Abstract
Background
A recent meta‐analysis of sodium–glucose cotransporter 2 (SGLT2) inhibitor outcome trials reported that SGLT2 inhibitors were associated with reduction in the risk of adverse composite kidney outcomes, with moderate heterogeneity across the trials; however, the endpoints were defined differently across the trials.
Hypothesis
The apparent heterogeneity of the meta‐analysis of kidney composite outcomes of SGLT2 inhibitor trials will be substantially reduced by using a consistent assessment of sustained ≥40% decline in eGFR/chronic kidney dialysis/transplantation/renal death across trials.
Methods
We performed a meta‐analysis of kidney composite outcomes from the four SGLT2 cardiovascular outcome trial programs conducted in general type 2 diabetes mellitus populations, which included, as a surrogate of progression to kidney failure, a sustained ≥40% decline in eGFR along with kidney replacement therapy and kidney death. The trials assessed were VERTIS CV (NCT01986881), CANVAS Program (NCT01032629 and NCT01989754), DECLARE‐TIMI 58 (NCT01730534), and EMPA‐REG OUTCOME (NCT01131676).
Results
Data from the trials comprised 42 516 individual participants; overall, 998 composite kidney events occurred. SGLT2 inhibition was associated with a significant reduction in the kidney composite endpoint (HR 0.58 [95% CI 0.51–0.65]) and with a highly consistent effect across the trials (Q statistic p = .64; I 2 = 0.0%).
Conclusions
Our meta‐analysis highlights the value of using similarly defined endpoints across trials and supports the finding of consistent protection against kidney disease progression with SGLT2 inhibitors as a class in patients with type 2 diabetes mellitus who either have established atherosclerotic cardiovascular disease or are at high cardiovascular risk with multiple cardiovascular risk factors.
Keywords: kidney disease, kidney failure, meta‐analysis, randomized clinical trials, SGLT2 inhibitor, type 2 diabetes mellitus
1. INTRODUCTION
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are medications initially developed to manage hyperglycemia in type 2 diabetes. Results from cardiovascular outcome trials (CVOTs) demonstrated that some of these agents significantly reduce the risk of major adverse cardiovascular and kidney events.1 In these trials, composite kidney outcomes were reported, comprising hard kidney outcomes of kidney death and kidney failure (kidney replacement therapy and end‐stage kidney disease), as well as surrogate measures of progression to kidney failure; i.e., doubling of serum creatinine (corresponding to a 57% decline in estimated glomerular filtration rate [eGFR]), or a 40% to 50% sustained decline in eGFR from baseline. One of the secondary endpoints from the ertugliflozin cardiovascular outcome trial (VERTIS CV) was a composite kidney outcome comprising doubling of serum creatinine/kidney dialysis/transplantation/kidney death, which had a hazard ratio (HR) (95% confidence interval [CI]) of 0.81 (0.63–1.04).2 A recent meta‐analysis of SGLT2 inhibitor outcome trials reported that SGLT2 inhibitors were associated with reduction in the risk of adverse composite kidney outcomes (HR 0.62, 95% CI 0.56–0.70), with moderate heterogeneity across the trials (Q statistic p = .09; I 2 = 49.7%).1
Previous meta‐analyses of kidney outcomes from SGLT2 inhibitor trials have not, however, used uniform definitions of “significant kidney function decline”, which have differed between the trials.1 The importance of using consistent definitions of eGFR decline has been emphasized by recent expert consensus statements, as thresholds for kidney function loss must be clinically relevant and also feasible over the 2–4 year time frame of clinical outcome trials.3, 4 Accordingly, our aim was to examine kidney composite outcome data from four SGLT2 inhibitor CVOTs using a uniform sustained ≥40% eGFR decline definition.3, 4
2. METHODS
The VERTIS CV trial (NCT01986881; protocol MK‐8835‐004) assessed the effects of ertugliflozin versus placebo in patients with type 2 diabetes and established atherosclerotic cardiovascular disease (ASCVD). The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. Based on analyses from a scientific workshop sponsored by the National Kidney Foundation and US Food and Drug Administration, which critically examined available data to determine whether alternative GFR‐based endpoints could be used in clinical trials,4 a pre‐specified exploratory analysis of VERTIS CV data was conducted that reported a significant risk reduction in a composite kidney outcome (sustained ≥40% decline in eGFR/chronic kidney dialysis/transplantation/renal death [HR 0.66, 95% CI 0.50–0.88]).5
We performed a meta‐analysis of kidney composite outcomes from four SGLT2 inhibitor CVOT programs, which included, as a surrogate of progression to kidney failure, a sustained ≥40% decline in eGFR along with kidney replacement therapy and kidney death. The analysis was conducted on the total patient population. The CVOTs assessed were VERTIS CV (NCT01986881),5 CANVAS Program (NCT01032629 and NCT01989754),6 DECLARE‐TIMI 58 (NCT01730534),7 and EMPA‐REG OUTCOME (NCT01131676).8 HRs and 95% CIs were pooled with the meta‐analysis performed across trials. A fixed‐effect meta‐analysis approach was used, with heterogeneity assessed using the Cochran Q test statistic and Higgins and Thompson I 2.9, 10 Heterogeneity was considered to be low, moderate, or high if I 2 was less than 25%, 25% to 75%, or greater than 75%, respectively. The HR and its 100 × (1–α) %CI were extracted from the publications of each individual study and converted to log(HR) and its SE before the meta‐analysis. The meta‐analysis was directly implemented on the natural log HR scale, with results exponentiated and reported on the original HR scale. The R package, metafor, version 3.6.2 (R Foundation), was used for all analyses and for forest plot generation. Two‐sided p values <.05 were considered significant.
3. RESULTS
Data from the four cardiovascular outcome study programs with 42 516 patients were included. The median follow‐up period for each trial ranged from 2.4 to 4.2 years. A summary of the key baseline characteristics of these trials can be found in Table 1. Overall, there were 998 patients with composite kidney events.5, 6, 7, 8 Of these, 947 patients had a sustained 40% decline in eGFR (Table 1). The results of meta‐analysis of effects of SGLT2 inhibitors on the hazard for the kidney composite are presented in Figure 1. Overall, SGLT2 inhibition was associated with a significant reduction in the kidney composite endpoint (HR 0.58, 95% CI 0.51–0.65) and with a highly consistent effect across the trials, with low heterogeneity (Q statistic p = 0.64; I 2 = 0.0%).
TABLE 1.
Key baseline characteristics, follow‐up, and number of patients with kidney events for the four SGLT2 inhibitor cardiovascular outcome trials identified for the meta analysis
| EMPA‐REG OUTCOME8, 13, 14 | CANVAS Program6, 15 | DECLARE‐TIMI 587, 16 | VERTIS CV2, 5 | |
|---|---|---|---|---|
| Number of patients | 7020 | 10 142 | 17 160 | 8246 |
| Mean age (years) | 63 | 63 | 64 | 64 |
| Female (%) | 29 | 36 | 37 | 30 |
| Caucasian (%) | 72 | 78 | 80 | 88 |
| ASCVD (%) | 100 | 66 | 41 | 100 |
| Mean HbA1c (%) | 8.1 | 8.2 | 8.3 | 8.2 |
| Mean BMI (kg/m2) | 31 | 32 | 32 | 32 |
| Mean SBP (mmHg) | 135 | 137 | 135 | 133 |
| Mean eGFR (ml/min/1.73 m) | 74 | 77 | 85 | 76 |
| Mean duration of follow‐up (years) | NA | 3.6 | NA | 3.5 |
| Median duration of follow‐up (years) | 3.1 | 2.4 | 4.2 | 3.0 |
| Number of patients with composite kidney events (n) | 186 | 249 | 365 | 198 |
| Sustained 40% decline in eGFR (n) | 175 | 239 | 341 | 192 |
| Kidney replacement therapy (n) | 25 | 18 | 25 | 12 |
| Kidney death (n) | NA | 3 | 16 | 2 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; SBP, systolic blood pressure.
FIGURE 1.
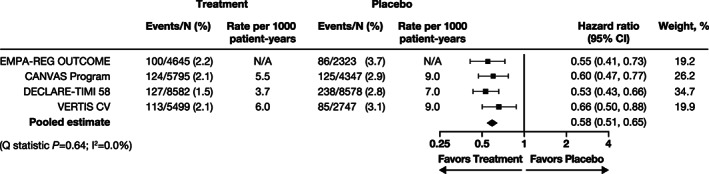
Effects of sodium–glucose cotransporter 2 inhibitors on the kidney composite outcome of sustained* ≥40% decline in eGFR from baseline, kidney replacement therapy, or kidney death. *In the EMPA‐REG OUTCOME and DECLARE‐TIMI 58 trials, 'sustained' was defined as a reduction that was present on at least two consecutive occasions ≥4 weeks apart. In the CANVAS Program and VERTIS CV trial, 'sustained' was defined as a reduction that was present on at least two consecutive occasions >30 days apart. CI, confidence interval; eGFR, estimated glomerular filtration rate. Weights are initially calculated as 1/variance of the hazard ratio and then standardized so that they sum up to 1. Studies with higher precision (i.e. smaller confidence intervals) will be given more weights
4. DISCUSSION
This meta‐analysis highlights the value of using similarly defined endpoints across trials. In previous meta‐analyses,1 individual trials used different definitions of significant eGFR loss as a surrogate for adverse kidney outcomes. In VERTIS CV, it was doubling of serum creatinine2; in EMPA‐REG OUTCOME,11 it was doubling of serum creatinine accompanied with an eGFR of ≤45 ml/min per 1.73 m2; in the CANVAS Program,6 it was a sustained ≥40% decline in eGFR; and in DECLARE‐TIMI 58,7 it was a sustained ≥40% decline in eGFR accompanied by an eGFR of <60 ml/min per 1.73 m2. In the current meta‐analysis, the surrogate endpoint of adverse kidney outcomes was sustained ≥40% decline in eGFR (in one trial, the decline in eGFR had to be accompanied with an eGFR of <60 ml/min per 1.73 m2).7 When using similar definitions, the results of the current meta‐analysis demonstrate a very low measure of heterogeneity, with an I 2 of 0.0% compared with 49.7% in the prior meta‐analysis using disparate endpoints.1
Based on recent literature, an international consensus on definitions of endpoints for clinical trials of kidney failure has emerged, coming to the conclusion that the preferred threshold for kidney function loss is a sustained ≥40% decline in eGFR from baseline.3 This threshold of kidney function loss is clinically meaningful, predictive of future need for kidney replacement therapy, and can be captured within the relatively brief timeframe of outcome trials.
SGLT2 inhibitors decrease the risk for kidney composite outcomes in CVOTs, with similar benefits reported in dedicated kidney outcome trials such as CREDENCE and DAPA‐CKD. Based on the results from the SGLT2 inhibitor CVOTs and kidney outcome studies, the Kidney Disease Improving Global Outcomes organization modified their 2020 guidelines for the management of chronic kidney disease in patients with diabetes, leading to the following recommendation “…glycemic management for patients with type 2 diabetes and chronic kidney disease should include lifestyle therapy, first‐line treatment with metformin and an SGLT2 inhibitor, and additional drug therapy as needed for glycemic control”.12
5. CONCLUSION
This meta‐analysis, using a consistent endpoint, supports the robustness of the finding of reduced kidney disease progression across the class of SGLT2 inhibitors in patients with type 2 diabetes either with established ASCVD or at high cardiovascular risk.
CONFLICT OF INTEREST
David Z.I. Cherney has received consulting fees or speaking honoraria, or both, from Bristol Myers Squibb, Novo Nordisk, Mitsubishis‐Tanabe, MAZE, Janssen, Bayer, Boehringer Ingelheim‐Eli Lilly, AstraZeneca, Merck & Co., Inc., Prometic, and Sanofi; and has received operating funds from Janssen, Boehringer Ingelheim‐Eli Lilly, Sanofi, AstraZeneca, and Merck & Co., Inc. Samuel Dagogo‐Jack has led clinical trials for AstraZeneca, Novo Nordisk, Inc., and Boehringer Ingelheim; has received fees from AstraZeneca, Boehringer Ingelheim, Janssen, Merck & Co., Inc., and Sanofi; and holds equity interests in Jana Care, Inc. and Aerami Therapeutics. Darren K. McGuire has had leadership roles in clinical trials for AstraZeneca, Boehringer Ingelheim, Eisai, Esperion, GlaxoSmithKline, Janssen, Lexicon, Merck & Co., Inc., Novo Nordisk, CSL Behring, and Sanofi USA; and has received consultancy fees from AstraZeneca, Boehringer Ingelheim, Lilly USA, Merck & Co., Inc., Pfizer, Novo Nordisk, Metavant, Afimmune, and Sanofi. Francesco Cosentino has received fees from Abbott, AstraZeneca, Bayer, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Novo Nordisk, and Pfizer; as well as research grants from Swedish Research Council, Swedish Heart & Lung Foundation, and the King Gustav V and Queen Victoria Foundation. Richard Pratley has received the following (directed to his institution): speaker fees from Novo Nordisk; consulting fees from Merck & Co., Inc., Novo Nordisk, Pfizer, Sanofi, Scohia Pharma Inc., and Sun Pharmaceutical Industries; and grants from Lexicon Pharmaceuticals, Hanmi Pharmaceutical Co., Novo Nordisk, Poxel SA, and Sanofi. Weichung J. Shih has received fees for an ertugliflozin advisory board of Merck & Co., Inc., Kenilworth, New Jersey, USA. Robert Frederich and Shuai Wang are employees and shareholders of Pfizer Inc. Mario Maldonado is an employee of MSD UK. He may own stock and/or stock options in Merck & Co., Inc., Kenilworth, New Jersey, USA. Jie Liu is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, New Jersey, USA. Christopher P. Cannon reports grants and personal fees from Pfizer Inc., Amgen, Boehringer Ingelheim, Bristol Myers Squibb, and Janssen; grants and personal fees from Merck & Co., Inc., during the conduct of the trial; grants from Daiichi Sankyo and Novo Nordisk; and personal fees from Aegerion, Alnylam, Amarin, Applied Therapeutics, Ascendia, Corvidia, HLS Therapeutics, Innovent, Kowa, Sanofi, Eli Lilly, and Rhoshan, outside the submitted work.
AUTHOR CONTRIBUTIONS
David Z.I. Cherney, Samuel Dagogo‐Jack, Darren K. McGuire, Richard Pratley, Francesco Cosentino, Mario Maldonado, Jie Liu, and Christopher P. Cannon substantially contributed to the conception, design, or planning of the study. Darren K. McGuire, Robert Frederich, Shuai Wang, and Christopher P. Cannon substantially contributed to the acquisition of the data. Samuel Dagogo‐Jack, Darren K. McGuire, Mario Maldonado, Jie Liu, Shuai Wang, and Christopher P. Cannon substantially contributed to the analysis of the data. David Z.I. Cherney, Samuel Dagogo‐Jack, and Mario Maldonado substantially contributed to the drafting of the manuscript. All authors substantially contributed to the interpretation of the results and critically reviewed and revised the manuscript for important intellectual content.
ACKNOWLEDGMENTS
The VERTIS CV study and this analysis were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, in collaboration with Pfizer Inc. Editorial support was provided by Moamen Hammad, PhD, MPharm, of Scion and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, in collaboration with Pfizer Inc., New York, USA. This analysis was presented in oral form at the virtual 56th European Association for the Study of Diabetes meeting, Vienna, Austria, September 21–25, 2020.
Mario Maldonado is the guarantor of this work and as such had full access to all the data from the trial and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Cherney DZI, Dagogo‐Jack S, McGuire DK, et al. Kidney outcomes using a sustained ≥40% decline in eGFR: A meta‐analysis of SGLT2 inhibitor trials. Clin Cardiol. 2021;44(8):1139–1143. 10.1002/clc.23665
Funding information Merck Sharp and Dohme; Pfizer
DATA AVAILABILITY STATEMENT
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
REFERENCES
- 1.DK MG, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta‐analysis. JAMA Cardiol. 2020;6(2):148–158. 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon CP, Pratley R, Dagogo‐Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425‐1435. [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Agarwal R, Herrington WG, et al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int. 2020;98(4):849‐859. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821‐835. [DOI] [PubMed] [Google Scholar]
- 5.Cherney DZI, Charbonnel B, Cosentino F, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64(6):1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Koitka‐Weber A, Cooper ME, et al. Choice of endpoint in kidney outcome trials: considerations from the EMPA‐REG OUTCOME® trial. Nephrol Dialysis Transplantation Off Pub European Dialysis Transplant Assoc European Renal Assoc. 2020;35:2103‐2111. [DOI] [PubMed] [Google Scholar]
- 9.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3/4):256‐266. [PubMed] [Google Scholar]
- 10.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 11.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 12.de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence‐based advances in monitoring and treatment. Kidney Int. 2020;98(4):839‐848. [DOI] [PubMed] [Google Scholar]
- 13.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira JP, Kraus BJ, Zwiener I, et al. Cardio/kidney composite end points: a post hoc analysis of the EMPA‐REG OUTCOME trial. J Am Heart Assoc. 2021;10(7):e020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691‐704. [DOI] [PubMed] [Google Scholar]
- 16.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA's data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.


