Abstract
Choline oxidase catalyzes the oxidation of choline to glycine betaine via betaine aldehyde in glycine betaine biosynthesis and betaine acts as an osmolyte. Choline oxidase has attracted a great deal of attention because of its wide application in clinical and its potential use in enzymatic betaine production. Therefore, the development of efficient methods for overexpression of choline oxidase will be very valuable. In the present study, the choline oxidase gene was amplified from a newly isolated Gram-positive soil Arthrobacter globiformis strain HYJE003 and was cloned into a pET expression vector. Furthermore, the culture conditions were optimized for overexpression of cloned choline oxidase gene in different hosts for periplasmic expression of the enzyme. Expression host system Rosetta-gami2(DE3)pLysS yielded more cell-free protein and 20 fold higher active enzyme compared to any other reported studies. Terrific Broth media were found to be yielding the highest cell biomass, by applying the optimized culture conditions and purification strategy 20,902 U of choline oxidase was produced with a specific activity of 95 U/mg. The optimum pH and temperature for the enzyme activity were found to be 7 and 37 °C, respectively. Finally, we have demonstrated efficient bioconversion of betaine using overexpressed and purified choline oxidase enzyme. The enzymatically produced betaine was estimated by the formation of betaine reineckate and we were able to produce 0.83 molar of betaine from one molar of choline chloride.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02960-z.
Keywords: Arthrobacter globiformis, Betaine, Choline, Choline oxidase, Cloning
Introduction
With the advances in genetic engineering today, the enzyme production, usage and applications are unlimited. Enzymes are explored for industrial-scale catalysts due to their diverse advantages in product selectivity, sensitivity, efficiency and lowest environmental toxicity. Choline oxidase is an oxidoreductase catalyzing two-step chemical reaction of four-electron oxidative conversion of choline to glycine betaine with betaine aldehyde as an intermediate. Betaine accumulation in the cytoplasm of the cells helps to prevent dehydration and plasmolysis in adverse hyperosmotic environmental conditions. Choline oxidase is the only enzyme that accepts either choline or the reaction intermediate, betaine aldehyde as a substrate in the oxidation of choline to betaine. On the other hand, betaine is a stable, non-toxic compound and the principle physiological role of betaine is an osmolyte and a methyl donor (Deole and Hoff 2020). As an osmolyte, betaine protects cells, inhibits hyperosmotic induced apoptosis, protects proteins and enzymes from ionic/osmotic stress. Betaine acts as a methyl donor in transmethylation, playing an essential role in cell membrane integrity, signal transduction, neurotransmitter synthesis, methionine cycle and embryonic development (Joselit et al. 2018). Dietary betaine supplementation plays a protective role against obesogenic diet-induced hepatic steatosis (Cordero et al. 2013), promotes growth, carcass characteristics, improves meat characteristics, weight gain and feed conversion ratio in domestic animals (Liu et al. 2019). Natural betaine is currently extracted and purified from beetroot, sugar beet and its derivatives (Rivoira and Studzinska 2017). Because of high betaine demand, it is chemically produced in the form of betaine hydrochloride (HCL). Recent comparative studies between natural betaine and betaine HCL clearly illustrate that the chemically synthesized betaine HCL is not as effective as the natural betaine (Amerah 2013).
Soil bacteria are considered as economical source of useful biocatalysts in numerous industrial processes. Screening, isolation and identification of new choline oxidase producing microorganisms are considered very important due to the range of applications that enzyme is involved in. A very well-established application of choline oxidase is its use in enzymatic determination of phospholipids, choline biosensor, mustard agent detection, measuring the cholinesterase inhibitory activities (Arduini et al. 2015) and in the development of transgenic plants with higher stress tolerance (Ahmad et al. 2008). Choline oxidase is also used for the development of biodegradable bleaching systems in detergent formulations to remove stains such as red wine, fruits, coffee and tea efficiently from the different fabric types (Dannacher 2006). In spite vast application of choline oxidase, there are very few studies that were reported on the source of active enzyme and effective ways of overexpression and purification methods. Choline oxidase was first isolated from rat liver in 1938 (Mann et al. 1938), Ikuta reported the purification of the enzyme from soil bacterium with 12.5 U/mg of protein through a traditional methodology (Ikuta et al. 1977). Production from the cell-free extract of fungi Cylindrocarpon didymum M-l revealed very limited amount of enzyme and involves very tedious methods of ammonium sulfate fractionation, ion exchange to gel filtrations column purification (Tani et al. 1979). Studies were also reported on the development of an osmotolerant phenotype from a betaine biosynthesis mutant E. coli strain by transforming the choline oxidase gene (Rozwadowski et al. 1991). Furthermore, most of the expression and purification methods reported are very tedious, involve traditional cloning, salting out the expressed protein, followed by multiple ion exchange or gel filtration chromatographic techniques. Most of the expressed choline oxidase was lost during the purification procedures and its overall activity was also decreased, because enzymes get exposed to a harsh environmental condition during the purification process. Overexpression from the Arthrobacter nicotianae lead to only a small amount of active enzyme and high yields of inclusion bodies (Ribitsch et al. 2010). Our previous study has demonstrated the oxidative potential of choline oxidase gene from different soil bacterial strains (Lokesha et al. 2019). The enzyme yield can be increased by strain improvement up to a certain extent or by cloning, overexpression and optimization of production conditions. In view of this in the present study, we have developed an effective, efficient yet simple straightforward method for the production of active choline oxidase enzyme. Furthermore, the produced enzyme was investigated for its application in enzymatic betaine production.
Materials and methods
Choline oxidase gene isolation and characterization
Choline oxidase gene from the previously isolated Arthrobacter globiformis strain HYJE003 was amplified using oligonucleotide primers containing restriction enzyme site for EcoR I-F_AATTGAATTCGATGCACATCGACAACATCGAG and Hind III-R_AGCTAAGCTTAGGCGAGGGCCGCGCTCA. The PCR was carried out with Eppendorf master cycler in the presence of 5% DMSO following cycling condition of 5 min at 94 °C, followed by 35 cycles of 1 min at 94 °C, 30 s at 55 °C, 2 min at 72 °C and a final extension of 10 min step at 72 °C in a total volume of 20 µL using 20 ng of template DNA and 2 U of Pfu Taq DNA polymerase (Promega, India). The resulting amplicon was then purified by agarose gel electrophoresis using the QIA quick Gel Extraction kit (Qiagen, India) following the manufacturer’s protocol. The amplified choline oxidase gene was then sequenced to confirm the gene sequence and the sequenced gene information was deposited at NCBI (National Center for Biotechnology Information) data base.
Cloning of choline oxidase gene into an expression vector
Both the amplified choline oxidase gene and pET22b(+) vector were digested with 10U EcoR I and 10U Hind III restriction enzyme (Thermo Fisher Scientific, India) in a double digestion reaction following manufacture protocol. The digested products were gel purified by QIA quick Gel Extraction column. The pET22b(+) plasmid was treated with 5U of calf intestine alkaline phosphatase (Thermo Fisher Scientific, India) for 2 h at 37 °C to minimize plasmid self-ligation. The choline oxidase gene (11 ng) was then ligated into pET22b(+) (100 ng) with a vector to insert ratio of 3:1 using 5U of T4 DNA ligase (Thermo Fisher Scientific, India) in a total volume of 10 μL by incubating at 16 °C for overnight in a water bath. From the ligation mixture, 5 µL was directly used for transformation into E. coli strain XL10-Gold ultracompetent cells by following the manufacture instruction. The transformation colonies were screened for the presence of choline oxidase gene using vector-specific T7 primers extension colony PCR amplification. From the positive clone, the plasmid was isolated, sequenced, initially transformed into E. coli strain of BL21(DE3) and later transformed into BL21(DE3)pLysS and Rosetta-gami2(DE3)pLysS (Thermo Fisher Scientific, India) competent cells by following the manufacture instruction. From the PCR confirmed colonies, glycerol stocks were prepared and stored for future use.
Optimization of expression conditions and purification of recombinant choline oxidase
The transformed cells from the glycerol stocks were plated on LB (Luria-Bertani) agar containing 50 μg/μL of ampicillin and incubated at 37 °C for overnight. Form the culture plate, single colony from each of the host strains was grown on 50 mL of LB medium containing 50 μg/μL of ampicillin on a rotary shaker (160 rpm) at 37 °C overnight. On the next day, 50 mL of starter culture was inoculated onto 1000 mL of LB medium containing 50 μg/μL of ampicillin and incubated under the same condition as mentioned above. Induction was performed by the addition of 0.5 mM IPTG (isopropyl β-d-1-thiogalactopyranoside) once the culture reached OD (optical density) of 0.6 at 600 nm (nanometer) and incubated at 160 rpm 37 °C for further 6 h. The cells were harvested by centrifuging at 8000 rpm at 4 °C for 10 min, later these cells were washed and resuspended in 100 mL of binding buffer (50 mM Tris-Hcl pH 8.0, 10 mM imidazole, 100 mM NaCl, 0.1 mM EDTA and 1 mM PMSF). The cells were disrupted by sonication at 40 kHz for 10 min, amplitude of 80% and cycles of 20 s. The cell lysate was centrifuged for 20 min at 13,000 rpm at 4 °C and 20 µL of the supernatant was loaded on the SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel) to check the expression level of the recombinant protein.
The choline oxidase enzyme present in the supernatant was purified by using 6× histidine amino acids tag at the C-terminal end of the expressed protein. Purification was performed in AKTA Explorer (GE Healthcare, India) FPLC (Fast Protein Liquid Chromatography) system using 5 mL of HisTrap HP column (GE Healthcare, India), the supernatant was loaded into a pre-equilibrated column with 10CV (Column Volume) of binding buffer. The non-specific bound proteins were washed off by running 10CV of wash buffer (50 mM Tris-Hcl, 100 mM NaCl, 20 mM imidazole, 0.1 mM EDTA and 1 mM PMSF). Finally, the column bound protein was eluted by gradient elution with elution buffer (50 mM Tris-Hcl, 50 mM NaCl, 500 mM Imidazole, 0.1 mM EDTA and 1 mM PMSF). The purified fractions were pooled together and buffer exchanged to 10 mM tris buffer.
Protein quantification was performed using Bradford assay and the enzymatic activity was measured by enzymatic assay. The assay mixture consists of 97 mM tris buffer pH 8.0, 0.14 M choline chloride, 33 µM EDTA, 2.2 mM KCl, 0.48 mM aminoantipyrine, 2.1 mM phenol and 5 U/mL of peroxidase. The enzymatic reaction was started by the addition of 50 µL purified choline oxidase into 3 mL of assay mixture and the increase in optical density at 500 nm was measured for 5 min in a spectrophotometer thermostatted at 37 °C. From the linear portion of the curve, the ΔOD was calculated for per minute and the experimental results were reported from the mean of three experiments ± SD (Standard deviation).
where 3.05 = Volume (in millilitres) of assay; df = Dilution factor; 12 = Millimolar extinction coefficient of Quinoneimine Dye at 500 nm under the conditions of the assay; 0.5 = μM of Quinoneimine Dye formed per μM of H2O2; 0.05 = Volume (in millilitre) of choline oxidase used in the assay.
One unit of choline oxidase activity was defined as the formation of 1 μM of choline to betaine aldehyde hydrogen per minute at pH 7.5 at 37 °C.
Effect of different culture media on the yield of active choline oxidase was determined by culturing Rosetta-gami2(DE3)pLysS harboring pET22-Choline oxidase gene in LB (Luria-Bertani), TB (Terrific Broth) and SB (Super Broth) medium. A single colony was inoculated into 50 mL of the medium and then 1000 mL of each medium was inoculated with pre-culture bacteria. The induction was done at 0.6 OD with 1 mM IPTG and incubated at 37 °C, 160 rpm for 6 h. The cultures were harvested and cell pellets were sonicated, the protein was purified from the cell lysate and recombinant enzyme activity was measured by performing enzyme assay as mentioned above.
Optimum culturing temperature and induction time was evaluated to produce more cell-free protein from the host strain Rosetta-gami2(DE3)pLysS-pET22-Choline oxidase. Conical flasks containing 1000 mL of TB-ampicillin medium was inoculated with 50 mL of overnight pre-culture and incubated at 200 rpm 25 °C, 30 °C and 37 °C. Induction was performed at 0.3, 0.6, 1.2 and 1.8 OD with 1 mM IPTG and cultures were harvested at four post-induction periods of 6, 8, 16, and 24 h. Cell pellets from all the cultures were collected and then the cells were disrupted by sonication. Enzyme activity assay was performed for purified enzyme fraction from the cell lysate supernatant.
Large scale recombinant choline oxidase was produced from Rosetta-gami2(DE3)pLysS-pET22-Choline oxidase under optimized conditions. Cells were harvested, lysed by sonication and the expressed choline oxidase was purified using 15 mL His tag column in the FPLC system. The purity of eluted fractions was evaluated by 10% SDS-PAGE, pure fractions were polled together and buffer exchanged against 10 mM tris buffer. The total protein and enzyme activity were established by Bradford method and enzymatic assay as mentioned above. The optimum temperature of recombinant choline oxidase activity was assayed at different temperature (20 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C and 80 °C). Similarly, optimum pH for recombinant enzyme activity was also determined by incubating the enzyme at 37 °C in buffers with 3, 4, 5, 6, 7, 8, 9, 10 and 11 pH condition and followed by the enzymatic assay as described above.
Betaine production using recombinant choline oxidase
The purified 8000 units of choline oxidase enzyme was immobilized to 20 mL of Ni-Sepharose resin and placed inside the rotating cylinder of SpinChem Rotating Bed Reactor (RBR). The RBR was placed in a 500 mL vessel containing 13.9 g of choline chloride dissolved in the 100 mL of 10 mM Tris-Hcl buffer (pH 8.0). 600 units of catalase was added to the enzymatic reaction and RBR was spun at 100 rpm at 37 °C for 12 h. As the RBR spins, a continuously circulating flow was developed in the reactor. The reaction solution is rapidly aspirated from the bottom of the vessel, percolated through the solid phase and quickly returned to the vessel. After 12 h of the incubation period, the immobilized enzyme was removed and the amount of betaine formed was determined by the formation of betaine reineckate (Bandelin 1953) and the amount of betaine was calculated from a standard curve obtained with pure betaine.
Results
Cloning and selection of expression host system
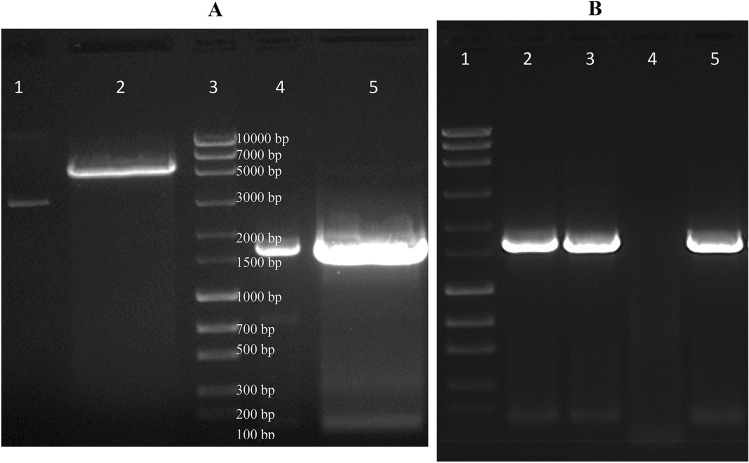
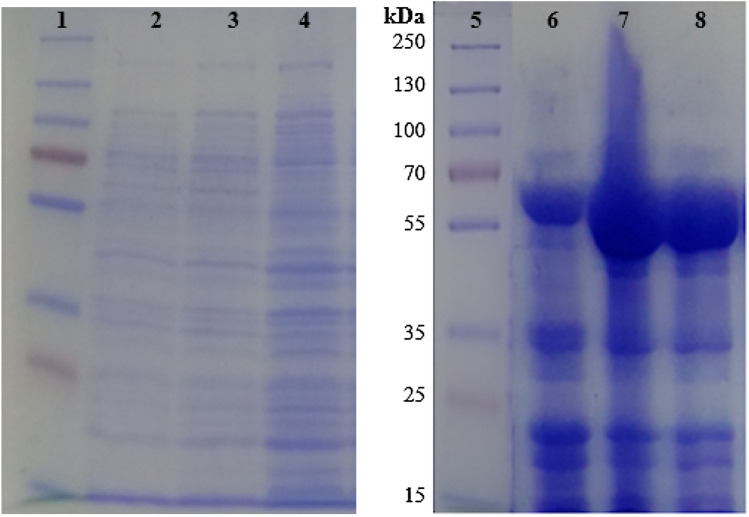
Amplified choline oxidase gene from the strain HYJE003 was sequenced and the gene sequence was deposited to NCBI database with Accession number MK988621. In the first step of protocol development for expression and purification of the choline oxidase gene, the gene was cloned into T7 promoter-driven overexpression vector pET22b(+) by double digestion and ligation (Fig. 1A). Gene sequencing data from the positive clone (Fig. 1B) confirmed the choline oxidase gene and the clone was transformed into BL21(DE3) expression host, but a significant amount of expressed protein was found in inclusion bodies (data not shown). Furthermore, the pLysS expression host yielded much higher expressed protein in cell-free extract than BL21(DE3) expression host. Harvested cells were loaded onto SDS PAGE gel (Fig. 2) and the recovered supernatant was used for enzyme purification through column chromatography. For the purified protein, an enzymatic assay was conducted, Rosetta-gami2(DE3)pLysS expression host cells yielded the highest level of active recombinant choline oxidase with 95 U/mg of specific activity in comparison with the expression host BL21(DE3) and BL21(DE3)pLysS (Table 1).
Fig. 1.
Restriction digestion and colony PCR images of PET22b (+) and choline oxidase gene. A 1-Un digested plasmid, 2-Double digested plasmid, 3-100 bp DNA Marker, 4-Un digested choline oxidase gene, 5-Double digested choline oxidase gene. B 1-DNA Marker, 2-Positive clone, 3-Positive clone, 4-Negative control and 5-Positive control
Fig. 2.
SDS-PAGE images of uninduced and induced cultures. 1-Protein marker, 2-Uninduced BL21(DE3), 3-Uninduced BL21(DE3)pLysS, 4-Uninduced Rosetta-gami2(DE3)pLysS, 5-Protein marker, 6-Induced BL21(DE3), 7-Induced BL21(DE3)pLysS, 8-Induced Rosetta-gami2(DE3)pLysS,
Table 1.
Summary of the recombinant choline oxidase purification from different E.coli strains
| Strains | Biomass (OD600) | Protein (mg) | Total activity (U) | Specific activity (U/mg) |
|---|---|---|---|---|
| BL21(DE3) | 4.8 | 98 | 4850 | 50 |
| BL21(DE3)pLysS | 3.7 | 120 | 6240 | 52 |
| Rosetta-gami2(DE3)pLysS | 2.9 | 96 | 9120 | 95 |
Culture media, expression optimization and large-scale enzyme production
The ability of Rosetta-gami2(DE3)pLysS harbouring pET22b(+)-Choline oxidase gene to produce soluble choline oxidase in different culture media (LB, TB and SB) was evaluated. The total cell biomass and enzyme obtained from different media are presented in Table S1. TB media were found to be generating the highest cell biomass and active enzyme in comparison with other culture media.
The culturing temperature, induction growth phase (early–mid–late exponential and stationary growth stages) and post-induction harvest period of Rosetta-gami2(DE3)pLysS harbouring pET22b(+)-Choline oxidase to produce choline oxidase was determined. The tabulated result in Tables S2, S3 indicates that the highest yield of active enzyme was obtained at a culture temperature of 37 °C, mid-exponential growth phase induction (0.6OD) and 16 h of the post-induction harvest period. Interestingly the highest cell biomass was observed when induced with IPTG at the stationary growth phase (OD of 1.8 at 37 °C); however, the enzyme yield was decreased.
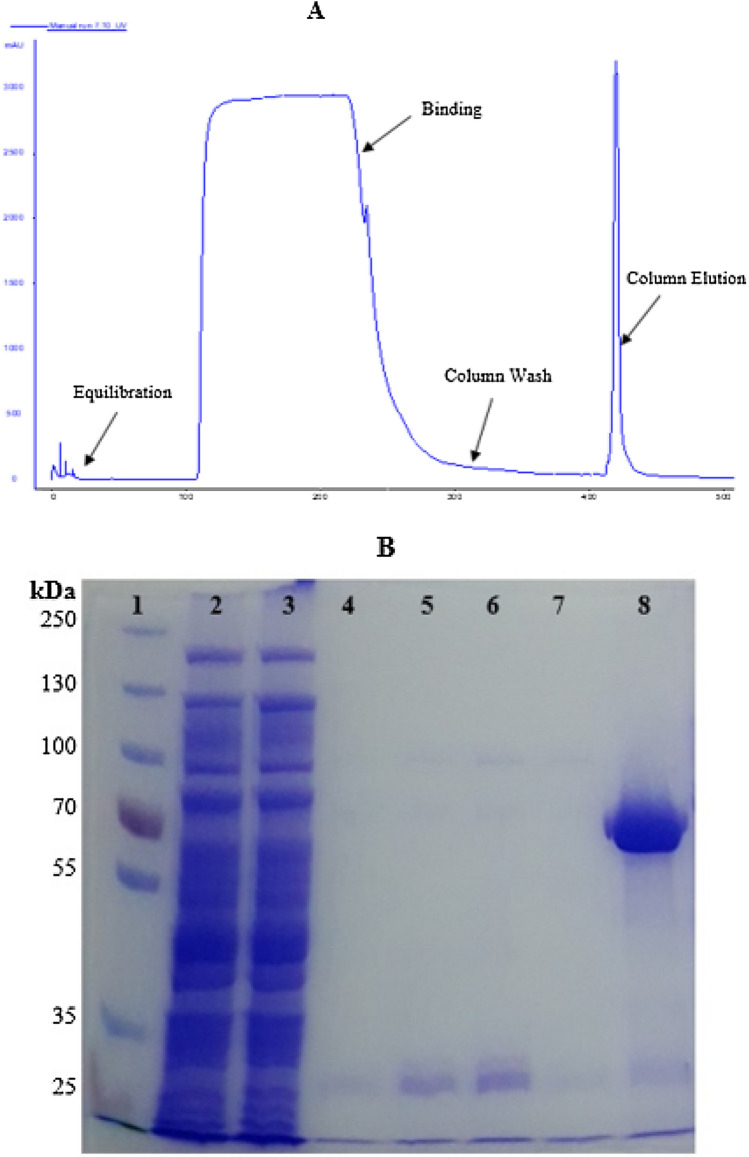
With the optimized condition of TB-Amp culture media, incubation at 37 °C, induction with 1 mM IPTG at 0.6 OD (at 600 nm) and 16 h post-induction harvest, the large-scale enzyme was produced from Rosetta-gami2(DE3) pLysS-pET22b-Choline oxidase. Overexpressed recombinant choline oxidase was purified by Ni-NTA column affinity chromatography. Highest amount of pure choline oxidase was eluted in 150 mM of imidazole (Fig. 3A), all the pure fractions were pooled and buffer exchanged with 10 mM tris buffer at pH 8 (Fig. 3B). After the enzyme purification, the total enzyme activity and total protein concentration were measured by enzymatic and Bradford assay. Finally, using the optimized conditions, 220 mg of protein with 95 U/mg specific active recombinant choline oxidase was produced from 1 L of the culture batch.
Fig. 3.
Purification of choline oxidase gene through AKTA FPLC and SDS-PAGE image. A Purification plot from AKTA FPLC system. B 1-Protein maker, 2 and 3-Column flow through at different time point, 4–7-Column wash at different time point, 8-Pooled fraction of purified choline oxidase
Enzymatic betaine production using purified choline oxidase
The optimum activity of the purified recombinant choline oxidase was determined at different temperatures and pH. The optimum temperature for choline oxidase activity was found to be 37 °C (Fig. 4A). However, the enzyme retained more than 60% of activity at temperatures range from 20 to 50 °C. Under various pH conditions tested, the maximal activity of the enzyme was observed at pH 7 (Fig. 4B). Enzymatic betaine production was carried out in RBR reactor using 8000U of recombinant choline oxidase enzyme. At the end of 12 h reaction, amount of betaine formed was assessed by the formation of betaine reineckate. Using purified enzyme, we were able to produce 0.83 molar of betaine from one molar of choline chloride (Table S4).
Fig. 4.
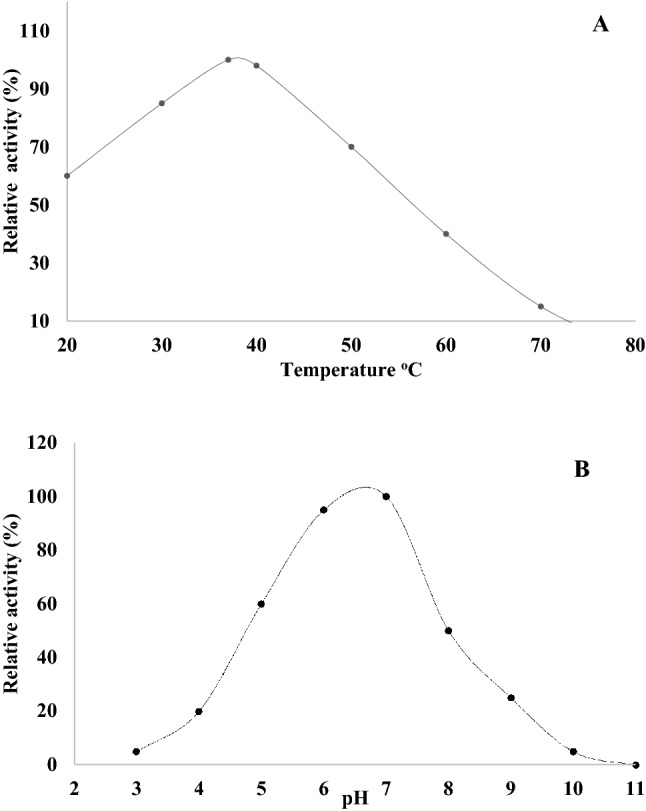
Effect of temperature and pH on the recombinant choline oxidase enzyme activity. A Effect of temperature: enzyme activity was assayed in tris buffer, pH 7.0 based on the H2O2 formation at mentioned temperature. B Effect of pH: Buffer system used-glycin-HCL (pH 3.0), citrate-sodium citrate (pH 4.0), CH3COOH–CH3COONa (pH 5.0), NaH2PO4–NaHPO4 (pH 6.0), Tris-HCL (pH 7.0–9.0) and Na2CO3 NaHCO3 (pH 10.0–11.0)
Discussion
Choline oxidase enzyme has a great importance in industrial and clinical application. The enzymes with high stability and overexpression could be very valuable. In the previous study, two strains of Arthrobacter from industrially polluted soil were isolated and their ability of betaine bioconversion was evaluated. The homologous expression of the choline oxidase from these isolated strains was found to be very inefficient and only able to convert less than 10% of fed choline chloride to betaine (Lokesha et al. 2019). In this view, choline oxidase gene from Arthrobacter globiformis strain HYJE003 was isolated and cloned into pET22(b+) for choline oxidase overexpression. In bacterial overexpression, the metabolic burden was usually observed in heterologous expression. A high rate of recombinant proteins was accumulated in insoluble aggregates as a consequence of host folding machinery and hydrophobic interaction constitute the key factor in the formation of inclusion bodies (Sorensen and Mortensen 2005; Ma et al. 2013). To overcome this, pelB leader sequence was deployed which consists of 22 N terminal amino acid residues, which direct the expressed protein to bacterial periplasm. Once the recombinant protein is in the periplasm, the pelB sequences were removed by signal peptidase, this enhances protein stability and reduces the formation of inclusion bodies. To obtain very high active protein, simplify the purification and minimize the harsh treatment of enzyme in purification procedure, 6× His tag labeling at the C-terminal end of the gene was deployed while cloning into the pET expression vector (Rosano German and Ceccarelli Eduardo 2014). Selection of the right expression host for overexpression of the recombinant protein is a very essential step, therefore, we evaluated the three expression hosts system such as BL21(DE3), BL21(DE3)pLysS and Rosetta-gami2(DE3)pLysS. Among the three host systems, the Rosetta(DE3)pLysS produced a very high level of active enzyme in comparison to the other host strains used in the study. E. coli hosts with DE3 strains are preferred for the expression of the recombinant enzyme in plasmid driven by T7 promoters. These strains contain a copy of T7 polymerase to efficiently transcribe the T7 promoter present upstream of the gene and pPARE plasmid which provides the tRNA genes for the rare codon in the expressing gene (Kaur and Kumar 2018).
The optimum cultural conditions and media, induction phase, condition of pre- and post-induction harvest will influence cell density and enhancing the recombinant protein production (Khow and Suntrarachun 2012). In view of this, all the cultural conditions were evaluated from different cultural media (LB, TB and SB), induction growth phase of early–mid–late exponential and stationary growth stages (0.3, 0.6, 1.2 and 1.8 OD) and the post-induction harvest period of 6, 8, 16, 24 h at three different incubation temperatures (25 °C, 30 °C and 37 °C) for overexpression of choline oxidase. As cultural conditions played a very important role in the expression of recombinant choline oxidase, induction at different phases of growth cycle revealed that early exponential phase biomass accumulation was lower than the stationary growth phase. When the induction was at early-stage bacterial metabolic resources were channeled for recombinant protein production and the lower cellular growth rate was observed and our results were also are in line with the previous reports (Sivashanmugam et al. 2009; Rosano German and Ceccarelli Eduardo 2014). Our finding also suggested that post-induction temperature and the induction time affect the yield of recombinant choline oxidase production and our result is also in line with previous reports (Khow and Suntrarachun 2012). Research data reveal that the media composition of TB, mid induction with 1 mM IPTG, post-harvest time of 16 h and incubation temperature of 37 °C yielded 20,902 U (95 U/mg) of choline oxidase.
Previously, Ribitsch and group (2010) had cloned the choline oxidase gene from Arthrobacter nicotianae into E coli and observed a high yield in inclusion bodies with a very small amount of active enzyme. Many overexpressed proteins in E. coli forms inclusion bodies, recovery of bioactive protein from them is one of the main hurdles during large-scale production. In addition, it needs a laborious purification process and that drastically affects the yield (Singh et al. 2015). To overcome this problem, pelB signal peptide was deployed in our cloning strategy; our result confirms that the use of pelB as leader sequence has significantly increased the yield of choline oxidase in the supernatant and a very negligible amount in inclusion bodies.
Various research groups have used homologous expression systems and traditional methodology for purification of choline oxidase. Ikuta and group used acetone and ammonium sulfate fractionations, ion exchange column followed by Sephadex G-200 column for purification of choline oxidase from Arthrobacter globiformis and reported 12.8 U/mg of protein from a two-litre culture batch (Ikuta et al. 1977). Tani and coworkers (1979) used ammonium sulfate fractionation, ion exchange, Sephadex G-150 column followed by hydroxylapatite treatment and second Sephadex G-150 column chromatography for purification of choline oxidase from Cylindrocarpon didymium M-1 and obtained 24 U/mg of choline oxidase. In a recent study, using ammonium sulfate fractionation, ion exchange, Sephacryl column followed by hydroxylapatite treatment, second Sephacryl S-200 column chromatography choline oxidase was purified from Fusarium oxysporum V2 and reported 32.1 U/mg of purified choline oxidase (Enokibara 2012). Gadda and coworkers used E. coil heterologous expression system for the overexpression of choline oxidase gene from Halomonas elongate. 1.8 U/mg of choline oxidase was purified using ammonium sulfate fractionation and ion exchange column chromatography (Gadda and McAllister Wilkins 2003). In another study, choline oxidase gene was isolated from Arthrobacter globiformis and cloned to pET20b(+) expression vector. Further choline oxidase enzyme was overexpressed in E. coli and expressed protein was purified using ammonium sulfate fractionation followed by DEAE-Sepharose column and 5.3 U/mg of enzyme was purified (Fan et al. 2004). In contrast, in our study, we have attached a His tag to recombinant protein and purified the recombinant protein using Ni-NTA affinity column chromatography and 220 mg of recombinant choline oxidase with 95 U/mg specific activity was obtained. Table 2 clearly illustrates that use of Rosetta(DE3)pLysS host system and cloning strategy of pelB and His tag had yielded 300% higher specific activity and 20-fold increase in total activity in comparison with earlier studies. In earlier studies, researchers have obtained choline oxidase gene with a molecular weight of 71 kDa (Ikuta et al. 1977) and 66 kDa (Ohta Fukuyama et al. 1980). In contrast, our SDS-PAGE result indicates that recombinant choline oxidase molecular weight is 60 kD. Therefore, the activity of enzyme was tested at various temperatures and pH and the result indicates that enzyme showed optimum activity at 37 °C with pH 7. Finally, we have investigated the synthesis of betaine using our recombinant choline oxidase, result confirms that 8000U of enzyme yielded 83% of betaine in 12 h.
Table 2.
Comparison of present and earlier reported study on choline oxidase gene expression and purification
| Gene source | Expression host | Total activity (unit) | Total protein (mg) | Specific activity (U/mg) | Study |
|---|---|---|---|---|---|
| A. globiformis | A. globiformis | 410 | 32 | 12.80 | Ikuta et al. (1977) |
| Cylindrocarpon didymium M-1 | Cylindrocarpon didymium M-1 | 1002 | 186 | 24.0 | Yoshiki et al. (1979) |
| Halomonas elongata | E.coli | 20 | 11 | 1.8 | Gadda and McAllister Wilkins (2003) |
| A. globiformis | E.coli | 940 | 185 | 5.3 | Fan et al. (2004) |
| Fusarium oxysporum V2 | Fusarium oxysporum V2 | 61 | 1.9 | 32.1 | Enokibara (2012) |
| A. globiformis strain HYJE003 | E.coli | 20,902 | 220 | 95.0 | Present study |
In conclusion, present study we had optimized the host cell growth parameter, pre- and post-induction culture condition and purification procedure for choline oxidase overexpression. Using the cloning strategy, optimized culture conditions and purification method the recombinant choline oxidase production was increased to 20-folds with 300% more specific active enzyme than any other reported studies. Further studies on enzyme kinetics, enzyme immobilization and reactor optimization will lead to the commercial application of this enzyme in large-scale betaine production.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 13 KB) Table S1: Effect of different type of culture media on choline oxidase gene expression in Rosetta-gami2(DE3)pLysS expression host.
Supplementary file2 (DOCX 13 KB) Table S2: Impact of pre-induction growth on choline oxidase expression.
Supplementary file3 (DOCX 13 KB) Table S3: Effect of post-induction incubation temperature and incubation period on choline oxidase expression.
Supplementary file4 (DOCX 13 KB) Table S4: Estimation of betaine through the spectrophotometer.
Acknowledgements
None.
Authors’ contributions
Conceptualization—SL, investigation—SL and YSRK, methodology—PSSG and SL, resources—PG, writing—review and editing—SL, HMA and YSRK.
Funding
None.
Availability of data and materials
All datasets generated or analyzed during this study are included in the manuscript and/or in the Supplementary Files.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
All the authors undersigned, give consent for the publication of identifiable details, which can include photographs and/or tables and/or details within the text (manuscript) to be published in the Journal.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Ahmad R, Kim MD, Back KH, Kim HS, Lee HS, Kwon SY, Murata N, Chung WI, Kwak SS. Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt and drought stresses. Plant Cell Rep. 2008;27(4):687–698. doi: 10.1007/s00299-007-0479-4. [DOI] [PubMed] [Google Scholar]
- Amerah AM. The differences between natural betaine and betaine hydrochloride. Int Poult Prod. 2013;22:11–13. [Google Scholar]
- Arduini F, Scognamiglio V, Covaia C, Amine A, Moscone D, Palleschi GA. Choline oxidase amperometric bioassay for the detection of mustard agents based on screen-printed electrodes modified with prussian blue nanoparticles. Sensors. 2015;15(2):4353–4367. doi: 10.3390/s150204353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelin P. The estimation of betaine and choline in mixtures. J Am Pharm Assoc. 1953;42(7):442–443. doi: 10.1002/jps.3030420715. [DOI] [PubMed] [Google Scholar]
- Cordero P, Gomez Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013;8(1):105–113. doi: 10.1007/s12263-012-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannacher J. Catalytic bleach most valuable applications for smart oxidation chemistry. J Mol Catal A Chem. 2006;251(1–2):159–176. doi: 10.1016/j.molcata.2006.02.031. [DOI] [Google Scholar]
- Deole R, Hoff WD. A potassium chloride to glycine betaine osmoprotectant switch in the extreme halophile Halorhodospira halophila. Sci Rep. 2020;10:3383. doi: 10.1038/s41598-020-59231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokibara S. Purification and characterization of an alkaliphilic choline oxidase of Fusarium oxysporum. Biosci Biotechnol Biochem. 2012;76(12):2219–2224. doi: 10.1271/bbb.120513. [DOI] [PubMed] [Google Scholar]
- Fan F, Ghanem M, Gadda G. Cloning, sequence analysis, and purification of choline oxidase from Arthrobacter globiformis: a bacterial enzyme involved in osmotic stress tolerance. Arch Biochem Biophys. 2004;421(1):149–158. doi: 10.1016/j.abb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Gadda G, McAllister Wilkins EE. Cloning, expression, and purification of choline dehydrogenase from the moderate halophile Halomonas elongata. Appl Environ Microbiol. 2003;69(4):2126–2132. doi: 10.1128/AEM.69.4.2126-2132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S, Imamura S, Misaki H, Horiuti Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J Biochem. 1977;82(6):1741–1749. doi: 10.1093/oxfordjournals.jbchem.a131872. [DOI] [PubMed] [Google Scholar]
- Joselit Y, Nanobashvili K, Jack Roberts C. Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr Diabetes. 2018;8:41. doi: 10.1038/s41387-018-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Kumar A. Strategies for optimization of heterologous protein expression in E. coli: roadblocks and reinforcements. Int J Biol Macromol. 2018;106:803–822. doi: 10.1016/j.ijbiomac.2017.08.080. [DOI] [PubMed] [Google Scholar]
- Khow O, Suntrarachun S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac J Trop Biomed. 2012;2(2):159–162. doi: 10.1016/S2221-1691(11)60213-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yuan Y, Sun C, Balasubramanian B, Zhao Z, An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9(8):506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokesha S, Ravi Kumar YS, Sonia Gaur, Sujan Ganapathy PS, Arjun HM, Gaur P. Arthrobacter strains from industrial polluted soil and its oxidative potential of choline oxidase gene. J Pure Appl Microbiol. 2019;13(3):1847–1854. doi: 10.22207/JPAM.13.3.62. [DOI] [Google Scholar]
- Ma P, Varela F, Magoch M, Silva AR, Rosário AL. An efficient strategy for small-scale screening and production of archaeal membrane transport proteins in Escherichia coli. PLoS ONE. 2013 doi: 10.1371/annotation/ba41a7db-2c22-4ffc-b603-526534594a51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PJ, Woodward HE, Quastel JH. Hepatic oxidation of choline and arsenocholine. Biochem J. 1938;32(6):1024–1032. doi: 10.1042/bj0321024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Fukuyama M, Miyake Y, Emi S, Yamano T. Identification and properties of the prosthetic group of choline oxidase from Alcaligenes sp. J Biochem. 1980;88(1):197–203. [PubMed] [Google Scholar]
- Ribitsch D, Winkler S, Gruber K, et al. Engineering of choline oxidase from Arthrobacter nicotianae for potential use as biological bleach in detergents. Appl Microbiol Biotechnol. 2010;87(5):1743–1752. doi: 10.1007/s00253-010-2637-9. [DOI] [PubMed] [Google Scholar]
- Rivoira L, Studzinska SM. New approaches for extraction and determination of betaine from Beta vulgaris samples by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409:5133–5141. doi: 10.1007/s00216-017-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano German L, Ceccarelli Eduardo A. Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol. 2014;5:172. doi: 10.3389/fmicb.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozwadowski KL, Khachatourians GG, Selvaraj G. Choline oxidase, a catabolic enzyme in Arthrobacter pascens, facilitates adaptation to osmotic stress in Escherichia coli. J Bacteriol. 1991;173(2):472–478. doi: 10.1128/jb.173.2.472-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Upadhyay V, Upadhyay AK. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;14:41. doi: 10.1186/s12934-015-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivashanmugam A, Murray V, Cui C, Zhang Y, Wang J, Li Q. Practical protocols for production of very high yields of recombinant proteins using Escherichia coli. Protein Sci. 2009;15(5):936–948. doi: 10.1002/pro.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact. 2005;4:1. doi: 10.1186/1475-2859-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani Y, Mori N, Ogata K, Yamada H. Production and purification of choline oxidase from Cylindrocarpon didymum M–1. Agric Biol Chem. 1979;43(4):815–820. doi: 10.1080/00021369.1979.10863527. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (DOCX 13 KB) Table S1: Effect of different type of culture media on choline oxidase gene expression in Rosetta-gami2(DE3)pLysS expression host.
Supplementary file2 (DOCX 13 KB) Table S2: Impact of pre-induction growth on choline oxidase expression.
Supplementary file3 (DOCX 13 KB) Table S3: Effect of post-induction incubation temperature and incubation period on choline oxidase expression.
Supplementary file4 (DOCX 13 KB) Table S4: Estimation of betaine through the spectrophotometer.
Data Availability Statement
All datasets generated or analyzed during this study are included in the manuscript and/or in the Supplementary Files.