Abstract
Background & aims
Sarcopenia, a loss of muscle mass, quality and function, which is particularly evident in respiratory muscles, has been associated with many clinical adverse outcomes. In this study, we aimed at evaluating the role of reduced muscle mass and quality in predicting ventilation weaning, complications, length of intensive care unit (ICU) and of hospital stay and mortality in patients admitted to ICU for SARS-CoV-2-related pneumonia.
Methods
This was an observational study based on a review of medical records of all adult patients admitted to the ICU of a tertiary hospital in Milan and intubated for SARS-CoV-2-related pneumonia during the first wave of the COVID-19 pandemic. Muscle mass and quality measurement were retrieved from routine thoracic CT scans, when sections passing through the first, second or third lumbar vertebra were available.
Results
A total of 81 patients were enrolled. Muscle mass was associated with successful extubation (OR 1.02, 95% C.I. 1.00–1.03, p = 0.017), shorter ICU stay (OR 0.97, 95% C.I. 0.95–0.99, p = 0.03) and decreased hospital mortality (HR 0.98, 95% C.I. 0.96–0.99, p = 0.02). Muscle density was associated with successful extubation (OR 1.07, 95% C.I. 1.01–1.14; p = 0.02) and had an inverse association with the number of complications in ICU (Β −0.07, 95% C.I. −0.13 - −0.002, p = 0.03), length of hospitalization (Β −1.36, 95% C.I. −2.21 - −0.51, p = 0.002) and in-hospital mortality (HR 0.88, 95% C.I. 0.78–0.99, p = 0.046).
Conclusions
Leveraging routine CT imaging to measure muscle mass and quality might constitute a simple, inexpensive and powerful tool to predict survival and disease course in patients with COVID-19. Preserving muscle mass during hospitalisation might have an adjuvant role in facilitating remission from COVID-19.
Keywords: Muscle mass, Muscle quality, Intensive care unit, Ventilator weaning, COVID-19
1. Background
Severe acute respiratory virus coronavirus 2 (SARS-CoV-2)-related disease (COVID-19) is currently widely diffuse around the globe, posing a persisting and unprecedented challenge to national health care systems [1]. Although severe pulmonary involvement is relatively uncommon during the course of COVID-19 in the general population, patients requiring mechanical ventilation (MV) and intensive care unit (ICU) admission might still be numerically relevant since SARS-COV-2 is highly contagious and shows a disproportionately high virulence in selected vulnerable subjects, such as older people [2,3]. Identifying factors predicting delayed weaning from respiratory support and hospital complications would optimise patients’ management and reduce COVID-19-related mortality [4]. Unfortunately, classic predictors of extubation are poorly performant in older people [[4], [5], [6], [7]]. Age-related modifications of the respiratory function (e.g. reduction in lung elastic recoil, diminished chest wall compliance and decreased vital capacity) [8] and sarcopenia in respiratory muscles could account for an increased risk of extubation failure in older people [5,[9], [10], [11]].
Sarcopenia is a progressive and generalised skeletal muscle disorder characterized by a decrease of muscle quality, quantity and function [[12], [13], [14], [15], [16]]. This process is particularly evident in respiratory muscles [1,15] where it impairs the ability to produce appropriate tidal volumes [17] and to perform high force expulsive airway clearance manoeuvres [18]. Sarcopenia has been associated with many clinical adverse outcomes [13,14,[19], [20], [21]], however only one study has evaluated the association between reduced muscle mass and extubation failure so far. This study included only 45 old surgical patients [22]. Acute diseases can aggravate chronic sarcopenia and prompt an acute muscle insufficiency even in young and middle-aged people [13,23]. Acute sarcopenia has been demonstrated in COVID-19 [24] patients but no study has yet tested a potential association between muscle characteristics and extubation failure in COVID-19 patients.
The primary objective of this study was to evaluate the role of muscle mass and quality in predicting ventilation weaning in patients with COVID-19 pneumonia admitted to ICU. As secondary objectives, we tested the role of muscle mass and quality as predictors of ICU and hospital stay, complications in ICU, and mortality. Finally, we analysed whether obesity and frailty could affect the association between muscle characteristics and outcomes.
2. Materials and methods
In this observational study, we reviewed the medical records of all adult patients (≥18 years old) with COVID-19 pneumonia who were admitted to the intensive care unit (ICU) of the San Raffaele Hospital, Milan, Italy between 25th February2020 and 1stMay 2020. Exclusion criteria were: i) chest computed tomography (CT) scan images without sections passing through the lumbar vertebrae; ii) low CT scan quality or presence or artefacts iii) no treatment with MV. This study was part of the COVID-BioB protocol (NCT04318366) [25] and was approved by the local Ethical Review Board. A convenience sample size was used due to the emergency setting of the first wave of the COVID-19 pandemic. Post-hoc analyses were eventually performed to assess statistical power. Demographic data, comorbidities, medications, vital signs, laboratory values at hospital admission, anthropometric parameters, SARS-CoV-2 real time polymerase chain reaction (PCR) nasopharyngeal swab test results, complications, length of ICU and hospital stay, extubation failure/success, ICU readmission, tracheostomy, and mortality, were extracted from the electronic patient data management system and from medical records.
Muscle mass was derived from the muscle cross-sectional area (CSA). Since muscle mass is closely related to body size, the absolute level of muscle mass was adjusted for squared height generating a skeletal muscle index (SMI) in cm/m2 [2]. Muscle quality was assessed by measuring muscle density and inter muscular adipose tissue (IMAT) from CT images at the lumbar vertebral regions. Routine CT images obtained during hospitalization were retrieved from the hospital Picture Archiving and Communication System (PACS) and stored on a secure computer system. The scans were analyzed by two trained radiology residents (EDG, ADP) supervised by a senior radiologist (GC) unaware of patient outcomes, using Slice-O-matic version 5.0 software (TomoVision, Montreal, Quebec, Canada), as described previously [[26], [27], [28]]. Skeletal muscle and abdominal adipose tissue areas were quantified on single-slice CT scan at the level of the first, second or third lumbar vertebra (L1, L2, L3). When available, slices passing through L3 were preferentially chosen, since muscle CSA has an optimal correlation with the whole-body composition at that level [27]. Alternative levels (L1, L2) were used in all other cases, based on their high overall accuracy compared to L3-centred estimation of sarcopenia [[25], [28], [29], [30], [31], [32], [33], [34], [35]]. Specific tissue demarcation using predefined thresholds in Hounsfield unit (HU), that is a radiological scale describing the density of tissues on CT scans, was performed by image analysis software. HU thresholds were set to −29 to +150 for muscle, −190 to −30 for intermuscular adipose tissue (IMAT) and subcutaneous adipose tissue, and −150 to −50 for visceral adipose tissue, according to the literature [36]. Tissue boundaries were corrected manually as needed. Cross-sectional areas were computed automatically by summing tissue pixels and multiplying by pixel surface area. Reduced muscle mass was defined using predetermined sex-specific and vertebral-level specific cut-off values [28]. Skeletal muscle density (SMD) was assessed by the mean muscle attenuation of all muscle visible in the CT slice analyzed, measured in HU [37]. Lower mean muscle attenuation indicates less dense muscle tissue with more lipid infiltration, e.g. lower SMD, while a higher mean muscle attenuation indicates denser muscle tissue with less lipid infiltration, e.g. higher SMD [37]. IMAT was assessed by identifying all visible adipose tissue within muscle fascia in cm2 [28]. Cut-offs for skeletal muscle radiation attenuation were defined according to vertebra levels according to literature data [28].
To summarise and measure patient complexity, we also created a Frailty Index (FI) by using the standardized criteria described by Searle et al. [38]. The variables used to generate the FI encompassed comorbidities, baseline assessment data and baseline blood test results at hospital admission. Each deficit included in the FI was scored 0 when absent and 1 when present. Thirty variables were used to compute the FI thus conferring it a sufficient robustness [38]. FI scores above 0.25 were classified as indicative of frailty [38].
2.1. Statistical analyses
Continuous variables were described with mean and standard deviation (SD) when normally distributed or with median and interquartile range [IQR] when data had a skewed distribution. Dichotomous variables were presented as number (N) and percentage (%). The distribution of categorical variables among patients with vs without extubation success were performed through the chi-squared test; continuous variables were compared with the Mann–Whitney test. Regression analyses were used to assess the role of muscle mass and quality as predictors of extubation, ICU complications, length of ICU stay and length of hospitalization. Cox regression analyses were used to assess the association between muscle characteristics and mortality (i.e. total mortality, mortality in ICU and in-hospital mortality). Analyses were repeated separately in young and older people to confirm the directions and magnitude of the findings. Moreover, we performed a correlation between body mass index (BMI) and muscle mass. Finally, we performed two multivariable regression models (the first one adjusted for age, sex and BMI and the second one adjusted for age, sex and FI) to evaluate whether obesity or frailty could have had an impact in the association between muscle characteristics and outcomes.
All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics and outcomes
Eighty-one patients were included in the study. The sample was composed mainly by males (87.7%) and the median age was 59.3 ± 11.9 years. Only 14 patients (17.3%) were frail (FI > 0.25). Table 1 illustrates the main characteristics of the study population. The median length of hospital stay was 37 days, with a median ICU permanence of 17 days. Ten patients were readmitted to ICU during their hospitalisation. Eight patients failed to be extubated at first attempt and 31 patients (38.3%) underwent tracheostomy. Sixty-six patients had at least one complication during their ICU stay. The most frequent complications were: bacteraemia (53.1%), need for red blood cell transfusion with more than two units (30.9%) and fungal infection (25.9%). Hospital mortality was 34.6% (28/81): 22 patients died in ICU and eight after being transferred to COVID-19 Medical Wards.
Table 1.
Main characteristics of the studied population.
| Variables | Total sample (N = 81) |
|---|---|
| Males: N (%) | 71 (87.7%) |
| Age (years): mean (SD) | 59.3 (SD 11.91) |
| Older peole (Age ≥65 years): N (%) | 29 (35.8%) |
| Ethnic group: N (%) | |
| White | 62 (76.5%) |
| Latin American | 13 (16%) |
| Asiatic | 2 (2.5%) |
| Smoke: N (%) | |
| Active | 2 (2.5%) |
| Former | 13 (16%) |
| Height (m): mean (SD) | 1.72 (SD 0.08) |
| Weight (kg): mean (SD) | 83.4 (SD 13.65) |
| BMI (kg/m2): mean (SD) | 28.3 (SD 4.74) |
| Obese (BMI >30 kg/m2): N (%) | 21 (25.9%) |
| Vertebral region: N (%) | |
| L1 | 26 (32.1%) |
| L2 | 26 (32.1%) |
| L3 | 29 (35.8%) |
| Muscle area (cm2): mean (SD) | 112.9 (SD 29.93) |
| Skeletal muscle index (cm2/m2): median (IQR) | 35.5 (IQR 28.9–43.6) |
| Reduced muscle mass: N (%) | 53 (65.4%) |
| Muscle density (HU): mean (SD) | 28.3 (SD 8.36) |
| Reduced muscle density: N (%) | 69 (85.2%) |
| Visceral adiposity (cm2): mean (SD) | 207.5 (SD 87.70) |
| IMAT (cm2): mean (SD) | 15.6 (SD 9.76) |
| Subcutaneus adiposity (cm2): mean (SD) | 164.1 (SD 64.54) |
| Total adiposity (cm2): mean (SD) | 387.2 (SD 127.77) |
| Comorbidities: N (%) | |
| Hypertension | 34 (42%) |
| Type 1 Diabetes | 1 (1.2%) |
| Type 2 Diabetes | 14 (17.3%) |
| COPD | 1 (1.2%) |
| Moderate to severe renal disease | |
| Liver disease | 3 (3.7%) |
| Ischemic heart disease | 5 (6.2%) |
| Cerebrovascular disease | 5 (6.2%) |
| Dementia | 1 (1.2%) |
| Solid tumor | 3 (3.7%) |
| Frailty Index: median (IQR) | 0.14 (IQR 0.07–0.21) |
| Frail patients: N (%) | 14 (17.3%) |
| Steroid use during hospitalization: N (%) | 1 (1.2%) |
3.2. Demographic and anthropometric features of patients who were successfully extubated
Thirty-two patients were successfully extubated at first attempt or without the need for tracheostomy. These patients were: less old (age 56.2 vs 63.0 years, p = 0.004), more obese (40.6% vs 16.7%, p = 0.02), with higher muscle CSA (121.8 vs 107.7 cm, p = 0.04), SMI (40.5 vs 31.8, p = 0.004) and density (32 HU vs 26.5 HU, p = 0.006) compared to those who failed extubation or who were tracheostomised. This yielded a post-hoc estimated study power of 81% (alpha = 0.0353) with the study sample size of 81 patients.
3.3. Muscle CSA and outcomes
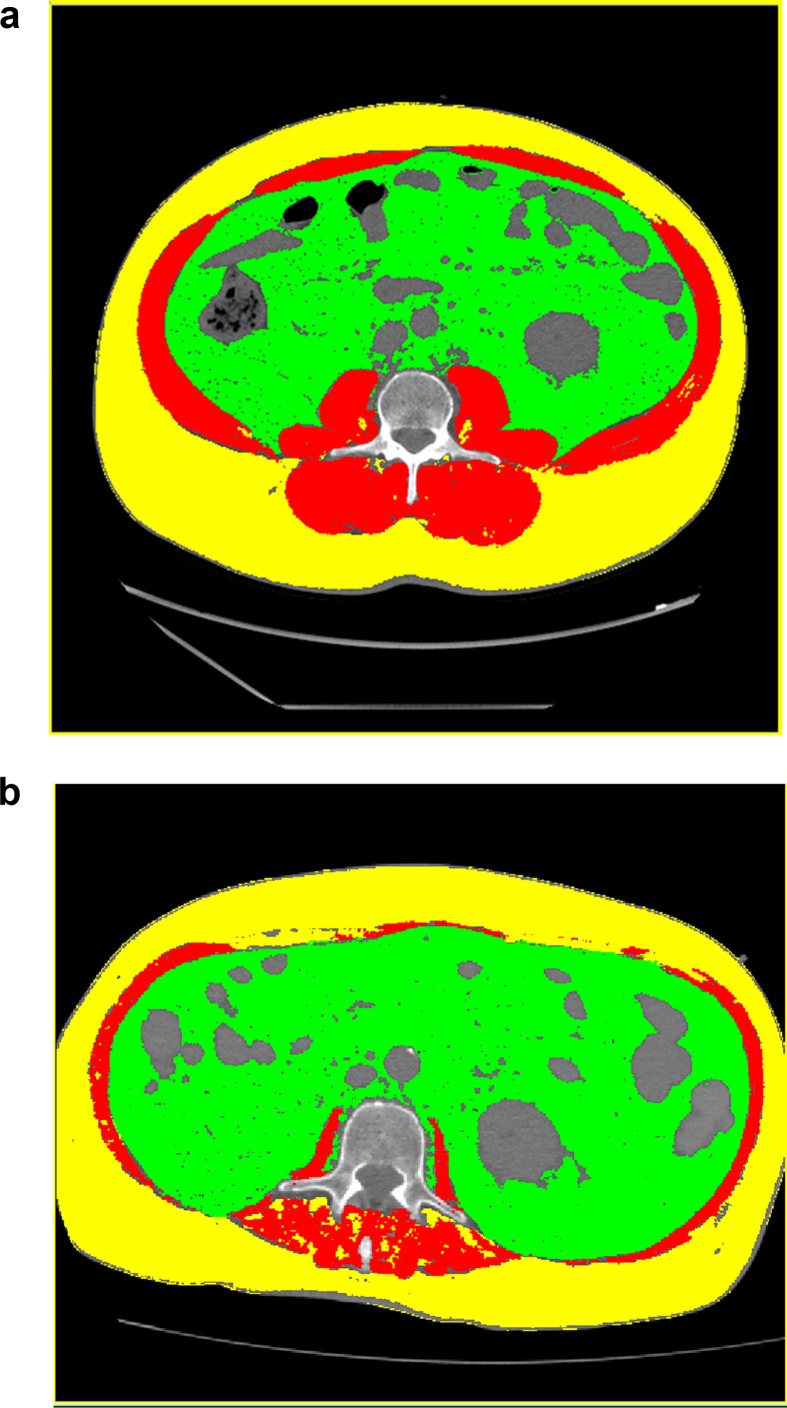
Muscle CSA predicted successful extubation (OR 1.02, 95% C.I. 1.00–1.03, p = 0.017). Moreover, muscle CSA was inversely associated to the development of complications during ICU stay (OR 0.97, 95% C.I. 0.95–0.99, p = 0.03), to the number of complications (Β −0.02, 95% C.I. −0.41 - −0.006, p = 0.01) during ICU stay and to hospital mortality (HR 0.98, 95% C.I. 0.96–0.99, p = 0.02). Figure 1 shows CT scans with a normal muscle mass and a patinet with a reduced muscle mass.
Fig. 1.
Comparison of CT scans of a normal and a patient with reduced muscle mass. Segmentation of muscles on CT images (red) using density thresholding. Axial images at the L3 level of a patient with normal (a) and reduced (b) muscle mass. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. SMI and outcomes
SMI predicted successful extubation (OR 1.06, 95% C.I. 1.01–1.1, p = 0.008). In addition, SMI was inversely associated with hospital mortality (HR 0.96, 95% C.I. 0.94–0.99, p = 0.002) and with mortality in ICU (HR 0.96, 95% C.I. 0.93–0.99, p = 0.008).
3.5. Muscle density and outcomes
Muscle density displayed an inverse association with the number of complications in ICU (Β −0.07, 95% C.I. −0.13 - −0.002, p = 0.03), with the length of hospital stay (Β −1.36, 95% C.I. −2.21 - −0.51, p = 0.002) and with in-hospital mortality (HR 0.88, 95% C.I. 0.78–0.99, p = 0.046).
3.6. Outcomes in young and older people
Magnitude and direction of findings were confirmed when analyses were repeated in young and older people (age ≥65) separately. In particular, we found a significant inverse association between SMI and in hospital mortality (HR 0.95, 95% C.I. 0.92–0.99, p = 0.04) and between SMI and ICU-mortality (HR 0.93, 95% C.I. 0.88–0.98, p = 0.01) in older people. We found a significant association between SMI and extubation success (OR 1.05, 95% C.I. 1.001–1.1, p = 0.04) in people aged <65 years. In people aged <65 years, inverse correlations were found between muscle CSA and the development of complications in ICU (OR 0.97, 95% C.I. 0.94–0.99, p = 0.04) and ICU mortality (HR 0.97, 95% C.I. 0.94–0.99, p = 0.04), muscle density and length of hospital stay (B −2.71, 95% C.I. −2.56 - −0.41, p = 0.009) and SMI and in-hospital mortality (HR 0.95, 95% C.I. 0.92–0.99, p = 0.02).
3.7. Influence of body mass index on outcomes
A moderate correlation between muscle mass and Body Mass Index (BMI) was found with the Pearson correlation (r = 0.3; p = p = 0.007). Multivariable binary regression analysis adjusted for age, sex and BMI showed that muscle density was significantly associated with successful extubation (adjusted OR 1.08, 95% C.I. 1.005–1.16, p = 0.036) and length of hospital stay (adjusted B −1.03, 95% C.I. −1.8 - −0.25, p = 0.01). SMI affected the length of ICU stay (adjusted B – 0.6; 95% C.I. −1.17 – - 0.03, p = 0.04) and the number of complications in ICU (adjusted B – 0.1, 95% C.I. −0.16 - −0.03, p = 0.005). Muscle CSA predicted the number of complications in ICU (adjusted B – 0.03, 95% C.I. −0.05 - −0.004, p = 0.02).
3.8. Influence of frailty on outcomes
In order to account for a possible role of frailty in modulating the clinical outcomes, a second multivariable model adjusted for age, sex and frailty index was built and tested. It found an inverse association between muscle CSA and a complicated ICU stay (adjusted OR 0.96, 95% C.I. 0.92–0.99, p = 0.008), muscle CSA and the number of complications in ICU (adjusted B – 0.3, 95% C.I. −0.43 - −0.10, p = 0.002), muscle CSA and the length of ICU (adjusted B −0.21, 95% C.I. −0.37 - −0.04, p = 0.014) and hospital stay (adjusted B −0.34, 95% C.I. −0.62 - −0.06, p = 0.02). In addition in this model, SMI predicted a successful extubation (adjusted OR 1.06, 95% C.I. 1.006–1.11, p = 0.027) and displayed an inverse association with in-hospital (adjusted HR 0.96, 95% C.I. 0.94–0.99, p = 0.01) and ICU (adjusted HR 0.96, 95% C.I. 0.93–0.99, p = 0.03) mortality. Moreover, we detected an inverse association between muscle density and the number of complications during ICU stay (adjusted B – 0.07, 95% C.I. −0.14–0.001, p = 0.05) and muscle density and length of hospital stay (adjusted B – 1.48, 95% C.I. −2.36 - −0.6, p = 0.001). Figure 2 illustrates the outcomes of univariable regression analyses and Table 2 shows the results of the multivariable regression models.
Fig. 2.
Outcomes of the univariable regression analyses. SMI = Skeletal Muscel Index. CSA = Cross Sectional Area.
Table 2.
Results of the multivariable regression models.
| Extubation success | |||
|---|---|---|---|
| Adjusted analysea | |||
| OR | 95% C.I. | p | |
| Muscle CSA | 1.02 | 0.99–1.04 | 0.22 |
| SMI | 1.03 | 0.96–1.1 | 0.47 |
| Muscle density | 1.08 | 1.005–1.16 | 0.036 |
| Adjusted analyseb | |||
| Muscle CSA | 1.02 | 0.99–1.04 | 0.12 |
| SMI | 1.06 | 1.006–1.11 | 0.027 |
| Muscle density |
1.07 |
1–1.15 |
0.05 |
|
Adjusted analyseb | |||
|
Any complication in ICU | |||
|
Adjusted analysea | |||
|
OR |
95% C.I. |
p |
|
| Muscle CSA | 0.97 | 0.94–1.004 | 0.09 |
| SMI | 0.91 | 0.81–1.001 | 0.053 |
| Muscle density |
0.96 |
0.88–1.05 |
0.44 |
|
Adjusted analyseb | |||
| Muscle CSA | 0.96 | 0.92–0.99 | 0.008 |
| SMI | 0.99 | 0.94–1.05 | 0.78 |
| Muscle density |
0.96 |
0.87–1.05 |
0.43 |
|
Adjusted analyseb | |||
|
Number of complications in ICU | |||
|
Adjusted analysea | |||
|
Β |
95% C.I. |
p |
|
| Muscle CSA | −0.03 | −0.05–−0.004 | 0.02 |
| SMI | −0.1 | −0.16–−0.03 | 0.005 |
| Muscle density |
−0.04 |
−0.11–0.03 |
0.23 |
|
Adjusted analyseb | |||
| Muscle CSA | – 0.3 | - 0.43 to −0.10 | 0.002 |
| SMI | −0.03 | −0.08–0.008 | 0.11 |
| Muscle density |
−0.04 |
−0.11–0.03 |
0.23 |
|
Adjusted analyseb | |||
|
Lenght of ICU stay | |||
| Adjusted analysea | |||
|
Β |
95% C.I. |
p |
|
| Muscle CSA | −0.2 | −0.39–0.0 | 0.05 |
| SMI | −0.6 | −1.17–−0.03 | 0.04 |
| Muscle density |
−0.4 |
−0.98–0.17 |
0.16 |
|
Adjusted analyseb | |||
| Muscle CSA | −0.21 | −0.37–−0.04 | 0.014 |
| SMI | −0.11 | −0.43–0.21 | 0.51 |
| Muscle density |
−0.47 |
−1.01–0.07 |
0.09 |
|
Adjusted analyseb | |||
|
Lenght of hospital stay | |||
| Adjusted analysea | |||
|
Β |
95% C.I. |
p |
|
| Muscle CSA | −0.2 | −0.48–0.08 | 0.17 |
| SMI | −0.61 | −1.41–0.2 | 0.14 |
| Muscle density |
−1.03 |
−1.8–−0.25 |
0.01 |
|
Adjusted analyseb | |||
| Muscle CSA | −0.34 | −0.62–−0.06 | 0.02 |
| SMI | −0.42 | −0.97–0.12 | 0.12 |
| Muscle density |
−1.48 |
−2.36–−0.6 |
0.001 |
|
Adjusted analyseb | |||
|
Hospital mortality | |||
|
Adjusted analysea | |||
| Muscle CSA | 0.98 | 0.96–1.13 | 0.56 |
| SMI | 0.97 | 0.9–1.03 | 0.33 |
| Muscle density |
1.02 |
0.96–1.1 |
0.55 |
|
Adjusted analyseb | |||
| Muscle CSA | 0.99 | 0.98–1.01 | 0.6 |
| SMI | 0.96 | 0.94–0.99 | 0.01 |
| Muscle density |
1.03 |
0.98–1.1 |
0.23 |
|
Adjusted analyseb | |||
|
ICU mortality | |||
|
Adjusted analysea | |||
| Muscle CSA | 0.99 | 0.96–1.01 | 0.31 |
| SMI | 0.98 | 0.92–1.06 | 0.68 |
| Muscle density |
1.02 |
0.96–1.1 |
0.48 |
|
Adjusted analyseb | |||
| Muscle CSA | 1.01 | 0.98–1.02 | 0.93 |
| SMI | 0.96 | 0.93–0.99 | 0.03 |
| Muscle density | 1.04 | 0.98–1.1 | 0.18 |
CSA cross-sectional area.
SMI skeletal muscle index.
Adjusted for: age, sex and body mass index.
Adjusted for: age, sex and frailty index.
4. Discussion
In this observational study, we found that, muscle mass and muscle density were associated with successful extubation, less complicated ICU stay and decreased mortality in patients intubated for COVID-19-related pneumonia. Muscle density also had an inverse association with the length of hospitalisation. Patients who had a successful extubation were younger and had more muscle mass than patients with extubation failure or need for tracheostomy. Consistent findings were observed after stratification into old and young people. Moreover, associations between muscle trophic parameters and outcomes were confirmed even after adjustment for BMI and FI.
We used two measures of muscle mass: CSA and SMI. Their association with clinical outcomes was similar thought slightly different. Both measures predicted successful extubation and hospital mortality; instead, only CSA was inversely associated with a complicated ICU stay and just SMI was inversely associated with ICU mortality.
The adjustment of CSA for height [2], that generates the SMI, allows the measure of relative muscle mass. Quantification of relative muscle mass is important since the amount of muscle mass is closely related to body size. Individuals with a larger body size should have larger muscle mass [39]. Indeed, people with similar CSA but different heights have different nutritional status [40]. Even if no consensus exists on which measure of muscle mass should be preferred, since SMI allows an evaluation of the adequacy of muscle mass it could be considered as a more accurate. Our findings are in line with the results of Woo et al. [22] who explored the role of reduced muscle mass (measured with CT scans) in predicting intensive care unit in adult surgical patients. Indirect confirmations of the association between muscle mass and ventilation weaning also came from the study by Salam et al. [41]. These latter authors showed that patients with inadequate cough responses, possibly due to sarcopenia in respiratory muscles, frequently needed reintubation after discontinuation of MV [41]. Moreover, Supinski et al. highlighted the possible role of sarcopenia (and in particular of diaphragm weakness) in patients who experience respiratory failure relapses despite improving lung parenchymal findings at imaging [20].
Sarcopenia has been associated with adverse outcomes, namely prolonged hospitalisation, complications during hospital stay, and mortality [12,13]. Our study confirms these findings and adds novel information on the potential role of muscle quality beside muscle mass. Specifically, we observed that muscle quality perturbations were predictive of extubation failure, length of hospitalization and in-hospital mortality in patients with severe COVID-19. Furthermore, pathological changes in muscle quality apparently precede muscle mass loss and might constitute an early, though relevant, pathogenic player and diagnostic marker of sarcopenia [12,13]. Consistently, alterations of muscle quality were more prevalent (85%) than alterations of muscle quantity (65%) in our cohort.
Our study highlights the importance of assessing muscle health in ICU patients, especially in light of the high prevalence of alteration detected in middle aged and non-underweight patient. Clinical tools based on objective measurement of muscle mass and quality loss during intensive care stay might thus be of paramount importance to minimise the risk of hospitalisation-related complications in critically ill individuals. CT imaging can be used to measure muscle mass and quality and might overcome potential underestimation biases in the evaluation of obese and overweight patients [42]. As CT scans are routinely performed in patients with severe respiratory manifestations, CT-derived measures of muscle trophism constitute simple and cheap tools to detect sarcopenia with no additional radiation burden for patients.
In this setting, it should, however, be highlighted that data about baseline muscle characteristics might be of particular importance to define the degree of acute deterioration caused before and during hospitalisation. COVID-19 is in fact an acute illness characterized by enhanced catabolism, which perturbs muscle homeostasis and increases muscle degradation [43,44]. Consistently, weight loss has been reported as a hallmark COVID-19 patients [44]. Therefore, COVID-19 patients are at high risk of developing acute sarcopenia. Acute sarcopenia augments patients’ vulnerability to stressors [45,46] and is associated with enhanced risk of developing adverse outcomes. Moreover, acute sarcopenia can evolve into chronic sarcopenia [47], which is in turn part of the spectrum of frailty [45].
Our results should be interpreted in light of some potential limitations. First, our study had a relatively small sample size. Enrolment issues due to the emergency context of the first pandemic wave, and monocentric design might account for this finding. In addition, we did not include any measure of muscle functionality, which prevents extensive considerations on potential generalisability of our findings.
Early identification of typical sarcopenic features at muscle imaging might prevent difficult ventilator weaning and associated adverse outcomes. In particular, early detection of reduced muscle mass and quality, especially in patients with an increased risk of acute muscle wasting during ICU stay [48], would allow sufficient time to implement corrective interventions such as adequate protein supply [49] and physical therapy for improving respiratory and peripheral muscle strength [50] possibly ultimately improving patient prognosis.
Without additional radiation exposure, muscle mass and quality analyses via existing chest CT scans would constitute an important prognostic tool in patients with COVID-19-related pneumonia and other critical ill settings.
Statement of authorship
All authors made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Acknowledgements
We thank our collaborators of the Intensive Care Unit Baiardo Redaelli Martina, Di Tommaso Nora, Alba Ada Carla, Sordoni Stella, Cuffaro Raffaele, Senarighi Giacomo, Palermo Paola, Barberio Cristina, Maimeri Nicolò, Faustini Carolina, Valsecchi Gabriele, Di Piazza Martina, Fresilli Stefano, Todaro Gabriele, Fedrizzi Monica.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. published online March 13. [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020 doi: 10.1016/S0140-6736(20)30627-9. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coplin W.M., Pierson J., Cooley K.D., Newell D.W., Rubenfeld G.D. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161:1530–1536. doi: 10.1164/ajrccm.161.5.9905102. [DOI] [PubMed] [Google Scholar]
- 5.El Solh A.A., Bhat A., Gunen H., Berbary E. Extubation failure in the elderly. Respir Med. 2004;98 doi: 10.1016/j.rmed.2003.12.010. 661e8. [DOI] [PubMed] [Google Scholar]
- 6.Cheng C.H., Shu K.H., Chuang Y.W., Huang S.T., Chou M.C., Chang H.R. Clinical outcome of elderly peritoneal dialysis patients with assisted care in a single medical centre: a 25 year experience. Nephrology. 2013;18 doi: 10.1111/nep.12090. 468–73. [DOI] [PubMed] [Google Scholar]
- 7.Thille A.W., Boissier F., Ben Ghezala H., Razazi K., Mekontso-Dessap A., Brun-Buisson C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med. 2015;43 doi: 10.1097/CCM.0000000000000748. 613–20. [DOI] [PubMed] [Google Scholar]
- 8.Su K.C., Tsai C.C., Chou K.T., Lu C.C., Liu Y.Y., Chen C.S., et al. Spontaneous breathing trial needs to be prolonged in critically ill and older patients requiring mechanical ventilation. J Crit Care. 2012;27 doi: 10.1016/j.jcrc.2011.06.002. 324 e1–7. [DOI] [PubMed] [Google Scholar]
- 9.Papanikolaou J., Makris D., Saranteas T., Karakitsos D., Zintzaras E., Karabinis A., et al. New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med. 2011;37 doi: 10.1007/s00134-011-2368-0. 1976–85. [DOI] [PubMed] [Google Scholar]
- 10.Savi A., Teixeira C., Silva J.M., Borges L.G., Pereira P.A., Pinto K.B., et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27 doi: 10.1016/j.jcrc.2011.07.079. 221 e1–8. [DOI] [PubMed] [Google Scholar]
- 11.Krieger B.P., Ershowsky P.F., Becker D.A., Gazeroglu H.B. Evaluation of conventional criteria for predicting successful weaning from mechanical ventilatory support in elderly patients. Crit Care Med. 1989;17:858–861. doi: 10.1097/00003246-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Writing group for the European working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 14.De Nardi P., Salandini M., Chiari D., Pecorelli N., Cristel G., Damascelli A., et al. Changes in body composition during neoadjuvant therapy can affect prognosis in rectal cancer patients: an exploratory study. Curr Probl Cancer. 2019 Nov 1:100510. doi: 10.1016/j.currproblcancer.2019.100510. [DOI] [PubMed] [Google Scholar]
- 15.Pecorelli N., Capretti G., Sandini M., Damascelli A., Cristel G., De Cobelli F., et al. Impact of sarcopenic obesity on failure to rescue from major complications following pancreaticoduodenectomy for cancer: results from a multicenter study. Ann Surg Oncol. 2018 Jan;25(1):308–317. doi: 10.1245/s10434-017-6216-5. Epub 2017 Nov 7. [DOI] [PubMed] [Google Scholar]
- 16.Pecorelli N., Carrara G., De Cobelli F., Cristel G., Damascelli A., Balzano G., et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016 Mar;103(4):434–442. doi: 10.1002/bjs.10063. Epub 2016 Jan 18. [DOI] [PubMed] [Google Scholar]
- 17.Carrara G., Pecorelli N., De Cobelli F., Cristel G., Damascelli A., Beretta L., et al. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr. 2017 Dec;36(6):1649–1653. doi: 10.1016/j.clnu.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Vang P., Vasdev A., Zhan W.Z., Gransee H.M., Sieck G.C., Mantilla C.B. Diaphragm muscle sarcopenia into very old age in mice. Physiol Rep. 2020 Jan;8(1) doi: 10.14814/phy2.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott J.E., Greising S.M., Mantilla C.B., Sieck G.C. Functional impact of sarcopenia in respiratory muscles. Respir Physiol Neurobiol. 2016 Jun;226:137–146. doi: 10.1016/j.resp.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Supinski G.S., Morris P.E., Dhar S., Callahan L.A. Diaphragm dysfunction in critical illness. Chest. 2018 Apr;153(4):1040–1051. doi: 10.1016/j.chest.2017.08.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greising S.M., Mantilla C.B., Medina-Martinez J.S., Stowe J.M., Sieck G.C. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol. 2015;309:L46–L52. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo H.Y., Oh S.Y., Lee H., Ryu H.G. Evaluation of the association between decreased skeletal muscle mass and extubation failure after long-term mechanical ventilation. Clin Nutr. 2019 Dec 14;19:33169–33173. doi: 10.1016/j.clnu.2019.12.002. pii: S0261-5614. [DOI] [PubMed] [Google Scholar]
- 23.Cesari M., Fielding R.A., Pahor M., Goodpaster B., Hellerstein M., van Kan G.A., et al. International Working Group on Sarcopenia Biomarkers of sarcopenia in clinical trials—recommendations from the international working group on sarcopenia. J Cachexia Sarcopenia Muscle. 2012 Sep;3(3):181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rovere-Querini P., Tresoldi C., Conte C., Ruggeri A., Ghezzi S., De Lorenzo R., et al. COVID-BioB Study Group Biobanking for COVID-19 research. Panminerva Med. 2020 Oct 19 doi: 10.23736/S0031-0808.20.04168-3. [DOI] [PubMed] [Google Scholar]
- 25.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.P., Albu J., et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 1985;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 26.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a populationbased study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 27.Mourtzakis M., Prado C.M., Lieffers J.R., Reiman T., McCargar L.J., Baracos V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 28.Derstine Brian A., Holcombe Sven A., Ross Brian E., Wang Nicholas C., Su Grace L., Wang Stewart C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018 Jul 27;8(1):11369. doi: 10.1038/s41598-018-29825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recio-Boiles A., Galeas J.N., Goldwasser B., Sanchez K., Man L.M.W., Gentzler R.D., et al. 2018 Jul. Enhancing evaluation of sarcopenia in patients with non-small cell lung cancer (NSCLC) by assessing skeletal muscle index (SMI) at the first lumbar (L1) level on routine chest computed tomography (CT) Support Care Cancer; pp. 2353–2359. 26(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim E.Y., Kim Y.S., Park I., Ahn H.K., Cho E.K., Jeong Y.M., et al. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support Care Cancer. 2016 Nov;24(11):4721–4726. doi: 10.1007/s00520-016-3321-0. [DOI] [PubMed] [Google Scholar]
- 31.Swartz J.E., Pothen A.J., Wegner I., Smid E.J., Swart K.M., de Bree R., et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016 Nov;62:28–33. doi: 10.1016/j.oraloncology.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Sun C., Anraku M., Karasaki T. Low truncal muscle area on chest computed tomography: a poor prognostic factor for the cure of early-stage non-small-cell lung cancer. Eur J Cardiothorac Surg. 2019 Mar 1;55(3):414–420. doi: 10.1093/ejcts/ezy324. [DOI] [PubMed] [Google Scholar]
- 33.Jung J., Lee E., Shim H., Park J.H., Eom H.S., Lee H. Prediction of clinical outcomes through assessment of sarcopenia and adipopenia using computed tomography in adult patients with acute myeloid leukemia. Int J Hematol. 2021 Jul;114(1):44–52. doi: 10.1007/s12185-021-03122-w. [DOI] [PubMed] [Google Scholar]
- 34.Sanders K.J.C., Degens J.H.R.J., Dingemans A.M.C., Schols A.M.W.J. Cross-sectional and longitudinal assessment of muscle from regular chest computed tomography scans: L1 and pectoralis muscle compared to L3 as reference in non-small cell lung cancer. Int J Chron Obstruct Pulmon Dis. 2019 Apr 3;14:781–789. doi: 10.2147/COPD.S194003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vangelov B., Bauer J., Kotevski D., Smee R.I. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr. 2021 Apr 29:1–14. doi: 10.1017/S0007114521001446. [DOI] [PubMed] [Google Scholar]
- 36.Aubrey J., Esfandiari N., Baracos V.E., Buteau F.A., Frenette J., Putman C.T., et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodpaster B.H., Kelley D.E., Thaete F.L., He J., Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 1985;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 38.Searle S.D., Mitnitski A., Gahbauer E.A., Gill T.M., Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher D., Visser M., De Meersman R.E., Sepúlveda D., Baumgartner R.N., Pierson R.N., et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1985;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 40.Bosy-Westphal A., Müller M.J. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease--there is need for a unified definition. Int J Obes (Lond) 2015 Mar;39(3):379–386. doi: 10.1038/ijo.2014.161. [DOI] [PubMed] [Google Scholar]
- 41.Salam A., Tilluckdharry L., Amoateng-Adjepong Y., Manthous C.A. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004;30:1334–1339. doi: 10.1007/s00134-004-2231-7. [DOI] [PubMed] [Google Scholar]
- 42.Damanti S., Colloca G.F., Ferrini A., Consonni D., Cesari M. Sarcopenia (and sarcopenic obesity) in older patients with gynecological malignancies. J Geriatr Oncol. 2021 Apr;12(3):467–469. doi: 10.1016/j.jgo.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Welch C., Greig C., Masud T., Wilson D., Jackson T.A. COVID-19 and acute sarcopenia. Aging Dis. 2020 Dec 1;11(6):1345–1351. doi: 10.14336/AD.2020.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pironi L., Sasdelli A.S., Ravaioli F., Baracco B., Battaiola C., Bocedi G., et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2021 Mar;40(3):1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson D., Jackson T., Sapey E., Lord J.M. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocheteau P., Chatre L., Briand D., Mebarki M., Jouvion G., Bardon J., et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun. 2015;6:10145. doi: 10.1038/ncomms10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanner R., Brunker L., Agergaard J., Barrows K., Briggs R., Kwon O.S., et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol. 2015;593:4259–4273. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 50.Kayambu G., Boots R., Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]