Abstract
Most animal species consist of two distinct sexes. At the morphological, physiological, and behavioural levels the differences between males and females are numerous and dramatic, yet at the genomic level they are often slight or absent. This disconnect is overcome because simple genetic differences or environmental signals are able to direct the sex-specific expression of a shared genome. A canonical picture of how this process works in insects emerged from decades of work on Drosophila. But recent years have seen an explosion of molecular-genetic and developmental work on a broad range of insects. Drawing these studies together, we describe the evolution of sexual dimorphism from a comparative perspective and argue that insect sex determination and differentiation systems are composites of rapidly evolving and highly conserved elements.
Introduction
Anisogamy is the definitive sex difference. The bimodality in gamete size it describes represents the starting point of a cascade of evolutionary pressures that have generated remarkable divergence in the morphology, physiology, and behaviour of the sexes [1]. But sexual dimorphism presents a paradox: how can a genome largely shared between the sexes give rise to such different forms? A powerful resolution is via sex-specific expression of shared genes. In the latter part of the 20th century, experiments in the fruit fly Drosophila melanogaster helped construct a canonical picture of the mechanisms through which this is achieved in insects. In this review, we discuss how recent discoveries at each stage of sex determination and differentiation both challenge and expand upon that canon.
The canonical view of insect sex determination and differentiation
In the canonical Drosophila sexual differentiation pathway [reviewed by 2,3], sex is largely defined at the level of the individual cell. Cell autonomy hinges on the ability of two autosomal transcription factors to produce sex-specific isoforms. Key among these factors is doublesex (dsx), which functions in a wide range of somatic tissues; the other, fruitless (fru), is mainly involved in sex-specific differentiation of the nervous system. The male and female isoforms of Dsx share a common DNA-binding domain but possess sex-specific C-termini. Thus, the two isoforms can have sex-biased [e.g. 4] or even opposite [e.g. 5] effects on the expression of their target genes.
In the canonical pathway, male isoforms of dsx and fru are produced by default, with female-specific isoforms requiring the splicing factor transformer (tra) and its partner transformer-2 (tra-2). Although tra-2 is active in the soma of both sexes, functional Tra protein is only produced in females. Female-specific splicing of tra is activated by Sex lethal (Sxl), a sex-determining master switch that also controls dosage compensation via its regulation of male-specific lethal 2 (msl-2). Sxl expression is activated by the dosage of several X-linked regulatory proteins, which in turn depends on the number of X-chromosomes [6]. Consequently, while D. melanogaster has X and Y chromosomes, it is not the presence of Y that specifies maleness, but rather the number of X’s – one in males, and two in females (Fig. 1).
Figure 1. Divergent primary sex determination signals in Diptera converge on sex-specific doublesex splicing.
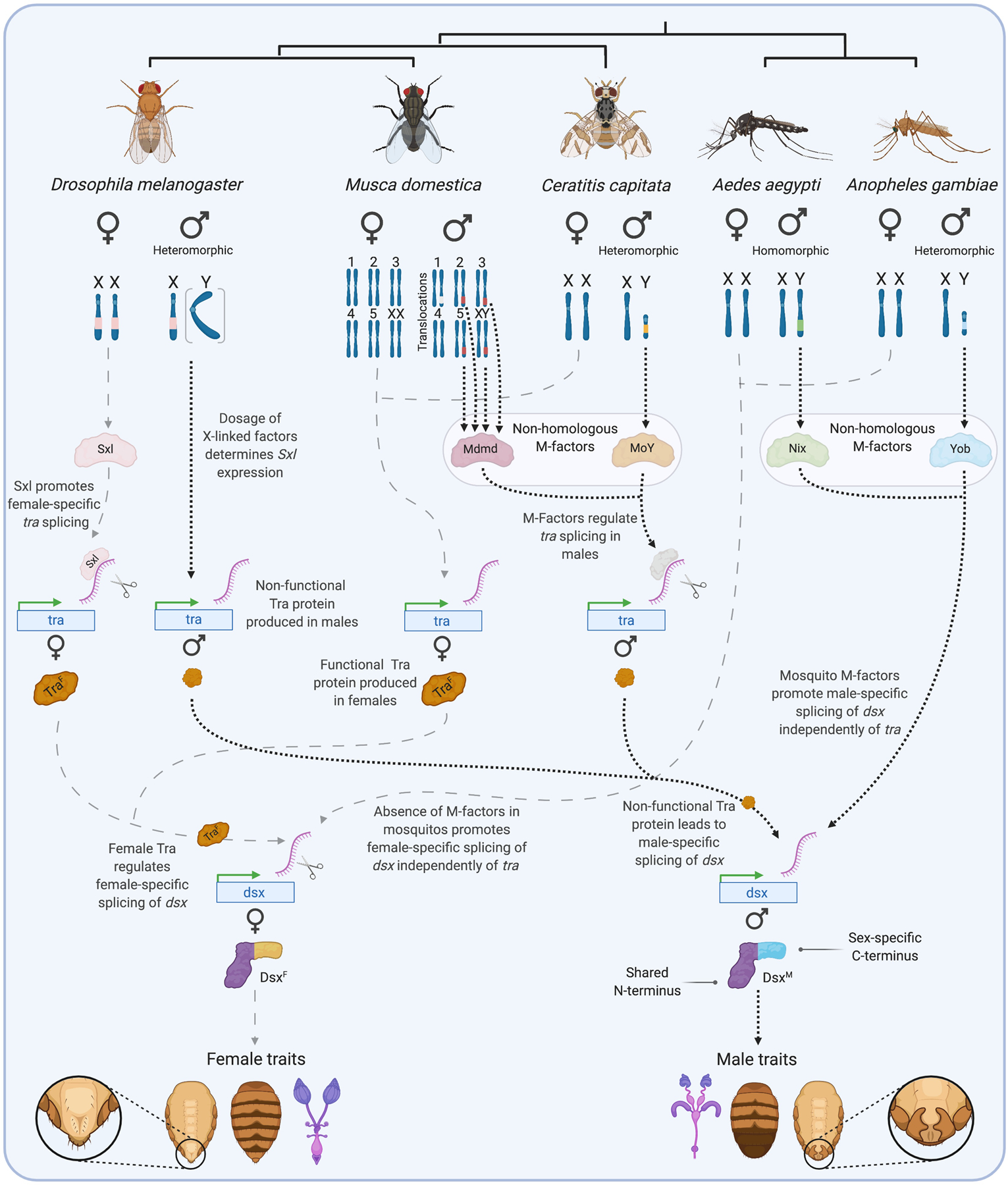
In the 5 Dipterans shown, sex is specified at the level of the individual cell by factors associated with sex (or proto-sex) chromosomes. These male- and female-defining chromosomes vary between species from being highly similar to each other (homomorphic) to highly divergent (heteromorphic) in morphology and gene content. In D. melanogaster, the number of X chromosomes determines the dosage of a set of X-linked factors that regulate the expression state of Sex lethal (Sxl). High dosage (XX) activates Sxl expression, the protein product of which promotes female-specific splicing of transformer (tra). The resulting female-specific isoform of Transformer protein (TraF) is required for the female-specific splicing of the transcription factor doublesex (dsx). Maleness is defined by the lower dosage of X-linked factors, rather than the presence of a Y-chromosome (e.g., X0 individuals are males). Having a single X chromosome leaves Sxl inactive in males, and the male-specific isoform of Transformer is produced (TraM). The presence of a premature stop codon renders TraM non-functional, which in turn leads to the production of the male-specific isoform of dsx. Musca domestica, Ceratitis capitata, Aedes aegypti, and Anopheles gambiae each use independently evolved (non-homologous) dominant M-factors to determine maleness. These are encoded on the Y-chromosome in most cases, but translocations to autosomes (turning them into proto-sex chromosomes) have been detected in different M. domestica populations. Whether the M-factor found on chromosome 1 in one population of M. domestica (shown in white) is a derived Mdmd sequence or an independently evolved M-factor remains unclear. In M. domestica and C. capitata, the presence of M-factors leads to the production of non-functional TraM and therefore, as in D. melanogaster, the production of the male-specific isoform of Dsx. No tra homolog has been found in Ae. aegypti or An. gambiae. Their M-factors, Nix and Yob respectively, are therefore presumed to determine the male-specific splicing of dsx by an as of yet unknown, tra-independent mechanism. The male and female isoforms of Dsx share a DNA-binding N-terminus but bear different C-termini, allowing them to regulate downstream target genes in a sex-specific manner, leading to the development of sex-specific traits. Figure created using BioRender.
Challenging the canon: rapid evolution of primary sex signals
Sex determination systems diversify rapidly among species [7]. Insects are no exception. Haplodiploid honeybees use zygosity at the sex-determining locus, booklice paternal genome elimination, and butterflies ZW chromosome systems with females as the heterogametic sex [8,9]. The speed and relative freedom with which sex determining signals evolve has been best studied in Diptera, where species are known to have gained and lost heteromorphic sex chromosomes, replaced original sex chromosomes with new ones, incorporated other chromosomal elements into the original sex chromosome, or transitioned from male to female heterogamety [10–13]. But it is not the sex chromosomes themselves that define sex, but rather the sex determining signals they encode. Indeed, evolution of new sex determining signals may initiate changes in sex chromosome structure as well as switches from old to new sex chromosomes.
Primary sex-determining signals have evolved many times independently and act via different mechanisms. For example, Drosophila’s system of measuring X-chromosome dosage via Sxl appears to be restricted to the Drosophilinae [14,15]. A phylogenetically diverse array of Dipterans instead use dominant male-determining genes (‘M-factors’), as in the case of the mosquitos Anopheles gambiae (Yob) and Aedes aegypti (Nix), the Medfly Ceratitis capitata (MoY), and the housefly Musca domestica (Mdmd) (Fig. 1). These M-factors are all unrelated to each other, reflecting their independent evolution [16–20]. Other non-homologous M-factors no doubt exist in other fly groups [13]. Where closely related species share a homologous M-factor, its sequence can diverge rapidly (e.g. Aedes Nix)[21]. In M. domestica, individuals can even vary in which chromosome encodes the M-factor – Mdmd has been detected on four of the six chromosomes (Y, II, III, and V) in different populations [16,22]. In most cases the origin of M-factors is unknown. An exception is Mdmd, which arose through the duplication and subsequent neofunctionalization of CWC22 (nucampholin), a spliceosomal factor gene [16]. Aedes Nix also encodes a potential splicing factor, suggesting this may be a common starting point for M-factors [18].
A pattern similar to the diversity of unrelated M-factors in Diptera may be found in Hymenoptera. Although all hymenopterans are haplodiploid, the ploidy signal is mediated by different genes and via different mechanisms. In honeybees, sex is determined zygotically by the csd locus, a paralog of tra [23]. But in the wasp Nasonia vitripennis, sex depends on the maternal imprinting of an unrelated gene, wom [24]. wom is a recently evolved chimeric gene, not found even in all species of the same family (Pteromalidae), suggesting that the proximate mechanisms of haplodiploid sex determination may be as varied as in the case of XY heterogametic systems. Rapid diversification of sex-determining signals may be due to intragenomic sexual conflict, sex ratio distorters, changing links between environmental conditions and sex-specific fitness, and other evolutionary factors [7]. The extent to which the rate of their diversification varies across taxa, and the reasons behind this variation, remain key questions for future work.
Challenging the canon: translating primary sex signals into the sex-specific splicing of dsx
Downstream, the story is different. Diverse sex determination inputs, from X chromosome dosage to M-factors to haplodiploidy, converge on the tra-dsx splicing cascade, which is present in early-branching insect clades like cockroaches and certainly ancestral to the Holometabola [25]. But even this deeply conserved mechanism is not universal. The entire order Lepidoptera have lost the tra gene, but maintain sex-specific dsx activity [26]. How, then, is the sex-specific splicing of dsx achieved? Studies of the silkworm Bombyx mori provide an answer. In this species, females are the heterogametic sex, bearing both Z and W chromosomes; males have two Zs. The Z-chromosome carries the Masculinizer (Masc) gene, which encodes a CCCH-tandem zinc finger protein that regulates maleness via its control of the sex-specific splicing of dsx [27,28]. The homologues of Masc in Trilocha varians and Plutella xylostella are similarly required for sex-specific splicing of dsx, suggesting deep conservation of this mechanism within Lepidoptera [29,30].
Masc functions by regulating the male-specific transcription of RNA-binding protein 3 (RBP3/Aret), which binds to one of the two dsx exons that are skipped in males and directly interacts with RBP1/Lark, which binds to the other [31]. The W chromosome encodes a dominant feminizing factor, a PIWI-interacting RNA (piRNA) produced from the Feminizer precursor [27]. Fem piRNA guides the assembly of a protein complex that suppresses Masc expression to promote the female-specific splicing of dsx [32]. piRNAs are thought to principally function in protecting the germline from transposons, which makes this derived role in Lepidopteran sex determination surprising. But while the participation of piRNAs appears novel, gene regulation by small RNAs during sex determination is not. Indeed, miR-1–3p appears to perform a role in the oriental fruit fly Bactrocera dorsalis that is opposite to that of Fem in silkworms [33]. miR-1–3p, which is transcribed at high levels in males, transduces an uncharacterized Y-linked M-factor signal to promote the canonical male-specific splicing of tra, which in turn converges on the conserved sex-specific splicing of dsx. The mechanistic simplicity and efficiency with which small RNAs can regulate the expression of their target genes may make them readily evolvable, and therefore common, intermediaries between rapidly evolving primary sex determination signals and regulators of dsx splicing.
tra has also not been detected in the genomes of a small number of non-Lepidopteran insect species, including Aedes, Anopheles, and other mosquitos [26]. If these species have lost tra, it remains to be seen how Nix, Yob, and other such M-factors control dsx splicing in its absence (Fig. 1).
Challenging the canon: not all insects rely on sex-specific dsx isoforms for sexual differentiation
dsx is an arthropod-specific paralog from the wider doublesex/mab-3 related (Dmrt) family of transcription factors [34]. Members of this ancient gene family appear to be the only conserved element of sexual differentiation pathways across Metazoa [35,36]. Despite this conservation, using sex-specific isoforms of a Dmrt gene to direct both male and female development is an insect innovation; vertebrates, nematodes, mites, and crustaceans instead use male-specific transcription of Dmrt genes to direct elements of male-specific development [36–40]. How did this transition from sex-specific transcription to the canonical sex-specific splicing of dsx occur?
Recent work suggests two key processes were at play [25]. Firstly, the expansion of dsx function from a “male gene” that overrides a default female pathway to a bifunctional switch actively required in both sexes [25,40]. Male and female dsx isoforms are present as far back in the insect phylogeny as cockroaches, but outside of the Holometabola the female isoforms appear dispensable for female differentiation [25,39,41]. Why female isoforms first evolved and how they later came to play critical functions in female sexual differentiation remains unknown. Secondly, while dsx function expanded, tra function narrowed. As in the canonical Drosophila pathway, basal insects such as cockroaches require tra for both female-specific differentiation and the sex-specific splicing of dsx. But they use tra differently. In these basal groups, tra’s role in female development is independent of dsx and does not involve the production of sex-specific tra isoforms [25]. Thus, tra appears to have transitioned from controlling female development via at least partly dsx-independent mechanisms to being a dedicated regulator of dsx. The selective forces behind these transitions, as well as any consequences that non-canonical variants of the tra-dsx cascade have for the manifestation of sexual dimorphism, remain significant outstanding questions.
Expanding the canon: changes in the expression and targets of dsx underlie the origin and diversification of sex-specific traits
Two processes are required for the evolution of sexually dimorphic traits in insects, and dsx is central to both (Fig. 2). One is the establishment of sex-specific identity in a previously monomorphic tissue. This process is facilitated by the cell-autonomous nature of dsx function: dsx transcription gives cells the capacity for sex-specific differentiation – but not all cells transcribe dsx [42–46]. From this sexual mosaicism emerges a prediction about the origin of new sexually dimorphic traits: by changing which cells express dsx, tissues can acquire (or lose) sex-specific functions. There is good evidence in support of this: the evolution of novel male-specific grasping structures in Drosophila legs, and the male-specific scent organs in Bicyclus butterflies, are both associated with the evolution of new spatial domains of dsx expression [43,47,48]. Localized upregulation of dsx also precedes the appearance of visible dimorphism in developing Trypoxylus dichotomus beetle horns, suggesting that the establishment of sexual identity by dsx early in the development of novel traits is critical to their dimorphic nature [45]. The evolutionary malleability in the spatiotemporal control of dsx expression that these studies demonstrate is afforded by modular enhancers. In Drosophila, several distinct enhancers have been identified that are collectively required for sex-specific development of leg sensory organs [49].
Figure 2. The origin and diversification of a new sex-specific trait.
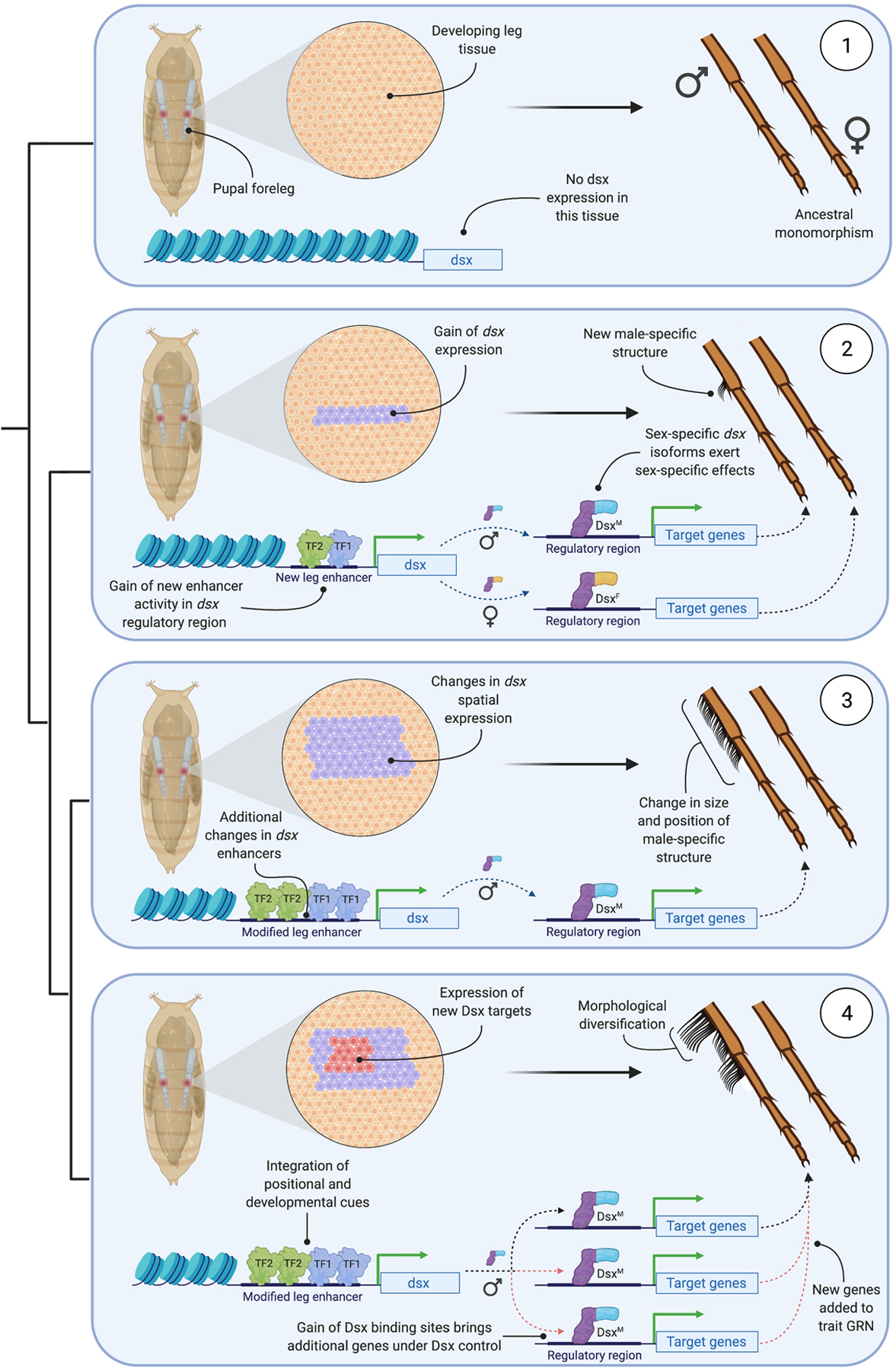
This schematic describes a four-part model for the origin and subsequent morphological diversification of a sex-specific structure, in this case a modified row of bristles (a ‘sex comb’) on the male Drosophila foreleg. Species 1 displays the ancestral state of monomorphism. Here, developing leg cells do not express the transcription factor doublesex (dsx) and therefore lack the capacity for sex-specific differentiation. In species 2, changes in the sequence of the regulatory region controlling dsx expression enable the binding of position- and stage-determining transcription factors (TF). These TFs activate dsx expression in a subset of leg cells during a particular developmental window. dsx is alternatively spliced to give rise to male- and female-specific isoforms (DsxM and DsxF), which bind to the regulatory regions of target genes via a shared DNA-binding domain and impart sex-specific effects on target gene expression through sex-specific C-termini. The localized, sex-specific regulation of gene expression that results enables the development of a novel structure only in males. In species 3, additional changes in the dsx enhancers generate changes in the binding of its upstream regulators. This leads to changes in the spatiotemporal pattern of dsx expression among developing leg cells, which in turn produces changes in the size and position of the male-specific structure. In species 4, Dsx has acquired new downstream target genes due to the gain of Dsx-binding sites in the regulatory regions of the new targets. Incorporation of the new targets into the gene regulatory network (‘GRN’) that controls the development of the male-specific structure leads to further morphological diversification. Figure created using BioRender.
Controlling the pattern of dsx expression in time and space lays the foundations for sexual dimorphism, but not the endpoint. The second process therefore is the establishment of a repertoire of dsx target genes. Work on the development of dung beetle (Onthophagus) horns suggests that this repertoire can expand and shift rapidly [40]. Moreover, it needn’t be the target genes that change, it can also be the direction of the regulatory effect conferred by dsx. A rare sex-reversal in the dimorphism of O. sagittarius horns appears to be driven by the two dsx isoforms swapping regulatory roles relative to the ancestral state: male dsx evolving from stimulating horn growth to repressing it, and female dsx evolving the reverse [50].
Genes can be added to or lost from the repertoire of dsx targets by the gain (or loss) of Dsx binding sites in their enhancers, or by structural changes in Dsx protein domains [51]. For example, transitions from sexual monomorphism to dimorphism (and vice versa) in the pheromone profile of Drosophilid flies have been partly driven by gain (and loss) of a Dsx binding site in the enhancer of the hydrocarbon-processing enzyme desat-F [4]. Because Dsx targets may be co-regulated by other transcription factors, multiple cues alongside sex, such as position and developmental stage, may be integrated. In the case of male-specific abdominal pigmentation in D. melanogaster, sexual dimorphism evolved via the gain of a Dsx binding site in the enhancer of bric á brac (bab), a gene that is also regulated by the position-specifying HOX gene Abd-b [5,52]. Combinatorial changes in the spacing, polarity, and number of transcription factor binding sites within bab enhancers are associated with inter- and intra-specific changes in the position and extent of sex-specific pigmentation across Drosophila species [5,53].
Changes in the targets and regulatory effects of Dsx are likely to represent a major channel through which sexually dimorphic traits diversify. The cell-autonomous nature of Dsx action, combined with the co-regulation of its downstream targets by cell-type and developmental stage specific transcription factors, affords a high level of modularity to the development of a given sex-specific trait. Such modularity may provide a high level of evolutionary lability, allowing different aspects of sexual dimorphism to evolve independently and, crucially, without disrupting conserved sexual differentiation programs in other tissues [53,54].
Expanding the canon: dsx, a master regulator of sex-limited intraspecific polymorphisms
Due to the modular control of its expression, a broad and evolving set of target genes, and the ability to switch roles between activator and suppressor, dsx can control wide-ranging morphological change within as well as between species. Some swallowtail butterflies (Papilio) have multiple discrete female morphs, some of which mimic the warning coloration of toxic model species, while the males are monomorphic. The differences between female morphs of P. polytes are controlled by different dsx alleles, which act as a switch between a default, male-like colour pattern and different mimetic morphs [55,56]. In P. polytes, the dsx-H allele controls wing coloration by activating “mimetic” genes that include Wnt1 and Wnt6, and repressing “non-mimetic” genes such as abd-a [57]. dsx mimicry alleles segregate within multiple Papilio species and show species-specific patterns of genetic differentiation [58–61]. This differentiation has been interpreted as pointing to independent evolutionary origins of dsx alleles in the genus Papilio [58,59]. However, the recent identification of dsx polymorphisms that are shared across Papilio species instead points to shared inheritance from a common polymorphic ancestor, rather than recurrent, convergent evolution at the same locus [60]. In contrast to incomplete lineage sorting, where ancestral polymorphisms are maintained across lineages, the pattern of genetic divergence observed among Papilio dsx alleles is best described by allelic turnover, where alleles from a polymorphic ancestor are subsequently replaced by their own allelic descendants [60]. Resolving the evolutionary history of these alleles is key to understanding the repeatability of dsx-dependent female-limited polymorphism. Indeed, evolutionary change in dsx is not the only route to female-limited mimicry polymorphism, as evidenced by the African mocker swallowtail (Papilio dardanus), where mimetic phenotypes are controlled by a polyalleic locus that contains the transcription factor genes engrailed and invected [62,63], and Hypolimnas misippus (Nymphalidae), where a novel, though unidentified, color patterning locus has been detected [64].
Challenging the canon: sexual differentiation affected by hormone signaling
Insects define sexual identity at the level of the individual cell, through cell-autonomous control of transcription and splicing. However, non-cell-autonomous, systemic hormonal inputs [reviewed by 65] are increasingly recognized as critical to the development and maintenance of some dimorphic traits [66,67]. For example, ecdysteroids and their receptors have been implicated in a variety of sex-specific processes in Drosophila, including ejaculate production, female post-mating gut growth, and courtship [66,68,69]. Available data currently support two mechanisms through which hormones can affect sexually dimorphic trait development (Fig. 3). Firstly, through sex differences in hormone titer (Fig. 3a). At present, the only conclusive demonstration of this mechanism comes from sex-specific seasonal wing patterns in the butterfly Bicyclus anynana [70]. Early in development, dry season morphs of both sexes express the Ecydsone Receptor (EcR) in a similar number of dorsal eyespot cells. Later, the titer of the hormone 20-hydroxyecdysone diverges between the sexes, inducing a corresponding divergence in the rate of division of eyespot cells that ultimately generates sex differences in eyespot size.
Figure 3. Hormonal inputs into insect sexual dimorphism.
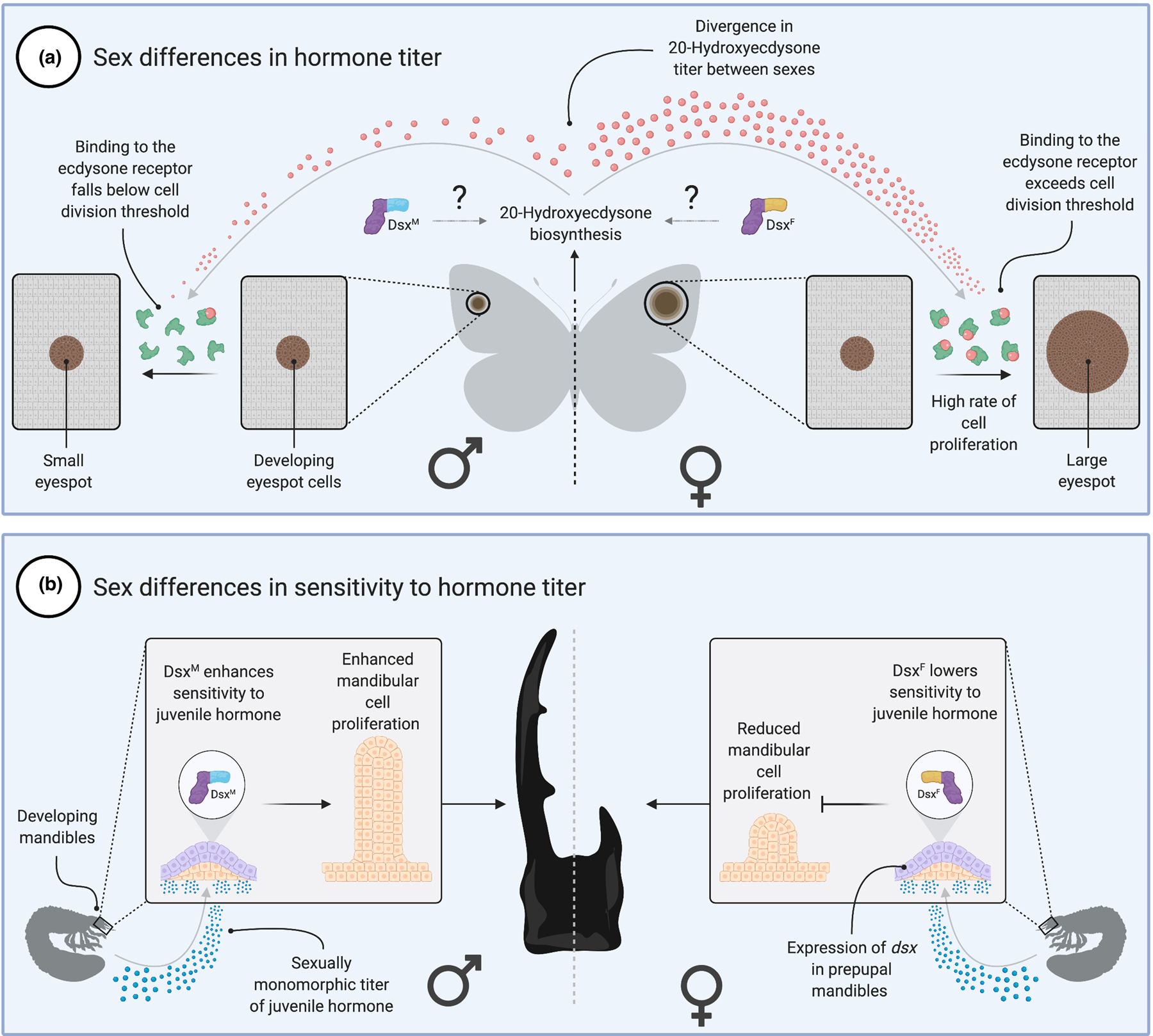
Two principal mechanisms exist through which hormones can deliver sex-specific effects in insects. (A) Sex differences in hormone titer. Developing eyespot cells in the butterfly Bicyclus anynana express ecdysone receptor. The titer of circulating 20-hydroxyecdysone in females leads to a binding threshold being exceeded, which causes the cells to proliferate and the eyespot to grow. The lower titer in males fails to exceed the binding threshold and the cells fail to proliferate. What generates the divergence in hormone titer is unclear, but one potential mechanism is the direct or indirect regulation of enzymes in the ecdysone biosynthesis pathway by DsxM and/or DsxF. (B) Sex differences in sensitivity to hormones. Expression of dsx in the developing prepupal mandibles of the stag beetle Cyclommatus metallifer changes the sensitivity of mandibular cell proliferation to juvenile hormone. DsxM increases sensitivity, leading to enlarged mandibles in males. DsxF reduces sensitivity, leading to small mandibles in females. Figure created using BioRender.
The second mechanism is through changes in the sensitivity of a developing tissue to a fixed hormone titer (Fig. 3b). Sex- and trait-specific sensitivity to insulin/IGF, juvenile hormone, and ecdysone signalling pathways is variously thought to underlie dimorphic horn and mandible growth in a number of beetle species [71–75]. Work in the stag beetle (Cyclommatus metallifer) has shown that sex-specific isoforms of dsx differentially regulate the sensitivity of mandible cells to juvenile hormone, promoting exaggerated growth in males and repressing it in females [74]. This illustrates the interplay between cell-autonomous and hormonal inputs into the development of sexually dimorphic traits. Rather than serving as alternative ways of generating sexual dimorphism, systemic hormones may act by co-regulating the target genes of dsx and tra. In other cases, the hormone titers themselves may be controlled via dsx- and tra-dependent mechanisms in hormone-secreting cells.
Conclusion
A canonical view of sex determination and differentiation in insects emerged from work on D. melanogaster. But as we broaden our taxonomic sampling, this canon is repeatedly challenged and expanded. The evolutionary history of insect sexual development increasingly appears to conform to the developmental hourglass model: while sex-determining signals and downstream target genes diverge rapidly, doublesex acts as a conserved linchpin, defining and expanding sex-specific identity into new tissues to dramatic and beautiful effect.
Acknowledgements
This work was supported by a Long-Term Fellowship from the Human Frontier Science Program Organization awarded to B.R.H. (LT000123/2020-L) and NIH grant R35GM122592 to A.K. We thank Antonia Monteiro and an anonymous reviewer for their comments on the manuscript.
Funding
Funding was received for this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict of interest exists.
Intellectual Property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
References
- 1.Parker GA: The Sexual Cascade and the Rise of Pre-Ejaculatory (Darwinian) Sexual Selection, Sex Roles, and Sexual Conflict. Cold Spring Harb Perspect Biol 2014, 6:a017509–a017509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millington JW, Rideout EJ: Sex differences in Drosophila development and physiology. Curr Opin Physiol 2018, 6:46–56. [Google Scholar]
- 3.Camara N, Whitworth C, Van Doren M: Chapter 3 The Creation of Sexual Dimorphism in the Drosophila Soma. In Current Topics in Developmental Biology. 2008:65–107. [DOI] [PubMed]
- 4.Shirangi TR, Dufour HD, Williams TM, Carroll SB: Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol 2009, 7:e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, Carroll SB: The Regulation and Evolution of a Genetic Switch Controlling Sexually Dimorphic Traits in Drosophila. Cell 2008, 134:610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson JW, Quintero JJ: Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol 2007, 5:2821–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman T-L, Hahn MW, Kitano J, Mayrose I, Ming R, et al. : Sex Determination: Why So Many Ways of Doing It? PLoS Biol 2014, 12:e1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodson CN, Hamilton PT, Dilworth D, Nelson CJ, Curtis CI, Perlman SJ: Paternal genome elimination in Liposcelis booklice (Insecta: Psocodea). Genetics 2017, 206:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gempe T, Beye M: Function and evolution of sex determination mechanisms, genes and pathways in insects. BioEssays 2011, 33:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andere AA, Pimsler ML, Tarone AM, Picard CJ: The genomes of a monogenic fly: views of primitive sex chromosomes. Sci Rep 2020, 10:15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicoso B, Bachtrog D: Numerous Transitions of Sex Chromosomes in Diptera. PLoS Biol 2015, 13:e1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicoso B, Bachtrog D: Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 2013, 499:332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meisel RP, Olafson PU, Adhikari K, Guerrero FD, Konganti K, Benoit JB: Sex chromosome evolution in muscid flies. G3 Genes, Genomes, Genet 2020, 10:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traut W, Niimi T, Ikeo K, Sahara K: Phylogeny of the sex-determining gene Sex-lethal in insects. Genome 2006, 49:254–262. [DOI] [PubMed] [Google Scholar]
- 15.Cline TW, Dorsett M, Sun S, Harrison MM, Dines J, Sefton L, Megna L: Evolution of the drosophila feminizing switch gene Sex-lethal. Genetics 2010, 186:1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16. Sharma A, Heinze SD, Wu Y, Kohlbrenner T, Morilla I, Brunner C, Wimmer EA, Van De Zande L, Robinson MD, Beukeboom LW, et al. : Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science (80-) 2017, 356:642–645. Sharma et al. identify the dominant male-determining gene of the house fly Musca domestica (Mdmd). This recently-evolved gene acts upstream of the widely conserved transformer/doublesex splicing cascade, demonstrating that new sex-determining signals can co-opt deeply conserved sexual differentiation pathways. Mdmd evolved by duplication and neofunctionalization of a generic splicing factor, suggesting that the tra/dsx pathway may predispose insects to evolving sex-determining mechanisms based on alternative splicing. Mdmd can translocate from the Y chromosome to different autosomes, potentially facilitating the evolution of new sex chromosomes.
- *17. Meccariello A, Salvemini M, Primo P, Hall B, Koskinioti P, Dalíková M, Gravina A, Gucciardino MA, Forlenza F, Gregoriou ME, et al. : Maleness-on-the-Y (MoY) orchestrates male sex determination in major agricultural fruit fly pests. Science (80-) 2019, 365:1457–1460. Meccariello et al. identify the dominant male-determining gene of the Medfly Ceratitis capitata (MoY). Importantly, this gene is unrelated to the male-determining gene Mdmd previously identified in Musca domestica by Sharma et al. (2017), and appears to be limited to the Tephritidae family. However, like Mdmd, MoY acts by regulating the sex-specific splicing of tra – although the exact molecular function of MoY remains to be determined.
- 18.Hall AB, Basu S, Jiang X, Qi Y, Timoshevskiy VA, Biedler JK, Sharakhova MV, Elahi R, Anderson MAE, Chen XG, et al. : A male-determining factor in the mosquito Aedes aegypti. Science (80-) 2015, 348:1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krzywinska E, Dennison NJ, Lycett GJ, Krzywinski J: A maleness gene in the malaria mosquito Anopheles gambiae. Science (80-) 2016, 353:67–69. [DOI] [PubMed] [Google Scholar]
- 20.Aryan A, Anderson MAE, Biedler JK, Qi Y, Overcash JM, Naumenko AN, Sharakhova MV, Mao C, Adelman ZN, Tu Z: Nix alone is sufficient to convert female Aedes aegypti into fertile males and myo-sex is needed for male flight. Proc Natl Acad Sci U S A 2020, 117:17702–17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, Jin B, Li X, Zhao Y, Gu J, Biedler JK, Tu ZJ, Chen XG: Nix is a male-determining factor in the Asian tiger mosquito Aedes albopictus. Insect Biochem Mol Biol 2020, 118:103311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamm RL, Meisel RP, Scott JG: The evolving puzzle of autosomal versus Y-linked male determination in Musca domestica. G3 Genes, Genomes, Genet 2015, 5:371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW: The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 2003, 114:419–429. [DOI] [PubMed] [Google Scholar]
- **24. Zou Y, Geuverink E, Beukeboom LW, Verhulst EC, van de Zande L: A chimeric gene paternally instructs female sex determination in the haplodiploid wasp Nasonia. Science (80-) 2020, 370:1115–1118. Zou et al. identify the gene (wom) that mediates haplodiploid sex determination in the parasitoid wasp Nasonia vitripennis. wom is unrelated to the csd locus that was shown to mediate haplodiploid sex in honeybees (Beye et al. 2003); moreover, csd acts zygotically, whereas wom acts via sex-specific parental imprinting. This contrast suggests that the molecular mechanisms of haplodiploid sex determination in Hymenoptera may turn out to be as diverse as the mechanisms of male-heterogametic (XY) sex in Diptera.
- **25. Wexler J, Delaney EK, Belles X, Schal C, Wada-Katsumata A, Amicucci MJ, Kopp A: Hemimetabolous insects elucidate the origin of sexual development via alternative splicing. Elife 2019, 8. This paper attempts to reconstruct the evolutionary history of the canonical insect sexual differentiation pathway. In the ancestral condition, dsx is expressed only in males and promotes male-specific traits; in the derived condition found in the Holometabola, dsx actively promotes both male and female differentiation via alternatively spliced isoforms. Wexler et al. show that hemimetabolous insects orders represent different stages in the transition from the transcription-based to the splicing-based mode of sexual development. They suggest that the canonical tra/dsx pathway evolved via merger between expanding dsx function (from males to both sexes) and narrowing tra function (from a broad-range splicing factor to a dedicated regulator of dsx).
- 26.Geuverink E, Beukeboom LW: Phylogenetic distribution and evolutionary dynamics of the sex determination genes doublesex and transformer in insects. Sex Dev 2014, 8:38–49. [DOI] [PubMed] [Google Scholar]
- **27. Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, Arai Y, Ishihara G, Kawaoka S, Sugano S, Shimada T, et al. : A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509:633–636. This is a foundational work that showed that in the silkworm Bombyx mori (which, like other Lepidoptera, has female-heterogametic, ZW mode of sex determination), female sex is specified by the dominant Fem locus located on the W chromosome. However, Fem is not a conventional protein-coding gene; rather, it encodes a Piwi-interacting RNA (piRNA). The Fem piRNA silences the Masc gene, which is located on the Z chromosome and promotes male development via male-specific dsx splicing; thus, Fem activity leads to female-specific dsx splicing and female development. This highly unusual mechanism shows that a wide variety of sex determination systems can all act by regulating sex-specific dsx splicing.
- 28.Sakai H, Sumitani M, Chikami Y, Yahata K, Uchino K, Kiuchi T, Katsuma S, Aoki F, Sezutsu H, Suzuki MG: Transgenic Expression of the piRNA-Resistant Masculinizer Gene Induces Female-Specific Lethality and Partial Female-to-Male Sex Reversal in the Silkworm, Bombyx mori. PLoS Genet 2016, 12:e1006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Kiuchi T, Kawamoto M, Shimada T, Katsuma S: Identification and functional analysis of a Masculinizer orthologue in Trilocha varians (Lepidoptera: Bombycidae). Insect Mol Biol 2015, 24:561–569. [DOI] [PubMed] [Google Scholar]
- 30.Harvey-Samuel T, Norman VC, Carter R, Lovett E, Alphey L: Identification and characterization of a Masculinizer homologue in the diamondback moth, Plutella xylostella. Insect Mol Biol 2020, 29:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31. Zheng ZZ, Sun X, Zhang B, Pu J, Jiang ZY, Li M, Fan YJ, Xu YZ: Alternative splicing regulation of doublesex gene by RNA-binding proteins in the silkworm Bombyx mori. RNA Biol 2019, 16:809–820. Although sexually dimorphic development in Lepidoptera, as in other holometabolous insects, depends on sex-specific dsx splicing, all examined Lepidoptera lack tra, the key regulator of dsx splicing in other Holometabola. Zheng et al. identify a group of RNA-binding proteins that promote male-specific dsx splicing in the silkworm Bombyx mori. One of these proteins itself has sex-specific isoforms produced under the control of the Masc gene, which promotes male development in Bombyx. This work suggests that Lepidopteran sexual differentiation is still based on a cascade of alternative splicing, but a new set of regulators has taken over this function from the ancestral transformer.
- 32.Li Z, You L, Yan D, James AA, Huang Y, Tan A: Bombyx mori histone methyltransferase BmAsh2 is essential for silkworm piRNA-mediated sex determination. PLoS Genet 2018, 14:e1007245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33. Peng W, Yu S, Handler AM, Tu Z, Saccone G, Xi Z, Zhang H: miRNA-1–3p is an early embryonic male sex-determining factor in the Oriental fruit fly Bactrocera dorsalis. Nat Commun 2020, 11:932. Peng et al. show that an autosomal microRNA, miRNA-1–3p, promotes male development in the Tephritid fly Bactrocera dorsalis by downregulating tra, which is required for female-specific development. In combination with the work in Bombyx mori and other Lepidopterans, this report suggests that gene regulation by small RNAs may be a relatively common theme in insect sex determination.
- 34.Wexler JR, Plachetzki DC, Kopp A: Pan-metazoan phylogeny of the DMRT gene family: a framework for functional studies. Dev Genes Evol 2014, 224:175–181. [DOI] [PubMed] [Google Scholar]
- 35.Kopp A: Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet 2012, 28:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matson CK, Zarkower D: Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet 2012, 13:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Li F, Yu K, Xiang J: Identification and characterization of a doublesex gene which regulates the expression of insulin-like androgenic gland hormone in Fenneropenaeus chinensis. Gene 2018, 649:1–7. [DOI] [PubMed] [Google Scholar]
- 38.Pomerantz AF, Hoy MA: Expression analysis of Drosophila doublesex, transformer-2, intersex, fruitless-like, and vitellogenin homologs in the parahaploid predator Metaseiulus occidentalis (Chelicerata: Acari: Phytoseiidae). Exp Appl Acarol 2015, 65:1–16. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y, Kobayashi K, Watanabe H, Iguchi T: Environmental Sex Determination in the Branchiopod Crustacean Daphnia magna: Deep Conservation of a Doublesex Gene in the Sex-Determining Pathway. PLoS Genet 2011, 7:e1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledón-Rettig CC, Zattara EE, Moczek AP: Asymmetric interactions between doublesex and tissue- and sex-specific target genes mediate sexual dimorphism in beetles. Nat Commun 2017, 8:14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuo JC, Hu QL, Zhang HH, Zhang MQ, Jo SB, Zhang CX: Identification and functional analysis of the doublesex gene in the sexual development of a hemimetabolous insect, the brown planthopper. Insect Biochem Mol Biol 2018, 102:31–42. [DOI] [PubMed] [Google Scholar]
- 42.Robinett CC, Vaughan AG, Knapp J-M, Baker BS: Sex and the Single Cell. II. There Is a Time and Place for Sex. PLoS Biol 2010, 8:e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka K, Barmina O, Sanders LE, Arbeitman MN, Kopp A: Evolution of sex-specific traits through changes in HOX-dependent doublesex expression. PLoS Biol 2011, 9:e1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hempel LU, Oliver B: Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol 2007, 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45. Morita S, Ando T, Maeno A, Mizutani T, Mase M, Shigenobu S, Niimi T: Precise staging of beetle horn formation in Trypoxylus dichotomus reveals the pleiotropic roles of doublesex depending on the spatiotemporal developmental contexts. PLOS Genet 2019, 15:e1008063. Morita et al. show that localized expression of dsx in the developing head horns of rhinoceros beetles precedes the onset of sexually dimorphic horn formation. Moreover, they find that the regulatory role played by dsx in modelling the developing horn, such as driving tissue growth, death, or movement, depends on both the spatial and temporal context. Alongside work on Drosophila, this study further suggests that region-specific expression of dsx may be an essential and general precursor to the development of sexually dimorphic traits in insects.
- 46.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF: Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci 2010, 13:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice G, Barmina O, Hu K, Kopp A: Evolving doublesex expression correlates with the origin and diversification of male sexual ornaments in the Drosophila immigrans species group. Evol Dev 2018, 20:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48. Prakash A, Monteiro A: Doublesex Mediates the Development of Sex-Specific Pheromone Organs in Bicyclus Butterflies via Multiple Mechanisms. Mol Biol Evol 2020, 37:1694–1707. Prakash and Monteiro show that the spatial regulation of dsx underlies the development of male-specific scent organs in Bicyclus butterflies. Moreover, they show that dsx controls the sexually dimorphic development of different sub-elements of these organs via different modes: some structures require the male-specific dsx isoform, while others develop by default but are repressed by the female dsx isoform. The lability in dsx expression and function provides a mechanism for the striking divergence that a single sexually dimorphic trait can show between species.
- *49. Rice GR, Barmina O, Luecke D, Hu K, Arbeitman M, Kopp A: Modular tissue-specific regulation of doublesex underpins sexually dimorphic development in drosophila. Dev 2019, 146:dev178285. Rice et al. identify cis-regulatory sequences that control the precise spatio-temporal expression of dsx that underlies the development of the sex comb, a male-specific leg ornament displayed by some Drosophila species. They also show that a different enhancer controls the development of sex-specific chemosensory organs, suggesting that modular control of dsx transcription allows insects to develop as mosaics of sexually dimorphic and monomorphic structures. Evolutionary changes in dsx enhancers can expand its expression into new tissues, thereby conferring the sexual identity upon which sexually dimorphic development is based.
- 50.Kijimoto T, Moczek AP, Andrews J: Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns. Proc Natl Acad Sci 2012, 109:20526–20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baral S, Arumugam G, Deshmukh R, Kunte K: Genetic architecture and sex-specific selection govern modular, male-biased evolution of doublesex. Sci Adv 2019, 5:eaau3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopp A, Duncan I, Carroll SB: Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 2000, 408:553–559. [DOI] [PubMed] [Google Scholar]
- 53.Rogers WA, Salomone JR, Tacy DJ, Camino EM, Davis KA, Rebeiz M, Williams TM: Recurrent Modification of a Conserved Cis-Regulatory Element Underlies Fruit Fly Pigmentation Diversity. PLoS Genet 2013, 9:e1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo SD, Baker BS: Constraints on the evolution of a doublesex target gene arising from doublesex’s pleiotropic deployment. Proc Natl Acad Sci 2015, 112:E852–E861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunte K, Zhang W, Tenger-Trolander A, Palmer DH, Martin A, Reed RD, Mullen SP, Kronforst MR: doublesex is a mimicry supergene. Nature 2014, 507:229–232. [DOI] [PubMed] [Google Scholar]
- 56.Nishikawa H, Iijima T, Kajitani R, Yamaguchi J, Ando T, Suzuki Y, Sugano S, Fujiyama A, Kosugi S, Hirakawa H, et al. : A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat Genet 2015, 47:405–409. [DOI] [PubMed] [Google Scholar]
- *57. Iijima T, Yoda S, Fujiwara H: The mimetic wing pattern of Papilio polytes butterflies is regulated by a doublesex-orchestrated gene network. Commun Biol 2019, 2:257. Female-limited Batesian mimicry in the swallowtail butterfly Papilio polytes is controlled by a tightly linked cluster of genes (the H locus) that includes dsx. Iijima et al. show that the mimetic (H) and non-mimetic (h) alleles at this locus regulate several transcription factors and signaling molecules in opposite directions in the developing wing, resulting in a binary switch between mimetic and non-mimetic color patterns. The mechanism that limits mimicry to females is not entirely clear, but could depend on lower expression of dsx in males (and in hh females) compared to HH and Hh females.
- 58.Iijima T, Kajitani R, Komata S, Lin C-P, Sota T, Itoh T, Fujiwara H: Parallel evolution of Batesian mimicry supergene in two Papilio butterflies, P. polytes and P. memnon. Sci Adv 2018, 4:eaao5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komata S, Lin C-P, Iijima T, Fujiwara H, Sota T: Identification of doublesex alleles associated with the female-limited Batesian mimicry polymorphism in Papilio memnon. Sci Rep 2016, 6:34782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *60. Palmer DH, Kronforst MR: A shared genetic basis of mimicry across swallowtail butterflies points to ancestral co-option of doublesex. Nat Commun 2020, 11:6. Female-limited Batesian mimicry is present in many species of the genus Papilio (swallowtail butterflies), and alternative dsx alleles are associated with mimetic and non-mimetic wing color patterns in several of those species. Does this reflect an ancestral polymorphism, or independent origin of mimetic dsx alleles in different species? The work of Palmer and Kronforst suggests that the answer may be “a bit of both”: dsx-dependent mimicry was likely present in the last common ancestor of four distantly related Papilio species, but the shared ancestral alleles have been largely replaced by their own allelic descendants.
- 61.Zhang W, Westerman E, Nitzany E, Palmer S, Kronforst MR: Tracing the origin and evolution of supergene mimicry in butterflies. Nat Commun 2017, 8:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timmermans MJTN, Baxter SW, Clark R, Heckel DG, Vogel H, Collins S, Papanicolaou A, Fukova I, Joron M, Thompson MJ, et al. : Comparative genomics of the mimicry switch in Papilio dardanus. Proc R Soc B Biol Sci 2014, 281:20140465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63. Timmermans MJTN, Srivathsan A, Collins S, Meier R, Vogler AP: Mimicry diversification in Papilio dardanus via a genomic inversion in the regulatory region of engrailed – invected. Proc R Soc B Biol Sci 2020, 287:20200443. In an important counterpoint to other studies of Papilio swallowtail butterflies, this report shows that female-limited mimicry is not always controlled by dsx. In the African mocker swallowtail P. dardanus, this trait is controlled instead by a chromosomal region that includes the paralogous transcription factors engrailed and invected. What limits the action of this “supergene” to females remains to be determined.
- 64.VanKuren NW, Massardo D, Nallu S, Kronforst MR: Butterfly Mimicry Polymorphisms Highlight Phylogenetic Limits of Gene Reuse in the Evolution of Diverse Adaptations. Mol Biol Evol 2019, 36:2842–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frederik Nijhout H: Insect Hormones. Princeton University Press; 1994. [Google Scholar]
- 66.Prakash A, Monteiro A: Molecular mechanisms of secondary sexual trait development in insects. Curr Opin Insect Sci 2016, 17:40–48. [DOI] [PubMed] [Google Scholar]
- 67.Bear A, Monteiro A: Both cell-autonomous mechanisms and hormones contribute to sexual development in vertebrates and insects. BioEssays 2013, 35:725–732. [DOI] [PubMed] [Google Scholar]
- 68.Leiblich A, Hellberg JEEU, Sekar A, Gandy C, Mendes CC, Redhai S, Mason J, Wainwright M, Marie P, Goberdhan DCI, et al. : Mating induces switch from hormone-dependent to hormone-independent steroid receptor–mediated growth in Drosophila secondary cells. PLOS Biol 2019, 17:e3000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCracken AW, Adams G, Hartshorne L, Tatar M, Simons MJP: The hidden costs of dietary restriction: Implications for its evolutionary and mechanistic origins. Sci Adv 2020, 6:eaay3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **70. Bhardwaj S, Prudic KL, Bear A, Dasgupta M, Wasik BR, Tong X, Cheong WF, Wenk MR, Monteiro A: Sex differences in 20-hydroxyecdysone hormone levels control sexual dimorphism in Bicyclus anynana wing patterns. Mol Biol Evol 2018, 35:465–472. Bhardwaj et al. provide the first conclusive evidence that sexual divergence in the titer of a hormone can drive sexual dimorphism in an insect trait. They show that females of a seasonal morph of the butterfly Bicyclus anynana have higher levels of circulating 20-hydroxyecdysone during larval development compared to males. This higher level leads to increased proliferation in the cells that give rise to the wing eyespot, resulting in larger eyespots in females.
- 71.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC: A Mechanism of Extreme Growth and Reliable Signaling in Sexually Selected Ornaments and Weapons. Science (80-) 2012, 337:860–864. [DOI] [PubMed] [Google Scholar]
- 72.Okada Y, Katsuki M, Okamoto N, Fujioka H, Okada K: A specific type of insulin-like peptide regulates the conditional growth of a beetle weapon. PLOS Biol 2019, 17:e3000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *73. Lavine MD, Gotoh H, Hayes A, Corley Lavine L: The Insulin Signaling Substrate Chico and the Ecdysone Response Element Broad Both Regulate Growth of the Head Horns in the Asian Rhinoceros Beetle, Trypoxylus dichotomus. Integr Comp Biol 2019, 59:1338–1345. Lavine et al. provide evidence that the insulin signalling pathway – a pathway with known sensitivity to nutritional inputs – is a key mediator of the sexually dimorphic and condition-dependent growth of rhinoceros beetle horns. They also show for the first time that horn growth in this species is mediated by the ecdysone signalling pathway. These discoveries pave the way for elucidating the crosstalk between hormonal pathways and cell-autonomous regulatory mechanisms that collectively mediate the development of sexually dimorphic traits.
- **74. Gotoh H, Miyakawa H, Ishikawa A, Ishikawa Y, Sugime Y, Emlen DJ, Lavine LC, Miura T: Developmental Link between Sex and Nutrition; doublesex Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles. PLoS Genet 2014, 10:e1004098. Gotoh et al. show that the expression of dsx has sex-specific and opposing effects on mandible growth in the stag beetle Cyclommatus metallifer: male dsx isoforms stimulate exaggerated mandible growth, while female dsx isoforms suppress it. Loss of dsx expression leads to intermediate mandible growth. The authors further show that dsx appears to achieve these effects by modulating the sensitivity of developing mandible cells to juvenile hormone. This work illustrates the interplay between cell-autonomous and hormonal inputs into the development of sexually dimorphic traits.
- 75.Gotoh H, Cornette R, Koshikawa S, Okada Y, Lavine LC, Emlen DJ, Miura T: Juvenile Hormone Regulates Extreme Mandible Growth in Male Stag Beetles. PLoS One 2011, 6:e21139. [DOI] [PMC free article] [PubMed] [Google Scholar]


