Abstract
Background:
Carpal osteochondral fragmentation and subsequent post-traumatic osteoarthritis (PTOA) are leading causes of wastage in the equine athlete. Identification of synovial fluid biomarkers could contribute to the diagnosis and understanding of osteoarthritis (OA) pathophysiology.
Objective:
The aim of this study was to identify differentially expressed metabolic and glycosylation pathways in synovial fluid from healthy horses and horses with naturally occurring carpal OA.
Study design:
Cross-sectional, in vivo metabolomics and glycomics study.
Methods:
In cohort 1, carpal synovial fluid (n = 12 horses; n = 6 healthy, n = 6 OA) was analysed using high-resolution liquid chromatography mass spectrometry (LC-MS). In cohort 2 (n = 40 horses; n = 20 healthy, n = 20 OA), carpal synovial fluid was analysed using lectin microarrays and a lubricin sandwich ELISA.
Results:
Metabolomic analysis identified >4900 LC-MS features of which 84 identifiable metabolites were differentially expressed (P < .05) between healthy and OA joints, including key pathways related to inflammation (histidine and tryptophan metabolism), oxidative stress (arginine biosynthesis) and collagen metabolism (lysine metabolism). Principle Component Analysis and Partial Least Squares Discriminant Analysis demonstrated separation between healthy and OA synovial fluid. Lectin microarrays identified distinct glycosylation patterns between healthy and OA synovial fluid, including increased Core 1/Core 3 O-glycosylation, increased α−2,3 sialylation and decreased α−1,2 fucosylation in OA. O-glycans predominated over N-glycans in all synovial fluid samples, and synovial fluid lubricin was increased in OA joints as compared to controls.
Main limitations:
The sample size in cohort 1 was limited, and there is inherent variation in severity and duration of joint injury in naturally occurring OA. However, LC-MS identified up to 5000 unique features.
Conclusions:
These data suggest new potential diagnostic and therapeutic targets for equine OA. Future targeted metabolomic and glycomic studies should be performed to verify these results. Lectin microarrays could be investigated as a potential screening tool for the diagnosis and therapeutic monitoring of equine OA.
Keywords: horse, glycomics, lectin microarray, lubricin/proteoglycan 4, mass spectrometry, metabolomics, O-glycosylation
1 |. INTRODUCTION
Osteochondral fragmentation is a common joint pathology that leads to subsequent post-traumatic osteoarthritis (PTOA) in horses. Osteochondral fragments can arise as a result of acute trauma or from cyclic overload, resulting in cartilage damage and release of inflammatory debris within the joint.1 In order to prevent inflammation and continued injury to the joint, subtle changes to articular cartilage, subchondral bone and synovium would ideally be detected prior to osteochondral fragmentation or development of osteoarthritis (OA). However, early detection of OA remains a challenge in all species, including horses, as radiographic changes are slow to develop.2 Synovial fluid biomarkers may enable detection of early joint disease prior to radiographic changes and could improve monitoring of OA progression, response to treatment and identification of novel therapeutic targets for joint disease.3
Several studies have investigated changes in the abundance of proteins in osteoarthritic synovial fluid;4,5 however, disease biomarkers can also arise from glycomic and metabolomic profiling. Glycosylation of synovial fluid proteins has never been systematically described in any species. Metabolomics shows promise for the early diagnosis and monitoring of OA progression in humans.6 Metabolic pathways related to inflammation,7 energy metabolism in synovial fluid8 and oxidative stress in serum9 are commonly deranged in both human OA and rheumatoid arthritis (RA). Thus far, equine synovial fluid metabolomic analyses have been restricted to targeted lipidomics studies,10 differentiation of septic and nonseptic joint pathologies11 and metabolomic assessment of fetlock palmar osteochondral disease (POD) in parallel with MRI.12 A targeted lipidomics study investigating eicosanoid release in lipopolysaccharide-induced equine synovitis revealed a reduction in inflammatory mediators, including prostaglandin E2, following treatment with meloxicam.10 1H-nuclear magnetic resonance (NMR) spectroscopic analysis has been able to identify multiple differentially expressed metabolites in synovial fluid from septic and nonseptic equine joint pathologies. Multivariate separation was driven primarily by higher glucose, in addition to other metabolites such as higher acetate, glycine, citrate, creatinine and alanine and lower glycylproline in cases with OA or osteochondrosis when compared to sepsis.11 1H-NMR spectroscopy of equine synovial fluid from clinical cases with MRI-diagnosed POD lesions found glucose and lactate to be the most influential metabolites between POD and control joints, though statistical significance was not achieved.12 Analysis of synovial fluid is of particular interest for investigation of joint diseases such as OA since it is directly in contact with the tissues of interest, including the synovial membrane and articular cartilage surface, thereby providing site-specific metabolic data as compared to blood or urine.
Synovial fluid glycosylation has not been extensively investigated in the context of OA for any species; however, protein glycosylation has been studied in other disease processes such as cancer,13 autoimmune disease14 and infectious disease.15 Protein glycosylation, a common post-translational modification, is the enzymatic addition of carbohydrates to proteins. Glycans, the carbohydrate portion of proteins, can play important roles in modulating protein binding, stability and downstream functions, including immune recognition. Lectin microarray analysis, developed in 2005,16 has been utilised extensively to study glycosylation in a multitude of complex biological samples including cells,17 tissues,18 synovial fluid19 and sera.20 Evaluation of glycosylation in synovial fluid has mainly focused on specific proteins,21 predominantly restricted to human patients, with the majority of studies investigating RA as compared to OA. For example, lectin microarray analysis of matrix metalloprotease-3 (MMP-3) from synovial fluid showed higher levels of O-linked sialic acid in RA than OA.19 In addition, glycosylation of equine synovial fluid lubricin has been reported.21 However, there are no reports on global glycosylation changes in synovial fluid as a function of OA.
Synovial fluid contains abundant hyaluronic acid (HA) and lubricin, the two primary cartilage lubricating molecules, which significantly contribute to the glycoprofile of synovial fluid.22 HA is a polysaccharide composed of repeating units of the disaccharide D-glucuronic acid and N-acetyl-D-glucosamine, and lubricin is a threonine-rich glycoprotein that undergoes extensive O-linked glycosylation. A recent study evaluating synovial fluid lubricin in horses with OA or osteochondral fragmentation identified 11 lubricin-associated glycans using quantitative proteomics, western blotting and ELISAs.21 Lubricin from synovial fluid in joints with osteochondral fragments had increased monosialylated Core 1 O-glycans and decreased disialylated Core 1 O-glycans.21 In a study evaluating lubricin from synovial fluid using LC-MS of two human OA patients, 90%−95% of the total ion counts were monosialylated and disialylated Core 1 O-glycans, whereas sulfation was only identified in 4%−8% of Core 2 O-glycans.22
The objective of this study was to identify differentially expressed metabolites and global protein glycosylation in synovial fluid obtained from healthy and osteoarthritic equine carpal joints using untargeted metabolomics and glycomics approaches. The ultimate goal is to identify metabolic and glycosylation profiles which could improve the overall understanding of OA pathophysiology as well as improve early disease detection, enable monitoring of disease progression and response to therapy and/or lead to identification of novel therapeutic targets for OA.
2 |. MATERIALS AND METHODS
2. 1 |. Sample population
2.1.1 |. Metabolomics study (cohort 1)
This study was designed as a case-control study. Cohort 1 consisted of 12 horses, including healthy (n = 6) and OA (n = 6) cases (Table S1A). All horses were racing breeds (n = 11 Thoroughbreds and n = 1 Standardbred) in active race training or racing immediately prior to presentation for surgery. OA cases were randomly selected from a large population of carpal OA samples matched by age, sex and breed as closely as possible to healthy samples. These samples consisted of equine clinical patients that presented to the Cornell University Equine Hospital for carpal arthroscopy with clinical and radiographic evidence of carpal osteochondral fragments from 2013 to 2017. The OA group (n = 5 Thoroughbreds and n = 1 Standardbred) consisted of 2 females and 4 castrated males, and horses ranged in age from 2 to 6 years with a median age of 3 years. Horses were housed in 12 × 12 ft box stalls in the hospital prior to and immediately after surgery with ad libitum water and grass/timothy hay mixture, supplemented with grain as needed. Radiographic evaluation of both carpi was performed prior to surgery, and joints were evaluated based on the presence of osteophytes, enthesophytes, osteoproliferation, joint space narrowing or chronic fracture lines, with additional arthroscopic scoring used to corroborate the radiographic score, as previously described.23,24 All OA joints were affected by osteochondral fragmentation (n = 4 radial carpal bone and n = 2 third carpal bone) with associated articular cartilage degeneration. Horses were treated with prophylactic peri-operative antimicrobials, including the administration of penicillin or penicillin and gentamicin in combination, at the discretion of the surgeon performing the procedure. All OA horses were induced with ketamine and diazepam and treated with peri-operative phenylbutazone for analgesia.
Healthy control horses were exracehorses donated for reasons unrelated to musculoskeletal disease, including poor performance and upper airway pathology. The healthy group were all Thoroughbreds (n = 6), including 2 females and 4 castrated males, and ages ranged from 3 to 5 years with a median age of 4.5 years. Horses were housed in 12 × 12 ft box stalls with paddock turnout, ad libitum water and grass/timothy hay mixture, and supplemented with grain as needed. Horses were de-wormed with ivermectin following an initial quarantine period of 2 weeks. Horses were free of any clinical evidence of carpal disease, including joint effusion or reduced range of motion, and only horses with an American Association of Equine Practitioners (AAEP) lameness score of ≤1 were included. Absence of carpal osteochondral fragmentation or evidence of OA was confirmed with pre-operative radiographs and corroborated with arthroscopic scoring. Synovial fluid was collected from both middle carpal joints, while horses were under general anaesthesia, similar to cases, immediately prior to arthroscopic evaluation. Control horses were treated with peri-operative antimicrobials, including the administration of trimethoprim sulfamethoxazole, and all horses were induced with ketamine and diazepam and treated with peri-operative phenylbutazone for analgesia.
2.1.2 |. Glycomics study (cohort 2)
The second cohort of 40 horses included healthy (n = 20) and OA (n = 20) cases (Table S1B). The OA cases consisted of both equine clinical surgical cases (n = 14) and cases donated for euthanasia due to moderate-severe carpal OA (n = 6) from 2013–2018. This group included Thoroughbreds (n = 15), Standardbreds (n = 2) and Quarter Horses (n = 3) and consisted of 13 females, 4 castrated males and 3 intact males. The horses ranged in age from 2 to 19 years with a median age of 3 years. Surgical cases were housed, medicated, and evaluated similarly to Cohort 1. Cases that presented for euthanasia had synovial fluid collected immediately following humane euthanasia induced via barbiturate overdose, and cartilage and osteoarthritic lesions were confirmed and documented by gross dissection of the carpal joints. All OA joints were affected by osteochondral fragmentation (n = 8 radial carpal bone, n = 6 distal radius, n = 3 intermediate carpal bone, n = 2 third carpal bone and n = 3 horses with generalised OA secondary to multiple osteochondral fragments), and cartilage degenerative changes were noted on either arthroscopic evaluation or postmortem examination.
Healthy cases consisted of horses donated for euthanasia for research projects unrelated to forelimb musculoskeletal pathology from 2013 to 2018. The healthy group contained Thoroughbreds (n = 9), Standardbreds (n = 3), light-breed crossbreds (n = 6), one Warmblood, and one Quarter Horse. The control group consisted of 11 females, 6 castrated males and 1 intact male with ages ranging from 2 to 11 years and a median age of 5 years. Horses had synovial fluid collected immediately following humane euthanasia induced via barbiturate overdose, and the absence of cartilage and osteoarthritic lesions was confirmed and documented by gross dissection of the carpal joints.
2.2 |. Sample collection
2.2.1 |. Metabolomics study (cohort 1)
Carpal synovial fluid samples were obtained from middle carpal joints (MCJ) via synoviocentesis immediately prior to arthroscopic procedures. Only one SF sample was evaluated per horse. All horses underwent synoviocentesis via an 18G 3 1/2 inch needle using a dorsolateral approach to the MCJ with the horse in dorsal recumbency under general anaesthesia, following clipping and a sterile preparation with chlorhexidine gluconate and alcohol. In OA cases, the synovial fluid volume obtained ranged from 3 to 8.5 mL (median: 5.3 mL). In healthy horses, the synovial fluid volume was the maximum volume obtainable through synoviocentesis and typically ranged from 4 to 10 mL. Synovial fluid was immediately transferred to a 15 mL polypropylene tube on ice, followed by centrifugation at 4000 g for 5 minutes. Supernatants were decanted into 2 mL aliquots and stored at −80°C until sample analysis.
2.2.2 |. Glycomics study (cohort 2)
Carpal synovial fluid samples were obtained from the antebrachiocarpal (ACJ) (n = 17 total; n = 8 OA and n = 9 control) or MCJ (n = 23 total; n = 12 OA and n = 11 control) via synoviocentesis immediately prior to arthroscopic procedures as described in Cohort 1, or immediately following euthanasia. Only one SF sample was evaluated per horse. If more than one sample was available for a single horse, the sample was selected by using a random number generator. The maximum synovial fluid volume attainable was collected via an 18G 3 1/2 inch needle using a dorsolateral approach to the ACJ or MCJ. In OA cases, the synovial fluid volume obtained ranged from 1.5 to 10 mL (median: 5 mL), and in healthy cases, the synovial fluid volume obtained ranged from 3 to 9 mL (median: 5.5 mL). Synovial fluid was immediately transferred to a 15 mL polypropylene tube on ice, followed by centrifugation at 4000 g for 5 minutes. Supernatants were decanted into 2 mL aliquots and stored at −80°C until sample analysis.
2.3 |. Synovial fluid metabolomics
Operators were blinded as to group allocation until after data collection was completed. Synovial fluid samples were thawed on ice over a period of 20 minutes. Once thawed, samples were vortexed for approximately 1 minute. Samples (200 μL) were transferred to 1.7 mL Eppendorf tubes, and 800 μL MeOH (1:4) was added to each synovial fluid sample. Samples were centrifuged, and the supernatant was dried. The samples were resuspended with 50% acetonitrile (ACN) at 0°C, centrifuged and dried down for final reconstitution in 25% ACN for liquid chromatography-mass spectrometry (LC-MS) analysis.
Chromatographic separation was performed on a Vanquish UHPLC system with a SeQuant ZIC pHILIC column (5 μm, 2.1 × 150 mm) coupled to a Q Exactive™ HF Mass Spectrometer (Thermo Fisher Scientific). The mobile phase consisted of (A) 10 mM ammonium acetate in Water pH = 9.8 and (B) acetonitrile. The gradient was as follows: 0–15 minutes, 90%−30% solvent B; 15–18 minutes, isocratic 30% solvent B; 18–19 minutes, 30%−90% solvent B; 19–27 minutes, 90% solvent B; followed by 3 minutes of re-equilibration of the column before the next run. The flow rate was 250 μL/min, and the injection volumes were set to 2 μL. To avoid possible bias, the sequence of injections was randomised.
All of the samples were analysed by negative and positive electrospray ionisation (ESI) in full scan MS mode. Nitrogen as sheath, auxiliary and sweep gas was set at 50, 8, and 1 U, respectively. Data were acquired under resolving power of 120 000 (at m/z 200); automatic gain control target, 3e6 ions; maximum injection time, 100 ms; scan range, 67–1005 m/z; spray voltage, 3.50 kV; and capillary temperature, 275°C.
ESI ± data-dependent MS/MS spectra were generated on a quality control (QC) sample containing equal amount of each sample which was required and used for identification and normalisation in quantitation data analysis. The QC sample was run at the beginning of batch runs, in the middle of every five to eight runs and at the end of runs. MS/MS data were acquired with a full scan followed by top 15 MS/MS scans with resolving power of 15 000 (at m/z 200); automatic gain control target, 1e5 ions; maximum injection time, 50 ms; isolation window, 0.4 m/z; and stepped NCE 20, 30 and 40. The acquired raw files, including raw QC samples, were processed using Compound Discoverer 2.1, an untargeted metabolomics workflow with putative annotation through in-house mass list and databases including ChemSpider, Human Metabolome database (HMDB), Metlin, Kyoto Encyclopaedia of Genes and Genomes (KEGG) and mzCloud were used for processing the raw data and for compound annotation. The software parameters for alignment were 5 ppm mass tolerance for the adaptive curve model and 0.5 minutes maximum shift allowed. For detecting unknown compounds, 5 ppm mass tolerance for detection was used along with 30% intensity tolerance, 3 for the signal to noise threshold and 2 × 106 as minimum peak intensity.
2.4 |. Metabolomics data filtering
The resulting data yielded a combined 4900 LC-MS features from both positive and negative modes. The process of filtering these features started with removing all features that could not be annotated by Compound Discover 2.1. Removing all those listed as “similar to,” which had a molecular mass that could not be accounted for with the removal or addition of an oxygen or hydrogen group, further narrowed the LC-MS features. Finally, all replicate samples were evaluated. Any features in replicate samples that had significantly different retention times (>1 minute) were removed. In some cases, there appeared to be two groups based on retention time within one metabolite. In this case, the known polarity of the metabolite was compared to the retention time. When metabolites were detected in both negative and positive ion modes, those with higher intensity and higher S/N were selected. The remaining annotated metabolites (n = 436) were analysed using databases such as ChemSpider, HMDB, Metlin, KEGG and mzCloud. In order to minimise the effects of confounding metabolites related to diet or administered medications, such associated metabolites were removed from the study. This resulted in a total of 223 metabolites for further analysis.
2.5 |. Synovial fluid glycomics
Again, operators were blinded as to group allocation until after data collection was completed. Synovial fluid samples from 20 OA joints and 20 healthy carpal joints were thawed on ice, vortexed and diluted to 2 mg/mL in phosphate buffered saline (PBS, pH 7.4). To digest hyaluronic acid, 2 units of hyaluronidase (EMD Millipore) was added to the diluted sample (50 μL) and incubated at 37°C for 1 hour. Post-digestion, 25 μg of protein (based on original sample concentration, determined by DC Protein Assay, Bio-Rad), was labelled with 10 μg of NHS-activated Alexa Fluor 555 (Thermo Fisher). A reference sample consisting of 6.25 μg from each sample (250 μg total, pooled reference) was labelled with 100 μg of NHS-activated Alexa Fluor 647 (Thermo Fisher). For both reactions, the labelling mixture was adjusted to a final concentration of 0.1 mol/L NaHCO3 (final pH ~ 8.5). After 30 minutes, the reaction was stopped by adding 2 M Tris buffer (pH = 6.8) to a final concentration 0.25 mol/L Tris. Labelled samples were desalted using Zeba Spin Desalting Plates (7K MWCO, Thermo Fisher) per manufacturer’s instructions. Lectin microarrays were printed as previously described25 (Table S2). Labelled samples (5 μg) were mixed with 5 μg of labelled reference and diluted to a final volume of 150 μL PBST (final concentration: 0.1% v/v Tween-20, pH = 7.4) and added to the arrays. After 2 hours, the samples were removed and arrays were rinsed with PBST (0.005% v/v Tween-20, 3×, 5 minutes). The slides were then gently rinsed with ultrapure water, centrifuged until dry and scanned with a Genepix 4400A (Molecular Devices) in the Cy3 (ex/em 532/550–600 nm) and Cy5 (ex/em 635/655–695 nm) channels. Data were extracted and analysed using Genepix Pro 7 software as previously described.26 Probes whose SNR < 5 for >90% of the samples were excluded. For the remaining probes, the intensity of each probe in each fluorescence channel was normalised to the median of the intensities of all probes.
2.6 |. Lubricin ELISA
A sandwich ELISA was performed to quantify equine synovial fluid lubricin as previously described,24 with slight modification. Samples from cohort 2, which matched the glycomics analysis, were used with the exception of two control horses with an inadequate volume of fluid (Table S1B). Synovial fluid samples were diluted 1:1000 in PBS. The PBS-diluted synovial fluid samples and serial dilutions of purified equine synovial fluid lubricin as standards were loaded onto 96-well plates coated with 10 μg/mL of peanut agglutinin (MilliporeSigma) for 20 minutes at room temperature and overnight at 4°C. Lubricin was detected with a mouse antihuman lubricin monoclonal antibody (mAb 9G3, MABT401, MilliporeSigma) at a dilution of 1:2500 in PBS+3% bovine serum albumin (BSA) and goat antimouse IgG-HRP (AP181P, MilliporeSigma) at 1:4000 in PBS+3% BSA. Absorbance was measured at 450 nm with a reference at 540 nm using a SPARK 10M microplate reader (TECAN).
2.7 |. Data analysis
2.7.1 |. Metabolomics
Statistical analysis of the derived metabolites was performed using MetaboAnalyst 4.0. Peak intensity data were analysed as unpaired data sets, a missing value estimate was performed removing features >50% missing values and replaced by 1/5 of the minimum positive value of each variable and interquantile range was utilised for data filtering. The metabolites were then normalised by log transformation and Pareto scaling for multivariate analysis. As previously described,8,27 multivariate analysis was performed using an orthogonal projections to latent structures-discriminant analysis (OPLA-DA) followed by principal component analysis (PCA). Pathway analysis was performed on the filtered metabolites with significant differences between healthy and OA groups using MetaboAnalyst 4.0 and referencing the KEGG metabolic pathway database. Pathway enrichment was determined by hypergeometric test and reported with an FDR-adjusted P-value.
2.7.2 |. Glycomics
Glycomics lectin microarray data analysis was performed in Excel. Multivariant and univariant analyses were performed, as well as an unpaired t-test between healthy and OA joints and between N-glycan and O-glycan signals for all samples. Lubricin ELISA concentration data were first analysed for normality using a Shapiro–Wilk test, followed by an unpaired t test using JMP 14 software (SAS).
3 |. RESULTS
3.1 |. Univariant and multivariant comparisons of OA to control joints
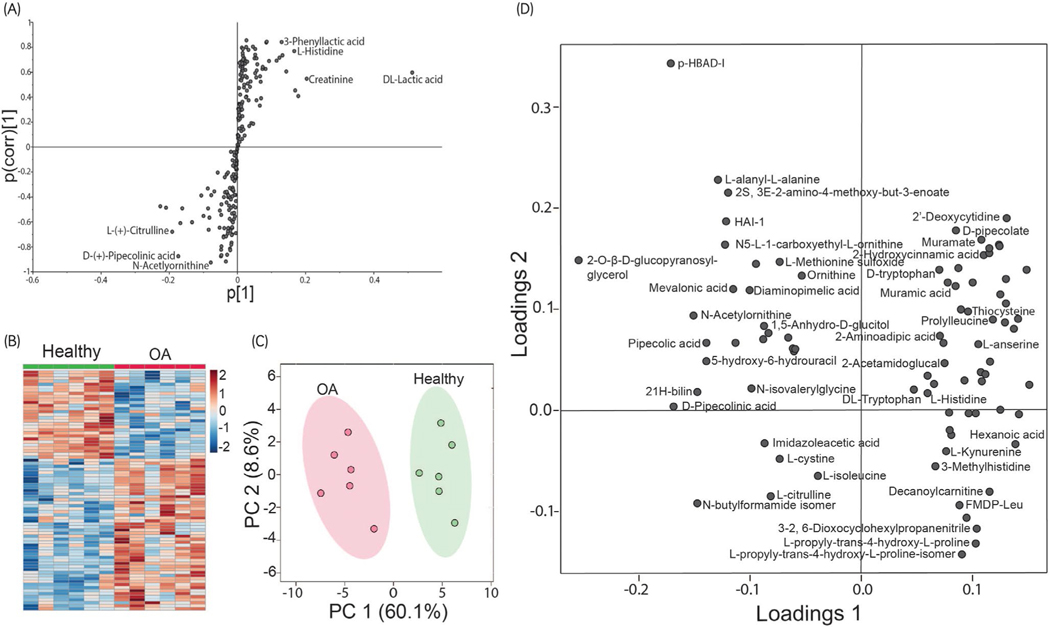
Analysis of synovial fluid revealed several metabolites with differences between healthy and OA joints. Once filtered, as described above, SIMCA-P 15.0 (Umetrics) was used to perform an orthogonal projection to latent structures-discriminant (OPLA-DA) analysis of the metabolites. The resulting s-plot (Figure 1A) consists of plotting the modelled correlation (Pcorr) against the model covariance (P).
FIGURE 1.
Comparison of the metabolome between healthy and osteoarthritis (OA) synovial fluid. (A) OPLS-DA loading S-plot to identify metabolites that are increased or decreased in OA. The model covariance represents the magnitude of the difference between healthy and OA of a metabolite, and the model correlation represents the reliability of the difference between healthy and OA. The metabolites situated in the extremes of the S-plot, in either positive or negative covariance, represent possible discriminating variables between healthy and OA synovial fluid samples. (B) Heat map of relative changes between metabolites (P < .05). Increases in OA are indicated in red, and decreases in OA are indicated in blue; the darker the colour, the larger the difference in concentration. (C) PCA score plot formed two distinct clusters of metabolites segregating healthy (green) and OA (red) between principal component 1 and principal component 2. Shading represents the 95% confidence interval. (D) Loading plots of the first two principal components. For clarity, some metabolite names were excluded from the figure
The metabolites were further filtered for those with significant differences (P < .05) between healthy and OA joints (Table S3), resulting in 84 metabolites with a fold change of OA to healthy ranging from 0.4 to 3.0. A univariant analysis demonstrated differences between healthy and OA joints. The resulting heat map (Figure 1B) revealed 79 metabolites that differed between healthy and OA joints (P < .05), with 50 increased in OA and 29 decreased in OA. When an FDR correction was applied, 28 metabolites remained different between healthy and OA joints (q < 0.05) (Table S3).
To map the metabolic changes between control and OA groups, PCA was performed for five principal components (PC). This unsupervised method allows for dimensionality reduction and resulted in two distinct groups segregating healthy and OA equine synovial fluid when PC1 (accounted for 58.8% of the variation between healthy and OA) was plotted against PC2 (8.1% variation) (Figure 1C) with no outliers. Additionally, a loading plot was analysed to identify the relative contributions and relationships between the metabolites for PC1 and PC2 (Figure 1D).
3.2 |. Metabolic pathway analysis
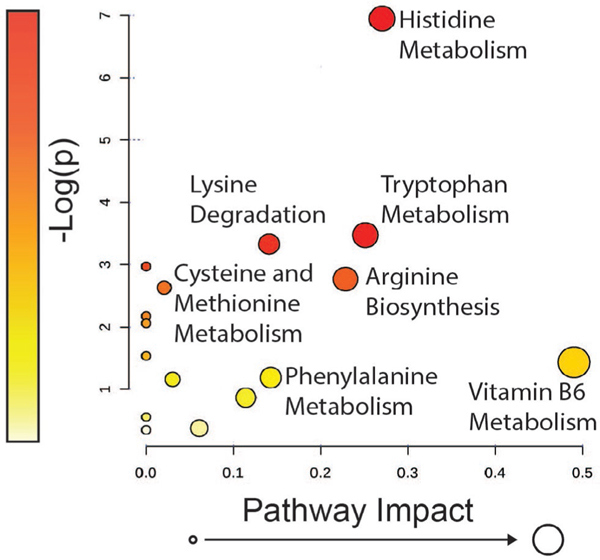
To identify metabolic pathways that were differentially regulated in OA, metabolites that differed significantly (P < .05) between healthy and OA joints underwent pathway analysis. Only the metabolites matched with KEGG pathway codes (n = 50) could be analysed via this method, resulting in 16 pathways containing metabolites that were altered in OA. When FDR correction was applied, none of the pathways were significantly altered in OA. Due to this, uncorrected p-values were used for the remainder of the analyses. In addition to being ranked by P-value, metabolites were assessed by pathway impact (Figure 2). P-value and impact factor were weighed equally to determine the pathways of focus for this study. For each pathway, a match statistic indicates the number of altered metabolites out of the total number of metabolites, and their respective identities, associated with that particular pathway (Table S4). The most significantly altered pathway was histidine metabolism (P = .001), followed by tryptophan metabolism (P = .03) and lysine degradation (P = .04). The top 10 pathways were summarised and ranked by P-value (Table 1).
FIGURE 2.
Pathway over representation analysis showing all matched pathways according to P-value (pathway enrichment analysis) and pathway impact (pathway topology analysis) derived from KEGG pathway codes. The P-value is indicated by colour ranging from yellow as least significant to red as most significant. Pathway impact is indicated by circle size where the pathways with the lowest impact factor have the smallest circle to those with the highest impact factor having the largest circle
TABLE 1.
The table indicates the top 10 pathways identified using pathway enrichment analysis and pathway impact ranked by P-value, as well as FDR adjusted P-value
| Pathway name | Match status | P-value | FDR P-value | Impact |
|---|---|---|---|---|
| Histidine metabolism | 4/16 | <.01 | .09 | 0.3 |
| Tryptophan metabolism | 4/41 | .01 | .3 | 0.3 |
| Lysine degradation | 3/25 | .04 | 1 | 0.1 |
| Aminoacyl-tRNA biosynthesis | 4/48 | .05 | 1 | 0 |
| Arginine biosynthesis | 2/14 | .07 | 1 | 0.2 |
| Cysteine and methionine metabolism | 3/33 | .08 | 1 | 0.02 |
| D-Arginine and D-ornithine metabolism | 1/4 | .1 | 1 | 0 |
| beta-Alanine metabolism | 2/21 | .1 | 1 | 0 |
| Valine, leucine and isoleucine biosynthesis | 1/8 | .2 | 1 | 0 |
| Vitamin B6 metabolism | 1/9 | .2 | 1 | 0.5 |
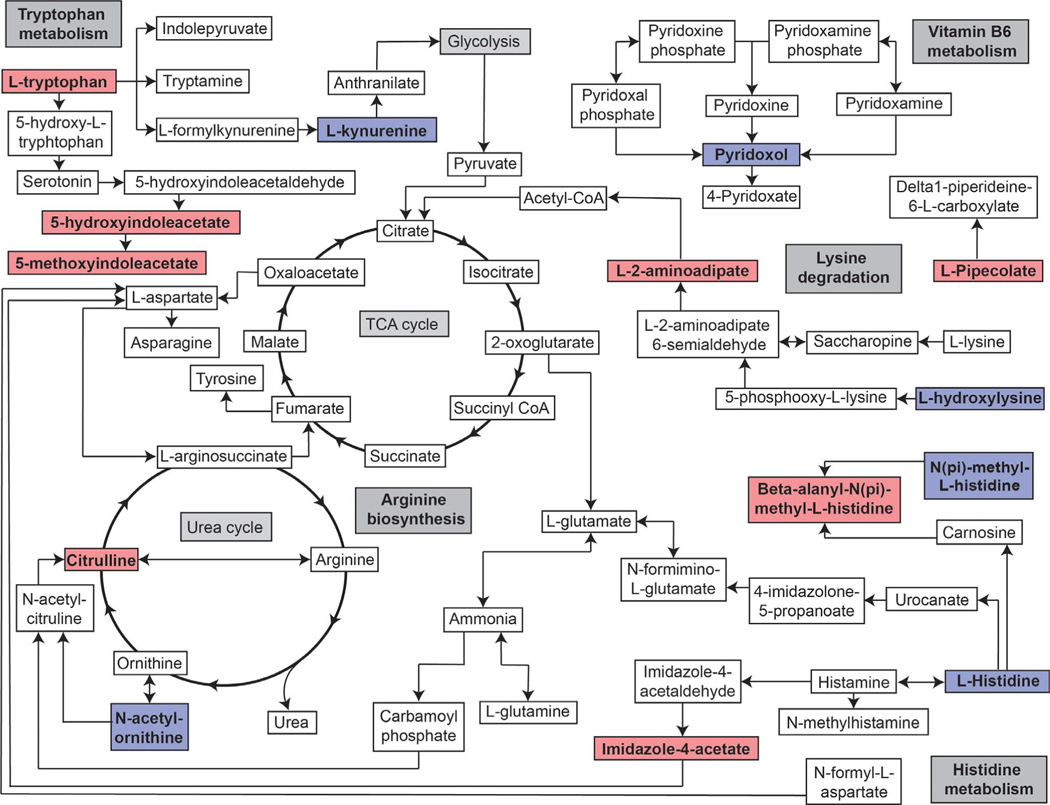
Pathway analysis does not account for directionality or the degrees of differences between healthy and OA synovial fluid metabolites. The resulting pathways from this analysis were therefore incorporated into one diagram (Figure 3) to allow for visualisation of this data with the added indication of directionality of the individual metabolites. There were 34 metabolites that were unable to link to a KEGG pathway for analysis (Table S5). These metabolites were manually analysed using HMDB, PubChem, MetaCyc and ChemSpider.
FIGURE 3.
Pathway map indicating the directionality of metabolites recognised to impact the top five KEGG pathways. Metabolites which were increased in OA are indicated in red, and those which were decreased are indicated in blue
3.3 |. Glycan analysis
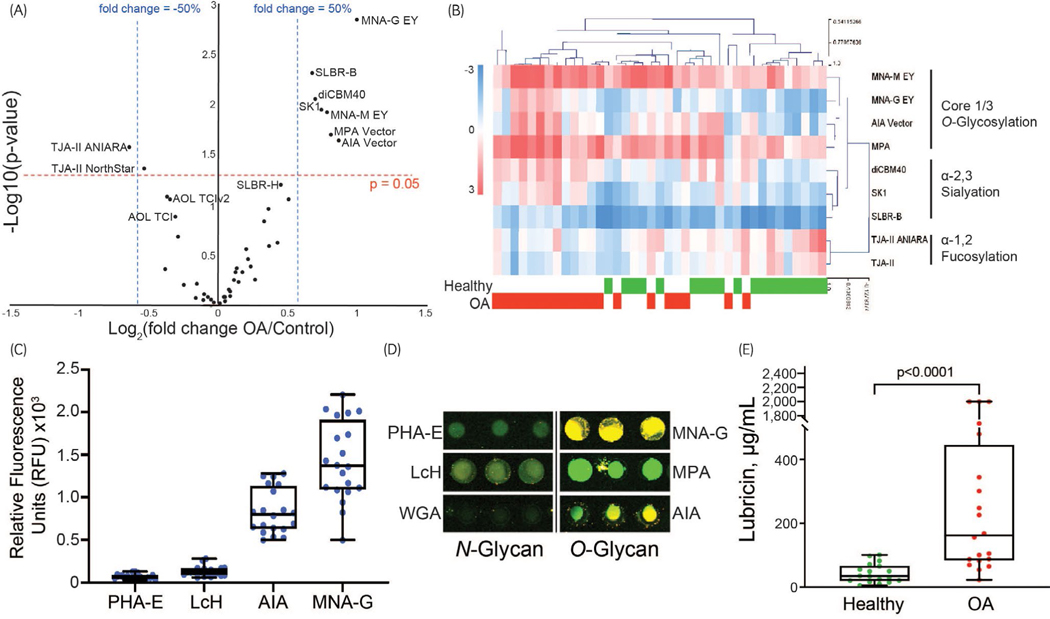
Lectin microarrays demonstrated differences in glycosylation patterns between OA and healthy synovial fluid samples. A univariant analysis was performed, with lectins showing a P < .05 and a fold change of ±50% relative fluorescence considered significant (Figure 4A). There were three notable changes in glycosylation identified by lectin microarrays (Figure 4B): increased Core 1/Core 3 O-glycosylation (lectins: MNA-M, MNA-G, AIA and MPA), increased α−2,3 sialylation (diCBM40, SK1, SLBR-B) and decreased α−1,2 fucosylation (TJA-II). Lower signal levels for lectins specific to core N-glycans when compared to O-glycans in both healthy and OA synovial fluid samples (Figure 4C,D).
FIGURE 4.
Glycomic analysis between healthy and osteoarthritis (OA) synovial fluid. (A) The volcano plot indicates eight lectins (including duplicate lectins from different sources) that differed significantly between healthy and OA synovial fluid; six with a fold change greater that 50%; one with a fold change greater than −50% and one with a fold change less than −50%. (B) Heat map of the relative changes (logarithmic, base = 2) between the eight lectins identified in the volcano plot. (C) Fluorescence intensity values for four lectins showing an overall predominance of O-glycans (AIA and MNA-G) to N-glycans (PHA-E and LcH) in both healthy and OA synovial fluid samples. (D) Dual colour lectin microarray image of a representative healthy synovial fluid sample, demonstrating the predominance of O-glycans. (E) Lubricin ELISA showed increased concentrations of lubricin in equine OA synovial fluid
Lubricin sandwich ELISAs confirmed a significant increase (P < .05) in synovial fluid lubricin in OA samples (Figure 4E). For three samples, the Abs 450 of 1:1000 diluted SF samples read out of the standard range (maximum 2 μg/mL); therefore, lubricin concentration was reported as the maximum standard value (2 μg/mL). The difference between the mean of the control samples (43.54 ± 7.0 μg/ mL) and the mean of the OA samples (471.7 ± 153.2 μg/mL) was approximately tenfold.
4 |. DISCUSSION
Distinct differences were observed in metabolite and glycan expression between healthy and OA equine synovial fluid. Metabolomics identified key pathways altered in OA, including inflammation (histidine and tryptophan metabolism), oxidative stress (arginine biosynthesis) and collagen metabolism (lysine degradation). The overall glycosylation patterns in synovial fluid were distinct when comparing OA to healthy joints. The increased α−2,3 sialylation and Core 1/3 O-g lycans observed could be due to the increase in synovial fluid lubricin, as these glycans are known to be dominant on that protein and increased sialylation has been observed with OA.21 Sialylation and fucosylation are terminal modifications and can occur on both N-and O-glycans. Interestingly, we observed stronger levels of signals from lectins specific to core O-glycan structures when compared to N-glycans, in both healthy and OA, arguing that the O-glycoproteome is more highly represented in this biofluid. These findings could be used to motivate future targeted studies of the O-linked glycoproteome in equine synovial fluid. An enhanced understanding of how synovial fluid metabolites and glycans are altered in equine OA could lead to development of novel treatments, earlier detection and improved monitoring of response to OA therapeutics.
One of the goals of this study was to gain a broader understanding of the glycan composition of equine synovial fluid. Few studies have investigated synovial fluid glycan composition using lectin microarrays, and to the authors’ knowledge, no studies have investigated OA synovial fluid using lectin microarrays. Lectin microarrays enable rapid, untargeted analysis of complex biological fluids, such as synovial fluid, which can then be followed by targeted analysis utilising methods such as LC-MS. O-glycans have previously been identified on the surface of healthy equine articular cartilage using lectin staining and confocal microscopy, with particularly strong signals for the lectin PNA, which binds to the primary disaccharide of Core 1 O-glycans, and weaker signals for the lectins S-WGA and PHA.24 Mass spectrometry of human articular knee cartilage found that OA chondrocytes expressed nonsialylated, monosialylated and disialylated N-glycans and Core 2 O-glycans.28 In contrast, we observe Core 1/3 O-glycosylation and α−2,3 sialyation in equine OA synovial fluid. Lectin microarrays cannot distinguish between Core 1 and Core 3 O-glycans since these lectins bind to both epitopes. Since previous studies identified Core 1 O-glycans in synovial fluid,21 it is likely that Core 1 O-glycans represent the majority of the Core 1/3 O-glycosylation identified in equine carpal OA, especially since Core 3 O-glycans have generally been associated with gastrointestinal tract mucins.29 Several of the metabolites that could not be linked with pathways were related to bacterial metabolism, suggesting that investigation of the microbiome in equine OA could be an important area for future study.
Lubricin, a synovial fluid glycoprotein with extensive Core 1 O-glycosylation and α−2,3 sialylation, has been reported to increase in experimental and naturally occurring carpal OA,24 tarsal impact injury,30 and full-thickness cartilage defect models.30 Increases in synovial fluid lubricin are also reported in human intra-articular fracture31 and end-stage OA.32 Targeted glycomics studies of purified lubricin from equine joints have revealed more Core 1 O-glycans in OA and osteochondral fragmentation as compared to normal joints and fewer Core 2 O-glycans in OA and osteochondral fragmentation as compared to normal joints.21 In purified lubricin samples from human RA and OA synovial fluid, a high abundance of modified Core 1 O-glycans and Core 2 O-glycans was identified but was not compared to healthy joints.33 Increases in neuraminic acid (sialic acid) were detected in OA synovial fluid using both LC-MS and lectin microarrays in the current study.
Along with altered glycosylation, the most significantly altered metabolic pathways in equine OA were inflammatory pathways, including increased tryptophan and histidine metabolism in equine OA joints. Although these pathways did not remain significant after FDR correction, this is likely a reflection of the small sample size as acknowledged in previous synovial fluid metabolomic studies.34 Alterations in inflammatory pathways have been reported in human RA, OA7 and in rodent OA models,35 and tryptophan metabolism has been studied as a biomarker for RA using urine tryptophan metabolites.36 Histamine was not altered in equine OA; however, imidazole-4-acetate, which has histamine as a precursor, was significantly elevated in OA joints. Mast cells, which express histidine decarboxylase (HDC), synthesise histamine from histidine, and increased expression of both HDC and histamine have been demonstrated in human OA cartilage.37 Additionally, inflammation causes tissue-specific depletion of vitamin B6, required for amino acid, glucose and lipid metabolism, and vitamin B6 was decreased in equine OA. Vitamin B6 depletion has been associated with severity of RA symptoms.38
Finally, oxidative stress and collagen metabolism pathways were altered in equine OA joints. Specifically, arginine biosynthesis was impacted by upregulation of citrulline and downregulation of ornithine identified by LC-MS. Arginine biosynthesis, when influ-enced by anti-inflammatory cytokines, forms ornithine which, in combination with urea, forms polyamines for cellular proliferation and proline for collagen synthesis. However, in the presence of pro-inflammatory cytokines, arginine biosynthesis forms nitrogen oxide which can combine with citrulline to form reactive nitrogen oxygen species (RNOS).39 Subsequent oxidative stress leads to damage to lipids, proteins and DNA.9 Lysine degradation was also increased in equine OA joints. Lysine is critical for type II collagen formation, comprising approximately 3%−4% of collagen by total amino acids. Lysine must be substantially modified by hydroxylation and other post-translational modifications prior to incorporation into collagen, and decreased L-hydroxylysine may represent decreased type II collagen synthesis.40 On the other hand, collagen degradation also results in increased glycosylated hydroxylysine which might be an arthritogenic stimulus for T cells in inflammatory arthritis.41 Degradation of type II collagen is a hallmark of OA, resulting in inferior cartilage mechanical properties and loss of proteoglycan retention.42
Limitations of this study included the relatively small cohort size for the metabolomics analysis; however, this cohort size is similar to previous equine metabolomics studies using LC-MS.43,44 Although an advantage of the LC-MS approach used in this study was the identification of an extensive number (>4900) of features and 436 annotated metabolites, some of the statistically significant metabolites were not linked to KEGG pathways and could not be included in the pathway analysis. Many metabolites which differed between healthy and OA joints could not be identified or were identified but have an unknown biological role at this time. The extent of crossover between the findings from metabolomics and glycomics increased confidence in the findings. Two high motion carpal joints commonly affected by osteochondral fragmentation and subsequent OA, including the ACJ and MCJ, were used for the glycomics analyses. In addition, data about injury duration and prior treatment with intra-articular medication were unavailable for the majority of horses included in this study.
5 |. CONCLUSION
Distinct differences in metabolite and glycan expression were found between healthy and OA equine synovial fluid. Inflammation, oxidative stress and collagen degradation were the most significantly altered pathways between healthy and OA joints. Lectin microarrays found significantly higher expression of O-glycans than N-glycans in all synovial fluid samples, with increased Core 1/3 O-glycosylation and sialylation in OA synovial fluid. Targeted analysis is required to further understand the composition of synovial fluid O-glycans and to determine whether there are correlations with increased synovial fluid lubricin in OA. Future targeted analyses should investigate whether metabolites linked to inflammatory, oxidative and collagen metabolism pathways could serve as biomarkers for early detection of equine OA or could be targeted for OA therapies. Finally, the results of this study will be able to guide future analyses of molecules identified in OA progression or, possibly, therapy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the clinicians at the Cornell University Equine Hospital, including Drs Alan Nixon, Norm Ducharme, Lisa Fortier and Jon Cheetham, and the Cornell Veterinary Biobank for providing synovial fluid samples. The authors would like to acknowledge Carolyn Shurer for assisting with the initial synovial fluid methanol extraction for LC-MS.
Funding information
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH K08AR068469 (HR) and the Cornell Veterinary Biobank under NIH grant (R24 GM08291).
ETHICAL ANIMAL RESEARCH
The study was approved by the Institutional Animal Care and Use Committee (IACUC 2005–0151 and 2011–0027).
Footnotes
INFORMED CONSENT
Owners of horses with osteoarthritis gave consent for their animals’ inclusion in the study. Explicit informed consent for enrolment of client-owned horses as controls in this study was not stated, but horse owners gave general permission for research.
DATA ACCESSIBILITY STATEMENT
The data that support the findings of this study are available from open access repositories. The LCMS raw metabolomics data are available at MetaboLights (doi: 10.1093/nar/gkz1019; https://www.ebi.ac.uk/metabolights/MTBLS2466), and the lectin microarray data are available at Synapse (https://doi.org/10.7303/syn24628878).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/evj.13440.
The abstract is available in Chinese in the Supporting Information section of the online version of this article
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTERESTS
No competing interests have been declared.
REFERENCES
- 1.McIlwraith CW. Arthroscopic surgery for osteochondral chip fragments and other lesions not requiring internal fixation in the carpal and fetlock joints of the equine athlete: what have we learned in 20 years? Clin Tech Equine Pract. 2002;1(4):200–10. [Google Scholar]
- 2.McIlwraith CW, Kawcak CE, Frisbie DD, Little CB, Clegg PD, Peffers MJ, et al. Biomarkers for equine joint injury and osteoarthritis. J Orthop Res. 2018;36:823–31. [DOI] [PubMed] [Google Scholar]
- 3.McIlwraith CW, Clegg PD. Science in brief: report on the Havemeyer Foundation workshop on equine musculoskeletal biomarkers— current knowledge and future needs. Equine Vet J. 2014;46:651–3. [DOI] [PubMed] [Google Scholar]
- 4.Skiöldebrand E, Ekman S, Mattsson Hultén L, Svala E, Björkman K, Lindahl A, et al. Cartilage oligomeric matrix protein neoepitope in the synovial fluid of horses with acute lameness: a new biomarker for the early stages of osteoarthritis. Equine Vet J. 2017;49:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JR, Smagul A, Simpson D, Clegg PD, Rubio-Martinez LM, Peffers MJ. The synovial fluid proteome differentiates between septic and nonseptic articular pathologies. J Proteomics. 2019;202:103370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen R, Lundstedt T, Trygg J. Chemometrics in metabolomics—a review in human disease diagnosis. Anal Chim Acta. 2010;659(1–2):23–33. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JR, Chokesuwattanaskul S, Phelan MM, Welting TJ, Lian LY, Peffers MJ, et al. 1H NMR metabolomics identifies underlying inflammatory pathology in osteoarthritis and rheumatoid arthritis synovial joints. J Proteome Res. 2018;17:3780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson AK, Rawle RA, Wallace CW, Brooks EG, Adams E, Greenwood MC, et al. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr Cartil. 2019;27:1174–8 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27:339–4 4. [DOI] [PubMed] [Google Scholar]
- 10.De Grauw JC, Van De Lest CHA, Van Weeren PR. A targeted lipidomics approach to the study of eicosanoid release in synovial joints. Arthritis Res Ther. 2011;13:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JR, Phelan MM, Clegg PD, Peffers MJ, Rubio-M artinez LM. Synovial fluid metabolites differentiate between septic and nonseptic joint pathologies. J Proteome Res. 2018;17:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham RJTY, Anderson JR, Phelan MM, Cillan-G arcia E, Bladon BM, Taylor SE. Metabolomic analysis of synovial fluid from thoroughbred racehorses diagnosed with palmar osteochondral disease using magnetic resonance imaging. Equine Vet J. 2020;52:384–9 0. [DOI] [PubMed] [Google Scholar]
- 13.Silsirivanit A. Glycosylation markers in cancer. Adv Clin Chem. 2019;89:189–213. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xu J, Li M, Zeng X, Wang J, Hu C. Aberrant glycosylation in autoimmune disease. Clin Exp Rheumatol. 2020;38:767–76. [PubMed] [Google Scholar]
- 15.Alter G, Ottenhoff THM, Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Semin Immunol. 2018;39:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilobello KT, Krishnamoorthy L, Slawek D, Mahal LK. Development of a lectin microarray for the rapid analysis of protein glycopatterns. ChemBioChem. 2005;6:985–9. [DOI] [PubMed] [Google Scholar]
- 17.Pilobello KT, Slawek DE, Mahal LK. A ratiometric lectin microarray approach to analysis of the dynamic mammalian glycome. Proc Natl Acad Sci USA. 2007;104:11534–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagatsuma T, Nagai-Okatani C, Matsuda A, Masugi Y, Imaoka M, Yamazaki K, et al. Discovery of pancreatic ductal adenocarcinoma-related aberrant glycosylations: a multilateral approach of lectin microarray-based tissue glycomic profiling with public transcriptomic datasets. Front Oncol. 2020;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeshita M, Kuno A, Suzuki K, Matsuda A, Shimazaki H, Nakagawa T, et al. Alteration of matrix metalloproteinase-3 O-glycan structure as a biomarker for disease activity of rheumatoid arthritis. Arthritis Res Ther. 2016;18:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y, Han P, Wang T, Ren H, Gao L, Shi P, et al. Stage-associated differences in the serum N-and O-glycan profiles of patients with non-small cell lung cancer. Clin Proteomics. 2019;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svala E, Jin C, Rüetschi U, Ekman S, Lindahl A, Karlsson NG, et al. Characterisation of lubricin in synovial fluid from horses with osteoarthritis. Equine Vet J. 2017;49:116–23. [DOI] [PubMed] [Google Scholar]
- 22.Estrella RP, Whitelock JM, Packer NH, Karlsson NG. The glycosylation of human synovial lubricin: implications for its role in inflammation. Biochem J. 2010;429:359–67. [DOI] [PubMed] [Google Scholar]
- 23.Kamm JL, Nixon AJ, Witte TH. Cytokine and catabolic enzyme expression in synovium, synovial fluid and articular cartilage of naturally osteoarthritic equine carpi. Equine Vet J. 2010;42:8:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reesink HL, Watts AE. Lubricin/proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthritis Cartilage. 2017;25:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilobello KT, Agrawal P, Rouse R, Mahal LK. Advances in lectin microarray technology: optimized protocols for piezoelectric print conditions. Curr Protoc Chem Biol. 2013;5:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppolu S, Wang L, Mathur A, Nigam JA, Dezzutti CS, Isaacs C, et al. Vaginal product formulation alters the innate antiviral activity and glycome of cervicovaginal fluids with implications for viral susceptibility. ACS Infect Dis. 2018;4:1613–2 2. Available from 10.1021/acsinfecdis.8b00157 [DOI] [PubMed] [Google Scholar]
- 27.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-t raumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–9 . Available from 10.1002/jor.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toegel S, Bieder D, André S, Altmann F, Walzer SM, Kaltner H, et al. Glycophenotyping of osteoarthritic cartilage and chondrocytes by RT-q PCR, mass spectrometry, histochemistry with plant/ human lectins and lectin localization with a glycoprotein. Arthritis Res Ther. 2013;15:R147. Available from 10.1186/ar4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao N, Bergstrom K, Fu J, Xie B, Chen W, Xia L. Loss of intestinalO-glycans promotes spontaneous duodenal tumors. Am J Physiol Gastrointest Liver Physiol. 2016;311:G74–8 3. Available from 10.1152/ajpgi.00060.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peal BT, Gagliardi R, Su J, Fortier LA, Delco ML, Nixon AJ, et al. Synovial fluid lubricin and hyaluronan are altered in equine osteochondral fragmentation, cartilage impact injury, and full-t hickness cartilage defect models. J Orthop Res. 2020;38:1826–35. Available from 10.1002/jor.24597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballard BL, Antonacci JM, Temple-Wong MM, Hui AY, Schumacher BL, Bugbee WD, et al. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Joint Surg Am. 2012;94:e64. Available from 10.2106/jbjs.k.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62:2680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali L, Flowers SA, Jin C, Bennet EP, Ekwall A-KH, Karlsson NG. The O-glycomap of lubricin, a novel mucin responsible for joint lubrication, identified by site-specific glycopeptide analysis. Mol Cell Proteomics. 2014;13:3396–409. Available from 10.1074/mcp.m114.040865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson AK, Rawle RA, Wallace CW, Adams E, Greenwood MC, Bothner B, et al. Global metabolomic profiling of human synovial fluid for rheumatoid arthritis biomarkers. Clin Exp Rheumatol. 2019;37:393–9. [PubMed] [Google Scholar]
- 35.Haudenschild DR, Carlson AK, Zignego DL, Yik JH, Hilmer JK, June RK. Inhibition of early response genes prevents changes in global joint metabolomic profiles in mouse post-traumatic osteoarthritis. Osteoarthr Cartil. 2019;27:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flinn JH, Price JM, Yess N, Brown RR. Excretion of tryptophan metabolites by patients with rheumatoid arthritis. Arthritis Rheum. 1964;7:201–10. [DOI] [PubMed] [Google Scholar]
- 37.Tetlow LC, Woolley DE. Histamine stimulates the proliferation of human articular chondrocytes in vitro and is expressed by chondrocytes in osteoarthritic cartilage. Ann Rheum Dis. 2003;62(10):991–4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson AK, Rawle RA, Wallace CW, Brooks EG, Adams E, Greenwood MC, et al. Characterization of osteoarthritis phenotypes by global metabolomic profiling of human synovial fluid. bioRxiv. 2018. 10.1101/395020 [DOI] [Google Scholar]
- 39.Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaohua Z, Jinlong Z, Jian X, Liang R, Liang L, Yiwu Z. Long-t ime fulvic acid supplementation modulates hydroxylysyl glycosylation of collagen in mice. J Huazhong Univ Sci Technol. 2004;24:427–9. [DOI] [PubMed] [Google Scholar]
- 41.Williams RO. Collagen-i nduced arthritis in mice: a major role for tumor necrosis factor-α . Methods Mol Biol. 2007;361:265–84. [DOI] [PubMed] [Google Scholar]
- 42.Crowley DC, Lau FC, Sharma P, Evans M, Guthrie N, Bagchi M, et al. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int J Med Sci. 2009;6:312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein DJ, McKeever KH, Mirek ET, Anthony TG. Metabolomic response of equine skeletal muscle to acute fatiguing exercise and training. Front Physiol. 2020;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter J, Huwiler F, Fortes C, Grossmann J, Roschitzki B, Hu J, et al. Analysis of the equine “cumulome” reveals major metabolic aberrations after maturation in vitro. BMC Genom.. 2019;20:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.