Abstract
Among the hallmarks of cancer is the ability of neoplastic cells to evade and suppress immune surveillance to allow their growth and evolution. Nowhere is this as apparent as in multiple myeloma, a cancer of antibody-producing plasma cells, where a complex interplay between neoplastic cells and the immune microenvironment is required for the development and progression of disease. Decades of research has led to the discovery of a number of therapeutic agents, from cytotoxic drugs to genetically engineered cells that mediate their anti-myeloma effects at least partially through altering these immune interactions. In this review we discuss the history of immunotherapy and current practices in multiple myeloma, as well as the advances that promise to one day offer a cure for this deadly disease.
Introduction
Targeting of the immune system in multiple myeloma has been a long-standing therapeutic goal with early approaches utilizing immune stimulants such as interferon(1) and allogeneic hematopoietic stem cell transplantation with its accompanying graft versus myeloma effects(2). While associated with significant toxicity, limited reports of long-term remissions have buoyed hopes that immune therapies could one day cure multiple myeloma. Over the past two decades these approaches have continued to evolve with the advent of immune modulatory agents, monoclonal antibodies, and engineered cellular therapies that have dramatically improved survival for multiple myeloma patients (Figure 1). In addition, our understanding of how even some cytotoxic agents, such as proteasome inhibitors, affect and may cooperate with immune targeted agents has created new synergistic avenues with which to explore combinatorial therapy.
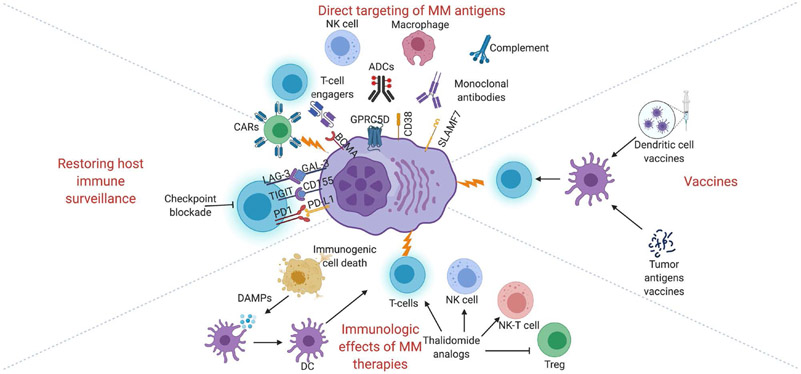
Figure 1.
Schematic overview of immune therapies in multiple myeloma.
Monoclonal antibodies recognize tumor antigens leading to inhibition of normal signaling or function of receptors, direct induction of apoptosis, antibody-directed cellular phagocytosis, antibody directed cellular cytotoxicity, complement-dependent cytotoxicity, immune stimulation, and via direct delivery of toxic payloads to tumor cells in the case of antibody-drug conjugates (ADCs). Chemotherapeutics such as bortezomib can induce immunogenic cell death leading to release of damage-associated molecular patterns (DAMPs) and stimulation of dendritic cells (DCs). CAR, chimeric antigen receptor; NK, natural killer; Treg, regulatory T-cell. Created with BioRender.com.
Immune dysfunction and the niche in multiple myeloma
Normal plasma cells develop and grow within a supportive niche in the bone marrow surrounded by stromal and immune cells(3). Neoplastic plasma cells tend to retain many features of their normal progenitors, including a dependence on interactions with this bone marrow microenvironment, a complex network that includes endothelial cells, osteoblasts, osteoclasts, plasmacytoid dendritic cells, tumor associated macrophages, myeloid-derived suppressor cells, and bone marrow stromal cells, that supports both normal hematopoiesis and the neoplastic cells (Figure 2)(4). These cells secrete growth factors such as transforming growth factor β (TGFβ)(5) and vascular endothelial growth factor (VEGF)(6) that promote angiogenesis, and cytokines such as interleukin-6 (IL-6), IL-17, and tumor necrosis factor (TNF) that support adhesion and growth of plasma cells, as well as protect them from the effects of cytotoxic therapies(7). The ability of neoplastic plasma cells to grow outside this supportive environment is associated with the development of extramedullary disease and plasma cell leukemia and portends a more aggressive clinical course, often occurring in the later stages of disease evolution. This may also act as an escape mechanism from immune-targeted therapies, allowing malignant plasma cells to grow and proliferate in immune-privileged spaces or in large extramedullary tumors that restrict access to monoclonal antibodies and T-cell engagers.
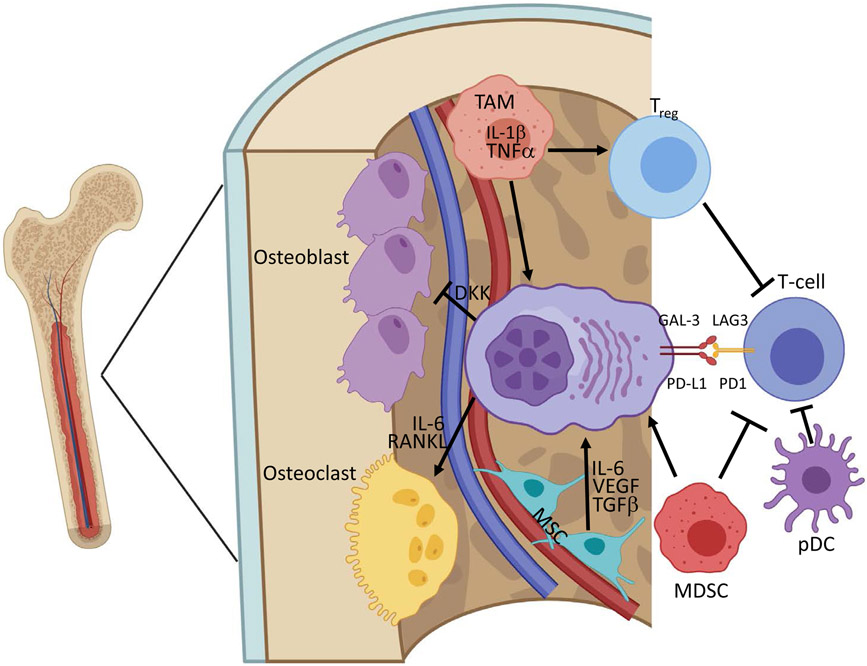
Figure 2.
Myeloma cells grow and evolve within a supportive bone marrow microenvironment.
Mesenchymal stromal cells (MSCs) and tumor associated macrophages (TAMs) secrete supportive cytokines and growth factors that promote growth of myeloma cells. T-regulatory cells, myeloid-derived suppressor cells (MDSCs), and plasmacytoid dendritic cells (pDC) suppress natural immune targeting and protect malignant plasma cells from therapy. Myeloma cells suppress normal osteoblasts and promote osteoclast-mediated bone turnover to disrupt the normal microenvironment. Created with BioRender.com.
Myeloma cells growing in the bone marrow are also surrounded by a matrix of T-, B- and NK-cells that have the potential to recognize and eliminate tumor cells(8). A complex interplay exists in which these components of the immune system are rendered inactive, or exhausted(9). This occurs via direct mechanisms such as upregulation of immune checkpoint proteins such as PD-L1(10), CD155 (the ligand for TIGIT) and GAL-3 (the ligand for LAG3), as well as via dysregulation of bone marrow stromal cells, which in turn suppress T- and B- lymphocytes(11). This evasion of surveillance by the innate and adaptive immune system is crucial to disease development, and may also contribute to the immunocompromised states seen in patients with multiple myeloma(12). Strategies to reverse this immune tolerance and restore anti-myeloma immunity are a major research focus.
Immunogenic cell death
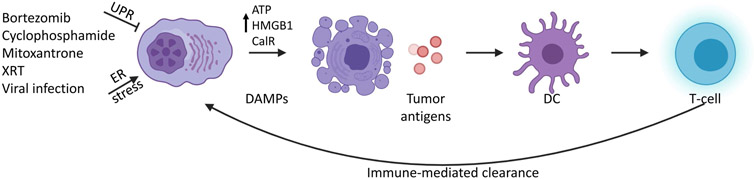
Most classical chemotherapeutic agents cause DNA damage and/or cell cycle arrest, eventually leading to apoptosis. In contrast, during immunogenic cell killing, cell death is preceded by upregulation of damage-associated molecular patterns (DAMPs), including signaling proteins such as Calreticulin and HMGB1 and release of ATP, which can stimulate macrophages and dendritic cells to take up and present tumor neoantigens (Figure 3)(13,14). These dendritic cells, in turn, promote activation of T- and B-cells, leading to improved recognition of tumor cells and immune-mediated clearance. It remains unclear exactly why some agents trigger immunogenic cell death, but activation of the unfolded protein response (UPR) and increased ER stress are major determinants(15). Proteasome inhibitors such as bortezomib(16) are potent inhibitors of protein turnover, activators of the UPR, and a mainstay of multiple myeloma therapy. They have previously been shown to increase dendritic cell exposure to tumor antigens(16), and more recently been demonstrated to induce immunogenic cell death(17). Thus, even some anti-myeloma drugs without a direct link to immune cell function can potentially stimulate immunogenic mechanisms of cancer therapy and provide a scientific rationale for combining proteasome inhibitors with other immune-modulating agents.
Figure 3.
Schematic of immunogenic cell death.
XRT, X-ray therapy; UPR, unfolded protein response; DAMP, damage-associated molecular pattern; DC, dendritic cell. Created with BioRender.com.
Thalidomide analogs
Thalidomide and its analogs, lenalidomide and pomalidomide, are a cornerstone of multiple myeloma therapy. At least two additional thalidomide analogs, iberdomide(18) and CC-92480(19), are in clinical development. These agents function via a novel mechanism in which they recruit neosubstrates to the E3 ubiquitin ligase substrate adaptor CRBN, leading to their eventual polyubiquitylation and proteasomal degradation(20). In multiple myeloma cells, degradation of the core lymphoid transcription factors IKZF1 and IKZF3 promotes apoptosis. Degradation of IKZF3 in T-cells leads to upregulation of IL-2 and inhibition of TNFα production in activated monocytes, which gave rise to the moniker immunomodulatory imides (IMiDs®)(21). Newer agents appear to overcome resistance at least partly by inducing deeper and more robust degradation of IKZF1 and IKZF3(22,23). Thalidomide analogs have also been proposed to inhibit angiogenesis and alter the bone marrow niche(24), and may have additional targets and functions(25). While there are good correlative and model system data supporting all of these immune modulatory effects, it remains unclear to what extent this is important in mediating their therapeutic efficacy, as there are no malignancies in which thalidomide analogs are active but do not also have direct cytolytic activity.
Data supporting combinatorial synergy with other anti-myeloma agents are more robust. While the combination of lenalidomide and bortezomib has been proposed to act synergistically(26), these agents are actually predicted to have opposing functions as bortezomib inhibits the proteasome, a key mediator of lenalidomide-induced neosubstrate degradation(20,27). Their combinatorial efficacy could be due to independent actions as they are dosed on very different schedules, or due to enhanced immune functions in the context of immunogenic cell death.
Lenalidomide has also been proposed to have synergistic effects in combination with dexamethasone(28), histone deacetylase inhibitors(29), JAK/STAT inhibitors suchs as ruxolitinib(30), and monoclonal antibodies(31). Lenalidomide is known to enhance NK-cell function(32), likely via upregulation of IL-2, which may be important for its activity in combination with the monoclonal antibodies elotuzumab(33) and daratumumab(31), and which are discussed further below.
Antigenic targets in multiple myeloma
Most novel immune based therapies take advantage of tumor specific antigens that can be directly targeted. Ideal therapeutic targets would be easily accessible (the cell surface is preferred), expressed at a high level or only in malignant plasma cells to allow for specificity, and required for cell viability, such that deletion or downregulation of the target leading to drug resistance cannot readily occur. Finally, an ideal target would be expressed on all malignant cells, including disease initiating stem cells. Significant work has gone into identifying targets that meet some, but not all, of these criteria, which include CD38, CS1, BCMA, GPRC5D, FCRL5, CD138, immunoglobulin light chain, and ICAM1, among many others (Table 1). Efforts are ongoing to identify additional antigens that specifically mark myeloma cells and thus might provide targets either alone, or in combination, that will allow for increased selectivity while minimizing immune and off-target toxicity.
Table 1.
Antigenic targets in multiple myeloma. SLAMF7, SLAM family member 7; BCMA, B-cell maturation antigen; APRIL, a proliferation-inducing ligand; BAFF, B-cell activation factor; GPRC5D, G protein-coupled receptor, class C group 5 member D; FCRL5, Fc receptor-like 5; ICAM1, Intercellular adhesion molecule 1.
| Antigen: | Normal function: | Expression: |
|---|---|---|
| CD38 | Ecto-enzyme (converts NAD+ to adenosine)(103) Modulates local immune function(104) Response to bacterial infections(104) Marker of immune activation(105) |
Mature B-cells Plasma cells T-cells NK cells HSCs Erythrocytes(106) |
| CS (SLAMF7) | Homophilic interactions leading to activation of NK-cell cytotoxicity(107) Aberrantly expressed on malignant plasma cells |
NK cells Malignant plasma cells(51) CD8+ cells Dendritic cells Activated monocytes |
| BCMA | Member of TNF receptor superfamily Binding to APRIL or BAFF leads to activation of NF-κB pathway(108) Promotes plasma cell survival and proliferation(109) Cleaved into soluble form by γ-secretase(110) |
Mature B-cells Plasma cells Plasmacytoid dendritic cells |
| GPRC5D | Unknown | Malignant plasma cells(111) Hair follicle |
| FCRL5 | Transmembrane protein homologous to Fc receptor(112) Binds IgG and stimulates B-cells(113) Located on chromosome 1q21 (often amplified in myeloma)(114) |
Malignant plasma cells(115) |
| CD138 | Cell surface protein involved in interactions with microenvironment Expression required for myeloma cell growth and survival(116) |
Plasma cells(117) |
| Ig κ | Immunoglobulin light chain Expressed on cell surface |
Mature B-cells Plasma cells(118) |
| ICAM1 | Leukocyte interactions with endothelial cells and transmigration(119) Overexpressed in myeloma cells due to hyperactivation of NF-κB pathway(120,121) |
Endothelial cells B-cells Plasma cells |
Monoclonal antibodies
Monoclonal antibodies have been a mainstay of the treatment of other B-cell malignancies such as non-Hodgkin lymphoma since the development of rituximab, but have only made their way to multiple myeloma in the last decade. These agents target cell surface proteins expressed at high levels or exclusively on malignant plasma cells and exert their cytotoxic effects through a variety of mechanisms (Figure 1).
Anti-CD38 monoclonal antibodies
There are two FDA-approved monoclonal antibodies targeting CD38, daratumumab and isatuximab. Daratumumab is a humanized immunoglobulin G1 (IgG1) antibody(34) with potent activity in multiple myeloma. In relapsed/refractory patients it demonstrated a 29% overall response rate as a single agent(35), and its addition to either lenalidomide or bortezomib in combination with dexamethasone lead to significant improvements in progression free survival in two large randomized phase 3 trials(36,37). Mechanistic synergy between lenalidomide and daratumumab has also been proposed(31), although the combination of daratumumab with either lenalidomide or bortezomib result in similar response rates, suggesting that potential mechanistic synergy observed in the lab could arise from different mechanisms (amplification of immunogenic cell death for instance) or may not translate into significant clinical advantages. More recently, daratumumab has been introduced into the newly diagnosed setting where its combination with lenalidomide and dexamethasone has shown excellent activity and tolerability and is thus a preferred option for older or unfit patients(38). Finally, quadruplet induction regimens with daratumumab, lenalidomide, a proteasome inhibitor and dexamethasone have demonstrated extraordinary rates of MRD negativity (51% versus 20% with RVd alone) and may soon become established as a standard of care for fit newly diagnosed patients(39).
Isatuximab differs from daratumumab in that it is a chimeric IgG1 antibody that targets a unique epitope on CD38 and similar to daratumumab induces cell death through a variety of mechanisms including direct apoptosis(40), antibody directed cellular cytotoxicity (ADCC), and complement activation(41). Isatuximab also inhibits the enzymatic activities of CD38(42) and thus may alter the microenvironment to inhibit tumor growth(43). As a single agent, it has activity in relapsed/refractory multiple myeloma (ORR of 23%)(44) that is similar to daratumumab, and it was recently FDA-approved for use in combination with pomalidomide and dexamethasone, where it demonstrated a near doubling in median PFS as compared to pomalidomide and dexamethasone alone (11.5 vs 6.5 months)(45).
Mechanisms of resistance to these agents have not been fully worked out, but appear to involve downregulation or loss of the CD38 epitope from myeloma cells(46,47). There are ongoing efforts to identify strategies to upregulate or induce re-expression of CD38. Signaling through the JAK-STAT pathway downregulates CD38, something that can be reversed following treatment with the pan-JAK inhibitor ruxolitinib(48). Retinoids such as all-trans retinoic acid(49) and histone deacetylase inhibitors(50) have been shown to increase CD38 expression in cell line models and clinical trials are ongoing to explore these agents in patients.
Anti-CS1 monoclonal antibodies
Elotuzumab is a humanized IgG1 antibody that targets CS1 and showed no single agent anti-myeloma activity despite high expression on neoplastic plasma cells(51,52). However, when combined with lenalidomide and dexamethasone it demonstrated an increased ORR (79% vs 66%) and a PFS (median of 19.4 vs 14.9 months) and OS (4-year OS of 50% vs 43%) advantage when compared to lenalidomide and dexamethasone alone(53,54). When combined with pomalidomide and dexamethasone the ORR (53% vs 26%) and median PFS (10.3 vs 4.7 months) were significantly increased as compared to pomalidomide and dexamethasone alone(55). The synergy with thalidomide analogs has been proposed to be mediated by elotuzumab-induced upregulation of IL-2 in T-cells and stimulation of NK-cell mediated cellular cytotoxicity(56,57). Thalidomide analogs also enhance T- and NK-cell functions, likely by degradation of IKZF3 and subsequent upregulation of IL-2. Other combinations, such as with bortezomib and dexamethasone also appear to have activity, possibly mediated by enhancement of immunogenic cell killing(58).
Anti-BCMA monoclonal antibodies
Anti-BCMA antibodies are rapidly making their way into the clinic with the FDA-approval of the antibody-drug conjugate (ADC) balantamab mafadotin(59). In a highly refractory patient setting it demonstrated a 34% ORR, but with significant toxicity, primarily cytopenias and keratopathy related to the toxin conjugate monomethyl auristatin F (MMAF)(60). This toxicity may limit its widespread use, as it requires every 3-week ophthalmologic evaluation. Studies are ongoing to assess its safety and efficacy in combination with a number of other anti-myeloma agents. MEDI2228 is another anti-BCMA antibody fused to the toxin pyrrolobenzodiazepine with promising pre-clinical activity(61), and it is in early stage clinical trials(62). Interestingly, ophthalmic toxicity, albeit through a different mechanism than with belantamab mafadotin, was common.
Anti-ICAM1 monoclonal antibodies
Naked anti-ICAM1 antibodies have demonstrated minimal activity in multiple myeloma, with the best response seen being stable disease(63). However, recent development of an antibody conjugated to MMAF has shown promise in pre-clinical models, and is now entering clinical trials(64).
Anti-CD138 monoclonal antibodies
The anti-CD138 antibody VIS832 is capable of inducing direct cytotoxicity to multiple myeloma cells in vitro and produces synergistic activity when combined with thalidomide analogs or bortezomib(65). Whether CD138 targeted agents are safe and effective in patients has not yet been tested.
Cellular therapies
Cellular therapies harness and re-direct the patient’s adaptive cellular immune system to attack and treat the cancer.
T-cell engagers
Bispecific antibodies or bispecific T-cell engagers (BiTEs) are recombinant antibody fragments containing the Fab variable regions of two separate antigen recognition motifs fused together in one of a variety of conformations (Figure 4). These variations can produce differences in half-life and function(66). They act to bring into close proximity the two targets of the cognate halves of the molecule, and in so doing produce their therapeutic action. The most common pairing is a CD3 binding domain fused to a domain that binds a tumor antigen such as CD19 or BCMA. In so doing they function to recruit αβT-cells into close proximity with tumor cells, activating the TCR and leading to cellular cytotoxicity. Molecules can be designed that target other immune cells such as NK-cells(67) or γδT-cells(68) to induce different modes of cell killing, or even to bring together soluble extracellular proteins such as in the case of emicizumab. Thus, these are highly flexible modular drugs capable of a number of therapeutic functions.
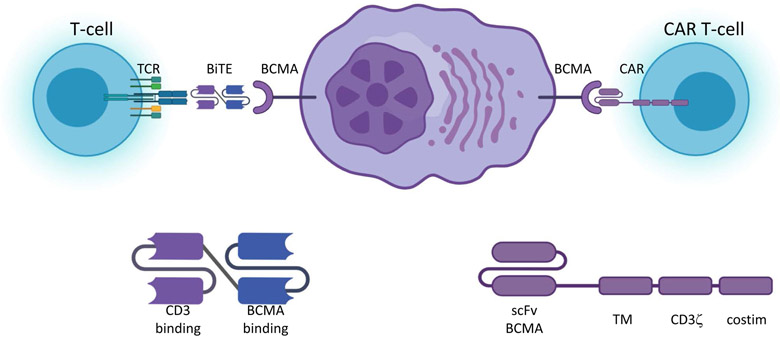
Figure 4.
Schematic of T-cell engagers and chimeric antigen receptors.
Bispecific T-cell engagers (BiTEs) have a CD3 binding moiety that can interact with the native T-cell receptor (TCR) fused to an antigen binding moiety that can target tumor antigens such as B-cell maturation antigen (BCMA) bringing T-cells into proximity of the tumor and inducing immune synapse and cytotoxicity. Chimeric antigen receptor (CAR) T-cells contain are genetically engineered to express a chimeric protein that includes an extracellular scFv domain capable of binding tumor antigens such as BCMA fused to a transmembrane (TM) domain, the CD3ζ stimulatory domain and a costimulatory (costim) domain, typically 4-1BB or CD28. Binding of the CAR to antigen leads to immune synapse formation and cytotoxicity. Created with BioRender.com.
In myeloma, the greatest experience has been reported with BCMA-targeted CD3 engagers. Early studies of AMG420 in patients with relapsed or refractory multiple myeloma demonstrated a 70% response rate at the maximally tolerated dose of 400ug/day, with five of ten patients achieving an MRD-negative complete response(69). The immune toxicity profile demonstrated mostly grade 2 CRS at this dose with increased rates of infection including two deaths. Despite these promising results, further development of this agent was abandoned due a short half-life that required continuous intravenous dosing. Studies are ongoing with modified agents, such as AMG701, that have a longer half-life and allow for every 3-week dosing. Multiple other T-cell engagers targeting BCMA are now in clinical trials(70,71).
Studies with bispecific antibodies targeting CD38, FCRL5 and GPRC5D among other targets are all ongoing, and are expected to report initial results in the coming year(66,72). Additional data will be needed in order to know how these agents compare to other molecularly-targeted drugs including ADCs and CAR T-cells and whether, and in what contexts, they can be safely and effectively combined with other anti-myeloma agents(73).
Chimeric antigen receptor T-cells
Adoptive cell transfer utilizing genetically engineered T-cells has been a highly successful approach inducing cures in multiple B-cell malignancies such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). These strategies have utilized autologous T-cells transduced with a chimeric T-cell receptor containing an extracellular domain targeting the tumor antigen fused to intracellular signaling domains (typically CD3ζ combined with either CD28 or 4-1BB costimulatory domains) that promote robust T-cell activation following antigen binding (Figure 4). Therapy involves apheresis of autologous T-cells, transduction with the engineered chimeric antigen receptor (CAR), followed by ex vivo growth and expansion. Following administration of lymphodepleting chemotherapy, typically a combination of fludarabine and cyclophosphamide, the cells are re-infused. For most currently available CARs, the process takes approximately 4-6 weeks from vein to vein. CD19 targeted CAR T-cells have produced durable remissions in up to half of patients with relapsed or refractory ALL and DLBCL and are now a mainstay of therapy for these patients. CD19 is not expressed on the majority of multiple myeloma cells, but it may be expressed on myeloma initiating cells, and there have been limited reports demonstrating efficacy of CD19-targeted CAR T-cells in this setting(74).
A far more promising target is anti-BCMA targeted CARs. Idecabtagene vicleucel (ide-cel, bb2121) is a BCMA-targeting CAR that is currently in phase 3 clinical trials, and is expected to obtain FDA-approval in 2021. Ide-cel has produced objective responses in 85% of patients, with complete response or better in 45% of patients(75). The median progression free survival was 11.8 months in this heavily pre-treated population, without any maintenance therapy. Grade 3 or higher hematologic toxicities occurred in the majority of patients. In addition, three quarters of patients developed CRS, although most was grade one or two. Severe neurologic toxicity was uncommon. Data for at least three other products have also been presented. These CARs all have similar structures and clinical profiles, although differences in the valency of the antigen binding domain (ciltacabtagene autoleucel, cilta-cel)(76) or humanization of the chimeric construct (CT053) may produce subtle differences in the activity, persistence, and toxicity profile of these different agents. What the specific differences are, and whether there are certain patient populations that may benefit more from one or another of these products, remains to be seen.
Unlike in other B-cell malignancies, multiple myeloma uniformly relapses following treatment with anti-BCMA CAR T-cells. The reasons for this are unclear, and mechanisms of resistance and relapse have not been well characterized, although it does appear that at least in some cases biallelic loss of BCMA may occur(77). The quality and makeup of the T-cell input and the effects multiple lines of prior therapy have on T-cell function may also be an important determinant of response and persistence(78). Whether T-cells should be banked early in disease is an important question in the field. Strategies are ongoing to develop approaches to improve outcomes with these therapies including upregulation of surface BCMA using γsecretase inhibitors(79), designing new CAR constructs that allow for more rapid manufacturing and improved persistence, introduction into other immune subsets such as γδT-cells(80) and NK cells(81), and the development of allogeneic “off the shelf” CARs. Future studies will likely involve novel combinations of CARs with standard therapies or other immune targeted agents to augment activity. Multi-targeting constructs to help decrease the chance for antigen escape and modified CARs to help overcome T-cell exhaustion are also being developed(82). These new approaches may at some point finally achieve the ultimate goal of curing patients with multiple myeloma.
Tumor vaccines and other cellular therapies
Somatic mutations in multiple myeloma cells can generate neoantigens capable of inducing anti-tumor immune responses, which may be associated with clinical responses(83) and are potential targets for cellular therapies. A variety of other cellular therapy approaches including tumor antigen vaccines, cytolytic T-lymphocytes, and allogeneic or autologous NK T-cell infusions have been tested or are in various states of clinical investigation(84,85). So far, these therapies have shown limited effectiveness, but they may have utility in selected disease states such as preventing progression from precursor states(86), in the setting of immune reconstitution following bone marrow transplantation or in combination with other immune modulatory drugs, immune checkpoint inhibitors, T-cell engagers or CARs.
Toxicities related to cellular therapies
Because cellular therapies lead to hyperactivation of the immune system, they tend to share a common set of toxicities. The most well characterized of these is hyperactivation of αβT-cells leading to the cytokine release syndrome (CRS)(87). This is mediated by release of high levels of inflammatory cytokines including IL-1(88) and IL-6(89), often associated with rapid T-cell expansion. A systemic inflammatory state akin to sepsis follows, marked by high fevers and capillary leak leading to hypoxemia and hypotension. The severity of symptoms can range from mild flu-like symptoms to fatal HLH-like macrophage activation syndrome(90). Treatment is focused on anti-cytokine agents such as IL-6 and IL-1 blockade, as well as T-cytolytic therapies including corticosteroids and chemotherapeutics(91). This immune hyper-inflammation can also produce neurotoxicity (ICANS, immune-effector cell associated neurotoxicity syndrome)(92), via a less well understood process but thought to be mediated by endothelial dysfunction. Almost all cellular therapies have the capability of producing some degree of CRS and/or ICANS, but the degree to which they do this can be quite variable, even with the same antigenic target and disease indication. There has also been limited correlation between the degree of inflammatory toxicities and clinical efficacy, and anti-inflammatory mitigation of CRS and ICANS does not appear to impair clinical efficacy.
Checkpoint blockade
Cells express a number of molecules on their surface to prevent immune-mediated destruction including PD-L1, GAL-3, and CD47 that are often upregulated in cancer. Inhibition of these immune checkpoints has been a highly successful therapeutic strategy in a variety of solid and hematologic malignancies(93). In multiple myeloma, despite elevated expression of PD-L1(10) and the importance of the immune microenvironment, checkpoint inhibitors have shown limited activity as single agents(94,95). More concerningly, early trials testing pembrolizumab, a PD1 inhibitor, in combination with lenalidomide and pomalidomide had to be stopped early due to increased mortality, possibly related to increased immune-related toxicity(96). Whether there is a role for PD1-targeted or other immune checkpoint therapies in specific clinical contexts such as after CAR T-cell therapy or in combination with other agents, especially those like bortezomib that induce immunogenic cell death, is an unanswered question in the field.
Immune therapies in the COVID era
The COVID-19 pandemic has created new challenges for cancer patients receiving therapy(97). Initial data has been reassuring that patients with hematologic malignancies undergoing therapy are not at higher risk of adverse outcomes from infection with the novel coronavirus as compared to those not receiving therapy(98) and that myeloma patients are capable of mounting an immune response(99). Among cancer patients in general there have been reports of worse outcomes among those receiving immune checkpoint targeted therapy as compared to conventional chemotherapy(100). Whether this extends to other immune based therapies is unclear, and most myeloma patients being treated with immune and cellular based therapies have few options for additional therapy. Their primary risk is morbidity and mortality related to disease progression(101); therefore the most efficacious possible therapy should be pursued without significant modification, aside from efforts to avoid infection(102). Whether the recently approved COVID-19 vaccines will have any unexpected toxicity and will be effective in this patient population also remains unknown, but we recommend that patients with multiple myeloma receive the vaccine as per CDC guidelines.
Conclusions
In summary, while the history of immune therapies in multiple myeloma is long, there remains significant work to be done in order to achieve their true promise. Thalidomide analogs, proteasome inhibitors, and CD38-targeted monoclonal antibodies make up the backbone of modern myeloma therapy, all of which have important immune-mediated mechanisms of action and have dramatically improved outcomes for patients. An increasing number of new monoclonal antibodies, T-cell engagers, and cellular therapies are rapidly making their way to the clinic. They have great potential to overcome constitutive genomic heterogeneity as well as ongoing DNA damage and clonal evolution underlying relapse of disease by achieving minimal residual disease negativity and restoring host anti-myeloma immunity. The optimal timing and sequencing of these agents to maximize efficacy with the overarching goal being cure is a key issue that will define the next decade in myeloma research.
Acknowledgements
Adam Sperling is supported by a grant from the National Cancer Institute (K08CA252174).
Footnotes
Conflicts of interest disclosure
Dr. Kenneth Anderson is a consultant for Pfizer, Amgen, Janssen, Gilead, and Precision Biosciences. He is also the scientific founder for Oncopep and C4 Therapeutics. The authors report no other conflicts of interest in this work.
References
- 1.Mellstedt H, Ahre A, Bjorkholm M, Holm G, Johansson B, Strander H. Interferon therapy in myelomatosis. Lancet 1979;1(8110):245–7 doi 10.1016/s0140-6736(79)90770-0. [DOI] [PubMed] [Google Scholar]
- 2.Crawley C, Lalancette M, Szydlo R, Gilleece M, Peggs K, Mackinnon S, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood 2005;105(11):4532–9 doi 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 3.Mendez-Ferrer S, Bonnet D, Steensma DP, Hasserjian RP, Ghobrial IM, Gribben JG, et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer 2020;20(5):285–98 doi 10.1038/s41568-020-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghobrial IM, Detappe A, Anderson KC, Steensma DP. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol 2018;15(4):219–33 doi 10.1038/nrclinonc.2017.197. [DOI] [PubMed] [Google Scholar]
- 5.Urashima M, Chauhan D, Uchiyama H, Freeman GJ, Anderson KC. CD40 ligand triggered interleukin-6 secretion in multiple myeloma. Blood 1995;85(7):1903–12. [PubMed] [Google Scholar]
- 6.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia 2001;15(12):1950–61 doi 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 7.Hao M, Zhang L, An G, Meng H, Han Y, Xie Z, et al. Bone marrow stromal cells protect myeloma cells from bortezomib induced apoptosis by suppressing microRNA-15a expression. Leuk Lymphoma 2011;52(9):1787–94 doi 10.3109/10428194.2011.576791. [DOI] [PubMed] [Google Scholar]
- 8.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19(11):1423–37 doi 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Smyth MJ, Martinet L. Cancer immunoediting and immune dysregulation in multiple myeloma. Blood 2020. doi 10.1182/blood.2020006540. [DOI] [PubMed] [Google Scholar]
- 10.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 2013;27(2):464–72 doi 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 11.Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. Ocul Surf 2016;14(2):121–34 doi 10.1016/j.jtos.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorrig R, Klausen TW, Salomo M, Vangsted A, Gimsing P. Risk factors for infections in newly diagnosed Multiple Myeloma patients: A Danish retrospective nationwide cohort study. Eur J Haematol 2019;102(2):182–90 doi 10.1111/ejh.13190. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17(2):97–111 doi 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 14.Serrano-Del Valle A, Anel A, Naval J, Marzo I. Immunogenic Cell Death and Immunotherapy of Multiple Myeloma. Front Cell Dev Biol 2019;7:50 doi 10.3389/fcell.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rufo N, Garg AD, Agostinis P. The Unfolded Protein Response in Immunogenic Cell Death and Cancer Immunotherapy. Trends Cancer 2017;3(9):643–58 doi 10.1016/j.trecan.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 2007;109(11):4839–45 doi 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulla A, Morelli E, Samur MK, Botta C, Hideshima T, Bianchi G, et al. Bortezomib Induces Anti-Multiple Myeloma Immune Response Mediated By Cgas/Sting Pathway Activation, Type I Interferon Secretion, and Immunogenic Cell Death: Clinical Application. Blood 2020;136:7–8 doi 10.1182/blood-2020-140356. [DOI] [Google Scholar]
- 18.Ye Y, Gaudy A, Schafer P, Thomas M, Weiss D, Chen N, et al. First-in-Human, Single- and Multiple-Ascending-Dose Studies in Healthy Subjects to Assess Pharmacokinetics, Pharmacodynamics, and Safety/Tolerability of Iberdomide, a Novel Cereblon E3 Ligase Modulator. Clin Pharmacol Drug Dev 2020. doi 10.1002/cpdd.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong L, Lamba M, Nunez M, Bauer D, Richardson PG, Bahlis NJ, et al. Dose- and Schedule-Dependent Immunomodulatory Effects of the Novel Celmod Agent CC-92480 in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020;136:47–8 doi 10.1182/blood-2020-137161. [DOI] [Google Scholar]
- 20.Kronke J, Hurst SN, Ebert BL. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology 2014;3(7):e941742 doi 10.4161/21624011.2014.941742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 2004;4(4):314–22 doi 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 22.Sperling AS, Burgess M, Keshishian H, Gasser JA, Bhatt S, Jan M, et al. Patterns of substrate affinity, competition, and degradation kinetics underlie biological activity of thalidomide analogs. Blood 2019;134(2):160–70 doi 10.1182/blood.2019000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matyskiela ME, Zhang W, Man HW, Muller G, Khambatta G, Baculi F, et al. A Cereblon Modulator (CC-220) with Improved Degradation of Ikaros and Aiolos. J Med Chem 2018;61(2):535–42 doi 10.1021/acs.jmedchem.6b01921. [DOI] [PubMed] [Google Scholar]
- 24.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A 1994;91(9):4082–5 doi 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hideshima T, Cottini F, Nozawa Y, Seo HS, Ohguchi H, Samur MK, et al. p53-related protein kinase confers poor prognosis and represents a novel therapeutic target in multiple myeloma. Blood 2017;129(10):1308–19 doi 10.1182/blood-2016-09-738500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das DS, Ray A, Song Y, Richardson P, Trikha M, Chauhan D, et al. Synergistic anti-myeloma activity of the proteasome inhibitor marizomib and the IMiD immunomodulatory drug pomalidomide. Br J Haematol 2015;171(5):798–812 doi 10.1111/bjh.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi CX, Kortum KM, Zhu YX, Jedlowski P, Bruins L, Braggio E, et al. Proteasome inhibitors block Ikaros degradation by lenalidomide in multiple myeloma. Haematologica 2015;100(8):e315–7 doi 10.3324/haematol.2015.124297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian Z, Zhang L, Cai Z, Sun L, Wang H, Yi Q, et al. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leuk Res 2011;35(3):380–6 doi 10.1016/j.leukres.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Ocio EM, Vilanova D, Atadja P, Maiso P, Crusoe E, Fernandez-Lazaro D, et al. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica 2010;95(5):794–803 doi 10.3324/haematol.2009.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berenson JR, To J, Spektor TM, Martinez D, Turner C, Sanchez A, et al. A Phase I Study of Ruxolitinib, Lenalidomide, and Steroids for Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res 2020;26(10):2346–53 doi 10.1158/1078-0432.CCR-19-1899. [DOI] [PubMed] [Google Scholar]
- 31.van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica 2011;96(2):284–90 doi 10.3324/haematol.2010.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hideshima T, Ogiya D, Liu J, Harada T, Kurata K, Bae J, et al. Immunomodulatory drugs activate NK cells via both Zap-70 and cereblon-dependent pathways. Leukemia 2021;35(1):177–88 doi 10.1038/s41375-020-0809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 2013;62(12):1841–9 doi 10.1007/s00262-013-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011;186(3):1840–8 doi 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 35.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016;387(10027):1551–60 doi 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 36.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016;375(14):1319–31 doi 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 37.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016;375(8):754–66 doi 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 38.Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med 2019;380(22):2104–15 doi 10.1056/NEJMoa1817249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood 2020;136(8):936–45 doi 10.1182/blood.2020005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu C, Song Z, Wang A, Srinivasan S, Yang G, Greco R, et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front Immunol 2020;11:1771 doi 10.3389/fimmu.2020.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno L, Perez C, Zabaleta A, Manrique I, Alignani D, Ajona D, et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin Cancer Res 2019;25(10):3176–87 doi 10.1158/1078-0432.CCR-18-1597. [DOI] [PubMed] [Google Scholar]
- 42.Deckert J, Wetzel MC, Bartle LM, Skaletskaya A, Goldmacher VS, Vallee F, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res 2014;20(17):4574–83 doi 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 43.Feng X, Zhang L, Acharya C, An G, Wen K, Qiu L, et al. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin Cancer Res 2017;23(15):4290–300 doi 10.1158/1078-0432.CCR-16-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin T, Strickland S, Glenn M, Charpentier E, Guillemin H, Hsu K, et al. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J 2019;9(4):41 doi 10.1038/s41408-019-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet 2019;394(10214):2096–107 doi 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 46.Krejcik J, Frerichs KA, Nijhof IS, van Kessel B, van Velzen JF, Bloem AC, et al. Monocytes and Granulocytes Reduce CD38 Expression Levels on Myeloma Cells in Patients Treated with Daratumumab. Clin Cancer Res 2017;23(24):7498–511 doi 10.1158/1078-0432.CCR-17-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Donk N, Usmani SZ. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front Immunol 2018;9:2134 doi 10.3389/fimmu.2018.02134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogiya D, Liu J, Ohguchi H, Kurata K, Samur MK, Tai YT, et al. The JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: therapeutic implications. Blood 2020;136(20):2334–45 doi 10.1182/blood.2019004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nijhof IS, Groen RW, Lokhorst HM, van Kessel B, Bloem AC, van Velzen J, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015;29(10):2039–49 doi 10.1038/leu.2015.123. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Guerrero E, Gotz R, Doose S, Sauer M, Rodriguez-Gil A, Nerreter T, et al. Upregulation of CD38 expression on multiple myeloma cells by novel HDAC6 inhibitors is a class effect and augments the efficacy of daratumumab. Leukemia 2020. doi 10.1038/s41375-020-0840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008;14(9):2775–84 doi 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012;120(3):552–9 doi 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med 2015;373(7):621–31 doi 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 54.Gavriatopoulou M, Terpos E, Dimopoulos MA. The extended 4-year follow-up results of the ELOQUENT-2 trial. Oncotarget 2019;10(2):82–3 doi 10.18632/oncotarget.26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med 2018;379(19):1811–22 doi 10.1056/NEJMoa1805762. [DOI] [PubMed] [Google Scholar]
- 56.Balasa B, Yun R, Belmar NA, Fox M, Chao DT, Robbins MD, et al. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-alpha pathways. Cancer Immunol Immunother 2015;64(1):61–73 doi 10.1007/s00262-014-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008;112(4):1329–37 doi 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jakubowiak A, Offidani M, Pegourie B, De La Rubia J, Garderet L, Laribi K, et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016;127(23):2833–40 doi 10.1182/blood-2016-01-694604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014;123(20):3128–38 doi 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol 2020;21(2):207–21 doi 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 61.Xing L, Lin L, Yu T, Li Y, Wen K, Cho S, et al. Anti-Bcma PBD MEDI2228 Combats Drug Resistance and Synergizes with Bortezomib and Inhibitors to DNA Damage Response in Multiple Myeloma. Blood 2019;134:1817 doi 10.1182/blood-2019-127163. [DOI] [Google Scholar]
- 62.Kumar SK, Migkou M, Bhutani M, Spencer A, Ailawadhi S, Kalff A, et al. Phase 1, First-in-Human Study of MEDI2228, a BCMA-Targeted ADC in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020;136:26–7 doi 10.1182/blood-2020-136375. [DOI] [Google Scholar]
- 63.Wichert S, Juliusson G, Johansson A, Sonesson E, Teige I, Wickenberg AT, et al. A single-arm, open-label, phase 2 clinical trial evaluating disease response following treatment with BI-505, a human anti-intercellular adhesion molecule-1 monoclonal antibody, in patients with smoldering multiple myeloma. PLoS One 2017;12(2):e0171205 doi 10.1371/journal.pone.0171205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherbenou DW, Su Y, Behrens CR, Aftab BT, Perez de Acha O, Murnane M, et al. Potent Activity of an Anti-ICAM1 Antibody-Drug Conjugate against Multiple Myeloma. Clin Cancer Res 2020;26(22):6028–38 doi 10.1158/1078-0432.CCR-20-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu T, Chaganty B, Lin L, Xing L, Ramakrishnan B, Wen K, et al. VIS832, a novel CD138-targeting monoclonal antibody, potently induces killing of human multiple myeloma and further synergizes with IMiDs or bortezomib in vitro and in vivo. Blood Cancer J 2020;10(11):110 doi 10.1038/s41408-020-00378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caraccio C, Krishna S, Phillips DJ, Schurch CM. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front Immunol 2020;11:501 doi 10.3389/fimmu.2020.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019;177(7):1701–13 e16 doi 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 68.Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with gammadelta T cells: many paths ahead of us. Cell Mol Immunol 2020;17(9):925–39 doi 10.1038/s41423-020-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J Clin Oncol 2020;38(8):775–83 doi 10.1200/JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 70.Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv 2020;4(18):4538–49 doi 10.1182/bloodadvances.2020002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verkleij CPM, Frerichs KA, Broekmans M, Absalah S, Maas-Bosman PWC, Kruyswijk S, et al. T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma. Oncotarget 2020;11(45):4076–81 doi 10.18632/oncotarget.27792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Stagg NJ, Johnston J, Harris MJ, Menzies SA, DiCara D, et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell 2017;31(3):383–95 doi 10.1016/j.ccell.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho SF, Lin L, Xing L, Li Y, Wen K, Yu T, et al. The immunomodulatory drugs lenalidomide and pomalidomide enhance the potency of AMG 701 in multiple myeloma preclinical models. Blood Adv 2020;4(17):4195–207 doi 10.1182/bloodadvances.2020002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med 2015;373(11):1040–7 doi 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019;380(18):1726–37 doi 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol 2018;11(1):141 doi 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai Y-T, Prabhala R, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nature Communications 2021;12(1):868 doi 10.1038/s41467-021-21177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garfall AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv 2019;3(19):2812–5 doi 10.1182/bloodadvances.2019000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, et al. gamma-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019;134(19):1585–97 doi 10.1182/blood.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straetemans T, Kierkels GJJ, Doorn R, Jansen K, Heijhuurs S, Dos Santos JM, et al. GMP-Grade Manufacturing of T Cells Engineered to Express a Defined gammadeltaTCR. Front Immunol 2018;9:1062 doi 10.3389/fimmu.2018.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020;59:102975 doi 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Larrea CF, Staehr M, Lopez AV, Ng KY, Chen Y, Godfrey WD, et al. Defining an Optimal Dual-Targeted CAR T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov 2020;1(2):146–54 doi 10.1158/2643-3230.bcd-20-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perumal D, Imai N, Lagana A, Finnigan J, Melnekoff D, Leshchenko VV, et al. Mutation-derived Neoantigen-specific T-cell Responses in Multiple Myeloma. Clin Cancer Res 2020;26(2):450–64 doi 10.1158/1078-0432.CCR-19-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenblatt J, Avigan D. Cellular Immunotherapy for Multiple Myeloma. Cancer J 2019;25(1):38–44 doi 10.1097/PPO.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 85.Boussi L, Niesvizky R. Advances in immunotherapy in multiple myeloma. Curr Opin Oncol 2017;29(6):460–6 doi 10.1097/CCO.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 86.Nooka AK, Wang ML, Yee AJ, Kaufman JL, Bae J, Peterkin D, et al. Assessment of Safety and Immunogenicity of PVX-410 Vaccine With or Without Lenalidomide in Patients With Smoldering Multiple Myeloma: A Nonrandomized Clinical Trial. JAMA Oncol 2018;4(12):e183267 doi 10.1001/jamaoncol.2018.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, Schlosser HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer 2018;6(1):56 doi 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24(6):731–8 doi 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6(224):224ra25 doi 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandler RD, Tattersall RS, Schoemans H, Greco R, Badoglio M, Labopin M, et al. Diagnosis and Management of Secondary HLH/MAS Following HSCT and CAR-T Cell Therapy in Adults; A Review of the Literature and a Survey of Practice Within EBMT Centres on Behalf of the Autoimmune Diseases Working Party (ADWP) and Transplant Complications Working Party (TCWP). Front Immunol 2020;11:524 doi 10.3389/fimmu.2020.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018;15(1):47–62 doi 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant 2020. doi 10.1038/s41409-020-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood 2013;121(5):734–44 doi 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ribrag V, Avigan DE, Green DJ, Wise-Draper T, Posada JG, Vij R, et al. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br J Haematol 2019;186(3):e41–e4 doi 10.1111/bjh.15888. [DOI] [PubMed] [Google Scholar]
- 95.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol 2016;34(23):2698–704 doi 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol 2019;6(9):e448–e58 doi 10.1016/S2352-3026(19)30109-7. [DOI] [PubMed] [Google Scholar]
- 97.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov 2020;10(6):783–91 doi 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020. doi 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang B, Van Oekelen O, Mouhieddine TH, Del Valle DM, Richter J, Cho HJ, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol 2020;13(1):94 doi 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26(8):1218–23 doi 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chari A, Samur MK, Martinez-Lopez J, Cook G, Biran N, Yong K, et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood 2020;136(26):3033–40 doi 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malard F, Mohty M. Management of patients with multiple myeloma during the COVID-19 pandemic. Lancet Haematol 2020;7(6):e435–e7 doi 10.1016/S2352-3026(20)30124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costa F, Dalla Palma B, Giuliani N. CD38 Expression by Myeloma Cells and Its Role in the Context of Bone Marrow Microenvironment: Modulation by Therapeutic Agents. Cells 2019;8(12) doi 10.3390/cells8121632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 2004;121(4):482–8 doi 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- 105.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood 1984;63(6):1424–33. [PubMed] [Google Scholar]
- 106.Chapuy CI, Nicholson RT, Aguad MD, Chapuy B, Laubach JP, Richardson PG, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion 2015;55(6 Pt 2):1545–54 doi 10.1111/trf.13069. [DOI] [PubMed] [Google Scholar]
- 107.Kumaresan PR, Lai WC, Chuang SS, Bennett M, Mathew PA. CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function. Mol Immunol 2002;39(1-2):1–8 doi 10.1016/s0161-5890(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 108.Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 2020;34(4):985–1005 doi 10.1038/s41375-020-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 2004;199(1):91–8 doi 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, et al. gamma-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 2015;6:7333 doi 10.1038/ncomms8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med 2019;11(485) doi 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis RS, Ehrhardt GR, Leu CM, Hirano M, Cooper MD. An extended family of Fc receptor relatives. Eur J Immunol 2005;35(3):674–80 doi 10.1002/eji.200425886. [DOI] [PubMed] [Google Scholar]
- 113.Dement-Brown J, Newton CS, Ise T, Damdinsuren B, Nagata S, Tolnay M. Fc receptor-like 5 promotes B cell proliferation and drives the development of cells displaying switched isotypes. J Leukoc Biol 2012;91(1):59–67 doi 10.1189/jlb.0211096. [DOI] [PubMed] [Google Scholar]
- 114.Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity 2001;14(3):277–89 doi 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 115.Ise T, Nagata S, Kreitman RJ, Wilson WH, Wayne AS, Stetler-Stevenson M, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia 2007;21(1):169–74 doi 10.1038/sj.leu.2404445. [DOI] [PubMed] [Google Scholar]
- 116.Moreaux J, Sprynski AC, Dillon SR, Mahtouk K, Jourdan M, Ythier A, et al. APRIL and TACI interact with syndecan-1 on the surface of multiple myeloma cells to form an essential survival loop. Eur J Haematol 2009;83(2):119–29 doi 10.1111/j.1600-0609.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 117.Sun C, Mahendravada A, Ballard B, Kale B, Ramos C, West J, et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget 2019;10(24):2369–83 doi 10.18632/oncotarget.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boux HA, Raison RL, Walker KZ, Hayden GE, Basten A. A tumor-associated antigen specific for human kappa myeloma cells. J Exp Med 1983;158(5):1769–74 doi 10.1084/jem.158.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Riet I, Van Camp B. The involvement of adhesion molecules in the biology of multiple myeloma. Leuk Lymphoma 1993;9(6):441–52 doi 10.3109/10428199309145751. [DOI] [PubMed] [Google Scholar]
- 120.Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood 2010;115(17):3541–52 doi 10.1182/blood-2009-09-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidmaier R, Morsdorf K, Baumann P, Emmerich B, Meinhardt G. Evidence for cell adhesion-mediated drug resistance of multiple myeloma cells in vivo. Int J Biol Markers 2006;21(4):218–22. [DOI] [PubMed] [Google Scholar]