Abstract
Using rigorous and clinically relevant experimental design and analysis standards, in this study, we investigated the potential of histone deacetylase (HDAC) inhibitors panobinostat and entinostat to enhance recovery of motor function after photothrombotic stroke in male mice. Panobinostat, a pan-HDAC inhibitor, is a FDA-approved drug for certain cancers, whereas entinostat is a class-I HDAC inhibitor in late stage of clinical investigation. The drugs were administered every other day (panobinostat—3 or 10 mg/kg; entinostat—1.7 or 5 mg/kg) starting from day 5 to 15 after stroke. To imitate the current standard of care in stroke survivors, i.e., physical rehabilitation, the animals run on wheels (2 h daily) from post-stroke day 9 to 41. The predetermined primary end point was motor recovery measured in two tasks of spontaneous motor behaviors in grid-walking and cylinder tests. In addition, we evaluated the running distance and speed throughout the study, and the number of parvalbumin-positive neurons in medial agranular cortex (AGm) and infarct volumes at the end of the study. Both sensorimotor tests revealed that combination of physical exercise with either drug did not substantially affect motor recovery in mice after stroke. This was accompanied by negligible changes of parvalbumin-positive neurons recorded in AGm and comparable infarct volumes among experimental groups, while dose-dependent increase in acetylated histone 3 was observed in peri-infarct cortex of drug-treated animals. Our observations suggest that add-on panobinostat or entinostat therapy coupled with limited physical rehabilitation is unlikely to offer therapeutic modality for stroke survivors who have motor dysfunction.
Keywords: Post-stroke recovery, Drug repurposing, Stroke pharmacotherapy, Pre-clinical trial, Neural repair, HDAC inhibitor
Introduction
Stroke continues to be a major cause of mortality and long-term functional disability worldwide (Wafa et al. 2020; Virani et al. 2020). Importantly, stroke-related deaths have been in decline during the last decade thanks to improved public education, advanced acute care facilities, and available acute treatment options. In contrast, the number of stroke survivors has been increasing with majority of them suffering long-term disabilities (Skolarus et al. 2016). Despite significant advances in understanding the pathophysiology of stroke, treatment options for combating it has been limited to acute intervention with either recombinant tissue plasminogen activator (rtPA, the only FDA-approved drug for this condition) or endovascular thrombectomy (Leng and Xiong 2019). Unfortunately, only a small fraction of patients (< 10%) qualify for these treatments due to a narrow therapeutic time-window and unwanted complications associated with these therapies (Leng and Xiong 2019). Because of this, in recent years, the scientific and clinical attention has been gradually shifting toward potential neurorestorative therapies to promote functional recovery in stroke survivors throughout post-acute and chronic phases of the disease (Carmichael 2016; Corbett et al. 2017). Many of the earlier studies focusing on neuroprotection have provided substantial knowledge regarding the complexity of stroke injury cascades and heterogenic interaction between stroke recovery mechanisms (Iadecola and Anrather 2011; Mattson 2003; Schaffer and Gage 2004; Berretta et al. 2014; Karamyan 2021; Selvaraj et al. 2016). These discoveries have paved the way for future stroke recovery studies and signified the importance of multi-targeted therapy. One of such targets are histone deacetylases or HDACs (Langley et al. 2009; Fessler et al. 2013; Sen 2015; Dietz and Casaccia 2010). HDACs are responsible for suppressing gene expression by removing acetyl groups from the lysine residues of histones to result in a more compact conformation that is not supportive for transcription initiation. Among many genes regulated by HDACs, a number are involved in stroke-related mechanisms, including cell survival, neuroinflammation, oxidative stress, BDNF biosynthesis, and neuronal plasticity (Langley et al. 2009; Fessler et al. 2013). Furthermore, alteration of histones 3 and 4 (H3 and H4) acetylation during acute and early post-acute phases of stroke have also been documented (Chen et al. 2012; Kassis et al. 2017). This knowledge has led to testing of small molecule HDAC inhibitors as neuroprotective or neurorestorative agents in rodent models of ischemic stroke and provided essential mechanistic insight about the potential function of HDACs in the post-stroke brain (Langley et al. 2009; Fessler et al. 2013). Importantly, as deliberated in more detail in the discussion section of this manuscript, majority of studies focusing on use of HDAC inhibitors for promoting neural repair and functional recovery after stroke lacked a number of translationally relevant considerations. Perhaps, chief among them is the combination of pharmacotherapy with physical rehabilitation, because the latter is currently the standard of clinical care for stroke survivors (Winstein et al. 2016). This is critical for preclinical studies too, because new drugs intended for post-stroke recovery are expected to be tested in combination with physical rehabilitation for anticipated therapeutic outcomes (Kwakkel et al. 2020). To this end, the aim of this study was to evaluate the effect of delayed pharmacological therapy by panobinostat or entinostat, coupled with limited physical rehabilitation, on recovery of motor function in mice after ischemic stroke. Panobinostat is a pan-HDAC inhibitor with highest potency compared to other pan-HDAC inhibitors and is approved for treatment of multiple myeloma (Atadja 2009). Entinostat is a selective Class-I HDAC inhibitor that is most potent in this class and has been studied in multiple solid tumors (Connolly et al. 2017). Our study is the first to directly evaluate and compare the dose-dependent effect of delayed panobinostat or entinostat therapy (starting on post-stroke day 5), coupled with limited physical rehabilitation (2 h daily voluntary wheel running, post-stroke days 9–42), on recovery of motor function in the mouse photothrombotic model of ischemic stroke. We applied rigorous and clinically relevant experimental design and analysis standards to test the efficacy of these therapeutic combinations in adult male mice. In this study, motor function was monitored in two tasks of spontaneous motor behaviors of the forelimb using grid-walking and cylinder tests. Our observations indicate that despite engaging with their pharmacological target in the brain, neither panobinostat nor entinostat facilitate motor recovery in mice after stroke. The results of both sensorimotor tests appear to be in line with another molecular-cellular outcome measure of post-stroke motor recovery—the number of parvalbumin-positive neurons in the ipsilateral medial agranular cortex (AGm), which were not substantially changed in drug-treated animals. These observations suggest that add-on panobinostat or entinostat therapy coupled with limited physical rehabilitation is unlikely to offer therapeutic modality for stroke survivors who have motor dysfunction.
Materials and Methods
Animals
For this study, 12–14-week-old (at the time of stroke surgery) male CD-1 mice, purchased from Charles River Laboratories, were used. The animals were maintained in 12-h light/dark cycle and had ad libitum access to food and water as approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee. All animals were habituated in the experimental room for ~ 2 weeks and were individually handled by two experimenters (~ 2 min once or twice daily) for at least a week before recording their baseline motor function in grid-walking and cylinder tests. Mice were housed individually in new home cages after stroke surgery.
Study Design and Treatments
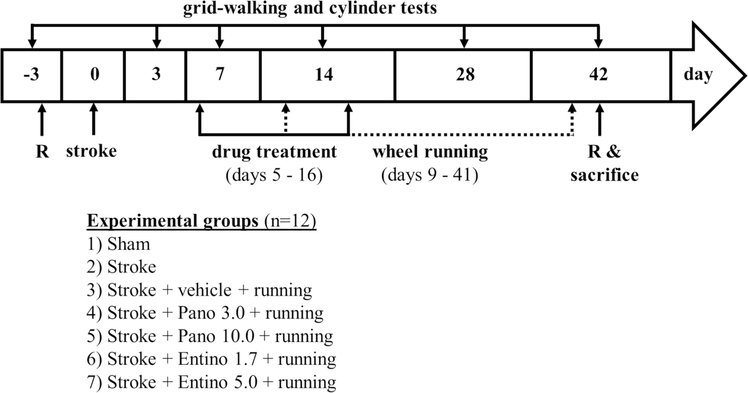
Selection of experimental groups (sham, stroke and drug treatments) and animals for post-experimental brain processing (fixing vs fresh tissue collection) was done randomly (https://www.random.org/lists/) (Jayaraman et al. 2020). Experimental groups (n = 12/group; Fig. 1) included “Sham” (no stroke or other treatments); “Stroke” (stroke but no drug treatment or physical exercise); “Vehicle” (stroke + vehicle treatment + physical exercise); “Pano 3.0” (stroke + 3 mg/kg panobinostat treatment + physical exercise); “Pano 10.0” (stroke + 10 mg/kg panobinostat treatment + physical exercise); “Entino 1.7” (stroke + 1.7 mg/kg entinostat treatment + physical exercise); “Entino 5.0” (stroke + 5 mg/kg panobinostat treatment + physical exercise). Panobinostat and entinostat (products P180500 and E559300, Toronto Research Chemicals) were administered intraperitoneally at 5 ml/kg volume (Al Shoyaib et al. 2019), every other day (~ 3 h before the dark cycle started), starting from post-stroke day 5 to 15. Both drugs were first dissolved in dimethyl sulfoxide (DMSO) (4.5% of the final mixture) and combined with polyethylene glycol (15)-hydroxystearate (Solutol), ethanol, and saline for injection at a ratio of 15:10:75 (Price et al. 2013). The vehicle control used in this study was combination of DMSO, Solutol, ethanol and saline in the same ratio used for the drugs. The doses of panobinostat (3 and 10 mg/kg) used in this study are within the same range used in other preclinical studies for HDAC modulation in rodents (Hennika et al. 2017; Meel et al. 2020). Likewise, the doses of entinostat (1.7 and 5 mg/kg) used in our study are analogous to earlier preclinical studies which used the drug in rodents (Ma et al. 2018; Zhang and Schluesener 2013). Notably, in the beginning of this study, we also used higher doses of both drugs (30 mg/kg panobinostat and 15 mg/kg entinostat); however, due to associated toxicity, we interrupted the experiments.
Fig. 1.
Schematic representation of the study design. Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat; R randomization
Every other day drug treatments were started on post-stroke day 5 to be outside of neuroprotective time-window (inflection point at ~ 3 days in this stroke model (Clarkson et al. 2010)), and completed on day 15. This drug regimen was selected to primarily modulate spontaneous endogenous plasticity and neurorestorative mechanisms at their activation after stroke through the beginning of their decline (Krakauer et al. 2012; Carmichael 2016), and to limit the potential side effects of the drugs upon chronic use (Hennika et al. 2017; Choi et al. 2019).
To imitate physical rehabilitation, voluntary wheel running was initiated in animal’s home cage starting from day 9 after stroke through the end of the study (at the start of the dark cycle, 2 h daily, 6 days per week with a break on the day before behavioral tests). Running wheels (Innowheel, Inno Vive) were custom equipped with an odometer to document the running distance. Additionally, running of each animal was video recorded during the last 3 weeks of the study to measure their running speed. Motor function of the experimental animals was evaluated during the light cycle (8–11 am), on days 3, 7, 14, 28 and 42 after stroke. Assignment of mice to a specific experimental group was concealed from experimenters.
In a separate set of experiments, mice were randomly assigned to the same experimental groups and treated like described above. These mice were euthanized on the next day after the last drug or vehicle treatment (post-stroke day 16) to obtain peri-infarct cortical tissue (1–2 mm) together with the infarct core for evaluation of histone acetylation (see below). These animals were not subjected to behavioral testing.
Ischemic Stroke Model
Photothrombotic model of ischemic stroke was used in this study three days after baseline evaluation of motor function according to our previously published procedure (Vijayan et al. 2019; Alamri et al. 2018). Briefly, after isoflurane anesthesia mice received Rose Bengal solution intraperitoneally (80 mg/kg) and 5 min later the right hemispheric primary motor cortical area (1.5 mm lateral from Bregma 0) was illuminated with cold light through intact skull for 15 min (2 mm diameter, fiber optic illuminator light source with halogen lamp). After completion of the surgery the mouse was transferred to a recovery chamber (~ 37 °C for 1.5 h) and then to an individual new cage. Sham control mice were subjected to the same procedure with the exception of light illumination. In this report, ‘affected forelimb’ represents the left (contralateral) forelimb, whereas ‘unaffected forelimb’ represents the right (ipsilateral) forelimb.
Motor Functional Tests
Motor function of the mice was evaluated using two well established behavioral tests, grid-walking and cylinder tests, which focus on spontaneous motor behavior of the forelimb and were described in detail in our previous publications (Alamri et al. 2018; Syeara et al. 2020). For grid-walking test each mouse was placed on an elevated wire grid, with 12 mm square wire mesh and 33 cm × 20 cm total area, and its exploratory behavior was video recorded for 5 min. Footfaults for each forelimb and total number of normal steps were counted, and the percent of affected and unaffected footfaults was calculated by: [(#affected or unaffected forelimb faults/#normal steps) × 100]. For cylinder test each mouse was placed in a clear acrylic cylinder (17 cm height and 10 cm diameter) and its exploratory rearing was video recorded for 5 min. Forelimb use symmetry index was then quantified by: [(#affected forelimb use − #unaffected forelimb use) / (#affected forelimb use + #unaffected forelimb use + #use of both forelimbs)].
Brain Collection and Infarct Size Evaluation
After the last set of behavioral tests mice were deeply anesthetized with isoflurane to either cardially perfuse with phosphate-buffered saline and 4% paraformaldehyde (PFA) for fixing and cryosectioning (immunostaining and infarct evaluation, n = 6/group), or to decapitate and dissect the brain for isolation of peri-infarct cortex and infract core (n = 6/group). The PFA-perfused brains were further incubated in 4% PFA overnight, followed by cryopreservation in 20 and 30% sucrose solutions, and coronal cryosectioning (50 μm thickness). To locate the infarction and quantify its volume, the brain sections were stained with Cresyl Violet followed by acquisition of digital images and volumetric analysis using ImageJ software (Syeara et al. 2020).
Immunoblotting and Immunofluorescence Staining
For immunoblotting, the collected cortical samples (1–2 mm of peri-infarct cortex together with infarct core; day 16 post-stroke mice) were homogenized in RIPA buffer containing protease and phosphate inhibitors (HALT Protease Inhibitor Cocktail and HALT Phosphatase Inhibitor Cocktail, products 1860932 and 78428, Thermo Fisher Scientific) (Jayaraman et al. 2020). The pellet of the homogenate, which largely consists of cell nuclei, was further processed in nuclear extraction solution (63 mM Tris buffer (pH 6.8), 2% SDS, 10% glycerol, 1 mM DTT, 1 mM PMSF, 1.6% protease inhibitor and 1.6% of phosphatase inhibitor) (Chopra et al. 2016), and the resulting protein extract was used for conventional Western Blotting as described elsewhere (Rashid et al. 2014; Wangler et al. 2012). The primary antibodies used for this experiment were rabbit anti-acetyl histone H3 (K9, K27, K14) antibodies (mixed at equal ratio, products 9649 T, 8173 T, 7627 T, Cell Signaling Technology), rabbit anit-histone H3 antibody (product 4499 T, Cell Signaling Technology) and mouse anti-β-actin antibody (product A5441, Sigma). The secondary antibodies used were goat anti-rabbit and anti-mouse IgGs (products 170–6515 and 170–6516, Bio-Rad Laboratories).
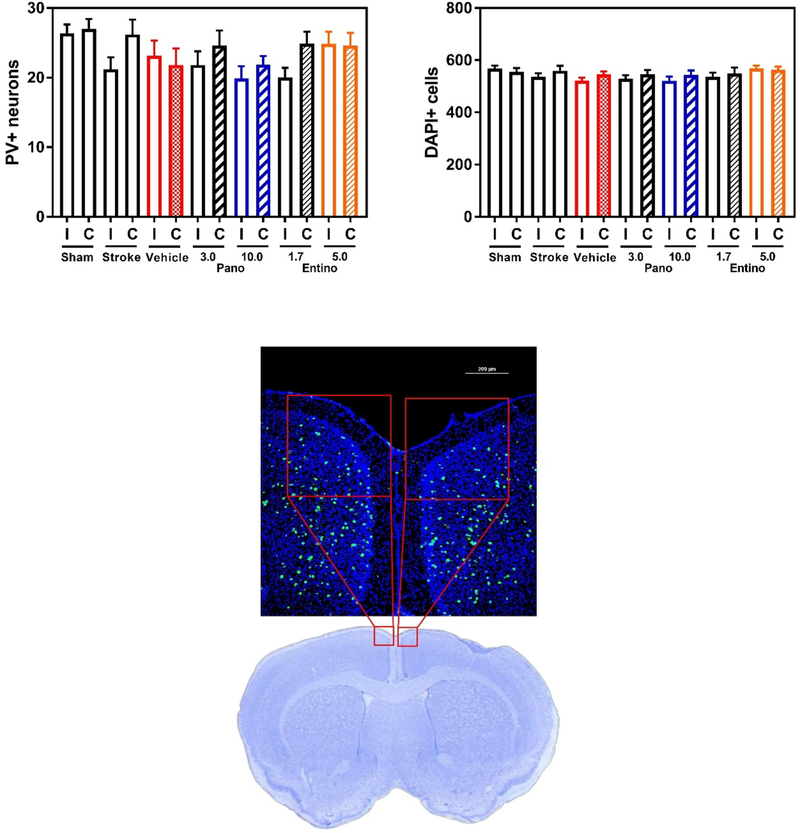
In case of immunostaining, free floating cryopreserved coronal sections were used to label parvalbumin containing cells (mouse anti-parvalbumin primary antibody, product P3088, Sigma-Aldrich and donkey anti-mouse AlexaFluor™ 488 secondary antibody, product A21202, Invitrogen) following a standard protocol (Jayaraman et al. 2020). Nikon A1R MP confocal microscope was used to acquire microscopic images of medial agranular cortex (AGm) within previously defined boundaries (Ng et al. 2015; Zeiler et al. 2013; Alamri et al. 2021) throughout the entire thickness of each brain section (z-stack imaging). Each z-stack image was saved at maximal projections as a 2D image, followed by selection of an AGm subarea (400 × 400 μm2, extending to medial and dorsal pial boundaries, Fig. 5) and counting of parvalbumin-positive cells by an experimenter blinded to the experimental groups. A cell was counted parvalbumin-positive if it had immunofluorescent label and a round shape. Two sections from each brain (~ 300 μm apart in rostral-caudal axis) were used in these experiments. DAPI-stained nuclear staining in the same brain sections was used to evaluate the total number of cells in these AGm regions of interest. For this, Analyze Particles function of ImageJ software (NIH) was used to automatically derive the number of DAPI-stained nuclei from each 400 × 400 μm2 subarea of AGm in ipsilateral and contralateral hemisphere.
Fig. 5.
Panobinostat or entinostat does not substantially affect the number of inhibitory interneurons in ipsilateral AGm. Top left panel, panobinostat- or entinostat-treated mice have comparable number of parvalbumin-positive (PV+) neurons in ipsilateral (I) and contralateral (C) medial premotor area (AGm, medial agranular cortex) (n = 6 per group; p > 0.05 for I vs. C comparisons within each experimental group). Top right panel, comparable number of DAPI-stained nuclei was documented within the same subareas of AGm in experimental groups. Bottom panel, a representative immunofluorescence confocal microscopy image of a mouse brain section on day 42 after stroke. PV signal is depicted in green and cell nuclei (stained with DAPI) in blue. Note that the representative Cresyl violet-stained brain section is shown to illustrate the imaged medial premotor area (red boxes). Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat
Data Analysis
Two-way repeated measures ANOVA followed by Dunnett’s post hoc tests were used to analyze data from motor behavior tests (Prism 7.05, GraphPad). For the rest of experimental data, two-tailed Student’s t-test was used to compare the means from two experimental groups (paired for body weight comparisons, un-paired for PV and DAPI staining comparisons), whereas one-way ANOVA (with Dunnett’s multiple comparisons test) was used to compare three or more groups. The statistical significance level was p < 0.05, values shown are mean ± standard error. Power analysis calculations were done using StatMate 2 (GraphPad). This was done after completion of the study to get more insight about sample size requirements to observe statistically significant difference between vehicle and drug-treated groups.
Results
Running Distance and Speed
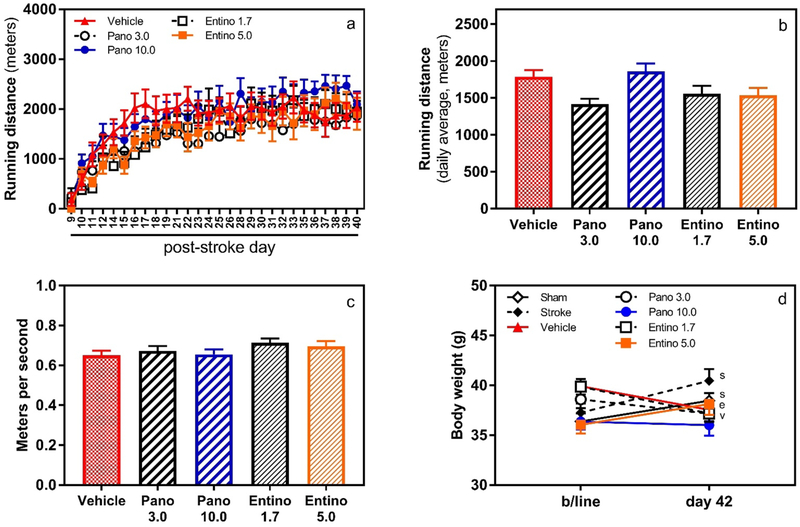
To imitate mild physical rehabilitation, all vehicle and drug-treated mice received access to a running wheel in their home cage for 2 h daily, from post-stroke day 9 to 41. Because of voluntary running, on average it took about 1 week (between post-stroke days 9 and 16) for the animals to gradually increase their covered distance and consistently run 1.5–2 km per session/day (Fig. 2a). No significant difference was observed in the running distance or speed of mice among experimental groups (Fig. 2b and c). Notably, wheel running prevented body weight gain in experimental animals, except 5 mg/kg entinostat group (Fig. 2d). Compared to baseline body weight values, high dose entinostat-treated mice recorded gain of ~ 2 g on post-stroke day 42, similar to “sham” and “stroke” groups.
Fig. 2.
Voluntary running distance and speed. Daily running distance (panel a) and total average running (panel b) of mice in experimental groups. Mice treated with panobinostat and entinostat covered similar distance of running compared to vehicle-treated animals. Panel c, speed of running in experimental groups did not differ significantly during the last three weeks of the study. Panel d, compared to their baseline body weight values, sham and stroke (sp = 0.032 and 0.0016, respectively) and 5 mg/kg entinostat-treated animals (ep = 0.025) gained 2–2.5 g weight by completion of the study. Vehicle and 1.7 mg/kg entinostat-treated animals (vp = 0.014 and ep < 0.001, respectively) lost ~ 2 g during the same period. N = 12/group in all panels; Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat
Motor Function in Grid-Walking Test
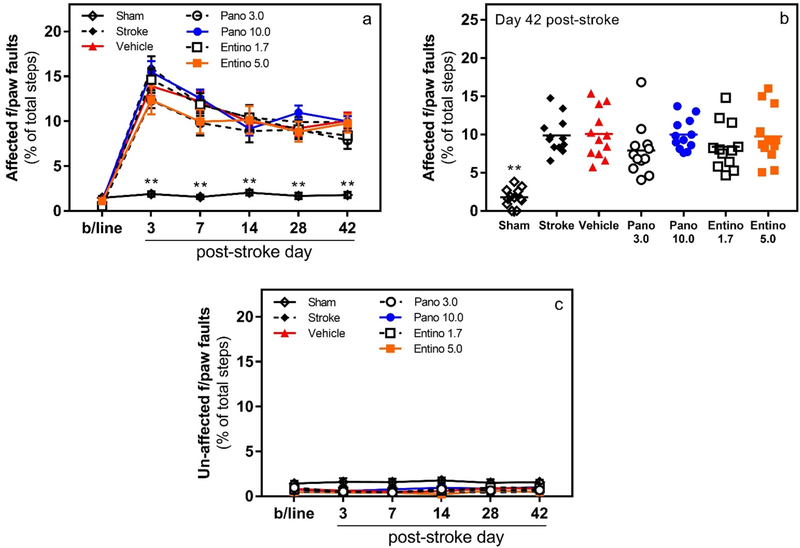
To evaluate the effect of panobinostat or entinostat, in combination with wheel running, on recovery of motor function after stroke, spontaneous motor function of the forelimb during gait was monitored in grid-walking test. As anticipated, focal cerebral stroke caused a significant and sustained deficit in the contralateral, i.e., affected, forelimb function of mice in the grid-walking test (Fig. 3a; n = 12/group; group × day interaction F (30, 385) = 7.523, p < 0.001). Within each stroke-affected experimental group, post hoc analyses with Dunnett’s correction revealed statistically significant differences in the affected forelimb function between baseline and corresponding post-stroke evaluation days (p < 0.001). As expected, in the sham-operated mice, no statistically significant difference was observed in the function of the affected forelimb between baseline and post-stroke evaluation days (p > 0.05). Gradual recovery of the impaired function was observed in all stroke-affected animals between post-stroke days 3 and 14, which, however, plateaued for all groups indicating lack of effects in the drug-treated animals. Within day comparisons of experimental groups (vehicle-treated vs others; post hoc analyses with Dunnett’s correction) showed statistically significant difference of the forelimb function only between vehicle-treated and sham-operated mice on all evaluation days (Fig. 3a and b; p < 0.001) except the baseline (p > 0.05). Lastly, similar comparisons done for the ipsilateral, i.e., unaffected, forelimb did not reveal statistically significant difference between any of the groups on any of the evaluation days (Fig. 3c; p > 0.05).
Fig. 3.
Lack of effect on motor recovery with panobinostat or entinostat treatment in grid-walking test. Panels a and b, following photothrombotic stroke mice treated with different doses of panobinostat or entinostat did not exhibit improvement in the affected forelimb motor function (i.e., number of footfaults; **p < 0.001 for sham vs. vehicle within day comparisons, n = 12/group). Panel c, no functional deficit was observed in the unaffected (i.e., ipsilateral) forepaw of mice in experimental groups (p > 0.05 for all within group and within day comparisons). Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat
Cylinder Test
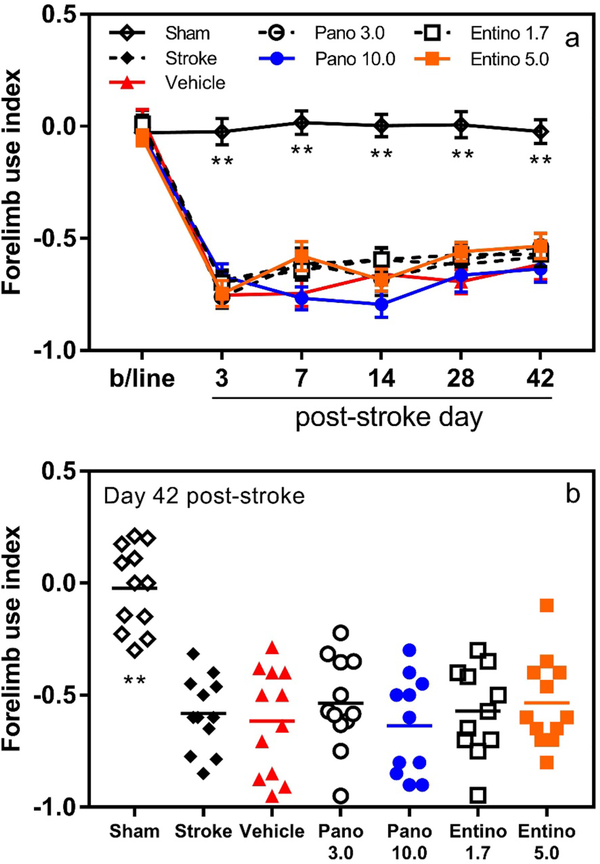
For the same purpose, we used cylinder test to monitor spontaneous motor function of the forelimb during rearing. Similar to observations in the grid-walking test, cylinder test revealed significant deficit in the function of the stroke-affected forelimb after stroke (Fig. 4a; n = 12/group; group × day interaction F (30, 385) = 6.676, p < 0.001). Post hoc analyses with Dunnett’s correction confirmed statistically significant differences for the affected forelimb function between baseline and corresponding post-stroke evaluation days for each stroke-affected experimental group (p < 0.001). As expected, no statistically significant difference was observed in the function of the affected forelimb between baseline and any post-stroke evaluation day within the sham group (p > 0.05). In this test too, gradual recovery of the impaired function was documented in all stroke-affected animals during the first 2 weeks after stroke, which, however, plateaued during the next 3–4 weeks. Within day comparisons between vehicle-treated and other experimental groups (post hoc analyses with Dunnett’s correction) showed statistically significant difference of the forelimb function only between vehicle and sham-operated groups on all evaluation days (p < 0.001) except the baseline (p > 0.05) (Fig. 4a and b).
Fig. 4.
Unaffected motor recovery with panobinostat or entinostat treatment in cylinder test. Panels a and b, the affected forelimb motor function of mice treated with panobinostat or entinostat was similar to that of vehicle-treated animals after photothrombotic stroke (**p < 0.001 for sham vs. vehicle within day comparisons; n = 12/group). Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat
Retrospective Power Analysis
To obtain more insight about the statistical power of the motor functional outcomes, we conducted further analysis of the obtained data and documented ~ 31% and 38% variability (SD/mean) for the vehicle-treated group on day 42 after stroke in grid-walking and cylinder tests, respectively. Similarly, the average variability for drug-treated groups was ~ 33% and 36%. Power analysis of these data revealed that 12 animals per experimental group should have allowed us to detect 40–45% difference between two groups (2-sided α-level of 0.05, 80% power).
Correlation of Parvalbumin-Positive Neurons in AGm with Motor Function
In addition to functional outcomes, we evaluated one molecular-cellular outcome measure of post-stroke motor recovery in experimental animals on day 42 after stroke. For this, the number of parvalbumin-positive neurons in medial premotor area (AGm, medial agranular cortex) of ipsilateral and contralateral hemispheres was evaluated using immunofluorescence labeling. Our observations revealed lack of statistically significant difference in the number of parvalbumin-positive inhibitory interneurons between the ipsilateral and contralateral AGm of the drug-treated mice (Fig. 5). In addition, no substantial difference in the sum of DAPI-stained nuclei was documented within the same subareas of AGm among experimental groups indicating similar number of cells in these regions of interest (Fig. 5).
Infarct Location and Volume
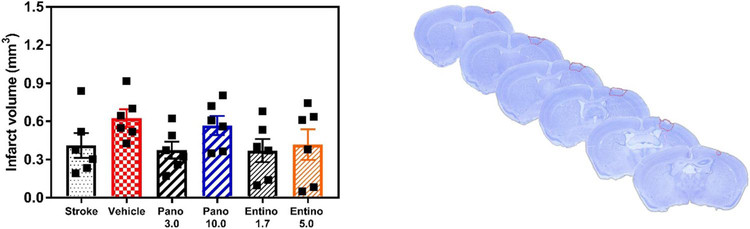
To assess the location of cortical infarction and its volume on day 42 after stroke, PFA-fixed coronal brain sections of all experimental groups were stained with Cresyl Violet, followed by histologic examination and volumetric measurements (Fig. 6). As anticipated, cerebral infarction involved the primary motor cortex in all stroke-affected experimental groups. Volumetric measurements did not reveal statistically significant differences in the infarct volume among the experimental groups (Fig. 6; p > 0.05, 0.37–0.62 mm3 average stroke volume).
Fig. 6.
Infarct location and volume. Left panel, volumetric assessment of brain infarction did not reveal statistically significant differences among experimental groups on day 42 after stroke (n = 6 per group; p > 0.05, vehicle vs. other groups). Right panel, a representative Cresyl Violet-stained mouse brain on day 42 after stroke, indicating location of infarction in the primary motor cortex. Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat
Brain HDAC Inhibition After Peripheral Administration of Panobinostat or Entinostat
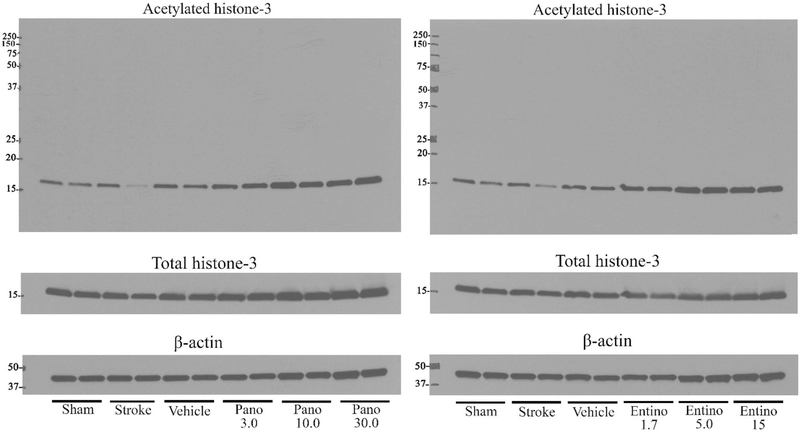
To verify the engagement of the primary pharmacological target in our experimental paradigm, in a separate set of experiments the animals were treated with the same doses of panobinostat or entinostat as described above and the brains were collected on the following day after the last dose to evaluate acetylation status of histone 3 using Western Blotting. Consistent with earlier reports (Ma et al. 2018; Meel et al. 2020), we observed dose-dependent effect of both drugs on acetylation status of histone 3 (Fig. 7 and Table 1), indicating that both drugs penetrate the brain and engage with their pharmacological target after peripheral administration.
Fig. 7.
Inhibition of brain histone deacetylases after peripheral administration of panobinostat or entinostat. Following photothrombotic stroke, mice were treated with panobinostat (3, 10 and 30 mg/kg) or entinostat (1.7, 5 and 15 mg/kg) every other day starting on post-stroke day 5 and one day after the last dosing animals were euthanized to obtain the peri-infarct cortical tissue (post-stroke day 16) for Western Blotting. Left and right panels, representative immunoblotting experiments documenting acetylation status of histone 3 after panobinostat (left panel) or entinostat (right panel) treatments. Pano 3.0 3 mg/kg panobinostat; Pano 10.0 10 mg/kg panobinostat; Pano 30.0 30 mg/kg panobinostat; Entino 1.7 1.7 mg/kg entinostat; Entino 5.0 5.0 mg/kg entinostat; Entino 15.0 15 mg/kg entinostat
Table 1.
Densitometry data of the presented Western Blotting experiments in Fig. 7
| Sham | Stroke | Vehicle | Pano 3.0 | Pano 10.0 | Pano 30.0 |
| 100 ± 4.8 | 74.2 ± 27 | 140 ± 7.3 | 185 ± 11.6 | 212 ± 13 | 217 ± 15.3 |
| Sham | Stroke | Vehicle | Entino 1.7 | Entino 5.0 | Entino 15.0 |
| 100 ± 6.6 | 83.6 ± 20 | 143 ± 6.6 | 208 ± 8.7 | 267 ± 17.8 | 229 ± 8.6 |
Relative values (%) are presented in relation to the signal documented for the sham group
Pano 3.0 3 mg/kg panobinostat, Pano 10.0 10 mg/kg panobinostat, Pano 30.0 30 mg/kg panobinostat, Entino 1.7 1.7 mg/kg entinostat, Entino 5.0 5.0 mg/kg entinostat, Entino 15.0 15 mg/kg entinostat
Discussion
Pharmacological therapy is considered to be one of the most promising approaches to meet the mounting need for treatments promoting post-stroke functional recovery (Carmichael 2016; Alamri et al. 2021). Pharmacotherapy is expected to be an add-on therapy to physical rehabilitation (Kwakkel et al. 2020), because the latter is the standard of care for stroke survivors with physical disabilities (Winstein et al. 2016). Based on this and a number of recent, failed clinical trials, there are increasing calls from experts around the world to include physical rehabilitation, i.e., current standard of care, in the study design of preclinical and clinical drug trials for post-stroke recovery (Cramer 2020; Kwakkel et al. 2020). Importantly, combination of pharmacotherapy with physical rehabilitation is also important for enhancing the effects of lower-intensity physical rehabilitation. This is because most survivors are unable to meet the requirement of high intensity and early rehabilitation after stroke, and therefore have very limited recovery (Nicholson et al. 2013; Krakauer et al. 2012). With this in mind, we designed the current study to evaluate the effect of delayed pharmacological therapy by panobinostat or entinostat, coupled with limited physical rehabilitation, on recovery of motor function in mice after ischemic stroke. Panobinostat and entinostat are pan- and selective Class-I HDAC inhibitors, which are the most potent drugs in their respective class and are used or being studied for various cancers (Connolly et al. 2017; Atadja 2009). Our rationale for focusing on HDAC inhibitors was because of a large body of literature indicating multifactorial signaling pathways in the stroke recovery process that are negatively modulated by HDAC-associated genes (Langley et al. 2009; Fessler et al. 2013).
In the current study, panobinostat or entinostat treatment started on post-stroke day 5, beyond the acute neuroprotective time-window and beginning of subchronic phase, and lasted until day 15 to include the period during which endogenous post-stroke brain plasticity is most active (Krakauer et al. 2012; Carmichael 2016; Alamri et al. 2021). Limited physiotherapy, which involved voluntary wheel running for 2 h daily, 6 days per week in animal’s home cage, started on post-stroke day 9 and lasted until the end of the study. Initiation of wheel running on day 9 after stroke is comparable to the time when most stroke survivors start some degree of regular physical rehabilitation (second week), which, however, is likely delayed and suboptimal (Lay et al. 2016; Fini et al. 2017). The daily average distance ran by mice in this study was 1.5–2 km during the 2 h rehabilitation period with no statistically significant difference between experimental groups (Fig. 2). This distance is fivefold to tenfold shorter compared to what mice run when having overnight access to the wheels (Al-Shoyaib, Karamyan, unpublished observations). Notably, this limited wheel running prevented body weight gain in all experimental groups, except 5 mg/kg entinostat group, which gained ~ 2.0 g over the course of our study, similar to “sham” and “stroke” groups (Fig. 2).
In this study, functional recovery of forelimb motor function was monitored during gait and exploratory rearing using grid-walking and cylinder tests, respectively. Both tests revealed that, with the used therapeutic regimen and doses, neither panobinostat nor entinostat facilitate motor recovery in mice after stroke (Figs. 3 and 4). Importantly, similar to an earlier observation (Alamri et al. 2021), the limited amount of wheel running alone was insufficient to promote motor recovery in mice.
As an additional outcome measure, we evaluated the number of parvalbumin-expressing neurons in the AGm area (medial premotor cortex) of ipsi- and contralateral hemispheres of the experimental animals. Parvalbumin-expressing neurons in AGm are primarily GABAergic inhibitory interneurons, and their reduced excitability and/or number is causally linked with motor learning and functional recovery after stroke (Ng et al. 2015; Alamri et al. 2021; Hakon et al. 2018). Our observations indicate lack of substantial difference in the number of parvalbumin-positive inhibitory interneurons or overall cells between the AGm areas of both hemispheres in drug-treated mice (Fig. 5). These data are in line with the results of grid-walking and cylinder tests indicating lack of functional recovery.
Because of these results, we sought to determine whether panobinostat and entinostat, at used doses and regimen, engage their pharmacological targets in the brain. For this, a separate cohort of animals was treated in the same way as the experimental groups noted above and 1 day after the last drug administration the brains of these animals were collected to measure the acetylation status of histone 3 in peri-infarct cortical tissue by Western Blotting. Our observations indicate dose-dependent effect of both drugs on acetylation status of histone 3 (Fig. 7 and Table 1) and are consistent with earlier reports (Ma et al. 2018; Meel et al. 2020) documenting the ability of both drugs to penetrate the brain and inhibit HDACs.
It is noteworthy, that there are several published experimental studies which evaluated the effects of selective and non-selective HDAC inhibitors in an ischemic stroke setting. The first study published in 2004 (Ren et al. 2004) used valproic acid (non-selective HDAC inhibitor, treatment started immediately after stroke and every 12 h thereafter) in rat transient middle cerebral artery occlusion (tMCAO) model and documented improved neurological function and decreased infarct volume on days 1 and 2 after stroke. Another study tested non-selective HDAC inhibitors valproic acid, sodium butyrate and trichostatin-A (TSA) in permanent MCAO model of stroke where the treatments were carried out immediately and 12 h after stroke, and improved stroke outcomes were documented 24 h later (Kim et al. 2007). This study included another cohort where sodium butyrate was administered immediately after stroke, every 12 h for the next 2 days and once daily thereafter. In this case too, improved stroke outcomes were documented on days 2, 4, 7 and 11 after stroke. Valproic acid was also used after transient global cerebral ischemia in rats, starting immediately after stroke and every 12 h until day 8, and documented improved spatial cognition on post-stroke days 6, 7 and 8 (Xuan et al. 2012). In a chronic treatment regimen with valproic acid, initiated immediately after tMCAO in rats and every 12 h thereafter until 14 days, Wang and colleagues observed significant improvement of motor learning and coordination on post-stroke days 7 and 14 (Wang et al. 2012). However, in a different study using both permanent and transient MCAO models, valproic acid was only able to significantly improve neurological function in mice when administered as pretreatment, i.e., before stroke, but it failed to show benefits when administered immediately and 4 h after stroke (Qian et al. 2010). In another study using the rat tMCAO model, non-selective HDAC inhibitor suberoylanilide hydroxamic acid (SAHA, administered once daily) or selective class-IIa HDAC inhibitor MC1568 (administered once every other day) were used starting from 24 h after stroke until day 7 (Kassis et al. 2016). Motor and neurological function were assessed on post-stroke days 1, 7, 21 and 28 using grid-walking test and modified neurological severity score, and modest but statistically significant improved outcomes were documented with SAHA but not MC1568.
Notably, all of these studies evaluated the neuroprotective potential of HDAC inhibition because the drug treatments were initiated shortly after stroke induction and/or during the acute phase. The only exception is the study by Kassis et al. (2016) where SAHA treatment was initiated at 24 h after stroke. This time point is the threshold of the acute phase in rodent MCAO model of stroke and therefore it likely confirms the neuroprotective potential of these agents. Overall, the results of these experimental studies suggest that HDAC inhibitors have neuroprotective potential in rodent ischemic stroke models; however, they do not answer whether these agents directly promote neurorestorative mechanisms in the post-stroke brain.
Currently, there are only two experimental studies from the one research group that were designed to evaluate the potential of HDAC inhibitors to promote post-stroke recovery (Lin et al. 2017; Tang et al. 2017). These studies evaluated pan-HDAC inhibitors SAHA and TSA, selective class-I HDAC inhibitor MGCD0103 and class-IIa HDAC inhibitor TMP269 in the mouse photothrombotic model of stroke. In one study, all drugs were administered via daily, intra-cortical microinjections at different post-stroke time points (days 2–4 or 5–7 or 8–10) and motor function was assessed in grid-walking and cylinder tests starting 24 h after the last administration of the drug and extended up to 6 weeks after stroke. From these therapeutic time-window regimens, administration of drugs only during post-stroke days 5–7 showed improvement of motor function (Lin et al. 2017). In a parallel study the same investigators reported improvement of motor function in mice (assessed on post-stroke days 11, 18 and 25) after intra-cortical microinjection of pan-HDAC inhibitors SAHA or TSA between post-stroke days 4–11 (Tang et al. 2017). These two stroke recovery studies provide wealth of information about the functional role of HDACs in the post-acute stroke brain and their involvement in modulation of neurorestorative mechanisms. However, when it comes to translational relevance of these observations, two points are noteworthy. One is the intra-cortical administration of the HDAC inhibitors, which is not clinically feasible at the moment. And the second is the need to test these drugs in combination with physical rehabilitation, as deliberated above. Our study addresses these deficiencies but has its own set of limitations. The first limitation is that we did not test other administration regiments (e.g., shorter duration or bigger interval between subsequent doses) and therapeutic time-windows of panobinostat or entinostat, which may lead to different outcomes (Ma et al. 2018; Meel et al. 2020). Another point of consideration is the interaction of physical rehabilitation with HDAC inhibitors on a molecular level (Christie et al. 2008; Llorens-Martin et al. 2008; Elsner et al. 2011), and their combined effect on neuroplasticity and functional recovery after stroke (Kwakkel et al. 2020; Krakauer et al. 2012). Furthermore, our study does not provide information about the effect of panobinostat and entinostat in adult female and older animals (Banerjee et al. 2020; Jackson et al. 2019).
In this context it is also important to recognize that there are key differences between the MCAO and photothrombotic models of stroke in rodents. The former is the model of choice for neuroprotection studies since it usually yields to large subcortical and cortical strokes in the result of blood flow interruption in the middle cerebral artery (MCA). The MCA is targeted in this model because in humans obstruction of blood flow in the MCA occurs more often than in any other brain artery resulting in stroke (Fluri et al. 2015). The main disadvantages of the MCAO model is that it is quite invasive and leads to large strokes which often are equivalent of malignant strokes in humans. Because of these, mortality of animals in this model is high and it is very difficult to conduct chronic studies. On the contrary, the photothrombotic model is the least invasive stroke model with permanent occlusion, which results in a small stroke that can be targeted at the primary motor cortex leading to long-lasting motor deficit, and is the model of choice for stroke recovery studies (Corbett et al. 2017). The main disadvantage of this model is that brain infarction occurs because of simultaneous clot formation in multiple small blood vessels and capillaries, due to photoactivation of Rose Bengal dye and endothelial cell injury, rather than in one large artery as is the case in humans and the MCAO model. Whether these differences could have contributed in the outcomes of the present and previous studies is also something to take into consideration; however, most likely, the key difference lies between the neuroprotective vs. neurorestorative potential of HDAC inhibitors in a stroke setting.
In summary, this study is the first to directly evaluate and compare the dose-dependent effect of delayed panobinostat or entinostat therapy, in combination with limited physical rehabilitation, on recovery of motor function in mice after ischemic stroke. Our results indicate that these HDAC inhibitors do not facilitate motor recovery during the chronic phase of stroke, and hence, it is unlikely that add-on panobinostat or entinostat therapy could offer therapeutic modality for stroke survivors who have difficulty to engage in early, high-intensity physiotherapy.
Acknowledgements
This work was partly supported by a NIH research Grant (1R01NS106879).
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Research Involving Animals The research conducted in this study has been approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee (Protocol # 16019).
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al Shoyaib A, Archie SR, & Karamyan VT (2019). Intraperitoneal route of drug administration: Should it be used in experimental animal studies? Pharmaceutical Research, 37(1), 12. 10.1007/s11095-019-2745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamri FF, Shoyaib AA, Biggers A, Jayaraman S, Guindon J, & Karamyan VT (2018). Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behavioural Brain Research, 336, 250–255. 10.1016/j.bbr.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Alamri FF, Al Shoyaib A, Syeara N, Paul A, Jayaraman S, Karamyan ST, et al. (2021). Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke. Neural Regeneration Research, 16(7), 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atadja P (2009). Development of the pan-DAC inhibitor panobinostat (LBH589): Successes and challenges. Cancer Letters, 280(2), 233–241. 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Chokkalla AK, Shi JJ, Lee J, Venna VR, Vemuganti R, et al. (2020). Microarray profiling reveals distinct circulating miRNAs in aged male and female mice subjected to post-stroke social isolation. Neuromolecular Medicine. 10.1007/s12017-020-08622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta A, Tzeng YC, & Clarkson AN (2014). Post-stroke recovery: The role of activity-dependent release of brain-derived neurotrophic factor. Expert Review of Neurotherapeutics, 14(11), 1335–1344. 10.1586/14737175.2014.969242. [DOI] [PubMed] [Google Scholar]
- Carmichael ST (2016). Emergent properties of neural repair: Elemental biology to therapeutic concepts. Annals of Neurology, 79(6), 895–906. 10.1002/ana.24653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Zang XF, Pan J, Zhu XL, Chen F, Chen ZB, et al. (2012). Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clinical and Experimental Pharmacology and Physiology, 39(9), 751–758. 10.1111/j.1440-1681.2012.05729.x. [DOI] [PubMed] [Google Scholar]
- Choi SA, Lee C, Kwak PA, Park CK, Wang KC, Phi JH, et al. (2019). Histone deacetylase inhibitor panobinostat potentiates the anti-cancer effects of mesenchymal stem cell-based sTRAIL gene therapy against malignant glioma. Cancer Letters, 442, 161–169. 10.1016/j.canlet.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Chopra V, Quinti L, Khanna P, Paganetti P, Kuhn R, Young AB, et al. (2016). LBH589, a hydroxamic acid-derived HDAC inhibitor, is neuroprotective in mouse models of Huntington’s disease. Journal of Huntington’s Disease, 5(4), 347–355. 10.3233/jhd-160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, & Titterness AK (2008). Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Medicine, 10(2), 47–58. 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, & Carmichael ST (2010). Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Nature, 468(7321), 305–309. 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly RM, Rudek MA, & Piekarz R (2017). Entinostat: A promising treatment option for patients with advanced breast cancer. Future Oncology, 13(13), 1137–1148. 10.2217/fon-2016-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, et al. (2017). Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable translational working group. International Journal of Stroke: Official Journal of the International Stroke Society, 12(5), 462–471. 10.1177/1747493017711814. [DOI] [PubMed] [Google Scholar]
- Cramer SC (2020). Issues important to the design of stroke recovery trials. The Lancet Neurology, 19(3), 197–198. 10.1016/s1474-4422(20)30030-2. [DOI] [PubMed] [Google Scholar]
- Dietz KC, & Casaccia P (2010). HDAC inhibitors and neurodegeneration: At the edge between protection and damage. Pharmacological Research, 62(1), 11–17. 10.1016/j.phrs.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner VR, Lovatel GA, Bertoldi K, Vanzella C, Santos FM, Spindler C, et al. (2011). Effect of different exercise protocols on histone acetyltransferases and histone deacetylases activities in rat hippocampus. Neuroscience, 192, 580–587. 10.1016/j.neuroscience.2011.06.066. [DOI] [PubMed] [Google Scholar]
- Fessler EB, Chibane FL, Wang Z, & Chuang DM (2013). Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery. Current Pharmaceutical Design, 19(28), 5105–5120. 10.2174/1381612811319280009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini NA, Holland AE, Keating J, Simek J, & Bernhardt J (2017). How physically active are people following stroke? Systematic review and quantitative synthesis. Physical Therapy, 97(7), 707–717. 10.1093/ptj/pzx038. [DOI] [PubMed] [Google Scholar]
- Fluri F, Schuhmann MK, & Kleinschnitz C (2015). Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy, 9, 3445–3454. 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakon J, Quattromani MJ, Sjolund C, Tomasevic G, Carey L, Lee JM, et al. (2018). Multisensory stimulation improves functional recovery and resting-state functional connectivity in the mouse brain after stroke. Neuroimage Clinical, 17, 717–730. 10.1016/j.nicl.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennika T, Hu G, Olaciregui NG, Barton KL, Ehteda A, Chitranjan A, et al. (2017). Pre-clinical study of panobinostat in xenograft and genetically engineered murine diffuse intrinsic pontine glioma models. PLoS One, 12(1), e0169485. 10.1371/journal.pone.0169485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, & Anrather J (2011). Stroke research at a crossroad: Asking the brain for directions [Research Support, N.I.H., Extramural]. Nature Neuroscience, 14(11), 1363–1368. 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L, Li W, Abdul Y, Dong G, Baban B, & Ergul A (2019). Diabetic stroke promotes a sexually dimorphic expansion of T cells. Neuromolecular Medicine, 21(4), 445–453. 10.1007/s12017-019-08554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S, Al Shoyaib A, Kocot J, Villalba H, Alamri FF, Rashid M, et al. (2020). Peptidase neurolysin functions to preserve the brain after ischemic stroke in male mice. Journal of Neurochemistry, 153(1), 120–137. 10.1111/jnc.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamyan VT (2021). The role of peptidase neurolysin in neuroprotection and neural repair after stroke. Neural Regeneration Research, 16(1), 21–25. 10.4103/1673-5374.284904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis H, Shehadah A, Chopp M, & Zhang ZG (2017). Epigenetics in stroke recovery. Genes (Basel), 8(3), 89. 10.3390/genes8030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis H, Shehadah A, Li C, Zhang Y, Cui Y, Roberts C, et al. (2016). Class IIa histone deacetylases affect neuronal remodeling and functional outcome after stroke. Neurochemistry International, 96, 24–31. 10.1016/j.neuint.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong J-S, Chen P-S, & Chuang D-M (2007). Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. Journal of Pharmacology and Experimental Therapeutics, 321(3), 892–901. 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, & Wittenberg GF (2012). Getting neurorehabilitation right: What can be learned from animal models? Neurorehabilitation and Neural Repair, 26(8), 923–931. 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Meskers C, & Ward NS (2020). Time for the next stage of stroke recovery trials. The Lancet Neurology, 19(8), 636–637. 10.1016/S1474-4422(20)30218-0. [DOI] [PubMed] [Google Scholar]
- Langley B, Brochier C, & Rivieccio MA (2009). Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke, 40(8), 2899–2905. 10.1161/strokeaha.108.540229. [DOI] [PubMed] [Google Scholar]
- Lay S, Bernhardt J, West T, Churilov L, Dart A, Hayes K, et al. (2016). Is early rehabilitation a myth? Physical inactivity in the first week after myocardial infarction and stroke. Disability and Rehabilitation, 38(15), 1493–1499. 10.3109/09638288.2015.1106598. [DOI] [PubMed] [Google Scholar]
- Leng T, & Xiong ZG (2019). Treatment for ischemic stroke: From thrombolysis to thrombectomy and remaining challenges. Brain Circulation, 5(1), 8–11. 10.4103/bc.bc_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Dong J, Tang Y, Ni HY, Zhang Y, Su P, et al. (2017). Opening a new time window for treatment of stroke by targeting HDAC2. Journal of Neuroscience, 37(28), 6712–6728. 10.1523/JNEUROSCI.0341-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, & Trejo JL (2008). Growth factors as mediators of exercise actions on the brain. Neuromolecular Medicine, 10(2), 99–107. 10.1007/s12017-008-8026-1. [DOI] [PubMed] [Google Scholar]
- Ma K, Qin L, Matas E, Duffney LJ, Liu A, & Yan Z (2018). Histone deacetylase inhibitor MS-275 restores social and synaptic function in a Shank3-deficient mouse model of autism. Neuropsychopharmacology, 43(8), 1779–1788. 10.1038/s41386-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (2003). Excitotoxic and excitoprotective mechanisms: Abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Medicine, 3(2), 65–94. 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- Meel MH, de Gooijer MC, Metselaar DS, Sewing ACP, Zwaan K, Waranecki P, et al. (2020). Combined therapy of AXL and HDAC inhibition reverses mesenchymal transition in diffuse intrinsic pontine glioma. Clinical Cancer Research, 26(13), 3319–3332. 10.1158/1078-0432.Ccr-19-3538. [DOI] [PubMed] [Google Scholar]
- Ng KL, Gibson EM, Hubbard R, Yang J, Caffo B, O’Brien RJ, et al. (2015). Fluoxetine maintains a state of heightened responsiveness to motor training early after stroke in a mouse model. Stroke, 46(10), 2951–2960. 10.1161/STROKEAHA.115.010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S, Sniehotta FF, van Wijck F, Greig CA, Johnston M, McMurdo ME, et al. (2013). A systematic review of perceived barriers and motivators to physical activity after stroke. International Journal of Stroke, 8(5), 357–364. 10.1111/j.1747-4949.2012.00880.x. [DOI] [PubMed] [Google Scholar]
- Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, et al. (2013). Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models.” Science, 340(6135), 924. 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Qian YR, Lee M-J, Hwang S, Kook JH, Kim J-K, & Bae CS (2010). Neuroprotection by valproic acid in mouse models of permanent and transient focal cerebral ischemia. The Korean Journal of Physiology and Pharmacology, 14(6), 435–440. 10.4196/kjpp.2010.14.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M, Wangler NJ, Yang L, Shah K, Arumugam TV, Abbruscato TJ, et al. (2014). Functional up-regulation of endopeptidase neurolysin during post-acute and early recovery phases of experimental stroke in mouse brain. Journal of Neurochemistry, 129(1), 179–189. 10.1111/jnc.12513. [DOI] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, & Chuang DM (2004). Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: Potential roles of histone deacetylase inhibition and heat shock protein induction. Journal of Neurochemistry, 89(6), 1358–1367. 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Schaffer DV, & Gage FH (2004). Neurogenesis and neuroadaptation. Neuromolecular Medicine, 5(1), 1–9. 10.1385/NMM:5:1:001. [DOI] [PubMed] [Google Scholar]
- Selvaraj UM, Poinsatte K, Torres V, Ortega SB, & Stowe AM (2016). Heterogeneity of B cell functions in stroke-related risk, prevention, injury, and repair. Neurotherapeutics, 13(4), 729–747. 10.1007/s13311-016-0460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N (2015). Epigenetic regulation of memory by acetylation and methylation of chromatin: Implications in neurological disorders, aging, and addiction. Neuromolecular Medicine, 17(2), 97–110. 10.1007/s12017-014-8306-x. [DOI] [PubMed] [Google Scholar]
- Skolarus LE, Freedman VA, Feng C, Wing JJ, & Burke JF (2016). Care received by elderly US stroke survivors may be underestimated. Stroke, 47(8), 2090–2095. 10.1161/STROKEAHA.116.012704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeara N, Alamri FF, Jayaraman S, Lee P, Karamyan ST, Arumugam TV, et al. (2020). Motor deficit in the mouse ferric chloride-induced distal middle cerebral artery occlusion model of stroke. Behavioural Brain Research, 380, 112418. 10.1016/j.bbr.2019.112418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Lin YH, Ni HY, Dong J, Yuan HJ, Zhang Y, et al. (2017). Inhibiting histone deacetylase 2 (HDAC2) promotes functional recovery from stroke. Journal of the American Heart Association, 6(10), e007236. 10.1161/JAHA.117.007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan M, Alamri FF, Al Shoyaib A, Karamyan VT, & Reddy PH (2019). Novel miRNA PC-5P-12969 in ischemic stroke. Molecular Neurobiology, 56(10), 6976–6985. 10.1007/s12035-019-1562-x. [DOI] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. (2020). Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation, 141(9), e139–e596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, & Wang Y (2020). Burden of stroke in Europe: Thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke, 51(8), 2418–2427. 10.1161/STROKEAHA.120.029606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, et al. (2012). Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke, 43(9), 2430–2436. 10.1161/strokeaha.112.652545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangler NJ, Santos KL, Schadock I, Hagen FK, Escher E, Bader M, et al. (2012). Identification of membrane-bound variant of metalloendopeptidase neurolysin (EC 3.4.24.16) as the nonangiotensin type 1 (Non-AT1), non-AT2 angiotensin binding site. Journal of Biological Chemistry, 287(1), 114–122. 10.1074/jbc.M111.273052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. (2016). Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 47(6), e98–e169. 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Xuan A, Long D, Li J, Ji W, Hong L, Zhang M, et al. (2012). Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sciences, 90(11–12), 463–468. 10.1016/j.lfs.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O’Brien RJ, et al. (2013). Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke, 44(2), 483–489. 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, & Schluesener HJ (2013). Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. Journal of Neuropathology and Experimental Neurology, 72(3), 178–185. 10.1097/NEN.0b013e318283114a. [DOI] [PubMed] [Google Scholar]