Abstract
Yeast cell death is triggered when essential nutrients such as potassium and lipid are limited but ammonium is in excess. When ammonium and glucose were maintained at 100% of the normal concentration while all the other essential nutrients in yeast nitrogen base (YNB) were reduced to 2%, yeast growth was halted by ammonium toxicity. Yeast started to grow again when either ammonium was also reduced to 2% or gluconate was added, but simultaneously adding gluconate as well as reducing all the nutrients except glucose 50-fold revived yeast growth to a greater extent, i.e. a quarter of the normal growth. Gluconate, as well as formate and alginate, stimulated yeast growth by buffering the drop in pH. Yeast cells were seemingly more susceptible to low pH under the nutrient-limited conditions, entering the stationary phase at pH higher than that of the normal condition. Carboxylate salts may prove a cost-efficient replacement for large proportions of the essential nutrients as yeast cells, in the presence of 2 mg ml−1 gluconate, could still achieve nearly 90% of the normal growth when cultured in only 10% of the normal YNB concentration.
Keywords: Ammonium toxicity, Carboxylate, Gluconate, Nutrient limitation, Yeast growth
Introduction
A basic yeast medium contains a nitrogen source, a carbon source, and essential nutrients such as salts, vitamins, and trace elements. Ammonium, often in the form of ammonium sulphate, continues to be the preferred nitrogen source for yeast culture due to its low cost compared to other sources such as amino acids, as well as its ability to support rapid growth (Godard et al. 2007). High levels of ammonium, however, can lead to toxicity and reduce plant growth (Esteban et al. 2016; Britto and Kronzucker 2002) whereas bacteria are highly resistant to ammonium toxicity (Muller et al. 2006). A study on the physiological role of potassium in Saccharomyces cerevisiae has, surprisingly, shown ammonium to be toxic under potassium limitation, probably due to ammonium leakage into the cell via potassium channels (Hess et al. 2006). Subsequently, excess ammonium has been observed to reduce yeast survival in lipid-limited fermentation (Tesniere et al. 2013). High levels of nitrogen are also found to trigger cell death when yeast is starved of micronutrients such as oleic acid, ergosterol, pantothenic acid, and nicotinic acid, as yeast may not be able to respond appropriately to nutrient limitations not naturally encountered (Duc et al. 2017).
The growth of yeast cells is also influenced by the medium’s pH, which drops rapidly as the cells enter the exponential phase. Maintaining the pH at 4.6 during cultivation or raising the pH to 4.6 at the exponential phase has been reported to more than double the growth of S. cerevisiae (Thomas et al. 2002). Alternatively, adding acetic acid or lactic acid induces a similar effect when the initial pH is adjusted to 4.5 (Thomas et al. 2002). Both pH adjustment and medium buffering likewise enhance yeast survival (Fabrizio et al. 2004; Burtner et al. 2009). Several organic acids including acetic, malic, citric, pyruvic, and oxalic acids are found to accumulate during yeast culture and contribute to the drop in pH, and acetic acid has been singled out as the only acid that exerts a deleterious effect on S. cerevisiae viability rather than low pH alone (Burtner et al. 2009). As its intracellular transport and metabolism by yeast are repressed in the presence of glucose, acetic acid is well-known to trigger programmed cell death in yeast since its protonated form can enter the cell by simple diffusion, dissociate inside the cell and lead to cytoplasmic acidification at low pH (Ludovico et al. 2001; Cassio et al. 1987; Giannattasio et al. 2013).
While screening for the effects of uronates, which are negatively charged sugars with a carboxyl group, on S. cerevisiae growth, we encountered their ability to promote cell growth under limiting concentrations of nutrients especially those in yeast nitrogen base (YNB). By optimising the medium conditions, we observed that yeast cells could overcome ammonium toxicity and low pH to increase growth. These results also raise the possibility of replacing a large proportion of essential nutrients with cheaper carboxylate salts, and hence reducing the costs of yeast cultivation for industrial applications.
Materials and methods
Yeast strain and growth conditions
Saccharomyces cerevisiae strain S288C was cultured in the yeast extract–peptone–dextrose (YPD) medium at 30 °C and 150 rpm for 48 h. Cells were then collected by centrifugation, washed twice with water, and resuspended in water to an OD600 of 1.0. For growth in the optimum condition, 100 μl of the cells was transferred into 10 ml of a minimal medium containing 20.0 g l−1 glucose, 5.0 g l−1 ammonium sulphate, and 1.7 g l−1 yeast nitrogen base without ammonium sulphate and amino acids (YNB). Nutrient-limited media were prepared by reducing ammonium sulphate from 5.0 g l−1, considered 100%, to 0.5 (10%), 0.25 (5%) or 0.1 g l−1 (2%); or YNB from 1.7 g l−1 (100%) to 0.17 (10%), 0.085 (5%) or 0.034 g l−1 (2%). Cell growth was measured in triplicate every 12 h up to 48 h. For pH experiments, the pH of the media was measured every 24 h and raised to 4.5 at 24 h by the addition of 1.0 N NaOH. A standard curve of the absorbance against the colony-forming unit per ml (CFU ml−1) was plotted by using culture under the optimum condition at 0, 6, 9, 12, and 15 h. The growth at the optimum condition was taken as 100%.
Carboxylate treatments
Sodium gluconate and sodium formate were dissolved in water and filtered. Sodium gluconate was added to the nutrient-limited media to a final concentration of 0.2, 0.5, 1.0, 2.0 or 5.0 mg ml−1, while sodium formate to a final concentration of 10 mM. Sodium alginate was first dissolved in water, then precipitated with 60% ethanol to remove any oligosaccharides and dried. The powder was dissolved in water, filtered, and added to a nutrient-limited medium to a final concentration of 2 mg ml−1.
Results
Ammonium toxicity at low YNB levels
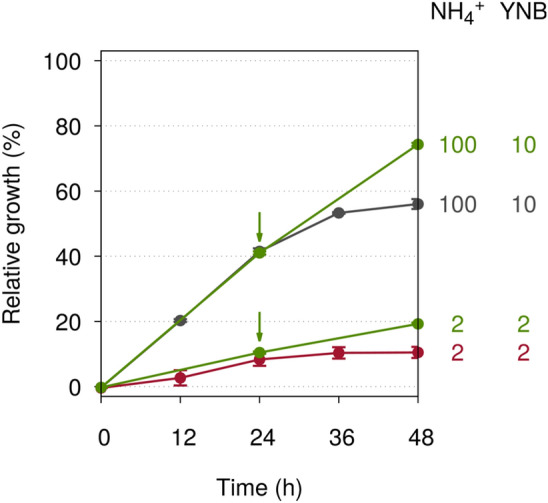
Under the optimum tested condition where the concentrations of all the nutrients were considered 100%, i.e. 20.0 g l−1 glucose, 5.0 g l−1 ammonium sulphate, and 1.7 g l−1 YNB without ammonium sulphate and amino acids, S. cerevisiae started entering the stationary phase within 24 h and its growth reached an OD600 of 1.75 at 48 h, or about 4.8 × 107 CFU ml−1 which was taken as 100% (Fig. 1). When both the carbon and nitrogen sources were maintained at 100% while all the vitamins, trace elements and salts in YNB were reduced to either 10% or 5% of the optimum concentration, yeast growth dropped respectively to 56.0% and 30.8% of the optimum level and halted around 0.2% when YNB was further reduced to 2% (Fig. 1). The drop in growth could be triggered by nutrient insufficiency or ammonium toxicity, which has been observed when ammonium levels are high while the levels of certain essential nutrients are low (Hess et al. 2006; Santos et al. 2012; Wang et al. 2003; Tesniere et al. 2013; Duc et al. 2017).
Fig. 1.

Relative growth of S. cerevisiae under varying concentrations of ammonium (NH4+) and YNB. As the YNB concentration was reduced from the optimum condition, taken as 100%, while the ammonium concentration was maintained at 100% (black lines), yeast growth declined and halted at 2% YNB. When the ammonium concentration was also reduced accordingly (red lines), yeast cells started to grow again at 2% ammonium and 2% YNB
To confirm this, the concentration of ammonium was reduced accordingly together with YNB. When the concentrations of ammonium and YNB were simultaneously reduced to either 10% or 5% of their optimum concentrations, no improvement in yeast growth was observed with the relative growth at 50.4% and 30.2% respectively (Fig. 1), indicating that either the limiting nutrients could support no further growth or ammonium was still toxic at these levels. Interestingly, in contrast to the halted growth at 100% ammonium, yeast started to grow when both ammonium and YNB were reduced to 2% and achieved 10.5% of the optimum growth at 48 h (Fig. 1). This signified that ammonium was indeed toxic to yeast under the limiting YNB level, and the toxicity could be reduced by lowering ammonium concentration.
Enhancement of yeast growth with carboxylates
Instead of lowering the ammonium concentration, the addition of gluconate to the growth-halting condition of 100% ammonium and 2% YNB was also observed to stimulate growth. While at 0.2 mg ml−1 yeast growth was barely stimulated with a relative growth of 0.7%, at 1.0 mg ml−1 yeast growth was driven up to 8.1% of the optimum level (Fig. 2a). Yeast growth was nevertheless already at 10.5% by simply reducing ammonium to 2%, and it more than doubled to 26.0% when gluconate was added at 2.0 mg ml−1 although no further increase was observed at 5.0 mg ml−1 with 23.0% of relative growth (Fig. 2b). These results also indicated that at a mere fiftieth, i.e. 2%, of the optimum concentrations, ammonium and YNB were able to support about a quarter of the optimum growth in the presence of gluconate.
Fig. 2.
Effects of ammonium concentration and carboxylate addition at 2% YNB. a Addition of gluconate (GlcA) (blue lines) revived yeast growth halted at 100% ammonium, and at 1.0 mg ml−1 gluconate promoted yeast growth to 8.1% of the optimum growth. b Simultaneous ammonium reduction and gluconate addition (blue lines) stimulated growth even further to as high as 26.0%. c Both formate and alginate, at 10.0 mM and 2.0 mg ml−1 respectively, also had a similar stimulatory effect on yeast growth
Although gluconate contains both hydroxyl and carboxyl groups, replacing it with the simplest carboxylate, formate, confirmed that gluconate’s effect on yeast growth was due to the carboxyl group. Similar to gluconate, which at 2.0 mg ml−1 was about 9.2 mM, addition of 10.0 mM formate was also able to enhance yeast growth to 21.4% when both ammonium and YNB were at 2% (Fig. 2c). Alginate at 2.0 mg ml−1, or 9.3 mM using a molecular weight of 216.12 g mol−1, likewise promoted yeast growth to 24.6% (Fig. 2c), which also indicated that the effect of these carboxylates could be extracellular as yeast is unable to degrade the polysaccharide.
At 100% ammonium and 10% YNB, similarly, supplement of 2.0 mg ml−1 gluconate stimulated growth to 86.8% compared to the 56.0% without gluconate, with the cells continuing growing in the exponential phase even after 24 h (Fig. 3a). The addition of 5.0 mg ml−1 gluconate, however, did not improve growth further at 84.2% (Fig. 3a). Although the addition of 2.0 mg ml−1 gluconate likewise promoted growth to 71.5% from 50.4% without gluconate when ammonium was also reduced to 10%, the increase of 21.1% in growth was less than the increase of 30.8% at 100% ammonium (Fig. 3b). Yeast growth was also improved to 50.5% by gluconate addition when both ammonium and YNB concentrations were lowered to 5% (Fig. 3c).
Fig. 3.
Effects of ammonium concentration and gluconate addition at 5 and 10% YNB. Addition of gluconate (GlcA) at 2.0 or 5.0 mg ml−1 improved yeast growth (blue lines) when only the YNB level was lowered to 10% (a), or when both ammonium and YNB were lowered to 10% (b) or 5% (c)
Effects of pH on yeast growth
The effects of gluconate, formate, and alginate on yeast growth under nutrient-limited conditions could possibly be attributed to the buffering capacity of their carboxyl groups which have a pKa below 4. At the optimum condition, the pH of the culture quickly dropped from 4.43 to 2.32 and remained almost constant after 24 h with marginal growth (Table 1). At 100% ammonium and 10% YNB, similarly, after dropping from 4.53 to 2.72 at 24 h the pH did not drop further as growth declined. The addition of 2.0 and 5.0 mg ml−1 gluconate, on the other hand, raised the initial pH of the medium to 5.49 and 5.74 respectively, and the pH continued dropping as yeast continued to grow even after 24 h. Likewise, at 2% ammonium and 2% YNB, in the absence of gluconate the pH remained around 3.15 with marginal growth after 24 h, but in the presence of the sugar, the initial pH rose and then continued to drop beyond 24 h with continuous yeast growth.
Table 1.
pH of yeast culture media
| Concentration | pH | ||||
|---|---|---|---|---|---|
| Ammonium (%) | YNB (%) | Gluconate (mg ml−1) | 0 h | 24 h | 48 h |
| 100 | 100 | − | 4.43 | 2.32 | 2.29 |
| 100 | 10 | − | 4.53 | 2.72 | 2.62 |
| 4.69 | 2.83* | 2.80 | |||
| 100 | 10 | 2.0 | 5.49 | 3.70 | 3.11 |
| 100 | 10 | 5.0 | 5.74 | 4.29 | 3.79 |
| 2 | 2 | − | 4.91 | 3.15 | 3.12 |
| 5.02 | 3.31* | 3.70 | |||
| 2 | 2 | 2.0 | 5.71 | 4.53 | 4.37 |
| 2 | 2 | 5.0 | 5.99 | 4.96 | 4.78 |
*pH before adjustment to 4.5
These findings correlated with the observation that the addition of acetic acid or lactic acid could spur yeast growth when the initial pH of the medium was adjusted to 4.5 (Thomas et al. 2002). Raising the pH of a yeast culture during the stationary phase has been reported to initiate the second phase of growth too (Thomas et al. 2002). Indeed, when the pH of the culture at 100% ammonium and 10% YNB was raised to 4.5 at 24 h in the absence of gluconate, yeast continued to grow to 74.3% with the pH dropping back to 2.80 at 48 h in contrast to the 56.0% without pH adjustment (Fig. 4, Table 1). At 2% ammonium and 2% YNB, raising the pH at 24 h also spurred yeast growth to 19.3%, which almost doubled the 10.5% relative growth without pH adjustment (Fig. 4).
Fig. 4.
Growth enhancement by pH adjustment. Yeast growth was also enhanced (green lines) when the pH of the culture at 24 h was raised to 4.5 (arrows)
Discussion
Stunted yeast growth under nutrient limitations can easily, and sometimes erroneously, be attributed to the insufficiency of nutrients to support growth. This assumption seemed plausible when yeast growth was retarded after the concentration of YNB, which contained all the necessary vitamins, trace elements, and salts, was reduced from 100%, i.e. 1.7 g l−1, to 2–10% while the ammonium concentration was maintained at 100% or 5.0 g l−1 (Fig. 1). Nevertheless, ammonium toxicity was confirmed instead of nutrient insufficiency when yeast growth, which was halted at 100% ammonium and 2% YNB, was revived after ammonium was also reduced to 2% (Fig. 1). The relatively high ratio of ammonium to YNB could have triggered cell death, which has been observed in conditions with high ammonium levels but limiting levels of nutrients such as potassium (Hess et al. 2006), auxotrophic-complementing amino acids (Santos et al. 2012), or the micronutrients oleic acid, ergosterol, pantothenic acid or nicotinic acid (Duc et al. 2017; Tesniere et al. 2013; Wang et al. 2003).
Ammonium at 100% was highly toxic as compared to 2% when YNB was 2%, but presumably not so when YNB was 5% or 10% as reducing ammonium concentration did not enhance yeast growth any further (Fig. 1). By reducing the ammonium concentration 50-fold, a similar improvement in the survival of yeast cells starved of auxotrophy-complementing amino acids has also been observed (Santos et al. 2012). The exact mechanism by which ammonium toxicity triggers cell death is unclear, although intracellular ammonium leakage through potassium channels (Hess et al. 2006) and the inability of yeast to initiate a stress response against unnatural nutrient limitations (Duc et al. 2017) have been suggested. Interestingly, ammonium toxicity could also be alleviated by the addition of gluconate alone (Fig. 2a), although simultaneously reducing the ammonium level and adding gluconate, or other carboxylates such as formate and alginate had a far greater effect on yeast growth (Fig. 2b, c).
It is, however, unclear how gluconate could attenuate ammonium toxicity. It was not expected to serve as a carbon source since the yeast cells were supplemented with 20.0 g l−1 glucose under all the conditions. As a weak acid with a pKa of 9.2, only a minute fraction of the ammonium ions in the medium would convert to ammonia which could be utilised by yeast. The addition of sodium gluconate caused an increase in pH and shifted the equilibrium towards more ammonia conversion. However, the reduction in ammonium concentration would be minuscule — the increase of about one pH unit upon addition of 2.0 mg ml−1 gluconate to the medium containing 100% ammonium and 10% YNB (Table 1), for instance, increased the calculated ammonia concentration severalfold, but at any pH below 6 more than 99.9% of the added ammonium sulphate would still remain as ammonium ions.
Carboxylates, on the other hand, could act as buffering agents at low pH. Organic acids such as acetic, malic, citric, pyruvic, and oxalic acid have been identified to accumulate and lead to medium acidification as more and more yeast cells divide and grow (Burtner et al. 2009). The effect of medium acidification was confirmed when increasing the pH to 4.5 with NaOH at 24 h was shown to have a stimulatory effect on yeast growth (Fig. 4 and Table 1), in agreement with the observation that pH adjustment can spur growth (Thomas et al. 2002). It has further been pinpointed that the accumulation of acetic acid, instead of an acidic environment alone, has a deleterious effect on yeast viability (Burtner et al. 2009). Undissociated acetic acid can diffuse into yeast cells and dissociate due to the higher intracellular pH, leading to the acidification of the yeast cytoplasm with adverse consequences. The added gluconate could accept a proton from acetic acid and convert it to harmless acetate, hence help lower the acetic acid concentration and its toxicity in the medium.
Although formic acid is more toxic to yeast than acetic acid (Burtner et al. 2009; Larsson et al. 1999), substituting gluconate with its conjugate base formate still had a similar stimulatory effect (Fig. 2c) by, perhaps, reducing the accumulated acetic acid concentration until the levels of both acids became intolerable. The lack of further growth at increasing gluconate to 5 mg ml−1 (Fig. 2b and 3a) could, at last, be likely attributed to nutrient insufficiency instead of gluconic acid toxicity, as similar carboxylic acids like malic acid and citric acid are not toxic even at 500 mM and pH 1.6 (Burtner et al. 2009) as probably their larger size impedes diffusion across yeast’s cell membrane (Gabba et al. 2020; Walter and Gutknecht 1984). In summary, yeast growth was greatly affected by both ammonium toxicity, particularly when the ammonium concentration was high while the other nutrients in YNB were limited, as well as medium acidification. At 100% ammonium, ammonium toxicity was especially severe at 2% YNB compared to 5% or 10% YNB, while medium acidification, most likely due to accumulation of acetic acid, seemed to retard growth in all the conditions (Fig. 1).
The growth characteristics of yeast cells also seemed to be influenced by nutrient levels. First, while yeast cells could grow until the pH dropped to as low as 2.3 under the optimum condition, yeast growth was already approaching stagnancy at higher pH around 2.7 or even 3.1 respectively at 100% ammonium and 10% YNB or 2% ammonium and 2% YNB (Table 1) although, as shown by gluconate addition, the media could still support further growth. Second, the growth-promoting effect of gluconate was already obvious during the exponential phase at 12 h (Figs. 2, 3), at which stage medium acidification was not expected to have proceeded significantly yet. Third, gluconate addition had the greatest effect when both ammonium and YNB were 2% — more than doubling the growth at the same condition without gluconate (Fig. 2b) — while at 100% ammonium and 10% YNB it increased only half of the growth achieved in the absence of gluconate (Fig. 3a). These findings also indicate that under lower nutrients levels, yeast cells might become more susceptible to low pH and enter the stationary phase at higher pH such as above pH 3 at 2% ammonium and 2% YNB — although the cells were expected to generate sufficient ATP from glucose to pump out H+ — and hence the medium buffering by gluconate might have a greater effect on stimulating cell growth at these conditions.
While more studies are necessary to investigate the exact relationship between limited nutrients and pH susceptibility, we have shown that ammonium toxicity and the pH of yeast culture can be modulated to promote yeast growth by simply reducing ammonium levels and adding carboxylate salts to the medium, which even rescued yeast culture from the brink of cell death at 100% ammonium and 2% YNB to a quarter of the optimum growth (Fig. 2). Further optimisation of culture conditions to replace a large proportion of the nutrients in YNB with much cheaper carboxylates, such as replacing 90% of YNB with 2 mg ml−1 gluconate to still achieve almost 90% of the optimum growth (Fig. 3a), may potentially contribute to cost-efficient cultivation of yeast for applications in the food, pharmaceutical, and feed industries. Moreover, the use of polysaccharides containing carboxyl groups such as alginate and pectin will not only avoid interference with yeast intracellular processes, but also enable easy removal of the sugar through precipitation with calcium.
Acknowledgements
This study was supported by Universiti Sains Malaysia RU grant (1001/PCCB/870036) for the URICAS programme.
Author contributions
AHT designed and analysed data. TYY and MQL performed experiments. All authors contributed to the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Tengku Yasmin Yusof and Melissa Qianyue Lian contributed equally to this work.
Contributor Information
Tengku Yasmin Yusof, Email: tengkuyasminputri@gmail.com.
Melissa Qianyue Lian, Email: melissalian95@student.usm.my.
Eugene Boon Beng Ong, Email: eugene@usm.my.
Aik-Hong Teh, Email: aikhong@usm.my.
References
- Britto DT, Kronzucker HJ. NH4+ toxicity in higher plants: a critical review. J Plant Physiol. 2002;159(6):567–584. doi: 10.1078/0176-1617-0774. [DOI] [Google Scholar]
- Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8(8):1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassio F, Leao C, van Uden N. Transport of lactate and other short-chain monocarboxylates in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 1987;53(3):509–513. doi: 10.1128/AEM.53.3.509-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc C, Pradal M, Sanchez I, Noble J, Tesniere C, Blondin B. A set of nutrient limitations trigger yeast cell death in a nitrogen-dependent manner during wine alcoholic fermentation. PLoS ONE. 2017;12(9):e0184838. doi: 10.1371/journal.pone.0184838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R, Ariz I, Cruz C, Moran JF. Review: mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016;248:92–101. doi: 10.1016/j.plantsci.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB, Longo VD. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166(7):1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabba M, Frallicciardi J, van Klooster J, Henderson R, Syga L, Mans R, van Maris AJA, Poolman B. Weak acid permeation in synthetic lipid vesicles and across the yeast plasma membrane. Biophys J. 2020;118(2):422–434. doi: 10.1016/j.bpj.2019.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio S, Guaragnella N, Zdralevic M, Marra E. Molecular mechanisms of Saccharomyces cerevisiae stress adaptation and programmed cell death in response to acetic acid. Front Microbiol. 2013;4:33. doi: 10.3389/fmicb.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard P, Urrestarazu A, Vissers S, Kontos K, Bontempi G, van Helden J, Andre B. Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 2007;27(8):3065–3086. doi: 10.1128/MCB.01084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Lu W, Rabinowitz JD, Botstein D. Ammonium toxicity and potassium limitation in yeast. PLoS Biol. 2006;4(11):e351. doi: 10.1371/journal.pbio.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant N-O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol. 1999;24(3):151–159. doi: 10.1016/S0141-0229(98)00101-X. [DOI] [Google Scholar]
- Ludovico P, Sousa MJ, Silva MT, Leao CL, Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147(Pt 9):2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- Muller T, Walter B, Wirtz A, Burkovski A. Ammonium toxicity in bacteria. Curr Microbiol. 2006;52(5):400–406. doi: 10.1007/s00284-005-0370-x. [DOI] [PubMed] [Google Scholar]
- Santos J, Sousa MJ, Leao C. Ammonium is toxic for aging yeast cells, inducing death and shortening of the chronological lifespan. PLoS ONE. 2012;7(5):e37090. doi: 10.1371/journal.pone.0037090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesniere C, Delobel P, Pradal M, Blondin B. Impact of nutrient imbalance on wine alcoholic fermentations: nitrogen excess enhances yeast cell death in lipid-limited must. PLoS ONE. 2013;8(4):e61645. doi: 10.1371/journal.pone.0061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KC, Hynes SH, Ingledew WM. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl Environ Microbiol. 2002;68(4):1616–1623. doi: 10.1128/aem.68.4.1616-1623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Gutknecht J. Monocarboxylic acid permeation through lipid bilayer membranes. J Membr Biol. 1984;77(3):255–264. doi: 10.1007/BF01870573. [DOI] [PubMed] [Google Scholar]
- Wang XD, Bohlscheid JC, Edwards CG. Fermentative activity and production of volatile compounds by Saccharomyces grown in synthetic grape juice media deficient in assimilable nitrogen and/or pantothenic acid. J Appl Microbiol. 2003;94(3):349–359. doi: 10.1046/j.1365-2672.2003.01827.x. [DOI] [PubMed] [Google Scholar]




