Abstract
Since the introduction of simultaneous liver-kidney transplantation (SLKT) in the 1960’s, the potential for immunological protection from the liver allograft to a simultaneously transplanted kidney has been recognized. Due to expanded indications and changes in allocation policies, there has been increased utilization of SLKT. Despite growing experience, a lack of consensus exists regarding the extent of the liver’s immunological privilege, the role for donor specific HLA antibody (DSA) and crossmatch testing, and appropriateness of modern immunosuppression protocols in SLKT recipients. This review provides a detailed analysis of SLKT outcomes in the context of these factors, suggesting that while the liver can reduce the incidence of antibody-mediated rejection, attention should be given to liver allograft function, previous failed transplants, and other risk factors in pretransplant risk assessment. Current methods of DSA and crossmatch testing in SLKT are also discussed, and the role of specific DSA (high mean fluorescence intensity antibody, C1q+ binding) and their potential importance in posttransplant risk assessment are examined. Finally, trends in SLKT immunosuppression are discussed, including the use of nondepleting agents for induction and de-escalating use of steroids for maintenance immunosuppression. Ongoing research including multicenter and/or randomized trials will be necessary to optimize immune-related outcomes in SLKT recipients.
Introduction
After early reports of simultaneous liver-kidney transplantation (SLKT) in the 1960s, transplant physicians began to recognize that the liver allograft could “protect” a simultaneously transplanted kidney from rejection and subsequent failure, leading to more frequent practice of the procedure.1,2 The implementation of the Model for End-Stage Liver Disease (MELD) criteria for liver graft allocation in 2002 led to another surge in the incidence of SLKT by greater than 500%.3 As more SLKTs have been performed, ongoing research and debate on multiple aspects of the procedure, including outcomes, donor-specific HLA antibodies (DSA) and crossmatch testing, and immunosuppression (IS) protocols has ensued. The precise nature and extent of the immunological privilege provided to the kidney by the liver has been a topic of interest, especially when placed in the context of DSA and crossmatch testing. Furthermore, the use of immunosuppressive regimens, in terms of induction and maintenance steroid use, has faced escalating scrutiny.
A lack of consensus in SLKT management protocols has led to center-to-center variability in anti-HLA testing (e.g. occurrence, result significance in risk assessment, timing, and repetition) and immunosuppressive regimens (both induction and maintenance).4,5,6 The broader role for crossmatch, DSA testing pretransplant and posttransplant, and immunosuppression approaches has not been established for SLKT. To understand the contemporary practice of SLKT, this review has been prepared, focusing on the following subtopics:
Overview of DSA, crossmatch testing, and immunosuppression in liver and kidney transplantation.
Positive crossmatch, DSA, and immunosuppression regimens and their potential to negatively impact SLKT outcomes.
Reassessing the impact of positive crossmatch, DSA, and immunosuppression regimens on SLKT outcomes.
Reconciling the evidence for DSA, crossmatch testing, and IS regimens in SLKT.
Recommendations for future studies.
Methods of Review
A literature review was conducted in order to find current and past articles pertaining to SLKT outcomes when factoring in preformed and de novo DSA, crossmatch testing, and immunosuppression regimens. The purpose of this search was to gain an understanding of how the paradigm around SLKT outcomes has shifted since the procedure’s conception in light of these other factors. Google Scholar and PubMed databases were searched using a mixture of the following keywords: simultaneous liver kidney transplantation, DSA, crossmatch, induction and maintenance immunosuppression, transplant outcomes, antibody testing, transplant failure, graft rejection, HLA, corticosteroid therapy, and transplant guidelines. References from each article were also reviewed to identify additional relevant articles. The search was completed between May-September 2020.
Section 1: Overview of DSA, crossmatch testing, and immunosuppression in liver and kidney transplantation.
Anti-HLA antibody and crossmatch testing techniques have evolved through the past half century, in parallel with the expansion of clinical solid organ transplantation. The assessment of HLA compatibility between an organ donor and recipient is paramount to the optimization of posttransplant outcomes.7,8 For kidney transplant candidates, the serum is first tested for the presence of ‘panel reactive antibodies’ (PRA), originally based on a panel of 60–100 individuals with unique, known HLA profiles that represent the level of sensitization existing pretransplant. A high pretransplant PRA (reported as a percent) is associated with a lower likelihood of being matched with a suitable donor. This method has been refined by the use of calculated PRA (cPRA), whereby recipient HLA frequencies are entered into the Organ Procurement and Transplantation Network (OPTN) system, and the likelihood of donor-recipient incompatibility is calculated based on HLA types found in more than 12 000 donors.9 Preexisting anti-HLA antibodies in the candidate can be detected through solid phase technology, whereby their serum is incubated with screening beads coated with purified HLA molecules and combined with an anti-IgG antibody, whose signal is measured by either flow cytometry or a Luminex analyzer.10 Each bead may be coated with a single recombinant HLA antigen (‘single antigen beads’ or SAB) or with a cluster of native class I or II antigens purified from transformed B cell lines.11 These solid phase assays have the capability to rapidly determine the existence, specificity, and potential strength of the HLA antibodies within the recipient serum.
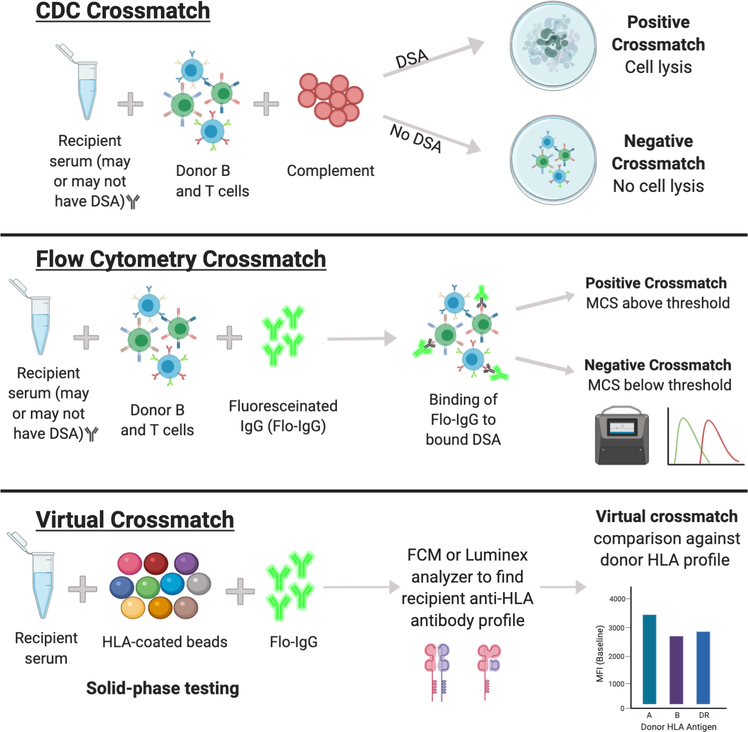
In terms of the crossmatch, there are 2 main assays: Complement-Dependent Cytotoxicity (CDC) and flow cytometry (Figure 1). The CDC crossmatch is a cell-based assay that involves mixing recipient serum with donor lymphocytes, followed by exposure to complement and a vital dye to visualize a potential cytotoxic reaction. A ‘positive CDC crossmatch’ results if there is cellular lysis of donor cells due to recipient derived, complement-fixing antibodies. The flow cytometry crossmatch (FCXM) involves incubating donor lymphocytes with recipient serum, followed by fluorescein-labelled anti-IgG that will specifically bind to and identify T and B cells that are bound with recipient antibody. A ‘positive flow cytometry crossmatch’ results if there is a MESF (molecules of equivalent soluble fluorochrome) shift beyond a predetermined threshold. In terms of sensitivity, the flow cytometry method is more reliable, detecting around 80% of DSA.12 A third option, the so called ‘virtual crossmatch,’ involves the comparison of recipient anti-HLA antibodies detected by solid phase technology (as described previously) against HLA antigens expressed by the prospective donor. A virtual crossmatch is interpreted as ‘positive’ if the detected anti-HLA antibodies meet thresholds that are predictive of an adverse immunologic response. Depending on the degree of resolution of HLA typing, the virtual crossmatch may be a superior method of crossmatching in that it can be completed quickly and can quantitatively identify particular DSA as a measure of MFI, especially HLA Class II DSA, which has been more closely associated with negative transplant outcomes (Table 1).12 The results of these crossmatch tests are interpreted in the context of both T- and B-cells, which is significant as T-cells express mainly HLA Class I, while B-cells, in virtue of their capacity as antigen presenting cells, express both HLA Class I and Class II.10
Figure 1. Crossmatch testing protocols in simultaneous liver-kidney transplantation.
CDC, complement-dependent cytotoxicity; DSA, donor specific antibody; IgG, immunoglobulin G; MCS, median channel shift.
Table 1.
Comparison of crossmatch testing methods.46
| CDC crossmatch | Flow crossmatch | Virtual crossmatch | |
|---|---|---|---|
|
| |||
| Time | 2–4 hours | 6–8 hours | Minutes |
| Prospective or retrospective | Usually prospective | Usually prospective | Prospective or retrospective |
| Sensitivity | Low | Intermediate | High |
| HLA antibody specificity determined | No | No | Yes |
| Live cells | Required | Required | Not required |
CDC, complement-dependent cytotoxicity.
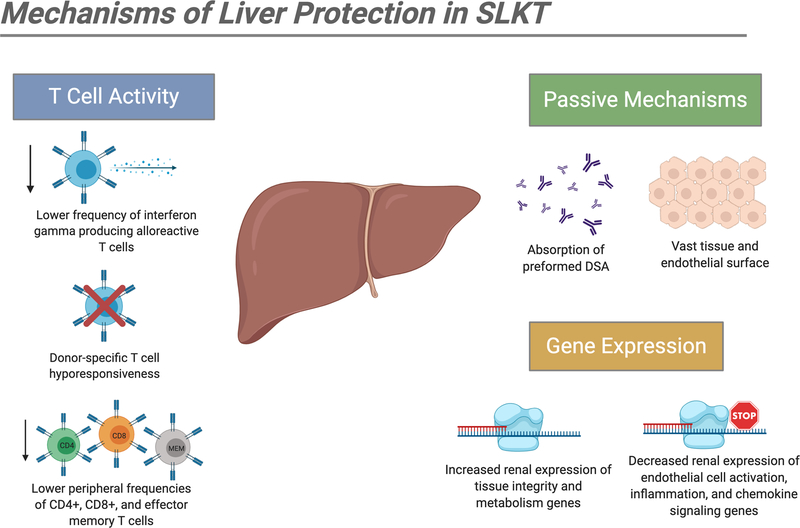
In kidney transplantation, antibody-mediated rejection (AMR) is the leading cause of graft loss and dysfunction, with both preexisting and de novo DSA being linked to acute and chronic rejection.13,14 AMR in kidney transplantation is manifested through microvascular inflammation (glomerulitis and peritubular capillaritis) rich in cells of the innate immune system. Because this inflammation is not controlled effectively by the current IS agents that target lymphocytes, the best option is the avoidance of high DSA titers and positive crossmatch. It is therefore no surprise that DSA and crossmatch testing is widely used in the pretransplant and posttransplant assessment of kidney graft recipients as well as kidney allocation schemes. On the other hand, the liver is well known for its unique immunologic privilege mediated by a multitude of mechanisms, including Kupffer cell elimination of immune complexes, regeneration aided by its vast endothelial surface area, and diminished endothelial expression of MHC Class II antigens (summarized in Figure 2).15 Accordingly, DSA and crossmatch testing is not routinely considered for liver transplant candidates due to the rarity of acute AMR and graft loss in this population.16,17
Figure 2. Potential mechanisms of liver allograft immunomodulation for simultaneous kidney transplants.
DSA, donor specific antibody; SLKT, simultaneous liver-kidney transplantation.
Typical immunosuppression protocols following kidney and liver transplants accommodate their respective immunologic risk. Liver transplant alone (LTA) patients often require less rigorous immunosuppressive regimens following transplant when compared to other solid organ recipients.6,18 Early cellular rejection episodes in LTA patients mandate significantly less immunosuppression compared to similar episodes in KTA patients.19 Furthermore, approximately half of LTA patients do not receive any induction immunosuppression at the time of transplant, in contrast to the widespread use of nondepleting (interleukin-2 receptor antagonist (IL2RA)) and depleting (rabbit antithymocyte globulin (r-ATG)) agents in KTA patients.18,20 The conservative employment of induction agents for LTA has been driven in part by the well-recognized risk for serious infections and malignancies with depleting induction therapy.21
When considering SLKT, the relative risks and approaches to immunosuppression for each organ have lead to heterogenous practice patterns. Since the procedure’s conception by Sir Royal Calne in the 1960’s, numerous studies have demonstrated the liver’s ability to convey immunologic tolerance to kidney grafts transplanted from the same donor.1,2,22,23,24 What remains a topic of controversy is the extent of this conferred protection as it pertains to preformed and de novo DSA, the impact of positive crossmatch testing, and choice of induction and maintenance immunosuppression.
Section 2: Positive crossmatch, DSA, and immunosuppression regimens and their potential to negatively impact SLKT outcomes.
The initial human SLKT studies performed after Calne’s experiments demonstrated decreased renal rejection episodes and improved kidney graft survival, postulating 2 main methods of liver immunologic protection: reversal of pretransplant positive crossmatches to negative crossmatches after the procedure and resorption of preformed DSA in the recipient serum (Figure 2).2,24 These studies provided early evidence that the presence of the accessory liver graft could increase the likelihood of good immunologic outcomes in the kidney.
As continued exploration of SLKT occurred, a second epoch of studies with a different tone emerged, suggesting that positive DSA and crossmatch results before or after the procedure could be associated with worse kidney outcomes. A 2013 analysis of 86 SLKT recipients by O’Leary et al reported an increased risk of kidney graft loss, liver graft loss, and overall mortality in patients with preformed Class II DSA.25 These results were complemented by studies showing a rapid decline in Class I DSA titers post-SLKT with persistence of Class II DSA, suggesting that the liver favors the absorption of HLA Class I antibodies.26 It has been hypothesized that this differential clearance can be attributed to the divergent expression of Class I and Class II HLA within liver allograft.26 Tissue HLA distribution studies after liver transplantation have demonstrated greater expression of Class I than Class II HLA on allograft hepatocytes.27
In the setting of the crossmatch, similar studies have suggested that patients who test positive prior to the combined liver-kidney transplant may be more susceptible to negative graft outcomes than those who test negative. A single-center SLKT study by Ong et al reported that the 3-year posttransplant survival rate in positive flow cytometry crossmatch patients was 71%, compared to 85% for negative flow cytometry crossmatch patients.28 Furthermore, the incidence of acute kidney rejection in the positive crossmatch cohort was 28%, in contrast with 10% in the negative crossmatch cohort.28 The presence of a strongly positive FCXM (mean channel shift 4 times above the positive cut-off) along with the presence of multiple high MFI (>10 000) DSA was also associated with graft dysfunction and loss.28 A registry study of 2484 post-SLKT patients found that allosensitization was associated with a 16% higher rate of kidney allograft loss and a 20% higher rate of mortality, although it was noted that this analysis was biased in its data collection.29,30
These studies suggested that the liver may not be as immunologically protective as was previously believed. It must be emphasized, however, that these studies had limitations. For one, the immunosuppressive management protocols were not standardized and not based on currently accepted regimens. O’Leary et al reported that at 3 months posttransplant, only 59% of patients were on tacrolimus, 37% were on cyclosporine, and 96% were receiving steroids.25 Additionally, these articles did not factor in other potentially confounding parameters like liver allograft status, time on dialysis, and previous transplants, all of which commonly impact graft outcomes and patient mortality, resulting in less well-rounded and conclusive analyses.
The use of induction and maintenance immunosuppression in the context of SLKT remains a topic of discussion as well. For the purpose of SLKT, although there is significant variation in immunosuppression regimens between centers, the overall paradigm tends to mirror that of LTA rather than KTA in light of the liver’s potential protective effects.6,18 In terms of induction immunosuppression, the use of IL2RA for SLKT has rapidly increased in the last decade, while r-ATG has remained relatively uncommon.18,31 While the purpose of IL2RA use is often to delay introduction of calcineurin inhibitors as part of renal-sparing immunosuppression protocols, recent data have suggested that this approach may be unnecessary.32 A multivariate analysis from 2019 reported that no reduction in rejection or mortality was observed with the use of induction therapy (r-ATG, IL2RA) in SLKT patients; in fact, administration of r-ATG was associated with a higher risk of mortality than no administration.18
A similar picture is painted for maintenance immunosuppression post-SLKT. Although there is extensive center-level variation regarding the choice of long-term IS for SLKT recipients (particularly in terms of steroid use), the hallmark of SLKT maintenance immunosuppression is the calcineurin inhibitor tacrolimus, and the most widespread discharge regimen involves triple therapy consisting of tacrolimus, steroids, and an antimetabolite.33 A recent registry study examining 4184 SLKT patients found that the implementation of steroid-sparing regimens increased in incidence from 16.1% at discharge to 88.0% 5-years posttransplant.33 Steroid inclusive regimens at 1-year posttransplant have been associated with increased risk of liver failure and patient mortality when compared to steroid sparing regimens, with no change in risk of kidney allograft loss.33 These findings corroborate previous single-center reports, which have demonstrated more successful results following steroid withdrawal in SLKT recipients when compared to KTA patients, with no differences in renal allograft function, rejection rates, or overall survival between the 2 cohorts.34 The implications of these results are especially important in light of the fact that infections are the leading cause of death in SLKT patients.35,36 These data suggest that it is important to avoid over immunosuppression in SLKT recipients. For both induction and maintenance immunosuppression, excessive administration of unnecessary IS may be a contributor to, rather than protection against, SLKT morbidity and mortality.
Section 3: Reassessing the impact of positive crossmatch, DSA, and immunosuppression regimens on SLKT outcomes.
Contemporary examination of SLKT outcomes has clarified features of the protective consequences of simultaneous liver graft transplantation. In a report from Mayo Clinic involving 68 patients, the incidence of both acute and chronic alloantibody-mediated injury in SLKT recipients was lower in comparison to matched kidney transplant alone (KTA) recipients, regardless of pretransplant DSA status.37 Preformed DSA, even at high titers, resolved in most SLKT recipients within the first 4 months.37 The only exceptions were observed in patients who had dysfunction of the liver allograft. Mechanistic investigations by the same group have demonstrated lower peripheral frequencies of alloreactive CD4+, CD8+, effector memory, and interferon-γ producing T-cells in SLKT patients when compared to KTA patients, suggesting that the presence of a liver allograft from the same donor can induce a hypo-responsive alloimmune response in the recipient.38 Furthermore, the SLKT procedure has been shown to result in reduced expression of endothelial cell activation and inflammation genes and increased expression of tissue integrity and metabolism genes within the renal allograft (Figure 2).39 Thus, it is likely that multiple beneficial immune-related mechanisms confer protection for the kidney graft following SLKT.
In terms of the relative significance of DSA and crossmatch results, recent data has suggested that these 2 factors constitute only one component of the total risk stratification for a potential SLKT recipient. Piñeiro et al conducted an extensive evaluation of the effects of immunological risk factors and comorbidities (positive DSA and crossmatch testing, Hepatitis C infection, time on dialysis, and previous renal transplant) on SLKT outcomes and observed that while acute kidney rejection occurred more frequently in “high immune risk” patients, overall renal graft function and survival were not impacted.30 Furthermore, the only “high immune risk” factor that did impact kidney outcomes was superimposed immunological burden due to previous kidney transplant.30 The presence of pretransplant DSA did not prove to have such an effect, as all Class II DSA disappear or decrease significantly on follow up post-SLKT.30 Along the same lines, Leca et al reported that although presensitization was associated with a higher rate of acute kidney rejection episodes, all rejection episodes were mild and did not have an impact on renal graft function overall.40 Most importantly, their sensitized and un-sensitized cohorts had relatively equal incidences of Hepatitis C infection, time on dialysis, and previous kidney transplants.30,40 These contemporary studies are particularly relevant as they took into consideration the effects of additional, potentially harmful covariables and suggest that one should take a more holistic approach to risk stratification when considering SLKT, rather than focusing exclusively on DSA and crossmatch data.
For SLKT immunosuppressive regimens, while prior studies have suggested negative outcomes following over immunosuppression, other studies have highlighted the lack of effect of IS on patient outcomes. For induction IS, a 2020 registry study found that the type of induction immunosuppression, whether T-cell depletion, IL2RA, or no induction, did not affect patient mortality or allograft loss.41 These results correspond with previous reports, which demonstrate superior kidney transplant outcomes in SLKT patients when compared to KTA patients, regardless of induction immunotherapy choice.36,42 In terms of maintenance IS, the same 2020 registry study found that the continued use of steroids in long term IS may actually be protective against graft failure and patient death.41 The lack of consensus in these reports highlights the necessity for randomized trials to gather more conclusive data on this topic for SLKT recipients and transplant specialists.
Section 4: Reconciling the evidence for DSA, crossmatch testing, and IS regimens in SLKT
We have outlined the contrasting findings of several studies for DSA and crossmatch results and immunosuppression protocols in SLKT. Although it is difficult to draw definitive conclusions, differences may be reconciled by looking at the details and aims of these specific studies. O’Leary et al, Ong et al, and others found that positive crossmatch and preformed DSA were associated with negative SLKT outcomes, including graft loss and patient mortality. However, these studies included patients given nonstandardized and outdated immunosuppression protocols. Furthermore, they did not consider the impact of previous transplants, time on dialysis, liver allograft dysfunction, and other confounding variables, which may have been also been elevated disproportionally in the sensitized cohort.30 More recent studies by Taner et al and Pineiro et al conducted thorough analyses with direct comparisons of outcomes between SLKT and KTA patients in the context of DSA and investigations into multiple high-risk factors and their long-term impact. These studies conclude that SLKT patients are indeed more immunologically advantaged than KTA patients due to the presence of the liver allograft, regardless of similar pretransplant DSA status. Several potential mechanisms of liver protection are also outlined, including upregulation of anti-inflammatory gene transcription and inhibition of destructive immune cell subsets (Figure 2). Additionally, the significance of DSA and crossmatch assays may only be one, perhaps less significant, factor in the larger assessment of SLKT vulnerability that includes liver graft status, prior immunologic burden, and dialysis longevity. Collectively, these data suggest that a healthy liver allograft, transplanted in the context of other low-risk immune factors, has the potential to protect the kidney against many of the negative consequences of preformed and de novo posttransplant DSA.
That being said, DSA and crossmatch testing cannot be completely eliminated from the pretransplant evaluation. Indeed, DSA with specific, high-risk characteristics may be resistant to liver-mediated immunomodulation. A recent retrospective study involving 86 patients observed an especially high risk of negative outcomes in SLKT recipients who developed de novo C1q+ DSA, in terms of both all-cause mortality and composite outcomes.43 The incidence of de novo C1q+ DSA development has been suggested to be similar in both SLKT and KTA patients, implying that the liver may not provide as much protection against this IgG complement binding antibody.43 Although Class I DSA is generally considered to be less pathogenic than Class II DSA, a single-center analysis linked cases of AMR to multiple high MFI Class I DSA and complement fixing, non-HLA IgG antibodies.44 These combined data suggest that the detailed DSA analyses which assess potentially higher risk DSA subcategories (Class II, C1q+, high MFI) may still be beneficial, especially when assessed with other comorbidities.
In 2016, Schinstock et al outlined a new protocol for alloantibody testing prior to kidney transplantation.45 While acknowledging the variability and complexity of solid phase and crossmatch assays, this approach used contemporary data to risk stratify AMR posttransplant based on several factors including DSA, recent anti-CD20 treatment, the presence of auto-antibodies, and corroborative crossmatch testing into low, high, and uncertain categories.45 Based on this classification, an algorithm was created to help identify the steps that a physician should take following positive solid phase assay or positive crossmatch testing in KTA patients.45 Although there are still many knowledge gaps to address, this article was an effective first step in creating a guideline for clinical decision making. Employing a similar approach in SLKT recipients, assessing the implications of positive DSA and crossmatch, along with the effects of comorbidities including HCV status, dialysis time, prior transplants as sensitizing events, immunosuppressive therapy, and the status and quality of the liver allograft, would be of value. Presently, the clinical impact of fluctuating posttransplant DSA in SLKT remains to be determined. Larger, multicenter studies have the potential to clarify risk factors and facilitate the subsequent development of protocols that will integrate crossmatch and DSA results into SLKT management algorithm.
When considering immunosuppression for SLKT patients, the path forward is less clear. The use of nondepleting induction immunosuppression for SLKT has increased over the last decade, but recent data demonstrates that this may confer no real benefit and may even be harmful to transplant recipients. Corticosteroid therapy in long-term maintenance immunosuppression may impact liver allograft loss and patient mortality, especially when considering that infections remain the leading cause of death in SLKT recipients. Thus, the role for and potential benefit of induction immunosuppression and steroid-based maintenance in SLKT remains to be determined.
Conclusion: Recommendations for future studies
While SLKT outcomes has shifted and continued to improve with time and experience, contemporary studies have suggested that the liver is capable of protecting the kidney from acute and chronic AMR, even in the presence of both preformed and de novo DSA in most cases. It is necessary to further delineate the impact of factors including time on dialysis and prior failed transplants as sensitizing events on graft failure and loss and to define their role in risk assessment prior to SLKT. Additional consideration should be given to detailed DSA and crossmatch analyses, with special attention on potentially high-risk characteristics, such as C1q+ complement-binding and high MFI DSA. The SLKT landscape would greatly benefit from larger, multicenter studies that clarify the consequences of specific classes and characteristics of DSA and risk stratify these factors in the context of the previously mentioned considerations. Lastly, the ideal induction and maintenance immunosuppression regimen for SLKT has not been established. Furthermore, steroid-sparing regimens have become more prevalent as well, which may limit over-immunosuppression in SLKT. Controlled trials need to be conducted in order to optimize the regimens in the context of SLKT patients, which have unique risk factors when compared to LTA or KTA.
Acknowledgments
Figures were created using biorender.com.
Funding: J.E. is supported by a career development award (K08 CA245220-01) from the National Cancer Institute.
Abbreviations Page
- AMR
Antibody-Mediated Rejection
- CDC
Complement-Dependent Cytotoxicity
- DSA
Donor Specific Antibody
- FCXM
Flow Cytometry Crossmatch
- HCV
Hepatitis-C Virus
- IL2RA
Interleukin-2 Receptor Antagonist
- IS
Immunosuppression
- KTA
Kidney Transplant Alone
- LTA
Liver Transplant Alone
- MCS
Median Channel Shift
- MELD
Model for End-Stage Liver Disease
- MESF
Molecules of Equivalent Soluble Fluorochrome
- MHC
Major Histocompatibility Complex
- MFI
Mean Fluorescent Intensity
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel Reactive Antibody
- rATG
Rabbit Anti-thymocyte Globulin
- SAB
Single Antigen Beads
- SLKT
Simultaneous Liver-Kidney Transplantation
Footnotes
Conflicts of Interest/Disclosures: None.
References
- 1.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(5205):472–476. doi: 10.1038/223472a0 [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen A, Davies HF, Jamieson NV, et al. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59(6):919–921. doi: 10.1097/00007890-199503270-00025 [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant. 2019;19(Suppl 2):184–283. doi: 10.1111/ajt.15276 [DOI] [PubMed] [Google Scholar]
- 4.Yazawa M, Cseprekal O, Helmick RA, et al. Association between longer hospitalization and development of de novo donor specific antibodies in simultaneous liver-kidney transplant recipients. Ren Fail. 2020;42(1):40–47. doi: 10.1080/0886022X.2019.1705338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvalaggio PR, Graff RJ, Pinsky B, et al. Crossmatch testing in kidney transplantation: patterns of practice and associations with rejection and graft survival. Saudi J Kidney Dis Transpl. 2009;20(4):577–589. [PMC free article] [PubMed] [Google Scholar]
- 6.Nilles KM, Krupp J, Lapin B, et al. Incidence and impact of rejection following simultaneous liver-kidney transplantation. J Hepatol. 2015;62(2):340–345. doi: 10.1016/j.jhep.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 7.Opelz G, Wujciak T, Döhler B, et al. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev Immunogenet. 1999;1(3):334–342. [PubMed] [Google Scholar]
- 8.Konvalinka A, Tinckam K. Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol. 2015;26(7):1489–1502. doi: 10.1681/ASN.2014080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant. 2010;10(1):26–29. doi: 10.1111/j.1600-6143.2009.02927.x [DOI] [PubMed] [Google Scholar]
- 10.Mulley WR, Kanellis J. Understanding crossmatch testing in organ transplantation: a case-based guide for the general nephrologist. Nephrology. 2011;16(2):125–133. doi: 10.1111/j.1440-1797.2010.01414.x [DOI] [PubMed] [Google Scholar]
- 11.Gebel HM, Bray RA. HLA antibody detection with solid phase assays: great expectations or expectations too great? Am J Transplant. 2014;14(9):1964–1975. doi: 10.1111/ajt.12807 [DOI] [PubMed] [Google Scholar]
- 12.Peräsaari JP, Jaatinen T, Merenmies J. Donor-specific HLA antibodies in predicting crossmatch outcome: comparison of three different laboratory techniques. Transpl Immunol. 2018;46:23–28. doi: 10.1016/j.trim.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Zhang R Donor-specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol. 2018;13(1):182–192. doi: 10.2215/CJN.00700117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–399. doi: 10.1111/j.1600-6143.2011.03840.x [DOI] [PubMed] [Google Scholar]
- 15.Thomson AW, Vionnet J, Sanchez-Fueyo A. Understanding, predicting and achieving liver transplant tolerance: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2020;17(12):719–739. doi: 10.1038/s41575-020-0334-4 [DOI] [PubMed] [Google Scholar]
- 16.Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12(6):1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x [DOI] [PubMed] [Google Scholar]
- 17.O’Leary JG, Demetris AJ, Friedman LS, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14(4):779–787. doi: 10.1111/ajt.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbdulRahim N, Anderson L, Kotla S, et al. Lack of benefit and potential harm of induction therapy in simultaneous liver-kidney transplants. Liver Transpl. 2019;25(3):411–424. doi: 10.1002/lt.25390 [DOI] [PubMed] [Google Scholar]
- 19.Abrol N, Jadlowiec CC, Taner T. Revisiting liver’s role in transplant alloimmunity. World J Gastroenterol. 2019;25(25):3123–3135. doi: 10.3748/wjg.v25.i25.3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong A, Kim WR, Lake JR, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant. 2020;20(Suppl 1):193–299. doi: 10.1111/ajt.15674 [DOI] [PubMed] [Google Scholar]
- 21.MacConmara M, Nwadei I, Kirk AD. Immunosuppressive biologic agents. In: Busuttil RW and Göran BG. Transplantation of the Liver. 3rd ed. Elsevier Inc; 2015:1343–1353. doi: 10.1016/B978-1-4557-0268-8.00096-8 [DOI] [Google Scholar]
- 22.Calne RY, White HJO, Yoffa DE, et al. Observations of orthotopic liver transplantation in the pig. Br Med J. 1967;2(5550):478–480. doi: 10.1136/bmj.2.5550.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordier G, Garnier H, Clot JP, et al. Orthotopic liver graft in pigs. 1st results. Article in French. Mem Acad Chir (Paris). 1966;92(27):799–807. [PubMed] [Google Scholar]
- 24.Fung J, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20(1 Suppl 1):88–91. [PMC free article] [PubMed] [Google Scholar]
- 25.O’Leary JG, Gebel HM, Ruiz R, et al. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. 2013;13(4):954–960. doi: 10.1111/ajt.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dar W, Agarwal A, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11(4):841–847. doi: 10.1111/j.1600-6143.2011.03467.x [DOI] [PubMed] [Google Scholar]
- 27.Rouger P, Gugenheim J, Gane P, et al. Distribution of the MHC antigens after liver transplantation: relationship with biochemical and histological parameters. Clin Exp Immunol. 2008;80(3):404–408. doi: 10.1111/j.1365-2249.1990.tb03301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong SC, White J, Hauptfeld-Dolejsek V, et al. Outcomes in simultaneous liver kidney transplants in the setting of a positive crossmatch: a single center experience. Clin Transpl. 2016;32:119–125. [PubMed] [Google Scholar]
- 29.Askar M, Schold JD, Eghtesad B, et al. Combined liver-kidney transplants: allosensitization and recipient outcomes. Transplantation. 2011;91(11):1286–1292. doi: 10.1097/TP.0b013e3182184181 [DOI] [PubMed] [Google Scholar]
- 30.Piñeiro GJ, Rovira J, Montagud‐Marrahí E, et al. Kidney graft outcomes in high immunological risk simultaneous liver‐kidney transplants. Liver Transplant. 2020;26(4):517–527. doi: 10.1002/lt.25726 [DOI] [PubMed] [Google Scholar]
- 31.Verna EC, Farrand ED, Elnaggar AS, et al. Basiliximab induction and delayed calcineurin inhibitor initiation in liver transplant recipients with renal insufficiency. Transplantation. 2011;91(11):1254–1260. doi: 10.1097/TP.0b013e318218f0f5 [DOI] [PubMed] [Google Scholar]
- 32.Levitsky J, O’Leary JG, Asrani S, et al. Protecting the kidney in liver transplant recipients: practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016;16(9):2532–2544. doi: 10.1111/ajt.13765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weeks SR, Luo X, Toman L, et al. Steroid‐sparing maintenance immunosuppression is safe and effective after simultaneous liver‐kidney transplantation. Clin Transplant. 2020;34(10):e14036. doi: 10.1111/ctr.14036 [DOI] [PubMed] [Google Scholar]
- 34.Weber NK, Wiseman AC, Trotter JF. Corticosteroid elimination in simultaneous liver-kidney transplantation recipients. Clin Transplant. 2009;23(6):958–963. doi: 10.1111/j.1399-0012.2009.01051.x [DOI] [PubMed] [Google Scholar]
- 35.Hibi T, Nishida S, Sageshima J, et al. Excessive immunosuppression as a potential cause of poor survival in simultaneous liver/kidney transplantation for hepatitis C. Transpl Int. 2014;27(6):606–616. doi: 10.1111/tri.12303 [DOI] [PubMed] [Google Scholar]
- 36.Ruiz R, Kunitake H, Wilkinson AH, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141(8):735–741; discussion 741–742. doi: 10.1001/archsurg.141.8.735 [DOI] [PubMed] [Google Scholar]
- 37.Taner T, Heimbach JK, Rosen CB, et al. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int. 2016;89(4):909–917. doi: 10.1016/j.kint.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 38.Taner T, Gustafson MP, Hansen MJ, et al. Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year. Kidney Int. 2018;93(6):1465–1474. doi: 10.1016/j.kint.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 39.Taner T, Park WD, Stegall MD. Unique molecular changes in kidney allografts after simultaneous liver-kidney compared with solitary kidney transplantation. Kidney Int. 2017;91(5):1193–1202. doi: 10.1016/j.kint.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 40.Leca N, Warner P, Bakthavatsalam R, et al. Outcomes of simultaneous liver and kidney transplantation in relation to a high level of preformed donor-specific antibodies. Transplantation. 2013;96(10):914–918. doi: 10.1097/TP.0b013e3182a192f5 [DOI] [PubMed] [Google Scholar]
- 41.Kamal L, Yu JW, Reichman TW, et al. Impact of induction immunosuppression strategies in simultaneous liver/kidney transplantation. Transplantation. 2020;104(2):395–403. doi: 10.1097/TP.0000000000002768 [DOI] [PubMed] [Google Scholar]
- 42.Simpson N, Cho YW, Cicciarelli JC, et al. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS database. Transplantation. 2006;82(10):1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6 [DOI] [PubMed] [Google Scholar]
- 43.Yazawa M, Cseprekal O, Helmick RA, et al. Association between post-transplant donor-specific antibodies and recipient outcomes in simultaneous liver-kidney transplant recipients: single-center, cohort study. Transpl Int. 2020;33(2):202–215. doi: 10.1111/tri.13543 [DOI] [PubMed] [Google Scholar]
- 44.Rajalingam R, Chandran S, Cunniffe K, et al. Multiple high MFI Class I DSA can cause acute AMR of kidney allograft in simultaneous liver and kidney transplant recipients: outcome analysis from a single-center. Hum Immunol. 2015;76(Suppl):137. doi: 10.1016/j.humimm.2015.07.19025636570 [DOI] [Google Scholar]
- 45.Schinstock CA, Gandhi MJ, Stegall MD. Interpreting anti-HLA antibody testing data: a practical guide for physicians. Transplantation. 2016;100(8):1619–1628. doi: 10.1097/TP.0000000000001203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaughan J, Xu Q, Tinckam K. Detecting donor-specific antibodies: the importance of sorting the wheat from the chaff. Hepatobiliary Surg Nutr. 2019;8(1):37–52. doi: 10.21037/hbsn.2019.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]