Presentation of the case
The coronavirus disease 2019 (COVID-19) pandemic is now established on the African continent, with cases rapidly increasing in Malawi (1742 confirmed cases and 19 deaths as of 5 July 20201). Clinicians require guidelines, deliverable in the Malawi context, to effectively and safely treat patients for the best possible outcome. In Malawi, key public messages around social distancing, hand washing and shielding for at-risk individuals have been widely distributed by the Ministry of Health. However, it has not been possible to implement strict lockdown measures in Malawi due to the risk of widespread economic disruption, hunger, worsened food insecurity, risk of violence and mass political rallies2. Testing rates are low such that the number of confirmed cases in Malawi is likely to significantly under-represent the actual number of cases. As the epidemic unfolds, it is vital that doctors implement standardised case management guidelines to improve survival for patients who require hospital admission. The majority of patients hospitalised with COVID-19 require medical-ward level care, including provision of adequate oxygen3. Increased oxygen provision has been a major focus of COVID-19 preparedness activities in Malawi4.
We report a case of a 46-year-old male who was admitted to Queen Elizabeth Central Hospital on 17 June 2020, complaining of exertional dyspnoea after a 10-day history of dry cough and fevers. Patient consent was obtained for this case report. Other than obesity, he had no other significant medical problems and was HIV seronegative. He was a lifelong non-smoker and consumed 30–40 units of alcohol per week. Shortness of breath had appeared over a period of 2 days accompanied by pleuritic chest pain upon coughing. There was no recent foreign travel or contact with COVID-19 suspects. He was strongly suspected of COVID-19 disease due to reported symptoms of fever, dry cough and worsening shortness of breath and was admitted directly to a hospital ward specifically created for the isolation and treatment of such patients.
Staff received the patient wearing appropriate personal protective equipment (PPE: gloves, gown, visor and N95 mask) in line with international guidance5. He was transported into an isolation ward in the hospital by a family member and walked into the bespoke high dependency area. This ward was designed specifically for COVID-19 care delivery including PPE donning and doffing areas and infection control and prevention movement and cleaning measures. Negative pressure facilities were not available. Subsequently, family members were not permitted to visit the patient. This area included provision of piped oxygen and continuous monitoring equipment. The patient was clearly dyspnoeic upon arrival with a respiratory rate of 30 breaths/min and unable to speak in full sentences; accessory muscle use was not observed. His oxygen saturations were immediately measured in advance of further medical history and examination and demonstrated SpO2 of 68% and heart rate (HR) 110 beats/min (bpm) without supplemental oxygen. The patient was immediately treated with oxygen (15 L non-rebreather reservoir bag FiO2 ∼0.6) with improvement in SpO2 to 92% and HR 98 bpm. The patient had a normal blood pressure, 138/86 mmHg. The patient was orientated in time and place and did not demonstrate signs of confusion during the admission. The patient was afebrile but reported subjective fevers prior to admission.
Discussion of the case
Doctors applied context-specific medical guidelines based on the most up-to-date evidence. Ward rounds and subsequent care were delivered with appropriate PPE provided to staff. The patient was treated with 6 mg intravenous (IV) dexamethasone once daily (OD)6 for 10 days, prophylactic subcutaneous low molecular weight heparin (enoxaparin 60 mg OD)7 and empirical antibiotics (ceftriaxone 2 g OD) because it was not possible to exclude a co-existing bacterial pneumonia. Antibiotic prescription was in line with World Health Organization (WHO) guidance for severe cases of confirmed COVID-198. Antibiotics were discontinued after 7 days. Doctors assessed the patient as euvolaemic at presentation, and throughout his admission the patient was able to eat and drink independently and renal function was normal such that IV fluids were not prescribed and he was not catheterised. Initial chest X-ray (Figure 1A) demonstrated diffuse ground glass opacities and consolidations in keeping with COVID-19 pneumonia9. A reverse transcriptase polymerase chain reaction (RT-qPCR) test for severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) the following morning confirmed COVID-19 diagnosis with probable high viral load (CT N1 26.0, N2 26.4 and RP 25). This test screens for the nucleocapside (N)-gene of SARS-CoV-2 cycle threshold (CT) and also human ribonuclease protein (RP) as an internal control to assess extraction quality. Results were reported to statutory national and local bodies in line with Malawi Ministry of Health guidance and contact tracing initiated by the local District Health Office. A SARS CoV-2 serology test was strongly positive with an optical density of 3.5 (5 times above cut-off value of seropositivity). The full blood count results (Table 1) showed typical severe COVID-19 profile10, a neutrophil-to-lymphocyte ratio (NLR) of 5.4 at 2 days post admission to hospital. An NLR of greater than 4 at admission is associated with more severe outcome and is a predictor of admission to the intensive care unit (ICU)11. The patient remained hypoxic for 4 days, requiring 15 L oxygen to maintain SpO2 at 88–92% but subjectively began to feel better. During this time the patient continued to receive prescribed medications and received high-quality nursing care from staff trained specifically to treat COVID-19 patients. This included chest physiotherapy manoeuvres, including deep breathing exercises (wearing a mask to reduce aerosols) and encouragement of self-proning according to UK Intensive Care Society Guidelines12. The patient tolerated self-proning well and was able to remain in prescribed positions for up to 2 hours at a time, in line with guideline recommendations. Staff observed that oxygen saturations improved upon proning by 2–3%. On day four the SpO2 worsened to 86% and the respiratory rate increased. Treatment dose of enoxaparin (60 mg twice daily) was empirically commenced (gold standard CT pulmonary angiography not possible) for presumptive pulmonary embolism. Plans for continuous positive airways pressure support and mechanical ventilation were prospectively discussed with the anaesthetic team but these modalities of care were not indicated during the clinical course.
Figure 1.
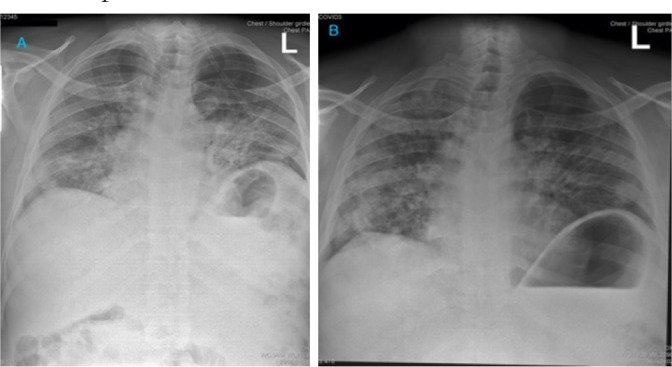
Paired chest X-ray findings on day 2 (A) and day 13 (B) in a patient admitted to hospital with severe COVID-19 infection. X-rays demonstrate diffuse bilateral alveolar shadowing and an incidental hiatus hernia.
Table 1.
Clinical laboratory blood results
| Normal range | Day 2 | Interpretation | Day 7 | Interpretation | |
| Full blood count | |||||
| Haemoglobin | 13.6–16.7 g/L | 16.2 | Normal | 15.6 | Normal |
| Haematocrit | 39.0–50.0 L/L | 47.8 | Normal | 47.1 | Normal |
| MCV | 82.0–98.0 fl | 95.4 | Normal | 96.6 | Normal |
| Platelets | 115–290 109/L | 447 | Abnormal | 499 | Abnormal |
| WBC | 2.8–7.2 109/L | 17.9 | Abnormal | 13.5 | Abnormal |
| Neutrophils | 0.9–4.2 109/L | 11.9 | Abnormal | 9.9 | Abnormal |
| Lymphocytes | 1.0–3.2 109/L | 2.2 | Normal | 2.2 | Normal |
| Monocytes | 0.15–0.58 109/L |
3.11 | Abnormal | 0.94 | Abnormal |
| Eosinophils | 0.02–0.79 109/L |
0.70 | Normal | 0.20 | Normal |
| Biochemistry | |||||
| Sodium | 126–145 mmol/L |
149 | Normal | 139 | Normal |
| Potassium | 3.5–4.3 mmol/L |
3.8 | Normal | 4.8 | Abnormal |
| Urea | 2.1–7.1 mmol/L |
8.4 | Abnormal | 6.9 | Normal |
| Creatinine | 53–115 µmol/L | 84 | Normal | 77 | Normal |
| Bilirubin (total) | 1.71–18.81 mg/dL |
9 | Normal | 5 | Normal |
| ALT | 8–32 IU/L | 63 | Abnormal | 87 | Abnormal |
| AST | 13–37 IU/L | 59 | Abnormal | 46 | Abnormal |
| GGT | 31–39 IU/L | 126 | Abnormal | 119 | Abnormal |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; MCV, mean corpuscular volume; WBC, white blood cell count.
The following day gas exchange had improved so oxygen was titrated to 10 L to achieve SpO2 94–96%, and the NLR had reduced to 4.5 (Table 1). The enoxaparin was reduced to a prophylaxis dose (40 mg OD) after clinical improvement was seen over 48 hours, in consideration of the long-term risk-benefit, given that a diagnosis of pulmonary embolism could not be confirmed. Subsequently, oxygen was titrated to no supplemental oxygen over the following week and the patient was discharged from hospital 10 days after admission (discharge saturation 91%). Discharge chest X-ray demonstrated partial resolution of findings (Figure 1B). The patient was advised to complete a period of self-isolation at home for 10 days after symptom onset plus at least 3 days without symptoms in line with WHO recommendations8, with ongoing pulmonary rehabilitation (telemedicine physiotherapy advice).
We present this case report as an example of successful management using standardised, evidence-based treatment guidelines to inform the future management of patients in the Malawi context. Successful management of severe hypoxic COVID-19 disease includes oxygen titrated to SpO2, self-proning to improve lung perfusion, dexamethasone (if not contraindicated), access to heparin and effective multidisciplinary teamwork.
References
- 1.Malawi Ministry of Health, author. COVID-19 National Information Dashboard. [cited 2020 Jun 30]. Available from: https://covid19.health.gov.mw/
- 2.Divala T, Burke RM, Ndeketa L, Corbett EL, MacPherson P. Africa faces difficult choices in responding to COVID-19. Lancet. 2020;395(10237):1611. doi: 10.1016/S0140-6736(20)31056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malikwa M. The Nation. 2020. Jun 21, Minster inaugurates oxygen plant at QECH. Available from: https://www.mwnation.com/minister-inaugurates-oxygen-plant-at-qech/ [Google Scholar]
- 5.Public Health England, author. COVID-19 personal protective equipment (PPE) [cited 2020 Jun 23]. Available from: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/covid-19-personal-protective-equipment-ppe.
- 6.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19 — Preliminary report. N Engl J Med. 2020 Jul 17; doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, author. Clinical management of COVID-19 interim guidance. [cited 2020 Jul 29]. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19.
- 9.Berlin DA, Gulick RM, Martinez FJ. Severe covid-19. N Engl J Med. 2020 May 15; doi: 10.1056/NEJMcp2009575. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 10.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccullo A, Borghetti A, Zileri Dal Verme L, Tosoni A, Lombardi F, Carcovich M, et al. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. 2020;56:106017. doi: 10.1016/j.ijantimicag.2020.106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bamford P, Bentley A, Dean J, Whitmore D, Wilson-Baig N. ICS guidance for prone positioning of the conscious COVID patient. 2020. [cited 2020 Jul 29]. Available from: https://icmanaesthesiacovid-19.org/news/ics-guidance-for-prone-positioning-of-the-conscious-covid-patient-2020.


