Abstract
Background
Effective treatment for Coronavirus Disease-2019 (COVID-19) is under intensive research. Nigella sativa oil (NSO) is a herbal medicine with antiviral and immunomodulatory activities, and has been recommended for the treatment of COVID-19. This study aimed to evaluate the efficacy of NSO treatment in patients with COVID-19.
Methods
All adult patients with mild COVID-19 symptoms presented to King Abdulaziz University Hospital, Jeddah, Saudi Arabia, were recruited for an open label randomized clinical trial (RCT). They were randomly divided into control or treatment groups, with the latter receiving 500 mg NSO (MARNYS® Cuminmar) twice daily for 10 days. Symptoms were daily monitored via telecommunication. The primary outcome focused on the percentage of patients who recovered (symptom-free for 3 days) within 14-days. The trial was registered at clinicaltrials.gov (NCT04401202).
Results
A total of 173 patients were enrolled for RCT. The average age was 36(±11) years, and 53 % of patients were males. The control and NSO groups included 87 and 86 patients respectively. The percentage of recovered patients in NSO group (54[62 %]) was significantly higher than that in the control group (31[36 %]; p = 0.001). The mean duration to recovery was also shorter for patients receiving NSO (10.7 ± 3.2 days) compared with the control group (12.3 ± 2.8 days); p = 0.001.
Conclusions
NSO supplementation was associated with faster recovery of symptoms than usual care alone for patients with mild COVID-19 infection. These potential therapeutic benefits require further exploration with placebo-controlled, double-blinded studies.
Abbreviations: COVID-19, Coronavirus Disease-2019; HCV, Hepatitis C Virus; HIV, Human Immunodeficiency Virus; IFNγ, Interferon-gamma; IL, Interleukin; ISARIC, International Severe Acute Respiratory and Emerging Infection Consortium; ITT, Intention-to-treat; KAUH, King Abdulaziz University Hospital; NSO, Nigella sativa L. oil; RCT, Randomized Controlled Trial; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; TNF, Tumour Necrosis Factor; WHO, World Health Organization
Keywords: COVID-19, SARS-CoV-2, Herbal medicine, Nigella sativa
1. Introduction
Coronavirus disease-2019 (COVID-19) was first recognized in China in December 2019. A novel betacoronavirus, designated as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)1 was found to be the responsible agent for this infection. SARS-CoV-2 has since spread throughout the world, causing an enormous pandemic and presenting major challenges to global health systems.1 In Saudi Arabia, COVID-19 has thus far been responsible for over 300,000 cases and 1500 fatalities.2
Fortunately, most COVID-19 patients are either asymptomatic or have a mild disease, which is usually managed by relieving constitutional symptoms through analgesics, antipyretics, hydration, and close monitoring for clinical deterioration.3 The most commonly reported symptoms include fever, cough, fatigue, anorexia, shortness of breath, myalgia, sore throat, nasal congestion, headache, diarrhea, nausea, vomiting, and loss of smell (anosmia).4 According to symptoms, disease severity is classified into mild, moderate, and severe.4 While about 80 % of COVID-19 patients develop mild to moderate disease, approximately 15 % develop a severe disease that requires oxygen support, and 5% develop a critical disease with complications.4 Several global studies have reported high mortality rates exceeding 20 % in hospitalized patients.5, 6, 7
As this pandemic continues, the search for an effective treatment has become a priority of scientific medical research.3 Currently, there are limited pharmacotherapeutic drugs effective against COVID-19. Therefore, complementary herbal medicines with their wealth of biologically active molecules are being considered for treatment against coronavirus infection.8 These therapies are widely used against respiratory ailments, and their efficacy towards flu symptoms has been reported.9 Particularly, Nigella sativa L is suggested as a potential phytomedicine owing to its several pharmacological activities such as anti-inflammatory, anti-viral, and immunomodulatory effects.10,11
N. sativa L., a well-known food spice with a high medicinal value, originates from different historical backgrounds.9 , 12 Several active compounds have been identified in N. sativa L. including thymoquinone.12 The safety profile, of thymoquinone has been documented in many clinical trials, including our previous trial with asthmatic patients.13 A recent systematic review of randomized clinical trials (RCTs) that used N. sativa L. showed no serious adverse effects on hepatic and renal function.14 Several preclinical and clinical studies have documented the antiviral activities of N. sativa L. against several viruses.15, 16, 17, 18, 19, 20, 21, 22, 23 An in vitro study showed that the coronavirus load decreased with N. sativa L.17 Some N. sativa L. compounds demonstrated potential inhibition of coronavirus replication in in silico models.24
The immune system comprises a complex cellular arrangement that defend against undesired intruders. However, well-coordinated action between its elements is essential for proper function.25 In several studies, N. sativa L. has been shown to exhibit immunostimulant effects through the induction of multiple cellular mediators and immune responses to eradicate infections.26, 27, 28, 29 Contrariwise, in studies on inflammatory conditions, N. sativa L. has demonstrated anti-inflammatory and immunomodulatory activities by reducing pro-inflammatory mediators.29 , 30 Moreover, analgesic, antipyretic, and bronchodilatation properties of N. sativa L. have also been reported.31, 32, 33
Based on these observations on the pharmacological activities of N. sativa, the potential therapeutic efficacy of N. sativa L. was proposed in COVID-19. This study aimed to explore the impact of N. sativa L. oil (NSO) supplementation in reducing the duration of symptoms and complications in symptomatic patients with mild COVID-19.
2. Methods
2.1. Trial design
The study was a prospective, two-arm, parallel-group, randomized, controlled, open-label, phase II clinical trial using oral NSO in adult patients with mild COVID-19. The trial was approved by the ethical committee of King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia (reference number 266-20). It was registered online at ClinicalTrials.gov Identifier: NCT04401202, https://clinicaltrials.gov/ct2/show/NCT04401202.
2.2. Participants
Consecutive adult patients aged 18 and above with mild COVID-19, presented to the emergency department and outpatient clinics at KAUH between May 1 and September 31, 2020, were recruited. Participants were isolated in the KAUH dorm or their homes. SARS-CoV-2 infection was confirmed in all patients via polymerase chain reaction test within one week of the onset of symptoms. Mild COVID-19 was defined as upper respiratory tract infection symptoms in the absence of clinical or radiological signs of pneumonia or hypoxia.4 All participants understood and agreed to comply with the planned study procedures. Baseline characteristics were recorded for participants, including age, sex, body mass index, smoking status, pre-enrollment days, and comorbidities.
Exclusion criteria included current history of pneumonia, severe illness requiring admission to the intensive care unit, severe chronic kidney disease (estimated glomerular filtration rate < 30 mL/min), end-stage renal disease requiring dialysis, elevated alanine transaminase or aspartate transaminase (> 5 times the upper limit of normal), pregnancy or breastfeeding, anticipated transfer within 72 h to another hospital from the study site, previous N. sativa L. treatment, or allergy to the study medication. Due to infection control policies, participants (or legally authorized representatives) provided verbal informed consent before randomization. The study followed the guidelines of the Declaration of Helsinki and Tokyo for humans.
2.3. Interventions and randomization
The web-based program (http://www.randomization.com) was used to generate a random allocation sequence. Centralized randomization was used to ensure allocation concealment. Participants were randomized in a 1:1 ratio to standard of care (control group) or standard of care with oral NSO (500 mg twice daily postprandial for 10 days; treatment group). This dose was recommended by the manufacturing company, and based on previous clinical trial on asthmatic patients.13 The duration was proposed based on several registered clinical trials on COVID-19 treatments.34 The NSO product was previously characterized with 0.7 % thymoquinone by high-performance liquid chromatography analysis at the UCL School of Pharmacy, London, UK.13 It is manufactured by the Good Manufacturing Practice (GMP) certified facility Marnys® (Cartagena, Spain; brand name: CUMINMAR; batch number: E365). Product quality assurance and licensing were performed by the Saudi Food and Drug Authority (26.861/MU). The NSO product was stored in a cool and dry place.
Prof. Tariq Madani generated the allocation sequence; Dr. Ali Atwah, Dr. Mazen Badawib, Dr. Meshari Alhamdane, and Dr. Reem Almalki enrolled participants; and Dr. Abdulrahman Koshak assigned participants to their respective groups. Subsequently, NSO was delivered to each patient of the treatment group at their isolation place. Compliance was monitored by the patients self-reporting their treatment intake through daily telecommunication and recorded in the follow-up questionnaire (Appendix 1). Symptoms and any potential side effects during the study were also monitored by daily telephone communication (Appendix 1). The standard of care was decided by the treating physicians and included antipyretics, antihistamines, and other drugs as per the Saudi Ministry of Health and the KAUH protocol.
2.4. Outcomes and follow-up
The primary outcome was the percentage of participants with clinical recovery within 14 days after randomization. Clinical recovery was defined as the absence of symptoms for three days. The secondary outcomes included the number of days for recovery, duration of each symptom, adverse drug reactions, and hospital admission due to disease complications.35 All outcomes were assessed by on-site investigators who were aware of the trial-group assignments. From the day of randomization, data were collected every day for 14 days in an electronic case report form including a pre-defined list of monitored symptoms (Appendix 1) via a structured telephone call to the participants or their legal representatives. The same investigator was responsible for data collection from all patients during the follow-up.
2.5. Sample-size calculation
To achieve a power of 80 % and a two-tailed p-value of <0.05 of an assumed 40 % difference between the proportion of patients with clinical cure among the NSO group in comparison with the control group, the sample size was estimated to be 200 patients assigned randomly into the two arms (i.e., 100 patients per arm) as calculated using G*Power version 3.1.9.2 (Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany).
2.6. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 23. The data normality was tested using the Shapiro–Wilk test and Q–Q plot. Means ± standard deviations of the normally distributed data were compared using an independent t-test. Non-normally distributed data were described as medians ± interquartile range and compared using the Mann–Whitney U test Intention-to-treat (ITT) analysis was used for the outcome analysis. Multiple regression substitution was used to impute missing outcome data, considering baseline values.
3. Results
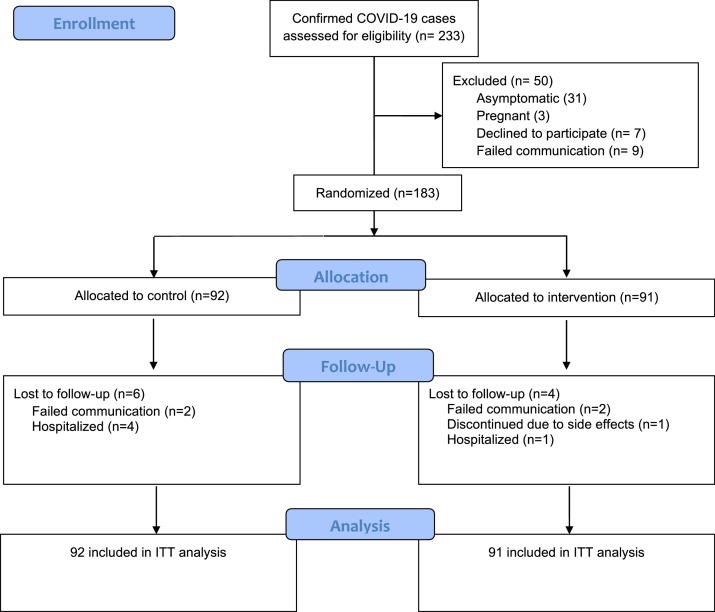
A total of 233 patients with confirmed COVID-19 were assessed for eligibility from May 1 to September 30, 2020 (Fig. 1 ). Fifty patients were excluded for the reasons described in Fig. 1. The remaining 183 patients were randomized into the NSO (91) and control groups (92). Subsequently, four patients from the NSO group and six patients from the control group were lost to follow-up for reasons shown in Fig. 1.
Fig. 1.
Flow diagram of patient’s enrollment.
A total of 183 patients with confirmed mild COVID-19 were included in the analysis. Their mean age was 36 ± 11 years. Ninety-seven (53 %) patients were male and 86 (47 %) were female. Their mean body mass index was 27.1 ± 5.6 kg/m2. Baseline characteristics of randomized patients are shown in Table 1 .
Table 1.
Baseline characteristics of randomized patients.
| Variable | Total (n = 183) | NSO group (n = 91) | Control group (n = 92) |
|---|---|---|---|
| Age, mean ± SD | 36 ± 11 | 35 ± 10 | 36 ± 12 |
| Male, n (%) | 97 (53 %) | 48 (53 %) | 49 (53 %) |
| Female, n (%) | 86 (47 %) | 43 (47 %) | 43 (47 %) |
| BMI, mean ± SD | 27.1 ± 5.6 | 27 ± 5.9 | 27.2 ± 5.4 |
| Smoking, n (%) | 21 (11 %) | 12 (13 %) | 9 (10 %) |
| Pre-enrollment days, mean ± SD | 3.7 ± 2.6 | 3.5 ± 2.1 | 4 ± 2.9 |
| Comorbidities | |||
| Obesity, n (%) | 45 (25 %) | 20 (22 %) | 25 (27 %) |
| Allergic rhinitis, n (%) | 28 (15 %) | 13 (14 %) | 15 (16 %) |
| Hypertension, n (%) | 16 (9%) | 13 (14 %) | 3 (3%) |
| Diabetes, n (%) | 14 (8%) | 11 (12 %) | 3 (3%) |
| Asthma, n (%) | 7 (4%) | 3 (3%) | 4 (4%) |
| Allergic Conjunctivitis, n (%) | 7 (4%) | 1 (1%) | 6 (6%) |
| Hypothyroidism, n (%) | 5 (3%) | 4 (4%) | 1 (1%) |
| Ezcema, n (%) | 4 (2%) | 3 (3%) | 1 (1%) |
| Food Allergy, n (%) | 4 (2%) | 1 (1%) | 3 (3%) |
| Allergic Sinusitis, n (%) | 3 (2%) | 1 (1%) | 2 (2%) |
| Migraine, n (%) | 2 (1%) | 2 (2%) | 0 |
| Hypercholesterolemia, n (%) | 1 (0.5 %) | 0 | 1 (1%) |
| Psoriasis, n (%) | 1 (0.5 %) | 1 (1%) | 0 |
| Ischemic heart disease, n (%) | 1 (0.5 %) | 0 | 1 (1%) |
| Osteoarthritis, n (%) | 1 (0.5 %) | 1 (1%) | 0 |
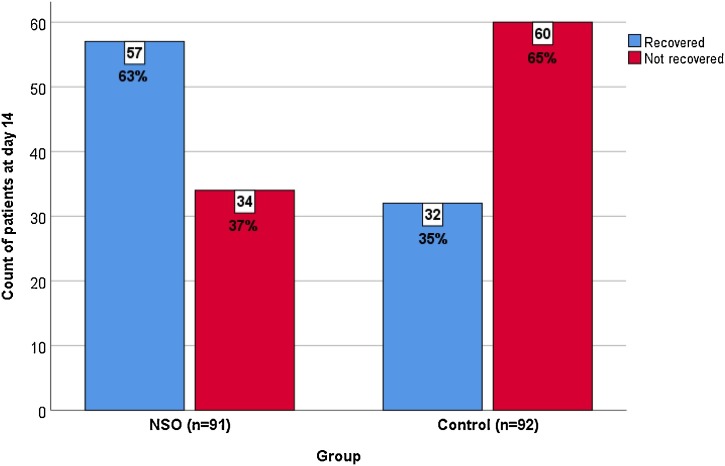
Of the total study patients (n = 183), 85 (46 %) patients recovered within the 14-day study period. The percentage of patients recovered in the NSO group was significantly higher than that in the control group (57 patients [63 %] versus 32 patients [35 %], respectively; P < 0.01) (Fig. 2 ). Additionally, the average number of days required for recovery in the NSO group was significantly less compared with those in the control group (10.7 ± 3.3 days versus 12.4 ± 2.9 days, respectively; P < 0.01).
Fig. 2.
The number of patients who recovered within the study period.
Table 2 shows the types and durations of COVID-19 symptoms reported by the participants. The most predominant symptoms were anosmia (56 %), cough (55 %), lethargy/fatigue (46 %), headache (46 %), myalgia (43 %), loss of appetite (34 %), and fever (34 %). NSO treated patients had a significantly shorter mean duration of chills (2.1 ± 1.0 versus 4.9 ± 3.4; P = 0.02), anosmia (8.5 ± 3.9 versus 10.4 ± 3.6; P < 0.01), runny nose (2.5 ± 1.6 versus 3.7 ± 2.5; P = 0.02), and loss of appetite (3.5 ± 2.9 versus 5.5 ± 3.1; P = 0.01) as compared to the control group. However, there were no significant changes found in the rest of symptoms’ duration between groups.
Table 2.
Frequency and duration of symptoms in 173 patients with mild COVID-19.
| Total frequency n (%) | NSO group |
Control group |
P-value of the symptom’s duration between groups | |||
|---|---|---|---|---|---|---|
| Frequency n (%) | Duration (days) Mean ± SD | Frequency n (%) | Duration (days) Mean ± SD | |||
| Anosmia | 123 (71) | 57 (66) | 8.5 ± 3.9 | 66 (77) | 10.4 ± 3.6 | <0.01 |
| Lethargy/Fatigue | 118 (68) | 62 (71) | 6.3 ± 4.1 | 56 (65) | 5.4 ± 3.2 | 0.18 |
| Cough | 116 (67) | 64 (74) | 7.3 ± 4.8 | 52 (60) | 8.2 ± 4.3 | 0.3 |
| Headache | 107 (62) | 57 (66) | 4.2 ± 3.4 | 50 (58) | 4.6 ± 3.3 | 0.55 |
| Myalgia | 99 (57) | 55 (63) | 5.2 ± 3.5 | 44 (51) | 4.6 ± 2.8 | 0.41 |
| Nasal congestion | 88 (51) | 44 (51) | 3.9 ± 2.8 | 44 (51) | 5.2 ± 3.6 | 0.06 |
| Fever | 85 (49) | 50 (57) | 2.8 ± 1.8 | 35 (41) | 3.6 ± 2.2 | 0.07 |
| Sore throat | 70 (40) | 40 (46) | 3.9 ± 2.8 | 30 (35) | 4.2 ± 3.2 | 0.73 |
| Runny nose | 68 (39) | 37 (43) | 2.5 ± 1.6 | 31 (36) | 3.7 ± 2.5 | 0.02 |
| Loss of appetite | 62 (36) | 35 (40) | 3.5 ± 2.9 | 27 (31) | 5.5 ± 3.1 | 0.01 |
| Arthralgia | 55 (31) | 34 (39) | 4.5 ± 3.5 | 21 (24) | 4.2 ± 3.4 | 0.74 |
| Sputum | 51 (29) | 27 (31) | 5.0 ± 4.0 | 24 (28) | 6.2 ± 4.4 | 0.30 |
| Sneezing | 48 (27) | 24 (28) | 2.9 ± 2.6 | 24 (28) | 3.5 ± 2.0 | 0.39 |
| Diarrhea | 46 (26) | 21 (24) | 2.8 ± 2.4 | 25 (29) | 2.7 ± 2.2 | 0.94 |
| Nausea | 41 (23) | 21 (24) | 3.3 ± 2.7 | 20 (23) | 4.5 ± 3.9 | 0.29 |
| Chest tightness | 36 (21) | 24 (28) | 5.2 ± 3.8 | 12 (14) | 3.6 ± 3.6 | 0.25 |
| Chills | 27 (16) | 16 (18) | 2.1 ± 1.0 | 11 (13) | 4.9 ± 3.4 | 0.02 |
| Shortness of breath | 26 (15) | 16 (18) | 2.9 ± 1.8 | 10 (12) | 3.4 ± 2.8 | 0.62 |
| Abdominal pain | 26 (15) | 13 (15) | 2.8 ± 3.4 | 13 (15) | 4.0 ± 2.7 | 0.32 |
| Chest pain | 17 (10) | 10 (11) | 2.9 ± 1.6 | 7 (8) | 4.7 ± 2.9 | 0.12 |
| Vomiting | 10 (6) | 6 (7) | 1.5 ± 1.2 | 4 (5) | 2.7 ± 1.7 | 0.21 |
Adverse events occurred in three patients with mild gastrointestinal symptoms of NSO group (3.4 %). Furthermore, four patients from the control group (4.6 %) were hospitalized during the study period due to disease complications (pneumonia [n = 1] and hypoxemia [n = 3]). However, only one patient from the NSO group (1.1 %) was hospitalized due to severe nausea and vomiting; these were suspected to be the aggravated adverse effects of NSO. Nearly all of the patients in the NSO group showed good compliance with the NSO treatment (consumed more than 90 % of their NSO capsules).
4. Discussion
In this study, NSO was found to be significantly associated with a higher likelihood of recovery from mild COVID-19 on day 14th. Almost 62 % of the patients with mild COVID-19 treated with NSO recovered compared to only 36 % of the patients in the control group. Furthermore, the average recovery time was significantly shorter in the NSO-treated group than in the control group by approximately 2 days.
The most common COVID-19 symptoms observed here include anosmia, cough, fatigue, lethargy, and headache, whereas the most common symptoms in the ISARIC Global COVID-19 report are fever, shortness of breath, cough, and fatigue/malaise.36 In this study, chills, anosmia, runny nose, and loss of appetite were significantly reduced in the NSO group in comparison with the control group after the treatment. This might be attributed to the anti-inflammatory effects of N. sativa L. Corticosteroids, potent anti-inflammatory drugs, have been used to eliminate inflammation in patients with post-infectious olfactory dysfunction.37
Some trials have been conducted to evaluate a specific therapeutic intervention to treat patients with mild COVID-19. However, high-quality data supporting interventions are limited. Additionally, there are concerns about the potential toxicity of experimental novel or repurposed medications.38 Hence, it was proposed that specific therapies for COVID-19 in the ambulatory setting should be limited to clinical trials.39
To our knowledge, this is the first RCT where the effects of NSO in patients with mild COVID-19 were observed. In contrast to the previously reported trials evaluating specific medications for mild COVID-19, our study revealed a significant benefit of NSO treatment for mild COVID-19 as it increased the likelihood of recovery and reduced the duration of illness. For example, hydroxychloroquine has received considerable attention with potential for COVID-19 treatment; however, it failed to demonstrate consistent benefits for patients with mild COVID-19.40 , 41 Bromhexine, a mucolytic agent used primarily for bronchopulmonary infections, likewise, failed to demonstrate therapeutic benefits for COVID-19 treatment.42 In comparison with other N. sativa L. studies pertaining to COVID-19, a retrospective study showed some benefits of using N. sativa L. in combination with other natural products.43 The study of Ashraf et al. has also reported promising therapeutic outcomes; however, they used a different treatment product: honey combined with whole N. sativa L seeds.44
Previous studies reported that in different grades of COVID‐19 severity, there were changes in release of leukocytes and pro-inflammatory cytokines 45 , 46 Therefore, therapies targeting the immune system and restricting the cytokine storm are worth investigating in COVID‐19 patients.47 N. sativa L. has immunoregulatory and anti-inflammatory effects via decreasing many pro-inflammatory cytokine responses.11 , 29 , 48 In addition, N. sativa L. may have therapeutic effects against immune disturbance, autophagy dysfunction, oxidative stress, ischemia, and inflammation in the COVID-19 associated comorbidities such as diabetes, cardiovascular disorders, and co-infections with bacterial and viral pathogens.47 The most common comorbidities found in this study were obesity, allergic disorders, smoking, hypertension, diabetes, and asthma. The significance of these diseases and treatment outcomes should be assessed in further studies.
Limitations of this study include restricting outcomes to clinical symptoms. Serological biomarkers (such as biochemical or hematological parameters) were not considered herein because of the regulations imposed by isolation policies and lack of sufficient funding. All symptom reports were based on a subjective assessment via telephone communication between the patient and the investigator. Moreover, the open design of the study may have introduced biases in assessing the response towards the treatment. The estimated sample size in the power calculation was not reached because the number of eligible cases decreased towards the end of the first pandemic wave. Although clinical symptoms showed that early administration of NSO did not worsen the immune response, the potential to aggravate the cytokine storm should be taken into consideration if NSO is administered at the later stages of the infection. The importance of timeframe towards the administrating of such immunomodulatory/anti-inflammatory agent in COVID-19 and its paradoxical effects require further analysis in future trials. Finally, the findings of present study cannot be generalized to other disease severities, age categories, treatment doses, or formulations.
5. Conclusion
Oral NSO dosage supplementation at 500 mg twice daily for 10 days in a sample of adult patients with mild symptoms of COVID-119 was associated with a higher percentage of recovery than usual care alone at day 14 of the illness. Additionally, faster recovery from COVID-19 symptoms and a lower hospitalization rate were observed with a low adverse effect profile. Specifically, NSO treatment had a pronounced effect on the duration of anosmia and runny nose. To our knowledge, this is the first RCT to demonstrate the effects of NSO on COVID-19 symptoms. Future larger double-blinded placebo-controlled studies using objective laboratory outcomes and including more patients with severe illness are required to verify the benefits of NSO for COVID-19 treatment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Abdulrahman E. Koshak: Conceptualization, Project administration, Writing - original draft, Writing - review & editing. Emad A. Koshak: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Abdullah F. Mobeireek: Methodology, Validation, Writing - review & editing. Mazen A. Badawi: Data curation. Siraj O. Wali: Methodology, Writing - review & editing. Husam M. Malibary: Data curation. Ali F. Atwah: Data curation, Writing - review & editing. Meshari M. Alhamdan: Data curation. Reem A. Almalki: Data curation. Tariq A. Madani: Conceptualization, Methodology, Resources, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
We acknowledge Marnys® for trial drug donation with no role in the conduct of the trial, the analysis, or the decision to submit the manuscript for publication. We thank Dr. Md Dilshad Manzar for the statistical consultation. We thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ctim.2021.102769.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.World Health Organization . 2020. Timeline of WHO’s response to COVID-19.https://www.who.int/news-room/detail/29-06-2020-covidtimeline Published 2020. (Accessed 6 August 2020) [Google Scholar]
- 2.Saudi Center for Disease Prevention and Control . 2020. Daily updates.https://covid19.cdc.gov.sa/daily-updates/ [Google Scholar]
- 3.Vijayvargiya P., Esquer Garrigos Z., Castillo Almeida N.E., Gurram P.R., Stevens R.W., Razonable R.R. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof) Mayo Clin Proc. 2020;95(7):1454–1466. doi: 10.1016/j.mayocp.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Clinical management of COVID-19.https://www.who.int/publications/i/item/clinical-management-of-covid-19 Published 2020. (Accessed 27 August 2020) [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA - J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Suleyman G., Fadel R.A., Malette K.M. Clinical Characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira D., Prieto-Garcia J.M., Boylan F. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharmacol. 2020;11:1479. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirvani H., Rostamkhani F., Arabzadeh E., Mohammadi F., Mohammadi F. Potential role of Nigella sativa supplementation with physical activity in prophylaxis and treatment of COVID-19: a contemporary review. Sport Sci Health. 2021;(May) doi: 10.1007/s11332-021-00787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khazdair M.R., Ghafari S., Sadeghi M. Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19. Pharm Biol. 2021;59(1):696–703. doi: 10.1080/13880209.2021.1931353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad A., Husain A., Mujeeb M. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koshak A., Wei L., Koshak E. Nigella sativa supplementation improves asthma control and biomarkers: a randomized, double-blind, placebo-controlled trial. Phyther Res. 2017;(January) doi: 10.1002/ptr.5761. [DOI] [PubMed] [Google Scholar]
- 14.Razmpoosh E., Safi S., Abdollahi N. The effect of Nigella sativa on the measures of liver and kidney parameters: a systematic review and meta-analysis of randomized-controlled trials. Pharmacol Res. 2020;156:104767. doi: 10.1016/j.phrs.2020.104767. [DOI] [PubMed] [Google Scholar]
- 15.Molla S., Abul M., Azad K. A review on antiviral effects of Nigella Sativa L. Pharmacologyonline. 2019;2:47–53. http://pharmacologyonline.silae.it (Accessed 18 March 2020) [Google Scholar]
- 16.Barakat A.B., Shoman S.A., Dina N., Alfarouk O.R. Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. seed extracts against in vitro herpes simplex and hepatitis A viruses infection. J Microbiol Antimicrob. 2010;2(3):23–29. [Google Scholar]
- 17.Ulasli M., Gurses S.A., Bayraktar R. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol Biol Rep. 2014;41(3):1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorra N., El-Berrawy M., Sallam S., Mahmoud R. Evaluation of antiviral and antioxidant activity of selected herbal extracts. J High Inst Public Heal. 2019;49(1):36–40. doi: 10.21608/jhiph.2019.29464. [DOI] [Google Scholar]
- 19.Oyero O.G., Toyama M., Mitsuhiro N. Selective inhibition of hepatitis c virus replication by alpha-zam, a nigella sativa seed formulation. Afr J Tradit Complement Altern Med. 2016;13(6):144–148. doi: 10.21010/ajtcam.v13i6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem M.L., Hossain M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22(9):729–740. doi: 10.1016/S0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 21.Onifade A.A., Jewell A.P., Adedeji W.A. Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr J Tradit Complement Altern Med. 2013;10(5):332–335. [PMC free article] [PubMed] [Google Scholar]
- 22.Onifade A.A., Jewell A.P., Ajadi T.A., Rahamon S.K., Ogunrin O.O. Effectiveness of a herbal remedy in six HIV patients in Nigeria. J Herb Med. 2013;3(3):99–103. doi: 10.1016/j.hermed.2013.04.006. [DOI] [Google Scholar]
- 23.Barakat E.M.F., El Wakeel L.M., Hagag R.S. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J Gastroenterol. 2013;19(16):2529–2536. doi: 10.3748/wjg.v19.i16.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshak A.E., Koshak E.A. Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies. Curr Ther Res - Clin Exp. 2020;93:100602. doi: 10.1016/j.curtheres.2020.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sultan M.T., Buttxs M.S., Qayyum M.M.N., Suleria H.A.R. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr. 2014;54(10):1298–1308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- 26.Mady W.H., Arafa A., Hussein A.S., Aly M.M., Madbouly H.M. Nigella sativa oil as an immunostimulant adjuvant in H5 based DNA vaccine of H5N1 avian influenza virus. Glob Vet. 2013;10(6):663–668. doi: 10.5829/idosi.gv.2013.10.6.73101. [DOI] [Google Scholar]
- 27.Haq A., Abdullatif M., Lobo P.I., Khabar K.S.A., Sheth K.V., Al-Sedairy S.T. Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacology. 1995;30(2):147–155. doi: 10.1016/0162-3109(95)00016-M. [DOI] [PubMed] [Google Scholar]
- 28.Gholamnezhad Z., Boskabady M.H., Hosseini M. Effect of Nigella sativa on immune response in treadmill exercised rat. BMC Complement Altern Med. 2014;14:437. doi: 10.1186/1472-6882-14-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majdalawieh A.F., Fayyad M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. 2015;28(1):295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Koshak A., Fiebich B., Koshak E., Heinrich M. Comparative anti-inflammatory/immunomodulatory effect of different extracts of medicinal plant Nigella sativa. European Academy of Allergy and Clinical Immunology Congress; Barcelona, Spain; 2015. EAACI Online Library http://eaaci.multilearning.com/eaaci/2015/barcelona/104874/emad.koshak.comparative.anti-inflammatory.immunomodulatory.effect.of.different.html?f=p6m3e814o10431. (Accessed 6 March 2017) [Google Scholar]
- 31.Al-Ghamdi M.S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76(1):45–48. doi: 10.1016/S0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 32.Gilani A.H., Aziz N., Khurram I.M., Chaudhary K.S., Iqbal A. Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): a traditional herbal product with multiple medicinal uses. J Pak Med Assoc. 2001;51(3):115–120. http://jpma.org.pk/full_article_text.php?article_id=2562 (Accessed 11 September 2016) [PubMed] [Google Scholar]
- 33.Boskabady M.H., Keyhanmanesh R., Khamneh S., Ebrahimi M.A. The effect of Nigella sativa extract on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. Clinics. 2011;66(5):879–887. doi: 10.1590/S1807-59322011000500027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babaei F., Mirzababaei M., Nassiri-Asl M., Hosseinzadeh H. Review of registered clinical trials for the treatment of COVID-19. Drug Dev Res. 2021;82(4):474–493. doi: 10.1002/ddr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medscape Drugs & Diseases . 2020. What are complications of patients with coronavirus disease 2019 (COVID-19)?https://www.medscape.com/answers/2500119-197504/what-are-complications-of-patients-with-coronavirus-disease-2019-covid-19 Published 2020. (Accessed 27 June 2021) [Google Scholar]
- 36.International Severe Acute Respiratory and Emerging Infections Consortium . 2020. ISARIC COVID-19 report: 08 June 2020. [Google Scholar]
- 37.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA - J Am Med Assoc. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration Coronavirus (COVID-19) update: FDA reiterates importance of close patient supervision for “off-label” use of antimalarial drugs to mitigate known risks, including heart rhythm problems. FDA-COVID-19-update. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-reiterates-importance-close-patient-supervision-label-use
- 39.U.S. Food and Drug Administration . 2020. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. FDA-COVID-19-update.https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or Published 2020. (Accessed 24 September 2020) [Google Scholar]
- 40.Skipper C.P., Pastick K.A., Engen N.W. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173(8):623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitjà O., Corbacho-Monné M., Ubals M. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahebnasagh A., Avan R., Saghafi F. Pharmacological treatments of COVID-19. Pharmacol Rep. 2020;1:3. doi: 10.1007/s43440-020-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Sayed S.M., Aboonq M.S., El Rashedy A.G. Promising preventive and therapeutic effects of TaibUVID nutritional supplements for COVID-19 pandemic: towards better public prophylaxis and treatment (a retrospective study) Am J Blood Res. 2020;10(5):266–282. http://www.ncbi.nlm.nih.gov/pubmed/33224571 (Accessed 27 June 2021) [PMC free article] [PubMed] [Google Scholar]
- 44.Ashraf S., Ashraf S., Ashraf M. Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): a multi-center placebo-controlled randomized clinical trial. medRxiv. 2020;2020(January) doi: 10.1101/2020.10.30.20217364. 10.30.20217364. [DOI] [PubMed] [Google Scholar]
- 45.Sun X., Wang T., Cai D. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS‐CoV‐2 infection: review of 3939 COVID‐19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islam M.N., Hossain K.S., Sarker P.P. Revisiting pharmacological potentials of Nigella sativa seed: a promising option for COVID-19 prevention and cure. Phyther Res. 2020;(October):1–16. doi: 10.31219/osf.io/56pq9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulyar M.Fe.A., Li R., Mehmood K., Waqas M., Li K., Li J. Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic. Phytomedicine. 2020;(July):153277. doi: 10.1016/j.phymed.2020.153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.