Abstract
Newly emerging synthetic cannabinoid compounds continue to be found in the designer drug market. They are often targeted as a “legal high” alternative to traditional cannabinoids via “darknet” markets and their increased potency and efficacy are becoming a growing concern internationally. The purpose of this study was to determine whether 4-CN-CUMYL-BUTINACA, 4F-MDMB-BINACA, 5F-AEB, 5F-CUMYL-P7AICA and EMB-FUBINACA exhibited similar behavioral effects as Δ9-tetrahydrocannabinol (Δ9-THC). Locomotor activity was assessed in an open-field assay using Swiss-Webster mice. Male Sprague-Dawley rats were trained to discriminate between IP injections of Δ9-THC (3 mg/kg) and vehicle. Following successful training, substitution tests for 4-CN-CUMYL-BUTINACA, 4F -MDMB-BINACA, 5F-AEB, 5F-CUMYL-P7AICA and EMB-FUBINACA were conducted. All of the test compounds decreased locomotor activity. 4-CN-CUMYL-BUTINACA (ED50= 0.26 mg/kg), 4F-MDMB-BINACA (ED50=0.019 mg/kg), 5F-CUMYL-P7AICA (ED50= 0.13 mg/kg) and EMB-FUBINACA (ED50 = 0.13 mg/kg) each fully substituted for the discriminative stimulus effects of the training dose of Δ9-THC, whereas 5F-AEB produced only a maximum of 67% drug-appropriate responding at 0.5 mg/kg. Higher doses produced piloerection, exophthalmos and convulsions. 4-CN-CUMYL-BUTINACA, 4F-MDMB-BINACA, 5F-CUMYL-P7AICA and EMB-FUBINACA are likely to produce similar subjective effects in humans as those produced by abused synthetic cannabinoids, and may therefore share similar abuse liability. In contrast, 5F-AEB may have a reduced abuse liability given its weaker THC-like discriminative stimulus effects, but may be more dangerous due to the adverse effects observed at doses needed to produce discriminative stimulus effects.
Keywords: cannabinoids, drug discrimination, locomotor activity
Cannabis has long been one of the most commonly used drugs to achieve psychoactive effects. In the last decade, synthetic cannabinoids have made a rapid and vast emergence as a popular alternative to the Δ9-tetrahydrocannabinol (Δ9-THC) found in cannabis (EMCDDA, 2018; WHO 2018). Synthetic cannabinoids tend to produce more powerful cannabimimetic effects than standard Δ9-THC (Bovens, 2017). They are usually described as “herbal incense” to naive buyers despite the serious abuse potential these drugs possess (Tai & Fantegrossi, 2014). The effects of these synthetic cannabinoids can range from confusion, anxiety, tachycardia and drowsiness to convulsions, extreme hypertension, and in some cases death (WHO, 2018). There are several growing concerns with synthetic cannabinoids that have been introduced in recent years, such as the abundance and ease of access for new and developing drugs (Tai & Fantegrossi, 2014).
The DEA has identified a set of 5 synthetic cannabinoid compounds of interest and requested tests of their abuse liability, 4-CN-CUMYL-BUTINACA (also known as CUMYL-4CN-BINACA, CUMYL-CYBINACA or SGT-78), 4F-MDMB-BINACA, 5F-EMB-PINACA (5F-AEB), 5F-CUMYL-P7AICA and EMB-FUBINACA. CUMYL-4CN-BINACA is widely trafficked (EMCDDA, 2017; DEA, 2019) and has been found in urine samples and seized plant material (Åstrand et al., 2018; Öztürk et al., 2018; Yeter, 2017; Yeter et al., 2018). It is a potent agonist at cannabinoid CB1 and CB2 receptors, binding and stimulating cAMP at nanomolar affinities (Kevin et al., 2019; Patel et al., 2020). Aerosolized CUMYL-4CN-BINACA produced dose-dependent increases in drug-appropriate responding in female mice trained to discriminate Δ9-THC to close to 80% (Wiley et al., 2019). CUMYL-4CN-BINACA also produced convulsions in mice (Kevin et al., 2019). There is one reported case of hyperthermia, rhabdomyolysis, and renal failure following dosing with CUMYL-4CN-BINACA (El Zahran et al., 2019). It is metabolized into cyanide, which may produce toxicity in multiple organ systems, including central nervous system, cardiovascular system, lungs, liver, and kidney. (Åstrand et al., 2018).
5F-CUMYL-P7AICA is also a potent CB1 agonist, binding and stimulating cAMP at nanomolar affinities, and producing hypothermia in mice (Banister et al., 2018; Patel et al., 2020). Its illicit use has been confirmed by its identification in urine samples (Steheli et al, 2019). Both CUMYL-4CN-BINACA and 5F-CUMYL-P7AICA are controlled in United States as Schedule I compounds (DEA, 2018). 4F-MDMB-BINACA has been identified in samples from seizures and samples from individuals (Haschimi et al., 2019; Krotulski et al., 2019; 2020; Norman, et a., 2020). 5F-AEB and EMP-FUBINACA have also been found in samples (Antonides et al, 2019) and bound to CB1 and CB2 receptors with nanomolar potencies (Doi et al., 2017). The two compounds were involved with a case of fatal intoxication (Adamowicz et al., 2019).
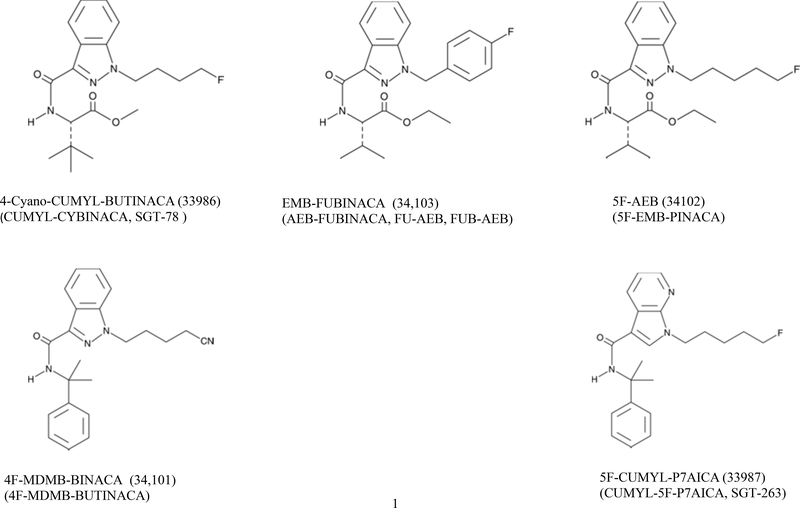
The purpose of the present study was to identify the potential abuse liability of CUMYL-4CN-BINACA, 4F-MDMB-BINACA, 5F-AEB, 5F-CUMYL-P7AICA and EMB-FUBINACA. Abuse liability is determined by a number of factors, including whether the chemical structure of the compound is closely related to those of any known substances of abuse, it has a pharmacological mechanism shared by any known substances of abuse, and it produces subjective effects similar to any known substances of abuse. Chemical structures are shown in Figure 1 and are closely related to a number of controlled synthetic cannabinoids. Four of the five compounds act as agonists at CB1 receptors as does Δ9-THC and the known synthetic cannabinoids. To date, no mechanistic testing of 4F-MDMB-BINACA has been published. Because little or no behavioral testing has been conducted with these compounds, 8-h tests for locomotor activity were conducted using multiple doses to identify the active time course and dose ranges of these compounds. To provide data on the whether the five compounds produce subjective effects similar to Δ9-THC, they were tested in rats trained to discriminate Δ9-THC. The drug discrimination assay is a well-validated animal model of the subjective effects of behaviorally-active compounds (Young, 2009; Horton et al., 2013).
Figure 1.
Chemical structures of the synthetic cannabinoid compounds tested in the present study.
Methods
Subjects
Male ND4 Swiss–Webster mice (n=232) were obtained from Envigo (Houston, TX) at approximately 8 weeks of age and maintained in the University of North Texas Health Science Center (UNTHSC) animal facility for two weeks prior to testing. Mice were housed 3–4 per cage on a 12:12-h light/dark cycle (lights on at 7:00 AM) and were allowed free access to food and water except during test sessions. Thirty-three male Sprague-Dawley rats were obtained from Envigo (Houston, TX). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 A.M.). Body weights were maintained at 320–350 g by limiting food to 15 g/day, which included the food received during sessions. Water was continuously available in the home cage. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor activity
Each study was conducted using 32 Digiscan locomotor activity testing chambers (40.5 X 40.5 X 30.5 cm) (Omnitech Electronics, Columbus OH) each housed within a sound-attenuating chamber that provided dim illumination. A panel of 16 infrared beams and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. Separate groups of 8 mice were injected with either vehicle (ethanol/Cremophor EL/0.9% saline 1:1:18) or a cannabinoid: Δ9-THC (2.5 – 25 mg/kg), 4-CN-CUMYL-BUTINACA (0.01–1 mg/kg), 4F-MDMB-BINACA (0.1–1 mg/kg), 5F-AEB (0.1–1 mg/kg), 5F-CUMYL-P7AICA (0.25–10 mg/kg, and EMB-FUBINACA (0.05–0.5 mg/kg) immediately prior to locomotor activity testing. Each dose range included doses that were without effect to those producing at least 50% depression compared to vehicle control. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, beginning at 8:00 AM (1 h after lights on). Behavioral observations were recorded on each mouse at 30, 120, and 480 min following the top dose of each test compound.
Discrimination procedures
Standard two-lever behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC-compatible computers via Med Associates interfaces (East Fairfield, VT). Response levers were positioned to the left and right of the food hopper. A houselight was centered over the hopper close to the ceiling and was illuminated only when the levers were active. The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data. Rats were first trained to discriminate Δ9-THC (3 mg/kg) from vehicle (ethanol/ Cremophor EL/0.9 % saline in a ratio of 1:1:18) using a two-lever choice methodology. Thirty minutes prior to the training sessions, rats received an injection of either vehicle or Δ9-THC and were subsequently placed in the behavior-testing chambers, where food (45-mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses (fixed-ratio 10) on a designated injection appropriate lever. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. Rats were used in tests of substitution of the experimental compounds once they had achieved nine of ten sessions at 85% or greater injection-appropriate responding for both the first reinforcer and the total session, which occurred after approximately 60 training sessions. The training sessions occurred on separate days in a double-alternating fashion (drug-drug-vehicle-vehicle-drug etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one vehicle and one drug session occurred between each test (drug-vehicle-test-vehicle-drug-test-drug etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions. During test sessions, both levers were active, such that ten consecutive responses on either lever led to reinforcement. For dose-effect experiments, sessions lasted until 20 reinforcers were obtained or for a maximum of 20 min. Each test compound was tested in a group of six rats using a repeated-measure design such that each rat was tested at all doses of a given drug. Δ9-THC was tested in a group of 15 rats. Vehicle (1 ml/kg) and Δ9-THC (3 mg/kg) controls were tested before the start of each compound evaluation. Doses of 4-CN-CUMYL-BUTINACA (0.01–1 mg/kg), 4F-MDMB-BINACA (0.1–1 mg/kg), 5F-AEB (0.1–1 mg/kg), 5F-CUMYL-P7AICA (0.25–10 mg/kg, and EMB-FUBINACA (0.05–0.5 mg/kg) were tested. A dose range was tested from no effect (< 20 % Δ9-THC-appropriate responding) to full effect (≥ 80 % Δ9-THC-appropriate responding or suppression of responding to less than 20% of vehicle control). Pretreatment times were based on the time of peak depression for each compound in the previous locomotor activity testing. 4F-MDMB-BINACA, 5F-CUMYL-P7AICA, 5F-AEB and EMB-FUBINACA were tested using a 15-min pretreatment. Δ9-THC and 4-CN-CUMYL-BUTINACA had a 30-min pretreatment.
Drugs
Δ9-Tetrahydrocannabinol, 4-CN-CUMYL-BUTINACA (1-(4-cyanobutyl)-N-(1-methyl-1-phenylethyl)-1H-indazole-3-carboxamide), 4F-MDMB-BINACA methyl (S)-2-(1-(4-fluorobutyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate), 5-fluoro-AEB (ethyl (1-(5-fluoropentyl)-1H-indazole-3-carbonyl)-L-valinate), 5F-CUMYL-P7AICA (1-(5-fluoropentyl)-N-(2-phenylpropan-2-yl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide) and EMB-FUBINACA (ethyl (1-(4-fluorobenzyl)-1H-indazole-3-carbonyl)-L-valinate) were provided by the National Institute on Drug Abuse Drug Supply Program. All drugs were dissolved in ethanol/Cremophor EL/0.9 % saline (in a ratio of 1:1:18) and were administered i.p. in a volume of 1 ml/kg. Cremophor EL was obtained from Sigma Aldrich (St. Louis, MO).
Data analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal depression of locomotor activity first appeared as a function of dose, was used for analysis of dose-response data and calculation of ED50 values. OriginGraph (OriginLab Corporation, Northampton, MA) was used to estimate the maximal depression (0 photocell counts) induced by each cannabinoid. The ED50 values were calculated by estimating the log dose producing ½ of maximal depression from the descending linear portion of the dose response curve. A two-way analysis of variance, with dose as a between groups factor and time as a within subject factor, was conducted on horizontal activity counts/10 min interval. Subsequently, a one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against the vehicle control using single degree-of-freedom F tests.
Drug discrimination data were expressed as the mean percentage (± standard error) of drug-appropriate responses occurring in each test period. The rate of responding was calculated by dividing the total number of responses for each rat tested by the session time. Response rate data are expressed as the mean (± standard error) of all rats tested. Because response suppression may compromise stimulus control, rats failing to complete at least ten responses during the test session were excluded from the analysis of the discriminative stimulus effects of that dose of test compound. If three or more of the rats did not complete the first fixed ratio at a given dose, the discrimination data for that dose is not shown. Graphs for percent drug-appropriate responding and response rate were plotted as a function of the dose of the test compound (log scale). Percent drug-appropriate responding was shown only if at least three rats completed the first fixed ratio, whereas all rats are shown for the response rate data. Full substitution was defined as ≥ 80 % drug-appropriate responding and not statistically different from the training drug. The potencies of 4-CN-CUMYL-BUTINACA, 4F-MDMB-BINACA, 5F-CUMYL-P7AICA and EMB-FUBINACA were calculated by fitting straight lines to the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). Straight lines were fitted to the linear portion of dose-effect curves, including not more than one dose producing < 20 % of the maximal effect and not more than one dose producing > 80 % of the maximal effect. Other doses were excluded from the analyses. Response-rate data were analyzed by one-way repeated- measure analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The criterion for significance was set a priori at p < 0.05.
Results
Locomotor Activity
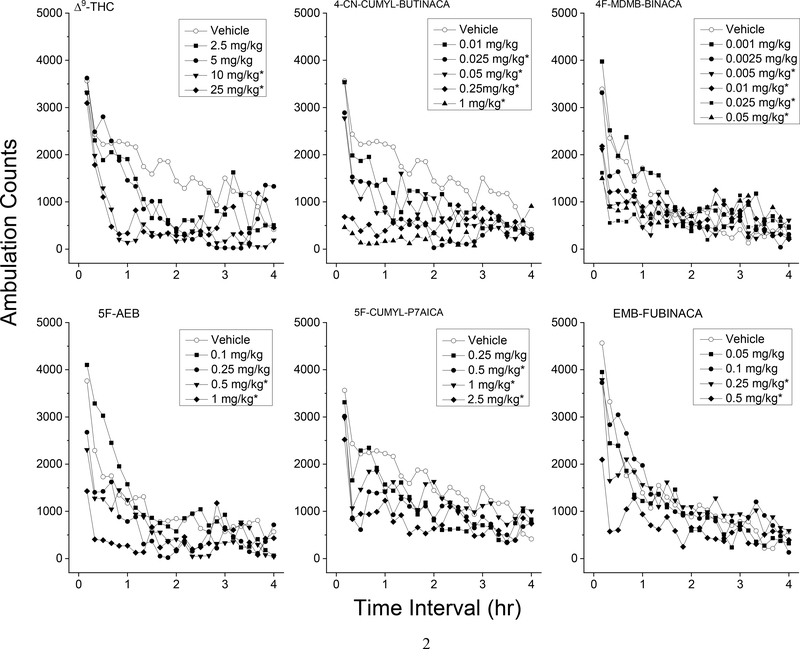
Treatment with Δ9-THC resulted in time- and dose-dependent depression of locomotor activity following 10 and 25 mg/kg (ED50=7.11±0.04 mg/kg). Depressant effects of 10 mg/kg occurred within 30 minutes following injection and lasted 210 minutes (Fig. 2). Potency data are shown in Table 1. Data in figure 2 represent only the first 4 h of locomotor activity. Peak effects were observed between 30–60 min. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated significant effects of Dose F(4,35)=11.6, p<.001, of 10-Minute Periods F(47,1645)=15.2, p<.001, and the interaction of Periods and Dose F(188,1645)=1.4, p=.001. A one-way analysis of variance conducted on horizontal activity counts for the 30–60 min time period (Fig. 3) indicated a significant effect of Dose F(4,35)=12.8, p<.001.
Figure 2. Locomotor activity time course.
Average horizontal activity counts/10 min as a function of time (10 min bins) and dose. Only data from the first four hours are shown. Data are from independent groups of 8 mice per dose. Asterisks indicate doses significantly different from vehicle during the time of peak effect (p<0.05).
Table 1.
Potencies of the test compounds in the drug discrimination (DD) and locomotor activity (LMA) assays. Average ED50 values are expressed in mg/kg ± standard error of the mean.
| Compound | DD | LMA | LMA/DD Ratio |
|---|---|---|---|
| Δ9-THC | 0.61±0.07 | 7.11±0.04 | 11.66 |
| 4-CN-CUMYL-BUTINACA | 0.049±0.08 | 0.032±0.12 | 0.71 |
| 4F-MDMB-BINACA | 0.019±0.08 | 0.012±0.14 | 0.63 |
| 5F-AEB | -- | 0.34±0.06 | -- |
| 5F-CUMYL-P7AICA | 0.085±0.33 | 0.45±0.06 | 5.29 |
| EMB-FUBINACA | 0.13±0.13 | 0.35±0.06 | 2.69 |
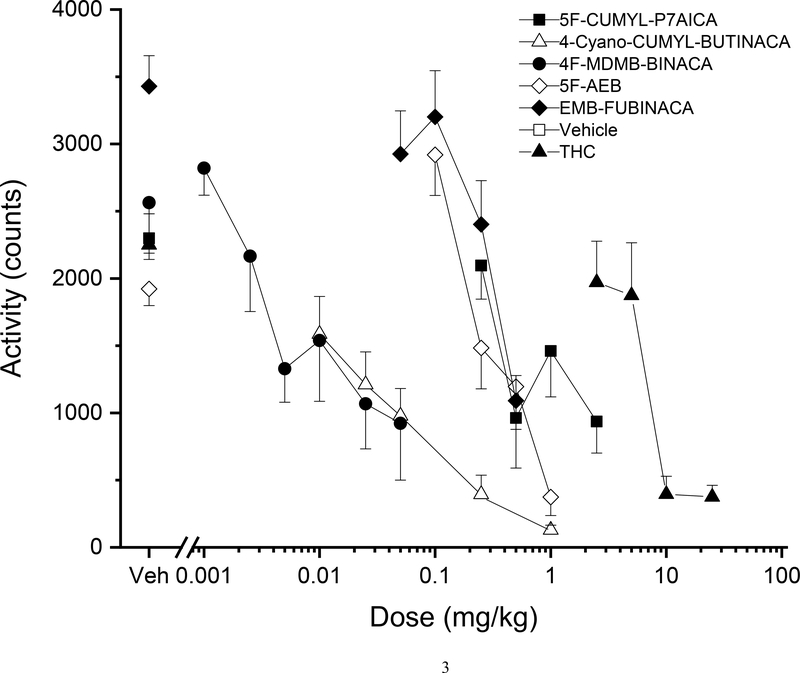
Figure 3. Locomotor activity dose effect.
Average horizontal activity counts/10 min (±SE) during the 30 min of peak effect as a function of dose for each test compound. Data are from independent groups of 8 mice per dose. Veh indicates vehicle controls. Asterisks indicate doses significantly different from vehicle during the time of peak effect (p<0.05).
4-CN-CUMYL-BUTINACA produced time- and dose-dependent depression of locomotor activity in doses from 0.01 to 1 mg/kg (ED50=0.032±0.12 mg/kg). Depressant effects occurred within 10–40 minutes following injection and lasted 40–180 minutes. Peak effects were observed between 30–60 min. There was a significant effect of Dose F(5,42)=6.8, p<.001, 10-Minute Periods F(47,1974)=10.3, p<.001, and interaction of Periods and Dose F(235,1974)=2.7, p<.001. Analysis of the period of maximal depressant effects (30–60 min) indicated a significant effect of dose F(5,42)=14.3, p<.001. Planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.01, 0.025, 0.05, 0.25, and 1 mg/kg. One out of 8 mice at 0.25 mg/kg and two out of 8 mice at 1 mg/kg exhibited convulsions at the 30 min observation time. Due to the likelihood of adverse effects, the 0.1 and 0.5 mg/kg doses of 4-CN-CUMYL-BUTINACA were not tested, and a lower dose range was examined (0.01 – 0.05 mg/kg). Bleeding from one eye was observed in 1 of 6 mice at the end of test session following 0.025 and 0.05 mg/kg.
Treatment with 4F-MDMB-BINACA resulted in time- and dose-dependent depression of locomotor activity in doses from 0.001 to 0.05 mg/kg (ED50=0.012±0.14 mg/kg). Depressant effects of 0.005 to 0.05 mg/kg occurred within 10 minutes following injection and lasted 60–70 minutes. Peak effects were observed between 0–30 min. There was not a significant effect of Dose F(6,49)=1.51, p=.196, but there was a significant effect for 10-Minute Periods F(47,2303)=17.59, p<.001, and a significant interaction of Periods and Dose F(282,2303)=1.37, p=.028. Analysis of the period of maximal depressant effects (30–60 min) indicated a significant effect of Dose F(6,49)=4.31, p=.0001; planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for the 0.005 to 0.05 mg/kg doses. Rigidity and convulsions were observed in 8 of 8 mice at 30 min following injection of 1 mg/kg 4F-MDMB-BINACA (data not shown). These adverse effects were not observed at 120 or 480 min.
5F-AEB produced depression of locomotor activity at 0.5 and 1 mg/kg (ED50=0.55±0.06 mg/kg). Depressant effects of 0.5 and 1 mg/kg occurred within 10 minutes following injection and lasted 40–80 minutes. Peak effects were observed between 0–30 min. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated a significant effect of Dose F(4,35)=3.68, p=.013, a significant effect for 10-Minute Periods F(47,1645)=20.38, p<.001, and a significant interaction of Periods and Dose F(188,1645)=2.26, p<.001. Analysis of the period of maximal depressant effects (30–60 min) indicated a significant effect of dose F(4,35)=13.83, p<.001, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.5 and 1 mg/kg and a significant stimulant effect during the first 30 minutes following 0.1 mg/kg 5F-AEB. Rigidity and convulsions were observed in 8 of 8 mice at 30 min following injection of 1 mg/kg 5F-AEB. These adverse reactions were not observed at 120 or 480 min.
5F-CUMYL-P7AICA produced time- and dose-dependent depression of locomotor activity in doses from 0.5 to 2.5 mg/kg (ED50=0.45±0.06 mg/kg). Depressant effects of 0.5 mg/kg occurred within 20 minutes following injection and lasted 60 minutes. Peak effects were observed between 10–40 min. There was a significant effect of Dose F(4,35)=3.32, p=.021, a significant effect for 10-Minute Periods F(47,1645)=13.42, p<.001, but no significant interaction of Periods and Dose F(188,1645)=0.986, p=.54. Analysis of the period of maximal depressant effects (10–40 min) indicated a significant effect of Dose F(4,35)=5.44, p=.002, and planned comparisons (a priori contrast) against the vehicle group showed a significant depressant effect for 0.5, 1, 2.5 and 10 mg/kg.
EMB-FUBINACA produced depression of locomotor activity at 0.25 and 0.5 mg/kg (ED50=0.35±0.036 mg/kg). Depressant effects of 0.25 and 0.5 mg/kg occurred within 10 minutes following injection and lasted 30–50 minutes. Peak effects were observed between 0–30 min. There was not a significant effect of Dose F(4,35)=1.7, p=.172, but there were significant effects for 10-Minute Periods F(47,1645)=27.86, p<.001, and the interaction of Periods and Dose F(188,1645)=1.56, p<.001. Analysis of the period of maximal depressant effects (30–60 min) indicated a significant effect of Dose F(4,35)=10.47, p<.001.
Drug Discrimination
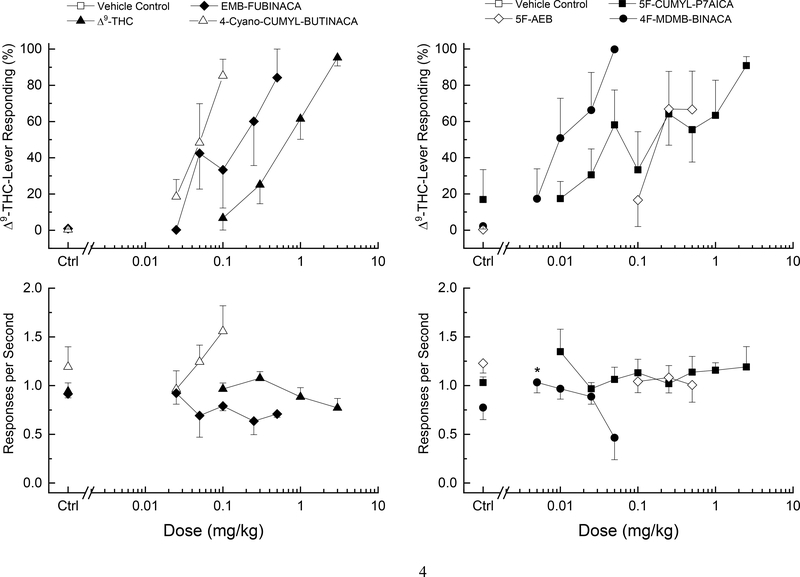
Δ9-THC (ED50=0.61±0.07 mg/kg) produced dose-dependent increases in drug-appropriate responding with a peak (95±5%) following the training dose of 3 mg/kg (Figure 4). 4-CN-CUMYL-BUTINACA (ED50=0.049±0.08 mg/kg), 4F-MDMB-BINACA (ED50=0.019±0.08 mg/kg), 5F-CUMYL-P7AICA (ED50=0.085±0.33 mg/kg) and EMB-FUBINACA (ED50=0.13±0.13 mg/kg) all fully substituted for the discriminative stimulus effects of Δ9-THC (Figure 4). Response rate was increased following 0.005 mg/kg 4F-MDMB-BINACA F(4,20)=4.48, p=.01. Response rate was not affected by Δ9-THC or the other test compounds. In contrast, 5F-AEB only produced maximum Δ9-THC-appropriate responding of 67±21% following 0.25 and 0.5 mg/kg. The same 4 of 6 rats selected the drug-appropriate lever following both doses. Response rate was not affected at these doses. A higher dose (1 mg/kg) produced convulsions in one rat; additional rats were not tested at that dose.
Figure 4.
Substitution for the discriminative stimulus effects of Δ9-tetrahydrocannabinol: Top panels show percentage of total responses (±SE) made on the drug-appropriate lever. Bottom panels show rate of responding (±SE) in responses per second (r/s). All of the cathinones fully substituted for the discriminative stimulus effects of Δ9-THC (≥80% drug-appropriate responding). n=6 unless otherwise shown, except Δ9-THC, n=15. Ctrl indicates vehicle and Δ9-THC control values. * indicates response rate different from vehicle control (p < 0.05).
Discussion
In the present study, the abuse liability of five synthetic cannabinoids identified by the DEA was evaluated using locomotor activity and drug discrimination. All five of the test compounds depressed locomotor activity similar to Δ9-THC, although the time courses of the compounds differed, with 5F-AEB and EMB-FUBINACA being relatively short-acting (1 – 1.5 h). In contrast, depressant effects of 4-CN-CUMYL-BUTINACA and 5F-CUMYL-P7AICA lasted close to 4 h, which was similar to Δ9-THC. All of the test compounds had a more rapid onset than did Δ9-THC, which may be of concern as short-onset, short-acting compounds have greater abuse liability.
Three of the test compounds (4-CN-CUMYL-BUTINACA, 4F-MDMB-BINACA, and 5F -AEB) produced convulsions at the higher doses tested. Retro-orbital bleeding was also observed in two mice following administration of mid-range doses (0.025 and 0.05 mg/kg) of 4-CN-CUMYL-BUTINACA. An earlier study noted 4-CN-CUMYL-BUTINACA produced convulsions in mice at doses similar to those observed in the present study (Kevin et al., 2019). Convulsions have been observed in humans taking synthetic cannabinoids recreationally (Gounder et al., 2020; Tait, et al., 2016) and the convulsant effects of at least some of the synthetic cannabinoids are mediated via CB1 receptors (Wilson et al., 2019). Of the 34 synthetic cannabinoids tested by this laboratory, only five have produced convulsions at the doses tested (Gatch and Forster, 2014; 2015; 2016, 2018; 2019), three of which are in the current study. The likelihood of a synthetic cannabinoid producing convulsions in vivo via CB1 receptor effects may be related to rate of metabolism (Wilson et al., 2019) or to other receptor mechanisms. For example, 4-CN-CUMYL-BUTINACA may be more dangerous due to the cyanide moiety attached at the end of the side chain which is cleaved off during metabolism and is able to enter systemic circulation (Åstrand et al., 2018). The convulsions and bleeding observed in the present study may be due at least in part to the toxic effects of cyanide.
Four of the five cannabinoid compounds (4-CN-CUMYL-BUTINACA, 4F-MDMB-BINACA, 5F-CUMYL-P7AICA and EMB-FUBINACA) fully substituted for the discriminative stimulus effects of Δ9-THC. This finding is in agreement with earlier work in which synthetic cannabinoids produce Δ9-THC-like discriminative stimulus effects (see review in Wiley et al., 2018). Further, aerosolized CUMYL-4CN-BINACA produced dose-dependent increases in drug-appropriate responding in female mice trained to discriminate Δ9-THC to close to 80%, the common cut-off for “full-substitution” (Wiley et al., 2019).
In contrast, 5F -AEB did not fully substitute for Δ9-THC, producing a plateau following 0.25 and 0.5 mg/kg with a peak effect of 67%. The low efficacy of 5-AEB is somewhat unusual for compounds flagged by DEA for testing. In our lab, 33 of the 34 synthetic cannabinoids tested have fully substituted at some dose (Gatch and Forster, 2014; 2015; 2016, 2018; 2019) However, there is evidence of at least one compound with CB1 receptor activity (EG-018) failing to substitute for Δ9-THC (Gamage et al., 2020). Higher doses of 5F-AEB produced convulsions and discrimination was not tested. Convulsions were not observed during the drug discrimination studies of 4-CN-CUMYL-BUTINACA or 4F-MDMB-BINACA, but lower doses were tested than in the locomotor activity studies.
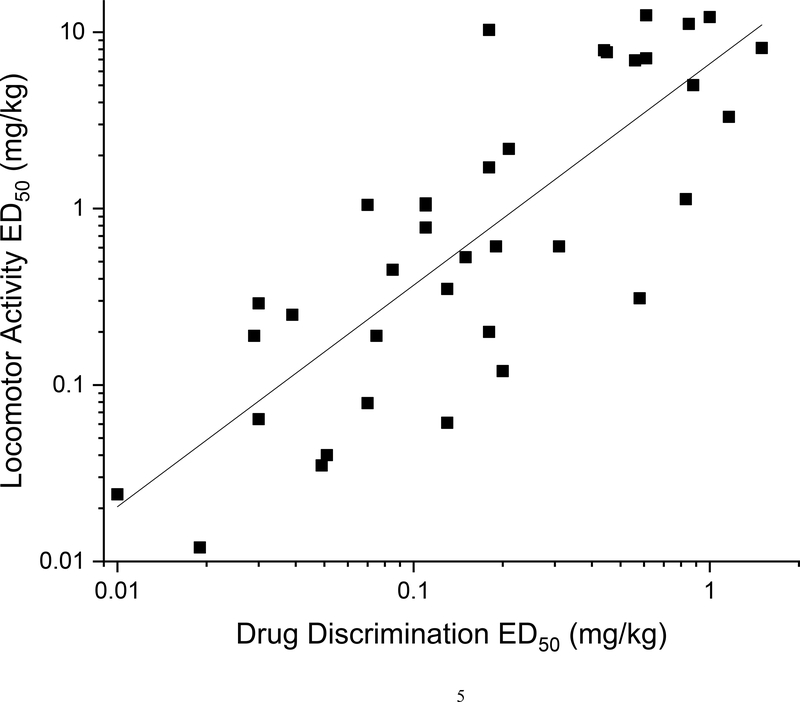
In our earliest studies of synthetic cathinones, we reported no correlation between the potencies of the test compounds in the locomotor activity and drug discrimination assays (Gatch and Forster, 2014; 2015). However, when we include 37 cannabinoid compounds tested in our laboratory in the regression, a weak correlation is observed between the potencies of the test compounds in the locomotor activity and drug discrimination assays (R2 of 0.41, p<0.001). The correlation is fairly weak. For example, of the 37 compounds, 28 were more potent in the drug discrimination assay, 9 were more potent in the locomotor activity assay and 3 compounds had similar potencies in the two assays. Further, in the present study, 4CN-CUMYL-BUTINACA produced stimulation of the rate of operant lever pressing in a dose range that produced depression of non-motivated locomotor activity.
Four of the five test compounds (4-CN-CUMYL-BUTINACA, 5F-CUMYL-P7AICA, 5F-AEB, and EMB-FUBINACA) have been reported to act as potent CB1 agonists (Banister et al., 2018; Doi et al., 2017; Kevin et al., 2019; Patel et al., 2020). 4-CN-CUMYL-BUTINACA and 5F-CUMYL-P7AICA were tested in functional assays (cAMP, β-arrestin) and were full agonists with EC50s of 8–9 nM (Banister et al., 2018; Kevin et al., 2019; Patel et al., 2020). Binding studies were conducted on the isomers of 5F-AEB and EMB-FUBINACA (Doi et al., 2017). Both compounds were potent in the nanomolar range, with the R-isomers being less potent at CB1 receptors than the S-isomers. It is difficult to make strong conclusions about relations between CB1 receptor affinity and relative potency in the behavioral assays, since different types of receptor tests were used, and the binding studies examined the stereoisomers whereas the behavioral studies tested the racemates. To date, mechanistic testing of 4F-MDMB-BINACA has not been published. The discriminative stimulus effects of cannabinoids have been shown to be mediated by CB1 receptors, with fairly high specificity (see review by Tanda, 2016), so it is likely that the behavioral effects of the present set of test compounds are mediated by CB1 receptors.
In conclusion, 4-CN-CUMYL-BUTINACA, 5F-CUMYL-P7AICA, 4F-MDMB-BINACA, and EMB-FUBINACA may have abuse liability similar to Δ9-THC. 4-CN-CUMYL-BUTINACA and 4F-MDMB-BINACA produced convulsions although at doses significantly higher than those needed to produce Δ9-THC-like discriminative stimulus effects. Nevertheless, these compounds may still be a substantial risk for street use. 5F-AEB may be less favored by recreational users due to its limited Δ9-THC-like discriminative stimulus effects; however, its pro-convulsant effects at doses needed to produce mild Δ9-THC-like discriminative stimulus effects may make it exceptionally dangerous for recreational use.
Figure 5.
Regression of potencies (ED50 values in mg/kg) in the locomotor activity and drug discrimination assays for 37 cannabinoid compounds tested in our laboratory.
Acknowledgments
Funding
This work was supported by the National Institutes of Health N01DA-18-8936.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- Adamowicz P, Meissner E, Maślanka M. (2019). Fatal intoxication with new synthetic cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clin Toxicol (Phila) 57: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Antonides LH, Cannaert A, Norman C, Vives L, Harrison A, Costello A, et al. (2019). Enantiospecific synthesis, chiral separation, and biological activity of four indazole-3-carboxamide-type synthetic cannabinoid receptor agonists and their detection in seized drug samples. Front Chem 16: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åstrand A, Vikingsson S, Lindstedt D, Thelander G, Gréen H, Kronstrand R. et al. (2018). Metabolism study for CUMYL-4CN-BINACA in human hepatocytes and authentic urine specimens: free cyanide is formed during the main metabolic pathway. Drug Test Anal 10: 1270–1279. [DOI] [PubMed] [Google Scholar]
- Banister S, Adams A, Kevin R, Macdonald C, Glass M, Boyd R, et al. (2018). Synthesis and pharmacology of new psychoactive substance 5F-CUMYL-P7AICA, a scaffold-hopping analog of synthetic cannabinoid receptor agonists 5F-CUMYL-PICA and 5F-CUMYL-PINACA. Drug Test Anal 11: 279–291. [DOI] [PubMed] [Google Scholar]
- Boven M, Bissig C, Staeheli S, Poetzsch M, Pfeiffer B, Kraemer T. (2017). Structural characterization of the new synthetic cannabinoids CUMYL-PINACA, 5F-CUMYL-PINACA, CUMYL-4CN-BINACA, 5F-CUMYL-P7AICA and CUMYL-4CN-B7AICA. Forensic Sci Int 281: 98–105. [DOI] [PubMed] [Google Scholar]
- Doi T, Tagami T, Takeda A, Asada A, Sawabe Y. (2018). Evaluation of carboxamide-type synthetic cannabinoids as CB1/CB2 receptor agonists: difference between the enantiomers. Forensic Toxicol 36: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice. (2018). Schedules of controlled substances: temporary placement of NM2201, 5F-AB-PINACA, 4-CN-CUMYL-BUTINACA, MMB-CHMICA and 5F-CUMYL-P7AICA into schedule I. Fed Regist 83: 24696–24701. [Google Scholar]
- El Zahran T, Gerona R, Morgan BW, Pomerleau AC. (2019). A novel synthetic cannabinoid (Cumyl-4-cyano-BINACA) resulting in hyperthermia, rhabdomyolysis, and renal failure in a 29-year-old patient: it’s not meningitis. Clin Toxicol (Phila) 57: 421–422. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2018: Trends and Developments. (2018). Publications Office of the European Union, Luxembourg. ISBN 978–92-9497–272-9. ISSN 2314–9086. doi: 10.2810/800331.TD-AT-18-001-EN-N. [DOI] [Google Scholar]
- Gamage T, Barrus D, Kevin R, Finlay D, Lefever T, Patel P. (2020). In vitro and in vivo pharmacological evaluation of the synthetic cannabinoid receptor agonist EG-018. Pharmacol Biochem Behav 193: 172918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Forster M. (2019). Cannabinoid-like effects of five novel carboxamide synthetic cannabinoids. Neurotoxicology 70: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Forster M. (2018). Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of five novel synthetic cannabinoids in rats. Psychopharmacology 253: 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Forster M. (2016). Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology 233: 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Forster M. (2015). Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids found on the gray market. Behav Pharmacol 26: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Forster M. (2014). Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav Pharmacol 25: 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounder K, Dunuwille J, Dunne J, Lee J, Silbert P, Lawn N. (2020). The other side of the leaf: seizures associated with synthetic cannabinoid use. Epilepsy Behav 104: 106901. [DOI] [PubMed] [Google Scholar]
- Haschimi B, Mogler L, Halter S, Giorgetti A, Schwarze B, Westphal F, et al. (2019). Detection of the recently emerged synthetic cannabinoid 4F-MDMB-BINACA in “legal high” products and human urine specimens. Drug Test Anal 11: 1377–1386. [DOI] [PubMed] [Google Scholar]
- Horton D, Potter D, Mead A. (2013). A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol 24: 410–436. [DOI] [PubMed] [Google Scholar]
- Kevin R, Anderson L, McGregor I, Boyd R, Manning J, Glass M, et al. (2019). CUMYL-4CN-BINACA is an efficacious and potent pro-convulsant synthetic cannabinoid receptor agonist. Front in Pharmacol 10: 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotulski A, Mohr A, Diamond F, Logan B. (2020). Detection and characterization of the new synthetic cannabinoid APP-BINACA in forensic casework. Drug Test Anal 12: 136–144. [DOI] [PubMed] [Google Scholar]
- Krotulski A, Mohr A, Kacinko S, Fogarty M, Shuda S, Diamond F, et al. (2019). 4F-MDMB-BINACA: A new synthetic cannabinoid widely implicated in forensic casework. J Forensic Sci 64: 1451–1461. [DOI] [PubMed] [Google Scholar]
- National Research Council. (2011). Guide for the care and use of laboratory animals, 8th ed. Washington, D.C. The National Academies Press. [Google Scholar]
- Norman C, Walker G, McKirdy B, McDonald C, Fletcher D, Antonides L, et al. (2020). Detection and quantitation of synthetic cannabinoid receptor agonists in infused papers from prisons in a constantly evolving illicit market. Drug Test Anal 12: 538–554. [DOI] [PubMed] [Google Scholar]
- Patel M, Manning J, Finlay D, Javitch J, Banister S, Grimsey N, et al. (2020). Signaling profiles of a structurally diverse panel of synthetic cannabinoid receptor agonists. Biochem Pharmacol 175: 113871. [DOI] [PubMed] [Google Scholar]
- Staeheli S, Steuer A, Kraemer T. (2019). Identification of urinary metabolites of the synthetic cannabinoid 5F-CUMYL-P7AICA in human casework. Forensic Sci Int 294: 76–79. [DOI] [PubMed] [Google Scholar]
- Tai S, Fantegrossi W. (2014). Synthetic cannabinoids: pharmacology, behavioral Effects, and abuse potential. Curr addict rep 1: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R, Caldicott D, Mountain D, Hill S, Lenton S. (2016). A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila) 54: 1–13. [DOI] [PubMed] [Google Scholar]
- Tanda G (2016). Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology (Berl) 233: 1845–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J, Owens R, Lichtman A. (2018). Discriminative stimulus properties of phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Curr Top Behav Neurosci 39: 153–173. [DOI] [PubMed] [Google Scholar]
- Wiley J, Lefever T, Glass M, Thomas B. (2019). Do you feel it now? Route of administration and Δ 9-tetrahydrocannabinol-like discriminative stimulus effects of synthetic cannabinoids in mice. Neurotoxicology 73: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Tai S, Ewing L, Crane J, Lockhart T, Fujiwara R, et al. (2019). Convulsant effects of abused synthetic cannabinoids JWH-018 and 5F-AB-PINACA are mediated by agonist actions at CB1 receptors in mice. J Pharmacol Exp Ther 368: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018). Critical review report: CUMYL-4CN-BINACA expert committee on drug dependence forty-first meeting. World Health Organization. [Google Scholar]
- Öztürk Y, Yeter O, Öztürk S, Karakus G, Ates I, Buyuk Y, et al. (2018). Detection of metabolites of the new synthetic cannabinoid CUMYL-4CN-BINACA in authentic urine samples and human liver microsomes using high-resolution mass spectrometry. Drug Test Anal 10: 449–459. [DOI] [PubMed] [Google Scholar]
- Yeter O (2017) Identification of the synthetic cannabinoid 1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)-1H-indazole-3-carboxamide (CUMYL-4CN-BINACA) in plant material and quantification in post-mortem blood samples. J Anal Toxicol 41:720–728. [DOI] [PubMed] [Google Scholar]
- Young R (2009). Drug discrimination in methods of behavior analysis in neuroscience. Buccafuso J. (editor). 2nd ed. Boca Raton. CRC Press, Taylor & Francis Group LLC. [PubMed] [Google Scholar]