Abstract
The aim of this work was to evaluate the technologies effect of cold extraction by centrifugation (CE) and ultrasound-assisted (US-CE) methods without adding water, on the avocado oil yield, nutritional composition, physicochemical characteristics, oxidative stability (oxidation temperature and time, besides activation energy) and accelerated shelf life regarding hexane extraction (control). The US-CE improved the physicochemical properties such as acidity, peroxides, and iodine indexes regarding CE and Control. US-CE improved the yield, nutritional quality of fatty acids, oxidative stability, shelf life, and ω-6/ω-3 ratio regarding CE. Furthermore, US-CE improved the ratio yield/time extraction of the oil and increased the oxidation temperature regarding control. The main advantage of oils extracted using CE and US-CE concerning control was higher oxidative stability. The most representative polyunsaturated fatty acids identified in all treatments were γ-linolenic and conjugated α-linolenic acids. α-linolenic acid was only detected in US-CE and control.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-021-00940-w.
Keywords: Avocado oil, Yield, Fatty acid, Omega-6/omega-3 ratio, Oxidative stability
Introduction
The avocado (Persea Americana Mill.) is an evergreen dicot plant of the Lauraceae family, covering approximately 45 genera and 2,850 species. Avocado fruit can be divided into three anatomical parts (peel, pulp, and seed). The yellowish edible pulp constitutes the largest proportion of the fruit (65%), while the peel (15%) and the seed (20%) represent the rest. The main variety of avocado consumed worldwide is ‘Hass’, and Mexico is the leading producer, exporter, and consumer, followed by the Dominican Republic, Peru, Indonesia, and Colombia (FAO 2020).
Avocado oil stands out as one of the products derived from the fruit with the highest values in the market, recognizable flavor and color, as well as to be potentially beneficial for human health. Moreover, it can be a good substitute for olive oil for its nutritional contribution and excellent taste. Avocado oil can be consumed either neat or added to salads. Also, it could be used as an ingredient in functional foods or in the formulation of meat products to decrease the color deterioration of these products and inhibit lipid and protein oxidation (Rodríguez-Carpena et al. 2012). It has excellent nutritional characteristics according to its contribution of unsaturated fatty acids (70% MUFA and 13% PUFA), including between 63 and 69% oleic acid, as well as 14% palmitic acid, vitamins, tocopherols, carotenoids, and polyphenolic compounds of great interest for their antioxidant and anti-inflammatory properties (Duque et al. 2012; Flores et al. 2019).
Among the most widely used technologies for avocado oil extraction at an industrial level are the traditional solvent method and the cold extraction method. In the first one, the fruits are dried at high temperatures, and then the oil is extracted by solvents, commonly hexane (Flores et al. 2019). This extraction process's disadvantages are the safety risk when using solvent since the hexane has carcinogenic and toxic properties, the use of high temperatures, and a long time of extraction (Tan et al. 2018a). Furthermore, the avocado oil extraction using organic solvents requires a subsequent refining process, and it is mainly intended for the cosmetic industry (Costagli and Betti 2015). Due to concerns related to environmental safety and health, the use of solventless extraction technologies is highly justified. One of the technologies that is friendly to health and the environment is the cold extraction method, where the oil is separated by centrifugal or pressure forces. This method was developed to reduce energy costs and minimize the contamination by organic solvents (Yang et al. 2018). This method uses water and some salts like CaCO3, CaSO4, or NaCl. However, the avocado oil's cold extraction process shows a lower yield regarding oil extraction using solvents, besides using temperatures lower to 40 °C (Costagli and Betti 2015; Martínez-Padilla et al. 2018).
On the other hand, the use of ultrasound-assisted cold extraction increased the yield and improved the oil quality. It is because of cavitation produced by sound waves with a frequency higher than the threshold of human hearing (> 20 kHz) (Franco y Bartoli 2019), which form compression and expansion cycles that, when they pass through an aqueous medium, break the cellular structure of the plant tissue with mechanical force, releasing intracellular components and improving mass transfer (Tan et al. 2018b). Ultrasound can increase the temperature of the study material, depending on the frequency and power used. Martínez-Padilla et al. (2018) used a treatment where they combined power of 150 W at 18 kHz with a power of 525 W at 40 kHz; hence, to avoid the increase in temperature (40–45 °C), they used ultrasonic pulses.
This work focus on the use of low power to maintain the temperature lower than 40 °C and does not use water to the extraction process in this work (do not use malaxation). This work hypothesizes that ultrasound of low frequency (20 kHz) and power of 187.5 W can increase the yield and quality of the oil regarding cold extraction or solvent extraction.
Therefore, the aim of this study was to evaluate the effect of cold extraction (CE), ultrasound-assisted (US-CE) without water added, and solvent extraction on the yield, composition nutritional, physicochemical characteristics, and oxidative stability.
Materials and methods
Conditioning of the raw material.
The ‘Hass’ avocado fruits were harvested at mid-season in a commercial orchard from the Venustiano Carranza community of the municipality of Tepic, Nayarit, Mexico. These fruits were considered as by-products due to their small size and appearance defects. These ones were kept at room temperature until full maturity, which was subjectively evaluated according to the pulp softening and the epicarp color (Gamble et al. 2010). The fruits were washed and disinfected with a solution of sodium hypochlorite (Alen, Santa Catarina, N.L., Mexico) at 2% for 10 min. Subsequently, the fruits were opened manually with a knife to remove the epicarp and the seed. The pulp was mixed in an industrial blender (International® model LI-5, Veca International S.A., Mexico City, Mexico) for approximately 30 s until homogenization. The paste was stored in vacuum-sealed polyethylene bags and frozen at − 20 °C until use.
Extraction technologies
Oil extraction with Soxhlet (control)
Avocado oil was extracted using the Soxhlet method, according to AOAC (2007). Hexane was used as the extraction solvent. The extraction time was six h at a heating temperature of 70 °C. The oil was recovered in a rotary evaporator (Büchi model R-3, Büchi labortechnik AG., Flawil, Switzerland).
Cold extraction by centrifugation
The separation of oil from the solid and liquid phases was carried out by placing 25 g of fresh sample in 50 mL reaction tubes to be centrifuged in an Eppendorf equipment model 5804R (Eppendorf AG, Hamburg, Germany) at 10,000 rpm (1,667 × g) and 40 °C for 10 min.
Ultrasound-assisted cold extraction (US-CE)
The ultrasound pretreatment was applied to the fresh avocado pulp previously prepared. The sonication was performed as follow: No water was added to the sonication process, the samples (200 g of pulp) were sonicated in an ultrasound device with temperature control Cole-Parmer 750 W model CPX750 (Cole-Parmer Instruments, Vernon Hills, IL, U.S.A.), which has an operating frequency of 20 kHz. A titanium probe with a diameter of 2.54 cm was used. The probe was immersed at 5 cm in the sample container in a 500 mL beaker. It was moved at a constant speed to get a homogeneous sonication and not limited by the avocado paste's viscosity since, at higher viscosity, the ultrasound propagation is less. The samples were sonicated at 187.5 W for five min. These conditions were previously optimized for oil extraction (data not shown). Samples were cooled in an ice-water bath during the sonication to dissipate most of the heat produced during the treatment.
Oil extraction yield
The oil extraction yield of fresh avocado pulp was determined by the ratio of the amount of oil extracted and the dry weight sample (avocado pulp). The yield was determined using Eq. 1.
| 1 |
Evaluation of the avocado oil physicochemical characteristics
Iodine index (YI)
The iodine index is an unsaturation measure of the oils. It was determined according to the Wijs method (AOCS 2009). The YI was recorded as g of iodine absorbed per 100 g of oil.
Acidity index (AI)
It is a measure of the amount of free fatty acids in the oil. The volumetric method was used to determine AI. The results were expressed as a percentage of oleic acid (AOCS 2009).
Peroxide index (PI)
The volumetric method used to quantify the PI is based on the determination of the substances in terms of milliequivalents of active oxygen per 100 g of the sample that oxidize potassium iodide (J.T. Baker, Phillipsburg, NJ, USA). The PI was expressed as milliequivalents of peroxide contained in a kg of oil (AOCS 2009).
Fatty acid profile
The fatty acid methyl esters (FAMEs) were prepared by cold methylation following the method described by Cert et al. (2000).
After the methylation of the fatty acids in avocado oil, it was introduced into a gas stream (carrier gas) before encountering the stationary phase. The chromatographic method described by Vickers (2007) was followed with modifications. 0.5 µL of the sample was injected into the gas chromatograph. The separation and determination of fatty acids were performed using a Brucker Gas Chromatograph model Scion 456-GC 8S (Scion Instruments Iberica, Spain) equipped with a column injector and flame ionization detector (FID). A capillary column Select FAME model CP 7420 (Agilent Technologies, 100 m long × 0.25 mm internal diameter × 0.25 µm thick) was used. The carrier gas was helium (Grupo Infra, Félix Guzmán, Edo. De Mex., Mexico) at a constant pressure of 10 psi. The oven temperature ramp started at an initial temperature of 180 °C, gradually increasing 2 °C/min until it reaches 250 °C, keeping it at that temperature for 10 min and ending the program. The injector temperature was 250 °C, and the detector was 280 °C. Once the fatty acids separation program was completed, each of them was identified by comparing the retention times in the samples with standards (Supelco 37 components FAME Mix, varied conc. in dichloromethane, part number CRM47885). The standards were subjected to the same chromatographic conditions. The results were expressed as a percentage of area under the curve of the total fatty acids analyzed (abundance).
Total soluble phenols
The determination of total soluble phenolic compounds was performed as reported by Singleton et al. (1999), with the Folin-Ciocalteu method. The results were expressed as mg of gallic acid equivalent per g of oil (mg GAE/g of oil).
Oxidative stability by differential scanning calorimetry (DSC)
The determination of the oxidation induction temperature (Tox) and the oxidation induction time (tox) are the measurement methods to assess oxidative stability by the differential scanning calorimetry (DSC) technique (Pardauil et al., 2011). The analysis was performed on a TA Instruent® DSC 250 device (New Castle, DE, USA). The oxidative stability assay was performed according the method described by Delgado et al. (2015).
The oxidative stability was performed with the Tox and tox obtained by the tangent method. This method consists of the extrapolation intersection of the baseline and the tangent of the exotherm. The tangent and baseline were recorded from the isothermal DSC thermogram. Data were analyzed using TA instrument® TRIOS software 4.3.1.39215.
Determination of activation energy (Ea)
Arrhenius model is one of the most accepted models for determining the effects of temperature on the increase in the chemical reaction rate, in which the temperature is incorporated into an exponential model of the rate constant (Eq. 2).
| 2 |
Applying logarithms to both sides of Eq. 2 gives the equation of a straight line with slope Ea/R (Eq. 3). This term can be evaluated to know the value of the activation energy.
| 3 |
where k is the reaction rate constant; A is the frequency factor; Ea is the activation energy (J/mol); R is the constant of the ideal gases (8.314510 J/K mol), and T is the absolute reaction temperature (K).
Accelerated shelf-life test
The general equation to estimate the accelerated shelf-life of avocado oils for the different treatments was obtained by using the Arrhenius equation and the specific values of tox and Tox.
The Arrhenius equation (Eq. 3) shows that the neperian logarithm of k (ln k) versus the reciprocal of the absolute temperature generates a straight line, whose slope is the activation energy divided by the ideal gas constant R.
Thus, by measuring k (rate constant) at three elevated temperatures, it can be extrapolated with a straight line at a lower temperature and predict the reaction rate constant at the lowest desired temperature (Riboh and Labuza, 1982), in this work at 30 °C.
Statistical analysis
Data were evaluated using an analysis of variance (ANOVA), and the specific differences among the treatments were determined with the Least Significant Differences (LSD) test with a p < 0.05. Statistical analysis was performed using Statgraphics Centurion XVI software (v16.1.03) (Statgraphics Technologies, Inc., VA, USA).
Results and discussion
Yield comparison among technologies
In the control treatment, the yield (66.57% dry base (d.b.)) was statistically higher (p < 0.05) compared to the CE (39% d.b.) and US-CE (45.38% d.b.) treatments (Fig. 1). The result in the control treatment could be due to the increase in the extraction temperature, which facilitated the mass transfer. Hence, the solvent completely penetrates the plant material by solubilizing the compounds related to hexane and facilitating extraction (Mostert et al. 2007). Carbohydrates and proteins are compounds of the avocado paste, which could encapsulate the oil or form an emulsion, difficulting the extraction process. Nevertheless, some variables used could destabilize these structures. Liu et al. (2019) concluded that the extraction temperature could change the ordered structure of starch granules and other carbohydrates from the evaluated matrix. Likewise, López-Ordaz et al. (2019) mention that the temperature and the prolonged extraction time of the oil can cause structural damage in proteins from the matrix. This can cause the carbohydrate and protein-based structures where the oil is stored in the matrix to be destabilized, which may improve the oil diffusivity in the avocado paste and the extraction yield.
Fig. 1.
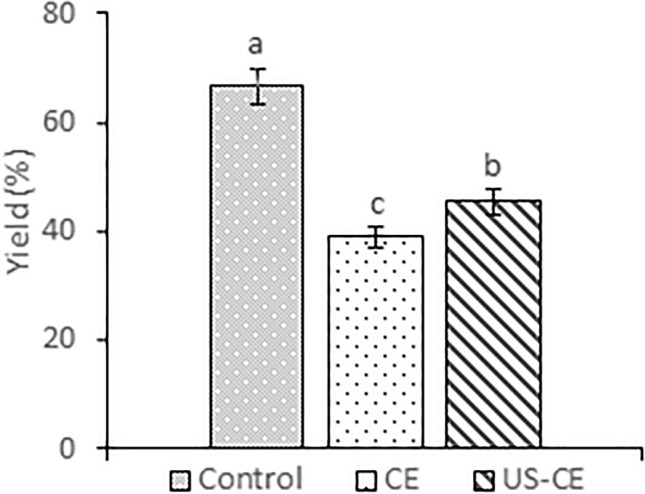
Rate of yield obtained for the different extraction treatments
The US-CE treatment showed a higher yield/extraction time ratio since the extraction time was 15 min, which was considerably shorter than the traditional Soxhlet method (6 h). Therefore, the advantage of this extraction method is that it reduces the extraction time and lowers production costs. Unlike the CE treatment, with the US-CE treatment, vibrations and acceleration between solid and liquid particles occurred under the ultrasonic action, increasing the diffusivity of the lipid fraction in the matrix. The impact of ultrasound on oil extraction could be explained by the rupture of the cell membrane and the consequent improvement of mass transfer phenomena (Puértolas and Martínez, 2015).
Physicochemical evaluation of avocado oil
The peroxide index (PI) determines the content of hydroperoxides and provides a measure of the oxidation of unsaturated fatty acids (Firestone 2013). The PI value for the US-CE treatment was lower than the PI of the control treatment (p < 0.05). However, there is no significant difference (p > 0.05) between the CE and US-CE treatments (Table 1). This result evidences the negative effect of the temperature applied in the control treatment on oil oxidation since a higher treatment temperature causes greater oil oxidation, which is reflected in the peroxide index. Yepes-Betancur et al. (2017) reported a PI of 5.56 mEq peroxide/kg for avocado oil obtained by a thermomechanical process. Duque et al. (2012) reported a PI of 16.87 mEq peroxide/kg for avocado oil obtained by supercritical fluids. The previously reported values were higher compared to those obtained in this work in the CE and US-CE treatments. Krumreich et al. (2018) reported different values depending on the extraction method used, which were found in a range of 3.3 to 7.4 mEq peroxide/kg, which were similar to those reported in this research with less sophisticated technologies. Krumreich et al. (2018) also mention that temperature is a relevant factor since it may confer ideal conditions for the activity of enzymes as lipooxygenases, which can increase PI.
Table 1.
Quality indexes of avocado oil obtained by the different treatments
| Treatments | PI (mEq peroxide/kg oil) | AI (% Oleic acid) | YI (g I2/g oil) |
|---|---|---|---|
| CE | 4.12 ± 0.13b | 1.19 ± 0.08a | 87.25 ± 1.32b |
| US-CE | 2.96 ± 0.97b | 0.49 ± 0.00b | 91.51 ± 1.66a |
| Control | 11.05 ± 0.40a | 1.31 ± 0.06a | 57.13 ± 1.92c |
CE: cold extraction; US-CE: ultrasound-assisted cold extraction; Control: extraction with hexane. PI: peroxide index; AI: acidity index; YI: iodine index. Values expressed in average ± standard deviation. The values followed by different letters in the same column differ statistically (p < 0.05)
The PI quality standards recommended by Woolf et al. (2009) are 4 mEq peroxide/kg for extra virgin oil as maximum and less than eight mEq peroxide/kg for virgin avocado oil. These values are within the by Firestone (2013), who mentions a maximum amount of 10 mEq peroxide/kg for avocado oil. Therefore, the PI for oils in optimal conditions must be low. In this study, the avocado oils obtained by US-CE and CE presented low oxidation of unsaturated fatty acids, so they are within the quality standards of extra virgin and virgin oils, respectively, while the oil extracted with solvent does not meet the recommended parameters.
The acidity index (AI) represents the amount of free fatty acids (FFA). It is an important quality factor widely used as a criterion for classifying oil into different commercial grades. The FFA determination is particularly important for industrial purposes. These can modify the organoleptic or physicochemical properties of the oil (Gharby et al. 2017). The value of AI expressed as a percentage of oleic acid for the US-CE treatment was lower (p < 0.05) compared to those obtained with the CE and control treatment (Table 1). The AI value of avocado oil obtained through the US-CE procedure (0.49% oleic acid) is a low-value, according to Woolf et al. (2009). It can be classified as extra virgin since its acidity is less than 0.5%. Some authors have reported AI values between 0.38 to 1.5% of oleic acid for avocado oil (Duque et al. 2012; Firestone 2013; Yepes-Betancur et al. 2017). The extraction conditions influence the level of oleic acid present in vegetable oil since some variables as the temperature can increase the activity of lipase enzymes (Krumreich et al. 2018), which hydrolyze triacylglycerides, thereby releasing fatty acid and increasing the AI.
The iodine index (YI) is a measure of the total number of double bonds present in fats and oils (Gharby et al. 2015). The YI value for the US-CE treatment was higher (91.51 ± 1.66 g I2/g of oil) compared to the CE and control treatments. Significant differences between treatments (p < 0.05) were found (Table 1). The results obtained for YI of the CE and US-CE treatments are relatively high values, an aspect related to a high percentage of unsaturated fatty acids. This may because, as previously stated, ultrasound can break down cellular structures such as membranes. These cell membranes are made up of phospholipids, which have unsaturated fatty acids in their structure (Colin and Jaillais 2020). Therefore, membrane rupture can increase the amount of unsaturated fatty acid in the oil.
Firestone (2013) mentions avocado oil should be in a YI range of 85–90 gI2/g of oil, so the oils obtained through US-CE and CE are within the established range for avocado oil. The amounts found in this work for avocado oil from US-CE and CE treatments exceed this value; furthermore, they are higher than those reported by other researchers for avocado oil (Duque et al. 2012; Yepes-Betancur et al. 2017; Krumreich et al. 2018).
Nutritional composition of oils obtained through control, CE, and US-CE treatments
Table 2 shows the relative concentrations of fatty acids of avocado oil corresponding to the CE, US-CE, and control treatments. The chromatograms are shown in figure S1. The total saturated fatty acids (SFA) of the cold extracted oil had a lower concentration than the ultrasound treated oil, and the one obtained with hexane (p < 0.05). The SFA presents in all treatments are palmitic and stearic acids. Palmitic acid was the most abundant in the CE and US-CE treatments (p < 0.05). Behenic and tricosanoic acids were found in higher proportions in the control treatment. Cicero et al. (2018) and Fernandes et al. (2018) reported lower SFA concentrations for avocado oil (16.93 and 18.64%, respectively) compared to this study.
Table 2.
FAME profile of avocado oil extracted by different treatments
| FAMEs | Acid | CE* | US-CE* | Control* |
|---|---|---|---|---|
| C14 | Myristic | Nd | 0.08 ± 0.07a** | 0.01 ± 0.03a |
| C16 | Palmitic | 24.17 ± 0.51ª | 22.51 ± 1.04a | 5.37 ± 0.48b |
| C16:1 | Palmitoleic | 15.71 ± 0.37ª | 14.46 ± 0.89a | 4.66 ± 0.22b |
| C17:1 | Heptadecenoic | Nd | Nd | 0.31 ± 0.17 |
| C18 | Stearic | 0.44 ± 0.07ª | 0.26 ± 0.09a | 1.39 ± 0.33a |
| C18:1 (trans) | Elaidic | Nd | Nd | 1.35 ± 0.20 |
| C18:1 (cis) | Oleic | 39.06 ± 0.41ª | 21.43 ± 2.72b | 9.25 ± 0.72c |
| C18:2 (cis) | Linoleic | Nd | 0.51 ± 0.24a | 0.91 ± 0.29a |
| C18:2 (trans) | Conjugated α-linolenic | 14.49 ± 0.40ª | 10.89 ± 0.38b | 6.93 ± 0.35c |
| C20 | Arachidic | Nd | 1.58 ± 0.42a | 1.46 ± 0.37a |
| C18:3 (n-6) | γ-linolenic | 0.78 ± 0.08ª | 2.22 ± 0.42a | 1.49 ± 0.33a |
| C18:3 (n-3) | α-linolenic (ALA) | Nd | 11.53 ± 0.93a | 4.18 ± 0.62a |
| C21 | Heneicosanoic | Nd | 2.24 ± 0.50a | 3.48 ± 0.18a |
| C20:2 | Eicosadienoic | Nd | 1.75 ± 0.44b | 6.33 ± 0.43a |
| C22 | Behenic | Nd | 1.49 ± 0.41b | 7.42 ± 0.29a |
| C22:1 | Erucic | Nd | 0.11 ± 0.11b | 6.56 ± 0.22a |
| C20:3 (n-6) | Dihomo-γ-linolenic | Nd | 1.58 ± 0.39b | 6.56 ± 0.22a |
| C20:3 (n-3) | Eicosatrienoic | Nd | 1.14 ± 0.34b | 7.06 ± 0.48a |
| C20:4 | Arachidonic | Nd | 1.84 ± 0.44a | 3.89 ± 0.32a |
| C23 | Tricosanoic | Nd | 1.25 ± 0.37b | 7.69 ± 0.34a |
| C22:2 | Docosadienoic | 0.01 ± 0.05b | 1.12 ± 0.35b | 5.20 ± 0.11a |
| C24 | Lignoceric | Nd | 1.17 ± 0.34a | 3.65 ± 0.36a |
| C20:5 (EPA n-3) | Eicosapentaenoic | Nd | 0.71 ± 0.26b | 1.86 ± 0.41a |
| C24:1 | Nervonic | Nd | 0.11 ± 0.11a | 2.97 ± 0.34a |
| C22:6 (n-3) | Docosahexaenoic | Nd | 0.01 ± 0.03 | Nd |
| SFAs | 24.60 ± 0.51b | 32.42 ± 0.46a | 30.47 ± 0.29a | |
| MUFAs | 54.77 ± 0.24ª | 36.10 ± 1.12b | 25.11 ± 0.65b | |
| PUFAs | 15.27 ± 0.41b | 33.30 ± 1.21ab | 44.41 ± 0.71a | |
| UFAs | 70.04 ± 0.43ª | 69.40 ± 0.46a | 69.52 ± 0.29a | |
| UFAs/SFAs | 2.87 ± 0.16ª | 2.28 ± 0.15b | 2.28 ± 0.09b | |
| ω-3 | Nd | 13.38 ± 0.92a | 13.11 ± 0.56a | |
| ω-6 | 15.29 ± 0.40b | 19.92 ± 0.79b | 31.31 ± 0.44a | |
| ω-6/ω-3 | 00.00 ± 0.00b | 1.99 ± 0.32a | 2.48 ± 0.22a |
CE (Cold extraction), US-CE (ultrasound-assisted cold extraction), Control (Hexane)
FAMEs, fatty acids methyl esters; SFAs, Saturated fatty acids; MUFAs, monounsaturated fatty acids; PUFAs, Polyunsaturated fatty acids; UFAs, unsaturated fatty acids; ω, omega fatty acid; nd, not detected
*Units in area percentage
**The values followed by different letters in the same line differ statistically (p < 0.05)
The avocado oil with the highest proportion of MUFAs (Table 2) was obtained by CE (p < 0.05). The oleic and palmitoleic acid were the MUFAs that presented the highest concentration in all treatments (p < 0.05). Fernandes et al. (2018) reported for ‘Hass’ avocado oil has a very similar amount of MUFAs (69.64%) compared to the oil obtained by CE. The importance of oleic acid in health is because it can reduce the risk of cardiovascular and cerebrovascular diseases (Krumreich et al. 2018).
The content of total PUFAs (Table 2) showed significant differences between CE and the rest of the treatments (p < 0.05); however, no significant differences were found between US-CE and control treatments (p > 0.05). Conjugated α-linolenic acids and γ-linolenic acids were found in all treatments. The CE treatment presented the highest proportion of conjugated α-linolenic acid (p < 0.05). The α-linolenic acid (ALA) followed by the conjugated α-linolenic acid were the most representative of the oil obtained with US-CE. Furthermore, the eicosatrienoic, conjugated α-linolenic and cis eicosanoic acids were the most abundant in the control oil.
Cicero et al. (2018) found a PUFA concentration on commercial avocado oil 15.96 ± 1.15%. This value is lower than those reported for oils treated with ultrasound and solvent; however, compared to the oil obtained by CE, they are very similar values. Likewise, Fernandes et al. (2018) reported a lower amount of the fatty acids mentioned (11.71%) for ‘Hass’ avocado oil.
Cold-pressed oils generally contain ALA, which is an essential dietary acid and cannot be synthesized in humans (Yepes-Betancur et al. 2017). However, in this work, CE was not detected, which stands out the importance of ultrasound-assisted method, since it increases the concentration of this fatty acid on the oil, improving its quality.
The data obtained for the ratio UFAs/SFAs showed that the oil extracted by CE had the highest proportion (Table 2), due to a higher content of UFAs (p < 0.05). The results published by Cicero et al. (2018) and Berasategi et al. (2012) for avocado oil (4.90 and 4.15, respectively) were higher than those found in this study; however, Donetti and Terry (2010) reported similar values in avocado fruits from South Africa (2.70 and 3.00) and Peru (2.00 and 2.20), in comparison with the results obtained in this study. According to Reddy et al. (2012), a high proportion of UFAs/SFAs is generally considered beneficial for human health.
The ratio of fatty acids ω-6/ω-3 for the CE treatment cannot be determinated because ω-3 was not detected. In the US-CE (1.99) and control (2.48) treatments, no significant difference between them was observed (Table 2). Fatty acid profiles reported by Berasategi et al. (2012) showed for the avocado oil a ratio ω-6/ω-3 of 14.05, which is a higher proportion to those found in this study. In contrast, Cicero et al. (2018), published values of less than 0.086 and 0.088 for olive and avocado oils, respectively.
In this regard, the ω-6/ω-3 ratio is essential to explain the PUFAs influence in the diet on the pathogenesis of diseases. A very high ω-6/ω-3 rate is considered harmful to human health, while a value closest to 1 is deemed to be protective against such pathologies (Russo, 2009).
The ω-3 fatty acid concentration of the US-CE and control treatments showed no statistically significant differences. In the oil of the CE treatment, the presence of these acids was not detected. The above can be explained because the effect of ultrasound breaks the cell membranes and organelles, being that the ω-3 and ω-6 acids are some of the constituents in the cell membrane (Simopoulos 2006). The absence of ω-3 acids in CE treatment may be due that the same degree of membrane rupture was not observed regarding ultrasound treatment. The consumption of ω-3 fatty acids improves vascular and cardiac hemodynamics, triglycerides, endothelial function, autonomous control, inflammation, thrombosis, and arrhythmia, reducing the risk of death associated with these types of pathologies (Russo 2009).
Concerning ω-6 fatty acids, the control treatment had a higher concentration (p < 0.05) than the CE and US-CE treatments. These results may be because the increase in temperature facilitated the transfer of mass, and the affinity of the solvent for fatty acids facilitated the extraction (Mostert et al. 2007). The US-CE treatment has the advantage over the control treatment of presenting a lower amount ω-6 fatty acids (p < 0.05) since the human metabolism of PUFA ω-6 produces inflammatory lipid mediators. At the same time, PUFA ω-3 is related to anti-inflammatory lipid mediators (Calder 2009).
Total soluble phenols
The oils obtained by the different procedures showed total soluble phenolic concentrations in the range of 61.10 ± 1.13 mg GAE/g to 131.96 ± 2.12 mg GAE/g. The control treatment presented the highest amount of phenols (131.96 ± 2.12) compared to the other treatments (p < 0.05), being twice as superior to the CE and US-CE treatments (61.10 ± 1.13 and 63.12 ± 1.02, respectively). In general, the differences may be due to the conditions of the extraction methodology used, such as solvent, centrifugal speed, acoustic waves power, time treatment, etc. During the oil extraction process, the control treatment was subjected to extraction with hexane; in this process, the non-polar and/or amphipathic phenolic compounds were concentrated. Subsequently, the amphipathic phenolic compounds were extracted through a methanolic extraction. CE and US-CE treatments showed only had one polar methanolic extraction from the oil.
Furthermore, Medina et al. (2006) mention that lignans are one of the main bioactive components present in oils. Some lignans found in plants are of low or medium polarity, and it is necessary to use a non-polar solvent to extract these compounds (Willför et al., 2006). Likewise, Pinelo et al. (2005) mention that an increase in temperature favors the phenols extraction by enhancing both the solubility of the solute and diffusion coefficient. They also found that phenolic compounds' stability and denaturation of membranes can happen at temperatures > 50 °C. Although the boiling point of hexane is 69 °C, this solvent is cooled in the Soxhlet equipment using a refrigerant at four °C, which could cause the hexane to reach a temperature close to 50 °C or less when it comes into contact with the avocado sample. For these reasons, the control oil could have a higher phenol amount than the other extraction methods.
Besides, temperature plays a crucial role in the extraction of these compounds, since it favors the increase in their diffusion and solubility coefficients by promoting the release of polyphenols due to the breakdown of cellular structures (Al-Farsi and Lee 2008). Tan et al. (2018b) reported a concentration of phenolic compounds of 130.17 mg GAE/g, which was similar to that obtained for the control treatment in this study, but higher than that obtained by the US-CE treatment.
Oxidative stability by differential scanning calorimetry (DSC)
Thermograms showed a rapid change in the heat flux, which is characteristic of an oxidation reaction that occurs when transferring a molecule of oxygen to an unsaturated fatty acid (Fig. 2). The oil obtained by ultrasound and cold extraction presented a higher Tox (p < 0.05) showing values of 177.33 and 172.85 °C, respectively, while the control oil showed a Tox of 141.44 °C. Ostrowska-Ligeza et al. (2010) found oxidation temperatures for olive oil between 168.1 and 178.0 °C under similar conditions to this work. Simon et al. (2000) found an oxidation temperature of 177.3 °C to sunflower oil and 189.7 °C to rapeseed oil. The above indicates that the avocado oil obtained by CE and US-CE has oxidative characteristics to olive and sunflower oils.
Fig. 2.
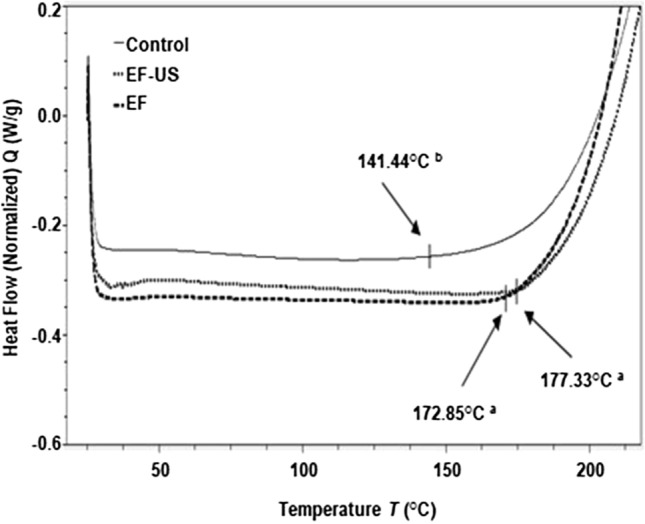
DSC oxidative thermogram to determine the oxidative induction temperature (Tox) of the avocado oils obtained by CE, US-CE, and control. The values followed by different letters differ statistically (p < 0.05)
The highest Tox shown by the oil treated with ultrasound is an advantage over the other treatments evaluated since a low Tox has been highly related to lipid oxidation under non-thermal conditions and the physicochemical composition of the oil (Qi et al. 2016) (Table 1). The oils treated by ultrasound and hexane showed no differences between the amount of saturated and unsaturated fatty acids (p > 0.05). Nonetheless, control oil had a higher phenols concentration, which could be heat-labile and start to rust at 144.44 °C and, therefore, lower thermo-oxidative stability. Wu et al. (2019) mention that some phenolic compounds can be degraded at fried temperature and produce more stable compounds; this may be the reason why the curve is not as sharp as in the other two treatments.
Kowalski et al. (2004) mention that Tox is the most suitable parameter for the determination of lipid oxidation under non-isothermal conditions because it is closely related to peroxide formation. On the other hand, the non-isothermal oxidation of the oils was carried out with excess oxygen; therefore, said oxidation was independent of its concentration, which can be treated as an apparent first-order reaction (Tan et al., 2001).
Table 3 shows the oxidative stability of the oils of the different treatments according to the tox in conditions of isothermal Tox. The ultrasound-treated oil (US-CE) was the one that presented the lowest tox to the different isothermal, followed by the cold extracted oil (CE), being the control treatment the one with the highest tox. These results indicate slow oxidation of control treatment, and it could be due to a higher concentration of phenolic compounds. Wu et al. (2019) mention that oils may contain phenolic compounds such as sesamol in sesame oil, which can be degraded to sesamolin by the effect of frying temperature. This is another phenolic compound, which has an excellent ability to trap free radicals, conferring greater stability thermal to oils.
Table 3.
Oxidative stability by DSC and estimation of the oils shelf life
| Tox (°C) |
tox (min) |
Ea (J/mol) |
Regression equation | R2 | Shelf-life at 30 °C (days) |
|
|---|---|---|---|---|---|---|
| CE | 155 | 3.94 ± 0.09* | 60,893.52 | ln k = −0.043 T + 8.0442 | 0.9999 | 0.60 |
| 140 | 6.98 ± 0.41 | |||||
| 125 | 14.72 ± 0.17 | |||||
| US-CE | 160 | 2.32 ± 0.34 | 99,466.00 | ln k = −0.0653 T + 11.558 | 0.9953 | 10.24 |
| 150 | 5.73 ± 0.14 | |||||
| 140 | 11.34 ± 0.19 | |||||
| Control | 135 | 15.29 ± 0.00 | 114,772.94 | ln k = −0.085 T + 14.217 | 0.9956 | 81.01 |
| 130 | 22.88 ± 1.75 | |||||
| 125 | 35.77 ± 0.00 |
CE (Cold extraction), US-CE (ultrasound-assisted cold extraction), Control (Hexane), DSC (Differential Scanning Calorimetry), Tox (oxidative induction temperature), tox (oxidative induction time), k (reaction rate constant), T (Reaction temperature), Ea (Activation energy), R2 (Correlation coefficient)
*Values expressed as average ± standard deviation
As mentioned earlier, the physicochemical composition influences the oxidative profile of the oils. Consequently, it can infer that the iodine index in the CE and US-CE treatments (Table 1) affected the tox, since, at a higher degree of unsaturation present in oils, these are more prone to oxidation than those containing smaller amounts, as is the case with the control treatment.
Tan et al. (2002) found oxidative induction time at 140 °C of 37.41 min for canola, 44.86 min for coconut, 21.45 min for corn, 7.53 min for grapeseed, 13.94 min for olive, 12.33 min for peanut, 8.17 min for safflower, 69.55 min for sesame, 20.0 min for soybean and 19.98 min for sunflower. According to the last, CE oil showed similar oxidative characteristics to grapeseed and safflower, while US-CE and control oils had similar characteristics to peanut and olive oils.
Table 3 shows the activation energy (Ea) values for the oils obtained by the different treatments. The CE treatment showed lower Ea, followed by US-CE, and finally, the control. Ea is the minimum energy necessary for a lipid oxidation reaction to occur. The amount of PUFA in the oil may influence these results (Table 2). Since at higher the concentration of these fatty acids, greater is the Ea necessary for the oxidation of lipids (Tan et al., 2001).
According to Torres et al. (2001), Ea for lipid oxidation reactions is in a range from 41,842 to 104,605 J/mol. The Ea values of the oils obtained by CE and US-CE are within said range, but not the control treatment, which had a higher value. This may be because the control treatment had a low YI value and high phenols concentration, which provides greater oxidative stability.
Table 3 shows the linear regression equations (derivated from Eq. 3) for each treatment. The regressions presented a high correlation index (r2 > 0.99). These equations are useful for predicting the oxidation of lipids at different temperatures; therefore, they were used to extrapolate the Tox at room temperature (30 °C) and predict oxidative stability according to the shelf life in accelerated conditions. The accelerated shelf life of the oil obtained in the CE was one that presents the least oxidative stability under excess oxygen conditions. However, the oil extracted with US-CE had 17 times greater stability. These results may be related to the low content of PI and AI reported in Table 1. In the case of the control, its higher shelf life can be due to its low iodine index value and its high concentration of total phenolic compounds, which can give it greater oxidative stability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to thank CONACyT for the scholarship granted to the first author (29432).
Declarations
Conflict of interest
All authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Farsi MA, Lee CY. Optimization of phenolics and dietary fiber extraction from date seeds. Food Chemistry. 108: 977-985 (2008) [DOI] [PubMed]
- AOAC. Official methods of analysis of AOAC international. 18th ed. Method 960.39. Association of Official Analytical Chemists. Washington, DC, USA (2007)
- AOCS. Official methods and recommended practices of the AOCS. 6th ed. Methods Tg 1–64, Ca 5a-40, Cd 8b-90. American Oil Chemists’ Society. Champaingn, IL, USA (2009)
- Berasategi I, Barriuso B, Ansorena D, Astiasarán I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chemistry. 132: 439-446 (2012) [DOI] [PubMed]
- Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 91(6), 791-795 (2009) [DOI] [PubMed]
- Cert A, Moreda W, Pérez-Camino MC. Methods of preparation of fatty acid methyl esters (FAME). Statistical assessment of the precision characteristics from a collaborative trial. Grasas y Aceites. 51: 447-456 (2000)
- Cicero N, Albergamo A, Salvo A, Bua GD, Bartolomeo G, Mangano V, Rotondo A, Stefano VD, Bella GD, Dugo G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Research International. 109: 517-525 (2018) [DOI] [PubMed]
- Colin LA, Jaillais Y. Phospholipids across scales: lipid patterns and plant development. Current Opinion in Plant Biology. 53: 1-9 (2020) [DOI] [PubMed]
- Medina E, de-Castro A, Romero C, Brenes M. Comparison of the concentrations of phenolic compounds in olive oils and others plant oils: Correlation with antimicrobial activity. Journal of Agriculture and Food Chemistry 54: 4954-4961 (2006) [DOI] [PubMed]
- Costagli G, Betti M. Avocado oil extraction processes: method for cold-pressed high-quality edible oil production versus traditional production. Journal of Agricultural Engineering. 46: 467 (2015)
- Delgado AE, Palacio O, Aperador W. Effect of butylated hydroxytoluene (BHT) on the oxidative stability of a lubricant based on sesame oil. Información Tecnológica 26: 81-88 (2015)
- Donetti M, Terry LA. Evaluation of factors affecting shelf-life and quality biomarkers of imported avocado fruit. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 934: 677-682 (2010)
- Duque AMR, Londoño-Londoño J, Álvarez DG, Paz YB, Salazar BLC. Comparación del aceite de aguacate variedad Hass cultivado en Colombia, obtenido por fluidos supercríticos y métodos convencionales: una perspectiva desde la calidad. Revista Lasallista de Investigación. 9: 151-161 (2012)
- FAO. Countries by commodity. Available from: http://www.fao.org/faostat/es/#rankings/countries_by_commodity_imports. Accessed June 25, 2020
- Fernandes GD, Gómez-Coca RB, Pérez-Camino MDC, Moreda W, Barrera-Arellano D. Chemical characterization of commercial and single-variety avocado oils. Grasas y Aceites. 69: 256 (2018)
- Firestone D. Physical and chemical characteristics of oils, fats and waxes (No. L-0671). AOCS Press. Urbana, IL, USA. (2013)
- Flores M, Saravia C, Vergara CE, Ávila F, Valdés H, Ortiz-Viedma J. Avocado oil: characteristics, properties, and applications. Molecules. 24: 2172 (2019) [DOI] [PMC free article] [PubMed]
- Franco A, Bartoli C. The ultrasounds as a mean for the enhancement of heat exchanger performances: an analysis of the available data. In Journal of Physics: Conference Series (Vol. 1224, No. 1, p. 012035). IOP Publishing. (2019)
- Gamble J, Harker FR, Jaeger SR, White A, Bava C, Beresford M, Stubbings B, Wohlers M, Hofman PJ, Marques R, Woolf A. The impact of dry matter, ripeness and internal defects on consumer perceptions of avocado quality and intentions to purchase. Postharvest Biology and Technology 57: 35-43 (2010)
- Gharby S, Harhar H, Guillaume D, Roudani A, Boulbaroud S, Ibrahimi M, Charrouf Z. Chemical investigation of Nigella sativa L. seed oil produced in Morocco. Journal of the Saudi Society of Agricultural Sciences. 14: 172-177 (2015)
- Gharby S, Harhar H, Bouzoubaa Z, Asdadi A, El-Yadini A, Charrouf Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. Journal of the Saudi Society of Agricultural Sciences 16: 105-111 (2017)
- Kowalski B, Ratusz K, Kowalska D, Bekas W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. European Journal of Lipid Sciences and Technology. 106: 165-169 (2004)
- Krumreich FD, Borges CD, Mendonça CRB, Jansen-Alves C, Zambiazi RC. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chemistry. 257: 376-381 (2018) [DOI] [PubMed]
- Liu XX, Liu HM, Li J, Yan YY, Wang XD, Ma YX, Qin GY. Effects of various oil extraction 677 methods on the structural and functional properties of starches isolated from tigernut (Cyperus esculentus) 678 tuber meals. Food Hydrocolloids 95: 262-272 (2019)
- López-Ordaz P, Chanona-Pérez JJ, Perea-Flores MJ, Sánchez-Fuentes CE, Mendoza-Pérez JA, Arzate-Vázquez I, Yáñez-Fernández J, Torres-Ventura HH. Effect of the extraction by thermosonication on castor oil quality and the microstructure oil of its residual cake. Industrial Crops and Products. 141: 111760 (2019)
- Martínez-Padilla LP, Franke L, Xu XQ, Juliano P. Improved extraction of avocado oil by application of sono-physical processes. Ultrasonics Sonochemistry. 40A: 720-726 (2018) [DOI] [PubMed]
- Mostert ME, Botha BM, Plessis LMD, Duodu KG. Effect of fruit ripeness and method of fruit drying on the extractability of avocado oil with hexane and supercritical carbon dioxide. Journal of the Science of Food and Agriculture. 87: 2880-2885 (2007)
- Ostrowska-Ligeza E, Bekas W, Kowalska D, Lobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: Dynamic differential scanning calorimetry and rancimat studies. European Journal of Lipid Science and Technology. 112: 268-274 (2010)
- Pardauil JJ, Souza LK, Molfetta FA, Zamian JR, Rocha-Filho GN, Da-Costa CEF. Determination of the oxidative stability by DSC of vegetable oils from the Amazonian area. Bioresource Technology. 102: 5873-5877 (2011) [DOI] [PubMed]
- Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. Journal of Agriculture and Food Chemistry. 53: 2111-2117 (2005) [DOI] [PubMed]
- Puértolas E, Martinez IM. Olive oil pilot-production assisted by pulsed electric field: impact on extraction yield, chemical parameters and sensory properties. Food Chemistry 167: 497-502 (2015) [DOI] [PubMed]
- Qi B, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study-assessing the influence of composition of vegetable oils on oxidation. Food Chemistry. 194: 601-607 (2016) [DOI] [PubMed]
- Reddy M, Moodley R, Jonnalagadda SB. Fatty acid profile and elemental content of avocado (Persea americana Mill.) oil effect of extraction methods. Journal of Environmental Science and Health Part B. 47: 529–537 (2012) [DOI] [PubMed]
- Riboh DL, Labuza TP. Effect of sine wave temperature cycling on thiamin loss in fortified pasta. Journal of Food Processing and Preservation. 6: 253-264 (1982)
- Rodríguez-Carpena JG, Morcuende D, Estévez M. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: effect on lipid composition, oxidative stability and quality traits. Meat Science. 90: 106-115 (2012) [DOI] [PubMed]
- Russo GL. Dietary n−6 and n−3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochemical Pharmacology. 77: 937-946 (2009) [DOI] [PubMed]
- Simon P, Koman L, Niklová I, Schmidt S. Analysis of the induction period of oxidation of edible oils by differential scanning calorimetry. Journal of the American Oil Chemists’ Society. 77(6): 639-642 (2000)
- Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedicine & Pharmacotherapy. 60: 502-507 (2006) [DOI] [PubMed]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Methods in Enzymology. Vol. 299. Pp 152–178. Academic Press. USA. (1999)
- Tan CP, Man YC, Selamat J, Yusoff MSA. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. Journal of American Oil Chemists’ Society. 78: 1133 (2001)
- Tan CP, Che-Man YB, Selamat J, Yussof MSA. Comparative studies of oxidative stability of edible oils by differential scanning calorimetry and oxidative stability index methods. Food Chemistry 76: 385-389 (2002)
- Tan CX, Chong GH, Hamzah H, Ghazali HM. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. The Journal of Supercritical Fluids. 135: 45–51 (2018a)
- Tan CX, Chong GH, Hamzah H, Ghazali HM. Optimization of ultrasound-assisted aqueous extraction to produce virgin avocado oil with low free fatty acids. Journal of Food Process Engineering. 41: e12656 (2018b)
- Torres A, Guerra M, Rosquete Y. Estimación de la vida útil de una fórmula dietética en función de la disminución de lisina disponible. Ciencia y Tecnología Alimentaria 21: 129-133 (2001)
- Vickers AK. High-efficiency FAMEs analyses using capillary GC. American Laboratory 39: 18 (2007)
- Willför SM, Smeds AI, Holmbom BR. Chromatographic analysis of lignans. Journal of Chromatography A. 1112: 64-77 (2006) [DOI] [PubMed]
- Woolf A, Wong M, Eyres L, McGhie T, Lund C, Olsson S, Wang Y, Bulley C, Wang M, Friel E, Requejo-Jackman C. Avocado oil. In Gourmet and health-promoting specialty oils. AOCS Press. Urbana, IL, USA. pp. 73-125. (2009)
- Wu G, Chang C, Hong C, Zhang H, Huang J, Jin Q, Wang X. Phenolic compounds as stabilizers of oils and antioxidative mechanisms under frying conditions: A comprehensive review. Trends in Food Science & Technology. 92: 33-45 (2019)
- Yang S, Hallett I, Rebstock R, Oh HE, Wolf AB, Kam R, Wong M. Cellular changes in “Hass” avocado mesocarp during cold‐pressed oil extraction. Journal of American Oil Chemists’ Society. 95: 229-238 (2018)
- Yepes-Betancur DP, Sánchez-Giraldo L, Marquez-Cardozo CJ. Extracción termomecánica y caracterización fisicoquímica del aceite de aguacate (Persea americana Mill. cv. Hass). Informador Técnico. 81: 75-85 (2017)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


