Abstract
Ceriporia lacerata (CL) is a species of white rot fungi. In this study, we have examined the beneficial effect of CL on scopolamine-induced memory impairment in mice. A freeze-dried CL mycelial culture broth was dissolved and orally administered to scopolamine-treated C57BL/6J mice followed by behavioral tests using the Y-maze, passive avoidance, and Morris water maze tasks. CL administration at a daily dose of 200 mg/kg body weight resulted in restoration of exploration reduction and improvement of associative and spatial learning and memory impairment in scopolamine-treated mice. Concomitantly, heme oxygenase-1 was highly expressed in the hippocampal region of CL-administered mice. Moreover, the ethanolic extract of CL significantly increased the transcriptional activity of antioxidant response element and attenuated the glutamate-induced cytotoxicity in HT22 mouse hippocampal neuronal cells. These findings suggest that the CL intake can confer a beneficial effect on learning and memory presumably through protecting hippocampal neuronal cells from oxidative stress-induced damage.
Supplementary information
The online version contains supplementary material available at 10.1007/s10068-021-00945-5.
Keywords: Ceriporia lacerata, Neuroprotection, Learning and memory, Hippocampal neuron, Heme oxygenase-1
Introduction
Learning and memory decline are both linked to normal aging and neurodegenerative disorders (Coyle and Puttfarcken, 1993; Finkel and Holbrook, 2000; Morris et al., 2006). It is generally accepted that oxidative stress is the major cause of the cognitive deficits associated with aging (Dey et al., 2017; Ghadrdoost et al., 2011; Logan et al., 2019; Pedersen et al., 2001; Yehuda et al., 2002). In this context, natural substances that upregulate cellular antioxidant/defense enzymes, and thus promote neuronal cell survival in the brain, have been studied from diverse sources, such as from a variety of vegetables and medicinal plants or their secondary metabolites (Dey et al., 2017; Dinkova-Kostova et al., 2018; Kanninen et al., 2009; Seo et al., 2016).
The health-promoting benefits of certain mushrooms and fungi are well-documented (Guo et al., 2012; Islam et al., 2019; Ma et al., 2018; Murcia et al., 2002). Bioactive components found in edible fungi include essential amino acids, vitamins, minerals, polysaccharides, and phenolic compounds (Heleno et al., 2015; Kozarski et al., 2014; Ma et al., 2018). In particular, some fungal extracts were reported to have neuroprotective effects while their active components have antioxidative properties (Islam et al., 2019; Lee et al., 2002; Phan et al., 2015; Salinaro et al., 2018; Wang et al., 2012).
Ceriporia is a genus of Basidiomycetes, which are wood-degrading fungi that cause white-rot (Jang et al., 2012). Among more than 30 species belonging to this genus, Ceriporia lacerata (CL) was first isolated from rotten wood in Japan and characterized as a filamentous fungus that produces white to buff, and dense nonsporulating colonies (Suhara et al., 2003). Over the last decade, CL has been studied from multiple perspectives, including its clinical significance as a possible human pathogen (Chowdhary et al., 2013, 2014; Singh et al., 2013a) and has an environmentally significant potential because of its wood-degrading, metal-adsorbing, and water-decolorizing capabilities (Lee et al., 2007; Li et al., 2015; Lin et al., 2011; Singh et al., 2013b; Wang et al., 2017). Recent studies have demonstrated that a submerged CL culture extract has the hyperglycemic effect in streptozotocin-induced diabetic rats (Kim et al., 2012, 2013). In recognition of its biological effectiveness, CL has been permitted as a health functional food ingredient by the Korean Ministry of Food and Drug Safety in 2018. In addition, a couple of studies revealed that CL contains certain bioactive flavonoids and sesquiterpenoids (Wang et al., 2013; Ying et al., 2013) which can strongly activate an nuclear factor E2-related factor 2 (Nrf2), a key regulator of cellular antioxidant response.
Herein, for the first time, we report the protective effect of a freeze-dried culture broth of CL mycelia against oxidative stress-induced cognitive impairment in mice. Learning and memory damage was induced by intraperitoneal injection of scopolamine which antagonizes muscarinic acetylcholine receptor, elevates reactive oxygen species in multiple regions of the brain (Budzynska et al., 2015; El-Khadragy et al., 2014; El-Sherbiny et al., 2003; Fan et al., 2005; Skalicka-Wozniak et al., 2018; Venkatesan et al., 2016), and thus gives rise to memory loss in animal models. In this study, mice were orally provided with the freeze-dried CL powder two weeks before scopolamine treatment and examined for their learning and memory abilities.
Materials and methods
Preparation of a freeze-dried culture broth of C. lacerata mycelia and its ethanolic extract
The mycelial culture of CL was harvested, freeze-dried, and ground to a fine powder by Fugenbio Co., Ltd. (Seoul, South Korea) and generously provided for a research purpose. For the in vivo study, powdered CL was dissolved in a vehicle containing 5% (v/v) Tween® 80 (Sigma-Aldrich, St. Louis, MO, USA) in sterilized saline (sodium chloride solution, Sigma-Aldrich) and administered to the mice. For in vitro examinations, powdered CL was extracted using various concentrations of ethanol (0%, 20%, 40%, 60%, 80%, and 100% (v/v) in deionized distilled water) at 25 °C with shaking (250 rpm) for 16 h. The extracts were filtered, rotary-evaporated, freeze-dried, and stored at –20 °C until used.
Measurement of free radical scavenging activities
The free radical scavenging activity of each extract was assessed by their capability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) or 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals as previously reported (Kedare and Singh, 2011; Kim et al., 2010). Vitamin C was used as a positive control.
Cell culture
A murine hippocampal neuronal cell line, HT22, was obtained from the Salk Institute (La Jolla, CA, USA) and grown as previously described (Oh and Lee, 2017). Briefly, the cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Welgene, Gyeongsan, South Korea) supplemented with 10% fetal bovine serum (FBS; Gibco™/Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin–streptomycin (Welgene), and maintained in a humidified CO2 incubator (Sanyo, Osaka, Japan). Cells were subcultured by trypsinizing at about 70% confluency with 0.05% trypsin-ethylene diamine tetraacetic acid solution (Welgene).
The HT22 cells that were transfected with antioxidant response element (ARE)-conjugated luciferase reporter plasmid (referred to as HT22-ARE cells) were maintained as previously described (Seo et al., 2016) and were used for the ARE-luciferase reporter assay.
Cell viability assay
HT22 cells were plated in a 96-well culture plate in the maintenance medium at a density of 5 × 103 cells per well. Cells were then treated with 5 mM glutamate in the absence and presence of CL extracts for 24 h. Subsequently, the relative cell viability was measured using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) as previously described (Oh et al., 2015). Cell viability was presented as the relative survival rate to the untreated control.
Animal experiment
The animal study was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Kyungpook National University (approval number: KNU-2019-0097).
Forty-eight C57BL/6J male mice (5-week old) were purchased from Daehan Biolink (Seoul, South Korea). Mice were maintained under a standard laboratory condition (temperature of 22 ± 2 °C, humidity 50 ± 5%, 12 h light–dark cycle with free access to tap water and chow). After a week of adaptation, mice with a body weight (BW) of 20 ± 2 g were subjected to behavioral tests, including the Y-maze, passive avoidance, and Morris water maze tasks as previously described (Kim et al., 2019; Seo et al., 2016).
A total of 48 mice were randomly assigned into six groups (8 mice per group). Each group was subjected to one of the following treatments: (1) oral administration of a vehicle only and no scopolamine injected; (2) oral administration with a vehicle and i.p. injection of scopolamine at 1 mg/kg BW/day; (3) oral administration of donepezil (Sigma-Aldrich) at 5 mg/kg BW/day and i.p. injection of scopolamine; (4) oral administration of CL at 200 mg/kg BW/day and i.p. injection of scopolamine; (5) oral administration of CL at 500 mg/kg BW/day and i.p. injection of scopolamine; or (6) oral administration of CL at 800 mg/kg BW/day and i.p. injection of scopolamine.
Mice were received the treatment every day for two weeks. Donepezil and freeze-dried CL powder were dissolved in a vehicle consisting of 5% (v/v) Tween-80 in isotonic saline and orally administered using an oral gavage needle. Mice were given either CL preparation or donepezil 1 h prior to performing a daily behavioral task. Scopolamine was intraperitoneally injected 30 min before the test session (Fig. S1A).
Fecal materials that were either present in the rectum or were excreted were collected from mice administered with dissolved CL preparation only at different dosages for two weeks and pooled in each group for further assay. The microbial DNA was extracted from the fecal samples using a FastDNA Spin kit for Soil (MP Biomedicals; Irvine, CA, USA) according to the manufacturer’s instructions. The fecal microbiota composition was analyzed using 16S ribosomal RNA gene sequencing which was conducted at ChunLab, Inc. (Seoul, South Korea) using an Illumina MiSeq sequencing system (San Diego, CA, USA).
Western blot analysis
Hippocampal tissues were removed from the sacrificed mice and homogenated for western blot analysis to detect the relative protein quantities of cytoplasmic heme oxygenase-1 (HO-1) and nuclear Nrf2 (Kim et al., 2019; Seo et al., 2016; Woo et al., 2017). The primary antibodies used in this study were rabbit anti-HO-1 (Abcam, Cambridge, UK), mouse anti-β-actin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit anti-Nrf2 (Abcam), and mouse anti-Lamin B (Santa Cruz Biotechnology). The secondary antibodies were anti-rabbit immunoglobulin G (IgG) or anti-mouse IgG (Santa Cruz Biotechnology) conjugated to horseradish peroxidase.
Statistical analysis
Significant differences among data sets were evaluated by one-way analysis of variance with Duncan's multiple range post hoc test using SPSS software (SPSS Inc., Chicago, IL, USA). Statistical differences were considered significant when the p-value was less than 0.05. Values are presented as means ± standard deviation (SD). Significant differences among means are indicated by different alphabetical letters.
Results and discussion
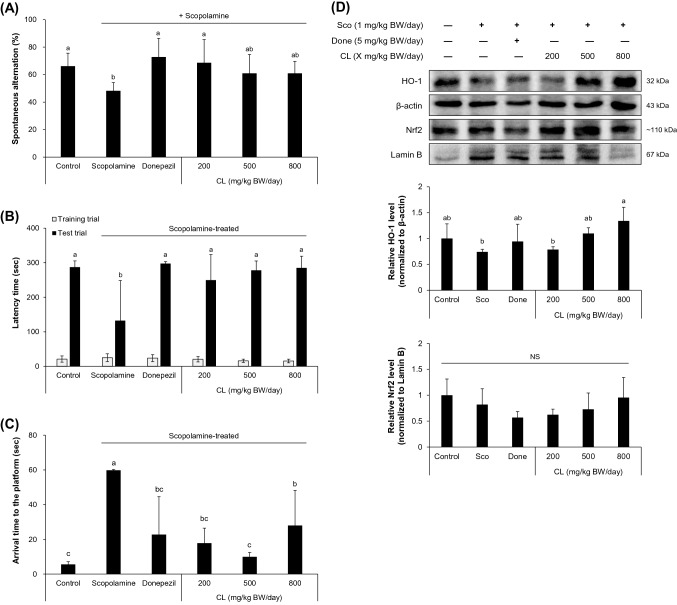
C57BL/6J mice were treated with powdered CL (at 200, 500, or 800 mg/kg BW/day) or donepezil (at 5 mg/kg BW/day, positive control) by oral administration on a daily basis for two weeks. Learning and memory damage was induced by intraperitoneal injection of scopolamine to mice. Scopolamine is a muscarinic cholinergic receptor antagonist that has been widely used to induce memory impairments in animal models (Ebert and Kirch, 1998; Klinkenberg and Blokland, 2010). Multiple studies have demonstrated that scopolamine treatment can produce reactive oxygen species, lower the activities of antioxidant enzymes, and induce lipid peroxidation, and thereby increase oxidative stress in the cerebral cortex and hippocampus in mice (Budzynska et al., 2015; El-Khadragy et al., 2014; El-Sherbiny et al., 2003; Fan et al., 2005; Skalicka-Wozniak et al., 2018; Venkatesan et al., 2016). In the present study, mice that received scopolamine treatment decreased spontaneous alternation in the Y-maze test (Fig. 1A), decreased latency time by staying in a bright chamber in the passive avoidance test (Fig. 1B), and increased the arrival time to the platform in the Morris water maze test (Fig. 1C). These findings suggest that scopolamine treatment induced impairment of working memory and also affected associative and spatial learning and memory in mice.
Fig. 1.
CL administration improved scopolamine-induced learning and memory impairment. Freeze-dried CL powder was dissolved in a vehicle (5% (v/v) Tween 80 in sterilized saline) and orally administered to C57BL/6J mice at designated doses. Scopolamine was intraperitoneally injected at 1 mg/kg BW/day 30 min after CL administration to induce memory impairment. (A–C) Memory improvement was assessed by the Y-maze task (A), passive avoidance task (B), and Morris water maze task (C). Values presented are means ± standard deviation (SD) from eight individual animals (n = 8) for (A) and n = 6 after the removal of outliers for (B–C). (D) Cytoplasmic HO-1 and nuclear Nrf2 protein levels were examined in the hippocampal tissue homogenates. Donepezil (5 mg/kg BW/day) was used as positive control. Sco, scopolamine; Done, donepezil; CL, Ceriporia lacerata; HO-1, heme oxygenase-1; Nrf2, nuclear factor E2-related factor 2. Values are means ± SD (n = 5). Different letters on the bars (a–b) denote statistically significant differences (p < 0.05). NS indicates no statistically significant difference among experimental groups
Donepezil is one of four US FDA-approved acetylcholinesterase (AChE) inhibitors and is commonly used for the management of Alzheimer’s disease (Shin et al., 2018; Zemek et al., 2014). Multiple studies have revealed that donepezil also causes suppression of oxidative stress in the brain (Saxena et al., 2008; Umukoro et al., 2014). Thus, we used donepezil as a positive control in a scopolamine-induced memory deficit mouse model. The behavioral tests demonstrated that the oral administration of powdered CL, even at the lowest dose, restored working memory performance (Fig. 1A), increased associative learning in the passive avoidance test (Fig. 1B), and enhanced spatial learning and memory in the Morris water maze test (Fig. 1C), with no significant differences in BW change among the experimental groups (Fig. S1B). The attenuation of scopolamine-induced cognitive impairment by powdered CL administration was comparable to the efficacy of donepezil, although a dose-dependent relationship was not clearly observed.
The results also implied that the effect of oral CL administration was likely related to the protection and/or restoration of hippocampal damage induced by scopolamine treatment. To address this possibility, hippocampal tissues were collected from mice after finishing behavioral examinations and analyzed for the expression levels of Nrf2 and HO-1 antioxidant proteins. Western blot analysis showed that the oral administration of powdered CL significantly increased the expression of cytoplasmic HO-1 with a slight but insignificant increase in nuclear Nrf2 level in the hippocampi of scopolamine-treated mice (Fig. 1D). This observation suggests that powdered CL intake may have a significant impact on HO-1-associated antioxidant and defense response in the hippocampal region and can result in subsequent improvements in terms of cognition-related behavioral performance.
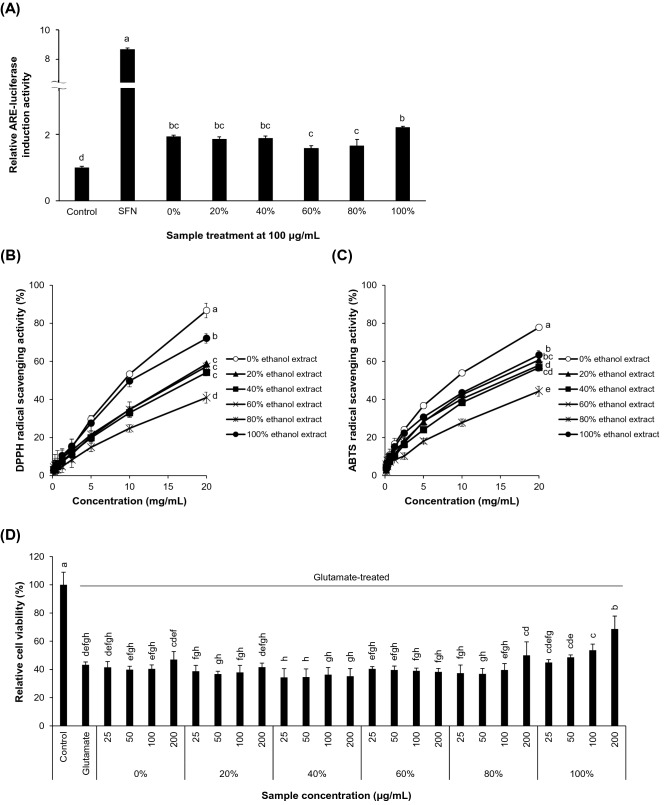
Nrf2 is a redox-regulating transcription factor that activates cellular antioxidant response, induces various antioxidant enzymes, and thus protects cells from intrinsic or extrinsic oxidative stressors (Itoh et al., 1997; Kensler et al., 2007). Considering the pro-oxidative feature of scopolamine in the mammalian brain (El-Sherbiny et al., 2003; Fan et al., 2005), it was conceivable that the alleviation of cognitive impairment by the intake of CL powder could be due to the restoration of the redox balance by antioxidant enzymes, including HO-1, in the hippocampal area. This theory was supported by observation from the ARE-luciferase reporter assay using the mouse hippocampal neuronal HT22 cells (Fig. 2A). Treatment with CL ethanol extracts resulted in an approximately two-fold activation of ARE where Nrf2 binds to initiate transcription of a group of genes involved in cellular antioxidant/defense response. In particular, 100% ethanol extract of CL was more potent for inducing ARE-luciferase activity compared to other extracts.
Fig. 2.
CL extracts possessed antioxidant and neuroprotective capabilities. (A) HT22-ARE cells were treated with CL extracts in various concentrations of ethanol (0%, 20%, 40%, 60%, 80%, and 100% (v/v) in water) at 100 µg/mL for 24 h. The activation of ARE was measured using a luciferase reporter assay. SFN, sulforaphane. (B − C) Radical scavenging capability was determined by DPPH (B) and ABTS+ assays (C). (D) HT22 cells were treated with the extracts of CL in various concentrations of ethanol at designated concentrations for 24 h followed by the treatment with 5 mM glutamate for an additional 24 h. Cell viability was measured by a CCK-8 assay. Values are presented as means ± SD (n = 3). Statistically significant differences are indicated by different alphabetical letters (p < 0.05)
The antioxidant effect of 100% ethanol extract of CL was emphasized by its radical scavenging capabilities in a concentration-dependent manner (Fig. 2B–C) and through its cytoprotective effect against glutamate-induced cytotoxicity in HT22 cells (Fig. 2D). Glutamate, an excitatory neurotransmitter, can cause excitotoxicity and oxidative stress (Behl et al., 1997; Sagara and Schubert, 1998; Tobaben et al., 2011). As HT22 cells do not express functional ionotropic glutamate receptors (He et al., 2019), the neurotoxicity of glutamate in HT22 cells is presumably attributed to oxidative stress (Fukui et al., 2009). Thus, the results demonstrated that the CL ethanol extract possessed radical scavenging and ARE-activating activities. It suggests that CL extract can activate Nrf2/ARE/HO-1 axis and subsequently lower the intracellular oxidative stress in hippocampal neurons. However, the detailed molecular mechanisms of action and active compounds need to be elucidated.
Interestingly, CL extract obtained from extraction with pure water exhibited the highest radical scavenging capability and significant ARE-inducing activity, despite of little protective effect against glutamate-induced HT22 cytotoxicity. In addition, our unpublished data showed that donepezil, at a concentration of 10 µg/mL, inhibited the AChE activity by about 65% and CL water extract at 200 µg/mL inhibited it by about 18%, while other extracts failed to affect its activity (data not shown). This implies that water extract may contain antioxidative and AChE inhibitory components. Thus, further studies to clarify these active components are warranted.
Certain flavonoids (such as dimethylchalcone) and tremulane sesquiterpenoids (such as ceriponols) were isolated from CL and determined to be potent bioactive components (Shan et al., 2012; Ying et al., 2013). However, the biological functions of these flavonoids and sesquiterpenoids in vivo are yet to be investigated.
Based on our current understanding of the interrelationship between cognitive function and gut microbial community (Magnusson et al., 2015; Mahowald et al., 2009; Proctor et al., 2017), we examined microbial composition in the excreted and rectal fecal samples obtained from powdered CL-administered mice. Consistent with published reports (Kang et al., 2014; Liu et al., 2019), it was found that three Phyla, including Bacteroidetes, Firmicutes, and Proteobacteria, were dominant (Fig. S2) in both rectal and excreted feces. However, excreted feces contained these Phyla at a concentration of about 38-fold higher than in rectal feces (data not shown). The identified bacterial counts were about three-fold higher than in rectal feces (Fig. 3A), resulting in a higher Chao 1 index compared to rectal feces (Fig. 3B). These findings indicated that the majority of microbiota were excreted. Specifically, the proportions of the major Phyla were comparable between the two fecal samples (Fig. S2A–B). The Simpson and Shannon indices revealed that the diversity of microbial composition was slightly, but insignificantly, increased by administration of freeze-dried CL preparation (Fig. 3C–D).
Fig. 3.
CL administration caused alteration of fecal microbiota compositions. Excreted or rectal feces were collected from the mice administered with powdered CL only at the designated doses. The fecal microbiota compositions were analyzed through 16S ribosomal RNA gene sequencing. (A) The number of microbial species in the fecal samples was identified. OTUs, operational taxonomic units. (B − D) Microbial diversity are presented as Shannon’s index (B), Simpson’s index (C), and Chao1 index (D). (E) The ratio of Firmicutes to Bacteroidetes was calculated. Con, control; CL, Ceriporia Lacerata. Values are presented as means ± SD from duplicated experimental batches. Statistically comparison was separately performed for the two fecal sample groups. Statistically significant differences are indicated by different alphabetical letters above each bar (p < 0.05). Capital case (A–B) and lower case (a–b) denote a statistical difference from each other within a set of rectal and excreted fecal sample groups, respectively
Moreover, powdered CL administration considerably reduced Bacteroidetes and increased Firmicutes in the excreted feces, compared to the controls, while it rarely affected the abundance of Phyla in rectal feces (Figs. 3E and S2C–D). According to numerous reports, the ratio of Firmicutes to Bacteroidetes (F/B ratio) is linked to various health conditions, including obesity, diabetes, inflammation, and cognitive dysfunction (Liu et al., 2019; Magnusson et al., 2015; Mariat et al., 2009). Considering that the intake of energy nutrients, such as fat and sucrose, can increase the F/B ratio in fecal microbial composition (Kang et al., 2014; Magnusson et al., 2015), it is speculated that the elevated F/B ratio in excreted fecal samples from CL-administered mice compared to the control group may be attributed to the extra supply of energy and nutrients contained in the CL preparation. In addition, multiple studies have shown that a decrease in the F/B ratio in intestinal microbiota is associated with aging, cognitive decline, and Alzheimer’s disease (Liu et al., 2019; Mariat et al., 2009; Saji et al., 2019; Vogt et al., 2017).
Interestingly, powdered CL administration significantly increased the richness, diversity, and F/B ratio from excreted fecal microbiota; however, rectal microbiota exhibited only a subtle alteration in the control group. The microbiome in excreted feces is solely and partially correlated with that found in the gut mucosa and also the indigenous gut microbiome resists to reset their colonization (Suez et al., 2018; Zmora et al., 2018). In this context, powdered CL intake was unlikely to have a substantial impact on gut microbial community. Thus, the cognitive-improving effect of CL powder would presumably not be associated with specific changes in gut microbial composition and diversity. Further systemic and metabolomic analyses may aid in understanding how CL administration leads to cognitive enhancement.
In conclusion, the oral administration of a freeze-dried preparation of CL mycelial culture improved cognitive function in scopolamine-induced learning and memory impairment in a mouse model, and its extract exerted neuroprotective effects on hippocampal-derived neuronal cells. Further studies are required for identifying the CL-derived bioactive components responsible for cognitive enhancement.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure S1. (A) Animal experimental scheme. (B) Body weight changes of mice during the whole experimental period. Supplementary Figure S2. The relative abundance of microbiota analyzed from rectal and excreted fecal samples acquired from powdered CL-administered mice. (A–B) The proportion of three major microbial phyla in rectal feces (A) and excreted feces (B) obtained from control mice. (C–D) The relative abundance of the three phyla in rectal feces (C) and excreted feces (D) from CL-administered mice at a dose of 200, 500 or 800 mg/kg BW/day, as opposed to control mice. Statistically significant differences are indicated by an asterisk in comparison to the control group within a set of phyla (p < 0.05).. Supplementary file 1 (PDF 467 kb)
Acknowledgements
This research was funded by the “Food Functionality Evaluation program” under the Ministry of Agriculture, Food, and Rural Affairs and partly by the Korea Food Research Institute (2019), Republic of Korea.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sujin Lee, Email: drg725@naver.com.
Ji Sun Lim, Email: lzsunny@daum.net.
Hyun Seok Yun, Email: tkdldjstm011@naver.com.
Yoonsu Kim, Email: yunsu531@gmail.com.
Soojung Jeong, Email: okok9625@naver.com.
Seong Deok Hwang, Email: sd.hwang@fugenbio.co.kr.
Jong Won Kim, Email: jongwon.kim@fugenbio.com.
Jisun Oh, Email: j.oh@knu.ac.kr.
Jong-Sang Kim, Email: vision@knu.ac.kr.
References
- Behl C, LezoualcH F, Trapp T, Widmann M, Skutella T, Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology. 2015;232:931–942. doi: 10.1007/s00213-014-3728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Agarwal K, Kathuria S, Singh PK, Roy P, Gaur SN, de Hoog GS, Meis JF. Clinical significance of filamentous basidiomycetes illustrated by isolates of the novel opportunist Ceriporia lacerata from the human respiratory tract. Journal of Clinical Microbiology. 2013;51:585–590. doi: 10.1128/JCM.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Kathuria S, Agarwal K, Meis JF. Recognizing filamentous basidiomycetes as agents of human disease: A review. Medical Mycology. 2014;52:782–797. doi: 10.1093/mmy/myu047. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dey A, Bhattacharya R, Mukherjee A, Pandey DK. Natural products against Alzheimer's disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnology Advances. 2017;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Kostov RV, Kazantsev AG. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS Journal. 2018;285:3576–3590. doi: 10.1111/febs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U, Kirch W. Review - Scopolamine model of dementia: electroencephalogram findings and cognitive performance. European Journal of Clinical Investigation. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- El-Khadragy MF, Al-Olayan EM, Abdel Moneim AE. Neuroprotective effects of Citrus reticulata in scopolamine-induced dementia oxidative stress in rats. CNS & Neurological Disorders Drug Targets. 2014;13:684–690. doi: 10.2174/1871527313666140618105404. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary Eel D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacology, Biochemistry and Behavior. 2003;76:525–533. doi: 10.1016/j.pbb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neuroscience Letters. 2005;374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. European Journal of Pharmacology. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. European Journal of Pharmacology. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Guo YJ, Deng GF, Xu XR, Wu S, Li S, Xia EQ, Li F, Chen F, Ling WH, Li HB. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food & Function. 2012;3:1195–1205. doi: 10.1039/c2fo30110e. [DOI] [PubMed] [Google Scholar]
- He JT, Xu L, Yang L, Sun CX. Anti-oxidative effects of catechins and theaflavins on glutamate-induced HT22 cell damage. RSC Advances. 2019;9:21418–21428. doi: 10.1039/C9RA02721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleno SA, Martins A, Queiroz MJRP, Ferreira ICFR. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chemistry. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- Islam T, Ganesan K, Xu BJ. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. International Journal of Medicinal Mushrooms. 2019;21:237–251. doi: 10.1615/IntJMedMushrooms.2019030079. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2 small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jang Y, Choi HE, Lim YW, Lee JS, Kim JJ. The first report of Ceriporia lacerata (Phanerochaetaceae, Basidiomycota) in Korea. Mycotaxon. 2012;119:397–403. doi: 10.5248/119.397. [DOI] [Google Scholar]
- Kang SS, Jeraldo PR, Kurti A, Miller MEB, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, Fryer JD. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Molecular Neurodegeneration. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yla-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. Journal of Food Science and Technology. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayash N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Suh HJ, Kim JH, Park S, Joo YC, Kim JS. Antioxidant activity of glyceollins derived from soybean elicited with Aspergillus sojae. Journal of Agricultural and Food Chemistry. 2010;58:11633–11638. doi: 10.1021/jf102829z. [DOI] [PubMed] [Google Scholar]
- Kim JE, Kim HJ, Lee SP. Hyperglycemic effect of submerged culture extract of Ceriporia lacerata in streptozotocin-induced diabetic rats. Food Science and Biotechnology. 2012;21:1685–1693. doi: 10.1007/s10068-012-0224-9. [DOI] [Google Scholar]
- Kim JH, Park YK, Kim JE, Lee SP, Kim BC, Jang BC. Crude extract of Ceriporia lacerata has a protective effect on dexamethasone-induced cytotoxicity in INS-1 cells via the modulation of PI3K/PKB activity. International Journal of Molecular Medicine. 2013;32:179–186. doi: 10.3892/ijmm.2013.1364. [DOI] [PubMed] [Google Scholar]
- Kim S, Oh J, Jang CH, Kim JS. Improvement of cognitive function by Gochujang supplemented with tomato paste in a mouse model. Food Science and Biotechnology. 2019;28:1225–1233. doi: 10.1007/s10068-019-00565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neuroscience and Biobehavioral Reviews. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kozarski MS, Klaus AS, Niksic MP, Van Griensven LJLD, Vrvic MM, Jakovljevic DM. Polysaccharides of higher fungi: biological role, structure and antioxidative activity. Hemijska Industrija. 2014;68:305–320. doi: 10.2298/HEMIND121114056K. [DOI] [Google Scholar]
- Lee IK, Yun BS, Han G, Cho DH, Kim YH, Yoo ID. Dictyoquinazols A, B, and C, new neuroprotective compounds from the mushroom Dictyophora indusiata. Journal of Natural Products. 2002;65:1769–1772. doi: 10.1021/np020163w. [DOI] [PubMed] [Google Scholar]
- Lee JW, Gwak KS, Park JY, Park MJ, Choi DH, Kwon M, Choi IG. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. Journal of Microbiology. 2007;45:485–491. [PubMed] [Google Scholar]
- Li XN, Li AR, Long MZ, Tian XJ. Equilibrium and kinetic studies of copper biosorption by dead Ceriporia lacerata biomass isolated from the litter of an invasive plant in China. Journal of Environmental Health Science and Engineering. 2015;13:37. doi: 10.1186/s40201-015-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, He XB, Han GM, Tian QJ, Hu WY. Removal of crystal violet from aqueous solution using powdered mycelial biomass of Ceriporia lacerata P2. Journal of Environmental Sciences-China. 2011;23:2055–2062. doi: 10.1016/S1001-0742(10)60643-2. [DOI] [PubMed] [Google Scholar]
- Liu P, Wu L, Peng GP, Han YQ, Tang RQ, Ge JP, Zhang LJ, Jia LF, Yue SQ, Zhou K, Li LJ, Luo BY, Wang BH. Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behavior and Immunity. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Logan S, Royce GH, Owen D, Farley J, Ranjo-Bishop M, Sonntag WE, Deepa SS. Accelerated decline in cognition in a mouse model of increased oxidative stress. Geroscience. 2019;41:591–607. doi: 10.1007/s11357-019-00105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma GX, Yang WJ, Zhao LY, Pei F, Fang DL, Hu QH. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Science and Human Wellness. 2018;7:125–133. doi: 10.1016/j.fshw.2018.05.002. [DOI] [Google Scholar]
- Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R, Perrone A, Bermudez LE. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. 2015;300:128–140. doi: 10.1016/j.neuroscience.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, Cantarel BL, Coutinho PM, Henrissat B, Crock LW, Russell A, Verberkmoes NC, Hettich RL, Gordon JI. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariat D, Firmesse O, Levenez F, Guimaraes VD, Sokol H, Dore J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiology. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia MA, Martinez-Tome M, Jimenez AM, Vera AM, Honrubia M, Parras P. Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. Journal of Food Protection. 2002;65:1614–1622. doi: 10.4315/0362-028X-65.10.1614. [DOI] [PubMed] [Google Scholar]
- Oh J, Jeon SB, Lee Y, Lee H, Kim J, Kwon BR, Yu KY, Cha JD, Hwang SM, Choi KM, Jeong YS. Fermented red ginseng extract inhibits cancer cell proliferation and viability. Journal of Medicinal Food. 2015;18:421–428. doi: 10.1089/jmf.2014.3248. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee NK. Schizandrin reduces cytoplasmic TDP-43 accumulation in hippocampal neuronal cells. Biotechnology and Bioprocess Engineering. 2017;22:9–13. doi: 10.1007/s12257-016-0656-9. [DOI] [Google Scholar]
- Pedersen WA, Wan RQ, Mattson MP. Impact of aging on stress-responsive neuroendocrine systems. Mechanisms of Ageing and Development. 2001;122:963–983. doi: 10.1016/S0047-6374(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Phan CW, David P, Naidu M, Wong KH, Sabaratnam V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: diversity, metabolite, and mechanism. Critical Reviews in Biotechnology. 2015;35:355–368. doi: 10.3109/07388551.2014.887649. [DOI] [PubMed] [Google Scholar]
- Proctor C, Thiennimitr P, Chattipakorn N, Chattipakorn SC. Diet, gut microbiota and cognition. Metabolic Brain Disease. 2017;32:1–17. doi: 10.1007/s11011-016-9917-8. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Schubert D. The activation of metabotropic glutamate receptors protects nerve cells from oxidative stress. Journal of Neuroscience. 1998;18:6662–6671. doi: 10.1523/JNEUROSCI.18-17-06662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Toba K, Sakurai T. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Scientific Reports. 2019;9:1008. doi: 10.1038/s41598-018-38218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinaro AT, Pennisi M, Di Paola R, Scuto M, Crupi R, Cambria MT, Ontario ML, Tomasello M, Uva M, Maiolino L, Calabrese EJ, Cuzzocrea S, Calabrese V. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer's disease and Alzheimer-linked pathologies: modulation by nutritional mushrooms. Immunity & Ageing. 2018;15:8. doi: 10.1186/s12979-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. European Journal of Pharmacology. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Seo JY, Ju SH, Oh J, Lee SK, Kim JS. Neuroprotective and cognition-enhancing effects of compound K isolated from red ginseng. Journal of Agricultural and Food Chemistry. 2016;64:2855–2864. doi: 10.1021/acs.jafc.5b05789. [DOI] [PubMed] [Google Scholar]
- Shan WG, Liang DE, Ying YM, Zhan ZJ. Two new tremulane sesquiterpenoids from Ceriporia lacerate, an endophytic fungus of Huperzia serrata. Journal of Chemical Research. 2012;36:365–366. doi: 10.3184/174751912X13361273882991. [DOI] [Google Scholar]
- Shin CY, Kim HS, Cha KH, Won DH, Lee JY, Jang SW, Sohn UD. The effects of donepezil, an acetylcholinesterase inhibitor, on impaired learning and memory in rodents. Biomolecules & Therapeutics. 2018;26:274–281. doi: 10.4062/biomolther.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Kathuria S, Agarwal K, Gaur SN, Meis JF, Chowdhary A. Clinical significance and molecular characterization of nonsporulating molds isolated from the respiratory tracts of bronchopulmonary mycosis patients with special reference to basidiomycetes. Journal of Clinical Microbiology. 2013;51:3331–3337. doi: 10.1128/JCM.01486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Doshi A, Pancholy A, Pathak R. Biodiversity in wood-decay macro-fungi associated with declining arid zone trees of India as revealed by nuclear rDNA analysis. European Journal of Plant Pathology. 2013;136:373–382. doi: 10.1007/s10658-013-0172-0. [DOI] [Google Scholar]
- Skalicka-Wozniak K, Budzynska B, Biala G, Boguszewska-Czubara A. Scopolamine-induced memory impairment is alleviated by xanthotoxin: Role of acetylcholinesterase and oxidative stress processes. ACS Chemical Neuroscience. 2018;9:1184–1194. doi: 10.1021/acschemneuro.8b00011. [DOI] [PubMed] [Google Scholar]
- Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Brik RB, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–1423. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- Suhara H, Maekawa N, Kaneko S, Hattori T, Sakai K, Kondo R. A new species, Ceriporia lacerata, isolated from white-rotted wood. Mycotaxon. 2003;86:335–347. [Google Scholar]
- Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death and Differentiation. 2011;18:282–292. doi: 10.1038/cdd.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umukoro S, Adewole FA, Eduviere AT, Aderibigbe AO, Onwuchekwa C. Free radical scavenging effect of donepezil as the possible contribution to its memory enhancing activity in mice. Drug Research. 2014;64:236–239. doi: 10.1055/s-0033-1357126. [DOI] [PubMed] [Google Scholar]
- Venkatesan R, Subedi L, Yeo EJ, Kim SY. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochemistry International. 2016;99:133–146. doi: 10.1016/j.neuint.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Gut microbiome alterations in Alzheimer's disease. Scientific Reports. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu YM, Cao W, Yao KW, Liu ZQ, Guo JY. Anti-inflammation and antioxidant effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metabolic Brain Disease. 2012;27:159–165. doi: 10.1007/s11011-012-9282-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Yao LY, Lu YH. Ceriporia lacerata DMC1106, a new endophytic fungus: Isolation, identification, and optimal medium for 2',4'-dihydroxy-6'-methoxy-3',5'-dimethylchalcone production. Biotechnology and Bioprocess Engineering. 2013;18:669–678. doi: 10.1007/s12257-012-0846-z. [DOI] [Google Scholar]
- Wang N, Chu YL, Wu FA, Zhao ZL, Xu XY. Decolorization and degradation of Congo red by a newly isolated white rot fungus, Ceriporia lacerata, from decayed mulberry branches. International Biodeterioration & Biodegradation. 2017;117:236–244. doi: 10.1016/j.ibiod.2016.12.015. [DOI] [Google Scholar]
- Woo Y, Oh J, Kim JS. Suppression of Nrf2 activity by chestnut leaf extract increases chemosensitivity of breast cancer stem cells to paclitaxel. Nutrients. 2017;9:760. doi: 10.3390/nu9070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiology of Aging. 2002;23:843–853. doi: 10.1016/S0197-4580(02)00074-X. [DOI] [PubMed] [Google Scholar]
- Ying YM, Shan WG, Zhang LW, Zhan ZJ. Ceriponols A-K, tremulane sesquitepenes from Ceriporia lacerate HS-ZJUT-C13A, a fungal endophyte of Huperzia serrata. Phytochemistry. 2013;95:360–367. doi: 10.1016/j.phytochem.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, Kuca K. Outcomes of Alzheimer's disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opinion on Drug Safety. 2014;13:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB, Federici S, Cohen Y, Linevsky R, Rothschild D, Moor AE, Ben-Moshe S, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Shapiro H, Pevsner-Fischer M, Sharon I, Halpern Z, Segal E, Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174:1388–1405. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. (A) Animal experimental scheme. (B) Body weight changes of mice during the whole experimental period. Supplementary Figure S2. The relative abundance of microbiota analyzed from rectal and excreted fecal samples acquired from powdered CL-administered mice. (A–B) The proportion of three major microbial phyla in rectal feces (A) and excreted feces (B) obtained from control mice. (C–D) The relative abundance of the three phyla in rectal feces (C) and excreted feces (D) from CL-administered mice at a dose of 200, 500 or 800 mg/kg BW/day, as opposed to control mice. Statistically significant differences are indicated by an asterisk in comparison to the control group within a set of phyla (p < 0.05).. Supplementary file 1 (PDF 467 kb)