Abstract
Introduction
Biological sex is an increasingly recognized factor driving clinical and structural heterogeneity in Alzheimer's disease, but its role in the behavioral variant of frontotemporal dementia (bvFTD) is unknown.
Methods
We included 216 patients with bvFTD and 235 controls with magnetic resonance imaging (MRI) from a large multicenter cohort. We compared the clinical characteristics and cortical thickness between men and women with bvFTD and controls. We followed the residuals approach to study behavioral and cognitive reserve.
Results
At diagnosis, women with bvFTD showed greater atrophy burden in the frontotemporal regions compared to men despite similar clinical characteristics. For a similar amount of atrophy, women demonstrated better‐than‐expected scores on executive function and fewer changes in apathy, sleep, and appetite than men.
Discussion
Our findings suggest that women might have greater behavioral and executive reserve than men, and neurodegeneration must be more severe in women to produce symptoms similar in severity to those in men.
Keywords: cognitive reserve, diagnosis, frontotemporal dementia, magnetic resonance imaging, neuroimaging, progression, resilience, survival
1. BACKGROUND
The behavioral variant of frontotemporal dementia (bvFTD) is a clinical syndrome characterized by progressive personality and behavioral change along with social, cognitive, and functional deterioration.1, 2 This syndrome is the most frequent presentation of frontotemporal lobar degeneration (FTLD). In contrast with typical Alzheimer´s disease (AD), patients with bvFTD show an anterior pattern of neurodegeneration with selective involvement of the frontotemporal cortex.2, 3, 4 These structures are critical nodes for the sustainment of executive function, language, and social cognition.
Structural imaging biomarkers, such as magnetic resonance imaging (MRI), are powerful tools for the in vivo study of neurodegenerative diseases. The identification of frontotemporal atrophy on MRI can increase diagnostic certainty of underlying FTLD in bvFTD, but patterns of atrophy and progression are heterogeneous.5, 6 The observed variability in the patterns of atrophy, together with variable clinical presentation and rates of longitudinal decline, hampers accurate predictions of treatment effects using clinical or imaging outcome measures.7 A better understanding of the factors determining clinical and structural heterogeneity is an essential step for the development of precision medicine approaches in neurodegenerative dementias.8, 9
Biological sex is an increasingly recognized factor driving clinical and structural heterogeneity at the single‐subject level, and the importance of sex‐related differences has been recently highlighted as a research priority in the study of neurodegenerative dementias.8, 10, 11, 12, 13 To date, most studies investigating sex differences in neurodegenerative dementias have focused on AD.14 However, some studies in FTLD syndromes indicate that sex could influence clinical presentation and diagnosis.8, 15, 16, 17, 18, 19 For instance, in large cohorts of FTLD, patients presenting with bvFTD included a higher proportion of men, whereas patients presenting the non‐fluent variant of primary progressive aphasia had a higher proportion of women (72% and 28% of men, respectively, in the largest pathology proven cohorts).3, 20 Under normal conditions, sex‐related differences in brain structure have been reported consistently in the frontotemporal cortex with higher cortical thickness in women.21, 22 Moreover, the observed difference in the ability to cope with pathology and sustain cognitive function (known as cognitive reserve) can also be modulated by sex.19, 23
RESEARCH IN CONTEXT
Systematic review: The authors used PubMed to identify previous studies examining sex differences in the behavioral variant of frontotemporal dementia (bvFTD). The impact of sex in the clinical presentation, longitudinal decline, and cortical thickness of bvFTD has not been investigated previously.
Interpretation: Women with bvFTD showed increased atrophy burden in frontotemporal regions compared to men despite similar clinical characteristics. For a given amount of atrophy, women with bvFTD demonstrated better‐than‐expected scores on executive function and fewer changes in apathy, sleep, and appetite than men. Our findings extend previous research on sex differences in cognitive reserve in neurodegenerative dementias.
Future direction: Women with bvFTD might have a greater ability to cope with frontotemporal neurodegeneration. Future studies should examine the specific mechanisms underlying the observed differences and consider biological sex for the design of research studies and clinical trials in bvFTD and other frontotemporal lobar degeneration syndromes.
In this multicenter study, we aimed to characterize the impact of sex on clinical presentation, longitudinal decline, and cortical thickness in bvFTD. We further explored the existence of sex differences in cognitive reserve by following the residuals approach to operationalize reserve as having better cognition and fewer behavioral changes than predicted by cortical thickness (a proxy of pathology). To do this, we modeled the relationship between cognition or behavior with cortical thickness and used each individual's residual as a proxy of their reserve.19, 24 We anticipated three possible scenarios: (1) men and women with bvFTD may show similar clinical presentation, progression, and burden of frontotemporal atrophy; (2) men and women with bvFTD may show different patterns of atrophy, reflecting differences in disease severity or clinical characteristics at diagnosis; and (3) men and women with bvFTD may show similar clinical features at diagnosis with significant differences in the atrophy burden.
2. METHODS
2.1. Study population
Participants with bvFTD were recruited from two cohorts, at three different centers: 149 at the University of California, San Francisco Memory and Aging Center (MAC, San Francisco, California, USA), and 67 at the Catalan Frontotemporal Dementia Initiative (CATFI; 52 at Hospital de Sant Pau, and 15 at the Hospital Clinic de Barcelona, Barcelona, Spain). Figure 1 shows a flowchart of the sample composition. Inclusion criteria for bvFTD participants were: (1) meeting the International Behavioral Variant FTD Criteria Consortium (FTDC) revised guidelines for the diagnosis of bvFTD25 and, (2) having an MRI available for analysis at the time of diagnosis. To avoid the inclusion of bvFTD “phenocopies” (bvFTD caused by non‐neurodegenerative conditions), we excluded 19 bvFTD participants classified as “possible bvFTD” without evidence of progressive deterioration or an alternative diagnosis during follow‐up (Figure 1). All patients underwent complete clinical history taking, physical examination, neuropsychological evaluation, and structural brain imaging. Neuropathological evaluation and genetic analyses were also available in a subgroup of bvFTD participants (Supplementary Methods).3, 26, 27 A total of 225 age‐matched healthy controls from the two cohorts were also included as imaging controls (168 UCSF and 57 CATFI). All healthy controls had normal cognitive performance according to normative data and did not have any neurologic, psychiatric, or other major medical illnesses.2, 28 Written informed consent was obtained from all participants or their assigned surrogate decision‐makers, and local institutional review boards for human research approved the study.
FIGURE 1.
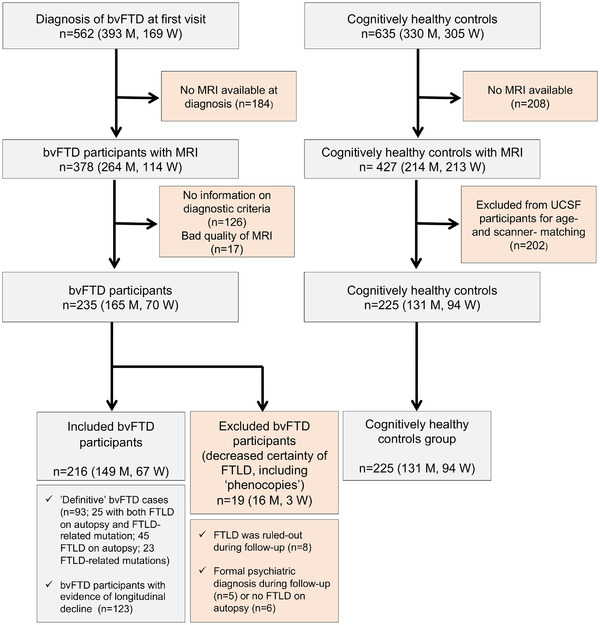
Flowchart of the sample composition. Abbreviations: bvFTD, behavioral variant of frontotemporal dementia; CATFI, Catalan Frontotemporal Dementia Initiative; FTLD, frontotemporal lobar degeneration; M, men; MRI, magnetic resonance imaging; UCSF, University of California San Francisco; W, women
2.2. Measures of disease severity
At presentation, the Clinical Dementia Rating Scale Sum of Boxes (CDRsb) and the CDR Dementia Staging Instrument PLUS National Alzheimer's Coordinating Center Behavior and Language Domains (CDR plus NACC FTLD) were recorded as the main measures of disease severity.29, 30 During follow‐up, longitudinal CDRsb and CDR plus NACC FTLD were also recorded.
2.3. Measures of cognitive function
The Mini‐Mental State Examination (MMSE) was recorded in all centers as a general measure of cognitive impairment.31 The MMSE was also recorded during follow‐up. Detailed cognitive functioning was assessed using two previously published neuropsychological protocols.2, 28 We selected cognitive measures from each protocol measuring parallel neuropsychological constructs, and we defined four main neuropsychological domains (memory, executive functioning, language, and visuospatial functioning) as in previous multicenter studies.32, 33 Details on the neuropsychological protocols can be found in Supplementary Methods and Table S2.
2.4. Behavioral measures
We recorded the specific features of bvFTD diagnostic criteria present at diagnosis. We selected the frequency‐by‐severity product of the Neuropsychiatric Inventory (NPI) and the total score of the NPI (sum of the frequency‐by‐severity scores) as measures of behavioral changes independent of diagnostic criteria recorded in all centers.34 Because the frequency‐by‐severity product of the NPI could be influenced by the characteristics of the informant (gender, values, and so on) we also considered the count of abnormal behaviors (herein, NPI behavior count) as a measure of behavioral burden less dependent on the informants' characteristics. Finally, we included the Geriatric Depression Scale (GDS) to quantify symptoms of depression at baseline.35
2.5. Progression and survival analyses
We used linear mixed‐effects analyses controlling for age, sex, baseline frontotemporal atrophy, and genetic status (presence or absence of an FTLD‐related mutation) to predict a longitudinal change as measured by CDRsb (256 measurements:183 at baseline and 73 at year 1) and CDR plus NACC FTLD (212 measurements: 145 at baseline and 67 at year 1). We included genetic status (mutation carrier vs no carrier) as a covariate because mutations have been related to the rate of clinical decline in FTLD syndromes.36, 37 We used a compound symmetry covariance matrix in all linear‐mixed models, and we included random intercepts to account for the effect of baseline values. A term for biomarker by time interaction was used to study the association between the baseline biomarker level and the outcome slope (eg, CDR+NACC/FTLD‐SB) over time.
For survival analyses, we designed a Cox regression model to account for known factors affecting survival in bvFTD. Patients alive at analysis were censored at the date of their last visit. We first introduced age at diagnosis, genetic status, disease severity (CDRsb), and education as covariates.36, 38 Next, in a second Cox regression model, we added sex to test if the previous model was significantly improved.
2.6. MRI
2.6.1. MRI acquisition
The images were acquired on scanners from seven different manufacturers using different imaging protocols (Table S1). Magnetic field strength varied between 1.5 T (n = 48 scans), 3.0 T (n = 385 scans), and 4.0 T (n = 27 scans). For additional information on the MRI quality control, please refer to Supplementary Methods.
2.6.2. MRI processing
MRIs were processed with the CAT12 toolbox within SPM12 (running in MATLAB r2019b).39, 40 It is notable that we performed cortical thickness analyses instead of volume methods, because the cortical thickness is not significantly affected by total intracranial volume, a measure that is known to differ between men and women (Supplementary Methods).41 We obtained total intracranial volume and white matter hyperintensities volumes, as implemented in CAT12. Finally, we calculated the mean frontotemporal cortical thickness by averaging the mean cortical thickness of all the frontal and temporal regions in the Desikan atlas.
2.7. Group comparison of cortical thickness
We performed separate group‐comparisons analyses in men and women following the recommendation for the study of sex‐related differences in neurodegenerative dementias.8 We introduced age, education, and MRI scanner as covariates in between‐group (bvFTD vs healthy controls) cortical thickness comparisons. For group comparisons involving healthy controls, we considered a significant statistical threshold of P < .05, family‐wise error (FWE)–corrected, using an extent threshold of the expected vertices per cluster. To assess the consistency of results across cohorts, we performed secondary analyses in UCSF and CATFI subgroups, separately. Due to the relatively small sample size in the CATFI cohort, we applied a less‐restrictive statistical threshold in the secondary analyses of this subgroup (P < .05, with false discovery rate [FDR] correction and an extent threshold of the expected vertices per cluster). Next, we calculated Cohen's d as a measure of the effect size of cortical thinning. Cohen's d takes into account sample size P‐values and allows the comparison of the effect size between groups of different sizes. Effect size computation was restricted to cortical regions showing statistically significant differences (according to predefined statistical thresholds) between bvFTD and healthy controls. Next, we determined the net effect size by subtracting the observed effect size in men to the one observed in women.
2.8. Interaction between group and sex
In a separate multiple regression model, we tested sex x group (bvFTD vs healthy control) interaction on cortical thickness in all the participants (Supplementary Methods and Figure S1).
2.9. Cognitive and behavioral reserve analyses
Next, we investigated the existence of sex differences in cognitive and behavioral reserve by following the residuals approach.19, 24 First, we fitted different linear regression models with each of the cognitive z‐scores and NPI scores as the response variable and age, education, and frontotemporal cortical thickness as predictors. This model provided an individual's predicted cognition/behavior scores for a certain level of atrophy. Here, we operationalized “reserve” as having better function than is predicted by the cortical thickness (a proxy of pathology) and used each individual's residual as a proxy of their reserve. This procedure was done for each cognitive composite and each NPI frequency x severity scores as the response variable. Robust Welch t tests with bias‐corrected accelerated bootstrapping on 1000 samples were then performed to test for a difference in residuals (ie, reserve) between women and men.
2.10. Other statistical analyses
Data were explored for normality using the Kolmogorov‐Smirnov test. Between‐group differences in baseline characteristics were assessed using t test, analysis of variance (ANOVA), or Kruskal‐Wallis test for continuous variables and chi‐square test for categorical data. We computed the effect size (partial Eta Squares) in analysis of covariance (ANCOVA) analyses of the difference in cognitive composites between bvFTD participants and healthy controls after adjusting by age and education (Table S5). To obtain robust confidence intervals, we estimated 95% confidence intervals (95% CIs) with bootstrapping‐based methods (bias‐corrected and accelerated for 1000 samples). Statistical significance for all tests was set at 5% (α = .05), and all statistical tests were two sided. All analyses were performed using SPSS 25 (Armonk, NY: IBM Corp.).
3. RESULTS
Age at MRI and education were similar between men and women with bvFTD and between participants with bvFTD and healthy controls (Table 1). Of note, age at disease onset and measures of disease severity (CDRsb and CDR plus NACC FTLD) were similar between men and women with bvFTD (Table 1). An FTLD mutation was identified in 22% of bvFTD participants (28 C9orf72, 12 GRN, 6 MAPT, and 2 TARDBP). FTLD was confirmed on autopsy in 32% (19 FTLD‐tau [including 3 MAPT], 43 FTLD‐TAR DNA‐binding protein 43 [FTLD‐TDP; including 15 C9orf72 and 6 GRN], and 8 FTLD‐FUS. But the proportion of bvFTD participants with pathology‐proven FTLD or FTLD mutations was similar between men and women (Table 1). Within bvFTD participants with pathology‐proven FTLD, we observed a higher frequency of FTLD‐Fused in Sarcoma (FTLD‐TDP; Table 1). Particularly, FTLD‐TDP type B was more frequent in women than in men with FTLD (Table S9).
TABLE 1.
Characteristics of the participants
| bvFTD | HC | |||||
|---|---|---|---|---|---|---|
| bvFTD‐men n = 149 (69%) | bvFTD‐women n = 67 (31%) | All bvFTD n = 216 | HC‐men n = 131 (58%) | HC‐women n = 94 (42%) | All HC n = 225 | |
| Demographics and disease severity | ||||||
| Age at MRI (y) | 63.3 (10.4) | 63.5 (9.6) | 63.3 (10.1) | 62.4 (14) | 61 (10.5) | 61.8 (12.6) |
| Age at disease onset (y) | 57.7 (10.9) | 58.4 (9.8) | 57.9 (10.5) | – | – | – |
| Education (y) | 14.9 (4.2) | 14.8 (4.5) | 14.8 (4.3) | 15.5 (3.6) | 15.1 (4.1) | 15.4 (3.8) |
| CDRsb* | 6.6 (3.4) | 6.7 (3.5) | 6.6 (3.4)† | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1)† |
| CDR plus NACC FTLD‡, | 8.7 (4.1) | 9.0 (4.1) | 8.8 (4.1)† | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1)† |
| Cohort, No. (UCSF/CATFI) | 99/50 | 50/17 | 149/67 | 111/20 | 57/37 | 168/57 |
| Diagnostic certainty | ||||||
| Time of follow‐up (y) | 2.1 (2.0) | 1.4 (1.4) | 1.9 (1.9) | – | – | – |
| FTLD mutation,§ % | 19 | 30 | 22 | – | – | – |
| FTLD confirmed on autopsy, % | 36 | 25 | 32 | – | – | – |
| Neuropathological diagnosis,¶ No. of major neuropathological categories |
16 FTLD‐Tau 29 FTLD‐TDP 8 FTLD‐FUS |
3 FTLD‐Tau 14 FTLD‐TDP 0 FTLD‐FUS |
19 FTLD‐Tau 43 FTLD‐TDP 8 FTLD‐FUS |
– | – | – |
| Behavioral measures | ||||||
| Number of bvFTD diagnostic features* | 4.6 (1.2) | 4.5 (1.4) | 4.6 (1.2)† | 0 (0) | 0 (0) | 0 (0)† |
| Delusions, % | 11 # | 26 # | 16† | 0 | 0 | 0† |
| Hallucinations, % | 8 | 13 | 9† | 0 | 0 | 0† |
| Dysphoria/Aggression, % | 56 | 56 | 56† | 5 | 6 | 6† |
| Anxiety, % | 39 | 34 | 37† | 2 | 2 | 2† |
| Euphoria/Elation, % | 47 | 53 | 49† | 0 | 0 | 0† |
| Apathy/Indifference, % | 93 | 89 | 92† | 2 | 1 | 1† |
| Disinhibition, % | 78 # | 92 # | 83† | 0 | 0 | 0† |
| Irritability/Lability, % | 47 | 48 | 47† | 2 | 3 | 3† |
| Aberrant motor behavior, % | 64 | 72 | 66† | 0 | 0 | 0† |
| Sleep changes, % | 48 | 42 | 46† | 5 | 0 | 3† |
| Appetite changes, % | 81 | 76 | 80† | 7 | 5 | 3† |
| NPI total score | 40.3 (21.5) | 41.6 (20.3) | 40.7 (21.0)† | 2.7 (5.2) | 2.3 (4.5) | 2.5 (4.9) |
| NPI behavior count | 6.2 (2.4) | 6.5 (2.4) | 6.3 (2.4)† | 0.2 (0.7) | 0.1 (0.6) | 0.2 (0.6) |
| Geriatric depression scale | 9.3 (6.8) | 9.7 (7.2) | 9.4 (6.9)† | 3.9 (3.7) | 4.9 (5.4) | 4.3 (4.5) |
| Cognitive measures | ||||||
| MMSE | 23.8 (6.3) # | 21.4 (7.4) # | 23.1 (6.7)† | 29.1 (0.9) | 28.8 (1.3) | 29.0 (1.1)† |
| zMemory | −0.4 (0.8) | −0.6 (0.9) | −0.5 (0.8)† | 0.6 (0.5) | 0.7 (0.5) | 0.6 (0.5)† |
| zLanguage | −0.6 (0.8) | −0.9 (0.9) | −0.7 (0.9)† | 0.6 (0.4) | 0.6 (0.4) | 0.6 (0.4)† |
| zExecutive functions | −0.7 (0.7) | −0.7 (0.9) | −0.7 (0.7)† | 0.5 (0.5) | 0.5 (0.6) | 0.5 (0.5)† |
| zVisuospatial | −0.4 (1.1) | −0.7 (1.7) | −0.5 (1.3)† | 0.3 (0.5) | 0.3 (0.4) | 0.3 (0.4)† |
| Imaging characteristics | ||||||
| Quality of MRI,** | 80 (7) | 86 (1) | 82 (6)† | 84 (3) | 84 (2) | 84 (3)† |
| Total intracranial volume, mL | 1487 # | 1335 # | 1440 (153) | 1503 # | 1328 # | 1430 (135) |
| White matter hyperintensities, mL | 6.0 (10.5) | 4.2 (3.4) | 5.5 (8.9)† | 2.8 (4.0) # | 2.1 (3.2) # | 2.5 (3.7)† |
NPI measures were available in 389 (88%) of participants. All cognitive measures were available in at least 85% of participants.
Abbreviations: bvFTD, behavioral variant of frontotemporal dementia; CATFI, Catalan Frontotemporal Dementia Initiative; CDR plus NACC FTLD, CDR Dementia Staging Instrument PLUS National Alzheimer's Coordinating Center Behavior and Language Domains; CDRsb, Clinical Dementia Rating scale sum of boxes; MMSE, Mini‐Mental State Examination; MRI, magnetic resonance image; NPI, Neuropsychiatric Inventory; UCSF, University of California San Francisco.
CDRsb was available in 183 (85%) bvFTD participants and 188 (84%) of healthy controls.
Difference between all bvFTD group and HC group (P < .05).
CDR plus NACC FTLD was available in 145 (67%) of bvFTD participants and 127 (56%) of healthy controls.
A total of 48 FTLD‐related mutations were identified: 28 C9orf72 (15 with neuropathology available), 12 GRN (six with neuropathology available), six MAPT (three with neuropathology available) and two TARDBP.
The proportion of FTLD‐TDP was higher in women with FTLD diagnosis than in men (82% vs 55%, P < .05, Bonferroni adjusted). For additional details on the neuropathological findings please refer to Table S9.
Difference between men and women (P < .05, Bonferroni adjusted).
Imaging quality rating obtained from CAT12 software (percentages closer to 100% indicating a better quality of MRI).
Women and men showed a similar frequency of bvFTD diagnostic features (Figure 2A). In addition, on average, men and women showed the same number of features of the diagnostic criteria at diagnosis (Mann‐Whitney U = 3.404; P = .737). Disinhibition and delusions were more frequently reported (present vs absent) in women than in men with bvFTD (Table 1). However, we only observed higher frequency x severity scores on women in delusions (Figure 2B). The NPI total score, the NPI behavior count, and the GDS score were similar between men and women with bvFTD (Table 1).
FIGURE 2.
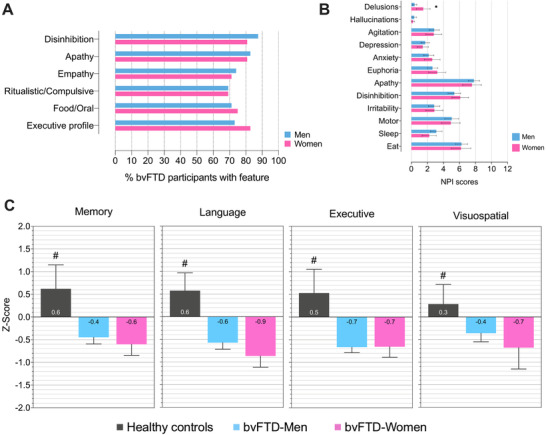
Women and men with bvFTD have similar behavioral and cognitive impairment at diagnosis. Behavioral and cognitive characteristics of bvFTD participants by sex. (A) Percentage of bvFTD participants with features of diagnostic criteria at diagnosis. Of note, no differences between the frequency of bvFTD diagnostic features were noted between men and women. (B) NPI frequency x severity scores by sex. (C) Cognitive composites by sex. *Different between men and women (P < .05, Bonferroni adjusted); #Superior to both bvFTD‐Men and bvFTD‐Women (P < .05). Error bars represent 95% confidence intervals. Abbreviations: bvFTD, behavioral variant of frontotemporal dementia; NPI, neuropsychiatric inventory
Next, we compared the cognitive profile between men and women with bvFTD. As expected, bvFTD participants were more impaired than age‐ and education‐matched healthy controls in all four domains. Detailed information on full the neuropsychologic testing for each cohort can be found in Table S3 to S4. However, no differences were noted between men and women in any cognitive domain in the bvFTD group (Figure 2C). Of note, the MMSE scores were lower in women with bvFTD compared to men. However, the effect size of the difference was small (Table S5).
As shown in Figure 3A, disease progression over 1 year of follow‐up was similar between men and women with bvFTD. Time was associated with a longitudinal increase in CDR plus NACC FTLD (estimate increase of 2.5 points per year, 95% CI 0.23 to 4.8). Sex was not an independent predictor of the rate of CDR plus NACC FTLD change (Time x sex interaction, P = .807), or changes of MMSE or CDRsb.
FIGURE 3.
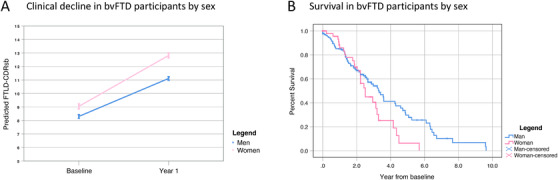
Men and women with bvFTD show similar progression and survival. (A) CDR plus NACC FTLD estimates were obtained from linear mixed‐effects models adjusted for age and sex. For illustrative purposes, we display the results for men and women (blue and pink lines, respectively). Error bars represent 95% confidence intervals. (B) Kaplan‐Meier Survival Curves for men and women with bvFTD. Abbreviations: bvFTD, behavioral variant of frontotemporal dementia
Overall survival from diagnosis in the bvFTD group was similar compared to previous studies (mean survival of 3.5 years 95% CI 3.0 to 4.0). Figure 3B shows Kaplan‐Meier survival curves in men and women with bvFTD. Mean survival in women was 2.7 years (95% CI 2.2 to 3.2) and mean survival in men 3.8 years (95% CI 3.2 to 4.3) from diagnosis. The difference in survival times between men and women with bvFTD was not statistically significant (Log Rank [Mantel Cox]: chi‐square = 2.982, P = .082). To take into account other factors that are known to affect survival in bvFTD, we designed different Cox regression models adjusted by age, education, disease severity at diagnosis (as measured by CDR plus NACC FTLD), and mutation status. We found that years of education (1.087, 95% CI 1.016 to 1.163, and P = .016) and CDR plus NACC FTLD (1.099, 95% CI 1.027 to 1.176, and P = .006) at diagnosis were independently associated with survival. However, the addition of sex did not improve the model and was not an independent predictor of survival (Table S8).
Men with bvFTD showed reduced cortical thickness in frontotemporal structures when compared to healthy controls of the same sex (Figure 4A). Similarly, women with bvFTD showed reduced cortical thickness in frontotemporal structures when compared to healthy controls of the same sex (Figure 4B). However, we observed larger effect sizes than in men, as illustrated in Figure 4C. Of note, we found similar results when we performed separate analyses in each cohort (Figure S1). Next, we directly compared cortical thickness between men and women with bvFTD and found that women with bvFTD had reduced cortical thickness compared to men with bvFTD in several frontotemporal regions of both hemispheres (Figure S2). It is important to note that we found a significant group (bvFTD or healthy control) by sex interaction in frontotemporal structures, indicating that, at diagnosis, women with bvFTD had lost more frontotemporal gray matter than men with bvFTD after accounting for age, education, MMSE, and MRI scan (Figure 4D and Figure S3).
FIGURE 4.
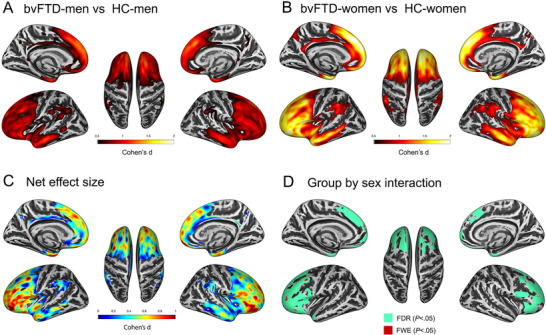
Women with bvFTD have higher atrophy than men at diagnosis. (A) Group difference in cortical thickness between men with bvFTD (bvFTD‐men) and healthy controls (HC) in the whole cohort. Only the effect sizes of regions with statistically significant results (family‐wise error [few], P < .05) are shown. (B) Group difference in cortical thickness between women with bvFTD (bvFTD‐women) and HC in the whole cohort. Only the effect sizes of regions with statistically significant results (FWE, P < .05) are shown. (C) We show the net effect size illustrating the greater degree of gray matter loss in women with bvFTD compared to men. The net effect size was obtained by subtracting the effect size map showed in panel B (effect size of cortical thinning in women with bvFTD compared to healthy controls of the same sex) from the effect showed in panel A (effect size of cortical thinning in men with bvFTD compared to healthy controls of the same sex). Only the effect sizes of regions with statistically significant results (FWE, P < .05) are shown. Higher values represent a higher net effect size favoring bvFTD‐women (ie, women have a higher effect size for the observed cortical thinning). (D) Colored regions denote areas where we observed a significant group by sex interaction (in green FDR, P < .05, and in red FWE, P < .05). In this model, age at MRI, education, MMSE, and MRI scan were introduced as covariates. Right hemisphere surfaces are shown on the right half of each panel. Abbreviations: bvFTD, behavioral variant of frontotemporal dementia; HC, healthy control; FDR, false discovery rate; FWE, family‐wise error; MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging
To more directly evaluate potential behavioral and cognitive reserve differences by sex, we took the residuals approach. We first fitted multiple linear regression models with NPI and cognitive z‐scores as the response variables and with age, education, and cortical thickness as predictors (Table S6). For illustrative purposes, Figure 5 shows the relationship between apathy NPI score, executive z‐score, and frontotemporal cortical thickness (Figure 5A and D). The sex‐comparison of the residuals obtained in these linear regression models suggests that women had lower‐than‐expected apathy (t(348) = 2.5, P = .012, Figure 5B) and higher‐than‐expected executive z‐score (t(352) = −2.1, P = .039, Figure 5D) than men, given their frontotemporal atrophy. We also observed lower‐than‐expected sleep and appetite change NPI scores in women compared to men (t(361) = 3.3, P = .001; and t(333) = 2.3, P = .020, respectively). Finally, we observed lower‐than‐expected NPI total scores and NPI behavior counts in women (t(349) = 2.2, P = .026 and t(349) = 2.2, P = .029, respectively), but we did not observe a significant difference in the residuals for the rest of the NPI and cognitive scores (Table S7).
FIGURE 5.
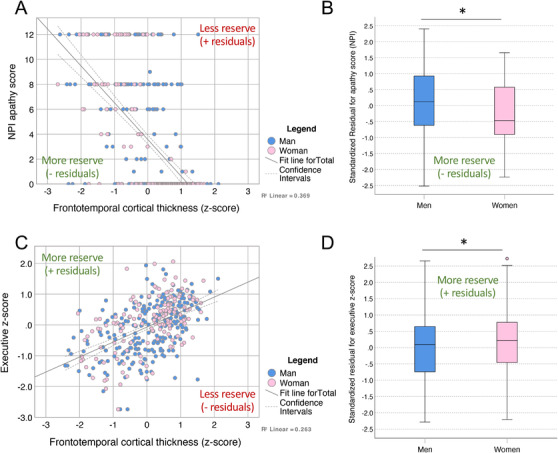
Women with bvFTD have higher behavioral and cognitive reserve than men. (A) Scatter plot between NPI apathy score (higher values represent more apathy) and frontotemporal cortical thickness (negative values represent higher atrophy). (B) Comparison of predicted residuals for NPI apathy score between men and women. Residuals were obtained in a general linear model with NPI apathy score as response variable and age, education, and frontotemporal cortical thickness as predictors. (C) Scatter plots between executive z‐score (negative values represent worst performance) and frontotemporal cortical thickness (negative values represent higher atrophy). (D) Comparison of predicted residuals for executive z‐score between men and women. Residuals were obtained in a general linear model with NPI apathy score as response variable and age, education, and frontotemporal cortical thickness as predictors. * P < .05 (Robust Welch t test with bias‐corrected accelerated bootstrapping on 1000 samples). Abbreviations: bvFTD, behavioral variant of frontotemporal dementia; NPI, Neuropsychiatric Inventory
4. DISCUSSION
In this multicenter study we explored the impact of biological sex on the clinical presentation, prognosis, and cerebral structure of bvFTD, a neurodegenerative syndrome characterized by the selective vulnerability of frontal and temporal lobes.3, 4 We found that women with bvFTD showed a higher burden of frontal atrophy at diagnosis compared to men despite very subtle differences in cognitive and functional impairment, progression, and survival. Next, we followed the residuals approach to investigate sex‐related differences in reserve. We found that for a given amount of atrophy, women performed better‐than‐expected on executive function and displayed fewer general behavioral changes, and particularly fewer changes in apathy, sleep, and appetite than men. This observation suggests that women with bvFTD have higher behavioral and executive reserve than men. Although previous works have reported significant sex differences in Alzheimer's disease (or AD), this is the first study investigating this important topic in bvFTD, one of the first causes of early onset dementia and the first clinical presentation of FTLD.
We found that at diagnosis, women with bvFTD had significantly more atrophy than men, both in the whole sample and when segregating participants in two cohorts. It is important to note that we first performed stratified analyses by sex as recommended in recent consensus paper for the investigation of sex differences.8 By doing this, we avoided the potential mitigation of sex‐related differences in cerebral structure when introducing sex as a covariate in structural analyses.8 We next investigated if the observed sex differences in cortical thinning could be related to disease severity at diagnosis. Indeed, previous observations suggested that the diagnosis of bvFTD could be delayed in women because of higher misdiagnosis with psychiatric diseases compared to men.16 However, we found similar age at disease onset and similar cognitive performance between men and women with bvFTD in both cohorts. The sole difference for cognitive measures was found for the MMSE (discussed below). It is notable that both disease severity measured by CDR plus NACC FTLD, clinical decline, and survival were similar between men and women. Taken together our results suggest that, in our sample, men and women with bvFTD were diagnosed at a similar disease stage and showed a similar trajectory after diagnosis. Additional prospective studies are needed to more precisely define the role of sex in the early identification of FTLD syndromes (ie, need for sex‐adjusted norms) and the effect of sex on prognosis. For this purpose, large multicenter cohorts of both sporadic FTLD syndromes and FTLD‐related mutation carriers would be of particular interest.42, 43
Despite similar diagnostic features at diagnosis, men and women displayed some subtle differences. We replicated previous observations of lower MMSE scores in women with bvFTD.2 However, the effect size of the observed difference in MMSE scores was small (inferior to 0.2). Because the MMSE combines language, memory, and visuospatial tasks, we hypothesize that this combination may have an additive effect to unveil small differences in general cognition between men and women with bvFTD that were not statistically significant when assessing independent tests or cognitive domains. We also found that a slightly higher frequency of delusions and disinhibition in women with bvFTD.2 However, only delusions (and not disinhibition) showed a higher frequency by severity score in women. This finding could be related to the higher frequency of FTLD‐TDP in women with bvFTD in our sample. However, a recent study evidenced a higher frequency of hallucinations (and not delusions) in FTLD‐TDP compared to other FTLD subtypes.44 Moreover, a higher frequency of delusions has also been observed in women with AD.45 Although sex may play a role in the higher frequency of delusions, more studies are needed to explore the neural and pathological substrate of delusions in neurodegenerative dementias.
The inclusion of a large group of age‐ and sex‐matched healthy controls allowed us to study the specific contribution of the diagnostic group (healthy control vs bvFTD), sex, and the interaction between group and sex on frontotemporal thickness. We found a significant sex by group interaction in frontotemporal regions after accounting for the effect of age, education, MMSE, and MRI scan. This is a critical finding of the study, indicating that women have lost more cortical thickness at the moment of bvFTD diagnosis than men after accounting for potential confounding factors. These results, together with the observed null to small effect sizes in the cognitive features and longitudinal decline between men and women with bvFTD, suggested that women could better tolerate frontotemporal pathology. To further test this hypothesis, we followed the residuals approach as a proxy to investigate potential sex differences in reserve. We showed that, for a given amount of atrophy, women with bvFTD had better‐than‐expected scores on executive function and less than expected apathy, sleep, and appetite behavior changes. We interpret these results as indirect evidence of higher brain reserve in women. However, the concept of reserve encompasses different dynamic processes that could also play a significant role in the observed differences.19 It should be noted that many of these processes cannot be captured with structural MRI. Thus, other functional biomarkers and imaging techniques are needed to investigate the specific mechanisms underlying sex‐related differences in cognitive reserve in bvFTD and other FTLD syndromes characterized by prominent frontotemporal neurodegeneration.
Regardless of the specific mechanism, our results open a new window to the study of executive and behavioral reserve in neurodegenerative dementias and add to previous evidence in FTLD indicating the importance of other sociobehavioral proxies of reserve to improve the diagnosis and prognosis of FTLD syndromes. The concept of brain reserve emerged from the observation of a mismatch between cerebral pathology and cognitive changes in AD.23, 46 Previous studies in AD have identified the anterior cingulum as an important region for resilience.47 Because an anterior pattern of neurodegeneration characterizes the bvFTD syndrome, the mechanisms and structures supporting reserve in bvFTD may be different than in AD, a disease characterized by a posterior pattern of neurodegeneration.
4.1. Strengths and limitations
This study has some limitations. First, most participants in this study did not have pathological confirmation of neurodegenerative disease. However, the diagnosis of bvFTD was made by expert clinicians based on established criteria, and participants with decreased diagnostic certainty of underlying FTLD were excluded to avoid the inclusion of participants with the so‐called “phenocopy syndrome.” Of note, the survival of included bvFTD participants was similar to that reported in other large studies after excluding non‐neurodegenerative causes of bvFTD.48 Second, in this study, we focused on biological sex, but more studies are needed to understand the role of gender (understood as a social construct) in the diagnosis of bvFTD and other neurodegenerative dementias. Finally, the rating of behavioral change was based on questionnaires administered to the caregiver, and it is possible that some of the informants' characteristics (including biological sex) could have meaningfully influenced the ratings. However, we also obtained significant results with the NPI behavior count, a measure that is less dependent on the subjective assessment of symptom severity. Moreover, our main results were not limited to behavioral measures, since we also found evidence for higher executive reserve in women. Future studies should examine the effect of biological sex and gender of the informant on NPI ratings and assess the impact of using clinician‐based instruments (such as the NPI‐clinician version).49
5. CONCLUSION
We describe striking differences in frontotemporal atrophy between men and women with bvFTD despite showing less evident differences in baseline clinical characteristics and trajectories. It is notable that our results also support the existence of sex‐related differences in the ability to cope with frontotemporal neurodegeneration and add to the growing evidence highlighting the importance of considering sex for the design of research studies and clinical trials in neurodegenerative dementias.
DECLARATION OF INTEREST
II‐G reports no disclosures; KBC reports no disclosures; SBE reports no disclosures; EA‐U reports no disclosures; AW reports no disclosures; YC reports no disclosures; SYG reports no disclosures; AMS reports no disclosures. DA declares a filed patent application (WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease) and participated in advisory boards from Fujirebio‐Europe and Roche Diagnostics and received speaker honoraria from Fujirebio‐Europe, Nutricia, and Krka Farmacéutica S.L. JF reports no disclosures; RB reports no disclosures; JC reports no disclosures; MFI reports no disclosures; AB‐S reports no disclosures; AL reports no disclosures; LTG reports no disclosures; KP reports no disclosures; KPR reports no disclosures; JHK reports no disclosures. GDR reports receiving grants from the NIH and Tau Consortium during the conduct of the study; grants from Avid Radiopharmaceuticals, Eli Lilly and Company, GE Healthcare, and Life Molecular Imaging outside the submitted work; and personal fees from GE Healthcare, Axon Neurosciences, Merck, Eisai, Roche, and Genentech outside the submitted work. AB receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer's Drug Discovery Foundation, and the Alzheimer's Association. He has served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Arvinas, Asceneuron, Ionis, Lundbeck, Novartis, Passage BIO, Samumed, Third Rock, Toyama, and UCB; and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche, and TauRx. WWS reports no disclosures; VES reports no disclosures; MGT reports no disclosures; BLM reports no disclosures. RSV has served at scientific advisory boards from Wave pharmaceuticals and Ionis‐Biogen. DCP reports no disclosures. AL has served at scientific advisory boards from Fujirebio‐Europe, Nutricia, Biogen, and Roche Diagnostics and has filed a patent application of synaptic markers in neurodegenerative diseases. HJR reports no disclosures.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank the patients and their relatives for their support for this study. The Catalan frontotemporal initiative (CATFI) was funded by the Health Department of the Government of Catalonia (grant PERIS SLT002/16/00408 to AL and RS‐V). This work was supported by NIH grants AG019724, AG032306, AG045390, NS092089, AG045333, AG056749, AG062422, AG061253 (AMS), and AG058752 (KBC), the Larry. L. Hillblom Foundation (2018‐A‐025‐FEL (AMS), 2017‐A‐004‐FEL (KBC), 2014‐A‐004‐NET(JHK)), and the Association for Frontotemporal Degeneration. LTG is supported by NIH K24AG053435. DCP funded by R01AG059794. This study was also supported by the Fondo de Investigaciones Sanitario (FIS), Instituto de Salud Carlos III (PI14/01126 and PI17/01019 to JF, PI13/01532 and PI16/01825 to RB, PI18/00335 to MCI, INT19/00016 and PI18/00435 to DA, PI15/01618 to RR, PI14/1561 and PI18/00326 to J.C., AC14/00013 to RS‐V, and PI17/01896 to AL), the CIBERNED program (Program 1, Alzheimer Disease to AL and SIGNAL study, www.signalstudy.es), the “Marató TV3” grant (20141210 to JF, 044412 to RB, and 20143810 to RS‐V), by Generalitat de Catalunya (2014SGR‐0235 to AL, PERIS SLT006/17/125 to DA, and SLT006/17/00119 to JF), by BBVA foundation (grant to AL), and by a grant from the Fundació Bancaria La Caixa to RB. II‐G and AB‐S are supported by the Global Brain Health Institute (Atlantic Fellows for Equity in Brain Health) and II‐G was supported the Rio Hortega grant (CM17/00074) from Instituto de Salud Carlos III. SB‐E is the recipient of Emili Letang post‐residency research grant from Hospital Clínic de Barcelona. MFI acknowledges support from the Sysley D'Ornano and Jérôme Lejeune Foundations.
Illán‐Gala I, Casaletto KB, Borrego‐Écija S, et al. Sex differences in the behavioral variant of frontotemporal dementia: A new window to executive and behavioral reserve. Alzheimer's Dement. 2021;17:1329–1341. 10.1002/alz.12299
REFERENCES
- 1.Elahi FM, Miller BL. A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol. 2017;13:457‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranasinghe KG, Rankin KP, Lobach IV, et al. Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage. Neurology. 2016;86:600‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140:3329‐3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong A, Toledo JB, Honnorat N, et al. Heterogeneity of neuroanatomical patterns in prodromal Alzheimer's disease: links to cognition, progression and biomarkers. Brain. 2017;140:735‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmonds EC, Delano‐Wood L, Clark LR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false‐positive diagnostic errors. Alzheimers Dement. 2015;11:415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranasinghe KG, Rankin KP, Pressman PS, et al. Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol. 2016;73:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.for the Women's Brain Project and the Alzheimer Precision Medicine Initiative , Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, et al, for the Women's Brain Project and the Alzheimer Precision Medicine Initiative . Sex differences in Alzheimer disease — the gateway to precision medicine. Nat Rev Neurol. 2018;14:457‐469. [DOI] [PubMed] [Google Scholar]
- 9.Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. The Lancet Neurology. 2017;16:661‐676. [DOI] [PubMed] [Google Scholar]
- 10.Snyder HM, Asthana S, Bain L, et al. Sex biology contributions to vulnerability to Alzheimer's disease: a think tank convened by the Women's Alzheimer's Research Initiative. Alzheimers Dement. 2016;12:1186‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer's disease: a call to action. Alzheimers Dement. 2018;14:1171‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauvais‐Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet North Am Ed. 2020;396:565‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferretti MT, Martinkova J, Biskup E, et al. Sex and gender differences in Alzheimer's disease: current challenges and implications for clinical practice: position paper of the Dementia and Cognitive Disorders Panel of the European Academy of Neurology. Eur J Neurol. 2020;27:928‐943. [DOI] [PubMed] [Google Scholar]
- 14.Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Clin Res. 2016;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossink FT, Dols A, Kerssens CJ, et al. Psychiatric diagnoses underlying the phenocopy syndrome of behavioural variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2016;87(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 16.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72:126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onyike CU, Diehl‐Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25:130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyle‐Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736‐1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern Y, Arenaza‐Urquijo EM, Bartrés‐Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018:S1552526018334915. 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol. 2017;81:430‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruigrok ANV, Salimi‐Khorshidi G, Lai M‐C, et al. A meta‐analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotze M, Domin M, Gerlach FH, et al. Novel findings from 2,838 adult brains on sex differences in gray matter brain volume. Sci Rep. 2019;9:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arenaza‐Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90:695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Digma LA, Madsen JR, Rissman RA, et al. Women can bear a bigger burden: ante‐ and post‐mortem evidence for reserve in the face of tau. Brain Communications. 2020;2:fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illan‐Gala I, Montal V, Borrego‐Ecija S, et al. Cortical microstructure in the behavioural variant of frontotemporal dementia: looking beyond atrophy. Brain. 2019;142:1121‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcolea D, Clarimon J, Carmona‐Iragui M, et al. The Sant Pau Initiative on Neurodegeneration (SPIN) cohort: a data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement. 2019;5:597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412‐2414. [DOI] [PubMed] [Google Scholar]
- 30.Miyagawa T, Brushaber D, Syrjanen J, et al. Utility of the global CDR plus NACC FTLD rating and development of scoring rules: data from the ARTFL/LEFFTDS Consortium. Alzheimers Dement. 2020;16:106‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. Mini‐mental state. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 32.Ossenkoppele R, Pijnenburg YAL, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Brain. 2015;138:2732‐2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Illan‐Gala I, Pegueroles J, Montal V, et al. APP‐derived peptides reflect neurodegeneration in frontotemporal dementia. Ann Clin Transl Neurol. 2019;6:2518‐2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308‐2308. [DOI] [PubMed] [Google Scholar]
- 35.Jongenelis K, Pot AM, Eisses AMH, et al. Diagnostic accuracy of the original 30‐item and shortened versions of the Geriatric Depression Scale in nursing home patients. Int J Geriat Psychiatry. 2005;20:1067‐1074. [DOI] [PubMed] [Google Scholar]
- 36.Caswell C, McMillan CT, Xie SX, et al. Genetic predictors of survival in behavioral variant frontotemporal degeneration. Neurology. 2019;93:e1707‐e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal S, Ahmed RM, D'Mello M, et al. Predictors of survival and progression in behavioural variant frontotemporal dementia. Eur J Neurol. 2019;26:774‐779. [DOI] [PubMed] [Google Scholar]
- 38.Massimo L, Xie SX, Rennert L, et al. Occupational attainment influences longitudinal decline in behavioral variant frontotemporal degeneration. Brain Imaging and Behavior. 2019;13:293‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336‐348. [DOI] [PubMed] [Google Scholar]
- 40.Seiger R, Ganger S, Kranz GS, Hahn A, Lanzenberger R. Cortical thickness estimations of FreeSurfer and the CAT12 toolbox in patients with Alzheimer's disease and healthy controls: cortical thickness of FreeSurfer and CAT12. J Neuroimaging. 2018;28:515‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarz CG, Gunter JL, Wiste HJ, et al. A large‐scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. NeuroImage: Clinical. 2016;11:802‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen HJ, Boeve BF, Boxer AL. Tracking disease progression in familial and sporadic frontotemporal lobar degeneration: recent findings from ARTFL and LEFFTDS. Alzheimers Dement. 2020;16:71‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavares TP, Mitchell DGV, Coleman KK, et al. Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2020;91:975‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarioni M, Gami‐Patel P, Timar Y, et al. Frontotemporal dementia: correlations between psychiatric symptoms and pathology. Ann Neurol. 2020. ana.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karttunen K, Karppi P, Hiltunen A, et al. Neuropsychiatric symptoms and Quality of Life in patients with very mild and mild Alzheimer's disease: neuropsychiatric symptoms and Quality of Life in patients with very mild and mild Alzheimer's disease. Int J Geriatr Psychiatry. 2011;26:473‐482. [DOI] [PubMed] [Google Scholar]
- 46.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. The Lancet Neurology. 2012;11:1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arenaza‐Urquijo EM, Przybelski SA, Lesnick TL, et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. 2019;142:1134‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcin B, Lillo P, Hornberger M, et al. Determinants of survival in behavioral variant frontotemporal dementia. Neurology. 2009;73:1656‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory‐Clinician rating scale (NPI‐C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22:984‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


