Abstract
Free radical accumulation in the body will cause oxidative stress damages including the renal damage. Schisandrae Sphenantherae Fructus (Schisandra), a traditional Chinese herbal medicine, has been used throughout the world. Anwulignan, a monomer extracted from Schisandra, has been shown in our previous studies to possess antioxidant and protective effects on the liver, brain and spleen damages in the aging mice. However, its effect on the renal damage caused by aging is not clear. This study showed that anwulignan could significantly increase the kidney index, the creatinine clearance, the activities of superoxide dismutase, catalase and glutathione peroxidase; reduce the urinary protein concentration, the serum urea nitrogen and creatinine content, the content of malondialdehyde and 8-hydroxylated deoxyguanosine in the renal tissue; and improve the renal tissue damage. Moreover, anwulignan increased the production of Nrf2, HO-1 and NQO1 proteins and decreased the production level of Keap1 protein in the renal tissue in the d-galactose induced aging mice. These results suggest that anwulignan significantly alleviates the renal damage by its antioxidant effect through regulating the production of Nrf2/ARE pathway-related proteins in the renal tissue in the d-galactose induced aging mice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-021-00951-7.
Keywords: Anwulignan, Aging, Kidney, Oxidative stress, Nrf2
Introduction
The aging population is increasing in the world (Chen and Huang, 2019; Yang et al., 2019; Zeng and Li, 2019). In the aging process, a gradual accumulation of free radicals will cause the oxidative stress damages to the functions and structures of the cells, tissues, organs and body. These consequences, in turn, lead to further loss of antioxidant capacity and aging aggravation (Denham, 1956; Gerschman et al. 1954). Kidney is one of the important target organs of aging and then undergoing the atrophy, tissue structure changes and function decline, which seriously affect the quality of life of the elderly people (Gao et al., 2019). Therefore, researchers have paid more and more attentions to the development of the anti-aging drugs and health foods.
Schisandrae Sphenantherae Fructus (Schisandra) is the mature dry fruit of Schisandra sphenanthera Rehd. et Wils. Ge Hong, an ancient Chinese scholar in Jin Dynasty, described in the book of Baopuzi-Internal Chapter that “Yimenzi has taken Schisandra for 16 years and his skin color looked like one of a teenage girl”, indicating that Schisandra should have the remarkable anti-aging effect (Ge, 2019). Modern pharmacological studies show that lignans and polysaccharides are the main active ingredients of Schisandra. Because of their antioxidant and anti-aging effects, they are often used as ideal raw materials for health foods (Chen et al., 2018a; Chen et al., 2018b; Liu et al., 2018; Yang et al., 2018). Anwulignan is a unique component among the lignan monomer compounds from Schisandra. Our previous studies showed that anwulignan had a strong antioxidant capacity both in vitro and in vivo, and a significant protective effect on the liver, brain and spleen in aging mice induced by d-galactose (d-gal) (Li et al., 2018; Yu et al., 2019). To wonder whether anwulignan has a protective effect against the renal damage in aging mice, in this study, d-gal induced aging mice were used to observe the effect, and Nrf2/ARE antioxidant pathway was taken as the target to explore its related mechanism, so as to provide an experimental evidence for the development of anwulignan as an ingredient of anti-aging drugs or health foods.
Materials and methods
Materials and instruments
Clean-grade and healthy ICR male mice, weighing 18 ± 2 g, were provided by Changchun Yisi Experimental Animal Technology Co., Ltd (Changchun, China). The mice were raised in separate cages and kept for 12 h:12 h day and night cycle, with a free access to food and water. All animal experiments were approved by Ethical Committee of Beihua University (Jilin, China). All procedures were conducted in accordance with the guidelines for the care and use of laboratory animals (China).
Anwulignan was purchased from Chengdu Pufeide Biotechnology Co., Ltd. (Chengdu, China). d-gal was purchased from Sigma Reagent Co., Ltd. (St. Louis, MO USA). Saline was purchased from Jilin Cornell Pharmaceutical Co., Ltd. (Jilin, China). Sodium carboxymethylcellulose was purchased from Shandong Weifang Lite Composite Material Co., Ltd. (Shandong, China). Skimmed milk powder was purchased from BD company (New York, NJ, USA). Protein lysis buffer was purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China). SOD, MDA, CAT, 8-OH-DG, GSH-Px, BUN, CRE and urine protein quantitative kits were purchased from Nanjing Jiancheng Biotechnology Co., Ltd. (Nanjing, China). Nrf2, HO-1, NQO1, Keap1 and HRP Goat anti rabbit LGG (H + L) were purchased from ABclonal Company (Boston,Massachusetts, USA). PVDF membrane was purchased from Amersham Hybond Company (New York; NJ, USA). Full-automatic enzyme labeling instrument was purchased from Swiss Tech Company (Männedorf, Switzerland). EPS 300 electrophoresis system was purchased from Tanon Co., Ltd. (Shanghai, China). Western blot electrophoresis apparatus was purchased from Bio-Rad Company (Hercules, CA, USA).
Animal grouping and administration
Mice were randomly divided into 5 groups: control group (CON, sodium carboxymethylcellulose solution by gavage, saline by subcutaneous injection at the back of the neck), model group (MOD, sodium carboxymethylcellulose solution by gavage, 220 mg/kg d-gal by subcutaneous injection at the back the neck), and low-, medium- and high-dose of anwulignan groups (1, 2 and 4 mg/kg anwulignan by gavage, respectively, 220 mg/kg d-gal by subcutaneous injection at the back of the neck. The mice were administered with the corresponding agents once a day for 42 days.
The urine of mice in 24-h was collected separately one day before the end of the experiment for the detection of the urinary protein. The blood samples were collected by removing eyeballs from the mice under anesthetized with ether 30 min after the last administration of anwulignan and were centrifuged at 4 °C to obtain the supernatant. The kidneys of mice were removed and weighed. The kidneys of three mice randomly selected from each group were soaked in formalin solution, with replaced once daily successively for 3 days. The pathological changes of the kidneys were analysed after HE staining.The kidney injury score was assessed by determining the tubular atrophy and degeneration, pyelonephrosis, renal papillary necrosis, interstitial inflammation, and fibrous hyperplasia according to the scoring criteria described previously(Debelle et al., 2002; Gu et al., 2019a; Gu et al., 2019b) (0 = none, 1 = 10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%).The remaining kidneys were stored in a − 80 refrigerator for the measurement of antioxidant indicators and proteins.
Measurement of kidney index
The kidney index was calculated according to the following Eq. 1.
| 1 |
Examination of renal functions
Thirty minutes after the last administration of anwulignan, the mice were anesthetized with ether and their blood samples were taken. The blood samples were centrifuged at 3100×g for 10 min, and the supernatants were collected for the determination of BUN (blood urea nitrogen) and CRE (creatinine). Twenty four-hour urine of mice was used to detect protein, CRE contents, and to calculate Ccr (creatinine clearance rate) according to the following Eq. 2.
| 2 |
All the renal function indexes were detected according to the instructions of the kits.
Detection of renal antioxidant-related indicators
After the kidney tissue homogenate was prepared with 1:9 saline, the activity of SOD (superoxide dismutase), CAT (catalase), GSH-Px (glutathione peroxidase), 8-OHdG (8-hydroxylated deoxyguanosine) and the content of MDA (malondialdehyde) in the supernatant were determined according to the instructions of the kits.
Detection of renal Nrf2/ARE pathway-related protein production
The kidney tissue was added with the lysis buffer for the preparation of the kidney tissue homogenate with 1:9 buffer, and then centrifuged at 9300×g for 40 min at 4 °C. The supernatant was used to detect the protein content by Bradford method. The sample was loaded on 10% SDS-PAGE gel and separated at 120 V for 120 min by a Bio-Rad Bole protein electrophoresis apparatus (USA). Then, the electrophoresis gel was transferred onto a PVDF membrane. The PVDF membrane was immersed in the blocking buffer containing 5% skimmed milk powder and shaken by a low-speed shaker at room temperature for 2 h. The membrane was incubated with the first antibodies at 4 °C overnight, and then washed with TBST and incubated with the second antibody HRP-Goat anti-rabbit IgG (H + L) (the ratio of the second antibody to TBST was 1:5000). The specific antibodies used included Nrf2 (nuclear factor E2 related factor 2), HO-1 (heme oxygenase-1), Keap1 (Kelch like ECH associated protein 1), NQO1 (quinone redox enzyme antibody) (The ratio of the second antibody to TBST was 1:1000), and GAPDH (The ratio of the second antibody to TBST was 1:50,000), and the western blot was scanned and quantified with ImageJ software.
Statistical analysis
The data from the different groups were expressed as mean ± SD (standard deviation). Analysis of variance and statistical analysis were carried out by using multiple comparison test (One-way ANOVA). p < 0.05 was considered statistically significant.
Results and discussion
Results
Effects of anwulignan on the kidney index and histopathology
Kidney index can directly reflect the state of kidney. The results (Fig. 1A) showed that the kidney index in MOD group was significantly lower than that in CON group (p < 0.01), and compared with that in MOD group, the kidney index of mice was significantly higher in anwulignan-treated groups (p < 0.05, p < 0.01), suggesting that anwulignan can significantly increase the kidney index of d-gal-induced aging mice.
Fig. 1.
Effects of anwulignan on the kidney index and histopathology in mice. Note A Effect of anwulignan on the kidney index (mean ± SD, n = 10), *p < 0.05; **p < 0.01; B Effect of anwulignan on the kidney injury score. C Effect of anwulignan on the renal histopathology (HE100X and 400X). Abbreviations: CON, blank control group; MOD, model group. Black arrow: glomeruli; Green arrow: capillary lumen; Red arrow: epithelial cell of renal tubules
In order to evaluate the effect of anwulignan on the histopathology of kidney tissue of aging mice induced by d-gal, the pathological changes of kidney tissue under a light microscope in HE (Hematoxylin–Eosin) staining. As shown in Fig. 1B, in CON group, the kidney tissue was stained evenly, the glomeruli were in a round shape, the cells in the glomeruli were arranged orderly, the renal tubules were arranged orderly, the epithelial cells in the proximal convoluted tubules were in a high columnar shape, in MOD group, the volume of glomeruli decreased, the renal tubules were arranged regularly, the proximal convoluted tubules became shorter and cubic, and the epithelial cells of distal convoluted tubules were still in a cubic shape, indicating that the glomeruli in MOD group were atrophic, anwulignan, alleviated the changes of glomeruli and renal tubules obviously, the glomerulus volume of mice was close to normal, the exudates in the renal capsule cavity disappeared, and the epithelial cells in the proximal convoluted tubules were nearly to normal in 4 mg/kg anwulignan group, suggesting that anwulignan could alleviate the renal damage.
The kidney injury showed that, in comparison to that of CON group (0.1 ± 0.5), the renal tissue damage score of D-gal group was significantly higher (2.8 ± 0.1, p < 0.01, n = 10), whereas, those of 2 mg/kg and 4 mg/kg anwulignan groups were lower (2.1 ± 0.2, p < 0.05; and 1.8 ± 0.2, p < 0.01, respectively). These results were consistent with the pathological changes of the renal tissue.
Effects of anwulignan on renal functions
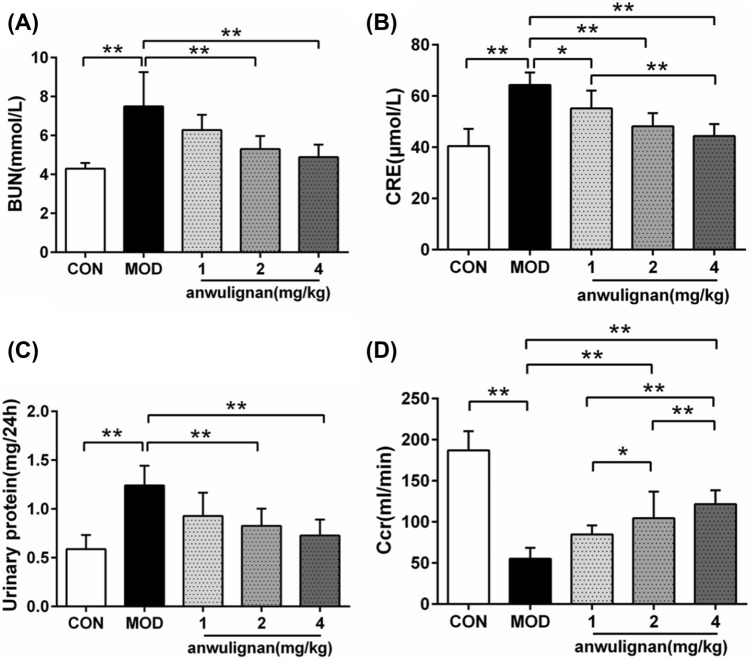
CRE, BUN, urinary protein and Ccr are classic indexes used to evaluate the renal function and damage. As shown in Fig. 2, 220 mg/kg of d-gal could significantly increase the serum CRE (from 40.46 ± 6.70 μmol/L to 64.33 ± 4.85 μmol/L) (p < 0.01), BUN (from 4.29 ± 0.29 mmol/L to 7.45 ± 1.75 mmol/L) (p < 0.01) and urinary protein (from 0.59 ± 0.15 mg/24 h to 1.24 ± 0.2 mg/24 h) (p < 0.01), and reduce the value of Ccr (from 0.065 ± 0.008 to 0.019 ± 0.005 ml/min) (p < 0.01); Anwulignan, significantly improved the above renal function indexes in 2 and 4 mg/kg (p < 0.01), and decreased the serum CRE level in 1 mg/kg significantly (p < 0.05). The serum CRE level of mice in the 4 mg/kg group was significantly lower than that in the 2 mg/kg group (p < 0.01), and the serum Ccr of mice in the three anwulignan-treated groups increased with the dose increase (p < 0.01), suggesting that anwulignan could significantly dose-dependently improve the renal function in d-gal-induced aging mice.
Fig. 2.
Effects of anwulignan on renal functions in d-gal-induced aging mice. Note A Effect of anwulignan on the serum BUN; B Effect of anwulignan on the serum CRE; C Effect of anwulignan on the urinary protein; D Effect of anwulignan on the Ccr. Mean ± SD, n = 10. *p < 0.05; **p < 0.01. Abbreviations: CON, blank control group; MOD, model group
Effects of anwulignan on antioxidation-related indicators in the kidney tissue
In order to evaluate the effect of anwulignan on the antioxidative indicators of the kidney in D-gal-induced aging mice, the activities of SOD, CAT, GSH-Px and the contents of MDA and 8-OHdG in the renal tissue of mice were detected. The results are shown in Fig. 3. Compared with those in CON group, the activities of SOD, CAT and GSH-Px decreased significantly (p < 0.01), and the levels of MDA and 8-OHdG increased significantly (p < 0.01) in MOD group; anwulignan at 1 mg/kg could significantly increase the activity of CAT, and reduce the content of MDA and 8-OHdG (p < 0.01, p < 0.05), while at 2 and 4 mg/kg, anwulignan could significantly improve all the above indexes (p < 0.01, p < 0.05). These data indicate that anwulignan could significantly increase the activity of antioxidant enzymes and reduce the content of lipid peroxidation products in the renal tissue of d-gal-induced aging mice to alleviate the oxidative damage.
Fig. 3.
Effects of anwulignan on antixidation-related indexes in the kidney tissue of mice. Note A Effect of anwulignan on the activity of SOD; B Effect of anwulignan on the activity of GSH-Px; C Effect of anwulignan on the activity of CAT; D Effect of anwulignan on the content of MDA; E Effect of anwulignan on the content of 8-OH-dG. Mean ± SD, n = 10. *p < 0.05, **p < 0.01. Abbreviations: CON, blank control group; MOD, model group
Effects of anwulignan on the production of Nrf2/ARE signaling pathway-related proteins in the kidney tissue
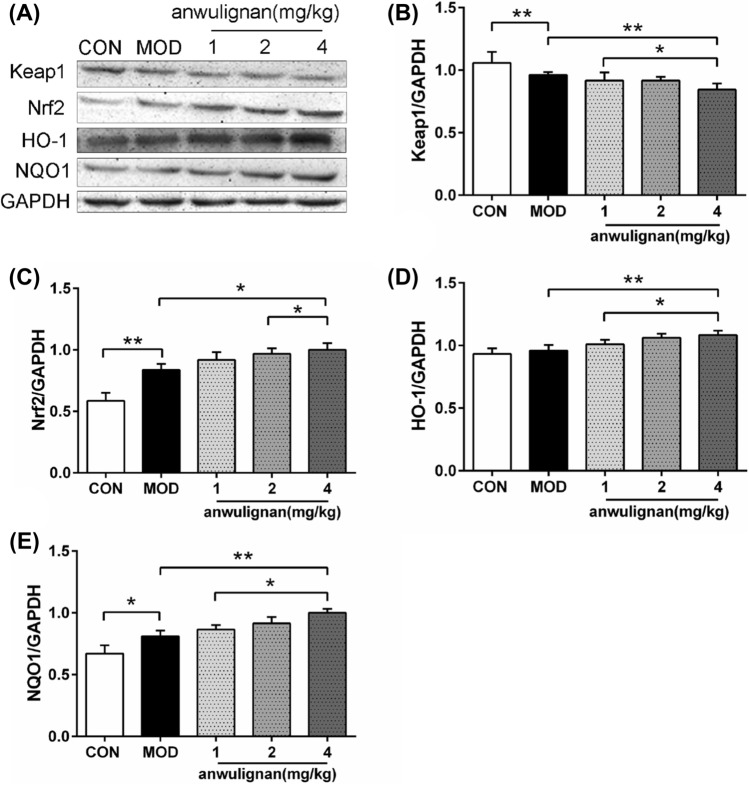
Nrf2/ARE signaling pathway plays an important role in antioxidation. In order to study the potential anti-oxidation mechanism of anwulignan, we observed the production of the Nrf2/ARE signal pathway-related proteins Keap1, Nrf2, HO-1 and NQO1 in the renal tissue of mice. The experimental results are shown in Fig. 4. Compared with those in CON group, the expression levels of Nrf2, HO-1 and NQO1 proteins was significantly higher (p < 0.01, p < 0.05), and that of Keap1 protein was significantly lower (p < 0.01) in MOD group. Compared with those in MOD group, the expression levels of Nrf2, HO-1 and NQO1 proteins was significantly increased (p < 0.01 or p < 0.05), and that of Keap1 proteins was significantly decreased (p < 0.01) in 4 mg/kg anwulignan group. The comparison among the three doses of anwulignan showed dose-dependent trend.
Fig. 4.
Expression levels of Nrf2, HO-1, NQO1 and Keap1 in the kidney tissue of mice. Note A Electrophoresis image of Keap1, Nrf2, HO-1 and NQO1 protein; B Keap1/GAPDH bar chart; C Nrf2/GAPDH bar chart; D HO-1/GAPDH bar chart; E NQO1/GAPDH bar chart. Mean ± SD, n = 3. *p < 0.05; **p < 0.01. Abbreviations: CON, blank control group; MOD, model group
Discussion
Studies have shown that a long-term high-dose injection of d-gal will cause an excessive production of galactitol, which is difficult to be metabolized and then accumulated in the body likely to stimulate the body to produce excessive ROS, resulting in the oxidative stress. It is such an oxidative stress that can make variety of damages to multiple organs in the body, so this model is commonly used as aging animal model for laboratory research (Chen et al., 2018a; Chen et al., 2018b; Cui et al., 2020; Jiao et al., 2018; Yi and Yang 2003). In our study, after the administration of d-gal for 42 days, the kidney index of mice decreased and the renal function declined significantly, showing an obvious damage of the kidney. However, anwulignan could significantly increase the kidney index and improve the renal pathological damage in D-gal induced aging mice, with a significant protective effect against the renal damage. To evaluate the renal functions, the classical indexes, such as CRE, BUN, urinary protein and Ccr were measured (Wei et al., 2019). The present results showed that anwulignan could significantly reduce the levels of CRE, BUN and urinary protein, and increase the level of Ccr in d-gal treated mice, suggesting that anwulignan could enhance the renal excretion function of d-gal induced aging mice, showing a significant renal protection. In addition, high-dose anwulignan showed more potent effect on the levels of CRE, Ccr and Nrf2/ARE signaling pathway related proteins than low-dose anwulignan, suggesting that there is a dose-dependent trend in its effect.
In 1956, Denham Harman proposed the theory of free radical aging: "degenerative diseases related to aging are attributed to the harmful effects of free radicals on cell components and connective tissue" (Jose, 2019). Subsequently, more and more studies have confirmed that oxidative damage is the direct cause of the aging process (Gu et al., 2019a; Gu et al., 2019b). MDA, a metabolite of the peroxidation of membrane lipids under the action of free radicals, and 8-OHdG, an oxidative product produced by DNA oxidative damage, are commonly used to evaluate the body’s oxidative damage and antioxidant function (Xu and Zhang 2019; Zhe et al., 2020). SOD, a kind of dismutase widely distributed in various tissues, is one of the main components of enzymes related to anti-lipid peroxidation to maintain the balance of oxidation–reduction, so it is one of the main index reflecting the antioxidant capacity of the body (Yang et al., 2020a; Yang et al., 2020b). CAT catalyzes the decomposition of H2O2 into H2O and O2 (Wen et al., 2020). GSH-Px can effectively scavenge free radicals (Yi et al., 2020). In this study, the above mentioned antioxidant indexes were observed, and the results showed that anwulignan can significantly reduce the content of MDA and 8-OHdG, and significantly increase the activity of SOD, CAT, and GSH-Px in the kidney tissue. It was also found in our previous studies that anwulignan could protect the liver, brain and spleen of aging mice induced by d-gal, and the effects of anwulignan on antioxidation-related indicators, such as MDA and SOD were consistent with those in this study (Gao et al., 2018; Zhao et al., 2020a; Zhao et al., 2020b). The results suggest that anwulignan may play its protective role against the renal damage of aging mice induced by d-gal through its antioxidant effect.
Nrf2/ARE signaling pathway is one of the most important antioxidant regulatory pathways (Liu et al., 2020). Keap1 protein functions as a substrate adaptor for a Cul3-RING dependent E3 ubiquitin ligase complex and targets Nrf2 for proteasome mediated degradation under homeostatic conditions (Yang et al., 2020a; Yang et al., 2020b). In an oxidative damage, Nrf2 is separated from Keap1, and the free Nrf2 moves to the nucleus to activate the expression of downstream antioxidant enzymes of ARE, so as to reduce the degree of injury. Nrf2 is the core of cell defense mechanism, and can indicate the oxidative stress state of the body. HO-1, an important antioxidant enzyme in the body, can participate in the antioxidant process of cells and tissues to protect against the kidney damage (Zhao et al., 2020a; Zhao et al., 2020b). NQO1 can inhibit the reduction of electron and reduce the generation of ROS through its own enzyme activity, and the increase of NQO1 level is related to the decrease of susceptibility to oxidative damage (Hu et al., 2016). When the body is under an oxidative stress, the Nrf2 anchored in the cytoplasm by binding to Keap1 is separated, then the increased free Nrf2 moves to the nucleus to increase the production of its downstream HO-1 or NQO1, so as to inhibit the oxidative damage (Lu et al., 2016; Zhao et al., 2020a; Zhao et al., 2020b). Interestingly, we also found that the Nrf2-HO-1 signaling pathway was activated by anwulignan as well as d-gal. As discussed in our previous study (Gao et al., 2018), d-gal is also an oxidative stress stimulus and can activate Nrf2 and its downstream products. However, the beneficial effects from the activation of Nrf2 and HO-1 by d-gal could not overcome its harmful effects from the accumulation of galactitol, whereas anwulignan, through its up-regulation of the expression of Nrf2, HO-1 and NQO1, but without harmful effect, might play the protective role against the oxidative damage, finally protecting against the renal damage induced by d-gal.
Anwulignan could significantly alleviate the renal damage induced by d-gal in mice by regulating the production of Nrf2/ARE pathway-related proteins in the renal tissues and cells to play its antioxidant role.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.This research was funded by Jilin Provincial Department of science and Technology (Grant Nos. 20170309006YY, 20200201521JC, 20200404053YY and 20200404022YY), Jilin Science and technology innovation development plan project (Grant No. 20190601177), Jilin provincial health and Family Planning Commission (Grant No. 2018J089, 2019J046), Jilin Provincial Development and Reform Commission (Grant No. 2020C033-2) and Jilin Administration of traditional Chinese Medicine (Grant No. 2020121).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunna Liu, Email: 2915759289@qq.com.
Huijiao Lin, Email: 1961646210@qq.com.
Liu Jiawei, Email: ljw2289379062@163.com.
Yao Wang, Email: 1838184714@qq.com.
Chunmei Wang, Email: wangcm74@126.com.
Jinghui Sun, Email: sunjinghui2008@126.com.
Chunyan Yu, Email: chunyanyu@163.com.
Ying Dong, Email: 95838596@qq.com.
Wenyue Zhuang, Email: wenyuezhuang@163.com.
Shu Jing, Email: yitongjingshu@163.com.
Jianguang Chen, Email: chenjg@beihua.edu.cn.
He Li, Email: yitonglh@126.com.
References
- Chen HM, Huang SS. The challenge of global population aging and China's response. China National Conditions and Strength. 2019;1:32–34. [Google Scholar]
- Chen F, Chen SH, Liu HY, Xiu LL, Liu DN, Gao J, Li N, Zhang C, Zhong GS. Analysis on formula characteristics of health food with auxiliary protective function in chemical liver injury. Chinese Traditional and Herbal Drugs. 2018;49:1703–1709. [Google Scholar]
- Chen Q, Yu P, Zhang J, Wu ZP. Effect of Puerarin on apoptosis gene expression in aging rats model induced by D-galactose. The Chinese Journal of Clinical Pharmacology. 2018;34:707–709. [Google Scholar]
- Cui CS, Shi QP, Xu N, Qi B, Wang RJ, Yang Y, Zhang Y, Zhao DQ, Liu L. Effects of compatible or single application of aqueous extract of ginseng and aqueous extract of sika deer antler on D-galactose-inducing aging mice. Chinese Journal of Gerontology. 2020;40:370–373. [Google Scholar]
- Debelle FD, Nortier JL, De Prez EG, Garbar CH, Vienne AR, Salmon IJ, Deschodt-Lanckman MM, Vanherweghem JL. Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. Journal of American Society Nephrology. 2002;13:431–436. doi: 10.1681/ASN.V132431. [DOI] [PubMed] [Google Scholar]
- Denham H. Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Gao JQ, Yu ZP, Jing S, Jiang WH, Liu C, Yu CY, Wang Sun J H, C M, Chen J G, Li H, Protective effect of Anwulignan against D-galactose induced hepatic injury through activating p38 MAPK-Nrf2-HO-1 pathway in mice. Clinical Interventions in Aging. 2018;13:1859–1869. doi: 10.2147/CIA.S173838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YY, Tang XF, Li XX, Chen YH, Ma ZT, Yu P, Liu RH. Effects of yin-yang replenishing chinese herbs on liver and kidney function, blood fat and serum bone turnover factor in aged rats. Journal of Liaoning University of Traditional Chinese Medicine. 2019;21:52–55. [Google Scholar]
- Ge H. Outer Chapter of Baopuzi. Art Panorama. 2019;1:1–2. [Google Scholar]
- Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Gu LF, Wang YG, Yang GL, et al. Ribes diacanthum Pall (RDP) ameliorates UUO-induced renal fibrosis via both canonical and non-canonical TGF-β signaling pathways in mice. Journal of Ethnopharmacology. 2019;231:302–310. doi: 10.1016/j.jep.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Gu LL, Cai WM, Jin QL, Zhang ZF, Wang JG, Zhang XY. Effect of sanghuang decoction on oxidative damage induced by D-galactose in mice. Chinese Journal of Modern Applied Pharmacy. 2019;36:2144–2148. [Google Scholar]
- Hu L F, Wang Y, Ren R J, Huo H R, Sun J H,.Li H, M,, Zhu Y Y, Tan Y Q. Anti-oxidative stress actions and regulation mechanisms of Keap1-Nrf2/ARE signal pathway. Journal of International Pharmaceutical Research, 43: 146-152+166 (2016)
- Jiao SM, Fu SX, Wen LX, Yan ZLSM. Action mechanism of losartan on human mesangial cell senescence induced by angiotensin II. Medical & Pharmaceutical Journal of Chinese People's Liberation Army. 2018;30:1–4. [Google Scholar]
- Jose V. The free radical theory of frailty: Mechanisms and opportunities for interventions to promote successful aging. Free Radical Biology & Medicine. 2019;134:690–694. doi: 10.1016/j.freeradbiomed.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Li X, Gao JQ, Yu ZP, Jiang WH, Sun W, Yu CY, Sun JH, Wang CM, Chen JG, Jing S, Li H. Regulatory effect of Anwulignan on the immune function through its antioxidation and anti-apoptosis in d-galactose-induced aging mice. Clinical Interventions in Aging. 2020;15:97–110. doi: 10.2147/CIA.S237601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li H, Wang CM, Sun JH, Ghen JG. Application and analysis of schisandra chinensis in domestic health foods from 1996 to 2016. Journal of Beihua University (Natural Science) 2018;19:191–196. [Google Scholar]
- Liu L, Zhang JW, Zhang XY. Effect of vitexin on Nrf2/ARE pathway in alleviating oxidative stress in rats with acute cerebral ischemia-reperfusion. Chinese Traditional and Herbal Drugs. 2020;51:1287–1293. [Google Scholar]
- Lu ZG, Huang JB, Liu Y, Lv XQ. Effect of Xingnaojing injection on keap1-nrf2 / are oxidative stress pathway in acute cerebral infarction. Guangdong Medical Journal. 2016;37:3127–3129. [Google Scholar]
- Wei FF, Wang WJ, He QH, Zhang B. A study of the kidney protective effects of haematococcus pluvialis power on alcoholic injury in mice. Life Science Research. 2019;23:122–127. [Google Scholar]
- Wen QL, Yin Z, Luo Y, Cai DZ. Effect and significance of total triterpenes of euonymus fortunei (Turcz.) hand-mazz on the expression of TAOC, CAT, and GSH-Px in serum and tissues of mice with D-galactose induced senescence. Hainan Medical Journal. 2020;31:413–417. [Google Scholar]
- Xu T, Zhang XM. Research progress of oxidative stress, 8-OHdG and hepatitis b related nephritis. Today Nurse. 2019;26:23–26. [Google Scholar]
- Yang YY, Chen L, Xu L, Liang YM, Wang B. Differentiation of schisandrae chinensis fructus and s. sphenanthera fructus by determining content of lignanoids. Journal of Liaoning University of Traditional Chinese Medicine. 2018;20:69–74. [Google Scholar]
- Yang CB, Lian XY, Shen PH, Wang BY, Liu WT, Li Q. Anti-aging effect of xiaHei-grape anthocyanin in brain, kidney and spleen of aging mice induced by d-galactose. Henan Medical Research. 2019;28:2113–2117. [Google Scholar]
- Yang GM, Hong SJ, Wang YH, Shen BY, Yu H, Zeng XF, Li LH. Progress of research in the regulation of Keap1/Nrf2/p62, NLRP3 inflammasome and autophagy. Chinese Journal of Comparative Medicine. 2020;3:1–5. [Google Scholar]
- Yang ZM, Shen YH, Wang ZW, Huang XZ, Ding XW. Effect of mulberry leaf alkaloid on kidney of d-galactose-induced oxidative damage in mice. Food Science. 2020;3:1–7. [Google Scholar]
- Yi W Y, Yang Q. Effect of baoshen decoction on the morphology changesof rat with experimental senile kidney. Chinese Journal of Traditional Medical Science and Technology, 10: 82-83+64 (2003)
- Yi GF, Din JU, Zhao F, Liu XQ. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Function. 2020;11:2725–2737. doi: 10.1039/C9FO01466G. [DOI] [PubMed] [Google Scholar]
- Yu Z P, Jing S, Gao J Q, Liu C, Li N, Yu C, Sun J, Wang C, Chen J, Li H. Anwulignan improves d-galactose-induced learning and memory impairment via regulating P38 mapk-nrf2-ho-1 pathway in mice. Natural Product Communications, 14: 1-6 (2019)
- Zeng TT, Li XZ. Research progress of aging mechanism and anti-aging mechanism of acupuncture. Lishizhen Medicine and Materia Medica Research. 2019;30:1457–1459. [Google Scholar]
- Zhao YD, Zhang TS, Ma J, Zhang WW, Yi Y, Gu B, Wang HQ, Xuan Y. Astragaloside alleviates particulate matter 2.5-induced oxidative injury of renal tubular epithelial cells by regulating the kelch-like ech-associated protein 1-nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway. Journal of Anhui University of Chinese Medicine. 2020;39:69–75. [Google Scholar]
- Zhao Y, Zhang W, Cui Y, Yin HS. Therapeutic effect of eye on cerebral ischemia-reperfusion injury and its effects on Keap1, Nrf2 and HO-1. Journal of Liaoning University of Traditional Chinese Medicine. 2020;3:1–11. [Google Scholar]
- Zhe LT, Zhao CH, Chen CH, Tang ML, Duan CD, Liu WW, Zhang XM. Anti-aging effect of oleuropein on aging mice induced by d-galactose. Chinese Journal of Traditional Medical Science and Technology. 2020;27:37–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.