Abstract
Objective:
The aim of this study was to evaluate health behaviors among colorectal cancer (CRC) patients and their at-risk relatives prior to undergoing genetic counseling and testing for Lynch syndrome and to examine associations between health risk behaviors and specific demographic and psychological variables.
Methods:
Participants included CRC patients (n=319) and their cancer-unaffected relatives (n=110) who were enrolled in studies regarding Lynch syndrome genetic testing. Prior to undergoing genetic counseling or testing, participants completed a questionnaire including measures of demographic characteristics, health behaviors, cancer screening practices (Pap test, clinical breast exam, and mammogram), and psychological distress.
Results:
Unaffected participants scored higher on a risk behavior index (RBI) than CRC patients (1.7 (SD=1.0) vs. 1.4 (SD=.09); p<.01). All female participants underwent cancer screening at rates similar to national data. Higher RBI scores were associated with being male, having less education, and age younger than 50 years old.
Conclusions:
We identified several health behaviors for potential intervention, including smoking, alcohol use, and diet. Genetic counseling offers a promising avenue for education and risk behavior reduction in persons at increased risk for cancer due to a familial or genetic predisposition, and a teachable moment to introduce lifestyle modifications.
Keywords: Lynch syndrome, hereditary non-polyposis colorectal cancer (HNPCC), health behaviors, cancer screening, genetic counseling
Introduction
Lynch syndrome, or hereditary non-polyposis colorectal cancer (HNPCC), is an autosomal dominant condition characterized by a predisposition to several adult-onset cancers, most commonly colorectal cancer (CRC). Lynch syndrome is caused by mutations in DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2) that can be identified through clinical genetic testing [1-5]. MMR mutation carriers have a higher lifetime risk for several cancers compared with the general population; for example, CRC risk ranges from 74% to 82% for men and from 30% to 54% for women, and women have a 40% to 60% risk of endometrial cancer [6, 7]. Lynch syndrome confers modestly increased lifetime risks for other malignancies, including ovarian, stomach, small bowel, hepatobiliary tract, pancreatic, urinary tract, brain, and skin cancers [6, 7]. Consequently, individuals with Lynch syndrome are advised to follow high-risk management recommendations including annual colonoscopy (often initiated at age 20-25 years) and screening for endometrial cancer and other cancers associated with the syndrome [6]. Data support the efficacy of prophylactic hysterectomy and oophorectomy for gynecological cancer risk reduction [8].
Several lifestyle behaviors are known or suggested to increase risk for sporadic cancers that also occur in Lynch syndrome. Smoking, alcohol consumption, fat intake and red meat consumption have been associated with increased risk for CRC [9-20]. Diets high in fruits and vegetables and low in red meat consumption may reduce CRC risk [17-21]; additionally, dietary fiber has been considered protective, although more recent studies show conflicting results [22, 23]. Obesity is considered a risk factor for both CRC and endometrial cancer [24, 25], and physical activity may reduce CRC risk [26-28]. While studies point to the potential benefit of adopting healthy lifestyle behaviors to improve CRC prevention and control both for cancer survivors and those without a personal history of cancer [29-31], relatively little is known about the relationship between lifestyle behaviors with Lynch syndrome-associated cancer risks. The limited available data suggest that certain lifestyle factors, such as diet and smoking, may play a role in modifying cancer risk conferred by the highly penetrant MMR mutations that cause Lynch syndrome [32-34]. Although further research is needed in this area, there is expert agreement that advocating the potential benefits of dietary and lifestyle modifications to persons with Lynch syndrome is important to maintaining overall health [6]. Individuals affected with Lynch syndrome also are advised to follow screening recommendations for nonsyndrome cancers, such as Pap smears, clinical breast exams, and mammograms for women.
Previous research on health behaviors in Lynch syndrome has focused primarily on CRC and endometrial screening adherence and use of preventive surgery [35-37]. There are scant data regarding other health and prevention behaviors among persons at risk for Lynch syndrome. In studies that evaluated hypothetical responses to learning that one carried a gene mutation increasing susceptibility to CRC, results suggested that individuals may be willing to make healthy lifestyle behavior changes as a result of learning their carrier status [38, 39]. even if found to be noncarriers [39]. These data suggest that individuals who undergo genetic testing for hereditary cancers may be motivated to improve their health and preventive behaviors [38, 39].
A limited number of studies have described lifestyle and preventive behaviors among individuals at risk for hereditary cancers, and most have focused on familial breast cancer. Risk behavior profiles (including smoking, physical activity, sun protection, diet, and alcohol intake) among women at increased breast cancer risk who underwent genetic counseling indicated that 41% reported one risk factor, 25% two risk factors, and 8% three or more risk factors; however, women’s overall risk behavior profiles were generally better compared to the general population [40]. Prevalence of screening behaviors was largely consistent with recommendations for women at increased risk for breast and ovarian cancer. [40]. Some studies have reported no association between having a strong family history of breast cancer and improved lifestyle behaviors [41, 42], while others have found that the diagnosis of breast cancer in a first-degree relative (FDR) motivated female relatives to make improvements in some behaviors [43, 44].
Genetic counseling for hereditary cancer risk may offer a teachable moment for encouraging improvements in lifestyle and prevention behaviors [45]. However, little is known about health behaviors of individuals who are at risk for Lynch syndrome. Our study sought to fill this gap in knowledge by examining the lifestyle behaviors of families at risk for Lynch syndrome. The aim of this study was to evaluate health behaviors among a sample of CRC patients and their at-risk relatives who were undergoing genetic counseling and testing for Lynch syndrome and to examine associations between health risk behaviors and specific demographic and psychological measures. Although this study was exploratory in nature, we hypothesized that younger persons, those with less education, higher affective distress, and unaffected FDRs would have a greater number of health risk behaviors.
Methods
Participants
This study was approved by the Institutional Review Board at The University of Texas M. D. Anderson Cancer Center. The study population included 429 adults who were 18 years of age or older and who participated in concurrent studies from 1995-2002 involving Lynch syndrome genetic counseling and testing. Eligible CRC patients were recruited from gastrointestinal cancer clinics at M.D. Anderson and had a cancer family history that met the Amsterdam II criteria for Lynch syndrome [46] or otherwise suggested an increased risk of carrying a MMR mutation (e.g., CRC diagnosed at age ≤45 years; multiple relatives and/or generations affected with syndrome-related cancers). Biological relatives of mutation-positive CRC patients who were at a 25% or 50% risk of carrying a mutation also were eligible. In this analysis, affected participants included persons with a prior diagnosis of any cancer excluding nonmelanoma skin cancer, and unaffected participants included those with no personal history of cancer.
Study Procedures
Data were collected as part of a longitudinal study of psychosocial outcomes associated with Lynch syndrome genetic counseling and testing, and study procedures have been described in detail elsewhere [47-49]. Genetic counseling and testing were provided at no cost to study participants as part of a concurrent companion protocol. Data for the present study were collected using questionnaires that were administered by telephone prior to genetic counseling and testing.
Measures
The questionnaire for this study has been described previously [47-49], and includes the following measures.
Demographics.
Demographic factors obtained included age, sex, race/ethnicity, education, marital status, household income, religious affiliation, and the presence of biological children.
Psychological variables.
The following psychological variables were assessed using validated measures:
Depression. The Center for Epidemiological Studies Depression Scale is a well-validated, 20-item scale that measures frequency and intensity of symptoms of depression during the preceding week.[50]
Anxiety. The State-Trait Anxiety Inventory (STAI) is a 40-item scale that is used to measure state (i.e., transitory) and trait (i.e., stable) anxiety [51].
Social support. Social support was measured using the Social Support Questionnaire (short form developed by Sarason et al. [52]. It is a 12-item scale with two dimensions: SSN, number of people available for support, and SSS, satisfaction with that source of support.
Quality of Life (QOL). QOL was measured using the Ferrans and Powers Quality of Life Index (QLI) [53]. The QLI measures satisfaction across four life domains, including health and functioning, psychological and spiritual, socioeconomic, and family subscales.
Risk behaviors.
Smoking was assessed using standardized items from the Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System (BRFSS)[54]. For the purposes of this study, participants were asked whether they had smoked in the preceding 7 days, smoked at least 100 cigarettes in their lifetime, their age at initiation of smoking, the number of years as a daily smoker, and the average number of cigarettes/day. Alcohol consumption was assessed using three questions: 1) have you consumed 12 or more alcoholic beverages such as beer, wine, or liquor in your lifetime; 2) at what age did you start drinking alcoholic beverages; and 3) approximately how many drinks of beer, wine, or liquor do you have in an average week? Fiber and fat consumption were assessed using a question from the Working Well Trial [55] that asked how high in fat/fiber the overall diet was, with responses ranging from very low to very high on a 5-point scale.
Screening behaviors.
Screening utilization for non-syndrome cancers, specifically Pap smear, mammogram, and clinical breast exam, was queried for female participants. Items were adapted from the BRFSS [54] and assessed the date of the most recent exam as well as number of lifetime mammograms.
Statistical Analysis
We characterized health risk behaviors and screening practices among affected participants (n=319) and their unaffected relatives (n=110) and examined the associations between the health risk behaviors and specific demographic and psychological measures. A comparison of health risk behaviors with gender, race, education and age was made using contingency tables and chi-square analysis. Correlations among family members with regard to health risk behaviors were also evaluated. Statistical significance was defined as p<0.05. All analyses were performed using SAS 9.1 and SPSS for Windows 12.0.
Intraclass correlation coefficients (ICCs) were calculated to determine the correlation in risk behaviors among members of the same family. Families with 2 or more members were included in the analysis. ICCs were calculated using methods and formulas for binary outcome as specified by Snijders and Bosker [56]. ICC values were 0.15 for smoking, 0 for alcohol consumption, 0.05 for high-fat diet and 0.04 for low fiber consumption.
A classification of subjects based on psychological variables (depression, anxiety, QOL, and social support) was defined by cluster analysis (based on similar analytic methods described in Gritz et al., 2005 [49]). For both affected and unaffected participants, two well-defined clusters were identified in terms of the Euclidean distance between the cluster centers. These two clusters were then used as a basis for comparing health risk. Clusters 1 and 2 were characterized as low distress and high distress, respectively. Cluster 2, the high distress cluster, had significantly higher mean scores on depression and anxiety and lower mean scores on QOL, SSN, and SSS [57].
A risk behavior index (RBI) was computed on the basis of current smoking status, alcohol consumption of more than 14 drinks per week, high-fat diet, and lack of high fiber in the diet. Scores varied from 0 (no risk behaviors) to 4 (all risk behaviors). This method for computing risk indices has been utilized in other studies [40, 57].
A random regression model was used to model the RBI scores for predicting risk for all participants. Risk scores were regressed on affected status, demographic variables (sex, race, education, and age), and psychological distress, designated by distress cluster. Family of origin was entered as a random effect to adjust for potential correlation of risk within families. Regression coefficient estimates, standard errors, F statistics and p values were used to summarize the results. Reference categories for the categorical variables were female sex, white race, education above high school, and age 50 years or older.
Results
Demographic Profile
Table 1 shows participants’ demographic characteristics. Compared to unaffected participants, those who were affected were older, more likely to be White and male, and a greater percentage reported a Protestant religious affiliation. Both groups were similar with respect to education, marital status, income, and number of biological children.
Table 1.
Participants’ demographic characteristics by personal cancer history (affected vs. unaffected)
| Characteristic | Affected n=319 % (n) |
Unaffected n=110 % (n) |
|---|---|---|
| Age ≥50 yrs** | 60% (192) | 22% (24) |
| White race* | 87% (278) | 77% (85) |
| Education | ||
| ≤HS | 32% (103) | 28% (31) |
| Some college | 31% (98) | 29% (32) |
| College degree or higher | 37% (118) | 42% (46) |
| Female sex* | 49% (157) | 65% (71) |
| Married marital status | 76% (242) | 67% (74) |
| Annual household income <$50 K | 58% (183) | 60% (65) |
| Religion** | ||
| Catholic | 25% (79) | 24% (26) |
| Protestant | 64% (203) | 47% (52) |
| Jewish | 1% (4) | 0% (0) |
| Other | 10% (32) | 29% (32) |
| ≥1 Biological children | 83% (212) | 75% (81) |
p<.05,
p<.01
Risk Behavior Index (RBI)
Figure 1 depicts the distribution of the RBI scores for affected and unaffected participants. Approximately 10% of affected and 20% of unaffected participants reported 3 or more risk behaviors, and 20% and 12% reported zero risk behaviors, respectively. Chi-square analysis revealed a statistically significant difference between the risk distributions of the 2 groups (χ2 (3, N = 429) = 11.7; p=.02). In the affected group, the mean number of risk behaviors was 1.4 (SD=0.9), the median risk was 1.0, and the modal risk was 2.0. In the unaffected group, the mean risk was 1.7 (SD=1.0), the median risk was 2.0, and the modal risk was 2.0.
Figure 1.
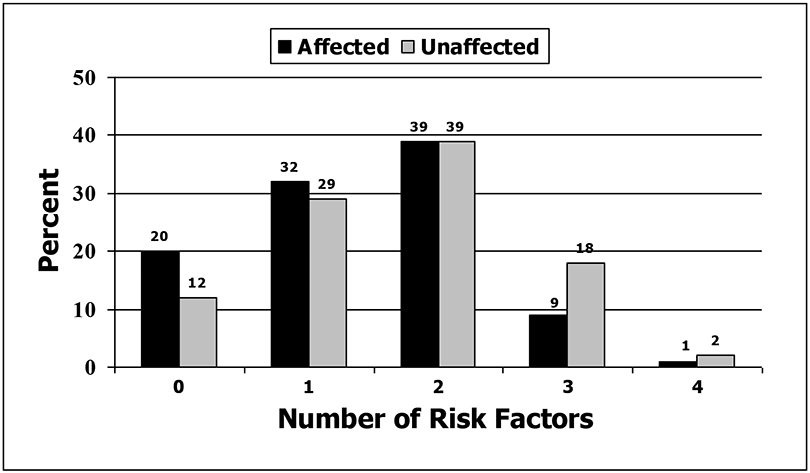
Percentage of affected and unaffected participants with each Risk Behavior Index score
Screening and Health Behaviors
For the screening variables (Pap smear, clinical breast exam, and mammograms), the observed frequencies for both affected and unaffected women were similar to national BRFSS data as shown by the 95% confidence limits (Table 2). Similar to national data, the majority of female participants had undergone screening during the preceding two years.
Table 2:
Cancer screening practices compared with standardized national survey data
| Pap Smear (Females) | |||
|---|---|---|---|
| Affected (n=157) | Unaffected (n=71) | BRFSS* (2002) | |
| % (95% CI) | % (95% CI) | % | |
| Past year | 60.3% (50, 70) | 64.3% (50, 78) | 70.5% |
| Past 2 years | 13.5% (0, 28) | 15.7% (0, 37) | 12.6% |
| Past 3 years | 7.1% (0, 22)) | 5.7% (0, 28) | 4.3% |
| Over 3 years ago | 19.1% (5, 33) | 14.3% (0, 36) | 11.4% |
| Clinical Breast Exam (Females) | |||
| Affected (n=157) | Unaffected (n=71) | BRFSS* (2002) | |
| % (95% CI) | % (95% CI) | % | |
| Past year | 76.2% (69,84) | 69.6% (57, 83) | 75.7% |
| Past 2 years | 11.6% (0, 26) | 15.9% (0, 38) | 12.9% |
| Past 3 years | 4.5% (0, 20) | 4.3% (0, 27) | 4.2% |
| Over 3 years ago | 7.7% (0, 23) | 10.1% (0, 32) | 2.8% |
| Females by age group who ever had a mammogram | |||
| Age (years) | Affected (n=157) | Unaffected (n=71) | BRFSS* (2002) |
| % (95% CI) | % (95% CI) | % | |
| < 40 | 41.9% (15, 69) | 27% (0, 55) | 23.4% |
| 40-49 | 91.2% (81, 100) | 81.3% (60, 100) | 83.2% |
| ≥ 50 | 92.4% (86, 98) | 88.9% (74, 100) | 92.7% |
Behavioral Risk Factor Surveillance System. 2002 CDC median of 50 states (including Washington, DC).
Table 3 shows the prevalence of health risk behaviors among affected and unaffected participants. Current smoking was less prevalent among affected participants compared with unaffected participants (p<.001; age-adjusted p<.01) and national data. Alcohol consumption of more than 14 drinks per week was less prevalent in female participants than reported in the general population. A higher percentage of affected participants reported following a low-fat diet than did unaffected participants although the results were not statistically significant at the p<.05 level, and a statistically significant lower percentage of both affected and unaffected participants reported consuming a high-fiber diet compared with national data.
Table 3:
Lifestyle risk behaviors of affected and unaffected participants by sex, compared with national survey data
| Smoking Prevalence: % (95% Confidence Limits) currently smoking | |||
|---|---|---|---|
| Males | Females | Total | |
| Affected (N=319) * | 11.1% (6.3, 15.9) | 14.6% (9.1, 20.1) | 12.9% (9.2, 16.6) |
| Unaffected (N=110) * | 28.2% (14.1, 42.3) | 29.6% (19.0, 40.2) | 29.1% (20.6, 37.6) |
| 2002 CDC BRFSS median of 50 states (incl. DC): Adults ≥ 18 years old** | 25.7% | 20.8% | 23.2% |
| Alcohol Consumption : % having >14 drinks/wk (of total study population) | |||
| Males | Females | Total | |
| Affected (N=319) | 11.1% (6.2, 15.9) | 4.5% (1.3, 7.7) | 7.8% (4.9, 10.7) |
| Unaffected (N=110) | 12.8% (2.3, 23.3) | 2.8% (0.0, 6.6) | 6.4% (1.8, 11.0) |
| 2002 CDC BRFSS median of 50 states (incl. DC): Adults ≥ 18 years old** | 8.4% | 7.8% | 7.8% |
| Fat Consumption: % on a low-fat diet | |||
| Males | Females | Total | |
| Affected (N=319) | 36.4% (29.0, 43.8) | 43.3% (35.6, 51.1) | 39.8% (34.4, 45.2) |
| Unaffected(N=110) | 23.1% (9.9, 36.3) | 35.2% (24.1, 46.3) | 30.9% (22.2, 39.5) |
| USDA CSFII (% with total fat intake ≤30% of calories) *** | 29.4% | 36.8% | 32.9% |
| Fiber Consumption: % on a high-fiber diet | |||
| Males | Females | Total | |
| Affected (N=319) | 43.2% (35.6, 50.8) | 43.9% (36.1, 50.8) | 43.3% (37.9, 48.7) |
| Unaffected (N=100) | 41.0% (25.6, 56.4) | 32.4% (21.5, 43.3) | 35.5% (26.6, 44.4) |
| USDA CSFII (% with adequate fiber intake) ** | 61.7% | 59.4% | 60.6% |
(p<0.001, affected vs. unaffected)
Behavioral Risk Factor Surveillance System
USDA CSFII: Continuing survey of food intake by individuals (adults ≥20 years old: 1994-1996). Based on self-assessment of nutrient intake[72].
Among affected participants, a higher percentage of those 50 years and older were currently not smoking compared with those less than 50 years old (9% vs. 18%; p<.05) (data not reported in a table). Significant differences also were observed between alcohol consumption and sex, with a higher percentage of males consuming more than 14 drinks per week compared with females (11% vs. 4%; p<.05). More affected participants with some college reported following a low-fat diet compared to those with less education (44% vs. 30%; p<.05), as did those who were 50 years and older compared to those less than 50 years old (45% vs. 32%; p<.05). A higher percentage of White affected participants reported following a high-fiber diet compared with those who were nonwhite (46% vs. 27%; p<.05). In addition, a higher percentage of affected participants who were 50 years and older reported a high-fiber diet compared with those who were younger (49% vs. 36%; p<.05).
Among unaffected participants, smoking status was associated with education, as a higher percentage of those without any college education were current smokers compared with those with at least some college education (48% vs. 22%; p<.05; age-adjusted p<.05) (data not reported in a table).
RBI, demographic characteristics and psychological distress
In univariate analysis, nonwhite race, age less than 50 years, and high distress were significantly associated with a higher RBI score in the affected group, while less education was associated with higher RBI scores in the unaffected group (Table 4).
Table 4.
Risk Behavior Index (RBI) score for affected and unaffected participants by demographics and distress cluster
| Characteristic | Affected (n=319) Mean (SD) |
Unaffected (n=110) Mean (SD) |
|---|---|---|
| Sex | ||
| Male | 1.3 (0.9) | 1.8 (1.1) |
| Female | 1.4 (0.9) | 1.6 (.09) |
| Race | ||
| White | 1.3 (0.9)* | 1.7 (1.0) |
| Nonwhite | 1.7 (1.0) | 1.8 (0.9) |
| Education | ||
| ≤ High school | 1.5 (0.9) | 2.0 (0.8)* |
| > High school | 1.3 (0.9) | 1.6 (1.0) |
| Age | ||
| < 50 | 1.6 (0.9)* | 1.7 (1.0) |
| ≥ 50 | 1.2 (0.9) | 1.5 (0.8) |
| Distress Cluster | ||
| High | 1.5 (0.9)* | 1.9 (1.0) |
| Low | 1.3 (0.9) | 1.6 (0.9) |
p<0.05
Multivariate Analysis
A random regression model (Table 5) was used to model the RBI scores for predicting risk behaviors for all participants. Higher RBI scores were significantly associated with being male, having lower education, and being less than 50 years of age.
Table 5.
Random regression model predicting the Risk Behavior Index scores
| Variable | Estimate | SE | F value | P value | Higher risk index associated with |
|---|---|---|---|---|---|
| Intercept | 1.2 | <.0001*** | |||
| Affected status | −0.2 | 0.1 | 3.32 | .0709 | • Unaffected |
| Sex | 0.2 | 0.1 | 4.17 | .0432* | • Male |
| Race | 0.2 | 0.1 | 2.40 | .1238 | • Nonwhite |
| Education | 0.2 | 0.1 | 4.09 | .0451* | • Less education(≤HS) |
| Age | 0.3 | 0.1 | 10.2 | .0017** | • Younger age (≤50 years old) |
| Distress | 0.2 | 0.1 | 2.38 | .1252 | • High distress |
p<0.05
p<0.01
p<0.001
In exploratory analysis, interactions between race and education as well as sex and distress cluster were included in the model to determine whether specific subgroups had higher risk scores. These tests were treated as hypothesis-generating and interpreted with caution. When the interaction between race and education was included in the model, results were found to be significant (F=10.2, p<.001). When adjusted for affected status, age, sex, and distress cluster, the estimated mean risk scores for nonwhite participants with higher education were significantly higher than mean scores for white participants with higher education (1.9 (SE=0.2) vs. 1.4 (SE=0.1), p<.05). The interaction between sex and distress cluster was not significant.
Discussion
An important benefit of genetic testing for Lynch syndrome is the ability to offer targeted recommendations for cancer risk reduction in MMR mutation carriers. Data are limited regarding the impact of environmental or lifestyle factors on risk for Lynch syndrome-associated cancers. Nonetheless, following general guidelines for diet, tobacco and alcohol use, and preventive behaviors is considered important to maintaining overall health and quality of life in both cancer survivors and unaffected persons from families affected by Lynch syndrome [6, 30, 31, 58], as these persons may be at risk for non-syndrome cancers as well as other chronic diseases.
Prior to genetic counseling and testing, 60% of unaffected participants and 50% of CRC patients in our study reported two or more risk behaviors, including ones that are amenable to intervention such as smoking, alcohol use, and dietary behaviors. CRC patients in our study had a lower mean RBI score compared with cancer-unaffected participants. This difference may reflect improvements in lifestyle behaviors in response to a CRC diagnosis, which is consistent with other studies of cancer survivors [59, 60]. The prevalence of risk behaviors among unaffected participants is comparable with other studies that included populations at increased risk for CRC [57]. Among our participants, screening rates for breast and cervical cancer also were comparable to those observed in the general population. Our data suggest that members of families at risk for hereditary CRC may benefit from improvement in health risk behaviors.
Persons who seek genetic testing may be motivated by the desire to learn about strategies for managing high cancer risk [35], and also may be receptive to making other health behavior changes [38, 39]. Those at risk for hereditary cancers may include cancer survivors as well as unaffected persons who themselves may have experienced one or more relatives’ cancer diagnoses. Although persons may make positive health behavior changes in response to a cancer diagnosis in a relative [43], it is not known whether such changes are sustained over time. In addition, having an awareness of one’s family cancer history may not be sufficient to motivate persons to adopt healthy lifestyle behaviors [41, 42]. Findings from qualitative research indicate that women who are at increased risk for breast cancer, due to their BRCA1/BRCA2 mutation carrier status or strong family cancer history, may be motivated to make positive changes in health behaviors [61, 62]. However, this research also suggests that women may not necessarily engage in health behaviors specifically to reduce breast cancer risk but rather for general health benefits, and that the relevance of lifestyle behaviors to breast cancer risk may be unclear [61] . This issue is salient to Lynch syndrome, given the limited data on how lifestyle behaviors moderate cancer risk in MMR mutation carriers, and points to the need to better understand how individuals make health behavior decisions in the context of genetic risk information. Research in this area is relevant to highly penetrant conditions such as Lynch syndrome, as well as to emerging uses of genetic testing for common, lower prevalence conditions involving multiple-risk alleles [63, 64].
Genetic counseling may offer a teachable moment for identifying and addressing needs for health behavior changes in persons at risk for Lynch syndrome [65]; however, the role of genetic counseling in accomplishing this task has not been systematically evaluated. A study that examined outcomes after BRCA1/BRCA2 genetic testing found no changes in diet, vitamin use or physical activity at 6 months following results disclosure [66] and concluded that counseling and testing had little impact on health behaviors. The authors suggested that the 6-month post-disclosure measurement point may not have allowed enough time to observe changes in health behaviors. Individuals may undergo genetic counseling primarily to learn about genetic risk information for themselves and their families, and may not expect to receive counseling on lifestyle behaviors and cancer risk [61]. Although it is considered appropriate to address health behaviors that may affect cancer risk during genetic counseling [67], there is no consensus as to how this information should be conveyed or the extent to which providers attempt to do so. Providers may be reluctant to emphasize health behavior changes during cancer genetic counseling due to the lack of evidence-based recommendations, a perceived higher priority of other information to be provided during a time-limited visit, and concerns about the potential psychosocial impact of providing lifestyle behavior information [68]. Nonetheless, genetic counseling may serve as a conduit for referring individuals to interventions aimed at health-promoting lifestyle behaviors. Indeed, interventions that target multiple risk factors have been effective among populations at risk for CRC, and can result in the improvement of multiple health behaviors [69]. As data emerge regarding the significance of lifestyle behaviors on cancer risk in Lynch syndrome, future work should evaluate optimal approaches for incorporating guidance about lifestyle behaviors into genetic counseling for Lynch syndrome, as well as potential barriers for doing so.
This study had several limitations. We were unable to examine changes in lifestyle behaviors over time. These data only allowed us to examine associations among our variables using baseline data collected prior to genetic testing and counseling and prior to notification of genetic risk status based on MMR mutation testing. Further, our study participants may have represented a selected population that was willing to engage in research regarding genetic counseling and testing for Lynch syndrome, which may have in turn reflected a more positive orientation toward health-promoting behaviors in general. Another limitation is that diet was measured using single-item measures instead of a food frequency questionnaire, and physical activity was not assessed, thus future studies should include a more comprehensive collection of dietary and physical activity data.
To the best of our knowledge, this is the first study of health risk behaviors in a population of CRC patients and unaffected first-degree relatives at risk for Lynch syndrome. We identified several areas of potential intervention among persons at risk for Lynch syndrome, including smoking, alcohol use, and diet. Although the risks associated with Lynch syndrome cancer are significant, MMR mutation carriers remain at risk for other cancers not associated with the syndrome as well as chronic diseases, as do those who are found not to have a mutation or whose test results are of uncertain significance. Thus, adherence to lifestyle behaviors that are consistent with guidelines advocated by public health agencies (such as the CDC [54], the American Cancer Society [70], and the United States Preventive Services Task Force [71]) for tobacco and alcohol use, diet and physical activity, and early detection practices is important for these individuals. Genetic counseling and continued interaction with health care professionals may offer opportunities to assess, advise, and provide referral or direct interventions on these health behaviors. Further studies need to be undertaken to educate and intervene with individuals with Lynch syndrome and their family members about important lifestyle risk factors and to evaluate the adoption of healthy lifestyle behaviors that may potentially lower the risk of other cancers and comorbid diseases as well as increase overall quality of life.
References
- 1.Nicolaides NC, Papadopoulos N, Liu B, et al. (1994) Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371: 75–80. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos N, Nicolaides NC, Liu B, et al. (1995) Mutations of GTBP in genetically unstable cells. Science 268: 1915–1917. [DOI] [PubMed] [Google Scholar]
- 3.Fishel R, Lescoe MK, Copeland NG, et al. (1993) The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 4.Bronner CE, Baker SM, Morrison PT, et al. (1994) Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368: 258–261. [DOI] [PubMed] [Google Scholar]
- 5.Palombo F, Gallinari P, Iaccarino I, et al. (1995) GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science 268: 1912–1914. [DOI] [PubMed] [Google Scholar]
- 6.Lindor NM, Peterson GM, Hadley DW, et al. (2006) Recommendations for the care of individuals with an inherited predisposition to Lynch Syndrome: A systematic review Journal of the American Medical Association 296: 1507–1517. [DOI] [PubMed] [Google Scholar]
- 7.Lynch H, Lynch JF, Lynch PM, Attard T (2008) Hereditary colorectal cancer syndromes: Molecular genetics, genetic counseling, diagnosis, and management. Familial Cancer 7: 27–39. [DOI] [PubMed] [Google Scholar]
- 8.Schmeler KM, Lynch HT, Chen L, et al. (2006) Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch Syndrome. New England Journal of Medicine 354: 261–269. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E (2001) An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention 10: 725–731. [PubMed] [Google Scholar]
- 10.Colangelo LA, Gapstur SM, Gann PJ, Dyer AR (2004) Cigarette smoking and colorectal carcinoma mortality in a cohort with long-term follow-up. Cancer 100: 288–293. [DOI] [PubMed] [Google Scholar]
- 11.Luchtenborg M, Weijenberg MP, Kampman E, et al. (2005) Cigarette smoking and colorectal cancer: APC mutations, hMLH1 expression, and GSTM1 and GSTT1 polymorphisms. American Journal of Epidemiology 161: 806–815. [DOI] [PubMed] [Google Scholar]
- 12.Cho E, Smith-Warner SA, Ritz J, et al. (2004) Alcohol intake and colorectal cancer: A pooled analysis of 8 cohort studies. Annals of Internal Medicine 140: 603–613. [DOI] [PubMed] [Google Scholar]
- 13.Mizoue T, Inoue M, Tanaka K, et al. (2006) Tobacco smoking and colorectal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Japanese Journal of Clinical Oncology 36: 25–39. [DOI] [PubMed] [Google Scholar]
- 14.Peppone LJ, Mahoney MC, Cummings KM, et al. (2008) Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. Journal of Cancer Research and Clinical Oncology 134: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slattery ML, Edwards S, Curtin K, SchaVer D, Neuhausen S (2003) Associations between smoking, passive smoking, GSTM-1, NAT2, and rectal cancer. Cancer Epidemiology, Biomarkers & Prevention 12: 882–889. [PubMed] [Google Scholar]
- 16.World Cancer Research Fund American Institute for Cancer Research (1997) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 17.Beresford SA, Johnson KC, Ritenbaugh C, et al. (2006) Low-fat dietary pattern and risk of colorectal cancer: The Women’s Health Initiative randomized controlled dietary modification trial. Obstetrical & Gynecological Survey 61: 456–458. [DOI] [PubMed] [Google Scholar]
- 18.International Agency for Research on Cancer (2003) Fruits and Vegetables. International Agency for Research on Cancer, World Health Organization. [Google Scholar]
- 19.Kimura Y, Kono S, Toyomura K, et al. (2007) Meat, fish, and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Science 98: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Zhang SM, Cook NR, et al. (2005) Dietary intakes of fruit, vegetables, and fiber, and risk for colorectal cancer in a prospective cohort of women (United States). Cancer Causes and Control 16: 225–233. [DOI] [PubMed] [Google Scholar]
- 21.Potter JD (1996) Nutrition and colorectal cancer. Cancer Causes and Control 7: 127–146. [DOI] [PubMed] [Google Scholar]
- 22.Park Y, Hunter DJ, Speigelman D, et al. (2005) Dietary fiber intake and risk of colorectal cancer: A pooled analysis of prospective cohort studies. Journal of the American Medical Association 294: 2849–2857. [DOI] [PubMed] [Google Scholar]
- 23.Schatzkin A, Mouw T, Park Y (2007) Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. American Journal of Clinical Nutrition 85: 1353–1360. [DOI] [PubMed] [Google Scholar]
- 24.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine 348: 1625–1638. [DOI] [PubMed] [Google Scholar]
- 25.Vainio H, Bianchini F (2002) Weight Control and Physical Activity. International Agency for Cancer Research Press. [Google Scholar]
- 26.Martinez ME, Giovannucci E, Spiegelman D, et al. (1997) Leisure-time physical activity, body size, and colon cancer in women: Nurses’ Health Study Research Group. Journal of the National Cancer Institute 89: 948–955. [DOI] [PubMed] [Google Scholar]
- 27.Samad AKA, Taylor RS, Marshall T, Chapman MAS (2004) A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Disease 7: 204–213. [DOI] [PubMed] [Google Scholar]
- 28.Slattery ML, Edwards S, Ma K, Friedman GD, Potter JD (1997) Physical activity and colon cancer: A public health perspective. Annals of Epidemiology 7: 137–145. [DOI] [PubMed] [Google Scholar]
- 29.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. (2006) Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. Journal of Clinical Oncology 24: 3535–3541. [DOI] [PubMed] [Google Scholar]
- 30.Courneya KS, Friedenreich CM, Quinney HA, et al. (2004) Predictors of adherence and contamination in a randomized trial of exercise in colorectal cancer survivors. Psychooncology 13: 857–866. [DOI] [PubMed] [Google Scholar]
- 31.Lynch BM, Cerin E, Owen N, Aitken JF (2007) Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes and Control 18: 735–742. [DOI] [PubMed] [Google Scholar]
- 32.Diergaarde B, Braam H, Vasen H, et al. (2007) Environmental factors and colorectal tumor risk in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 5: 736–742. [DOI] [PubMed] [Google Scholar]
- 33.Watson P, Ashwathnarayan R, Lynch HT, Roy HK (2004) Tobacco use and increased colorectal cancer risk in patients with hereditary nonpolyposis colorectal cancer (Lynch syndrome). Arch Intern Med 164: 2429–2431. [DOI] [PubMed] [Google Scholar]
- 34.Pande M, Lynch P, Hopper J, et al. (Manuscript Under Review) Smoking and colorectal cancer in Lynch syndrome: Results from the Colon Cancer Family Registry and The University of Texas M. D. Anderson Cancer Center. Clinical Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meiser B (2005) Psychological impact of genetic testing for cancer susceptibility: An update of the literature. Psychooncology 14: 1060–1074. [DOI] [PubMed] [Google Scholar]
- 36.Hadley DW, Jenkins JF, Dimond E, et al. (2004) Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol 22: 39–44. [DOI] [PubMed] [Google Scholar]
- 37.Hughes Halbert C, Lynch H, Lynch J, et al. (2004) Colon cancer screening practices following genetic testing for Hereditary Nonpolyposis Colon Cancer (HNPCC) mutations. Archives of Internal Medicine 164: 1881–1887. [DOI] [PubMed] [Google Scholar]
- 38.Ramsey S, Blough D, McDermott C, et al. (2009) Will knowledge of gene-based colorectal cancer disease risk influence quality of life and screening behavior? Public Health Genomics: March 2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodersen N, Sutton S, Goff S, Hodgson S, Thomas H (2004) Anticipated reactions to genetic testing for hereditary non-polyposis colorectal cancer susceptibility. Clin Genet 66: 437–444. [DOI] [PubMed] [Google Scholar]
- 40.Emmons K, Kalkbrenner K, Klar N, et al. (2000) Behavior risk factors among women presenting for genetic testing. Cancer Epidemiology Biomarkers and Prevention 9: 89–94. [PubMed] [Google Scholar]
- 41.Madlensky L, Flatt SW, Bardwell WA, Rock CL, Pierce JP (2005) Is family history related to preventive health behaviors and medical management in breast cancer patients? Breast Cancer Res Treat 90: 47–54. [DOI] [PubMed] [Google Scholar]
- 42.Madlensky L, Vierkant RA, Vachon CM, et al. (2005) Preventive health behaviors and familial breast cancer. Cancer Epidemiol Biomarkers Prev 14: 2340–2345. [DOI] [PubMed] [Google Scholar]
- 43.Lemon SC, Zapka JG, Clemow L (2004) Health behavior change among women with recent familial diagnosis of breast cancer. Prev Med 39: 253–262. [DOI] [PubMed] [Google Scholar]
- 44.Audrain J, Schwartz M, Herrera J, Goldman P, Bush A (2001) Physical activity in first-degree relatives of breast cancer patients. J Behav Med 24: 587–603. [DOI] [PubMed] [Google Scholar]
- 45.Biesecker BB, Marteau TM (1999) The future of genetic counseling: an international perspective. Nat Genet 22: 133–137. [DOI] [PubMed] [Google Scholar]
- 46.Lipton LR, Johnson V, Cummings C, et al. (2004) Refining the amsterdam criteria and bethesda guidelines: testing algorithms for the prediction of mismatch repair mutation status in the familial cancer clinic. J Clin Oncol 22: 4934–4943. [DOI] [PubMed] [Google Scholar]
- 47.Vernon SW, Gritz ER, Peterson SK, et al. (1997) Correlates of psychologic distress in colorectal cancer patients undergoing genetic testing for hereditary colon cancer. Health Psychology 16: 73–86. [DOI] [PubMed] [Google Scholar]
- 48.Vernon SW, Gritz ER, Peterson SK, et al. (1999) Intention to learn results of genetic testing for hereditary colon cancer. Cancer Epidemiol Biomarkers Prev 8: 353–360. [PubMed] [Google Scholar]
- 49.Gritz ER, Peterson SK, Vernon SW, et al. (2005) Psychological impact of genetic testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol 23: 1902–1910. [DOI] [PubMed] [Google Scholar]
- 50.Radloff LS (1977) The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1: 385–401. [Google Scholar]
- 51.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) State-Trait Anxiety Inventory for Adults. Palo Alto, CA: Mind Garden. [Google Scholar]
- 52.Sarason IG, Levine HM, Basham RB, Sarason BR (1983) Assessing social support: The social support questionnaire. J Pers Soc Psychol 1: 127–139. [Google Scholar]
- 53.Ferrans C, Powers M (1992) Psychometric assessment of the Quality of Life Index. Res Nurs Health 15: 29–38. [DOI] [PubMed] [Google Scholar]
- 54.Center for Disease Control (2009) Behavioral Risk Factor Surveillance System. http://www.cdc.gov/BRFSS/. May 18, 2009. [Google Scholar]
- 55.Abrams D, Boutwell W, Grizzle J, et al. (1994) Cancer control at the workplace: The Working Well Trial. Preventive Medicine 23: 15–27. [DOI] [PubMed] [Google Scholar]
- 56.Snijders TAB, Bosker RJ (1999) Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage Publications. [Google Scholar]
- 57.Emmons KM, McBride CM, Puleo E, et al. (2005) Prevalence and predictors of multiple behavioral risk factors for colon cancer. Prev Med 40: 527–534. [DOI] [PubMed] [Google Scholar]
- 58.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. (2006) Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 24: 3535–3541. [DOI] [PubMed] [Google Scholar]
- 59.Demark-Wahnefried W, Aziz N, Rowland J, Pinto B (2005) Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol 23: 5814–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satia J, Campbell M, Galanko J, et al. (2004) Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev 13: 1022–1031. [PubMed] [Google Scholar]
- 61.Rees G, Gaff C, Young M, Martin P (2007) Health beliefs and behaviors of women who have received genetic counseling for breast cancer. J Genet Couns 16: 457–468. [DOI] [PubMed] [Google Scholar]
- 62.Spector D (2007) Lifestyle behaviors in women with a BRCA1 or BRCA2 genetic mutation: An exploratory study guided by concepts derived from the Health Belief Model. Cancer Nurs 29: E1–10. [DOI] [PubMed] [Google Scholar]
- 63.McBride C, Brody L (2007) Point: Genetic risk feedback for common disease-time to test the waters. Cancer Epidemiol Biomarkers Prev 16: 1724–1726. [DOI] [PubMed] [Google Scholar]
- 64.McBride C, Alford S, Reid R, et al. (2008) Putting science over supposition in the arena of personalized genomics. Nat Genet 40: 939–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawson P, Flocke S (2009) Teachable moments for health behavior change: A concept analysis. Patient Educ Couns 76: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quach J, Porter K, Leventhal H (2009) Health behaviors among Ashkenazi Jewish individuals receiving counseling for BRCA1 and BRCA2 mutations. Fam Cancer: January 28, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Schneider K (2002) Counseling About Cancer - Strategies for Genetic Counseling. New York: Wiley-Liss, Inc. [Google Scholar]
- 68.Rees G, Young M, Gaff C, Martin P (2006) A qualitative study of health professionals’ views regarding provision of information about health-protective behaviors during genetic consultation for breast cancer. J Genet Couns 15: 95–104. [DOI] [PubMed] [Google Scholar]
- 69.Emmons K, McBride C, Puleo E, et al. (2005) Project PREVENT: A randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev 14: 1453–1459. [DOI] [PubMed] [Google Scholar]
- 70.American Cancer Society (2005) Colorectal Cancer Facts and Figures Special Edition 2005. American Cancer Society. [Google Scholar]
- 71.United States Preventive Services Task Force (2008) The Guide to Clinical Preventive Services. Agency for Healthcare Research and Quality. [Google Scholar]
- 72.Cook A, Friday J, Subar A (2004) Dietary Source Nutrient Database for USDA Survey Food Codes. http://www.ars.usda.gov/ba/bhnrc/fsrg/. May 18, 2009. [Google Scholar]


