Abstract
Background and Objectives:
Animal-assisted interventions (AAIs) are increasingly popular as treatments to reduce anxiety. However, there is little empirical evidence testing the mechanisms of action in AAIs, especially among adolescents. We examined whether two possible mechanisms, social interaction and/or physical contact with a therapy dog, might reduce anxiety during a social stressor.
Design and Methods:
To test these mechanisms, we randomly assigned 75 adolescents with low, middle, and high levels of social anxiety to complete a laboratory-based social evaluative stressor in one of three conditions: social interaction with a therapy dog (no physical interaction), social plus physical interaction with a therapy dog, or no interaction with a therapy dog. We measured self-reported anxiety and autonomic reactivity during the social stressor to assess the effects of contact with a therapy dog.
Results and Conclusions:
We found no evidence that the presence of a real dog, with or without the opportunity to touch it, reduced anxiety or autonomic reactivity or improved cognitive performance relative to the presence of a stuffed dog in the control condition, regardless of levels of preexisting social anxiety.
Keywords: social anxiety, animal-assisted therapy, human-animal interaction, social stress, emotion regulation
Introduction
Contact with animals in a variety of therapeutic and non-therapeutic settings can buffer responses to stress, and social stress in particular (Kerns et al., 2017; Polheber & Matchock, 2013). Such observations undergird the growing field of animal-assisted interventions (AAIs), which reflect the practice of incorporating animals into treatments to meet therapeutic goals (Jegatheesan et al., 2019). Initial evidence has demonstrated that AAIs can be effective in treating mental health symptoms in youth (Hoagwood et al., 2017; Jones et al., 2019), including anxiety (Barker et al., 2015).
AAIs are a particularly promising treatment option for adolescents; engaging adolescents in traditional therapeutic treatments such as psychotherapy can be challenging, due in part, to perceived stigma (Oetzel & Scherer, 2003). AAIs involve the use of trained therapy animals (frequently dogs, but other species are also common) who are integrated into a range of structured and unstructured therapeutic settings. Animal-assisted therapy (AAT), a structured form of AAI, is frequently used in mental health settings. Mental health professionals with specialized expertise can use AAT to deliver goal-oriented interventions with therapy animals to address socio-emotional, cognitive, and behavioral health outcomes (Jegatheesan et al., 2019). Therapy animals can help scaffold therapeutic activities; the nonjudgmental nature of animals has been frequently cited as a critical component of effectiveness in AAIs, providing motivation to engage in therapy (Jones et al., 2019) and promoting positive social engagement (Grandin et al., 2015).
However, despite the promise of AAIs, three key barriers limit their use as a treatment option for mental health problems like social anxiety in adolescents. First, although existing literature supports the scientific premise that animal contact can buffer anxiety, this effect has primarily been tested in healthy adults (Polheber & Matchock, 2013) and preadolescent children (Beetz et al., 2012; Kerns et al., 2017; O’Haire et al., 2015). There is a lack of AAI research in adolescents, particularly with regard to research designed to shed light on its utility for adolescents coping with social anxiety, a salient mental health challenge during this developmental period (Miers et al., 2013). Adolescents commonly experience social anxiety at both clinical and subclinical levels of social anxiety, and contact with a therapy animal may be an effective and easily delivered intervention for mitigating this anxiety.
Second, AAI research has suffered from methodological weaknesses that limit understanding if and when AAI can be effective (Crossman & Herzog, 2019). AAI research has traditionally relied heavily on self-report measurement, and it is critical that more studies of AAI use multiple outcome measures validated for use in human-animal interaction settings to capture a more robust understanding of the impact of AAI. AAI research should also employ random assignment, appropriate control groups, and other rigorous statistical and design approaches when testing interventions. Moreover, AAI research stands to benefit from greater adoption of transparent, open research practices, including preregistration of study designs and analysis plans, publishing null results, and justifying one’s sample size. Existing reviews of AAI (and AAT in particular) have consistently documented a need for more research before AAT becomes evidence-based practice (e.g., Hoagwood et al., 2016), and greater transparency will help achieve this goal.
Finally, the field of human-animal interaction more broadly lacks research documenting the specific mechanisms of action by which AAIs can potentially produce therapeutic outcomes. There have been repeated calls for research that addresses the specific processes driving different types of AAIs (Hoagwood et al., 2017; Kazdin, 2019). Isolating the effects of specific activities within AAIs is critical in understanding how to best design interventions for maximal therapeutic impact.
The objective of the present research was to examine the specific mechanisms by which interacting with a therapy dog might reduce anxiety in a way that addresses the above barriers to using AAI as a treatment for mental health symptoms. We considered two potential mechanisms: social support and physical touch.
Social support is broadly associated with beneficial psychological and physiological functioning (see Uchino et al., 1996), and can buffer the effects of laboratory-based stressors (Heinrichs et al., 2003). According to Social Baseline Theory (Coan & Sbarra, 2015), humans are inherently social, and evaluate challenges based on their ability to achieve goals. Part of this implicit calculation includes social relationships (e.g. friends, family, colleagues) that might be helpful in achieving goals. One possibility is that when a person interacts with an animal, a relationship is formed that becomes part of the person’s representation of social resources. For instance, being around a friendly dog might decrease the possibility of being surprised by another human or animal, thus reducing risk and decreasing anxiety. Existing research has shown a relationship between attachment to a pet (indicative of social support) and adaptive coping to stress (Mueller & Callina, 2014), but this mechanism has not been well explored in AAIs.
Another possible mechanism for anxiety reduction via AAI is physical touch. Since the famous “wire mother” studies by Harlow & Zimmerman (1959), researchers have explored the effects of physical touch, though the topic remains relatively understudied and has focused on interpersonal touch between two humans (for review, see Gallace & Spence, 2010). Research has found touch can reduce the experience of pain (Mancini et al., 2014) and influence heart rate, even in young children (Fairhurst et al., 2014). Less research has looked at how touching animals can influence anxiety, but initial evidence suggests that physical contact with a dog may be an important component of the interaction (Vormbrock & Grossberg, 1988). Increased physical touching of a dog is correlated with less of a cortisol response in children with attachment disorders during laboratory induced stressors (Beetz et al., 2012), and higher positive affect in preadolescent children completing a social stress task (Kerns et al., 2017). Moreover, initial research has suggested that social and physical contact with an animal was associated with lower anxiety than social contact without physical touch with a human friend (Polheber & Matchock, 2013), suggesting that physical touch may be an important additive factor for reducing anxiety.
To test the social support and physical touch mechanisms by which AAIs might reduce anxiety, we used stratified sampling to enroll 75 adolescents who ranged from low to high levels of pre-existing social anxiety. Participants completed a laboratory-based social evaluative stressor in one of three randomly-assigned conditions: 1) no therapy dog interaction (presence of a stuffed toy dog, our control condition; CO), 2) social interaction only (presence of a therapy dog but no physical interaction; SO); or 3) social interaction plus physical interaction (presence of and opportunity to touch a therapy dog; SP). We measured self-reported anxiety, autonomic activity, and cognitive performance.
We hypothesized that participants in the SP condition would have lower self-reported state anxiety, lower autonomic activity, and better cognitive performance than participants in the CO condition. We also had an exploratory hypothesis that self-reported state anxiety, autonomic activity, and cognitive performance errors for participants in the SO condition might lie in between those of participants in the CO and SP conditions. Our design further allowed us to gauge whether the anxiolytic effect of social and physical interaction with a dog might depend on pre-existing levels of social anxiety.
Methods
Participants
Participants were 75 adolescents ages 13–17 on a continuum of social anxiety who were recruited through convenience and snowball sampling via local libraries and youth centers, social media, and other public venues. Prior to participating in the experiment, youth participants were screened for level of social anxiety using the Social Anxiety Scale for Adolescents (SAS-A; La Greca & Lopez, 1998). We used stratified random sampling through REDCap version 8.6.5 to distribute the eligible participants who ranged across low (n = 18), mid (n = 22), and high (n = 35) social anxiety (as defined by SAS-A scores of >50 [high anxiety] and <36 [low anxiety] as recommended by La Greca, 1999) across the three experimental conditions (n = 25 in each condition). In the CO condition, n = 8 were low anxiety, n = 7 mid anxiety, n = 10 high anxiety; in the SO condition, n = 6 were low anxiety, n = 6 mid anxiety, n = 13 high anxiety; in the SP condition, n = 4 were low anxiety, n = 9 mid anxiety, n = 12 high anxiety. Exclusion criteria included fear of or allergy to dogs or adhesives (due to the wearable physiology sensor).
Participants were 76% (n = 57) female, 24% (n = 18) male; 5.3% (n = 4) identified as Hispanic or Latinx, 8.0% (n = 6) as Asian, 1.3% (n = 1) as Black or African American, 89.3% (n = 67) as White, and 4.0% (n = 3) as more than one race. The majority of participants owned at least one pet (77.3%; n = 58); 61.3% (n = 46) owned a dog, 36.0% (n = 27) owned a cat, 5.3% (n = 4) owned a horse, 21.3% (n = 16) owned fish, 2.7% (n = 2) owned birds, 6.7% (n = 5) owned a lizard, snake, turtle, or other reptile, 5.3% (n = 4) owned a guinea pig, hamster, or rodent, and 2.7% (n = 2) owned a rabbit. Pet owning participants reported high levels of attachment to their pets (M = 28.71, SD = 5.06; range 3–33) based on the Lexington Attachment to Pets Scale (Johnson et al., 1992), an 11-item Likert scale with item response options ranging from 0 (Strongly Disagree) to 3 (Strongly Agree), range 0 to 33.
Prior to confirmatory hypothesis testing, 7 participants were excluded due to protocol deviations, which included pausing or shortening the experiment due to participant stress (n = 4) or the participant requesting to move on to the next task prior to finishing the first one (n = 2), and touching the dog in the SO condition (n = 1). The final sample included 68 participants; 22 were in the SO condition, 25 in the SP condition, and 21 in the CO condition. Intent-to-treat analyses were performed with the 7 excluded participants and are included in the Supplementary materials.
Materials and Measures
Social Anxiety
The SAS-A was used to assess trait levels of social anxiety. The SAS-A was designed for use in adolescence and has been validated extensively across many samples and in multiple languages (Garcia-Lopez et al., 2011; La Greca & Lopez, 1998). The SAS-A contains 18 items that include three domains of social anxiety: fear of negative evaluation from peers (e.g., “I worry about being teased”), social avoidance and distress in new situations (e.g., “I worry about doing something new in front of others”), and generalized social avoidance (e.g., “I’m quiet when I’m with a group of people.”). The potential range of scores is 18 to 90, with higher scores signaling higher social anxiety. The SAS-A demonstrated excellent reliability in our sample (Cronbach’s α = .94).
Trier Social Stress Task for Children
Participants completed the Trier Social Stress Task for Children (TSST-C), which involved six distinct phases: baseline, anticipation, preparation, speech, mental math, and recovery (Buske-Kirschbaum et al., 1997). We used the TSST-C protocol developed by Stroud et al. (2009) for use in adolescents up to 17 years old. During the baseline rest period (20 minutes), participants watched a science documentary (with no animal content) on a digital tablet. At the conclusion of the baseline period, participants were told about the speech task (anticipation). Participants then had a five minute preparation period, and then were asked to speak on an academic topic for five minutes (e.g., history). They were then asked to complete a serial subtraction mental arithmetic task for an additional five minutes. Per the Stroud et al. (2009) protocol, the serial subtraction task was adjusted for age (13–14 year olds subtracted by 11s, 15–17 year olds subtracted by 17s). If an error was made, participants were instructed to restart from the beginning. At the completion of the speech and math tasks, participants had a 30-minute recovery period where they continued to watch the science documentary. The stuffed or real dog was present for the first 15 minutes of the recovery period. Throughout the TSST-C, participants were asked to self-report their anxiety levels; see Supplemental Materials for the study timeline.
Outcomes
To test the mechanism of action by which canine interaction reduces anxiety in adolescents, we assessed three outcomes: a) self-reported anxiety, b) autonomic physiological reactivity (electrodermal activity and heart rate), and c) cognitive performance (error rates and number of correct responses on mental math task).
Self-reported Anxiety.
Self-reported anxiety during the experiment was measured using the state scale of the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983), which is validated for use in adolescence, is commonly used with the TSST, and has consistently demonstrated excellent reliability and construct validity. We used the six-item short form of the STAI (Marteau & Bekker, 1992), which asks participants to rate how each of the six words reflects their feelings (calm, upset, relaxed, worried, tense, content). The short form was originally administered as a four-point scale, which we further modified to a three-point scale for feasibility in administering repeatedly over a short time period (e.g., I feel… very calm, calm, not calm). Self-reported anxiety was measured at six time points, during: (1) baseline, (2) anticipation (3) preparation, (4) speech, (5) mental math, and (6) recovery. Responses to the six items were used to create a sum score at each time point with a possible range of 3 to 18. Reliability across time points ranged from Cronbach’s α = .69 to .83).
Physiological Measurement.
Psychophysiological measurements (electrodermal activity [EDA] and heart rate [HR]) were recorded using Empatica E4 wristband sensors (Empatica; Cambridge MA; Garbarino et al., 2014). Participants wore a wristband on their non-dominant hand, and were instructed to minimize movement to reduce motion artifacts.
EDA was measured using 7cm long wire leads connecting the E4 wristband to disposable, pre- filled (0.5% chloride salt) Ag/AgCl (11mm inner diameter) Biopac electrodes (Goleta, CA) attached to the thenar and hypothenar eminences of the palm on the participant’s non-dominant hand. EDA was recorded at 4 Hz by the E4 sensor.
Heart rate was also collected via photoplethysmography from the E4 wristband. While recent work suggests the E4 has acceptable accuracy for measuring HR (Menghini et al., 2019), visual inspection and systematic analysis of our data suggested it did not reflect the expected increase during the TSST-C (Kudielka et al., 2004) nor was it influenced by the conditions of interest, possibility due to movement artifacts (Kleckner et al., 2020). Therefore, analysis of the heart rate data is reported in the Supplemental Materials.
Cognitive Performance.
As recommended by existing research with the TSST paradigm (e.g., Simeon et al., 2007), we measured cognitive performance during the mental arithmetic task by tracking the number of errors and lowest number reached by participants on the mental math task. Better performance was characterized by fewer errors and reaching a lower number during serial subtraction. To adjust for the different levels of subtraction based on age level, lowest number reached was operationalized by calculating the number of correct responses (a higher score indicating better performance). Number of errors ranged from 0 to 8, and number of correct answers ranged from 1 to 41.
Therapy Dogs and Handlers
All therapy dogs used in this study were registered through Tufts Paws for People, a community partner group of the national therapy animal organization Pet Partners. As part of their membership in these organizations, all dog handlers completed an 8-hr training course and their animals passed a rigorous evaluation to meet training, safety, and health standards (https://petpartners.org/standards/). The animals were bathed 24 hours prior to participating. In total, four dogs were used for this study, all under 30 pounds in weight (n = 3 female; n = 1 male), ranging from 8 to 13 years of age. All handlers were female and over the age of 30 years.
Procedure
All study procedures for human and animal subjects were approved by the Tufts University Social-Behavioral-Educational Research Institutional Review Board and Institutional Animal Care and Use Committee. Participants were compensated with a $10 gift card for the screening process, and a $75 gift card for the full experimental study to cover travel costs.
Interested participants were provided with a link to an online screening survey in which we obtained parental consent and youth assent. The online screener was used to assess pre-existing level of social anxiety to inform stratified randomization, as well as exclusion criteria. Eligible participants were then contacted with information regarding the full experimental study.
To test the mechanism by which interacting with dogs can reduce anxiety, eligible participants were randomly assigned to one of three conditions that varied the nature of the interaction: 1) no interaction control (CO), 2) social interaction (SO), or 3) social + physical interaction (SP). As previously noted, stratified randomization was used to create a balanced distribution of social anxiety across the three conditions.
After providing written parental consent and youth assent at the start of the experimental session, participants were seated in a chair and fitted to a wearable heart rate and electrodermal activity wristband device (Empatica E4), and were asked to complete the first self-report anxiety questionnaire and provide demographic and pet information. Participants in all three conditions then listened to an experimenter read a short, standardized description of a therapy dog and view a photo of the dog, in order to further control for the novelty effects of an animal stimulus. Following the animal description, either a real therapy dog or a stuffed dog entered the room with a handler.
In the control condition, to control for the novelty of an animal stimulus, a stuffed toy dog (Beetz et al., 2012) was placed in a chair next to participants and a person mirrored the role of a therapy dog handler. In both animal interaction conditions, one therapy dog and his or her handler accompanied the participant during all phases of the TSST-C. In the social interaction condition, the dog was placed on the floor to the side of the participant’s chair. Participants were told that the dog would be present, and they could socially interact with the dog at any point during the experiment (i.e., talking, look at), but they were not be permitted to physically touch the dog. In the social + physical interaction condition, the dog was seated on a wide chair next to the participant. Participants were told that the dog would be next to them during the study and that they could interact socially and touch the dog during the experiment as they wished. Animal handlers were trained to provide consistently minimal verbal contact (limited to the introductory period) with the participants to reduce the confounding effects of handler variability and distraction from the TSST-C tasks while still maintaining the general structure of an AAI. All dogs were on a 6’ leash for the entirety of the session. To simulate a typical AAI environment, the handler remained in proximity to the dog to monitor his/her behavior, but did not block the participant or interfere with the TSST-C tasks in any way. Control group participants were offered a visit with a therapy dog at the conclusion of their experimental session. All sessions were video recorded.
Data Analysis
Sample size calculation
To estimate the sample size, we used heart rate as the primary outcome and the STAI (state subscale) as the secondary outcome. At the time of the study pre-registration, there were no prior data with which to estimate the relative effect of the two intervention and control conditions on the proposed outcomes. Therefore, estimates were compiled from other research using HR as an indicator of stress response (e.g., Greenland et al., 1999). In this situation, the most conservative approach for sample size calculation is to assume a series of two-way tests. We used a mean of 75 beats per minute (bpm) with a SD of 12 bpm for baseline in all groups, assuming normal distribution, an equal number of subjects in each group, and a 0.05 significance level (alpha). With these assumptions, we estimated 90% power to detect a difference of 12 bpm between any two treatment groups with 23 subjects per group, which we round to 25 per group for a total of 75 subjects in the three treatment groups. To determine the power for the STAI state subscale, based on the STAI normative data (Spielberger et al., 1983), we use a mean of 35.5 with a SD of 10.5. Using the same approach as above for HR, we estimated 90% power to detect a difference of 10 between any two groups with a sample size of 25. Our study was, thus, design to yield adequate power to detect large effects. All sample size and power calculations were performed using PASS 13 (Hintze, 2014).
Physiological data processing
To analyze the psychophysiology data, activity was assessed over six time windows during the TSST: time 1 = beginning of study; time 2 = baseline period; time 3 = anticipation phase; time 4 = last 5 min of the TSST; time 5 = recovery 1; time 6 = recovery 2 (see Figure 1). Each time point was 5 minutes in duration. The event button on the E4 was used to mark the start of the TSST, and time points were identified and analyzed relative to the start of the TSST (e.g. the speech task time window was 5–10 minutes after the TSST started). Due to a temporary bug in an E4 software update, 10 participants had inaccurate event button times. To correct, video recordings were used to determine when the TSST took place.
Figure 1.
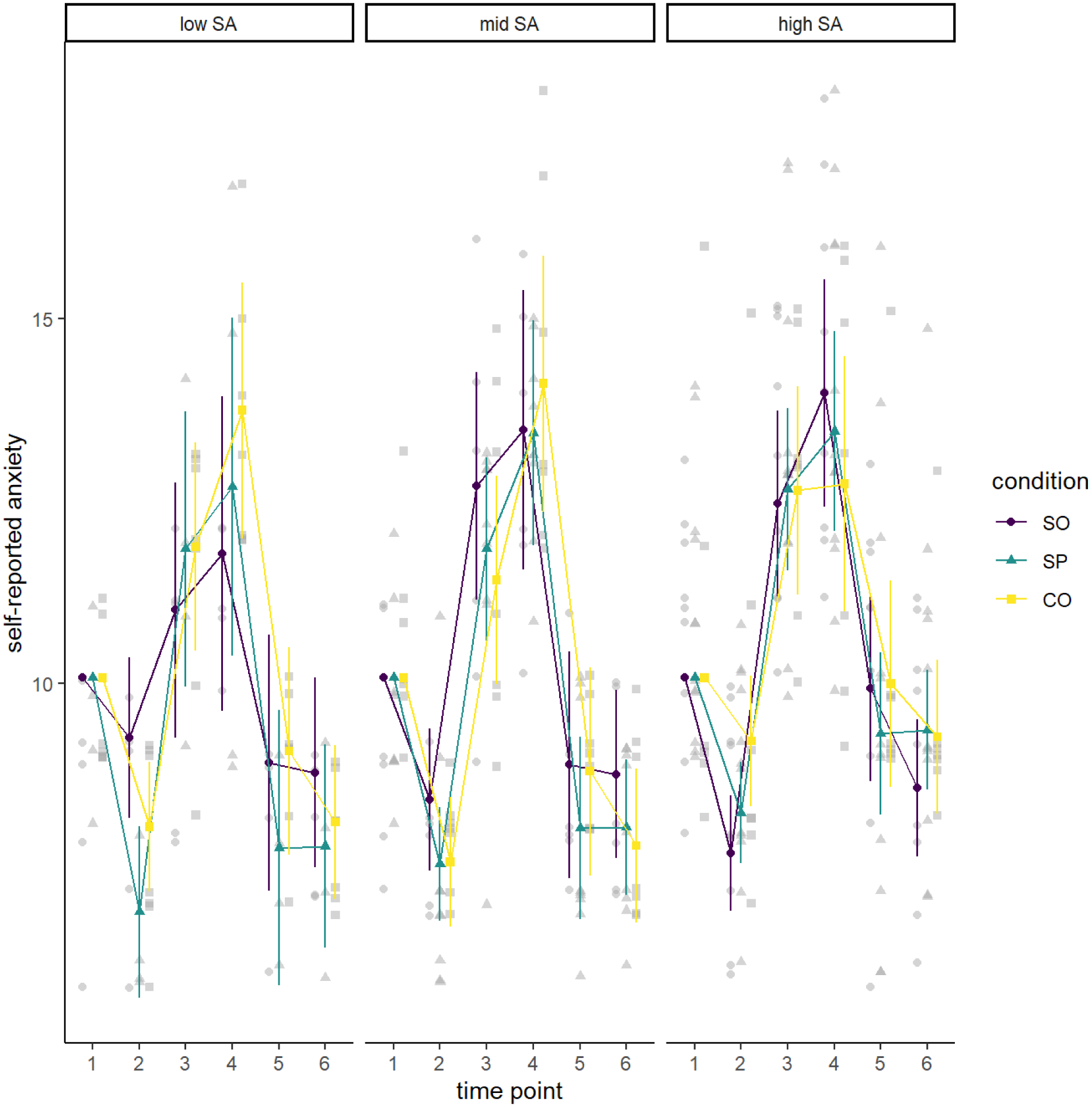
Self-reported Anxiety by Condition and Social Anxiety Level
Note. Values are estimated marginal means controlling for self-reported anxiety at the first baseline time point. The three panels reflect participants reporting low, mid, and high levels of social anxiety (SA) from left to right. In each panel, we show level of anxiety from shortly after consent (time 1) to just prior to the preparation phase of the TSST-C (second baseline; time 2) to just prior to the debriefing phrase (time 6). Lines correspond to the three experimental conditions (social only [SO], social and physical [SP] and control [CO]). Values are estimated marginal means controlling for self-reported anxiety at the first baseline, just after consent. The values were obtained in a three-way analysis of variance in self-reported anxiety treating social anxiety as a categorical variable (low, middle, high).
For some participants, the adhesive EDA sensors became detached from participants’ palms. This resulted in missing data where skin conductance was not measured. To identify participants with this issue, two researchers coded EDA for signal drop out during the six time windows described above. There was an 98.9% agreement rate (ties were resolved by discussion and consulting a third researcher for breaking ties), which resulted in 14 participants excluded from analysis of the EDA signal (3 of whom had already been excluded for protocol deviations).
Statistical Analyses
To test our confirmatory hypotheses, we conducted two three-way analyses of variance (ANOVA) with either self-reported anxiety (STAI) or autonomic reactivity (EDA) as the dependent variable. For both outcomes, there were two between-subjects factors, condition (social, social + physical, control) and social anxiety (SA; centered continuous predictor), and one within-subjects factor, time point (1, 2, 3, 4, 5, 6). We included baseline STAI or EDA at time point 1 as a centered continuous covariate. For cognitive performance, we conducted ANOVAs that treated condition as a between-subjects categorical factor and social anxiety as a centered continuous predictor.
Results
Self-Reported Anxiety
After 7 participants with a protocol deviation were excluded, and an additional 2 participants missing at least one STAI observation, we had 66 participants for analyses of self-reported anxiety; 20 were in the SO condition, 25 in the SP condition, and 21 in the CO condition. Within conditions, the number of participants in the low, mid, and high social anxiety groups ranged from 4 to 12.
Among participants included in analyses of state anxiety, mean state anxiety at the first baseline, immediately after consent, was 10.08 (SD = 1.71). Mean state anxiety at the second baseline, immediately prior to the preparation phase of the TSST-C, was 8.08 (SD = 1.62). Mean state anxiety across the four TSST-C time points (3 through 6) was 10.21 (SD = 1.43). The potential range of scores is 6 to 18, with higher scores representing higher anxiety. Mean social anxiety measured using the SAS-A was 47.92 (SD = 14.77).
As is evident in Figure 1, ANOVA results suggested that self-reported anxiety changed over time as expected during the TSST-C, an effect supported by a significant effect of time point, F(3.76, 222.09) = 144.13, MSE = 2.60, p <.001, . However, contrary to our hypotheses, this analysis did not reveal any statistically significant main or interactive effects involving condition. Table 1 summarizes all findings.
Table 1.
Three-way analysis of variance in self-reported anxiety (STAI) controlling for STAI at baseline
| Effect | F | MSE | p | |||
|---|---|---|---|---|---|---|
| Baseline STAI at Time 1 (control) | 49.23 | 1 | 59 | 5.78 | < .001 | .237 |
| Condition | 0.33 | 2 | 59 | 5.78 | .723 | .004 |
| Social Anxiety | 3.45 | 1 | 59 | 5.78 | .068 | .021 |
| Time | 144.13 | 3.76 | 222.09 | 2.60 | < .001 | .606 |
| Condition × Social Anxiety | 0.01 | 2 | 59 | 5.78 | .989 | .000 |
| Baseline STAI × Time | 4.33 | 3.76 | 222.09 | 2.60 | .003 | .044 |
| Condition × Time | 0.64 | 7.53 | 222.09 | 2.60 | .736 | .013 |
| Social Anxiety × Time | 0.67 | 3.76 | 222.09 | 2.60 | .607 | .007 |
| Condition × Social Anxiety × Time | 1.95 | 7.53 | 222.09 | 2.60 | .058 | .040 |
Note: Social anxiety was a centered continuous predictor. As reflected by the GG superscripts, non-integer degrees of freedom reflect Greenhouse-Geisser corrections for departure from sphericity. MSE = mean squared error; = generalized eta-squared
Autonomic Reactivity: Electrodermal Activity
After excluding 7 participants with a protocol deviation and 14 participants with EDA data that were not useable (3 of whom also had a protocol deviation), we had 57 participants for analyses of EDA; 20 were in the SO condition, 20 in the SP condition, and 17 in the CO condition. Within conditions, the number of participants in the low, mid, and high social anxiety groups ranged from 3 to 10.
Among participants included in analyses of EDA, mean EDA at the first baseline, a 5-minute period at the start of the session, was 4.94 μSiemens (SD = 2.78). Mean EDA at the second baseline, a 5-minute period immediately prior to the TSST-C preparation phase, was 5.55 (SD = 3.38). Mean EDA across the four TSST-C 5-minute time points from preparation phase to recovery was 7.29 (SD = 3). Mean social anxiety was 48.18 (SD = 15.71).
As is evident in Figure 2, EDA changed over time as expected during the TSST-C, an effect supported by a significant effect of time point, F(3.38, 169.14) = 81.01, MSE = 2.96, p <.001, . However, contrary to our hypotheses, this analysis did not reveal any statistically significant main or interactive effects involving condition. Table 2 summarizes all findings.
Figure 2.
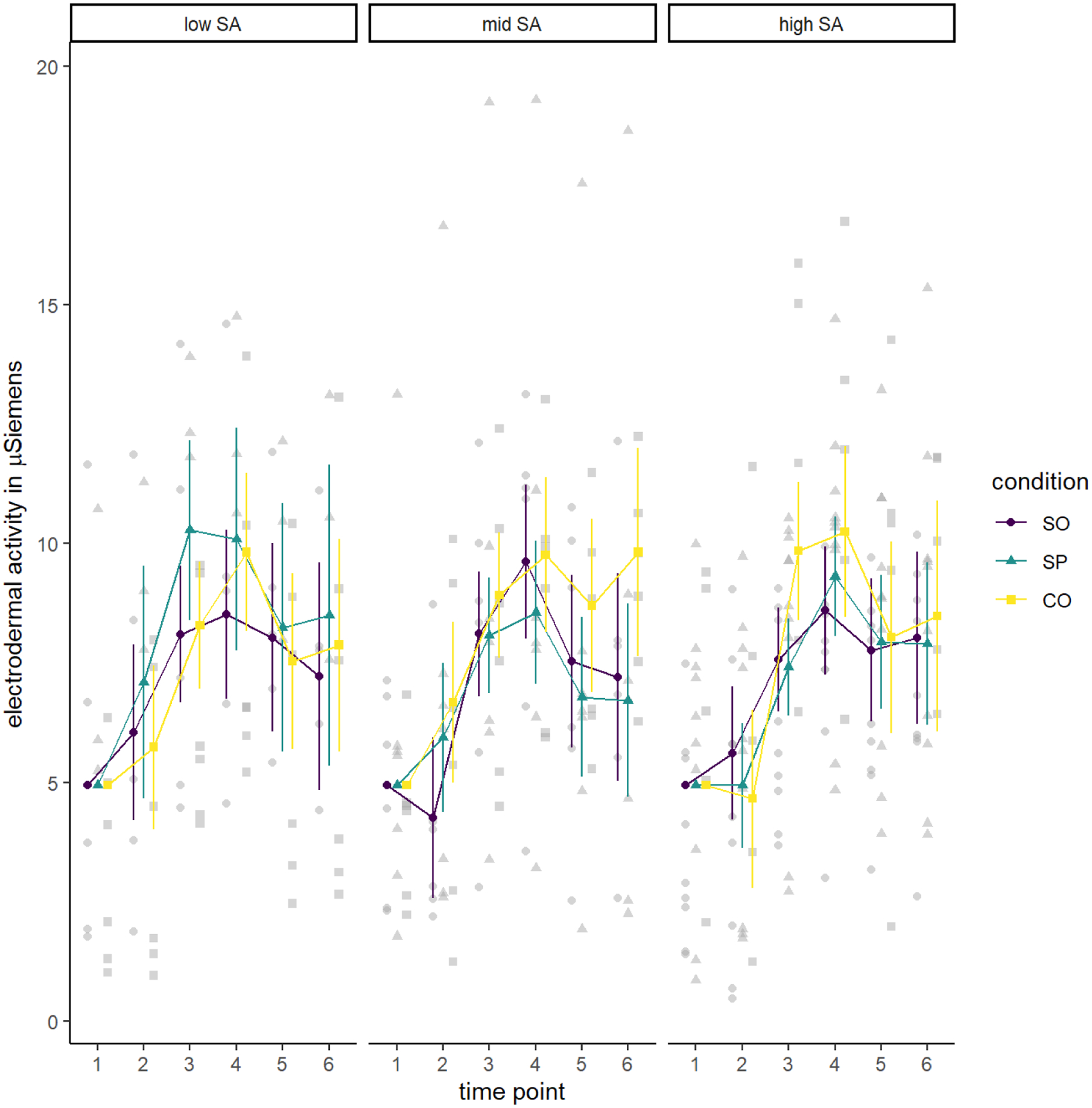
Electrodermal Activity by Condition and Social Anxiety Level
Note. The three panels reflect participants reporting low, mid, and high levels of social anxiety (SA) from left to right. Values are estimated marginal means controlling for electrodermal activity (EDA) at baseline (time 1), just after consent. In each panel, we show level of EDA from the first baseline, just after consent (time 1), to just prior to the preparation phase of the Trier Social Stress Task (TSST-C; time 2, −5–0 minutes), to just prior to the debriefing phase (time 6; +40–45 minutes). control). Lines correspond to the three experimental conditions (social only [SO], social and physical [SP] and control [CO]). Values are estimated marginal means controlling for EDA at baseline (time 1), just after consent. As in previous figures, the three panels reflect participants reporting low, mid, and high levels of social anxiety. In each panel, we show level of EDA from the first baseline, just after consent (time 1), to just prior to the preparation phase of the TSST-C (time 2, −5–0 minutes), to just prior to the debriefing phase (time 6; +40–45 minutes). Lines correspond to the three experimental conditions.
Table 2.
Three-way analysis of variance in electrodermal activity (EDA) controlling for EDA at baseline
| Effect | F | MSE | p | |||
|---|---|---|---|---|---|---|
| Baseline EDA at Time 1 (control) | 166.34 | 1 | 50 | 11.65 | < .001 | .642 |
| Condition | 1.34 | 2 | 50 | 11.65 | .271 | .028 |
| Social Anxiety | 0.36 | 1 | 50 | 11.65 | .549 | .004 |
| Time | 81.01 | 3.38 | 169.14 | 2.96 | < .001 | .428 |
| Condition × Social Anxiety | 1.32 | 2 | 50 | 11.65 | .276 | .028 |
| Baseline EDA × Time | 1.72 | 3.38 | 169.14 | 2.96 | .158 | .016 |
| Condition × Time | 0.84 | 6.77 | 169.14 | 2.96 | .549 | .015 |
| Social Anxiety × Time | 1.40 | 3.38 | 169.14 | 2.96 | .242 | .013 |
| Condition × Social Anxiety × Time | 1.18 | 6.77 | 169.14 | 2.96 | .319 | .021 |
Note: Social anxiety was a centered continuous predictor. As reflected by the GG superscripts, non-integer degrees of freedom reflect Greenhouse-Geisser corrections for departure from sphericity. MSE = mean squared error; = generalized eta-squared
Cognitive Performance
After excluding 7 participants with a protocol deviation, we had 68 participants; 22 were in the SO condition, 25 in the SP condition, and 21 in the CO condition. Within conditions, the number of participants in the low, mid, and high social anxiety groups ranged from 4 to 12. Mean social anxiety was 48.07 (SD = 15.5).
Among participants included in analyses of performance, participants achieved a mean of 13.82 (SD = 10.44) as the highest number of correct responses for the best attempt. Figure 3 demonstrates that there was not much variation in the highest number of correct responses as a function of condition or its interaction with social anxiety group. Indeed, contrary to our hypotheses, these analyses did not reveal any statistically significant main or interactive effects involving condition.
Figure 3.
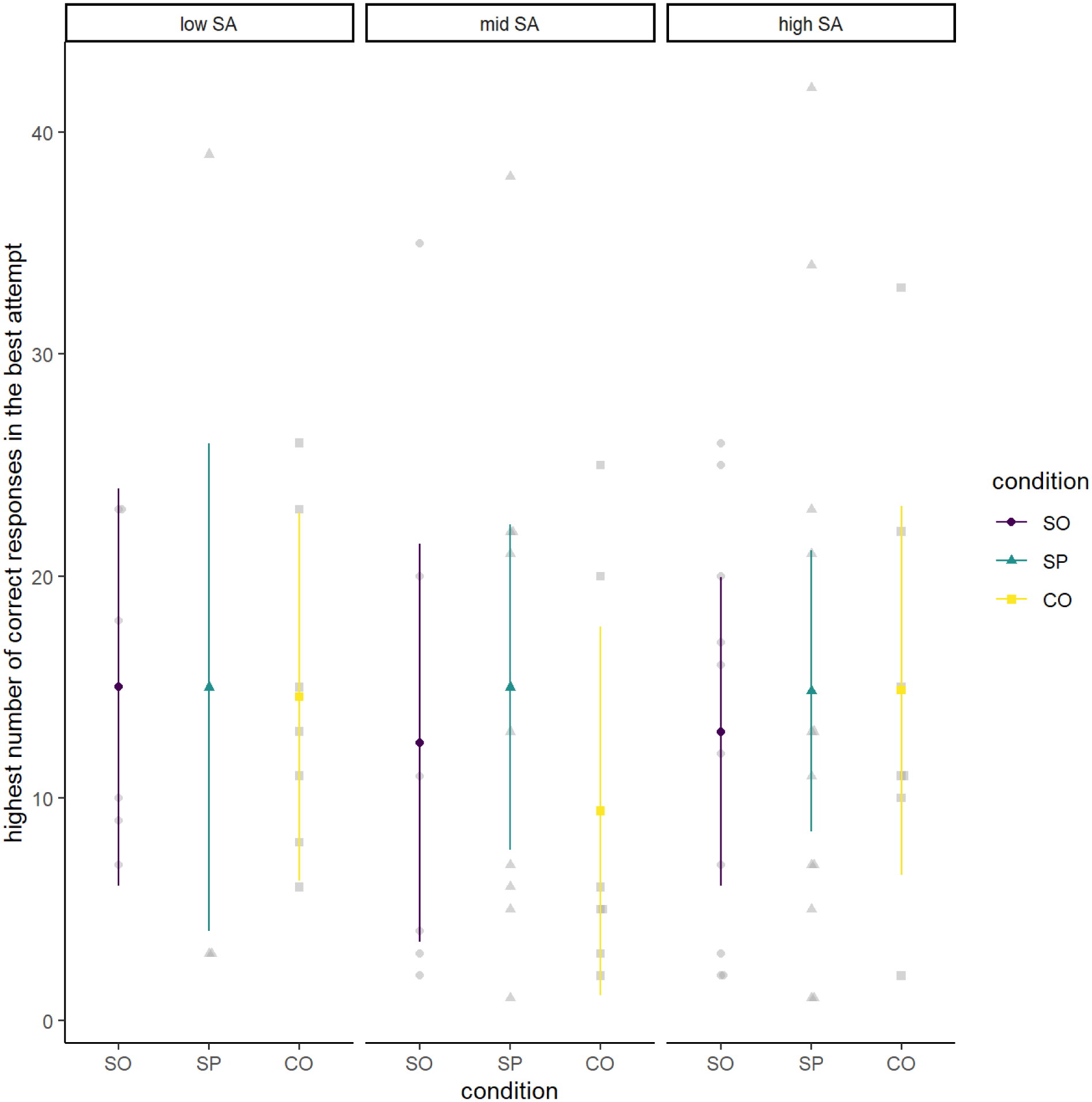
Cognitive Performance (Correct Responses) by Condition and Social Anxiety Level
Note. Cognitive performance (highest number of correct responses in the best attempt) by condition (social only [SO], social and physical [SP] and control [CO]) and social anxiety level; the three panels reflect participants reporting low, mid, and high levels of social anxiety (SA) from left to right.
Participants made a mean of 3.28 (SD = 2.11) errors during the cognitive performance task. Similarly, Figure 4 demonstrates that there was not much variation in number of errors during the TSST-C, and contrary to our hypotheses, our analyses did not suggest any statistically significant main or interactive effects involving condition. Table 3 summarizes all findings.
Figure 4.
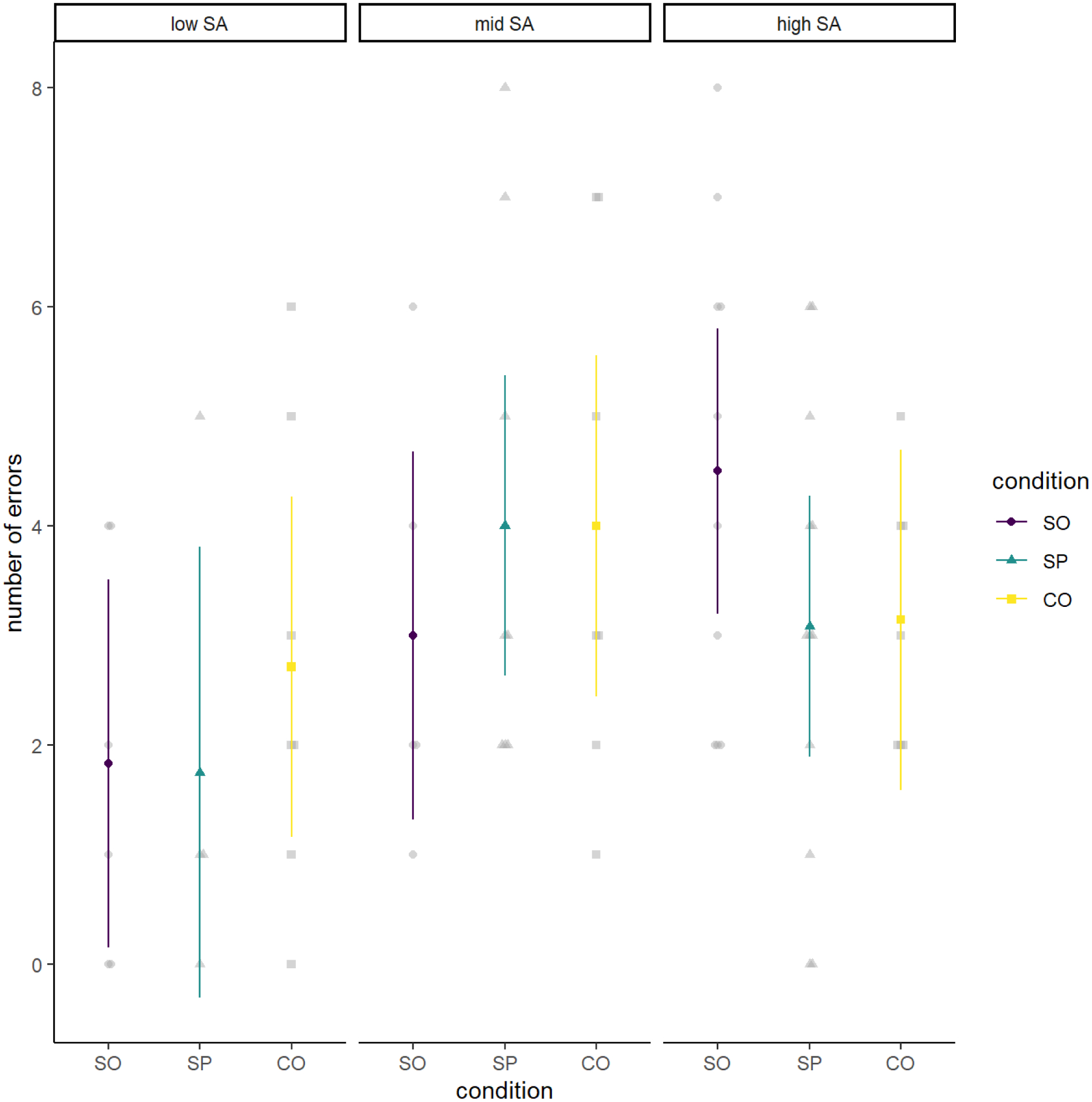
Cognitive Performance (Number of Errors) by Condition and Social Anxiety Level
Note. Cognitive performance (number of errors) by condition (social only [SO], social and physical [SP] and control [CO]) and social anxiety level; the three panels reflect participants reporting low, mid, and high levels of social anxiety (SA) from left to right.
Table 3.
Analysis of variance in cognitive performance (highest number of correct responses in best attempt and number of errors).
| Effect: Correct Responses | F | df 1 | df 2 | MSE | p | |
|---|---|---|---|---|---|---|
| Condition | 0.21 | 2 | 62 | 114.98 | .809 | .007 |
| Social Anxiety | 0.45 | 1 | 62 | 114.98 | .504 | .007 |
| Condition × Social Anxiety | 0.22 | 2 | 62 | 114.98 | .805 | .007 |
| Effect: Number of Errors | ||||||
| Condition | 0.02 | 2 | 62 | 4.42 | .975 | .001 |
| Social Anxiety | 2.15 | 1 | 62 | 4.42 | .148 | .034 |
| Condition × Social Anxiety | 1.01 | 2 | 62 | 4.42 | .371 | .031 |
Note: Social anxiety was a centered continuous predictor. MSE = mean squared error; = generalized eta-squared
Exploratory Analyses
Finally, we conducted exploratory Bayesian ANOVA to determine whether evidence favored the null hypothesis that condition would have no effect on outcomes over the alternative hypothesis that condition would have an effect. Indeed, these analyses revealed strong evidence in favor of the null hypothesis for self-reported anxiety and EDA, and moderate evidence in favor of the null hypothesis for cognitive performance. See the Supplementary Materials for details.
Discussion
As expected, the stress task (TSST-C) increased participants’ self-reported anxiety and autonomic reactivity measured via EDA. However, contrary to our hypotheses, there was no convincing evidence that the presence of a real dog, with or without the opportunity to touch it, reduced anxiety, autonomic reactivity, or increased cognitive performance relative to the presence of a stuffed dog in the control condition. This pattern of null effects was true regardless of level of social anxiety.
There are several potential interpretations of these findings. First, it may be that, contrary to our hypothesis, contact with a therapy dog does not have a meaningful effect on anxiety in socially stressful situations. The TSST-C was successful in inducing significant social anxiety, but it may be that such a short, controlled interaction with a dog is insufficient to reduce the effects of a significant stressor. Future research should explore separately if the context of the stressor (e.g., in a laboratory setting vs. a school setting) and/or the strength of the stressor could influence whether a therapy dog interaction supports coping. For example, several recent studies on animal-assisted “stress relief” programs in college settings have shown that in less controlled settings without a directly induced stressor, exposure to a therapy dog reduced overall self-reported anxiety (Pendry et al., 2018) and stress (Barker et al., 2016). However, results of physiological indicators of anxiety were mixed (Barker et al., 2016; Fiocco & Hunse, 2017; Pendry et al., 2018).
Another potential reason a dog did not reduce anxiety could be the lack of pre-existing relationship with the therapy dogs or their handlers. Given the relational nature of animal interactions, it may be that a more robust relationship between the adolescent and dog/handler is needed to attenuate the effects of social stressors. Recent research has shown that the presence of a pet dog (with whom the participant likely has a longer-term relationship) has increased positive affect during the TSST-C (Kerns et al., 2017). However, even Kerns et al. also found no differences in physiological measures of anxiety (heart rate variability). Future research should explore the differences between contact with a pet dog and contact with a therapy dog to assess if there are differential effects in the context of social anxiety. Interacting with one’s own pet may be more effective at reducing social anxiety than interacting with a novel dog.
In addition, the constraints of the TSST-C may have reduced any potential stress-reducing benefits of the therapy dog interaction. The interaction was relatively short, and due to the active nature of the stressor tasks, there were limited opportunities for the participants to interact with the therapy dog during the actual stressor. Furthermore, the interactions between the participants and the dog/handler were controlled within the laboratory setting in order to standardize the procedure, but these constraints may have reduced the ecological validity of the interaction and may not reflect a typical real-life interaction with a therapy dog. AAIs are delivered in myriad ways with different species of animals, varying activities, and in different contexts. Assessing these variations is critical in understanding when AAIs can be effective.
The role of pre-existing social anxiety level should also be examined further in future research. Although we explored the interaction between pre-existing social anxiety level and condition on outcomes, we did not have adequate power to fully test these relationships. Indeed, this study was adequately powered to detect large effects of condition on social stress reactions during the TSST-C, not interactions between condition and social anxiety. In fact, even for the basic condition effect across levels of social anxiety – where the analytic sample sizes were maximal, true between-condition effects would have had to be even larger to detect moderation by pre-existing social anxiety. The results from this study suggest that large effect sizes are perhaps not practical to expect, and future studies should use sample sizes that would allow for detection of small to medium sized main effects and interaction effects. In order to advance our understanding, larger sample sizes will be required (Crossman & Herzog, 2019). AAI research may benefit from a multi-laboratory approach to achieve adequate power to detect small-to-medium interactive effects.
The frequency, type, and duration of individual interactions between participants and therapy animals may also influence treatment efficacy within AAIs. Participants were not instructed how frequently to interact with the dogs, and therefore there may have been variation in the characteristics of the interactions. These mutual behaviors could be significant; for example, a therapy dog proactively initiating interaction may prompt an adolescent to engage with the dog and potentially reduce their anxiety. Sustained interaction may be facilitated by a therapy dog’s continued affiliative behaviors, creating a positive feedback loop that could increase the efficacy of the interaction. Future analysis should examine if specific behaviors and interaction between therapy dogs and individual participants are linked to anxiolytic effects.
Limitations & Conclusions
The participants in this study were drawn from a convenience sample with a high percentage of pet owners and limited racial/ethnic diversity. In general, individuals who have an affinity for animals may be more likely to participate in research on AAIs, which reduces the generalizability of the findings. For ethical reasons, we specifically excluded youth with fear of dogs, but pre-existing attitudes about animals may significantly contribute to the effects of AAIs. As noted above, this study was insufficiently powered to detect small or medium-sized main effects. Finally, this research was conducted with adolescents reporting a wide range of levels of social anxiety and we did not conduct diagnostic interviews to assess if anxiety met clinical thresholds. Future research should explore the use of AAIs in adolescents diagnosed with social anxiety disorder. This study did not find support for anxiolytic effects of therapy dogs during social stress. However, future research should explore the nature of the relationship between the participant and the dog, the type of interactions, and pre-existing characteristics that may moderate potential effects.
Supplementary Material
Acknowledgments:
The authors would like to thank the therapy dog teams from Tufts Paws for People for participating in this study.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under grant R03HD091892. REDCap use was supported by the National Center for Advancing Translational Sciences, under grant UL1TR002544.
Footnotes
Disclosure of interest: The authors report no conflicts of interest.
Data Availability Statement: The design and analysis plan for this study were preregistered on the Open Science Framework (https://osf.io/5nqus?view_only=1d714b919364402fbb88adb35f9dc945), and all data, analysis code, and study materials are publicly available via https://osf.io/w7k8p/?view_only=965f3d7390d7434895216fe8b88a2160. We report deviations from our preregistered protocol in the Supplementary Materials.
Trial registration: This clinical trial is registered on Clinicaltrials.gov NCT03249116; registered August 15, 2017.
References
- Barker SB, Barker RT, McCain NL, & Schubert CM (2016). A randomized cross-over exploratory study of the effect of visiting therapy dogs on college student stress before final exams. Anthrozoös, 29(1), 35–46. 10.1080/08927936.2015.1069988 [DOI] [Google Scholar]
- Barker SB, Knisely JS, Schubert CM, Green JD, & Ameringer S (2015). The effect of an animal-assisted intervention on anxiety and pain in hospitalized children. Anthrozoos, 28(1), 101–112. 10.2752/089279315x14129350722091 [DOI] [Google Scholar]
- Beetz A, Julius H, Turner D, & Kotrschal K (2012). Effects of social support by a dog on stress modulation in male children with insecure attachment. Frontiers in Psychology, 3(352), 1–9. 10.3389/fpsyg.2012.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, & Hellhamer D (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine, 59(4), 419–426. 10.1097/00006842-199707000-00012 [DOI] [PubMed] [Google Scholar]
- Coan JA, & Sbarra DA (2015). Social Baseline Theory: The social regulation of risk and effort. Current Opinion in Psychology, 1, 87–91. 10.1016/j.copsyc.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman MK, & Herzog H (2019). The research challenge: Threats to the validity of animal-assisted therapy studies and suggestions for improvement. In Fine AH (Ed.), Handbook on animal-assisted therapy: Theoretical foundations and guidelines for applying animal assisted interventions (5th ed., pp. 479–486). Elsevier Publishing. 10.1016/b978-0-12-815395-6.00031-6 [DOI] [Google Scholar]
- Fairhurst MT, Loken L, & Grossman T (2014). Physiological and behavioral responses to reveal 9-month-old infants’ sensitivity to pleasant touch. Psychological Science, 15(5), 1124–1131. 10.1177/0956797614527114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco AJ, & Hunse AM (2017). The buffer effect of therapy dog exposure on stress reactivity in undergraduate students. International Journal of Environmental Research and Public Health, 14(7), 707. 10.3390/ijerph14070707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallace A, & Spence C (2010). The science of interpersonal touch: An overview. Neuroscience & Biobehavioral Reviews, 34(2), 246–259. 10.1016/j.neubiorev.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Garbarino M, Lai M, Bender D, Picard RW, & Tognetti S (2014). Empatica E3 - A wearable wireless multi-sensor device for real-time computerized biofeedback and data acquisition. Paper presented at the Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare - “Transforming Healthcare Through Innovations in Mobile and Wireless Technologies”, MOBIHEALTH 2014. 10.4108/icst.mobihealth.2014.257418 [DOI] [Google Scholar]
- Garcia-Lopez LJ, Ingles CJ, Carcia-Fernandez JM, Hidalgo MD, Bernejo R, & Levpuscek MP (2011). Psychometric properties and clinical cutoff scores of the Spanish version of the Social Anxiety Scale for Adolescents. Journal of Personality Assessment, 93(5), 474–482. 10.1080/00223891.2011.594126 [DOI] [PubMed] [Google Scholar]
- Grandin T, Fine AH, O’Haire ME, Carlisle G, & Bowers CM (2015). The roles of animals for individuals with autism spectrum disorder. In Fine AH (Ed.), Handbook on animal-assisted therapy: Theoretical foundations and guidelines for applying animal assisted interventions (4th ed., pp. 225–236). Elsevier Publishing. 10.1016/b978-0-12-801292-5.00016-x [DOI] [Google Scholar]
- Greenland P, Daviglus ML, Dyer AR, Liu K, Huang C-F, Goldberger JJ, & Stamler J (1999) Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: The Chicago Heart Association Detection Project in industry. American Journal of Epidemiology,149, 853–862. 10.1093/oxfordjournals.aje.a009901 [DOI] [PubMed] [Google Scholar]
- Harlow HF, & Zimmerman RR (1959). Affectional response in the infant monkey: Orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science, 130(3373), 421–432. 10.1126/science.130.3373.421 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, & Ehlert U (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398. 10.1016/s0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Hintze J (2014). PASS 13. NCSS, LLC. www.nccss.com
- Hoagwood KE, Acri M, Morrissey M, & Peth-Pierce R (2017). Animal-assisted therapies for youth with or at risk for mental health problems: A systematic review. Applied Developmental Science, 21(1), 1–13. 10.1080/10888691.2015.1134267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegatheesan B, Beetz A, Ormerod E, Johnson R, Fine A, Yamazaki K, et al. (2019). The International Association of Human–Animal Interaction Organizations (IAHAIO) definitions for animal assisted intervention and guidelines for wellness of animals involved. In Fine AH (Ed.), Handbook on animal-assisted therapy: Theoretical foundations and guidelines for applying animal assisted interventions (5th ed., pp. 499–504). Elsevier Publishing. 10.1016/b978-0-12-815395-6.15001-1 [DOI] [Google Scholar]
- Jones MG, Rice SM, & Cotton SM (2019). Incorporating animal-assisted therapy in mental health treatments for adolescents: A systematic review of canine assisted psychotherapy. PloS one, 14(1), e0210761. 10.1371/journal.pone.0210761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2015). Methodological standards and strategies for establishing the evidence base of animal-assisted therapies. In Fine AH (Ed.), Handbook on animal-assisted therapy: Theoretical foundations and guidelines for applying animal assisted interventions (5th ed., pp. 451–464). Elsevier Publishing. 10.1016/b978-0-12-801292-5.00027-4 [DOI] [Google Scholar]
- Kerns KA, Stuart‐Parrigon KL, Coifman KG, van Dulmen MH, & Koehn A (2017). Pet dogs: Does their presence influence preadolescents’ emotional responses to a social stressor? Social Development, 27(1), 34–44. 10.1111/sode.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Feldman MJ, Goodwin MS, & Quigley KS (2020). Framework for selecting and benchmarking mobile devices in psychophysiological research. Behavior Research Methods, 1–18. 10.3758/s13428-020-01438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, & Kirschbaum C (2004). Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: The impact of age and gender. International Journal of Behavioral Medicine, 11(2), 116–121. 10.1207/s15327558ijbm1102_8 [DOI] [PubMed] [Google Scholar]
- La Greca AM (1999). Social Anxiety Scales for Children and Adolescents manual. University of Miami. [Google Scholar]
- La Greca AM, & Lopez N (1998). Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology, 26(2), 83–94. 10.1023/a:1022684520514 [DOI] [PubMed] [Google Scholar]
- Mancini F, Bauleo A, Cole J, Lui F, Porro CA, Haggard P & Iannetti GD (2014). Whole-body mapping of special acuity for pain and touch. Annals of Neurology, 75(6), 917–924. 10.1002/ana.24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, & Bekker H (1992). The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). British Journal of Clinical Psychology, 31(3), 301–306. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- Menghini L, Gianfranchi E, Cellini N, Patron E, Tagliabue M, & Sarlo M (2019). Stressing the accuracy: Wrist-worn wearable sensor validation over different conditions. Psychophysiology, 56(11). 10.1111/psyp.13441 [DOI] [PubMed] [Google Scholar]
- Miers AC, Blote AW, de Rooij M, Bokhorst CL, & Westenberg PM (2013). Trajectories of social anxiety during adolescence and relations with cognition, social competence, and temperament. Journal of Abnormal Child Psychology, 41, 97–110. 10.1007/s10802-012-9651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MK, & Callina KS (2014). Human-animal interaction as a context for thriving and coping in military-connected youth: The role of pets during deployment. Applied Developmental Science, 18(4), 214–223. 10.1080/10888691.2014.955612 [DOI] [Google Scholar]
- Oetzel KB, & Scherer DG (2003). Therapeutic engagement with adolescents in psychiatry. Psychotherapy: Theory, Research, Practice, Training, 40(3), 215–225. 10.1037/0033-3204.40.3.215 [DOI] [Google Scholar]
- O’Haire ME, McKenzie SJ, Beck AM, & Slaughter V (2015) Animals may act as social buffers: Skin conductance arousal in children with autism spectrum disorder in a social context. Developmental Psychobiology, 57(5), 584–595. 10.1002/dev.21310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, Carr AM, Roeter SM, & Vandagriff JL (2018). Experimental trial demonstrates effects of animal-assisted stress prevention program on college students’ positive and negative emotion. Human-Animal Interaction Bulletin, 6(1), 81–97. [Google Scholar]
- Polheber JP, & Matchock RL (2013). The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. Journal of Behavioral Medicine, 37, 860–867. 10.1007/s10865-013-9546-1 [DOI] [PubMed] [Google Scholar]
- Simeon D, Yehuda R, Cunill R, Knutelska M, Putnam FW, & Smith LM (2007). Factors associated with resilience in healthy adults. Psychoneuroendocrinology, 32(8–10), 1149–1152. 10.1016/j.psyneuen.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: performance versus peer rejection stressors. Development and Psychopathology, 21, 47–68. 10.1017/s0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, & Kiecolt-Glaser JK (1996). The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin, 119(3), 788–531. 10.1037/0033-2909.119.3.488 [DOI] [PubMed] [Google Scholar]
- Vormbrock JK, & Grossberg JM (1988). Cardiovascular effects of human-pet dog interactions. Journal of Behavioral Medicine, 11(5), 509–517. 10.1007/bf00844843 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


