOUND |
Streptococcus mitis, Streptococcus oralis, Streptococcus sanguinis |
| Richards et al., 2017 | Supragingival plaque | Each plaque sample was obtained by pooling material from at least two different tooth sites of similar health conditions using sterile periodontal curettes | Children; Ages 2–7 | Caries active with enamel lesions only (CAE) (no decayed teeth [DT] and ≥0 missing and filled teeth [MFT]), and caries active with at least two cavitated, unrestored dentin carious lesions (CA) (≥2 DT and ≥0 MFT) | n=55; | HOMINGS and 16s rRNA sequencing ; V3–V4; Illumina Miseq Sequencing; Statistical Significance: p<0.05 |
Streptococcus mutans ST-15, Bergeyella sp oral taxon 322 BE-02, Veillonella parvula, Streptococcus anginosus, Prevotella salivae, Prevotella maculosa, Megasphaera micronuciformis, Scardovia Genus probe GP-075, Selenomonas sp oral taxon 149 SE-13, Cryptobacterium curtum, Lactobacillus fermentum, Lactobacillus Genus probe 2 GP-045, Lactobacillus Genus probe 4 GP-047 |
Ottowia sp oral taxon 894 OT-02, Neisseria bacilliformis NE-01, TM7[G-3] sp oral taxon 351, Streptococcus Genus probe 3GP-128 |
| Schoilew et al., 2019 | Pooled Supragingival Plaque | The collection site was isolated with cotton rolls and gently air dried. Sterile curettes were used for sampling of supragingival plaque from healthy enamel on the buccal surface of the first and second maxillary molars. The samples were pooled in an empty and sterile 1.5 ml microcentrifuge tube and frozen (−25°C) until further analysis. | Adults; Ages 18–80 | Active Caries Lesions: cavitated lesions as well as white spot lesions with an opaque, chalky surface. Inactive Caries Lesions: white spots with an intact, smooth and glossy surface and brown spots. | n = 46; n = 19 subjects without caries experience (NH; DMFT = 0); n = 27 subjects with ‘caries experience’ (CE; DMFT > 0 [F(T)> 0; D(T)=0]) | 16s rRNA Amplicon Sequencing; V4; Illumina Miseq; Statistical Significance: p < 0.05; Benj amini-Hochberg corrected |
NONE FOUND |
Capnocytophaga ochracea, Corynebacterium matruchotii, Porphyromonas pasteri, Selenomonas noxia |
| Tanner et al., 2011 | Plaque samples were taken with sterile wooden toothpicks from the buccal and interproximal surfaces of molars to include plaque from carious lesions in the severe ECC children. | Each plaque sample was put in a 5-ml airtight vial containing 2 ml prereduced anaerobically sterilized (PRAS) Ringer’s solution (49) with 3-mm glass beads (44) and placed in insulated bags with frozen freezer blocks. Samples were transported to the microbiology laboratory and processed within 4 h of sampling. | Children; Ages 2–6 years old | Children with severe ECC (14) had extensive caries in the primary dentition that affected over 36% of tooth surfaces with an average of 4 pulpally involved teeth (21) and were scheduled for restorative treatment under general anesthesia. Caries-free children, determined by visual and radiographic examination, had no cavities or enamel white spot lesions, which can represent early stages of tooth enamel demineralization. | N = 82; Severe ECC: n = 42; caries-free children: n = 40 | Partial sequences for 16S rRNA gene. Isolate sequence compared with taxon sequences in HOMD; Statistical Significance: p<0.05 |
Actinomyces gerencseriae, Scardovia wiggsiae, Streptococcus cristatus, Streptococcus mutans, Veillonella parvula |
NONE FOUND |
| Teng et al., 2015 | Dental plaque biofilm | Children; Age around 4 | Microbiota with dmfs of zero were designated as “Healthy” (“H”); otherwise were as “Caries” (“C”), which consist of “low caries” (1 % dmfs < 6) and “severe caries” (dmfs >=6) | n = 50; (i) The “stay healthy” (H2H) group: n = 17 subjects (94 samples) maintained healthy state, with dmfs staying zero. (ii) The “caries-onset” (H2C) group: n = 21 subjects (120 samples) underwent the transition from healthy to caries-active state. (iii) The “caries-progression” (C2C) group: n = 12 subjects (70 samples) | 16S rRNA ; V1–V3; Pyrosequencing; Statistical Significance: p < 0.05 |
Streptococcus spp., Prevotella spp., Veillonella spp., |
NONE FOUND | |
| Xiao et al., 2018 | Supragingival dental plaque was collected from the whole dentition with a standard dental scaler. | Previously established methods (Grier et al., 2017; Merkley et al., 2015) were used to perform oral microbiome sequencing and related bioinformatics analysis. Total genomic DNA from clinical samples (100ul saliva and 200ul plaque suspension) was extracted using Quick- DNATM Fecal/Soil Microbe Miniprep Kit (ZymoResearch, Irvine, Ca) (Dardas et al., 2014; Merkley et al., 2015; Zhu et al., 2013) | Children Ages Unspecified: Average Age Children SECC = 4.0 +– 0.9, CF = 3.8 +– 1.6; | S-ECC was diagnosed per criteria of the American Academy of Pediatric Dentistry. The number of carious teeth and surfaces was charted with index variables of decayed, missing due to decay, or filled, according to the codes proposed by the World Health Organization’s Oral Health Surveys Basic Methods (1997). Plaque status for mothers and children were assessed separately according to criteria modified from Silness and Loe (1964) and Ribeiro Ade et al. (2002) |
n = 36 plaque samples; children S-ECC: n = 21 and children CF: n = 15 | 16s rRNA amplicon sequencing; V1–V3 ; Illumina; Statistical Significance: p<0.05 |
Candida albicans, Streptococcus mutans, Veillonella atypica, Veillonella dispar, Veillonella parvula |
S. oral taxon B66, Actinomyces viscosus, Actinomyces naeslundii, Actinomyces oris, Leptotrichia BU064 |
| Zhang et al., 2015 | Supragingival Plaque from surface of intact enamel and surface of a deep caries lesion in non healthy subjects, and from at least three sites (including posterior and anterior teeth) in healthy samples. | The samples were transported on ice to the laboratory and stored at−80°C until used. | Children; Ages 3–6 | Caries group subjects: had more than 3 cavitated caries teeth which includes at least one carious primary molar(codes 5 or 6 based on the classification of the carious status of the ICDAS); Healthy group subjects: did not have caries in any teeth (code 0 based on the classification of the carious of the ICDAS) | n = 20 (10 twin pairs); healthy individuals: n = 10; Caries Individuals: n = 10 | Pyrosequencing of the 16s rRNA gene amplicons; V3–V5; Pyrosequencing; Statistical Significance: p<0.05 | NONE FOUND | NONE FOUND |
| Zheng et al., 2018 | Supragingival plaque; Caries group samples were pooled from the lingual and proximal surfaces of decayed teeth were pooled and samples from the dental plaque in caries lesions were pooled. Samples from the caries-free group and the mothers group were collected from the labial, lingual and proximal surfaces of healthy teeth (including anterior and posterior teeth). | All supragingival plaque samples were obtained by scraping the tooth surfaces with a sterile metal excavator. The metal excavator was immersed in a sterile 1.5 ml eppendorf tube containing 1 ml of Ringer’s solution. The samples were transported on ice to the laboratory, and stored at −20°C until genomic DNA extraction. | Children; Ages 3–6 | Caries group: had two or more cavitated teeth; Caries-free group: did not have any carious teeth in their oral cavity | n = 31; Dental caries: n = 16 ; Caries-free: n = 15 | 16S rRNA amplicons; V3–V4 ; Illumina sequencing in the Human Oral Microbial Identification Next Generation Sequencing (NGS) HOMINGS Laboratory ; Statistical Significance: Benjamini-Hochberg, adjusted P-value <0.0001 |
Actinomyces massiliensis, Lactobacillus fermentum, Neisseria sicca, Prevotella oulorum, Streptococcus mutans, Veilonella dispar |
Actinomyces israelii, Capnocytophaga sputigena, Fusobacterium periodonticum, Haemophilus parainfluenzae, Leptotrichia sp ot 498, Porphyromonas sp ot 279 |
Table 2.
Characteristics of included salivary studies (n = 12)
| First Author, Year | Sample Type | Storage of Sample | Study Population | Caries Definition | Sample Size | Method of Analysis | Caries-Active Taxa | Caries-Free Taxa |
|---|---|---|---|---|---|---|---|---|
| Belstrøm et al., 2017 | Stimulated Saliva | Participant was instructed to chew and spit continuously for 3 min into a sterile plastic cup, after which time the collected saliva from the cup was stored at −80°C | Adults ; Ages 18–76; Unspecified | Caries was registered according to Moller and Poulsen as manifest caries on tooth and surface level expressed as DMFT (decayed, missed, filled tooth) and DMFS (decayed, missed, filled surfaces) | n=88; Healthy: n = 57; Caries: n = 31 | HOMINGS; bacterial identification was performed using HOMINGS.; V3–V4; Illumina Miseq Sequencing; Statistical Significance: Benjamini-Hochberg correction was used to control for multiple comparisons; For this analysis, an adjusted p-value of <0.0001 was considered statistically significant |
Bifidobacterium dentium, Lactobacillus salivarius, Streptococcus sp ot068, Parascardovia denticolens |
Fusobacterium periodonticum, Porphyromonas sp ot279, Bergeyella sp ot322, Alloprevotella sp ot914, Stomatobaculum sp ot097, Leptotrichia sp ot223, Lachnoanaerobacul um umeaense |
| Belstrøm, Paster, et al., 2016 | Stimulated Saliva | The subjects expectorated for 1 min, and the saliva was discarded. For an additional 3 min, subjects continued to expectorate, and the saliva was collected in a plastic cup and stored at −80°C. | Adults; Ages 22–70; Unspecified | Dental caries was defined as manifest untreated ≥3 surfaces caries | n=30; Periodiontis: n = 10 ; Dental caries: n = 10; Orally healthy: n = 10 | HOMINGS; DNA isolation was performed following specifications of the protocol: Pathogen_Universal_200 (Roche, Mannheim, Germany) (26), and the HOMInGs technique was used for microbial analysis; V3–V4; Illumina Miseq Sequencing; Statistical Significance: p<0.05 in combination with a FDR value of 0 was considered statistically significant |
Streptococcus mutans, Streptococcus Genus probe 4, Streptococcus Genus probe 1, Lactobacillus Genus probe 1, L. vaginalis, Actinomyces sp oral taxon 448, Veillonella sp oral taxon 917 |
NONE FOUND |
| Dashper et al., 2019 | Unstimulated Saliva | Unstimulated saliva, up to 5 mL, was collected from infants using a pipette and from children by passive drooling into a sterile tube. Maternal saliva was collected at the same time as their infant’s second clinical assessment at 7.7 months-of-age. The samples were rapidly frozen at −20 °C, stored and periodically transported to the Melbourne Dental School where they were stored at −80 °C in secure freezers until further analysis. | Children; ages 2 months to 4 years | Caries-Active: ECC as defined as one or more ICDAS II score of 2 or above Caries-Free: no clinically detectable disease |
N=134 children were characterized at six time-points from two months up to four years-of-age. | 16S rRNA gene sequencing; V4; Ion Amplicon Library Preparation Fusion Method; Statistical Significance: p <0.05 | 39 months of age Streptococcus mutans 48.6 months-of-age Streptococcus mutans Leptotrichia shahii, Scardovia wiggsiae, Leptotrichia IK040 Increased abundances for development of disease from 39 months-of-age (healthy) to 48.6 months-of age (diseased): Streptococcus mutans, Streptococcus sobrinus, Veillonella parvula |
39 months of age Fusobacterium periodonticum, Stomatobaculum longum, Bergeyella 602D02 48.6 months of age Prevotella shahii, Prevotella pallens, Stomatobaculum longum, Porphyromonas CW034 and Capnocytophaga AM20030 Decreased abundances for development of disease from 39 months-of-age (healthy) to 48.6 months-of age (diseased): Prevotella nigrescens, three species of Leptotricia and Actinobaculum 12B759 |
| Eriksson et al., 2018 | Supragingival biofilm collected from all accessible tooth surfaces; whole stimulated saliva | Supragingival biofilm was collected from all accessible tooth surfaces with sterile wooden toothpicks and pooled by subject into 100 μL of TE buffer (10mM Tris, 1mM ethylenediaminetetraac etic acid [EDTA], pH 7.6). Whole saliva collected for 3 min into ice-chilled steril test tubes while subjects chewed paraffin wax. All samples were stored at −80 °C. | Adults; Age 17; Unspecified | Numbers of tooth surfaces that had caries in the enamel or into the dentine, had a filling, or were missing were recorded from visual and radiographic examinations. Caries in the enamel was scored according to a visual color change or demineralization within the enamel on bitewing X-rays. Caries in the dentine was scored according to local breakdown in the enamel or a cavity or when demineralization extended into the dentine on bitewing X-rays. |
n = 154; 71 present caries (46%); 82 no caries. [This is confusing as the text says 154, but numbers in the table add to 153) | Multiplex 16S rDNA amplicon sequencing with HOMINGS protocol ; V3–V4 ; Illumina Miseq Sequencing; Statistical Significance: P ≤ 0.005 | Only S. mutans was significantly (p<=0.005) more abundant in saliva and tooth biofilm samples from subjects with caries than those from caries-free adolescents | NONE FOUND |
| Gussy et al., 2020 | Unstimulated saliva | Unstimulated saliva (1–5 mL) was collected at every wave from infants using a pipette and from children by passive drooling into a sterile tube. Saliva samples were frozen (−20 °C) and periodically transported to laboratory for long term storage at −80 °C. | Children; Ages 2 months-9 years | Caries-Active: d3–6mfs defined as decayed, missing and filled surface index with ICDAS II codes of 3 or greater (i.e. cavitation) | N=419 children were recruited at baseline and were invited to participate in follow-up studies over a period five years of data collection. At baseline, 86 had reported at least one surface tooth cavitation (d3–6mfs) | 16S rRNA gene sequencing; V4; Ion Torrent PGM massively-parallel-sequencer; Statistical Significance: p <0.05 | Risk for d3–6mfs was significantly higher among children whose saliva sample sequencing over time showed higher percentages of Streptococcus mutans (IRR 1.39, 95 % CI 1.11–1.74, p = 0.005) | NONE REPORTED |
| Hurley et al., 2019 | Unstimulated Saliva and layers of dentinal caries | Caries samples were placed in a sterile 1.5-ml micro-centrifuge tube and transported to the laboratory, where they were frozen until further analysis and stored at −80 °C. Salivary swabs were placed in a collection tube, and stored at−80 °C. | Children; Age <60 months; Primary | For the caries-active group, caries was recorded at the level of cavitation into dentine (cavitation level), using the WHO criteria, with the addition of visible non cavitated dentine caries as referenced by Whelton et al.; Caries-free children did not show clinical evidence of early pre-cavitation of caries or white spot lesions and had no history of treatment on any tooth surfaces | n=138; Severe Early Childhood Caries: n = 68; Caries-Free: n = 70 | 16S rRNA amplicon sequencing; V4–V5; Illumina MiSeq; Statistical Significance: Benjamini and Hochberg correction; p<0.05 |
Prevotella histicola, Streptococcus mutans, Veillonella dispar |
Alloprevotella tannerae, Haemophilus parainfluenzae, Leptotrichia buccalis, Prevotella salivae |
| Jiang et al., 2016 | Unstimulated saliva | The collected samples were quickly frozen on dry ice, transported to the laboratory within 2 h, and then stored at −80 °C until use | Children; Ages 3–4 ; Unspecified | The caries examination method and criteria recommended by the World Health Organization were followed. Decayed teeth were detected at the cavitation level. | N=40; Caries: n = 20; No Caries: n = 20 | 16S rRNA gene; V3–V4; Illumina Miseq ; Statistical Significance: p < 0.05 |
Rothia dentocariosa, Actinomyces graevenitzii, Veillonella sp. oral taxon 780, Prevotella salivae, Streptococcus mutans |
Fusobacterium periodonticum, Leptotrichia sp. Oral clone FP036 |
| Luo et al., 2012 | Saliva Sample (Subjects expectorated whole saliva) | Subjects expectorated whole saliva into 2-ml sterile Eppendorf microcentrifuge tubes during a 5-min period. All participants were instructed not to clean their teeth the evening and morning before sampling. Two hours before saliva sampling, the subjects were not to eat or drink. Sampling was performed by one examiner. These samples were quick frozen in liquid nitrogen and subsequently stored at −80°C until use | Children; Ages 6–8 ; Mixed | Caries-free: dmfs = 0; Caries-active: dmfs > 8 | n = 50; Caries-free: n = 20; Caries-active: n = 30 | 16S rRNA genes, sampled were assayed using HOMIM microarray; Statistical Significance: p<0.05 |
Bacteroidetes[G-2] sp _ot274, Capnocytophaga sputigena_ot775, Tannerella sp_ot286, Campylobacter showae_ot763, Campylobacter Cluster I_ot580_748_763, Selenomonas infelix_ot126 and related species ot479_ot481_ot639_ ot054, Parvimonas micra ot111, Leptotrichia hofstadii_ot224 |
Gemella haemolysans_ot626, Granulicatella elegans_ot596, Streptococcus infantis and sp clone FN042_ot065_638, Streptococcus mitis bv2 and sp clone FP064_ot069_398, Streptococcus sp clone F0042_ot067, Rothia dentocariosa_ot587 |
| Ortiz et al., 2019 | Non-stimulated saliva | Non-stimulated saliva specimens (1–2 ml) were collected from the oral cavity of enrolled participants using the OMNIgene-ORAL collection and stabilization kit for microbial DNA (DNA Genotek; OM-501). In some cases, especially for young children, sterile bulbs or sterile swabs were used to collect saliva from the oral cavity, prior to placement of saliva within the OMNIgene-ORAL stabilization reagent. OMNIgene-ORAL stabilizes samples at the point of collection until transport to the laboratory, and permits long-term storage up to 1 year under ambient temperatures. | Children; Ages 2–12; Primary and Mixed | Caries-active: exhibited DMFT scores of 1–15 at the time of appointment. Caries-free individuals: demonstrated no apparent evidence of carious lesions. | n = 85; Caries-Active: n = 64; Caries-Free: n = 21 | PCR amplification using V3–V4 16S rDNA-specific primers and next-generation sequencing ; V3–V4 ; Illumina sequencing in the Human Oral Microbial Identification Next Generation Sequencing (NGS) HOMINGS Laboratory ; Statistical Significance: Fold changes larger than 1.5, with the FDR adjusted p-value of < 0.05 were considered statistically significant |
Actinomyces gerencseriae, Aggregatibacter actinomycetemcomitans, Alloprevotella tannerae, Bacteroidetes[G-5] Genus probe, Bacteroidetes[G-5] sp HOT 511, Butyrivibrio sp HOT 455, Capnocytophaga haemolytica, Capnocytophaga sp HOT 902, Fusobacterium nucleatum, Lachnospiraceae[G-2] sp HOT 096, Lactococcus lactis, Leptotrichia Genus probe 3, Leptotrichia sp HOT 218, Leptotrichia sp HOT 498, Moraxella Genus probe 2, Mycoplasma salivarium, Neisseria flavescens, Peptococcus sp HOT 167, Peptostreptococcaceae [XI][G-1][Eubacterium] infirmum, Porphyromonas sp HOT 930, Prevotella denticola, Prevotella pleuritidis, Prevotella veroralis, Streptococcus Genus probe 2, Streptococcus sobrinus, Treponema denticola, Treponema Genus probe 4, Treponema lecithinolyticum, Treponema maltophilum, Treponema sp HOT 237, Treponema sp HOT 257 |
Actinomyces Genus probe 3, Actinomyces johnsonii, Actinomyces lingnae, Actinomyces massiliensis, Actinomyces odontolyticus, Actinomyces sp HOT 170, Actinomyces sp HOT 178, Actinomyces sp HOT 877, Aggregatibacter paraphrophilus, Bacteroidales[G-2] sp HOT 274, Campylobacter concisus, Campylobacter curvus, Campylobacter gracilis, Capnocytophaga Genus probe 2, Capnocytophaga gingivalis, Capnocytophaga leadbetteri, Capnocytophaga sp HOT 332, Capnocytophaga sputigena, Cardiobacterium Genus probe, Cardiobacterium hominis, Cardiobacterium valvarum, Catonella Genus probe, Corynebacterium durum, Corynebacterium Genus probe, Corynebacterium matruchotii, Fusobacterium Genus probe 4, Fusobacterium periodonticum, Gemella Genus probe, Gemella haemolysans, Gemella sanguinis, Granulicatella adiacens, Granulicatella elegans, Haemophilus Genus probe 2, Haemophilus parainfluenzae, Haemophilus pittmaniae, Lachnoanaerobacul um sp HOT 083, Lachnoanaerobacul um umeaense, Lachnospiraceae[G-3] sp HOT 100, Lactobacillus Genus probe 3, Lautropia mirabilis, Leptotrichia Genus probe 4, Leptotrichia sp HOT 219, Leptotrichia sp HOT 223, Leptotrichia sp HOT 392, Oribacterium sinus, Peptostreptococcace ae[XI][G-7] sp HOT 922, Porphyromonas catoniae, Porphyromonas pasteri |
| Wang et al., 2019 | Saliva (To minimize stimulation of salivation, saliva needed to be kept in the mouth for 3 min. Subjects were then instructed to drool into sterile cryogenic vials for 3 min) | Each saliva sample was pipetted into a sterile 1.5-ml Eppendorf tube, which was snap-frozen in liquid nitrogen and stored at −80°C | Children; Ages 3–5 months; Unspecified | Severe ECC: dmfs ≥ 8; Caries-Free: dmfs=0 | n = 44; Severe early childhood caries (ECC): n = 25; Caries-free: n = 19 | Metagenomics Analysis (gene cataloging); Illumina HiSeq/TruSeq; Statistical Significance: FDR < 0.1 |
Anaeroglobus geminatus, atopobium rimae, Bifidobacterium dentium, Cryptobacterium curtum, Dialister invisus, Eubacteriaceae bacterium ACC19, Lactobacillus salivarius, Olsenella uli, Oribacterium sp. Oral taxon 078, Parascardovia denticolens, Prevotella amnii, prevotella buccae, Prevotella denticola, prevotella multiformis, Prevotella multisaccharivorax, prevotella nigrescens, prevotella oris, prevotella sp. Oral taxon 317, Shuttleworthia satelles, Streptococcus mutans |
Candidatus Nitrospira defluvii, Erysipelotrichaceae bacterium 5_2_54FAA, Neisseria lactamica, Streptococcus australis |
| Xiao et al., 2018 | Nonstimulated whole saliva was collected from subjects through a saliva jet connected to a suction pump at least 2 h after any toothbrushing, eating, or drinking | Previously established methods (Grier et al., 2017; Merkley et al., 2015) were used to perform oral microbiome sequencing and related bioinformatics analysis. Total genomic DNA from clinical samples (100ul saliva and 200ul plaque suspension) was extracted using Quick- DNATM Fecal/Soil Microbe Miniprep Kit (ZymoResearch, Irvine, CA) (Dardas et al., 2014; Merkley et al., 2015; Zhu et al., 2013) | Children; Unspecified: Average Age Children SECC = 4.0 +– 0.9, CF = 3.8 +– 1.6; | S-ECC was diagnosed per criteria of the American Academy of Pediatric Dentistry. The number of carious teeth and surfaces was charted with index variables of decayed, missing due to decay, or filled, according to the codes proposed by the World Health Organization’s Oral Health Surveys Basic Methods (1997). Plaque status for mothers and children were assessed separately according to criteria modified from Silness and Löe (1964) and Ribeiro Ade et al. (2002) |
n=39; Severe Early Childhood Caries: 19; Caries-Free: 18 | 16s rRNA amplicon sequencing; V1–V3 ; Illumina; Statistical Significance: p<0.05 |
Streptococcus vestibularis, Streptococcus salivarius, Veillonella atypica, Veillonella dispar, Veillonella parvula |
Streptococcus ET G 4d04 |
| Yang et al., 2012 | Saliva (unspecified) | Two milliliters of saliva were collected from each human-host individual into a tube containing an equal volume of lysis buffer. Samples were stored at −80°C before high-salt DNA extraction | Adults; Ages 18–22 | Caries-active: DMFT ≥ 6; Healthy: DMFT=0; | n=45; Healthy: n = 26; Caries Active: n = 19 | 16s rRNA Amplicon Sequencing; V4–V5; Illumina; Statistical Significance: p < 0.1 |
Actinomyces sp., Aggregatibacter paraphrophilus, Capnocytophaga granulosa, Catonella morbi, Eggerthella lenta, Enterobacter sakazakii, Fusobacterium periodonticum, Gemella sanguinis, Haemophilus parainfluenzae, Haemophilus sp., Kingella denitrificans, Lachnospiraceae [G-1] sp., Lachnospiraceae [G-2] sp., Lachnospiraceae [G-4] sp., Lachnospiraceae [G-8] sp., Leptotrichia sp., Mobiluncus mulieris, Neisseria sp., Neisseria subflava, Oribacterium sp., Peptococcus sp., Peptoniphilus sp., Peptostreptococcaceae [XI][G-5] sp., Peptostreptococcaceae [XI][G-7] sp., Porphyromonas sp., Prevotella histicola, Prevotella melaninogenica, Prevotella oris, Prevotella sp., Prevotella veroralis, Propionibacterium propionicum, Treponema sp., Treponema vincentii, Veillonella dispar, Veillonella parvula |
NONE FOUND |
Species statistically significantly associated with caries in plaque and saliva
We tallied all species that were positively or negatively associated with dental caries in saliva and plaque with a p value ≤ 0.05 (Figure 2). Two studies found no species statistically significantly associated with either caries or oral health, and 4 studies found species significantly associated only with caries and 2 studies found species significantly associated only with oral health. Among the 24 articles reviewed, the most common statistically significant cariogenic species reported were Streptococcus mutans (14/24, 58.3%), Veillonella dispar (6/24, 25%), and Veillonella parvula (5/24, 20.8%). When limited to the 8 studies that corrected for multiple comparisons, Streptococcus mutans was associated with caries 4/8 (50%) studies and Veillonella dispar was associated with caries in 3/8 (37.5%). By contrast, Fusobacterium periodonticum and Haemophilus parainfluenzae were significantly associated with oral health in 5/24 (20.8%) and 3/24 (12.5%) studies, respectively. Among studies correcting for multiple comparisons, Fusobacterium periodonticum and Haemophilus parainfluenzae were significantly associated with oral health in 3/8 studies (37.5%).
Figure 2.
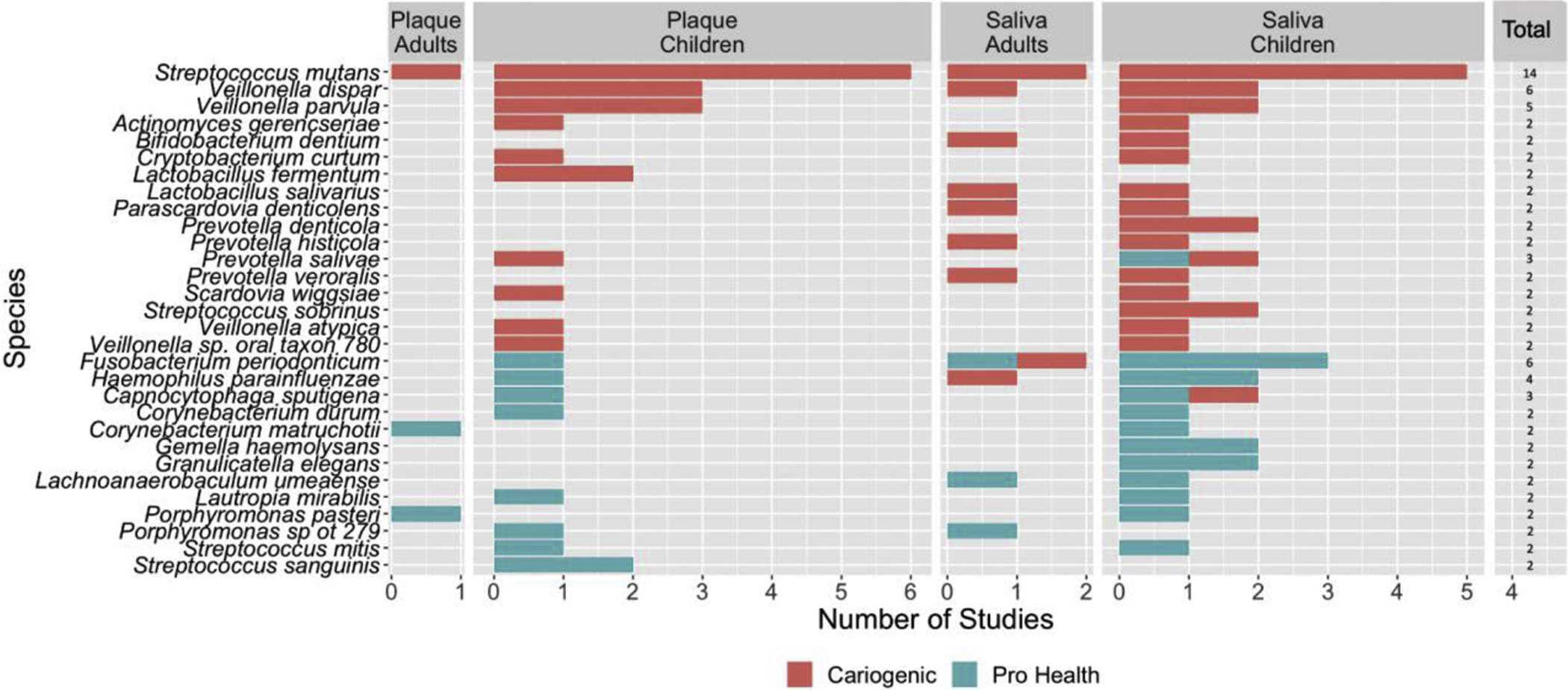
Number of studies that found statistically significant taxa identified as cariogenic (red) or oral health (green) from the total 24 studies found eligible in this scoping review, stratified by sample type and age group. Studies that looked at plaque in adults (n = 3), plaque in children (n = 9), saliva in adults (n = 4), saliva in children (n = 8). Note: Only taxa that were found to be statistically significant in at least 2/24 studies are presented.
We further analyzed papers to determine if there were differences in species identified by age or biologic specimen (saliva or plaque). Streptococcus mutans was statistically significantly associated with caries in 66.6% of the studies (6/9) conducted in children analyzing supragingival plaque and 62.5% of studies analyzing saliva (5/8). Among adults, Streptococcus mutans was statistically significantly associated with cariogenesis in 2/4 (50%) saliva studies and in 1/3 (33%) plaque studies.
Multiple taxa were significantly associated with the caries-free state in children but not consistently across studies. In 2/9 (22.2%) plaque studies in children, Streptococcus sanguinis was significantly associated with oral health. Among saliva studies in children, Fusobacterium periodonticum was significantly associated with oral health 3/8 studies (37.5%) and Granulicatella elegans, Gemella haemolysans, and Haemophilus parainfluenzae were statistically associated with oral health in 2/8 (25%) studies. Among adults, there was no overlap in species that were statistically significantly associated with oral health in either saliva or plaque studies for more than one study.
Among the studies conducted in children that corrected for multiple comparisons, S. mutans in plaque was significantly associated with caries in 2/2 (100%) and in saliva in 2/3 (66.7%). Prevotella denticola was significantly associated with caries in 2/3 (66.7%) of the children salivary studies. Only Haemophilus parainfluenzae was statistically significantly associated with the caries-free state in more than one study (2/3 (66.7%)).
Discussion
Overall Findings
In this scoping review of microbiome articles identifying bacterial species found more frequently among adults and children with caries compared to individuals that were caries-free (or vice-versa), the species associated with the caries presence or absence varied widely, with notable differences by age and whether plaque or saliva was analyzed. No species was statistically significantly associated with caries or the caries-free state (oral health) in all studies. However, Streptococcus mutans and Veillonella dispar were associated with caries in 14/24 studies (58.3%) and 6/24 (25%), respectively. The most common taxa significantly associated with oral health were found less frequently: Fusobacterium periodonticum (5/24, 20.8%) and Haemophilus parainfluenzae (3/24, 12.5%). These four taxa were also identified when the review was limited to studies that corrected for multiple testing in roughly the same proportions.
S. mutans is widely accepted as cariogenic; its cariogenicity is attributed to its superior acidogenic and aciduric potential (Aas et al., 2008; Fakhruddin et al., 2019; Mattos-Graner et al., 2000). Further, S. mutans is the main producer of insoluble glucans, a key component of cariogenic biofilms. Insoluble glucans enable S. mutans and other aciduric-cariogenic bacteria to bind to teeth and thrive (Bowen et al., 2018). Reasons for a lack of association of S. mutans with caries in all studies may be due to differences in study design, conduct or analysis or reflect true differences by study population or disease process. Most plaque studies reviewed did not collect plaque separately from carious lesions but pooled samples from all teeth. Caries is a site-specific disease (Richards et al., 2017); pooling plaque can attenuate the true differences between a carious lesion and healthy tooth (Banas & Drake, 2018). The oral microbiome changes between childhood to adulthood (Gomez & Nelson, 2017; Papaioannou et al., 2009; Ribeiro et al., 2017). Thus, the bacterial communities leading to caries may also be somewhat different. Further, bacterial composition changes with disease progression; some studies included enamel lesions and dentinal caries which have distinct microbiomes (Munson et al., 2004; Rôças et al., 2016). Therefore, some of the variability in associations with specific species may be explained by variation in when during the disease process samples were collected (Torlakovic et al., 2012).
Plaque bacteria are shed in the saliva (Shi et al., 2018), but the amount may vary by disease stage. It is also possible that saliva plays an ancillary role in cariogenesis or in preventing caries (Chen et al., 2020). While the studies reviewed varied in whether they used stimulated or unstimulated saliva, results of a validation suggest that for microbiome studies, the two are effectively equivalent (Daniel Belstrøm, Holmstrup, et al., 2016). There also may be multiple pathways to dental caries, and the processes leading to caries may depend on behavioral or physiologic factors associated with age, diet, or dentition.
While several studies reviewed reported associations between Veillonella dispar and dental caries, we found no previous studies suggesting a mechanism for this association. At least one report suggested that increased Veillonella dispar abundance is negatively associated with periodontitis (Papapanou et al., 2020). The consistent association of F. periodonticum with the caries-free state - observed in children and adults - is somewhat surprising, as this species is positively associated with periodontitis (Lee et al., 2003; Oettinger-Barak et al., 2014) and oral squamous cell carcinoma (Yang et al., 2018). However, one study included that tested saliva among adults (Yang et al., 2012), identified F. periodonticum as cariogenic. Haemophilus parainfluenzae is considered a pathobiont, and has been associated with respiratory disease (Middleton et al., 2003). Although 4 studies found significant associations of Haemophilus parainfluenzae with oral health, one study among adults flagged it as cariogenic.
This review is limited by variations in samples used, study population, and how bacteria were detected and classified to species. DNA extraction strategy and 16S rRNA hypervariable regions can influence the results of oral microbiota biodiversity profiling. Based on a mock community existing of five oral bacterial species in equal abundance where different hypervariable regions were compared, Teng et al. showed that the hypervariable regions targeting V3–V4 and V4–V5 seemed to produce more reproducible results than V1–V3 (Teng et al., 2018). Differences in region of the 16S rRNA gene amplified, methods of resolving taxa to the species level, and choice of reference database may have led to conflicting results, particularly for species associated with oral health. In addition, all but two studies collected samples at only a single point in time, and thus were unable to identify which species contributed to caries incidence and disease progression. The lack of longitudinal studies of the oral microbiome leading to caries is a major gap in the literature. Finally, a major limitation of all studies was they were limited to bacterial species. Candida spp. are found frequently in the mouth and are associated caries (Ramadugu et al., 2020; Jin Xiao et al., 2018). There is a well-described interaction between S. mutans and C. albicans that is stimulated by the presence of sugars. This interaction results in the development of an extracellular matrix that provides binding sites for S. mutans and enables C. albicans to colonize the tooth surface (Koo et al., 2018).
Nonetheless, this review revealed some general insights. All 4 taxa significantly associated with presence of a caries lesion or the caries-free state across all studies reviewed or when limited to studies correcting for multiple testing were detected in plaque and saliva. This suggests that studies using saliva can provide insights into the multiple pathways to cariogenesis. Further, although the most proximal cause of caries might be found in dental plaque (Marsh et al., 2011), saliva might contribute to or thwart cariogenesis.
Supplementary Material
Highlights.
Streptococcus mutans was associated with caries in 58.3% of studies reviewed
No single species was associated with caries or oral health across all studies
Species associated with caries or oral health varied with age
Species associated with caries or oral health varied by biological specimen
Testing saliva can provide insights into microbes causing caries
Acknowledgements
The authors thank the Center for Oral Health Research in Appalachia (COHRA) whose ongoing research motivated this review.
Funding
The National Institutes of Health, R01DE014899-17 provided salary support for the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests
The authors declare no conflicts
Availability of data and material
Provided in the supplementary material
Contributor Information
Deesha Bhaumik, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, United States.
Divya Manikandan, University of Michigan College of Literature, Science, and the Arts, Ann Arbor, Michigan, United States.
Betsy Foxman, Center of Molecular and Clinical Epidemiology of Infectious Diseases, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, United States.
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, & Paster BJ (2008). Bacteria of dental caries in primary and permanent teeth in children and young adults. Journal of Clinical Microbiology, 46(4), 1407–1417. 10.1128/JCM.01410-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello M, Marques J, Cen L, Mittermuller B, Huang A, Chaichanasakul Tran N, Shi W, He X, & Schroth RJ (2017). Microbiome associated with severe caries in canadian first nations children. Journal of Dental Research, 96(12), 1378–1385. 10.1177/0022034517718819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JL, & Edlund A (2019). Exploiting the oral microbiome to prevent tooth decay: Has evolution already provided the best tools? Frontiers in Microbiology, 9, 3323. 10.3389/fmicb.2018.03323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan M, Simmonds RS, & Tagg JR (2000). Dental caries is a preventable infectious disease. Australian Dental Journal, 45(4), 235–245. 10.1111/j.1834-7819.2000.tb00257.x [DOI] [PubMed] [Google Scholar]
- Banas JA, & Drake DR (2018). Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health, 18(1), 1–8. 10.1186/s12903-018-0595-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Holmstrup P, Fiehn NE, Kirkby N, Kokaras A, Paster BJ, & Bardow A (2017). Salivary microbiota in individuals with different levels of caries experience. Journal of Oral Microbiology, 9(1), 1270614. 10.1080/20002297.2016.1270614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm Daniel, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, & Paster BJ (2016). Comparative analysis of bacterial profiles in unstimulated and stimulated saliva samples. Journal of Oral Microbiology, 8(1), 30112. 10.3402/jom.v8.30112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm Daniel, Paster BJ, Fiehn NE, Bardow A, & Holmstrup P (2016). Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. Journal of Oral Microbiology, 8(1), 1–7. 10.3402/jom.v8.30170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, & Koo H (2018). Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends in microbiology, 26(3), 229–242. 10.1016/j.tim.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, & Jiang W (2014). Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Frontiers in Microbiology, 5, 508. 10.3389/fmicb.2014.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jiang Q, Yan G, & Yang D (2020). The oral microbiome and salivary proteins influence caries in children aged 6 to 8 years. BMC Oral Health, 20(1), 295. 10.1186/s12903-020-01262-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashper SG, Mitchell HL, Lê Cao KA, Carpenter L, Gussy MG, Calache H, Gladman SL, Bulach DM, Hoffmann B, Catmull DV, Pruilh S, Johnson S, Gibbs L, Amezdroz E, Bhatnagar U, Seemann T, Mnatzaganian G, Manton DJ, & Reynolds EC (2019). Temporal development of the oral microbiome and prediction of early childhood caries. Scientific Reports, 9(1), 1–12. 10.1038/s41598-019-56233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus VC, Shikder R, Oryniak D, Mann K, Alamri A, Mittermuller B, Duan K, Hu P, Schroth RJ, & Chelikani P (2020). Sex-based diverse plaque microbiota in children with severe caries. Journal of dental research, 99(6), 703–712. 10.1177/0022034520908595 [DOI] [PubMed] [Google Scholar]
- Eriksson L, Lif Holgerson P, Esberg A, & Johansson I (2018). Microbial complexes and caries in 17-year-olds with and without Streptococcus mutans. Journal of Dental Research, 97(3), 275–282. 10.1177/0022034517731758 [DOI] [PubMed] [Google Scholar]
- Fakhruddin KS, Ngo HC, & Samaranayake LP (2019). Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Diseases, 25(4), 982–995. 10.1111/odi.12932 [DOI] [PubMed] [Google Scholar]
- Gomez A, & Nelson KE (2017). The oral microbiome of children: development, disease, and implications beyond oral health. Microbial Ecology, 73(2), 492–503. 10.1007/s00248-016-0854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussy M, Mnatzaganian G, Dashper S, Carpenter L, Calache H, Mitchell H, Reynolds E, Gibbs L, Hegde S, Adams G, Johnson S, Amezdroz E, & Christian B (2020). Identifying predictors of early childhood caries among Australian children using sequential modelling: Findings from the VicGen birth cohort study. Journal of Dentistry, 93, 103276. 10.1016/j.jdent.2020.103276 [DOI] [PubMed] [Google Scholar]
- Hurley E, Barrett MPJ, Kinirons M, Whelton H, Ryan CA, Stanton C, Harris HMB, & O’Toole PW (2019). Comparison of the salivary and dentinal microbiome of children with severe-early childhood caries to the salivary microbiome of caries-free children. BMC Oral Health, 19(1), 13. 10.1186/s12903-018-0693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Takeshita T, Kageyama S, Matsumi R, Asakawa M, Shibata Y, Sugiura Y, Ishikawa K, Takahasi I, & Yamashita Y (2019). Identification of initial colonizing bacteria in dental plaques. Clinical Science and Epidemiology, 4(5), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Gao X, Jin L, & Lo ECM (2016). Salivary microbiome diversity in caries-free and caries-affected children. International Journal of Molecular Sciences, 17(12). 10.3390/ijms17121978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jiang YT, Li CL, & Liang JP (2011). Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresis. Microbial Ecology, 61(2), 342–352. 10.1007/s00248-010-9753-z [DOI] [PubMed] [Google Scholar]
- Koo H, Andes DR, & Krysan DJ (2018). Candida–streptococcal interactions in biofilm-associated oral diseases. In PLoS Pathogens, 14(12), e1007342. 10.1371/journal.ppat.1007342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-W, Choi B-K, Yoo Y-J, Choi S-H, Cho K-S, Chai J-K, & Kim C-K (2003). Distribution of periodontal pathogens in Korean aggressive periodontitis. Journal of Periodontology, 74(9), 1329–1335. 10.1902/jop.2003.74.9.1329 [DOI] [PubMed] [Google Scholar]
- Luo AH, Yang DQ, Xin BC, Paster BJ, & Qin J (2012). Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Diseases, 18(6), 595–601. 10.1111/j.1601-0825.2012.01915.x [DOI] [PubMed] [Google Scholar]
- Marsh PD (2018). In sickness and in health-what does the oral microbiome mean to us? An ecological perspective. Advances in Dental Research, 29(1), 60–65. 10.1177/0022034517735295 [DOI] [PubMed] [Google Scholar]
- Marsh Philip D., Moter A, & Devine DA (2011). Dental plaque biofilms: Communities, conflict and control. Periodontology 2000, 55(1), 16–35. 10.1111/j.1600-0757.2009.00339.x [DOI] [PubMed] [Google Scholar]
- Mattos-Graner RO, Smith DJ, King WF, & Mayer MPA (2000). Wafer-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12- to 30-month-old children. Journal of Dental Research, 79(6), 1371–1377. 10.1177/00220345000790060401 [DOI] [PubMed] [Google Scholar]
- Middleton AM, Dowling RB, Mitchell JL, Watanabe S, Rutman A, Pritchard K, Tillotson G, Hill SL, & Wilson R (2003). Haemophilus parainfluenzae infection of respiratory mucosa. Respiratory Medicine, 97(4), 375–381. 10.1053/rmed.2002.1454 [DOI] [PubMed] [Google Scholar]
- Munson MA, Banerjee A, Watson TF, & Wade WG (2004). Molecular analysis of the microflora associated with dental caries. Journal of Clinical Microbiology, 42(7), 3023–3029. 10.1128/JCM.42.7.3023-3029.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDCR. (2018). Dental Caries (Tooth Decay). National Institute of Dental and Craniofacial Research. https://www.nidcr.nih.gov/research/data-statistics/dental-caries [Google Scholar]
- Oettinger-Barak O, Sela M, Sprecher H, & Machtei E (2014). Clinical and microbiological characterization of localized aggressive periodontitis: a cohort study. Australian Dental Journal, 59(2), 165–171. 10.1111/adj.12165 [DOI] [PubMed] [Google Scholar]
- Ortiz S, Herrman E, Lyashenko C, Purcell A, Raslan K, Khor B, Snow M, Forsyth A, Choi D, Maier T, & Machida CA (2019). Sex-specific differences in the salivary microbiome of caries-active children. Journal of Oral Microbiology, 11(1), 1653124. 10.1080/20002297.2019.1653124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou W, Gizani S, Haffajee AD, Quirynen M, Mamai-Homata E, & Papagiannoulis L (2009). The microbiota on different oral surfaces in healthy children. Oral Microbiology and Immunology, 24(3), 183–189. 10.1111/j.1399-302X.2008.00493.x [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Park H, Cheng B, Kokaras A, Paster B, Burkett S, Watson CWM, Annavajhala MK, Uhlemann AC, & Noble JM (2020). Subgingival microbiome and clinical periodontal status in an elderly cohort: The WHICAP ancillary study of oral health. Journal of Periodontology, 91(S1), S56–S67. 10.1002/JPER.20-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadugu K, Blostein F, Bhaumik D, Jiang W, Davis E, Salzman E, Srinivasan U, Marrs CF, Neiswanger K, McNeil DW, Marazita ML, & Foxman B (2020). Co-occurrence of yeast, streptococci, dental decay, and gingivitis in the post-partum period: results of a longitudinal study. Journal of Oral Microbiology, 12(1). 10.1080/20002297.2020.1746494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AA, Azcarate-Peril MA, Cadenas MB, Butz N, Paster BJ, Chen T, Bair E, & Arnold RR (2017). The oral bacterial microbiome of occlusal surfaces in children and its association with diet and caries. PLoS ONE, 12(7). 10.1371/journal.pone.0180621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards VP, Alvarez AJ, Luce AR, Bedenbaugh M, Mitchell ML, Burne RA, & Nascimento MM (2017). Microbiomes of sitespecific dental plaques from children with different caries status. Infection and Immunity, 85(8), 1–11. 10.1128/IAI.00106-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rôças IN, Alves FRF, Rachid CTCC, Lima KC, Assunção IV, Gomes PN, & Siqueira JF (2016). Microbiome of deep dentinal caries lesions in teeth with symptomatic irreversible pulpitis. PLOS ONE, 11(5), e0154653. 10.1371/journal.pone.0154653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoilew K, Ueffing H, Dalpke A, Wolff B, Frese C, Wolff D, & Boutin S (2019). Bacterial biofilm composition in healthy subjects with and without caries experience. Journal of Oral Microbiology, 11(1), 1633194. 10.1080/20002297.2019.1633194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Tian J, Xu H, Zhou Q, & Qin M (2018). Distinctions and associations between the microbiota of saliva and supragingival plaque of permanent and deciduous teeth. PloS ONE, 13(7), e0200337. 10.1371/journal.pone.0200337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, & Dewhirst FE (2011). Cultivable anaerobic microbiota of severe early childhood caries. Journal of Clinical Microbiology, 49(4), 1464–1474. 10.1128/JCM.02427-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Darveekaran Nair SS, Zhu P, Li S, Huang S, Li X, Xu J, & Yang F (2018). Impact of DNA extraction method and targeted 16S-rRNA hypervariable region on oral microbiota profiling. Scientific Reports, 8(1), 1–12. 10.1038/s41598-018-34294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Yang F, Huang S, Bo C, Xu ZZ, Amir A, Knight R, Ling J, & Xu J (2015). Prediction of early childhood caries via spatial-temporal variations of oral microbiota. Cell Host and Microbe, 18(3), 296–306. 10.1016/j.chom.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Torlakovic L, Klepac-Ceraj V, Øgaard B, Cotton SL, Paster BJ, & Olsen I (2012). Microbial community succession on developing lesions on human enamel. Journal of Oral Microbiology, 4(2012). 10.3402/jom.v4i0.16125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang S, Wu C, Chen X, Duan Z, Xu Q, Jiang W, Xu L, Wang T, Su L, Wang Y, Chen Y, Zhang J, Huang Y, Tong S, Zhou C, Deng S, & Qin N (2019). Oral microbiome alterations associated with early childhood caries highlight the importance of carbohydrate metabolic activities. MSystems, 4(6), 1–15. 10.1128/msystems.00450-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SSL, Wilczynski NL, & Haynes RB (2006). Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. Journal of the Medical Library Association, 94(4), 451–455. http://www.jisc.ac [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). Fact Sheet: Oral health. 10.1016/S0197-4572(88)80065-X [DOI]
- Xiao J, Grier A, Faustoferri RC, Alzoubi S, Gill AL, Feng C, Liu Y, Quivey RG, Kopycka-Kedzierawski DT, Koo H, & Gill SR (2018). Association between oral Candida and bacteriome in children with severe ECC. Journal of Dental Research, 97(13), 1468–1476. 10.1177/0022034518790941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Jin, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, Castillo DA, Campbell F, Davis J, Herzog K, Billings R, Kopycka-Kedzierawski DT, Hajishengallis E, & Koo H (2018). Candida albicans and early childhood caries: A systematic review and meta-analysis. Caries Research, 52(1–2), 102–112. 10.1159/000481833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Yeh YM, Yu HY, Chin CY, Hsu CW, Liu H, Huang PJ, Hu SN, Liao CT, Chang KP, & Chang YL (2018). Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Frontiers in Microbiology, 9, 862. 10.3389/fmicb.2018.00862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zeng X, Ning K, Liu KL, Lo CC, Wang W, Chen J, Wang D, Huang R, Chang X, Chain PS, Xie G, Ling J, & Xu J (2012). Saliva microbiomes distinguish caries-active from healthy human populations. ISME Journal, 6(1), 1–10. 10.1038/ismej.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chen Y, Xie L, Li Y, Jiang H, & Du M (2015). Pyrosequencing of plaque microflora in twin children with discordant caries phenotypes. PLoS ONE, 10(11), 1–14. 10.1371/journal.pone.0141310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zhang M, Li J, Li Y, Teng F, Jiang H, & Du M (2018). Comparative analysis of the microbial profiles in supragingival plaque samples obtained from twins with discordant caries phenotypes and their mothers. Frontiers in Cellular and Infection Microbiology, 8, 361. 10.3389/fcimb.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


