Abstract
The recreational drug 3,4-methylenedioxymethamphetamine (MDMA) has well documented prosocial effects and is currently under clinical investigation as a treatment for patients with PTSD, autism, and other conditions. Early clinical trials have found that MDMA-assisted therapy may have robust long-lasting therapeutic effects, yet the mechanism by which acute treatments produce these long-term effects is unclear. Sensitization to certain behavioral drug effects is a common rodent model used to assess long-lasting neurobiological adaptations induced by acute drug treatments. Nine independent experiments were undertaken to investigate if/and how mice sensitize to the prosocial effects of MDMA. When treated with 7.8 mg/kg MDMA and paired every other day for a week, MDMA-induced social interaction increased precipitously across treatment sessions. This previously unreported phenomenon was investigated and found to be heavily influenced by a social context and 5-HT2AR activation. Social sensitization did not appear to develop if mice were administered MDMA in isolation, and pretreatment with MDL100907, a selective 5-HT2AR antagonist, inhibited the development of social sensitization. However, when MDL100907 was administered to mice that had already been sensitized, it did not attenuate social interaction, suggesting that 5-HT2AR activity may be necessary for the development of social sensitization but not the expression of MDMA-induced social behavior. Additional investigation is warranted to further explore the phenomenon of social sensitization and to determine the underlying neurobiological mechanisms.
1. Introduction
3,4-methylenedioxymethamphetamine (MDMA) is a recreational drug (ecstasy, molly) that is under clinical investigation as a therapeutic adjunct for the treatment of post-traumatic stress disorder (PTSD), autism spectrum disorder, and other conditions (Mithoefer et al., 2016). MDMA shares some similarities with psychostimulants and hallucinogens but is generally regarded as a distinct, prototypically social drug (Kamilar-Britt and Bedi, 2015). Volunteers administered MDMA report increased feelings of friendliness and closeness towards others (Bedi et al., 2010; Hysek et al., 2013), they are more likely to choose to participate in social situations (Kirkpatrick et al., 2014), and they spend more time interacting with one another than those given placebo (Kirkpatrick and de Wit, 2014). MDMA also increases measures of trust, generosity, and empathy (Bershad et al., 2016). Although these prosocial effects are acute, they may contribute to the sustained therapeutic benefits that have been reported following MDMA-assisted therapy in patients with autism (Danforth et al., 2018) or PTSD (Mithoefer et al., 2018, 2013) in recent phase I and II clinical trials.
The idea that acute administration of a drug can have lasting effects on the brain and behavior has been extensively studied in the context of addiction (Robinson and Berridge, 2008). Rodents given repeated intermittent treatments with certain drugs of abuse, including MDMA, show an enhanced or “sensitized” behavioral and neurochemical response to subsequent drug treatments. Behavioral sensitization involves the enhancement of multiple discrete and largely dissociable behaviors, with locomotor behavior being studied most frequently (Robinson and Becker, 1986). Like many other recreational drugs, repeated MDMA treatments can produce long-lasting locomotor and neurochemical sensitization in rodents (Ball et al., 2011; Bradbury et al., 2012; Kalivas et al., 1998). However, sensitization to MDMA’s prosocial effects has not been previously reported. Sensitization to MDMA-induced social behavior could indicate that MDMA produces lasting neurobiological changes relevant to its social effects and provide a novel behavioral paradigm to investigate these changes. Such a model could inform not only the mechanisms of MDMA-induced prosocial behavior, but also the endogenous mechanisms that drive normal affiliative social behaviors (Heifets and Malenka, 2016).
A series of experiments were performed to determine if mice sensitize to the prosocial effects of MDMA and whether this is accompanied by sensitized locomotor and neurochemical responses. Next, the pharmacological underpinnings of social sensitization were assessed, focusing on the role of serotonin 2A receptors (5-HT2ARs). MDMA is a partial 5-HT2AR agonist (Nash et al., 1994), and 5-HT2AR activation has been implicated in the prosocial (Pitts et al., 2017) and certain therapeutic-like effects of MDMA (Young et al., 2017). 5-HT2ARs are also known to be involved in the development (Auclair et al., 2004; Wu et al., 2015) and expression (Ramos et al., 2005; Zayara et al., 2010) of sensitized locomotor and neurochemical responses to other drugs of abuse. Together these experiments provide the first demonstration that animals sensitize to the prosocial effects of MDMA.
2. Methods
2.1. Animals
Male Swiss Webster mice (Charles River Laboratories, Wilmington, MA) aged 7-10 weeks served as subjects in all experiments. Nine independent experiments were conducted, with a total of 172 mice (Table 1). All mice entered the study drug-naïve with no prior behavioral testing. Mice were housed five per cage in a temperature- and humidity-controlled colony room at the Yerkes National Primate Research Center with food and water available ad libitum. Lights were set to a 12-hour light/dark cycle. All experiments were performed at an ambient temperature of 22 ± 2°C during the lights-on phase. All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Experimental protocols were approved by the Emory University Institutional Animal Care and Use Committee.
Table 1.
Summary of Experiments
| Independent Experiments | Treatment Groups | Mice per group (pairs per group)a | Descriptive Figure |
|---|---|---|---|
| Social sensitization to MDMA | 3b | 10 (5) | Fig. 1a–d |
| Durability of sensitization | 2 | 12 (6) | Fig. 1e–f |
| Development of sensitization in isolated vs paired mice | 3 | 8 (4) | Fig. 1g |
| Locomotor sensitization | 2 | 6 | Fig. 2a |
| Neurochemical sensitization | 1 | 5 | Fig. 2b |
| M100 and development of sensitization | 2 | 8 (4) | Fig. 3a |
| M100 and expression of sensitization | 2 | 8 (4) | Fig. 3b |
| Effect of M100 on locomotor activity | 2 | 5 | Fig. 3c |
| DOI and expression of sensitization | 4 | 8 (4) | Fig. 3d |
Pairs of mice were the unit of analysis in social interaction experiments (n=pairs/group).
Social interaction for group 3 (Saline days 1,3,5; MDMA day 7) was only measured on day 7 (Fig. 1d).
2.2. Drugs
MDMA HCl was supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC); d-amphetamine hemisulfate and the 5-HT2AR agonist R(–)-2,5-Dimethoxy-4-iodoamphetamine HCl (DOI) were purchased from Sigma-Aldrich (St. Louis, MO). Each was dissolved in 0.9% sterile saline. The 5-HT2AR antagonist R(+)-MDL100,907 HCl (M100) was provided by Dr. Kenner Rice and was dissolved in sterile saline containing HCl, with NaOH added to restore the solution to physiological pH. All drugs were administered via intraperitoneal injection at a volume of 10 μl/g at previously established doses: 7.8 mg/kg MDMA (Curry et al., 2018), 2 mg/kg d-amphetamine (Lanteri et al., 2013), 1 mg/kg M100 (Brody et al., 2004), and 1 mg/kg DOI (Ross et al., 2006). All doses were calculated and are expressed as salts.
2.3. Behavioral Tests
2.3.1. Social Interaction Procedure
The social interaction test is a well validated measure of dyadic social behavior in male rodents (File and Seth, 2003) that is sensitive to the behavioral effects of MDMA (Curry et al., 2018; Morley and McGregor, 2000). After drug or vehicle treatments, each mouse was isolated in a clean cage for 25 minutes and then paired with an unfamiliar conspecific from the same treatment group for a 10-minute social interaction test in a clear 35 x 28 cm (unless otherwise noted) plexiglass arena. While in the testing arena, subjects were free to move around and interact, allowing a diverse range of observable behaviors. Tests were videotaped and scored using JWatcher by an observer blind to the experimental conditions. The durations of three behaviors were scored: anogenital investigation (sniffing the conspecific’s anogenital area), general investigation (non-anogenital sniffing, grooming, and following the conspecific), and adjacent lying (side-by-side contact or huddling, excluding climbing under/over the conspecific) (Morley et al., 2005). The durations of these behaviors were averaged for each pair of mice and then summed to produce a total social interaction score.
2.3.2. Social Sensitization to MDMA
To determine if mice sensitize to the prosocial effects of MDMA, mice were administered MDMA or saline (5 pairs/group) every 48 hours for a total of four treatment sessions. Due to high aggression within one saline-treated pair, the two mice had to be separated during the first test day and were removed from the study. To determine if increased social interaction following subsequent MDMA treatments was due to experience with the testing procedure, five pairs of mice were treated with saline during the first three social interaction tests and received MDMA on the forth test day (day 7). To determine the persistence of MDMA-induced social sensitization and its effect on off-drug social interaction, another cohort of mice (6 pairs/group) underwent the same sensitization procedure, receiving either saline or MDMA across four treatment sessions, each 48 hours apart. On day 11, four days after the last treatment session, mice were paired with an unfamiliar conspecific but did not receive drug treatments. On day 21, 14 days after the last treatment session, 7.8 mg/kg MDMA or saline was administered to determine if sensitization persisted after two weeks. Finally, to determine if social sensitization was dependent upon social interaction during the treatment sessions or if it would occur regardless of the social context, mice (4 pairs/group) received MDMA across three treatment sessions and were either isolated or paired for two hours after treatment, before being returned to their home cages. On day 7, mice were given MDMA, isolated for 25 minutes, and then paired in a novel 30 x 18 cm testing arena. Their social interaction was compared to mice receiving MDMA for the first time.
2.3.3. 5-HT2AR role in Social Sensitization
The role that 5-HT2ARs play in mediating social sensitization was assessed with several related experiments. First, to determine if activation of 5-HT2ARs is necessary for the development of social sensitization, mice (4 pairs/group) received pretreatments of M100 or saline 15 minutes before treatment with MDMA and subsequent social interaction testing. This procedure was repeated on days 3 and 5. On day 7, mice were given MDMA without a pretreatment and then tested for social interaction. To determine if 5-HT2AR activation was necessary for the expression of sensitized MDMA-induced social behavior, an additional cohort of mice (4 pairs/group) was given MDMA across three treatment sessions as above. On day 7 they were given a pretreatment of M100 or saline 15 minutes before MDMA. Social interaction was then tested as described above. Next, to determine if activation of 5-HT2ARs is sufficient to induce the development of sensitization, mice (4 pairs/group) were administered DOI, MDMA, or saline for three treatment and social interaction sessions as described above and then treated with MDMA or saline on day 7 for sensitization testing. One MDMA treated subject from the MDMA sensitization group escaped the testing arena during testing on day 7 and had to be excluded from analysis.
2.3.4. Locomotor Activity Testing
Ambulatory activity was tested in a 45 x 39 x 37 cm open field activity monitoring apparatus in a dark, enclosed space (San Diego Instruments, San Diego, CA). Accumulative beam breaks of adjacent photocells were recorded as the measure of locomotor activity. The locomotor activity of each subject was monitored for one hour immediately after drug administration. Mice (6/group) were tested for locomotor sensitization across four treatments of MDMA or saline, each administered 48 hours apart. To measure cross-sensitization, all mice were given an injection of d-amphetamine two weeks after the last MDMA or saline treatment. To assess off-target effects of M100 (e.g., sedation) that might interfere with behavior, locomotor activity was tested in a separate group of mice (5/group) for one hour following treatment with M100 or saline.
2.4. In Vivo Neurochemistry
2.4.1. Stereotaxic Surgery and Microdialysis
To determine if the MDMA dosing regimen employed above produced neurochemical sensitization, 5-HT overflow was measured in the nucleus accumbens (NAcc) via microdialysis. Eight mice were anesthetized and underwent stereotaxic surgery for implantation of unilateral guide cannulae, installed 1.9 mm anterior and 0.9 mm lateral to bregma. After surgery, mice were singly housed for 24 hours and then pair-housed for the duration of the experiment. Following at least five days of recovery, mice were administered MDMA every 48 hours across four treatment sessions. On each treatment day, mice were placed into a clean circular cage with access to food and water. 5-HT overflow was measured during the first and forth treatment sessions. CMA 7 dialysis probes with 1 mm membranes (CMA, Kista, Sweden) were connected via FEP tubing to a Hamilton syringe mounted on a motor-driven syringe pump. Probes were flushed with artificial cerebrospinal fluid (aCSF) for 30 minutes prior to insertion into the guide cannulae (Thrivikraman et al., 2013). The flow of aCSF was maintained at 1 μl/min for the duration of the experiment. After a five-hour equilibration period, sample collection and experimental manipulations began. Samples of dialysate were collected every 20 minutes into micro-centrifuge tubes within a CMA refrigerated sample collector. Three baseline samples were taken before treatment with MDMA, and seven samples were collected post-treatment. Due to a non-responsive site or loss of guide cannula during the course of the experiment, three mice were removed from analysis.
2.4.2. High-Performance Liquid Chromatography
The concentration of 5-HT in each sample was quantified via high-performance liquid chromatography (HPLC) with electrochemical detection using established methods (Murnane et al., 2010). The HPLC system was composed of a small-bore column (3.2 mm x 150 mm x 3 um; 70-0636; Thermo Scientific, Sunnyvale, CA), a Thermo Dionix Ultimate 3000 solvent delivery pump set to a flow rate of 0.6 ml/min, a 5020 ESA guard cell (+350 mV; Chelmsford, MA), and an ESA 542 autosampler. Detection was carried out with a dual-channel analytical cell (5014B, Thermo Scientific) and an ESA Coulochem III detector. The analytical cell’s oxidative channel was set to −150 mV and its reductive channel was set to +200 mV. The mobile phase was prepared with polished water and contained 90 mM Na2HPO4, 50 mM citric acid, 1.7 mM 1-octanesulfonic acid, 50μM EDTA, 10% acetonitrile, and was adjusted with phosphoric acid to a pH of 3. Data were acquired and analyzed using Chromeleon 6.8 software (Thermo Scientific).
2.5. Statistical Analysis
Data were analyzed with Prism (Graphpad, La Jolla, CA). For social interaction experiments, pairs of mice were the units for statistical analysis. Sensitization of social interaction and locomotor activity measured across multiple treatment days were assessed by two-factor ANOVAs with Sidak’s multiple comparisons tests used to determine pairwise differences between treatments and test days while controlling familywise error rates. The primary statistical criterion for social and locomotor sensitization was the interaction of test day and treatment. Because novel pairings of mice were used on each social interaction test day to eliminate familiarity as a confound, comparisons across days were treated as between-subjects. To assess within-subjects changes in the two longitudinal data sets, a secondary analysis was performed with individual mice as the units of comparison (see Supplementary Information). Sensitization of individual social behaviors within the two treatment groups and locomotor sensitization were assessed with two-factor mixed-design ANOVAs, and neurochemical sensitization was assessed with a two-factor repeated-measures ANOVA. Two-tailed Student’s t-tests or one-factor ANOVAs with a Tukey’s post-hoc test were used to assess group differences at single time-points. Alpha for all experiments was set at 5%. Error bars represent the standard error of the mean (SEM).
3. Results
3.1. Mice sensitize to the prosocial effects of MDMA
Pairs of rodents that are placed into an area where neither has established territorial control will engage in a variety of social behaviors. These include active social behaviors such as sniffing, following, and allogrooming, as well as passive social behaviors such as lying in close contact (de Angelis and File, 1979; File and Hyde, 1978). Initial treatment with MDMA did not affect the duration of social interaction between unfamiliar male mice compared to saline treated subjects. However, subsequent MDMA treatments steadily increased the duration of social interaction (Fig. 1a, Fig. S1), with a significant effect of treatment (F(1,28) = 115.2, p < 0.0001), day (F(3,28) = 50.1, p < 0.0001), and most importantly an interaction (F(3,28) = 29.32 p < 0.0001). Social interaction following MDMA treatment was significantly greater than controls on days 5 and 7 (p < 0.0001), and greater on these days than MDMA treatment on days 1 and 3 (p < 0.0001), and MDMA-induced social interaction was greater on day 7 than on day 5 (p = 0.0003). Both active and passive social behaviors were increased by MDMA across treatment sessions (Fig. 1b), with a significant effect of day (F(3, 16) = 82.75, p < 0.0001), behavior (F(2,32) = 122.8, p < 0.0001), and an interaction (F(6,32) = 12.44, p < 0.0001). General investigation and adjacent lying increased significantly across subsequent MDMA treatments, while anogenital investigation did not. General investigation was higher on days 5 and 7 than on days 1 and 3 (p < 0.0001), and adjacent lying was higher on day 7 than on days 1 (p = 0.0008), 3 (p = 0.001), and 5 (p = 0.0252). In contrast, mice treated with saline interacted at a relatively stable amount upon each pairing (Fig. 1c).
Fig. 1. Mice sensitize to the prosocial effects of MDMA.
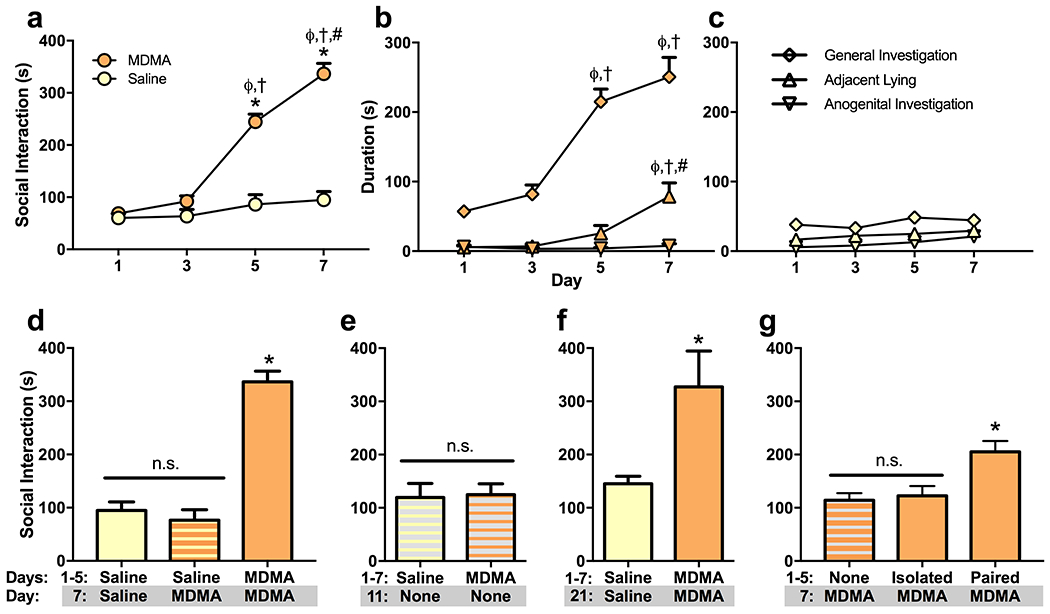
(a) Mice were treated every-other day with 7.8 mg/kg MDMA or saline and paired with a novel conspecific from the same treatment group for a 10-minute social interaction test. The duration of MDMA-induced social interaction increased precipitously across treatment sessions. (b) Total social interaction was the sum of three social behaviors: general investigation, adjacent lying, and anogenital investigation. The durations of general investigation and adjacent lying, but not anogenital investigation, increased across subsequent MDMA-treatment sessions. (c) In contrast, mice treated with saline interacted at a relatively stable amount upon each pairing. (d) Social sensitization to MDMA was not due to familiarity with the testing procedure. Mice that had been tested with saline on days 1, 3, and 5 did not display increased social interaction when treated with MDMA on day 7. (e) Sensitization of MDMA-induced social interaction did not affect off-drug social interaction when mice were paired without treatment. (f) Sensitization was long-lasting. When mice were treated again, following two weeks off-drug, MDMA-treated mice interacted significantly more than mice treated with saline. (g) A social context appears critical for the development of social sensitization. Mice that were paired during each treatment displayed significantly more social interaction when paired in a novel environment on test day 7 than did mice that had been isolated during the previous treatment sessions. Despite receiving MDMA during all four treatment sessions, these previously isolated mice displayed no more social interaction than mice receiving MDMA for the first time. Symbols indicate that social behavior was significantly different (p < 0.05) from day 1 (ϕ), day 3 (†), day 5 (#), or that treatment day’s control group(s) (*).
Social sensitization was not an artifact of familiarity with the testing procedure (F(2,11) = 60.04, p < 0.0001) (Fig. 1d). Mice treated with MDMA on day 7, after three prior saline treatments and social interaction tests, displayed no increase in social interaction relative to mice treated with saline (p = 0.7914), and they interacted significantly less than mice treated with MDMA on all four days (p < 0.0001). Sensitization did not affect off-drug social interaction (Fig.1e). There was no difference in social interaction between subjects with a treatment history of saline or MDMA when paired without treatment on day 11, four days following their last treatments (t(10) = 0.1591, p = 0.8767). However, sensitization was long lasting (Fig. 1f). Mice treated with MDMA on day 21, two weeks after their previous treatment sessions, engaged in significantly more social interaction than saline treated controls (t(10) = 2.646, p = 0.0245).
Social pairing during MDMA treatment sessions appeared crucial for the development of social sensitization (F(2,9) = 8.236, p = 0.0093) (Fig. 1g). Mice that were isolated during MDMA treatment sessions on days 1, 3, and 5 displayed significantly less social interaction when paired on day 7 than did mice that were socially paired during all four MDMA treatments (p = 0.0214). These previously isolated mice also displayed no more social interaction than did mice receiving MDMA for the first time on day 7 (p = 0.9417). Social sensitization, therefore, does not appear to be merely a product of MDMA treatment, but rather involves an interaction between the drug and the social environment in which it is given.
3.2. Limited evidence of locomotor or neurochemical sensitization
Ambulatory activity was measured across four treatment sessions with MDMA or saline and two weeks later with d-amphetamine (Fig. 2a). There was a significant effect of test day (F(3,30) = 4.688, p = 0.0084) and trends towards an effect of treatment (F(1,10) = 4.627, p = 0.0570) and an interaction (F(3,30) = 2.451, p = 0.0828). MDMA treated mice also had higher locomotor activity on day 7 than on day 1 (p = 0.0013). However, because the interaction of test day and treatment was not significant, the null hypothesis cannot be rejected. So, although locomotor sensitization might be occurring, the effect size is too small for this study to detect. Yet, there was also no locomotor cross-sensitization with d-amphetamine (t(10) = 0.1104, p = 0.9142), which is an effect that has previously been demonstrated as robust when mice are sensitized to the locomotor effects of MDMA (Bradbury et al., 2012; Lanteri et al., 2013). Thus, the effect of MDMA on locomotor sensitization in this paradigm remains unclear, and if occurring, appears substantially more modest than social sensitization. There was also no evidence of sensitized 5-HT release with this treatment regimen. 5-HT overflow was measured in the NAcc during the first and fourth MDMA treatment sessions (Fig. 2b). There was an effect of time (F(11,44) = 2.474, p = 0.0165) indicating that MDMA affected 5-HT overflow, but critically there was no effect of treatment day (F(1,4) = 0.04376, p = 0.8445). However, there was large variability in 5-HT overflow between subjects, limiting the interpretability of this finding.
Fig. 2. Limited evidence of locomotor and neurochemical sensitization.
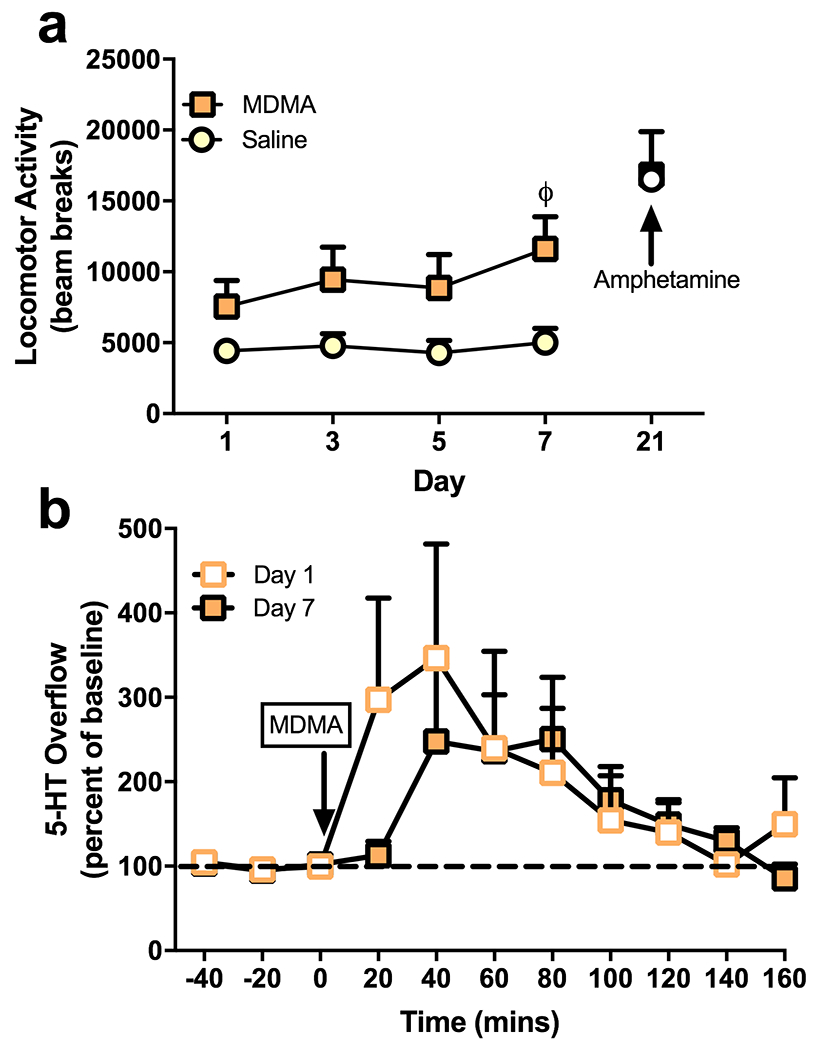
(a) The seven-day MDMA treatment regimen produced only limited evidence of locomotor sensitization. Locomotor activity was higher among MDMA-treated mice on day 7 than on day 1 (ϕ, p = 0.0013), and there was a trend towards an interaction between the treatments and test days. However, this trend was not large enough to reach the predefined level of significance. There was also no cross-sensitization with d-amphetamine (2 mg/kg). (b) There was no evidence that 5-HT release sensitized across treatment sessions. 5-HT overflow in the nucleus accumbens did not significantly differ between the first MDMA treatment given on day 1 and the fourth MDMA treatment given on day 7.
3.3. 5-HT2AR activity may be necessary but not sufficient for MDMA-induced social sensitization
Pretreatment with the selective 5-HT2AR antagonist M100 inhibited the development of social sensitization (Fig. 3a). There was a significant effect of pretreatment (F(1,24) = 9.257, p = 0.0056), a non-significant effect of day (F(3,24 ) = 1.406, p = 0.2653), and a significant interaction (F(3,24) = 3.129, p = 0.0444). Mice in the saline pretreatment group had significantly higher social interaction following MDMA treatment on day 7 than they did on day 1 (p = 0.0242), and on day 7 their social interaction was higher than in mice previously pretreated with M100 (p = 0.0358). However, MDMA-induced prosocial behavior was not attenuated in mice pretreated with M100 only on day 7 (t(6) = 1.31, p = 0.2380), indicating that while 5-HT2AR activity appears necessary for the development of sensitization it may not be critical for the expression of sensitized social behavior (Fig. 3b). Treatment with M100 did not significantly affect locomotor activity (t(8) = 0.8869, p = 0.401), indicating that changes in behavior following M100 were unlikely to have been caused by sedation (Fig. 3c).
Fig. 3. 5-HT2AR activity may be necessary but not sufficient for the development of social sensitization.
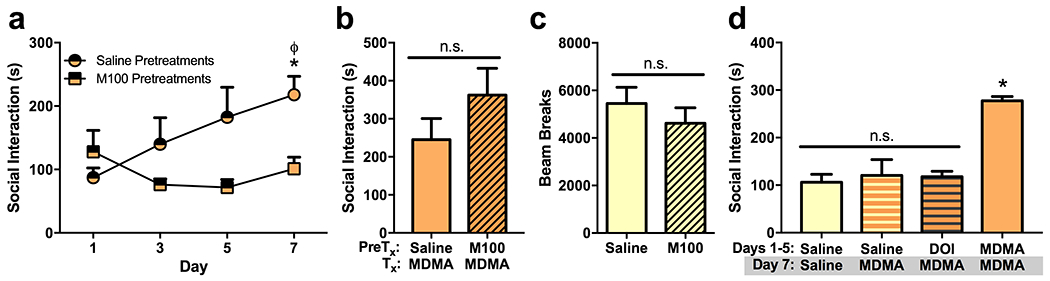
(a) Pretreatment with the 5-HT2AR antagonist M100 (1 mg/kg) on days 1, 3, and 5 inhibited the development of social sensitization in MDMA treated mice. On day 7 when mice were tested for social interaction without pretreatments, mice that had received M100 during the previous treatment sessions interacted significantly less than mice that had received saline pretreatments during those days. (b) When M100 was administered before MDMA on day 7 to mice that had received MDMA without pretreatments on the three previous test days, M100 did not attenuate MDMA-induced social interaction. Therefore, at this dose M100 does not appear to inhibit the expression of MDMA-induced social behavior in mice that are already sensitized. (c) M100 did not significantly affect locomotor activity relative to saline. (d) Treatment with the 5-HT2AR agonist DOI (1 mg/kg) during test days 1, 3, and 5 did not sensitize mice to MDMA-induced social interaction when it was administered on day 7. Treatment with a 5-HT2AR agonist therefore appears insufficient to sensitize mice to the prosocial effects of MDMA. Symbols indicate that social interaction was significantly different (p < 0.05) from day 1 (ϕ) or that treatment day’s control group(s) (*).
Administration of a 5-HT2AR agonist was insufficient to produce a later sensitized social response to MDMA. Mice were administered DOI on days 1, 3, and 5, and then received MDMA on day 7. Total social interaction on day 7 was compared to mice with a treatment history of saline receiving MDMA for the first time and to mice treated with MDMA or saline across all treatment sessions (Fig. 3d). There was a significant effect of treatment on day 7 (F(3,11) = 12.43, p = 0.0007). Mice previously treated with DOI displayed no more social interaction than mice treated with saline (p = 0.9775), and they displayed significantly less social interaction than mice treated with MDMA across all sessions (p = 0.0018). Thus, activation of 5-HT2ARs appears to be necessary but not sufficient for the development of social sensitization.
4. Discussion
The acute prosocial effects of MDMA have been well-characterized in humans and a growing number of studies have reported similar effects in other species (Kamilar-Britt and Bedi, 2015), including mice (Curry et al., 2018; Daza-Losada et al., 2009), rats (Ando et al., 2006; Morley and McGregor, 2000), and nonhuman primates (Ballesta et al., 2016; Pitts et al., 2017). In the present study, the prosocial effects of MDMA in mice increased precipitously across repeated intermittent treatments. This progressive enhancement of MDMA-induced social interaction appears to be a form of behavioral sensitization, wherein repeated administration of a drug results in increasingly amplified responses (Robinson and Becker, 1986). Much like locomotor sensitization, which has been extensively studied, social sensitization developed rapidly with an amplified response to the drug after just two or three intermittent treatments, the sensitized behavior did not persist off-drug, and yet it could be re-elicited by the drug even many days later. These similarities could indicate that social sensitization occurs in tandem with locomotor sensitization and that both phenomena share the same underlying neurobiological mechanism. However, the present findings do not support that conclusion. Although previous studies have demonstrated robust locomotor and neurochemical sensitization to MDMA (Bradbury et al., 2012; Kalivas et al., 1998; Varela et al., 2011), they utilized higher and/or more frequent dosing regimens than the present study where MDMA was administered only every other day. With this dosing regimen, there was only limited evidence that locomotor sensitization occurred and no cross-sensitization with d-amphetamine. Compared to the robust sensitization of social behavior that was observed, the effect on locomotor sensitization was more limited, with an effect size not large enough to detect as significant in the present study. In mice, locomotor sensitization to MDMA is reported to stem from neuronal adaptations that increase the quantity of 5-HT and NE released by MDMA (Lanteri et al., 2013). There was also no evidence of sensitized 5-HT release across treatments, and although NE release was not measured, we do not expect that its sensitization would differ from 5-HT, given the evidence that their sensitization is linked (Auclair et al., 2004; Lanteri et al., 2013). These findings suggest that social sensitization to MDMA may occur independently from locomotor and neurochemical sensitization, but additional studies will be necessary to clarify these results.
In addition to the acute prosocial effects of MDMA, there is also preliminary evidence that MDMA-assisted therapy may produce long-lasting therapeutic effects and personality changes (Mithoefer et al., 2016; Wagner et al., 2017). A small phase I clinical trial recently found that psychotherapy including two MDMA-treatment sessions significantly reduced social anxiety in autistic adults compared to psychotherapy with a placebo, and this reduction persisted for at least 6 months following the treatments (Danforth et al., 2018). The mechanism(s) by which an acute regimen of MDMA-assisted therapy can have long-lasting effects is unclear. It is possible that the mechanisms involved in the development and long-term persistence of social sensitization are also involved in MDMA’s long-lasting therapeutically relevant effects. 5-HT2ARs facilitate neural plasticity (Carhart-Harris and Nutt, 2017) and are important mediators of behavioral sensitization (Ago et al., 2008; Auclair et al., 2004; Zayara et al., 2010). In the present study, pretreatment with the selective 5-HT2AR antagonist M100 inhibited the development of social sensitization. Activation of these receptors may be necessary for social sensitization to develop, but stimulating these receptors with an agonist was insufficient on its own to produce a later sensitized social response to MDMA. This lack of sufficiency is not surprising given the purported relevance of other acute pharmacological effects of MDMA, such as increased serotonin and oxytocin release, in mediating the drug’s prosocial effects (Liechti and Vollenweider, 2001; Thompson et al., 2007). Furthermore, while 5-HT2AR activation appears necessary for the development of sensitization, it does not appear necessary for the expression of MDMA-induced social behavior. However, it is possible that higher or lower doses of DOI or M100 might affect MDMA differently. Interestingly, mice that were treated with MDMA using the same sensitization protocol, but without concomitant social pairing on days 1, 3, and 5, did not display sensitized social behavior when paired with a novel conspecific on day 7. Therefore, social sensitization to MDMA seems to result from a combination of acute prosocial drug-effects, 5-HT2AR activation to potentially facilitate neuroplasticity, and a social setting.
Behavioral sensitization to drugs of abuse has long been studied in the context of addiction (Robinson and Berridge, 2008). There is evidence that many drugs of abuse can produce adaptations within the mesocorticolimbic system that lead to sensitized behavioral responses and potentially increase the salience of drug associated cues (Robinson and Berridge, 2008; Steketee and Kalivas, 2011). Social interaction is a natural reward that engages some of the same brain circuits as abused drugs (Hung et al., 2017; Insel, 2003). Intriguingly, the neuropeptide oxytocin, which is released by MDMA (Hysek et al., 2013; Thompson et al., 2007), can mediate social reward in mice and alter synaptic plasticity in the NAcc (Dölen et al., 2013), producing alterations similar to those associated with drug-induced sensitization (Brebner et al., 2005; Thomas et al., 2001). Oxytocin has been proposed as a key neural signal that links social stimuli to the mesolimbic reward system and increases their salience (Hung et al., 2017; Ross and Young, 2009; Xiao et al., 2017). MDMA increases activation of many mesolimbic brain regions involved in reward (e.g. NAcc and VTA), but only when administered in a social context (Thompson et al., 2009). Thus, MDMA may increase social behavior, at least in part, by enhancing social reward (Ramos et al., 2015), and social sensitization may reflect the increasing salience of social stimuli.
There are several important limitations in the present study. All experiments were conducted with relatively small sample sizes. As previously mentioned, the locomotor and neurochemical sensitization experiments may have been insufficiently powered to detect genuine group differences. Furthermore, given the intriguing finding that social pairing was critical for the development of social sensitization, future studies are warranted to further dissect the role that a social context has on sensitization to MDMA’s effects. It is possible that social interaction during MDMA treatment would also increase locomotor and neurochemical sensitization. Another limitation of the present study is that only male mice were used. Future studies will be needed to determine if female mice also sensitize to the prosocial effects of MDMA. Lastly, a discussion of sensitization would be incomplete without also mentioning tolerance. With repeated administration of a drug, sensitization may develop to some effects while tolerance develops to others. The species, dose, and frequency of treatments all influence which of the two occurs, and in some cases initial sensitization may give way to tolerance (Stewart and Badiani, 1993). Although, in the present study, mice sensitized to the prosocial effects of MDMA, if higher or more numerous doses had been given, there is evidence to suggest that tolerance might have developed instead. In a separate study, rats given repeated binge doses of MDMA had decreased off-drug social interaction and a blunted prosocial response to subsequent MDMA treatments (Thompson et al., 2008). Even 12 weeks later, a higher dose of MDMA was required to produce the prosocial effects previously elicited by MDMA in these subjects. This kind of “chronic tolerance” has been described in heavy recreational MDMA users (Parrott, 2005, 2013). Tolerance to MDMA may develop due to depletion of 5-HT, potentially caused by MDMA neurotoxicity (Baumann et al., 2008; Jones et al., 2010). As clinical trials with MDMA move forward, it is important to consider how repeated treatments may produce different effects over time.
5. Conclusion
To our knowledge, this is the first report demonstrating sensitization to the prosocial effects of MDMA. Sensitization developed rapidly and persisted even following extended abstinence. Both 5-HT2AR activation and a social context appear necessary for the development of social sensitization. Social sensitization may represent a novel animal model to study the neural adaptations elicited by MDMA. Such adaptations may have a role in the remarkable durability of MDMA-assisted therapy’s putative therapeutic effects (Danforth et al., 2018; Mithoefer et al., 2018, 2013). Although changes in off-drug social behavior were not observed in the social interaction test, more sensitive measures should be explored in future studies to determine if acute MDMA treatments can effect long-lasting changes to murine social behavior. Identifying the neurobiological changes that are responsible for sensitization to MDMA’s prosocial effects should also be pursued. These changes may shed light on the endogenous biological systems that mediate social interaction and provide clues as to what has gone awry in disorders characterized by deficits in normal social behavior.
Supplementary Material
Acknowledgements
We thank the animal care and veterinary staff at the Yerkes National Primate Research Center (YNPRC) for maintaining the health and well-being of our research subjects. We also thank our research subjects for their contribution. Dr. Kevin Murnane, Carolyn McLaughlin, and Brian Zhao contributed to the collection of these data.
Funding
This work was supported by NIH grants T32 DA015040 (for DWC), K05 DA031246 (to LLH), and P51 OD011132 (to the YNPRC); and AFIP and FAPESP grant 2015/25482-3 (for LFB). A portion of this work was supported by the Intramural Research programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
References
- Ago Y, Nakamura S, Baba A, Matsuda T, 2008. Neuropsychotoxicity of Abused Drugs: Effects of Serotonin Receptor Ligands on Methamphetamine- and Cocaine-Induced Behavioral Sensitization in Mice. J. Pharmacol. Sci. J Pharmacol Sci 106, 15–211. doi: 10.1254/jphs.FM0070121 [DOI] [PubMed] [Google Scholar]
- Ando RD, Benko A, Ferrington L, Kirilly E, Kelly PAT, Bagdy G, 2006. Partial lesion of the serotonergic system by a single dose of MDMA results in behavioural disinhibition and enhances acute MDMA-induced social behaviour on the social interaction test. Neuropharmacology 50, 884–96. doi: 10.1016/j.neuropharm.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin J-P, 2004. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur. J. Neurosci 20, 3073–84. doi: 10.1111/j.1460-9568.2004.03805.x [DOI] [PubMed] [Google Scholar]
- Ball KT, Klein JE, Plocinski J. a, Slack R, 2011. Behavioral sensitization to 3,4-methylenedioxymethamphetamine is long-lasting and modulated by the context of drug administration. Behav. Pharmacol 22, 847–50. doi: 10.1097/FBP.0b013e32834d13b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta S, Reymond G, Pozzobon M, Duhamel JR, 2016. Effects of MDMA Injections on the Behavior of Socially-Housed Long-Tailed Macaques (Macaca fascicularis). PLoS One 11, 1–10. doi: 10.1371/journal.pone.0147136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB, 2008. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience 152, 773–84. doi: 10.1016/j.neuroscience.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H, 2010. Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol. Psychiatry 68, 1134–40. doi: 10.1016/j.biopsych.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Miller MA, Baggott MJ, de Wit H, 2016. The effects of MDMA on socio-emotional processing: Does MDMA differ from other stimulants? J. Psychopharmacol 30, 1248–1258. doi: 10.1177/0269881116663120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury S, Gittings D, Schenk S, 2012. Repeated exposure to MDMA and amphetamine: sensitization, cross-sensitization, and response to dopamine D(1)- and D (2)-like agonists. Psychopharmacol. doi: 10.1007/s00213-012-2726-9 [DOI] [PubMed] [Google Scholar]
- Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT, 2005. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–3. doi: 10.1126/science.1116894 [DOI] [PubMed] [Google Scholar]
- Brody SA, Conquet F, Geyer MA, 2004. Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology (Berl). 172, 187–195. doi: 10.1007/s00213-003-1635-3 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R, Nutt D, 2017. Serotonin and brain function: a tale of two receptors. J. Psychopharmacol 026988111772591. doi: 10.1177/0269881117725915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry DW, Young MB, Tran AN, Daoud GE, Howell LL, 2018. Separating the agony from ecstasy: R(−)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice. Neuropharmacology 128, 196–206. doi: 10.1016/j.neuropharm.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth AL, Grob CS, Struble C, Feduccia AA, Walker N, Jerome L, Yazar-Klosinski B, Emerson A, 2018. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology (Berl). doi: 10.1007/s00213-018-5010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daza-Losada M, Rodríguez-Arias M, Maldonado C, Aguilar MA, Guerri C, Miñarro J, 2009. Acute behavioural and neurotoxic effects of MDMA plus cocaine in adolescent mice. Neurotoxicol. Teratol 31, 49–59. doi: 10.1016/j.ntt.2008.07.005 [DOI] [PubMed] [Google Scholar]
- de Angelis L, File SE, 1979. Acute and chronic effects of three benzodiazepines in the social interaction anxiety test in mice. Psychopharmacology (Berl). 64, 127–9. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC, 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–84. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Hyde JRG, 1978. Can Social Interaction Be Used To Measure Anxiety? Br. J. Pharmacol 62, 19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P, 2003. A review of 25 years of the social interaction test. Eur. J. Pharmacol 463, 35–53. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Malenka RC, 2016. MDMA as a Probe and Treatment for Social Behaviors. Cell 166, 1–4. doi: 10.1016/j.cell.2016.06.045 [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, Malenka RC, 2017. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411. doi: 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME, 2013. MDMA enhances emotional empathy and prosocial behavior. Soc. Cogn. Affect. Neurosci 222, 293–302. doi: 10.1093/scan/nst161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, 2003. Is social attachment an addictive disorder? Physiol. Behav 79, 351–7. doi: 10.1016/S0031-9384(03)00148-3 [DOI] [PubMed] [Google Scholar]
- Jones K, Brennan KA, Colussi-Mas J, Schenk S, 2010. Tolerance to 3,4-methylenedioxymethamphetamine is associated with impaired serotonin release. Addict. Biol 15, 289–98. doi: 10.1111/j.1369-1600.2010.00217.x [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR, 1998. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology 18, 469–79. doi: 10.1016/S0893-133X(97)00195-4 [DOI] [PubMed] [Google Scholar]
- Kamilar-Britt P, Bedi G, 2015. The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neurosci. Biobehav. Rev 57, 433–446. doi: 10.1016/j.neubiorev.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H, 2014. MDMA: a social drug in a social context. Psychopharmacology (Berl). 232, 1155–1163. doi: 10.1007/s00213-014-3752-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H, 2014. Effects of MDMA and Intranasal Oxytocin on Social and Emotional Processing. Neuropsychopharmacology 39, 1–37. doi: 10.1038/npp.2014.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri C, Doucet EL, Hernández Vallejo SJ, Godeheu G, Bobadilla a-C., Salomon L, Lanfumey L, Tassin J-P, 2013. Repeated exposure to MDMA triggers long-term plasticity of noradrenergic and serotonergic neurons. Mol. Psychiatry 1–11. doi: 10.1038/mp.2013.97 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX, 2001. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum. Psychopharmacol 16, 589–598. doi: 10.1002/hup.348 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Grob CS, Brewerton TD, 2016. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. The Lancet Psychiatry 3, 481–488. doi: 10.1016/S2215-0366(15)00576-3 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Mithoefer AT, Feduccia AA, Jerome L, Wagner M, Wymer J, Holland J, Hamilton S, Yazar-Klosinski B, Emerson A, Doblin R, 2018. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. The Lancet Psychiatry 5, 486–497. doi: 10.1016/S2215-0366(18)30135-4 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, Michel Y, Brewerton TD, Doblin R, 2013. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J. Psychopharmacol 27, 28–39. doi: 10.1177/0269881112456611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KC, Arnold JC, McGregor IS, 2005. Serotonin (1A) receptor involvement in acute 3,4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 648–57. doi: 10.1016/j.pnpbp.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Morley KC, McGregor IS, 2000. (+/−)-3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) increases social interaction in rats. Eur. J. Pharmacol 408, 41–9. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL, 2010. Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J. Pharmacol. Exp. Ther 334, 642–50. doi: 10.1124/jpet.110.166595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA, 1994. Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphotidyl inositol turnover in cultured cells expressing 5-HTzA or 5-HTzc receptors 177, 111–115. [DOI] [PubMed] [Google Scholar]
- Parrott a C., 2005. Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J. Psychopharmacol 19, 71–83. doi: 10.1177/0269881105048900 [DOI] [PubMed] [Google Scholar]
- Parrott AC, 2013. Human psychobiology of MDMA or “Ecstasy”: an overview of 25 years of empirical research. Hum. Psychopharmacol 28, 289–307. doi: 10.1002/hup.2318 [DOI] [PubMed] [Google Scholar]
- Pitts EG, Minerva AR, Oliver EB, Kohn JN, Logun MT, Sulima A, Rice KC, Howell LL, 2017. 3,4-Methylenedioxymethamphetamine Increases Affiliative Behaviors in Squirrel Monkeys in a Serotonin 2A Receptor-Dependent Manner. Neuropsychopharmacology 1–10. doi: 10.1038/npp.2017.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Caminer A, Goodwin J, McGregor IS, 2015. Oxytocin and MDMA (‘Ecstasy’) enhance social reward in rats. Psychopharmacology (Berl). 232, 2631–2641. doi: 10.1007/s00213-015-3899-9 [DOI] [PubMed] [Google Scholar]
- Ramos M, Goñ i-Allo B, Aguirre N, 2005. Administration of SCH 23390 into the Medial Prefrontal Cortex Blocks the Expression of MDMA-Induced Behavioral Sensitization in Rats: An Effect Mediated by 5-HT 2C Receptor Stimulation and not by D 1 Receptor Blockade. doi: 10.1038/sj.npp.1300735 [DOI] [PubMed]
- Robinson TE, Becker JB, 1986. Enduring Changes in Brain and Behavior Produced by Chronic Amphetamine Administration: A Review and Evaluation of Anima1 Models of Amphetamine Psychosis. Brain Res. Rev 11, 157–198. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2008. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B. Biol. Sci 363, 3137–46. doi: 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ, 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol 30, 534–47. doi: 10.1016/j.yfrne.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JD, Herin DV, Frankel PS, Thomas ML, Cunningham KA, 2006. Chronic treatment with a serotonin(2) receptor (5-HT(2)R) agonist modulates the behavioral and cellular response to (+)-3,4-methylenedioxymethamphetamine [(+)-MDMA]. Drug Alcohol Depend. 81, 117–27. doi: 10.1016/j.drugalcdep.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW, 2011. Drug Wanting : Behavioral Sensitization and Relapse to Drug-Seeking Behavior 63, 348–365. doi: 10.1124/pr.109.001933.remains [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Badiani a., 1993. Tolerance and sensitization to the behavioral effects of drugs. Behav. Pharmacol doi: 10.1097/00008877-199308000-00003 [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC, 2001. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. doi: 10.1038/nn757 [DOI] [PubMed]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS, 2007. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience 146, 509–514. doi: 10.1016/j.neuroscience.2007.02.032 [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, McGregor IS, 2008. Reduced sensitivity to MDMA-induced facilitation of social behaviour in MDMA pre-exposed rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1013–21. doi: 10.1016/j.pnpbp.2008.01.014 [DOI] [PubMed] [Google Scholar]
- Thompson MR, Hunt GE, McGregor IS, 2009. Neural correlates of MDMA (“Ecstasy”)-induced social interaction in rats. Soc. Neurosci 4, 60–72. doi: 10.1080/17470910802045042 [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Kinkead B, Murray KE, Owens MJ, 2013. In vivo dialysis setup with a loop injection valve facilitates retrodialysis studies. J. Pharmacol. Toxicol. Methods 68, 217–24. doi: 10.1016/j.vascn.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Varela MJ, Brea J, Loza MI, Maldonado R, Robledo P, 2011. Sensitization to MDMA locomotor effects and changes in the functionality of 5-HT(2A) and D2 receptors in mice. Behav. Pharmacol 22, 362–9. doi: 10.1097/FBP.0b013e3283487346 [DOI] [PubMed] [Google Scholar]
- Wagner MT, Mithoefer MC, Mithoefer AT, MacAulay RK, Jerome L, Yazar-Klosinski B, Doblin R, 2017. Therapeutic effect of increased openness: Investigating mechanism of action in MDMA-assisted psychotherapy. J. Psychopharmacol 31, 967–974. doi: 10.1177/0269881117711712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang Y-M, Li G, Xu S, Dong L, Stackman RW, Zhang G, 2015. Activation of serotonin 5-HT2C receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci. Lett 607, 23–28. doi: 10.1016/j.neulet.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y, 2017. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 95, 368–384.e5. doi: 10.1016/j.neuron.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MB, Norrholm SD, Khoury LM, Jovanovic T, Rauch SAM, Reiff CM, Dunlop BW, Rothbaum BO, Howell LL, 2017. Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3,4-methylenedioxymethamphetamine (MDMA). Psychopharmacology (Berl). 1–13. doi: 10.1007/s00213-017-4684-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayara AE, McIver G, Valdivia PN, Lominac KD, McCreary AC, Szumlinski KK, 2010. Blockade of nucleus accumbens 5-HT2A and 5-HT2C receptors prevents the expression of cocaine-induced behavioral and neurochemical sensitization in rats. Psychopharmacology (Berl). 213, 321–335. doi: 10.1007/s00213-010-1996-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


