Abstract
For the past 20 years, the notion of bioterror has been a source of considerable fear and panic among people worldwide. In response to the terror attacks of 2001 in the United States (US), extensive research funding was awarded to bioterror-related pathogens. The global scientific legacy of this funding has extended into the present day, highlighted by the ongoing COVID-19 pandemic. Unsurprisingly, the surge in biodefense-related research and preparedness has been met with considerable apprehension and opposition. Here, we will briefly outline the history of modern bioterror threats and biodefense research, describe the scientific legacy of biodefense research by highlighting advances pertaining to specific bacterial and viral pathogens, and summarize the future of biodefense research and its relevance today. In sum, we seek to address the sizeable question: Have the last 20 years of investment into biodefense research and preparedness been worth it?
Keywords: biodefense, biological warfare, bioterrorism, anthrax, salmonella, Q fever, plague, Lassa virus, filoviruses, Ebola virus, Marburg virus
Introduction
Following the terror attacks on 9/11/2001 and the anthrax bioterror incident in the same year in the United States (US), the global scientific, clinical, and general perception of biosecurity and biodefense have been permanently altered. With the subsequent influx of biodefense research and preparedness funding1 a number of scientific advancements have been achieved. In this review, we briefly discuss the history of bioterrorism, define the entity of biodefense research, describe the legacy of modern biodefense funding, and deliberate the merits of these investments. Bioterrorism is defined by Price et al. as “attacks on civilians and non-military targets to incite fear among targeted groups to bend them to another’s political motives, whether that of an individual, a group, or a state2.” The history of bioterrorism and biological warfare are organically connected to scientific progress in infectious disease research3. What began as the deployment of infected materials into enemy territory4–6 before the knowledge of germ theory (BC-early 1800s) evolved into the production of large amounts of cultured organisms in the wake of modern microbiology (mid-1800s-early 1900s) and culminated in the targeted weaponization of pathogens from World War I on due to burgeoning microbiological and molecular methods (early 1900s-present)3,7. Despite international declarations banning the manufacture, stockpiling, and use of biological weapons5,8,9, these declarations had little effect, presumably due to the lack of any means of enforcement. Documented breaches include covert assassination attempts using biologic toxins by the former Soviet Union5, a large anthrax outbreak among Soviet citizens caused by a weapons manufacturing plant release10, and the expansion of an offensive biological warfare program in Iraq11.
With increased post-war access to and mastery of technology needed to produce and disseminate biological agents by civilians and terrorist organizations4, bioterrorism became a tangible threat. Unbound by national treaties or desires to conform with the international community, non-state sponsored terrorists emerged as bioterrorism participants in the 1970s, with less than successful results.12 In 1984 the Rajneeshee cult contaminated the salad bars of Oregon, US restaurants with Salmonella typhimurium in an attempt to affect an upcoming local election. These attacks resulted in 751 cases of enteritis and 45 hospitalizations13. Bioterrorism was further imparted to the forefront of the US public’s psyche in 2001 following several key events, including the World Trade Center attacks and the anthrax (aka Amerithrax) attacks. These events would inexorably alter global perceptions of terrorism, stimulate US nationalism, and increase notions of perceived threats14,15. On 9/11/2001 the Twin Towers of the World Trade Center in New York City, NY were razed following the hijacking of two aircraft. Potentiating these effects, a week later letters containing anthrax spores were discovered in the US leading to at least 22 individuals contracting anthrax, 11 of which were afflicted with pulmonary anthrax, the deadliest form of the disease. Amerithrax claimed the lives of five individuals, led to prophylactic treatment of nearly 10,000 individuals, and resulted in an immense economic burden. Both the World Trade Center attacks and the Amerithrax incident contributed to a shift in the American consciousness and a surge in global bioterrorism-related funding and research interest.
Post-2001 Biodefense Efforts
Prior to 2001, annual US biodefense funding totaled an estimated $700,000,00016. Following the incidents of 2001, the worldwide surge in biodefense-related funding was largely spurred by the realization that many countries were not prepared for bioterrorism attacks. The 2001 US Amerithrax attack revealed shortcomings in medical countermeasure availability through the Strategic National Stockpile (SNS), the laboratory response network system, public health infrastructure, and communication17. Many of the funding programs were associated with the US federal government. A $1,000,000,000 program was implemented in the US in 200218 in the form of a bioterrorism preparedness grants, biodefense research funding, and medical countermeasure stockpiling within the Department of Health and Human Services19. Additional notable post-2001 US biodefense funding efforts include the Department of Homeland Security’s (DHS) Biowatch Program (2001)20, The Centers for Disease Control and Prevention’s (CDC) preparedness program21, the DHS’s Project Bioshield (2004)22, the Biomedical Advanced Research and Development Authority (BARDA; 2006)23, and the National Bio and Agro Defense Facility (2014). These programs typically address matters outside of biodefense, such as public health, national and international security, and healthcare issues, adding to their broader impact. Total US biodefense funding dramatically increased from ~$700,000,000 in 2001 to ~$4,000,000,000 spent in 2002; the peak of funding in 2005 was worth nearly $8,000,000,000 and continued with steady average spending around $5,000,000,00016,24.
In 2019, the global biodefense market was valued at $12,200,000,000 and is expected to grow at a compound annual growth rate of 5.8% from 2020–2027, resulting in a projected market value of $19,800,000,000 in 202725. Factors such as sustained government and private funding resources driven by the looming threat of bioterrorism and the recent occurrence of natural outbreaks of bioterror-related pathogens including Coxiella burnetii, Ebola virus (EBOV), SARS-CoV-1, SARS-CoV-2, influenza, and Lassa virus are likely major contributors to the ever-expanding global biodefense market.
Modern Topics in Biodefense Research
Bioterror-Related Pathogens
Typically, pathogens useful for bioterrorism exhibit several shared qualities, including 1) severity of impact on public health/transmissibility, 2) potential for delivery as a weapon, 3) the need for specialized preventative measures/therapeutics/diagnostic techniques, and 4) the ability to generate fear and/or terror26. These attributes contribute to the pathogen’s potential for destruction and ease of use by terrorists on a susceptible population. Relevant agents include bacterial, fungal, and viral pathogens along with bacterial and plant-derived toxins. According to the criteria above, 67 select agents and toxins have been identified by the CDC as posing the potential for severe threat to human and animal health27. Internationally, the World Health Organization (WHO), the European Medicines Agency (EMA), and relevant organizations of other countries have identified key diseases of bioterror concern and these generally overlap28,29. Organisms frequenting these lists include Bacillus anthracis, Clostridium botulinum (toxin), Coxiella., Francisella tularensis, Yersinia pestis (Panel 1), Filoviruses (EBOV and Marburg virus), Lassa virus, and Variola major (Panel 2) among others. These core bioterror-related pathogens require specialized containment laboratories and have restrictions for research. Global numbers of Biosafety level (BSL) 3 and 4 containment laboratories have surged since 2001 and funding for specialized containment centers has been awarded. Accordingly, research output exploded from 2001–2020 most significantly for B. anthracis and EBOV compared to pre-2001 efforts and those for non-bioterror-related pathogens (Table 1).
Panel 1: Select Bacterial Pathogen-Specific Advancements Resulting from Biodefense Funding, 2001–2021.
- Bacillus anthracis
- 2007: An anthrax vaccine, BioThrax, was approved for use by the US FDA as PEP treatment for individuals exposed to B. anthracis.
- Clostridium botulium (botulinum toxin)
- 2004-present: Advances in botulinum toxin diagnostic testing were achieved beginning in 2004, characterized by immunoassays that yielded results in approximately 20 minutes, a dramatic improvement from the prior standard of hours to days38,128. Additional advancements in clinical and environmental diagnostics have been made, including more sophisticated immunoassays (e.g. ELISA, Luminex multiplex assay, immune-PCR, and microfluidic immunoassays), providing a quicker and more accessible alternative to the standard in vivo mouse lethality bioassay32.
- 2013: A heptavalent botulinum antitoxin therapeutic (HBAT) was approved for use by the US FDA as a PEP treatment for individuals exposed to botulinum toxin.
- Coxiella burnetii
- 2009: The development of axenic media129 enabled large-scale genetic manipulation of this bacteria.
- Fransicella tularensis
- 2016–2018: F. tularensis diagnostics progressed in 2016 when a rapid, point of care detection assay based on cartridge-based PCR was introduced. Expanding the utility of this diagnostic advance, a multiplex Luminex-based immunoassay was developed in 2018 capable of detecting not only F. tularensis but also B. anthracis and Y. pestis34
- Yersinia pestis
- 2018: A Plague vaccine, rF1-V, was approved for use by the US FDA as an Orphan drug for Plague PreP
Panel 2: Select Viral Pathogen-Specific Advancements Resulting from Biodefense Funding, 2001–2021.
- Filoviruses (Ebola and Marburg viruses)
- Lassa virus
- 2001-present: Ribavirin is used as treatment against Lassa fever with mixed results; Favipiravir is being investigated as additional treatment option130
- 2016-present: Coalition for Epidemic Preparedness Innovations (CEPI) is funding vaccine development efforts (www.cepi.net)
- SARS-CoV-2
- 2020-present: A flurry of vaccine development and deployment activity is taking place during the COVID-19 pandemic, resulting in the approval of multiple COVID-19 vaccinations globally and billions of vaccine doses being administered worldwide.
- 2020-present: Several repurposed therapeutics have been approved for SARS-CoV-2 PEP use in various countries around the world including remdesivir. Monoclonal antibody (mAb) development has also resulted in several SARS-CoV-2 mAbs receiving emergency PEP use approvals in the US.
- Variola major/minor
- 2003: Large amounts of Smallpox vaccine were incorporated into the US SNS, and were recently updated with the second-generation vaccines
- 2007: A newly developed Smallpox vaccine, ACAM2000 was licensed by the US FDA for PrEP.
- 2019: A non-replicating Smallpox vaccine by Jynneos™ is approved by the US FDA, EMA, and in Canada for PrEP of both Smallpox and Monkeypox.
Table 1.
Publications related to Bioterror Pathogens in PubMed
| Pathogen/Disease | Number of PubMed hits | ||
|---|---|---|---|
|
|
|||
| 1981–2000 | 2001–2020 | Fold change | |
| Bioterror-Related Pathogens - CDC Category A Bioterrorism Agents | |||
| Bacillus anthracis/anthrax | 641 | 5,112 | +7.98 |
| Clostridium botulinum toxin/Botulism | 295 | 760 | +2.58 |
| Ebola virus/Ebola hemorrhagic fever/Ebola virus disease | 227 | 4,102 | +18.07 |
| Francisella tularensis/Tularemia | 371 | 1,718 | +4.63 |
| Lassa virus/Lassa fever | 182 | 711 | +3.9 |
| Marburg virus/Marburg hemorrhagic fever | 163 | 945 | +5.8 |
| Variola major/Smallpox | 1,260 | 3,812 | +3.03 |
| Yersinia pestis/Plague | 599 | 2,074 | +3.46 |
| Non-Bioterror-Related Pathogens | |||
| Bordetella pertussis/Whooping cough | 933 | 2,023 | +2.17 |
| Borrelia burgdorferi/Lyme disease | 3,245 | 4,960 | +1.53 |
| Chlamydia trachomatis/Chlamydia | 6,985 | 8,799 | +1.26 |
| Corynebacterium diptheriae/Diphtheria | 719 | 814 | +1.13 |
| Cytomegalovirus/Cytomegalovirus disease | 14,176 | 20,998 | +1.48 |
| Herpes virus; Herpes | 19,750 | 26,812 | +1.36 |
| Human Immunodeficiency Virus/HIV AIDS | *55,878 | 110,169 | +1.97 |
| Measles Virus/Measles | 3,631 | 5,114 | +1.41 |
| Mycobacterium leprae/Leprosy or Hansen’s Disease | 2,430 | 2,194 | −0.9 |
| Vibrio cholerae/Cholera | 3,874 | 6,649 | +1.72 |
To account for fluctuations in general research output, non-bioterror-related pathogens were added based on their relative persistence from 1981–2021.
HIV was documented from 1982–2000 due to the time of discovery of this virus
Key Scientific Advances and Technologies
Key scientific advances related to biodefense funding encompass a variety of sectors including diagnostics, pre-exposure prophylaxis (PrEP) and vaccines, and postexposure prophylaxis (PEP) strategies. Diagnostic advances abounded for bioterror-related pathogens in the past two decades and were largely enabled by scientific advancements in assay design and molecular biology. Related research lead to the introduction of rapid PCR assays for clinical and environmental Anthrax samples 30,31, botulinum toxin 32,33, F. tularensis 34, and EBOV 35. Notably, in 2018 the utility of a F. tularensis point of care cartridge qPCR detection assay was expanded to include B. anthracis and Y. pestis 34. Several novel, field-deployable EBOV qPCR-based detection assays have been approved by the US FDA and the EMA to aid prevention of the next EBOV epidemic36. Luminex-based antigen or antibody detection assays have been introduced for EBOV, aiding this effort37. Advances in botulinum toxin diagnostic testing were achieved in 2004, leading to immunoassays that yield results in ~20 minutes, a dramatic improvement from the prior standard of hours to days38. Since 2004, additional advancements in clinical and environmental botulinum toxin diagnostics include more sophisticated immunoassays (e.g. ELISA, Luminex multiplex assay, microfluidic immunoassays), providing a quicker and more accessible alternative to the standard in vivo mouse lethality bioassay. Lastly, mass spectrometry has been harnessed for diagnostic purposes; the Endopep-MS assay detects enzymatic activity of the botulinum toxin and was refined in 2017, enabling the detection of multiple toxin serotypes in a multiplex fashion33.
PrEP and vaccine development has been a focus of many biodefense research programs and accordingly, many significant advances have been made resulting in vaccine licensures for B. anthracis, Y. pestis and EBOV. In 2015, the Animal Rule was invoked for the eventual approval of BioThrax for anthrax PEP by the US FDA in 201539. PEP vaccination involves a three-dose subcutaneous regimen plus antibiotic treatment, an improvement from the more extensive PrEP schedule39. Since 2015, research has been ongoing to improve BioThrax. Recent work has described advances such as 1) a new formulation of BioThrax including adjuvant that may reduce the PEP vaccine schedule to just one40 or two 41 doses, 2) multiple recombinant vaccine candidates42, one of which has demonstrated promising results in a human study43, 3) a next-generation live-attenuated spore vaccine based on bacterial mutagenesis44, and 4) dual purpose vaccines, first, a universal influenza/anthrax toxin vaccine that demonstrated protection against both pathogens in mice45 and, second, a recombinant anthrax/Plague vaccine46. The path to a usable PEP vaccine to combat anthrax, whether naturally occurring or deliberately released, has been accelerated by biodefense funding of the past two decades47,48.
A next-generation Y. pestis vaccine utilizing the adenovirus 5 platform has recently been developed in response to concerns regarding existing live-attenuated vaccines currently used in China, Mongolia, and numerous countries in Eastern Europe49. Iterations of combinatorial F1 (capsular antigen) and V (type 3 secretion system components) subunit vaccines have been developed by scientists around the globe and in 2017 the US FDA granted Orphan Drug designation to a rF1-V vaccine after the completion of three clinical trials (www.clinicaltrials.gov). The DynPort Vaccine Company, responsible for taking this rF1-V vaccine to market, reported clinical trial funding from the Chemical Biological Medical System-Joint Vaccine Acquisition Program and the US Department of Defense (DOD)50 highlighting the importance of biodefense funding in this advancement. Although elusive, vaccine development efforts have been promising for additional bioterror-related pathogens including: C. botulinum as a variety of new vaccine design strategies are being pursued, including DNA, recombinant, and viral vectored vaccines51,52 along with C. burnetii by way of LPS mimicking peptides53, genetically-altered WCVs54, and adjuvanted subunit vaccines55.
Another biodefense vaccine development success story relates to EBOV. Crucial early funding for EBOV vaccine development was primarily derived from the US DOD and the Public Health Agency of Canada (PHAC) in the absence of interest from the pharmaceutical sector56. In 201457, when the West African EBOV epidemic caused over 28,000 infections and 11,000 deaths58 and with EBOV spreading to Nigeria, Spain and the US through travel58, the world was reminded of the severe nature and the threat posed by EBOV58,59. International organizations worked together and enabled the accelerated evaluation of vaccines in clinical trials around the world60,61. Russia and China were the first nations to license their own EBOV vaccines62. The WHO approved the use of the live-attenuated EBOV vaccine Ervebo in 2019, which was licensed in the US and Europe63,64 and in the following year in several African countries65.
Advanced PEP strategies and treatments such as monoclonal antibody (mAb) and antitoxin therapy have been employed for numerous bioterror-related pathogens as a result of biodefense funding. C. botulinum mAbs have been developed as an alternative to sera66, B. anthracis mAbs were licensed for use by the US FDA in 2012 and 2016 for inhalational anthrax PEP67,68, and EBOV therapeutic strategies were developed and optimized with the most promising intervention being monoclonal antibody therapy69, resulting in FDA-approval of two antibody-based treatments70,71. In 2013, heptavalent, pepsin-cleaved botulism antitoxin (HBAT) was approved by the US FDA for pediatric and adult treatment of botulism72. This formulation represents an improved antitoxin due to its reduced capacity for severe allergic reactions73 compared to equine-sourced antitoxins used previously. HBAT has also demonstrated potential as a PEP treatment in non-human primates74, which could be particularly important in a bioterrorism scenario.
General Advancements in Biosecurity
Bioterrorism risk assessment has become more sophisticated in the past two decades, with the establishment of model frameworks capable of applying qualitative and quantitative parameters to establish risk, both in the case of and prior to a potential attack 75 (Panel 3). An improved pathogen bioterror risk scoring scheme was proposed in 2009 and considered not only the classical features of bioterror potential but also societal factors related to the environment of a potential attack76. Considerations such as availability of healthcare facilities, local incidence of the pathogen, and clinical picture of the disease caused by selected pathogens have been topics of mounting interest and concern.
Panel 3: Select General Advancements Resulting from Biodefense Funding, 2001–2021.
- Biocontainment
- Biocontainment technology and implementation has surged, with an explosion of biocontainment laboratories emerging worldwide, increased regulatory capabilities, and more sophisticated technologies.
- For field work, mobile biocontainment laboratories have been developed and have proven useful during outbreaks, such as that of EBOV in West Africa93 and advancements in high level containment transport allow for safer, more effective means of transportation of infected individuals.
- Clinical biocontainment units, also known as high-level containment care (HLCC), have recently been utilized during the 2014–2016 EBOV epidemic and are being established around the world to enable safe, high quality patient care.
- Biosurveillance
- Digital biosurveillance networks have been established as international early warning systems for unusual infections, oubreaks, and pandemics. These newly developed digital disease reporting databases include the WHO/Health Canada’s Global Outbreak Alert and Response Network (GPHIN), the EU’s BICHAT with Early Warning and Response System, various US government initiatives (e.g. the CDC’s National Syndromic Surveillance Program), and the privately funded ProMED-mail system; all have been well-received due to their efficacy87.
- Recent advances in biosurveillance harness wearable technology, smartphones, and advanced software88.
- Forensics
- The field of microbial forensics emerged in response to the Amerithrax events, and this burgeoning field has demonstrated utility within epidemiology, crime scene investigations, biocrime, and bioterror investigations133.
- Infrastructure development
- International Communication
- In the midst of the 2001 Amerithrax events, the US CDC set up an international emergency operations center, effectively communicating with health organizations and individuals from around the world enhancing the global reasponse134.
- The 2017 CELYLEX exercises led by Portugal demonstrated the importance of international cooperation and communication during a bioterror event.
- Healthcare
- Biodefense funds have been directed at improvement of healthcare infrastructure (e.g. the US Hospital Preparedness Program) but many of these funds have been reduced, likely due to lower urgency regarding the threat of bioterror.
- The National Ebola Virus Training and Education Center (NETEC) was founded in 2015 in the US, serving as a support program for clinical management of infectious disease patients96, offering training, resources, and education, with an overall objective of increasing competency among healthcare and public health workers in the event of an EBOV or other pathogen outbreak. Demonstrating its value beyond a bioterror attack, the NETEC has been an important source of COVID-19-related information by providing resources for the public and professionals alike.
- Public health
- Biodefense funds have been directed at improvement of public health infrastructure (e.g. the US’s Public Health Emergency Preparedness Program), particularly in the early 2000s.
- Supply chain/stockpiles
- Global medical stockpiles have been established and/or bolstered since 2001. The WHO maintains a pharmaceutical stockpile, which includes the EBOV and smallpox vaccines84. The US SNS was established in 2003 and has been rapidly expanding since then77, providing deployable medical stations, personal protective equipment, medical equipment, and pharmaceuticals for bioterror-related pathogens.
- Policy
- Appropriate policy is an imperative component of the response to public health emergencies of any kind. Accordingly, major changes in US law were proposed and have been adopted post-2001135. The WHO has also implemented major policy changes since 2001 related to bioterrorism, with some of the most current involving the establishment of a global emergency outbreak response fund.
- Risk Assessment
- Advancements in risk assessment have led to more representative and sophisticated methods to be used in preparatory and reactionary ways. Model frameworks capable of applying quantitative parameters and incorporating societal factors to risk establishment have been developed and refined75.
The rapid deployment of medical equipment and countermeasures has been a focus of global biodefense funding. The US SNS was established in 2003 and has been rapidly expanding since then resulting in the acquisition of a massive supply of antibiotics, antidotes, antitoxins, medications, medical supplies, and personal protective equipment (PPE) to be used to assist and supplement first response emergency efforts in the case of a national emergency, such as that caused by a bioterrorism event77 or naturally. The SNS appears to be a critical program78 whose utility is likely directly tied to the recent increase in US biodefense funding. With the mitigated threat of EBOV in Dallas, Texas79 in 2014, and the ZIKV pandemic 2015–2016 never taking root in the US80, an SNS deployment was not necessary. However, the SNS was deployed in 2020 during the COVID-19 pandemic, providing critical supplies, and prompting calls for a better integrated supply chain deployment system in the US81. Many of these concerns were sparked regarding ventilator and PPE availability82. These concerns indicated that the SNS can be improved for future events but was useful during the early stages of the pandemic83. The WHO has its own pharmaceutical stockpiles including doses of smallpox vaccine and EBOV vaccine84. In 2005, the WHO reported boasted stockpiles of antivirals in European countries with France and Switzerland reporting complete population coverage in case of an Influenza outbreak85. Stockpiling is certainly a useful approach to biosecurity but faces constraints such as space and funding.
Biosurveillance is a critical component of international and local biosecurity and involves the development and implementation of surveillance technologies to detect and mitigate biologic threats. Initial post-2001 biosurveillance pursuits like the US Biowatch initiative involved environmental detection. Since 2001, the US Biowatch program has received billions of US dollars of funding and has been plagued with concerns of questionable output and the use of antiquated technology86. In contrast, digital disease reporting databases such as the Global Outbreak Alert and Response Network (GOARN; a joint venture by the WHO and PHAC), the EU’s BICHAT with Early Warning and Response System, various US government initiatives (e.g. the CDC’s National Syndromic Surveillance Program), and the privately funded ProMED-mail system, have been well-received due to their efficacy. The ProMED-mail system enables rapid reporting of potential unusual disease outbreaks from around the world87. Recent advances in biosurveillance harness wearable technology, smartphones, and advanced software supported by biodefense funding88.
The field of infectious disease modeling has been impacted by biodefense funding, leading to advancements such as the ability to identify drivers of measles virus epidemic seasonality89. This work was enabled by the use of satellite imagery, which identified population density changes as a key factor in measles epidemic seasonality in Niger, expanding predictive capabilities for future outbreaks and improving interventions. This work was supported by the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Fogarty International Center within the US National Institutes of Health (NIH). RAPIDD has significantly contributed to the rapid advancement of the global field of infectious disease modeling90 and was established from biodefense funding in 200791.
Advancements in biocontainment have emerged as this field rapidly expands. In the past two decades, the global density of biocontainment laboratories has increased and in turn, advancements in laboratory design and methods have materialized92. For field work, mobile biocontainment laboratories have been developed and have proven useful during outbreaks, such as that of EBOV in Sierra Leone93. Clinical biocontainment and isolation have received much interest in the past 20 years, leading to increased implementation of these facilities around the world. Clinical biocontainment units, also known as high-level containment care (HLCC), were utilized during the 2014 EBOV epidemic. Patients transported from West Africa to HLCC units in their home countries experienced dramatically lower mortality rates (18%) than their non-HLCC hospitalized counterparts (64%)94. Since 2005, HLCC units have opened in the US and worldwide (e.g. China, France, Germany, Italy, the Netherlands, Norway, the Republic of Korea, Singapore, Spain, Switzerland, and the United Kingdom) and many have demonstrated utility by caring for patients infected with bioterror-related pathogens such as EBOV and SARS-CoV-295. Beyond HLLCs, serving as a support program for clinical management of infectious disease patients, the National Ebola Virus Training and Education Center (NETEC) was founded in 2015 in the US96. Demonstrating its value, the NETEC has been an important source of COVID-19-related information and resources for the general public and professionals alike. In summary, many advances in biosecurity have been made as a result of the past two decades of biodefense funding (Panel 3) and have likely better prepared us for infectious disease outbreaks, regardless of their origin.
Natural Outbreaks and the Impact of Biodefense Funding
While it may be difficult to quantify the practical impact of the post-2001 funding surge on biosecurity due to the rarity of bioterrorism-related events in the past two decades, the impact of biodefense funding on natural outbreaks of bioterror-related organisms is evident. In the 21st century the world had a first taste of a pandemic in 2003 when SARS-CoV-1 spread from Hong-Kong throughout the world resulting in 774 deaths in 26 countries97. In 2009, H1N1 influenza virus reemerged causing the swine flu pandemic costing over 250,000 people their lives98. The deployment of EBOV vaccines and therapeutics in clinical trials proved to be a supporting control measure in the fight against EBOV during the 2013–2016 epidemic99,100. Additionally, portable biocontainment and diagnostic advances also contributed to the management of EBOV patients100. While the global community managed relatively well during these events, likely due in part to prior biodefense efforts, they did not prepare us well for the “real thing”. The ongoing COVID-19 pandemic has demonstrated the devastating effects of a transmissible, mutable respiratory pathogen on the world’s population. Although not thought to be released intentionally, SARS-CoV-2 has spread globally and caused extreme morbidity, mortality, and economic losses. US biodefense funding bolstered the development of a mRNA vaccine platform rapidly deployed for COVID-19101. International cooperation between the US DoD’s Chemical and Biological Defense Program (CBDP) and the Republic of Korea has resulted in biological defense activities, coined Able Response, focused on communication between both countries, and leading to identification of response gaps, the application of new technologies, and improved infrastructure in the Republic of Korea101–103. The Able Response program and associated impact likely aided in the Republic of Korea’s successful COVID-19 response104. Unfortunately, the global response to COVID-19 has been far from ideal, exposing weaknesses in biosecurity and response systems in many nations105,106.
Arguments against biodefense funding
The surge of biodefense funding was met with scientific outcry and was not universally supported107. Principle arguments against biodefense funding include the absence of large-scale, successful bioterror events in modern times, perceived redirection of funds from more eminent, and research moral and safety reservations. Perhaps surprisingly, US NIH’s National Institute of Allergy and Infectious Disease (NIAID) biodefense and non-biodefense funding allocation rates remained relatively proportional from 2000–2006108. NIAID’s biodefense funding increased from 2001–2004 but subsided in the following year, a trend which was also generally reflected in non-biodefense efforts across the NIAID. Some have proposed justification for additional biodefense funding based on perceived insufficiencies in current funding efforts following declining funding in more recent years109. Indeed, the general funding focus seems to have shifted from biodefense to other health issues from 2001 to the present. The potential for bioterrorism targeting agricultural entities like plants and livestock has also been recognized110. While so far there is no example of intentional agriculture warfare, the release of rabbit pox to eradicate the European rabbit in Australia demonstrates the potential consequences of a bioterror attack on a mammalian species111. Therefore, biodefense research into biological attacks on agricultural targets has been funded by the US Defense Advanced Research Projects Agency (DARPA)112 but has not been received without controversy.
Some critics of biodefense funding argue that biodefense funds would be better spent targeting chronic infectious diseases and other public health threats. While these issues are certainly more imminent given the sporadic and uncertain nature of bioterrorism and emerging infectious disease outbreaks, we feel that complete diversion of biodefense funding would be remiss, given the enormity of the threat posed by bioterror-related agents. Additionally, if biodefense funding was diverted, there is no guarantee as to where it would end up. The benefits of biodefense research are wide reaching and help improve global biosecurity for infectious disease outbreaks of all kinds.
Another frequently cited argument against biodefense research is the risk of studying bioterror-related pathogens characterized by the potential for accidental or intentional release of these dangerous pathogens or even more lethal versions engineered in the lab. Funds allocated for classified biodefense research113,114 sparked controversy complicating funding efforts and public perception of biodefense research. While many bioterror-related pathogens must be studied in BSL-3 or 4 containment facilities, laboratory failures are certainly possible115. “Gain-of-function” (GOF) research is a topic of tenuous discussion among the scientific community. Biodefense research efforts have encompassed these sometimes-risky experiments and have led to growing oversight of applicable US federally-funded research which is controversial in its own right116. Even among biosecurity experts there is no consensus as to where the “red line” is for GOF research117. The GOF issue is more encompassing than biodefense-related research alone, impacting work on many infectious diseases. The entire enterprise of biodefense (and research on any dangerous pathogen) can be considered an exercise in cost-benefit analysis. With proper resources and oversight, biodefense and infectious disease research can continue to provide important advancements without posing a disproportionate risk. Specifically, we feel that GOF research should continue to be scrutinized and balanced based on appropriate risk analysis.
Looking toward the future
The modern legacy of post-2001 biodefense research is an asset now and will be in the future. Ongoing biodefense accomplishments will likely be affected by a number of factors, funding being an important one. The focus of future biodefense work will likely be broad, yet in an increasingly interconnected world, several concepts appear to be very important for future success. These concepts include 1) cyber bioterrorism, 2) international cooperation, and 3) embracing technology. The growth of technology undoubtably aided in the success of past biodefense research efforts. A concept proposed by Bernard, et al as a “cyber biowarfare framework”7 outlines the use of disinformation campaigns and cyber technologies to affect public health, even in the absence of a tangible biological weapon or agent of bioterror. As previously mentioned, the perceived threat of bioterrorism may be a large enough stimulus to have the desired effect of a true bioterrorism event, particularly in the case of a concurrent natural outbreak. The ongoing COVID-19 pandemic is a poignant example of this classification in action. The onslaught of disinformation and misinformation surrounding the pandemic has been amplified by access to social media. COVID-19 has been weaponized in a virtual sense, as fodder for false yet widely circulated theories claiming that SARS-CoV-2 was a biological weapon deployed by a variety of countries, depending on the target of the disinformation campaign7 or the 5G origin theory, leading to the destruction of 5G cell phone towers in the United Kingdom118. These campaigns are thought to have been created with the intent of increasing ongoing public health crises in target countries through the rise of distrust in public health responses, healthcare systems, and government officials; this strategy has been remarkably effective119. The intent of disinformation campaigns is typically to achieve financial gain, political gain, and simply to manipulate the masses for experimental purposes120. These motivations are strikingly similar to that of bioterrorism. The damage that can be conferred as a result of these campaigns are well documented not only in the case of the ongoing pandemic but also in the case of measles and anti-vaccination sentiment121,122. As illustrated by the COVID-19 pandemic, disinformation and misinformation can lead to widespread resistance surrounding basic public health measures (eg, mask-wearing and social distancing), public distrust, and hate crimes/acts of violence against targeted groups (eg, sentiments and acts against Asian-American and Pacific Islanders during COVID in the US123). These powerful new drivers of public health and epidemic responsiveness emphasize the need for continued research into biodefense and epidemic preparedness alongside the development of strategies to combat this cyber biowarfare framework.
International cooperation and collaboration is perhaps one of the most effective biodefense strategies. In 2017, Portugal conducted a biopreparedness training exercise with both national and international participation called CELULEX17. This exercise emphasized the importance of such drills to bolster international responsiveness efforts. The WHO and United Nations released a 2017 report outlining scenario-based gap analysis in response to chemical and/or biological attacks. Again, the report concluded that international and interagency collaboration is extremely difficult yet imperative for a successful response to a bioterror event. Advances in biosurveillance, particularly digital strategies that are not confined by national borders, have proven to be extremely effective in pandemic responsiveness and transparency. These technologies have been achieved due to biodefense funding and research and will likely continue to be improved, contributing to global biosecurity. Just as next-generation mRNA vaccines have aided in the fight against SARS-CoV-2, these promising technologies can continue to be embraced leading to future advancements in biodefense. Importantly, evaluation of existing biodefense capabilities will help us to prepare for potential future infectious disease and bioterror events. Highlighted events occurring in the past 20 years such as the EBOV epidemic and COVID-19 pandemic have allowed for many recent biodefense developments to be evaluated. While some newer advancements displayed remarkable utility such as rapid vaccine development platforms124, digital biosurveillance tools, and biocontainment facilities, others missed the mark and likely need significant improvement such as more antiquated biosurveillance techniques and the US’s SNS. Indeed, these advances are not airtight, and many improvements are yet to be made. Certainly, there is much work to be done to improve global biosecurity in the future, a task that will likely be perpetual.
Conclusion
Perhaps one of the strongest arguments in support of biodefense research is the multifaceted impact it has and can have on general infectious disease control. As demonstrated by the recent EBOV epidemic and COVID-19 pandemics, emerging and re-emerging infectious diseases are largely ignored outside of the biodefense funding arena. Biodefense funding impacted the trajectory of these events and likely mitigated significant morbidity, mortality, and economic losses.
Although the documented bioterrorism events of the past fifty years have caused morbidity, mortality, economic losses, and detriment to the public morale of targeted populations and beyond, a large-scale epidemic resulting from an act of bioterrorism has not been experienced in modern times. In the absence of a bioterror event, it is difficult to quantify the practical impact that biodefense funding has had on biosecurity and preparedness. Biosecurity evaluation tools such as the Global Health Security Index and the WHO’s Joint External Evaluation exist but have not been validated and have not proven to be useful in the face of real infectious events125. Estimated health consequences associated with simulated bioterrorism events are variable but generally suggest that these events could cause thousands of immediate deaths. The ongoing COVID-19 pandemic poignantly illustrates the enormous costs associated with an outbreak of a newly emerging, transmissible disease. Death tolls of this pathogen are still rising but have breached 3,750,000 lives. This sobering statistic emphasizes the reality that outbreaks of infectious diseases, whether naturally occurring or intentional, can cause enormous destruction. The potential devastation associated with future outbreaks, particularly those caused by bioterrorism, is especially sobering in the context of COVID-19’s effects. In an additive fashion, modern communicative technology (eg, social media) and disinformation campaigns pose serious threats to worldwide biosafety and biosecurity. The past two decades of focused biodefense research and preparedness are particularly relevant in this milieu. Biodefense-related research efforts have yielded many advancements and have contributed to the preemptive response to natural events such as the EBOV epidemic and the COVID-19 pandemic, which has likely saved many lives. Although much of the biodefense funding and resulting advancements originated in the US, global cooperation and efforts are necessary for optimal global biosecurity.
Advancements in biodefense have not addressed all concerns and needs but due to the highly nebulous nature of bioterror threats, a perfect system is unachievable. An argument may be made that adequate, tangible progress has not been made in the wake of two decades of biodefense funding, but the accomplishments outlined above challenge this assertion (Figure 1, Panels 1–3). With logical and targeted funding, biodefense-related research has contributed to advancements in protection against bioterrorism and related agents, as demonstrated by advancements in vaccines, therapeutics, preparedness, research facilities and infrastructure. However, further funding and support is needed to bolster global and local biosecurity. With new scientific technology and rapid advancements across many fields, future biodefense research should focus on modernized research and technology, as some past programs have seemingly neglected that. Like those deployed for COIVD-19, next generation diagnostics, therapeutics, and vaccines should be in the forefront of biodefense research objectives. Local and global public health infrastructure and communications also stand out as areas in need of significant improvement. Compared to an estimated $1 trillion plus cost of a bioterror event126and combined with tangible advancements as a result of relevant funding, biodefense funding efforts seem to be worthwhile, both in theory and in practice. We feel that biodefense research is essential to the safety and wellbeing of the global population, and historical targeted funding was a successful venture. Clearly, biodefense funding has assisted in the response to natural outbreaks of both newly emerging and reemerging pathogens, as demonstrated by the COVID-19 pandemic. Biodefense research is not a singular pursuit; it has the potential to impact many facets of infectious disease and public health and should not be abandoned127.
Figure 1. Visual Representation of the Legacy of Modern Biodefense Funding.
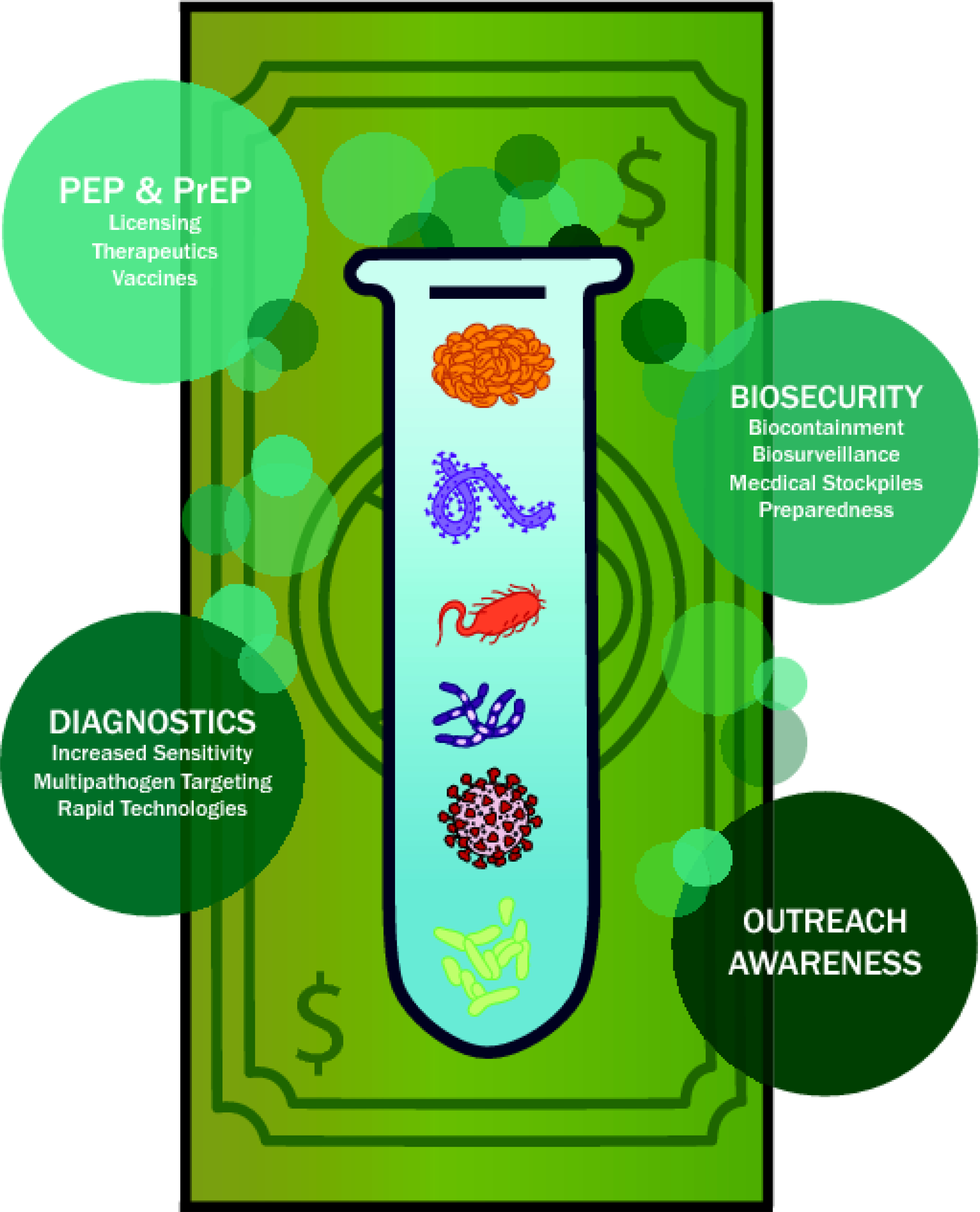
Significant advancements yielded by biodefense funding in a variety of scientific areas are listed. Key bioterror-related pathogens are depicted. PEP post-exposure prophylaxis; PrEP pre-exposure prophylaxis.
Acknowledgements
We thank Amanda Zito and Hazer Novich for assistance with figure production. This work was funded by the Intramural Research Program, NIAID, NIH.
Footnotes
Declaration of interests
The authors declare no conflicts of interest.
Search Strategy and Selection criteria
References for this review were identified through a search of PubMed and Google Scholar for articles published until May 26th 2021 by use of the terms “agriculture attack”, “biodefense”, “bioterror”, “biological warfare”, “biological weapon”, “classified research”, “anthrax”, and “Ebola virus”. Other relevant references were identified from key online resources (eg, WHO, CDC, FDA). Only articles published in English were included.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sell TK, Watson M. Federal agency biodefense funding, FY2013-FY2014. Biosecur Bioterror 2013; 11(3): 196–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price B, Price R. Chapter 59 - Terrorism and Warfare (Chemical, Biological, and Radioactive and Nuclear). In: Wexler P, Gilbert SG, Hakkinen PJ, Mohapatra A, eds. Information Resources in Toxicology (Fourth Edition). San Diego: Academic Press; 2009: 485–96. [Google Scholar]
- 3.Carus WS. The History of Biological Weapons Use: What We Know and What We Don’t. Health Secur 2015; 13(4): 219–55. [DOI] [PubMed] [Google Scholar]
- 4.Barras V, Greub G. History of biological warfare and bioterrorism. Clin Microbiol Infect 2014; 20(6): 497–502. [DOI] [PubMed] [Google Scholar]
- 5.Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM Jr. Biological warfare. A historical perspective. Jama 1997; 278(5): 412–7. [PubMed] [Google Scholar]
- 6.Patterson KB, Runge T. Smallpox and the Native American. Am J Med Sci 2002; 323(4): 216–22. [DOI] [PubMed] [Google Scholar]
- 7.Bernard R, Bowsher G, Sullivan R, Gibson-Fall F. Disinformation and Epidemics: Anticipating the Next Phase of Biowarfare. Health Security 2020; 19(1): 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frischknecht F The history of biological warfare. Human experimentation, modern nightmares and lone madmen in the twentieth century. EMBO Rep 2003; 4 Spec No(Suppl 1): S47–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuzmukhamedov B Convention on the Prohibition, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction. 2021. https://legal.un.org/avl2021).
- 10.Zilinskas MLRA. The Soviet Biological Weapons Program: A History. Cambridge, MA: Harvard University Press; 2012. [Google Scholar]
- 11.Zilinskas RA. Iraq’s biological weapons. The past as future? Jama 1997; 278(5): 418–24. [PubMed] [Google Scholar]
- 12.Oliveira M, Mason-Buck G, Ballard D, Branicki W, Amorim A. Biowarfare, bioterrorism and biocrime: A historical overview on microbial harmful applications. Forensic Sci Int 2020; 314: 110366–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedel S Biological warfare and bioterrorism: a historical review. Proc (Bayl Univ Med Cent) 2004; 17(4): 400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Brewer MB. What Does It Mean to Be an American? Patriotism, Nationalism, and American Identity after 9/11. Political Psychology 2004; 25(5): 727–39. [Google Scholar]
- 15.Bloch-Elkon Y TRENDS—PUBLIC PERCEPTIONS AND THE THREAT OF INTERNATIONAL TERRORISM AFTER 9/11. The Public Opinion Quarterly 2011; 75(2): 366–92. [Google Scholar]
- 16.McCarty A Changes in U.S. Biosecurity Following the 2001 Anthrax Attacks. J Bioterror Biodef 2018; 9(163). [Google Scholar]
- 17.Gursky E, Inglesby TV, O’Toole T. Anthrax 2001: observations on the medical and public health response. Biosecur Bioterror 2003; 1(2): 97–110. [DOI] [PubMed] [Google Scholar]
- 18.Frank E Funding the public health response to terrorism. BMJ 2005; 331(7516): 526–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CIDRAP. HHS to release $200 million in bioterrorism preparedness funds to states28 January 2002, 2002. https://www.cidrap.umn.edu/news-perspective/2002/01/hhs-release-200-million-bioterrorism-preparedness-funds-states (accessed 28 April 2021).
- 20.National Research Council. Overview of the BioWatch Program. Washington DC: National Academies Press; 2013. [Google Scholar]
- 21.Watson CR, Watson M, Sell TK. Public Health Preparedness Funding: Key Programs and Trends From 2001 to 2017. Am J Public Health 2017; 107(S2): S165–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell PK. Project BioShield: What It Is, Why It Is Needed, and Its Accomplishments So Far. Clinical Infectious Diseases 2007; 45(Supplement_1): S68–S72. [DOI] [PubMed] [Google Scholar]
- 23.Trull MC, du Laney TV, Dibner MD. Turning biodefense dollars into products. Nature Biotechnology 2007; 25(2): 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuler A Billions for biodefense: federal agency biodefense funding, FY2001-FY2005. Biosecur Bioterror 2004; 2(2): 86–96. [DOI] [PubMed] [Google Scholar]
- 25.Biodefense Market Size, Share, Growth, Trends, Company Analysis, Regional Insights and Forecast 2020 – 2027, 2020.
- 26.Noah DL, Huebner KD, Darling RG, Waeckerle JF. The history and threat of biological warfare and terrorism. Emerg Med Clin North Am 2002; 20(2): 255–71. [DOI] [PubMed] [Google Scholar]
- 27.Program FSA. Select Agents and Toxins. 2021. https://www.selectagents.gov (accessed 26 May 2021).
- 28.Eureopean Medicines Agency. Biological and chemical threats. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/biological-chemical-threats (accessed 26 May 2021).
- 29.World Health Organization. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug resistant bacterial infections, including tuberculosis, 2017.
- 30.Banada PP, Deshpande S, Russo R, et al. Rapid Detection of Bacillus anthracis Bloodstream Infections by Use of a Novel Assay in the GeneXpert System. J Clin Microbiol 2017; 55(10): 2964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentahir M, Ambroise J, Delcorps C, Pilo P, Gala JL. Sensitive and Specific Recombinase Polymerase Amplification Assays for Fast Screening, Detection, and Identification of Bacillus anthracis in a Field Setting. Appl Environ Microbiol 2018; 84(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirunavukkarasu N, Johnson E, Pillai S, et al. Botulinum Neurotoxin Detection Methods for Public Health Response and Surveillance. Front Bioeng Biotechnol 2018; 6: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalb SR, Moura H, Boyer AE, McWilliams LG, Pirkle JL, Barr JR. The use of Endopep-MS for the detection of botulinum toxins A, B, E, and F in serum and stool samples. Anal Biochem 2006; 351(1): 84–92. [DOI] [PubMed] [Google Scholar]
- 34.Mechaly A, Vitner E, Levy H, et al. Simultaneous Immunodetection of Anthrax, Plague, and Tularemia from Blood Cultures by Use of Multiplexed Suspension Arrays. J Clin Microbiol 2018; 56(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueroa DM, Kuisma E, Matson MJ, et al. Development and validation of portable, field-deployable Ebola virus point-of-encounter diagnostic assay for wildlife surveillance. One Health Outlook 2021; 3(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Food and Drug Administration. Ebola Preparedness and Response Updates from FDA. 2021. https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/ebola-preparedness-and-response-updates-fda (accessed 26 May 2021).
- 37.Ayouba A, Toure A, Butel C, et al. Development of a Sensitive and Specific Serological Assay Based on Luminex Technology for Detection of Antibodies to Zaire Ebola Virus. J Clin Microbiol 2017; 55(1): 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn-Yoon S, DeCory TR, Durst RA. Ganglioside-liposome immunoassay for the detection of botulinum toxin. Anal Bioanal Chem 2004; 378(1): 68–75. [DOI] [PubMed] [Google Scholar]
- 39.Longstreth J, Skiadopoulos MH, Hopkins RJ. Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: the first vaccine licensed using the FDA animal rule. Expert Rev Vaccines 2016; 15(12): 1467–79. [DOI] [PubMed] [Google Scholar]
- 40.Kachura MA, Hickle C, Kell SA, et al. A CpG-Ficoll Nanoparticle Adjuvant for Anthrax Protective Antigen Enhances Immunogenicity and Provides Single-Immunization Protection against Inhaled Anthrax in Monkeys. J Immunol 2016; 196(1): 284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins RJ, Kalsi G, Montalvo-Lugo VM, et al. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine 2016; 34(18): 2096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jelinski J, Terwilliger A, Green S, Maresso A. Progress towards the Development of a NEAT Vaccine for Anthrax II: Immunogen Specificity and Alum Effectiveness in an Inhalational Model. Infection and Immunity 2020; 88(8): e00082–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell JD, Clement KH, Wasserman SS, Donegan S, Chrisley L, Kotloff KL. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum Vaccin 2007; 3(5): 205–11. [DOI] [PubMed] [Google Scholar]
- 44.Chitlaru T, Israeli M, Rotem S, et al. A novel live attenuated anthrax spore vaccine based on an acapsular Bacillus anthracis Sterne strain with mutations in the htrA, lef and cya genes. Vaccine 2017; 35(44): 6030–40. [DOI] [PubMed] [Google Scholar]
- 45.Arevalo MT, Li J, Diaz-Arevalo D, et al. A dual purpose universal influenza vaccine candidate confers protective immunity against anthrax. Immunology 2017; 150(3): 276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao P, Mahalingam M, Zhu J, et al. A Bivalent Anthrax-Plague Vaccine That Can Protect against Two Tier-1 Bioterror Pathogens, Bacillus anthracis and Yersinia pestis. Front Immunol 2017; 8: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiffer JM, McNeil MM, Quinn CP. Recent developments in the understanding and use of anthrax vaccine adsorbed: achieving more with less. Expert Rev Vaccines 2016; 15(9): 1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark A, Wolfe DN. Current State of Anthrax Vaccines and Key R&D Gaps Moving Forward. Microorganisms 2020; 8(5): 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilgore PB, Sha J, Andersson JA, Motin VL, Chopra AK. A new generation needle- and adjuvant-free trivalent plague vaccine utilizing adenovirus-5 nanoparticle platform. NPJ Vaccines 2021; 6(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price JL, Manetz TS, Shearer JD, House RV. Preclinical safety assessment of a recombinant plague vaccine (rF1V). Int J Toxicol 2013; 32(5): 327–35. [DOI] [PubMed] [Google Scholar]
- 51.Rusnak JM, Smith LA. Botulinum neurotoxin vaccines: Past history and recent developments. Hum Vaccin 2009; 5(12): 794–805. [DOI] [PubMed] [Google Scholar]
- 52.Sundeen G, Barbieri JT. Vaccines against Botulism. Toxins (Basel) 2017; 9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng Y, Zhang Y, Mitchell WJ, Zhang G. Development of a lipopolysaccharide-targeted peptide mimic vaccine against Q fever. J Immunol 2012; 189(10): 4909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long CM, Beare PA, Cockrell DC, et al. Contributions of lipopolysaccharide and the type IVB secretion system to Coxiella burnetii vaccine efficacy and reactogenicity. NPJ Vaccines 2021; 6(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilkes AP, Albin TJ, Manna S, et al. Tuning Subunit Vaccines with Novel TLR Triagonist Adjuvants to Generate Protective Immune Responses against Coxiella burnetii. J Immunol 2020; 204(3): 611–21. [DOI] [PubMed] [Google Scholar]
- 56.Plummer FA, Jones SM. The story of Canada’s Ebola vaccine. CMAJ 2017; 189(43): E1326–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quirk EJ, Gheorghe A, Hauck K. A systematic examination of international funding flows for Ebola virus and Zika virus outbreaks 2014–2019: donors, recipients and funding purposes. BMJ Glob Health 2021; 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coltart CE, Lindsey B, Ghinai I, Johnson AM, Heymann DL. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Philos Trans R Soc Lond B Biol Sci 2017; 372(1721). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauss S Ebola research fueled by bioterrorism threat. CMAJ 2014; 186(16): 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feldmann H, Feldmann F, Marzi A. Ebola: Lessons on Vaccine Development. Annu Rev Microbiol 2018; 72: 423–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matz KM, Marzi A, Feldmann H. Ebola vaccine trials: progress in vaccine safety and immunogenicity. Expert Rev Vaccines 2019; 18(12): 1229–42. [DOI] [PubMed] [Google Scholar]
- 62.Marzi A, Mire CE. Current Ebola Virus Vaccine Progress. BioDrugs 2019; 33(1): 9–14. [DOI] [PubMed] [Google Scholar]
- 63.World HEalth Organization. Major milestone for WHO-supported Ebola vaccine. 2019. [Google Scholar]
- 64.Food and Drug Administration. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. 2019. [Google Scholar]
- 65.World Health Organization. Four countries in the African region license vaccine in milestone for Ebola prevention. WHO; 2020. [Google Scholar]
- 66.Snow DM, Riling K, Kimbler A, et al. Safety and Pharmacokinetics of a Four Monoclonal Antibody Combination Against Botulinum C and D Neurotoxins. Antimicrob Agents Chemother 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Food and Drug Administration. FDA approves new treatment for inhalation anthrax. FDA; 2016. [Google Scholar]
- 68.Tsai CW, Morris S. Approval of Raxibacumab for the Treatment of Inhalation Anthrax Under the US Food and Drug Administration “Animal Rule”. Front Microbiol 2015; 6: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong G, Kobinger GP. Backs against the wall: novel and existing strategies used during the 2014–2015 Ebola virus outbreak. Clin Microbiol Rev 2015; 28(3): 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hansen F, Feldmann H, Jarvis MA. Targeting Ebola virus replication through pharmaceutical intervention. Expert Opin Investig Drugs 2021; 30(3): 201–26. [DOI] [PubMed] [Google Scholar]
- 71.O’Donnell KL, Marzi A. Immunotherapeutics for Ebola Virus Disease: Hope on the Horizon. Biologics 2021; 15: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lonati D, Schicchi A, Crevani M, et al. Foodborne Botulism: Clinical Diagnosis and Medical Treatment. Toxins (Basel) 2020; 12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni SA, Brady MF. Botulism Antitoxin. StatPearls. Treasure Island (FL); 2021. [PubMed] [Google Scholar]
- 74.Kodihalli S, Emanuel A, Takla T, et al. Therapeutic efficacy of equine botulism antitoxin in Rhesus macaques. PLoS One 2017; 12(11): e0186892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radosavljevic V, Belojevic G. A new model of bioterrorism risk assessment. Biosecur Bioterror 2009; 7(4): 443–51. [DOI] [PubMed] [Google Scholar]
- 76.Pappas G, Panagopoulou P, Akritidis N. Reclassifying bioterrorism risk: are we preparing for the proper pathogens? J Infect Public Health 2009; 2(2): 55–61. [DOI] [PubMed] [Google Scholar]
- 77.Bullock JA, Haddow GD, Coppola DP. 9 - All-Hazards Emergency Response and Recovery. In: Bullock JA, Haddow GD, Coppola DP, eds. Homeland Security (Second Edition): Butterworth-Heinemann; 2018: 227–90. [Google Scholar]
- 78.Fitzpatrick S Why the Strategic National Stockpile isn’t meant to solve a crisis like coronavirus. 2020. https://www.nbcnews.com/health/health-care/why-strategic-national-stockpile-isn-t-meant-solve-crisis-coronavirus-n1170376 (accessed April 22 2021). [Google Scholar]
- 79.Chevalier MS, Chung W, Smith J, et al. Ebola virus disease cluster in the United States--Dallas County, Texas, 2014. MMWR Morb Mortal Wkly Rep 2014; 63(46): 1087–8. [PMC free article] [PubMed] [Google Scholar]
- 80.Musso D, Ko AI, Baud D. Zika Virus Infection - After the Pandemic. N Engl J Med 2019; 381(15): 1444–57. [DOI] [PubMed] [Google Scholar]
- 81.Handfield R, Finkenstadt DJ, Schneller ES, Godfrey AB, Guinto P. A Commons for a Supply Chain in the Post-COVID-19 Era: The Case for a Reformed Strategic National Stockpile. The Milbank Quarterly 2020; 98(4): 1058–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rich Branson M, Jeffrey R. Dichter M, Henry Feldman M, et al. The US Strategic National Stockpile Ventilators in Coronavirus Disease 2019. Critical Care: Special Features 2021; 159(2): 634–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenfieldboyce N Why Even A Huge Medical Stockpile Will Be Of Limited Use Against COVID-1914 March 2020, 2020. https://www.npr.org/sections/health-shots/2020/03/14/814121891/why-even-a-huge-medical-stockpile-will-be-of-limited-use-against-covid-19 (accessed.
- 84.World Health Organization. UNICEF, WHO, IFRC and MSF announce the establishment of a global Ebola vaccine stockpile. New York/Geneva; 2021. [Google Scholar]
- 85.Mounier-Jack S, Jas R, Coker R. Progress and shortcomings in European national strategic plans for pandemic influenza. Bull World Health Organ 2007; 85(12): 923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naylor BUS Bioterrorism-Detection Program Is Unreliable, Report Finds23 November 2015, 2015. https://www.npr.org/sections/thetwo-way/2015/11/23/457101931/u-s-bioterrorism-detection-program-is-unreliable-report-finds (accessed.
- 87.Madoff LC. ProMED-mail: an early warning system for emerging diseases. Clin Infect Dis 2004; 39(2): 227–32. [DOI] [PubMed] [Google Scholar]
- 88.Al-Zinati M, Alrashdan R, Al-Duwairi B, Aloqaily M. A re-organizing biosurveillance framework based on fog and mobile edge computing. Multimed Tools Appl 2020: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bharti N, Tatem AJ, Ferrari MJ, Grais RF, Djibo A, Grenfell BT. Explaining seasonal fluctuations of measles in Niger using nighttime lights imagery. Science 2011; 334(6061): 1424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeitvogel K Fogarty’s RAPIDD program has catalyzed the field of infectious disease modeling. Global Health Matters newsletter, 2016. https://www.fic.nih.gov/News/GlobalHealthMatters/may-june-2016/Pages/rapidd-infectious-disease-modeling.aspx (accessed 26 May 2021).
- 91.Nelson MI, Lloyd-Smith JO, Simonsen L, et al. Fogarty International Center collaborative networks in infectious disease modeling: Lessons learnt in research and capacity building. Epidemics 2019; 26: 116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shurtleff AC, Garza N, Lackemeyer M, et al. The impact of regulations, safety considerations and physical limitations on research progress at maximum biocontainment. Viruses 2012; 4(12): 3932–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Gong Y, Wang C, et al. Rapid deployment of a mobile biosafety level-3 laboratory in Sierra Leone during the 2014 Ebola virus epidemic. PLoS Negl Trop Dis 2017; 11(5): e0005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cieslak TJ, Kortepeter MG. A Brief History of Biocontainment. Curr Treat Options Infect Dis 2016; 8(4): 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flinn JB, Hynes NA, Sauer LM, Maragakis LL, Garibaldi BT. The role of dedicated biocontainment patient care units in preparing for COVID-19 and other infectious disease outbreaks. Infect Control Hosp Epidemiol 2021; 42(2): 208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kratochvil CJ, Evans L, Ribner BS, et al. The National Ebola Training and Education Center: Preparing the United States for Ebola and Other Special Pathogens. Health Secur 2017; 15(3): 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med 2003; 349(25): 2431–41. [DOI] [PubMed] [Google Scholar]
- 98.Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12(9): 687–95. [DOI] [PubMed] [Google Scholar]
- 99.Jacob ST, Crozier I, Fischer WA 2nd, et al. Ebola virus disease. Nat Rev Dis Primers 2020; 6(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andrew SA, Arlikatti S, Chatterjee V, Ismayilov O. Ebola crisis response in the USA: Communication management and SOPs. Int J Disaster Risk Reduct 2018; 31: 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Risks TCoS. Key U.S. Initiatives for Addressing Biological Threats. 2021. [Google Scholar]
- 102.Kim SS, Oh DW, Jo HJ, Chu C. Introduction of the Republic of Korea-the United States of America’s Joint Exercise Against Biothreats in 2013: Able Response 13. Osong Public Health Res Perspect 2013; 4(5): 285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tak S, Jareb A, Choi S, Sikes M, Choi YH, Boo HW. Enhancing ‘Whole-of-Government’ Response to Biological Events in Korea: Able Response 2014. Osong Public Health Res Perspect 2018; 9(1): 32–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeong E, Hagose M, Jung H, Ki M, Flahault A. Understanding South Korea’s Response to the COVID-19 Outbreak: A Real-Time Analysis. Int J Environ Res Public Health 2020; 17(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson M, Mckee M, Mossialos E. Covid-19 exposes weaknesses in European response to outbreaks. BMJ 2020; 368: m1075. [DOI] [PubMed] [Google Scholar]
- 106.Haffajee RL, Mello MM. Thinking Globally, Acting Locally - The U.S. Response to Covid-19. N Engl J Med 2020; 382(22): e75. [DOI] [PubMed] [Google Scholar]
- 107.Alcabes P The Bioterrorism Scare. The American Scholar 2004; 73(2): 35–45. [Google Scholar]
- 108.Fauci AS, Zerhouni EA. NIH Response to Open Letter. Science 2005; 308(5718): 49. [DOI] [PubMed] [Google Scholar]
- 109.Matheny J, Mair M, Mulcahy A, Smith BT. Incentives for biodefense countermeasure development. Biosecur Bioterror 2007; 5(3): 228–38. [DOI] [PubMed] [Google Scholar]
- 110.Wheelis M, Casagrande R, Madden LV. Biological attack on agriculture: Low-tech, high-impact bioterrorism. Bioscience 2002; 52(7): 569–76. [Google Scholar]
- 111.Goldfarb B A virus is taming Australia’s bunny menace, and giving endangered species new life17 February 2016, 2016. https://www.sciencemag.org/news/2016/02/virus-taming-australia-s-bunny-menace-and-giving-endangered-species-new-life (accessed 28 April 2021).
- 112.Reeves RG, Voeneky S, Caetano-Anolles D, Beck F, Boete C. Agricultural research, or a new bioweapon system? Science 2018; 362(6410): 35–7. [DOI] [PubMed] [Google Scholar]
- 113.Kahn LH. Biodefense research: can secrecy and safety coexist? Biosecur Bioterror 2004; 2(2): 81–5. [DOI] [PubMed] [Google Scholar]
- 114.Atlas RM. Biodefense research: an emerging conundrum. Curr Opin Biotechnol 2005; 16(3): 239–42. [DOI] [PubMed] [Google Scholar]
- 115.Patterson A, Fennington K, Bayha R, et al. Biocontainment laboratory risk assessment: perspectives and considerations. Pathogens and Disease 2014; 71(2): 102–8. [DOI] [PubMed] [Google Scholar]
- 116.Subbaraman N US officials revisit rules for disclosing risky disease experiments. Nature 2020. [DOI] [PubMed] [Google Scholar]
- 117.Boddie C, Watson M, Ackerman G, Gronvall GK. BIOSECURITY. Assessing the bioweapons threat. Science 2015; 349(6250): 792–3. [DOI] [PubMed] [Google Scholar]
- 118.Ahmed W, Vidal-Alaball J, Downing J, López Seguí F. COVID-19 and the 5G Conspiracy Theory: Social Network Analysis of Twitter Data. Journal of Medical Internet Research 2020; 22(5): e19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tagliabue F, Galassi L, Mariani P. The “Pandemic” of Disinformation in COVID-19. SN Compr Clin Med 2020: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.The Lancet Infectious Diseases. The COVID-19 infodemic. The Lancet Infectious Diseases; 2020; 20(8): 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benecke O, DeYoung SE. Anti-Vaccine Decision-Making and Measles Resurgence in the United States. Glob Pediatr Health 2019; 6: 2333794X19862949–2333794X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hussain A, Ali S, Ahmed M, Hussain S. The Anti-vaccination Movement: A Regression in Modern Medicine. Cureus 2018; 10(7): e2919-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Covid “hate crimes” against Asian Americans on rise. 2021. https://www.bbc.com/news/world-us-canada-56218684 (accessed April 19 2021).
- 124.Hodgson J The pandemic pipeline. Nat Biotechnol 2020; 38(5): 523–32. [DOI] [PubMed] [Google Scholar]
- 125.Haider N, Yavlinsky A, Chang Y-M, et al. The Global Health Security index and Joint External Evaluation score for health preparedness are not correlated with countries’ COVID-19 detection response time and mortality outcome. Epidemiology & Infection 2020; 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Biological Hafemeister D. and Weapons Chemical. Nuclear Proliferation and Terrorism in the Post-9/11 World. Cham: Springer International Publishing; 2016: 337–51. [Google Scholar]
- 127.Burnett JC, Panchal RG, Aman MJ, Bavari S. The rapidly advancing field of biodefense benefits many other, critical public health concerns. Discov Med 2005; 5(28): 371–7. [PubMed] [Google Scholar]
- 128.Sharma SK, Eblen BS, Bull RL, Burr DH, Whiting RC. Evaluation of lateral-flow Clostridium botulinum neurotoxin detection kits for food analysis. Appl Environ Microbiol 2005; 71(7): 3935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Omsland A, Cockrell DC, Howe D, et al. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc Natl Acad Sci U S A 2009; 106(11): 4430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hansen F, Jarvis MA, Feldmann H, Rosenke K. Lassa Virus Treatment Options. Microorganisms 2021; 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci 2020; 27(1): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai ST. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis 2005; 24(9): 583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oliveira M, Mason-Buck G, Ballard D, Branicki W, Amorim A. Biowarfare, bioterrorism and biocrime: A historical overview on microbial harmful applications. Forensic Sci Int 2020; 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Polyak CS, Macy JT, Irizarry-De La Cruz M, et al. Bioterrorism-related anthrax: international response by the Centers for Disease Control and Prevention. Emerging infectious diseases 2002; 8(10): 1056–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gable L, Hodge JG Jr. Public Health Law and Biological Terrorism. Beyond Anthrax 2008: 239–52. [Google Scholar]


