Abstract
Purpose
To compare the changes in subfoveal choroidal thickness (SFChT) in myopic children treated with 0.01% atropine, orthokeratology (OK), or their combination in myopic children, and to study the connection between increase in SFChT and axial length (AL) elongation.
Methods
This is a prospective, randomized, controlled study. A total of 67 children were included; 22 patients were randomly assigned to the SA group (patients with spectacles and 0.01% atropine), 24 patients were randomly assigned to the OK group (OK), and 21 patients were randomly assigned to the OKA group (OK and 0.01% atropine). Comprehensive ophthalmologic examinations were performed at baseline, 1 month, 6 months, and 12 months.
Results
After 1 month, SFChT increased by 5.41 ± 1.65 μm in the SA group, 17.46 ± 2.79 μm in the OK group, and 20.19 ± 2.18 μm in the OKA group (P = 0.00), whereas AL was not significantly increased. After 12 months, the changes of SFChT were not increased significantly compared with that at 1 month; AL increased by 0.20 ± 0.03 mm in the SA group, 0.28 ± 0.03 mm in the OK group, and 0.14 ± 0.03 mm in the OKA group (P = 0.00). The change in SFChT at 12 month was negatively correlated with the change in AL at 12 months.
Conclusion
The control of AL elongation was better in SA group than OK group. The increase in SFChT was best in OKA group, followed by OK group, and the changes were significant after only 1 month. In addition, the increase in SFChT may influence AL elongation and myopia progression.
Keywords: 0.01% Atropine, Orthokeratology, Myopia, Axial length, Subfoveal choroidal thickness
Introduction
The prevalence of myopia and high myopia has attracted worldwide attention, especially in East Asia [1]. Myopia is the most common refractive error, and is influenced by various factors, including environment, lifestyle, education, and heredity [2, 3]. Childhood-onset myopia is associated with the development of high myopia, which can result in several pathological complications such as macular degeneration, posterior sclera staphyloma, retinal detachment, and even blindness [4]; the visual impairment is usually irreversible. Therefore, it is critical to implement measures to control the development of myopia and to explore the underlying mechanisms of myopia.
The mechanisms of myopia are still not clear; a potential biological basis is eye growth. Many experiments with animals, including chicks [5, 6], macaque monkeys [7], and marmosets [8], have suggested that imposed defocus on the retina can result in an increase in SFChT and thus influence AL elongation. Similar results have been found in humans [9–11]. Changes in SFChT may play an important role in myopia progression.
OK is a very popular optical method to control myopia progression, and the specific mechanism of OK lens reducing the progression of myopia is unknown. Many previous studies have reported that the effects may result from peripheral retina defocus with an increase in high-order aberration through corneal epithelial redistribution [12–14]. Recently, several studies have reported that the use of OK lens can increase SFChT [15–17] and thus influence AL elongation. For example, Chen et al. [15] found increases in SFChT after short-term treatment with OK lenses. Li et al. [16, 17] demonstrated that the OK lens can induce significant increase in SFChT and retard AL elongation. Zhao et al. [18] showed that the combination of OK lenses and atropine resulted in a greater increase in SFChT than monotherapy with atropine.
Atropine is the effective pharmacological agent to retard myopia progression [19–21]. Many studies have demonstrated administration of 0.01% atropine with a low incidence of side effects and rebound during the washout period [20, 21]. A previous animal study demonstrated that atropine appears to block the M1 and M4 receptors in the retina and sclera that restrain axial elongation and reduce myopia progression by affecting the remodeling of sclera and reducing vitreous chamber growth [22]. And Li et al. [23] have shown that atropine mainly causes reduction in AL elongation. Some studies have reported that atropine can also thicken SFChT by hyperopic retina defocus in animals and humans [24–26]. However, no studies have compared the effects of 0.01% atropine, OK lenses, and their combination on long-term changes in SFChT and AL. The purpose of this study was to compare the effects of these three treatment regimens on long-term changes in SFChT and AL and to study the association of changes in SFChT with AL elongation.
Materials and methods
Patients
We included 75 children aged 8–12 years with myopia from the Second Affiliated Hospital of Dalian Medical University between June 2019 and September 2020. Before the experiment, we explained the expected benefits and potential risks of using 0.01% atropine and OK lenses to the parents of children and also informed the detailed methods and precautions. This study was reviewed and approved by the Institutional Review Board of the Second Affiliated Hospital of Dalian Medical University (Dalian, China) and adhered to the tenets of the Declaration of Helsinki. In addition, consent was obtained from the parents of patients. Explanation was provided to children using an easy-to-understand method before obtaining informed consent. Eight participants did not successfully complete the study during the follow-up visits: three because of difficulty in complying with the use of 0.01% atropine eyedrops, two because of keratitis, and three because of lost visits.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) cycloplegic spherical equivalent refraction (SE) at least − 1.00 diopters (D) and diopter of spherical within − 1.00 to − 6.00 DS in both eyes; (2) myopic astigmatism ≤ − 1.00 DC and less than or equal to half the spherical diopter; (3) anisometropia of no more than 1.50 D.
The exclusion criteria were as follows: (1) wearing contact lenses within 3 days of the start of examination; (2) children with ocular disorders such as glaucoma, cataract, keratopathy, strabismus, and amblyopia and systemic disorders such as cardiac and respiratory illnesses; (3) intraocular pressure (IOP) of > 21 mmHg and difference between the eyes of > 8 mmHg; (4) use of anticholinergic and cholinergic drugs that affect the evaluation of efficacy, such as atropine, pirenzepine, and pilocarpine, within the past 1 month; (5) use of other therapies that may affect the evaluation of efficacy within the past 3 months, such as wearing OK lenses and therapy of traditional Chinese medicine; (6) low birth weight (≤ 1500 g); and (7) history of hypersensitivity to atropine or anticholinergic drugs.
Methods
All subjects underwent comprehensive tests before treatment, including slit-lamp examination, visual acuity testing, autorefraction, intraocular pressure, AL, corneal topography and optical coherence tomography (OCT). We applied 1% atropine eye gel 3 times a day for 3 days for cycloplegic autorefraction. Cycloplegic spherical equivalent (SE) ≥ − 1.00 D was considered to indicate myopia. Subjects were prescribed 0.01% atropine eye drops to be applied once per night before bedtime in both eyes in both the SA and OKA groups. Subjects were instructed to wear OK lens on both eyes every night for at least 8 consecutive hours in both the OK and OKA groups. Additionally, OK lenses were worn 10 min after the use of 0.01% atropine in the OKA group. Some measurements, such as SE, AL, and SFChT, were taken after the use of tropicamide to induce sufficient mydriasis to induce the influence of accommodation. Further, 0.01% atropine is produced by Shenyang Xingqi pharmaceutical company (Shenyang, China), and there were no preservatives and the PH value between 3.5 and 4.0 in 0.01% atropine. The OK lens in this study are four-zone reverse-geometry lenses (Euclid Systems Ortho-K; Euclid System Corp., Herndon, USA) with BOSTON EQUALENS II (oprifocona) and a nominal Dk of 127 × 10–11 (cm2/s) (ml O2/ml_mmHg) (ISO/Fatt). Spectacles were rematched when the SE dropped by 0.5 D and the vision of wearing glasses decreased. OK lenses were rematched when the naked vision during the day reached < 0.5.
In this study, SE was measured using an autorefractor (KR-800, TOPCON, TOKYO, JAPAN). AL was measured using the non-contact Biometer (lenstar LS 900, HAAG-STREIT AG, KOENIZ, SWITZERLAND). SFChT was measured using the optical coherence tomography (CIRRUS HD-OCT, 5000, SINGAPORE). The average of five consecutive measurements by the same technician was used for analysis.
Statistical analysis
We applied SPSS 26.0 (IBM Corp., Armonk, NY) statistical analysis software for data analysis. All values were described by mean ± standard deviation. The comparisons of baseline age, SE, AL and SFChT and changes in AL and SFChT were used independent sample one-way ANOVA analyses. The Pearson's Chi-square test was used to analyze gender differences among groups. P < 0.05 was considered statistically significant. Stepwise multiple linear regression models were used to investigate the association between the change in SFChT at 12 months and the change in AL at 12 months. The standard β indicating the relative importance of each variable in comparable standardized units (z scores) was used to evaluate the importance of each variable in predicting weight. A two-tailed P value < 0.05 was considered to indicate statistical significance.
Results
A total of 75 eyes from 75 participants (right eyes) met the above criteria; 67 participants successfully completed the study. No statistically significant differences were found among the three groups in baseline age, gender, SE, AL, and SFChT (Table 1).
Table 1.
Demographic and biometric and subfoveal choroidal thickness measures (mean ± standard deviation) at baseline of three groups
| SA | OK | OKA | P values | |
|---|---|---|---|---|
| N | 22 | 24 | 21 | |
| Age (years) | 9.77 ± 1.27 | 10.13 ± 1.19 | 10.10 ± 1.22 | 0.571a |
| M:F | 10:12 | 13:11 | 11:10 | 0.826b |
| SE (D) | 3.62 ± 0.57 | 3.66 ± 0.60 | 4.07 ± 0.74 | 0.293a |
| AL (mm) | 24.91 ± 0.61 | 25.17 ± 0.52 | 25.29 ± 0.56 | 0.097a |
| SFChT (μm) | 240.64 ± 19.93 | 236.83 ± 16.78 | 235.14 ± 20.33 | 0.623a |
M: F male: female, SE spherical equivalent refractive error, AL axial length, SFChT subfoveal choroidal thickness
aIndependent sample one-way ANOVA analyses
bPearson's Chi-square test
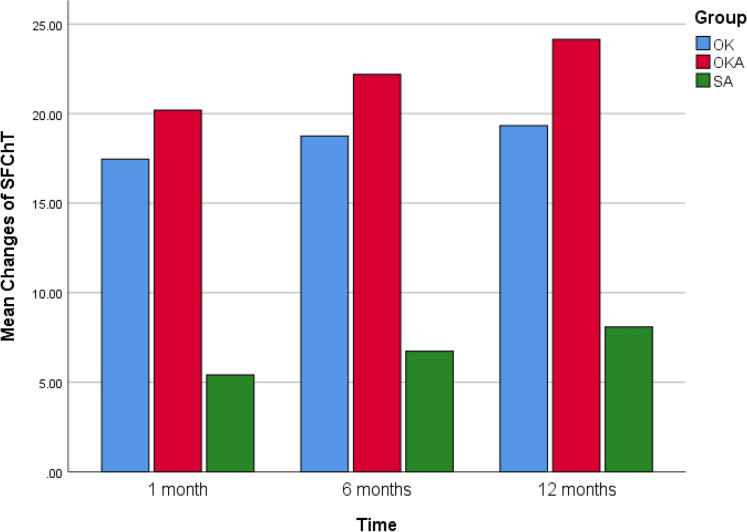
During follow-up visits, the rate of AL elongation was significantly reduced in the three groups, although the changes were not significant at 1 month. The reduction in AL elongation was greatest in the OKA group (0.14 ± 0.03 mm), followed by the SA group (0.20 ± 0.03 mm). However, the increase in SFChT in the three groups was significant after only 1 month, especially in the OKA group (20.19 ± 2.18 μm) and the OK group (17.46 ± 2.79 μm). There was no significant change in SFChT in the OK group after 12 months (19.33 ± 2.63 μm). The changes in SFChT in the SA group (5.41 ± 1.65 μm at 1 month, 8.09 ± 1.47 μm at 12 months) were also significant, although not enough obvious compared with the OK group and the OKA group (Table 2).
Table 2.
The changes of AL and SFChT within SA, OK and OKA group
| SA | OK | OKA | P values | |
|---|---|---|---|---|
| AL (mm) | ||||
| Change at 1 m | 24.94 ± 0.61 | 25.19 ± 0.51 | 25.31 ± 0.56 | 0.09a |
| Change over 1 m | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.99a |
| Change at 6 m | 25.03 ± 0.63 | 25.31 ± 0.54 | 25.38 ± 0.58 | 0.11a |
| Change over 6 m | 0.11 ± 0.02 | 0.14 ± 0.03 | 0.09 ± 0.02 | 0.00a |
| Change at 12 m | 25.12 ± 0.63 | 25.41 ± 0.59 | 25.43 ± 0.59 |
0.17a 0.17a |
| Change over 12 m | 0.20 ± 0.03 | 0.28 ± 0.03 | 0.14 ± 0.03 | 0.00a |
| SFChT (μm) | ||||
| Change at 1 m | 246.00 ± 21.30 | 254.29 ± 19.24 | 255.38 ± 22.19 | 0.27a |
| Change over 1 m | 5.41 ± 1.65 | 17.46 ± 2.79 | 20.19 ± 2.18 | 0.00a |
| Change at 6 m | 247.32 ± 21.08 | 255.71 ± 19.01 | 257.38 ± 22.04 |
0.23a 0.23a |
| Change over 6 m | 6.73 ± 1.42 | 18.75 ± 2.65 | 22.19 ± 2.01 | 0.00a |
| Change at 12 m | 248.73 ± 21.06 | 256.17 ± 19.03 | 259.29 ± 21.73 | 0.22a |
| Change over 12 m | 8.09 ± 1.47 | 19.33±2.63 | 24.14 ± 1.93 | 0.00a |
AL axial length, SFChT subfoveal choroidal thickness
aIndependent sample one-way ANOVA analyses
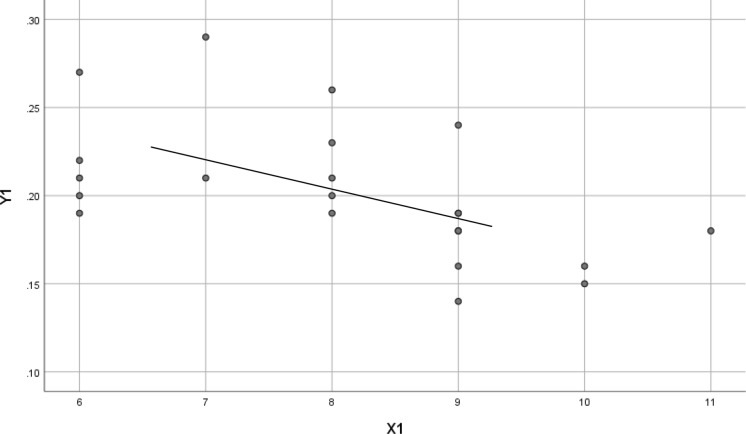
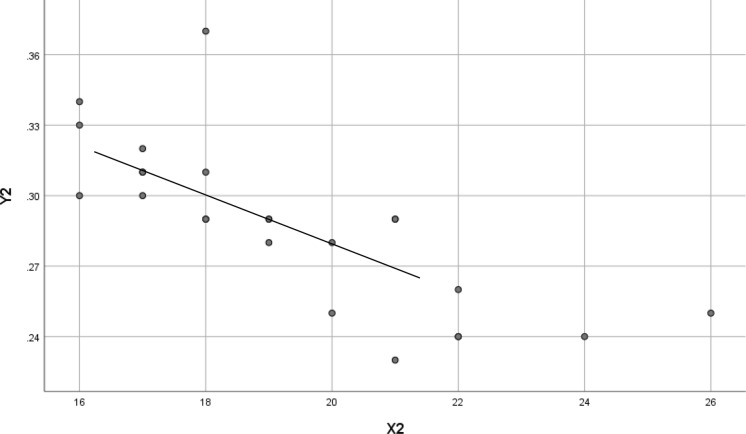
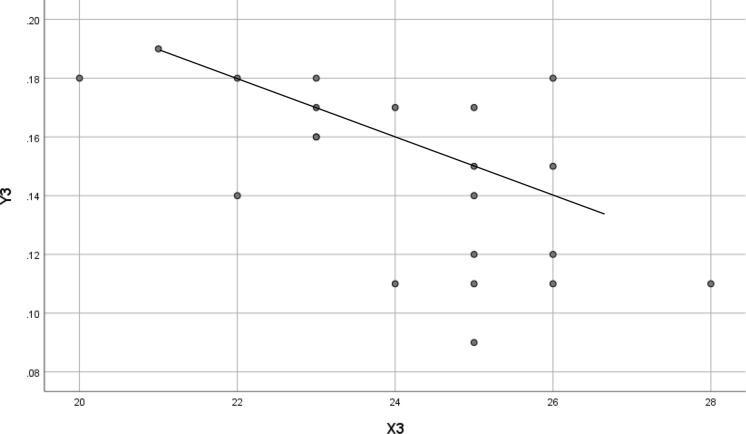
The scatter plots in Figs. 1, 2 and 3 show that the change in SFChT at 12 months was negatively associated with the change in AL at 12 months in the SA group (standard β − 0.544; t = − 2.903; P = 0.009), the OK group (standard β − 0.771; t = − 5.679; P < 0.001), and the OKA group (standard β,− 0.598; t = − 3.255; P = 0.004) (Figures 1, 2, and 3).
Fig. 1.
Scatter plots of 12-month change in AL and 12-month change in SFChT. X1 = Change in SFChT at 12 months in the SA group. Y1 = Change in AL at 12 months in the SA group. Standard β,− 0.544; t = − 2.903; P = 0.009
Fig. 2.
Scatter plots of 12-month change in AL and 12-month change in SFChT. X2 = Change in SFChT at 12 month in the OK group. Y2 = Change in AL at 12 months in the OK group. Standard β− 0.771; t = − 5.679; P < 0.001
Fig. 3.
Scatter plots of 12-month change in AL and 12-month change in SFChT. X3 = Change in SFChT at 12 month in the OKA group. Y3 = Change in AL at 12 months in the OKA group. Standard β, − 0.598; t = − 3.255; P = 0.004
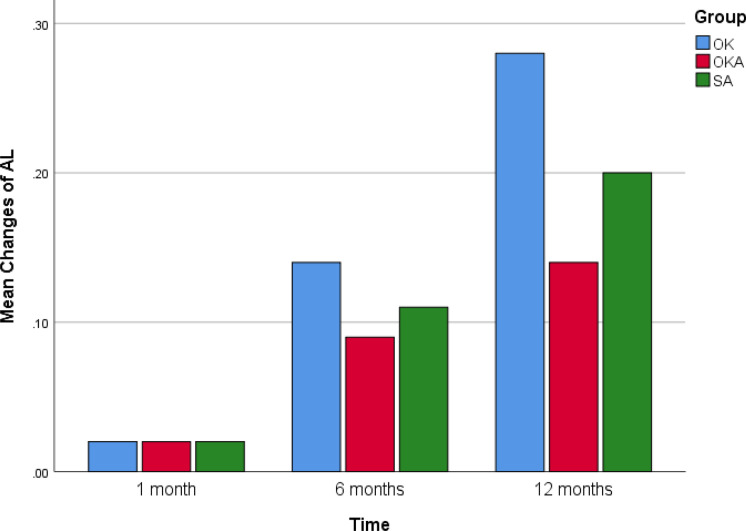
The bar graphic of Figs. 4 and 5 show the mean changes of AL and SFChT in OK, OKA and SA at 1 month, 6 months and 12 months (Figures 4 and 5).
Fig. 4.
Bar graphic of mean changes of AL
Fig. 5.
Bar graphic of mean changes of SFChT
Discussion
Myopia is a refractive error in which the refractive power is too large relative to the axial length of the eye, and uncorrected refractive error is the main cause of visual impairment [1, 27]. Various methods have been used to impede the progression of myopia, including the use of low-concentration atropine [28], pirenzepine, OK lenses [29], peripheral defocus modifying spectacle lenses, rigid gas-permeable contacts lenses, and soft multifocal contact lenses [30]. The uses of 0.01% atropine and OK lens are presently the most common and effective methods.
Previous studies demonstrated that the mechanism of myopia may be related to AL elongation and SFChT thinning. Treatment with 0.01% atropine, OK lenses, and their combination can effectively control the development of myopia and AL elongation, but the specific mechanism needs more research to confirm. Li et al. [23] showed that atropine mainly caused reduction in AL elongation. In this study, we also found that the reduction in AL elongation was greater in the SA group than in the OK group, i.e., the control of AL elongation was better with 0.01% atropine.
Zhang et al. [31] showed that administration of 1% atropine gel for 1 week led to a significant increase in SFChT (20.33 ± 28.41 μm), but this study only measured the effect of a high dose (1%) of atropine. In our study, we found that 0.01% atropine can also increase SFChT; therefore, we speculate that the effect of atropine on the SFChT may be mainly affected by the concentration of atropine. Zhao et al. reported that the increase in SFChT was greater with the combination of OK and atropine than with monotherapy with atropine after 1 month.
However, no previous study has compared the effects of 0.01% atropine, OK lenses, and the combination of them on the increase of SFChT and the change in AL elongation within 1 year. Our study showed that both 0.01% atropine and OK lenses effectively retarded AL elongation and increased SFChT. SFChT was significantly increased after only 1 month in the OK group and remained almost stable, and the increase in SFChT was also significant in the SA group.
The effects on control of AL elongation and increase in SFChT were greatest with the combination of 0.01% atropine and OK lenses; whether there is a superposition effect is unclear. Several studies [32–35] have also demonstrated that the combination of OK lenses and 0.01% atropine is more effective against myopia progression and AL elongation than monotherapy with OK lenses or 0.01% atropine.
This study also showed that the change in SFChT at 12 months was negatively associated with the change in AL at 12 months. Therefore, SFChT may influence AL elongation and myopia progression, but the mechanism is still unknown. We speculate that changes in SFChT may affect the oxygen supply and produce certain chemical substances to provide a sign of slowed axial elongation. Further animal experiments will be required to investigate this hypothesis.
There are also some limitations in this study. First, the change of SFChT may be influenced by the age, SE, accommodation and ethnic of myopia children. Second, our study only used 0.01% atropine, although other concentrations such as 0.5%, 0.1%, 0.05%, and 0.025% are also used clinically. Further studies are required to determine the effect on SFChT with different concentrations of atropine.
Author’s contribution
QH participated in the design of the study, carried out the study and drafted the manuscript and performed the statistical analyses. QZ has participated in the study’s coordination and has helped to draft the manuscript and has been involved in revising the manuscript carefully. All authors read and approved the final manuscript.
Funding
This study was funded by the Life Science Society of Liaoning.
Data availability
All data and material are available from supplementary material.
Declarations
Conflict of interest
The authors declare that they no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the second hospital of Dalian Medical University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consents were obtained from the subjects after explanation of the nature of the study.
Consent to publish
The parents of patients agreed to collect and publish the data of their children.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chia A, Chua W-H, Cheung Y-B, et al. Atropine for the treatment of childhood Myopia: safety and efficacy of 0.5%, 0.1% and 0.01% doses (atropine for the treatment of Myopia 2) Ophthalmology. 2012;119:347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmol. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Sander BP, Collins MJ, Read SA. Short-term effect of low-dose atropine and hyperopic defocus on choroidal thickness and axial length in young myopic adults. J Ophthalmol. 2019;2019:1–8. doi: 10.1155/2019/4782536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickla DL, Jordan K. Effects of time-of-day on inhibition of lens-induced myopia by quinpirole, pirenzepine and atropine in chicks. Exp Eye Res. 2019;181:5–14. doi: 10.1016/j.exer.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Williams KM, Bertelsen G, Cumberland P, et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology. 2015;122:1489–1497. doi: 10.1016/j.ophtha.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung LF, Wallman J, Smith EL. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41:1259–1269. [PubMed] [Google Scholar]
- 8.Wan L, Wei C-C, Chen CS, et al. The synergistic effects of orthokeratology and atropine in slowing the progression of myopia. J Clin Med. 2018;7:259. doi: 10.3390/jcm7090259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan IG, Ohno-Matsui K, Saw SM. Myopia Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 10.Bullimore MA, Johnson LA. Overnight orthokeratology. Cont Lens Anterior Eye. 2012;43(4):322–332. doi: 10.1016/j.clae.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita N, Konno Y, Hamada N, et al. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol. 2018;62:544–553. doi: 10.1007/s10384-018-0608-3. [DOI] [PubMed] [Google Scholar]
- 12.Li FF, Kam KW, Zhang Y, et al. Effects on ocular biometrics by 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression (LAMP) study. Ophthalmology. 2020;32:478–484. doi: 10.1016/j.ophtha.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Gallego P, Martinez-Garcia C, Perez-Merino P, et al. Scleral changes induced by atropine in chicks as an experimental model of myopia. Ophthalmic Physiol Opt. 2012;32:478–484. doi: 10.1111/j.1475-1313.2012.00940.x. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka T, Kakita T, Okamoto F, et al. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122:93–100. doi: 10.1016/j.ophtha.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, et al. Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Curr Eye Res. 2017;42(5):713–720. doi: 10.1080/02713683.2016.1221979. [DOI] [PubMed] [Google Scholar]
- 17.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vis Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)E0049-Q. [DOI] [PubMed] [Google Scholar]
- 18.Jin P, Zou H, Zhu J, et al. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol. 2016;168:164–176. doi: 10.1016/j.ajo.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Gong Q, Janowski M, Luo Mi, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135:624–630. doi: 10.1001/jamaophthalmol.2017.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott A, Read DA-C, Vincent SJ, et al. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015;56:3103–3112. doi: 10.1167/iovs.15-16446. [DOI] [PubMed] [Google Scholar]
- 21.Chiang ST-H, Phillips JR. Effect of atropine eye drops on choroidal thinning induced by hyperopic retinal defocus. J Ophthalmol. 2018;2018:1–6. doi: 10.1155/2018/8528315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102:855–8622. doi: 10.1136/bjophthalmol-2017-311266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiraoko T, Kakita T, Okamoto F, et al. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53:3913–3919. doi: 10.1167/iovs.11-8453. [DOI] [PubMed] [Google Scholar]
- 24.Tan Q, Ng AL, Cheng GP, et al. Combined atropine with orthokeratology for myopia control: study design and preliminary results. Curr Eye Res. 2019;44:671–678. doi: 10.1080/02713683.2019.1568501. [DOI] [PubMed] [Google Scholar]
- 25.Zhao W, Li Z, Hu Y, Jiang J, Long W, Cui D, Chen W, Yang X (2020) Short-term effects of atropine combined with orthokeratology (ACO) on choroidal thickness. Contact Lens Anterior Eye, undefined(undefined) 101348. 10.1016/j.clae.2020.06.006 [DOI] [PubMed]
- 26.Xiang F, He M, Morgan IG. The impact of parental myopia on myopia in Chinese children: population-based evidence. Optom Vis Sci. 2012;89(10):1487–1496. doi: 10.1097/OPX.0b013e31826912e0. [DOI] [PubMed] [Google Scholar]
- 27.Chia A, Lu Q-S, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2. Ophthalmology. 2016;123:391–399. doi: 10.1016/j.ophtha.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019;96(8):556–567. doi: 10.1097/OPX.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 29.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-C. [DOI] [PubMed] [Google Scholar]
- 30.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258. [PubMed] [Google Scholar]
- 31.Zhang Z, Zhou Y, Xie Z et al (2016) The effect of topical atropine on the choroidal thickness of healthy children [J]. Sci Rep 6(1):34936 [DOI] [PMC free article] [PubMed]
- 32.Chen Z, Xue F, Zhou J, et al. Effects of orthokeratology on choroidal thickness and axial length. Optom Vis Sci. 2016;93:1064–1071. doi: 10.1097/OPX.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Cui D, Hu Y, et al. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye. 2017;40:417–423. doi: 10.1016/j.clae.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Hu Y, Cui D, et al. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. 2019;97:454–459. doi: 10.1111/aos.13866. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Huang S, Zhou J, et al. Adjunctive effect of orthokeratology and low dose atropine on axial elongation in fast-progressing myopic childrenA preliminary retrospective study. Cont Lens Anter Eye. 2019;42:439–442. doi: 10.1016/j.clae.2018.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available from supplementary material.