Abstract
With the recent approval and widespread administration of the Pfizer-BioNTech, Moderna, and Janssen vaccines worldwide, incidence of severe Coronavirus Disease 2019 (COVID-19) infection has significantly decreased. In spite of their undisputed role in reducing the severity of the disease and reduction of the disease burden in the community, there have been case reports of serious side effects with these vaccines. We aim to describe a case report of myocarditis following administration of the Janssen vaccine in a healthy, young male and review the available literature on COVID-19 vaccine related myocarditis and its possible pathogenesis. This case and literature review notes a temporal association between COVID-19 vaccination and myocarditis. Despite these observations, the benefits of the vaccines far outweigh the risks of possible myocarditis.
Keywords: COVID-19, Myocarditis, Vaccine
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-COV)-2 infection and the consequent disease, Coronavirus Disease 2019 (COVID-19) have led to a global pandemic resulting in over 3.97 million deaths at the time of publication [1]. To mitigate transmissibility, three novel vaccines have been granted emergency use authorization (EUA) in the United States. Widespread use of these vaccines has resulted in a precipitous decline in COVID-19-associated hospitalizations and mortality. The two-dose mRNA based COVID-19 vaccines, BNT162b2 mRNA (Pfizer-BioNTech, New York NY) and the mRNA-1273 (Moderna, Cambridge MA), have been widely used globally and granted provisional FDA approval in the United States on December 2020 resulting in 180 million and 134 million vaccinations to date, respectively. The single-dose Janssen Ad26.COV2.S (Johnson and Johnson, New Brunswick NJ) COVID-19 vaccine, a recombinant, replication-incompetent human adenovirus type 26 vector, was authorized for use in the United States on February 27, 2021 and has been administered to more than eight million patients to date [2]. Through either mRNA or a viral vector, these vaccines lead to expression of the SARS-CoV-2 spike (S) antigen without virus propagation. An immune response elicited to the S antigen protects against COVID-19 [3]. Although adverse effects due to these vaccines are commonly limited to mild and transient symptoms, there have been reports of myocarditis associated with the BNT162b2 mRNA and the mRNA-1273 vaccines (Table 1 ). In this article, we present the second reported myocarditis case after the Janssen vaccine, and we review the current literature for post COVID-19 vaccine myocarditis.
Table 1.
Literature review of demographic and clinical characteristics of patients with myocarditis following various COVID-19 vaccination, December 2020 to July 4, 2021.a
| Publication |
Montgomery et al. [12] |
Larson & Ammirati et al. [22] |
Rosner et al. [17] |
Marshall et al. [23] |
Mouch et al. [24] |
Dickey et al. [25] |
Kim et al. [26] |
Mansour et al. [27] |
|---|---|---|---|---|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
| Age, median (range), y | 25 (20–51) | 29 (21–56) | 24 (19–39) | 17 (14–19) | 23 (16–45) | (17–37) | 30 (23–70) | 23 (21–25) |
| Sex | ||||||||
| Male | 23 (100) | 8 (100) | 7 (100) | 7 (100) | 6 (100) | 6 (100) | 3 (75) | 1 (50) |
| Female | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (50) |
| Proximate vaccine dose | ||||||||
| Second mRNA-1273 dose | 14 (61) | 2 (25) | 1 (14) | 0 | 0 | 2 (33) | 2 (50) | 2 (100) |
| Second BNT162b2-mRNA dose | 6 (26) | 6 (75) | 4 (57) | 7 (100) | 5 (83) | 4 (67) | 2 (50) | 0 |
| First mRNA-1273 dose | 2 (9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| First BNT162b2-mRNA dose | 1 (4) | 1 | 1 (14) | 0 | 1 (17) | 0 | 0 | 0 |
| Single Ad.26.COV2.S dose | 0 | 0 | 1 (14) | 0 | 0 | 0 | 0 | 0 |
| Time to symptom onset, mean (range), h | 50 (12–96) | 66 (48–96) | 93 (48–168) | 62 (48–96) | 104 (24–384) | 80 (48–96) | 66 (24–100) | 39 (30–48) |
| Troponin level | ||||||||
| Elevated | 23 (100) | 8 (100) | 7 (100) | 7 (100) | 6 (100) | 6 (100) | 4 (100) | 2 (100) |
| Not elevated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Electrocardiogram findingsb | ||||||||
| Abnormal | 19 (83) | 7 (88) | 5 (72) | 7 (100) | 6 (100) | 5 (83) | 4 (100) | 2 (100) |
| Normal | 4 (17) | 1 (12) | 2 (28) | 0 | 0 | 1 (17) | 0 | 0 |
| Echocardiogram findingsc | ||||||||
| LVEF <50% | 4 (17) | 2 (25) | 1 (14) | 1 (14) | 0 | 5 (83) | 1 (25) | 0 |
| LVEF ≥50% | 19 (83) | 6 (75) | 6 (86) | 6 (86) | 6 (100) | 1 (17) | 3 (75) | 2 (100) |
| Coronary artery imagingd | ||||||||
| Abnormal | 0 | 0 | 0 | 0 | NR | 0 | 0 | |
| Normal | 16 (70) | 5 (62) | 3 (43) | 0 | 2 (33) | NR | 1 (25) | 2 (100) |
| Not performed | 7 (30) | 3 (38) | 4 (57) | 7 (100) | 4 (67) | NR | 3 (75) | 0 |
| Cardiac MRIe | ||||||||
| Abnormal | 8 (35) | 8 (100) | 7 (100) | 7 (100) | 6 (100) | 6 (100) | 4 (100) | 2 (100) |
| Normal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not performed | 15 (65) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SARS-CoV-2 PCR findings at presentation | ||||||||
| Positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Negative | 19 (83) | 8 (100) | 6 (86) | 7 (100) | 6 (100) | 6 (100) | 3 (75) | 2 (100) |
| Not performed | 4 (17) | 0 | 1 (14) | 0 | 0 | 1 (25) | 0 | |
| Other viral testing at presentationf | ||||||||
| Positive | 0 | NR | 0 | 0 | NR | NR | 0 | 0 |
| Negative | 13 (57) | NR | 5 (72) | 7 (100) | NR | NR | 4 (100) | 2 (100) |
| Not performed | 10 (43) | NR | 2 (28) | 0 | NR | NR | 0 | 0 |
| History of prior SARS-CoV-2 infection | ||||||||
| Positive | 3 (13) | 1 (12) | 3 (43) | 0 | 0 | NR | 0 | 0 |
| Negative | 20 (87) | 7 (88) | 4 (57) | 6 (86) | 6 (100) | NR | 4 (100) | 2 (100) |
| Unknown | 0 | 0 | 0 | 1 (14) | 0 | NR | 0 | 0 |
| Publication |
D'Angelo et al. [28] |
Habib et al. [29] |
Muthukumar et al. [30] |
Shaw et al. [31] |
Nevet et al. [32] |
Vidula et al. [33] |
Park et al. [34] |
Ammirati et al. [35] |
|---|---|---|---|---|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
| Age, median (range), y | 30 | 37 | 52 | 21 (16–31) | 24 (20–29) | 19 (18–19) | 16 (15–16) | 56 |
| Sex | ||||||||
| Male | 1 (100) | 1 (100) | 1 (100) | 2 (50) | 3 (100) | 2 (100) | 2 (100) | 1 (100) |
| Female | 0 | 0 | 0 | 2 (50) | 0 | 0 | 0 | 0 |
| Proximate vaccine dose | ||||||||
| Second mRNA-1273 dose | 0 | 0 | 1 (100) | 0 | 0 | 1 (50) | 0 | 0 |
| Second BNT162b2-mRNA dose | 1 (100) | 1 (100) | 0 | 2 (50) | 3 (100) | 1 (50) | 1 (50) | 1 (100) |
| First mRNA-1273 dose | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 | 0 |
| First BNT162b2-mRNA dose | 0 | 0 | 0 | 1 (25) | 0 | 0 | 1 (50) | 0 |
| Single Ad.26.COV2.S dose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Time to symptom onset, mean (range), h | 72 | 72 | 72 | 210 (48–600) | 48 | 60 (24–96) | 60 (24–72) | 72 |
| Troponin level | ||||||||
| Elevated | 1 (100) | 1 (100) | 1 (100) | 4 (100) | 3 (100) | 2 (100) | 2 (100) | 1 (100) |
| Not elevated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Electrocardiogram findingsb | ||||||||
| Abnormal | 1 (100) | 1 (100) | 0 | NR | 3 (100) | 2 (100) | 2 (100) | 1 (100) |
| Normal | 0 | 0 | 1 (100) | NR | 0 | 0 | 0 | 0 |
| Echocardiogram findingsc | ||||||||
| LVEF <50% | 0 | 0 | 0 | 0 | NR | 1 (50) | 0 | 0 |
| LVEF ≥50% | 1 (100) | 1 (100) | 1 (100) | 4 (100) | NR | 1 (50) | 2 (100) | 1 (100) |
| Coronary artery imagingd | ||||||||
| Abnormal | 0 | 0 | 0 | NR | NR | 0 | NR | 0 |
| Normal | 1 (100) | 1 (100) | 1 (100) | NR | NR | 2 (100) | NR | 1 (100) |
| Not performed | 0 | 0 | 0 | NR | NR | 0 | NR | 0 |
| Cardiac MRIe | ||||||||
| Abnormal | 1 (100) | 1 (100) | 1 (100) | 4 (100) | 1 (100) | 2 (100) | 1 (50) | 1 (100) |
| Normal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not performed | 0 | 0 | 0 | 0 | 0 | 1 (50) | 0 | |
| SARS-CoV-2 PCR findings at presentation | ||||||||
| Positive | 0 | 0 | 0 | 0 | NR | 0 | 0 | 0 |
| Negative | 1 (100) | 1 (100) | 1 (100) | 4 (100) | NR | 2 (100) | 2 (100) | 1 (100) |
| Not performed | 0 | 0 | 0 | 0 | NR | 0 | 0 | 0 |
| Other viral testing at presentationf | ||||||||
| Positive | 0 | 0 | 0 | NR | NR | 0 | 0 | 0 |
| Negative | 1 (100) | 1 (100) | 1 (100) | NR | NR | 2 (100) | 2 (100) | 1 (100) |
| Not performed | 0 | 0 | 0 | NR | NR | 0 | 0 | 0 |
| History of prior SARS-CoV-2 infection | ||||||||
| Positive | 0 | 0 | 0 | 2 (50) | NR | NR | 0 | 1 (100) |
| Negative | 1 (100) | 0 | 1 (100) | 2 (50) | NR | NR | 2 (100) | 0 |
| Unknown | 0 | 1 (100) | 0 | 0 | NR | NR | 0 | 0 |
| Publication |
Garcia et al. [36] |
Deb et al. [37] |
Singh et al. [38] |
Minocha et al. [39] |
Cereda et al. [40] |
Albert et al. [41] |
Watkins et al. [42] |
|---|---|---|---|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
| Age, median (range), y | 39 | 67 | 24 | 17 | 21 | 24 | 20 |
| Sex | |||||||
| Male | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Female | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proximate vaccine dose | |||||||
| Second mRNA-1273 dose | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 |
| Second BNT162b2-mRNA dose | 1 (100) | 0 | 1 (100) | 1 (100) | 1 (100) | 0 | 1 (100) |
| First mRNA-1273 dose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| First BNT162b2-mRNA dose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Single Ad.26.COV2.S dose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Time to symptom onset, mean (range), h | 6 | 6 | 72 | 48 | 30 | 96 | 48 |
| Troponin level | |||||||
| Elevated | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Not elevated | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Electrocardiogram findingsb | |||||||
| Abnormal | 1 (100) | 1 (100) | 0 | 1 (100) | 1 (100) | 0 | 1 (100) |
| Normal | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 0 |
| Echocardiogram findingsc | |||||||
| LVEF <50% | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 |
| LVEF ≥50% | 1 (100) | 0 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Coronary artery imagingd | |||||||
| Abnormal | 0 | NR | NR | NR | 0 | 0 | 0 |
| Normal | 1 (100) | NR | NR | NR | 1 (100) | 1 (100) | 1 (100) |
| Not performed | 0 | NR | NR | NR | 0 | 0 | 0 |
| Cardiac MRIe | |||||||
| Abnormal | 1 (100) | NR | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Normal | 0 | NR | 0 | 0 | 0 | 0 | 0 |
| Not performed | 0 | NR | 0 | 0 | 0 | 0 | 0 |
| SARS-CoV-2 PCR findings at presentation | |||||||
| Positive | 0 | 0 | 0 | 0 | 0 | 0 | |
| Negative | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Not performed | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other viral testing at presentationf | |||||||
| Positive | 0 | 0 | 0 | 0 | 0 | 0 | NR |
| Negative | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | NR |
| Not performed | 0 | 0 | 0 | 0 | 0 | 0 | NR |
| History of prior SARS-CoV-2 infection | |||||||
| Positive | 0 | NR | 0 | 0 | NR | NR | NR |
| Negative | 1 (100) | NR | 0 | 1 (100) | NR | NR | NR |
| Unknown | 0 | NR | 1 (100) | 0 | NR | NR | NR |
Abbreviations: LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; mRNA, messenger RNA; PCR, polymerase chain reaction; NR not reported.
Adapted from Montgomery et al. [12].
Electrocardiogram findings included ST elevations, T-wave inversions, and nonspecific ST changes.
Echocardiogram findings reported as LVEF.
Coronary artery imaging included CT coronary angiography and coronary angiography.
All abnormal cardiac MRIs reportedly meeting criteria for myocarditis.
Testing varied in each case for other acute viral infections; panels included some or all of these pathogens: coxsackie viruses, cytomegalovirus, Epstein-Barr virus, hepatitis A virus, hepatitis B virus, hepatitis C virus, herpes simplex virus, human herpesvirus 6, HIV, influenza viruses, and parvoviruses.
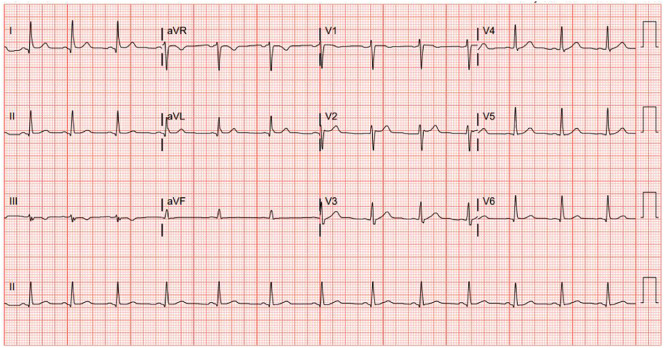
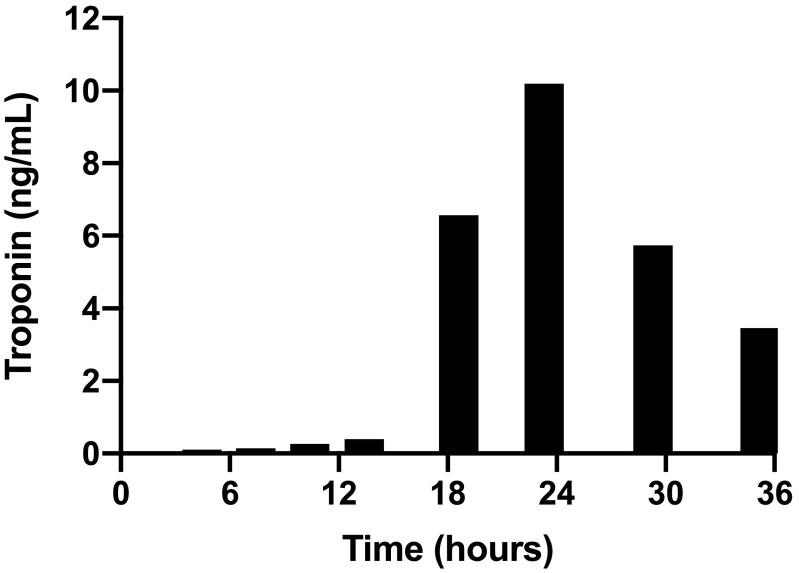
A previously healthy 33-year-old male presented to the emergency department with acute onset substernal chest pain. Two days prior, he had received the Janssen Ad26.COV2·S vaccine. Initially he noted myalgias and chills which resolved 24 h following vaccination. This was followed by a constant, retrosternal, non-radiating, non-exertional chest pain. The pain was not positional, pleuritic, nor exertional. His past medical history was significant for asthma and obstructive sleep apnea but no known cardiac history. He did not have any known allergies, and he was up-to-date with all standard vaccinations with no prior history of adverse reactions. He denied any sick contacts or exposure to COVID-19 patients in the weeks prior to vaccination or foreign travel. Upon admission, the patient was hemodynamically stable and afebrile. Electrocardiogram (ECG) showed normal sinus rhythm (Fig. 1 ) with normal intervals. Laboratory tests were remarkable for high-sensitivity (hs) troponin T 0.041 ng/mL (Normal range < 0.014 ng/mL). Complete blood cell count with differential was normal and C reactive protein was 40.4 mg/L (Normal range 3.0 mg/L). Over the course of 24 h, the troponin peaked to 10.2 ng/mL. (Fig. 2 ). A gadolinium enhanced cardiac magnetic resonance imaging showed a small focal area of myocarditis in the mid to apical lateral region of the left ventricle with a scar size of 2% (Fig. 3 ). Overall, systolic function of the left ventricle was normal with no hypokinesis noted. Myocarditis was presumed to be due to the vaccine administered due to the strong chronological association. The patient was treated symptomatically, and he endorsed significant improvement in his symptoms. He was discharged home in stable condition with close follow-up.
Fig. 1.
ECG at the time of admission noted normal sinus rhythm. Two additional ECGs at 6 h and 12 h after admission also showed normal sinus rhythm without PR interval, ST interval, or T wave changes.
Fig. 2.
Histogram of high-sensitivity Troponin T (ng/mL) at time of admission until discharge.
Fig. 3.
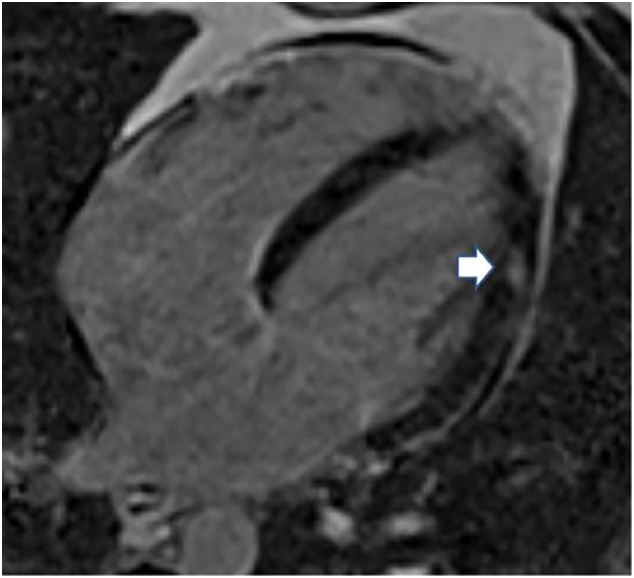
Cardiac magnetic resonance of a four-chamber, long-axis T2-weighted image demonstrating small focal edema (arrow) in the anterolateral wall of the left ventricle.
2. Methods
An extensive search of PubMed, Scopus, Web of Science, and Google Scholar were performed using the terms “myocarditis,” “COVID-19” and “vaccine” ranging from December 2020 to July 4, 2021. From 38 results, 23 articles were selected and analyzed constituting 11 case series and 12 case reports with a total of 81 patients. Moreover, the Centers for Disease Control and Prevention (CDC) and The Vaccine Adverse Event Reporting System (VAERS) databases were utilized for data regarding vaccination rates by age and episodes of reported myocarditis by vaccine manufacturers, respectively.
3. Results
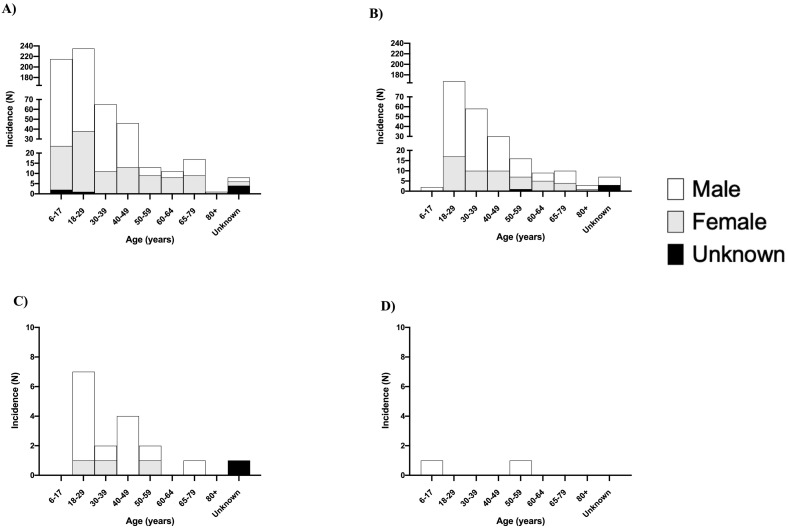
Out of 38 articles, 23 case reports and case series were selected for further analysis. These articles included 81 patients with 77 males who were primarily under the age of 30 (Fig. 4 ). Onset of myocarditis was noted in 71 individuals following their second mRNA COVID-19 vaccine; nine individuals following their first mRNA COVID-19 vaccine, of which six had a prior COVID-19 infection; and one individual had myocarditis following administration of the Janssen Ad26.COV2.S vaccine (Table 1). Whereas 31.8% of all individuals vaccinated were below the age of 40 years old, they constituted 80.7% of all myocarditis cases. Further, in individuals below the age of 29 years old, the observed cases of myocarditis were in excess of the expected cases (Table 2 ).
Fig. 4.
Histogram of incidence of myocarditis with vaccine types relative to gender and age patients incidence of myocarditis following immunization with various COVID-19 vaccine manufacturers as reported per VAERS from December 10, 2020 to July 4, 2021. A Pfizer-BioNTech, B Moderna, C Jansen, and D unknown manufacturer.
Table 2.
Numbers of fully vaccinated individuals stratified by age and expected vs observed cases of myocarditis stratified by age.
| No. of fully vaccinated individuals per CDCa |
No. of myocarditis case per VARESb |
|||||
|---|---|---|---|---|---|---|
| Age group | N (%) | Total % | Age group | Expected Nc | Observed N (%) | Total % |
| 6–17 | 6,293,191 (4.3) | 31.8% | 6–17 | 5–52 | 218 (23.4) | 80.7% |
| 18–24 | 11,232,667 (7.7) | 18–29 | 9–94 | 410 (43.9) | ||
| 25–39 | 28,587,841 (19.7) | 30–39 | 24–238 | 125 (13.4) | ||
| 40–49 | 20,452,763 (14.1) | 14.1% | 40–49 | 17–170 | 80 (8.6) | 8.6% |
| 50–64 | 38,171,118 (26.3) | 26.3% | 50–64 | 32–318 | 52 (5.6) | 5.6% |
| 65–74 | 23,793,960 (16.4) | 27.7% | 65–79 | 20–198 | 28 (3.0) | 3.4% |
| 75+ | 16,406,810 (11.3) | 80+ | 14–137 | 4 (0.4) | ||
| Unknown | 4702 (0) | 0.0% | Unknown | 0 | 16 (1.7) | 1.7% |
Fully vaccinated (vaccine manufacturer unspecified) individuals as of July 4, 2021.
As of July 4, 2021.
Expected number of cases based on a US annual background incidence rate of 1–10 per 100,000 person-years assuming presentation 30 days post vaccination.
4. Discussion
The commonly reported side effects for the COVID-19 vaccines are usually mild and self-limited, including pain at the injection site, headache, fatigue, muscle aches and nausea. Most of these side effects occur within 1–2 days following vaccination and resolve within 1–2 days [4]. Among the more serious adverse events reported, thrombotic and thromboembolic events were observed in the lower extremities, chest, and cerebral vasculature with use of the Janssen COVID-19 vaccine, which resulted in an FDA amendment to the EUA on April 23, 2021 limiting distribution to individuals 18 years of age and older [[5], [6], [7]]. Further, the Israeli Ministry of Health had initially reported on cases of myocarditis following mRNA COVID-19 vaccination [8]. This was a significant observation as prior assessment of other vaccines, as reflected in the US Vaccine Adverse Effects Reporting System (VAERS) from 1990 to 2018, identified 620,195 cases of vaccine side effects with 0.1% of the case associated with myopericarditis which was most reported with the smallpox vaccine [9].
Analysis of the VARES data (Table 2) for episodes of myocarditis following COVID-19 vaccination was significant for an increased prevalence of myocarditis in males, which is consistent with prior findings; however, the rates of myocarditis relative to the background rates in the general US population, which is estimated at 1 to 10 cases per 100,000 person-years, was much higher than expected [[10], [11], [12], [13]]. Individuals below the age of 40 years old, who accounted for 32% of the total full vaccination, resulted in 81% of the myocarditis case in VARES as of July 4, 2021 [14] (Table 2). This led to the FDA revising the patient and provider fact sheets on June 25, 2021 for the mRNA vaccine due to increased risk of myocarditis and pericarditis [7]. Although incidences of myocarditis were not reported during the clinical trials of the vaccine manufacturers, this may be due to the limited size of the trial population who received the vaccines before its authorization [4,15,16]. As there is widespread vaccine distribution, increasing cases of myocarditis have been observed with the mRNA vaccines. Since the Janssen vaccine has a similar end product as the mRNA vaccines, further cases of myocarditis with the Janssen vaccine may be expected. Currently, the Janssen vaccine accounts for 12.4 million vaccines compared to the 133 million and 180 million vaccines of Moderna and Pfizer-BioNTech, respectively [10].
Further, observationally it was noted that a diagnosis of myocarditis was more common following the second dose of the mRNA vaccines in individuals without a prior history of COVID-19 infection whereas for individuals with a prior COVID-19 infection, myocarditis occurred following the first dose (Table 1). Interestingly, both our patient and the patient reported by Rosner et al., developed myocarditis following vaccination with the single dose of Janssen vaccine despite no prior reported COVID infection [17].
Although, the mechanism of COVID-19 induced myocarditis remains poorly understood, it is postulated that myocarditis is a complication of the immune response rather than direct injury of the myocardiocytes through viral binding [18]. Specifically, either a severe cytokine release syndrome leading to a systemic inflammatory response or overactivation of the autoimmune system with interferon mediated hyperactivation of innate and adaptive immune system may play a central role [19,20]. This may explain why the SARS-COV-2 virus is commonly found in the lungs of infected patients but detected less commonly in the heart [19]. Further, an immune inflammatory response rather than direct viral infection of the myocardium is consistent with the mechanism of myocarditis noted in smallpox vaccinia-associated myocarditis [21].
This case was limited in that a viral pathology panel was not performed to rule out other etiologies of viral myocarditis. As the patient did not have a preceding viral prodrome or recent exposures, there was initially a low suspicion for other viral etiologies. Further, as noted in the presented case as well as the literature review, the patients improved with supportive treatment and a myocardial biopsy was not necessary for further investigation of the viral etiology. A further limitation is that the literature review consists of case reports and case series which are self-reported; this may have a likelihood of bias with weak inferences to the general population. As the vaccine-associated myocarditis cases are a very rare occurrence, we are currently restricted to these observation studies.
Despite the current temporal relationship of COVID-19 vaccination and myocarditis, it is prudent to be cognizant of the efficacy of these vaccines and its clear demonstrable benefits, both for the individual immunized as well as the community. Although the observed cases of myocarditis are higher in young males, it is still an exceedingly rare possible adverse effect of vaccination.
5. Conclusion
Our case highlights the second possible case of vaccine induced myocarditis in an individual following administration of the Janssen vaccine. Further our literature review noted that a temporal relationship between myocarditis and COVID 19 vaccination was commonly seen in young males and following the second mRNA COVID-19 vaccine except in those with a prior COVID-19 infection, where an occurrence of myocarditis was seen after the first mRNA COVID-19 vaccine. These findings are observational and we hypothesize that the myocarditis may be related to COVID-19 vaccination as a complication of the immune response. Despite these observations, we reiterate that though we may see more cases of myocarditis following vaccination as millions are vaccinated worldwide, it is still a rare phenomenon for which the benefits of the vaccines far outweigh the risks.
Declaration of competing interest
No disclosures to be made by the authors.
Acknowledgments
Acknowledgments
The author would like to thank Dr Mark A Iler for the MRI image acquisition.
Sources of funding
No external sources of funding to be disclosed by the authors.
References
- 1.Coronavirus (COVID-19) deaths - statistics and research. Our world in data n.d. https://ourworldindata.org/covid-deaths (accessed July 4, 2021).
- 2.Shay D.K. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021:70. doi: 10.15585/mmwr.mm7018e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos R., Rutten L., van der Lubbe J.E.M., Bakkers M.J.G., Hardenberg G., Wegmann F., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. Npj Vaccines. 2020;5:1–11. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commissioner O of the. Janssen COVID-19 vaccine. FDA; 2021. [Google Scholar]
- 5.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commissioner O of the. COVID-19 vaccines. FDA; 2021. [Google Scholar]
- 8.Surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and May 2021 (including). GOVIL n.d. https://www.gov.il/en/departments/news/01062021-03 (accessed July 4, 2021).
- 9.Su J.R., McNeil M.M., Welsh K.J., Marquez P.L., Ng C., Yan M., et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 10.CDC COVID Data Tracker. Centers for disease control and prevention 2020. https://covid.cdc.gov/covid-data-tracker
- 11.Gubernot D., Jazwa A., Niu M., Baumblatt J., Gee J., Moro P., et al. U.S. population-based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine. 2021;39:3666–3677. doi: 10.1016/j.vaccine.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuntz J., Crane B., Weinmann S., Naleway A.L., Vaccine Safety Datalink Investigator Team Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine. 2018;36:1524–1527. doi: 10.1016/j.vaccine.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VAERS - Data n.d. https://vaers.hhs.gov/data.html (accessed July 4, 2021).
- 15.Ad J. Vaccines and Related Biological Products Advisory Committee Meeting February 26, 202 n.d.:62.
- 16.Covid P-B. PFIZER-BIONTECH COVID-19 VACCINE (BNT162, PF-07302048) VACCINES AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE BRIEFING DOCUMENT n.d.:92.
- 17.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;6:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [CIRCULATIONAHA.121.055891] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansueto G., Niola M., Napoli C. Can COVID 2019 induce a specific cardiovascular damage or it exacerbates pre-existing cardiovascular diseases? Pathol Res Pract. 2020;216 doi: 10.1016/j.prp.2020.153086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami R., Sakamoto A., Kawai K., Gianatti A., Pellegrini D., Nasr A., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawalha K., Abozenah M., Kadado A.J., Battisha A., Al-Akchar M., Salerno C., et al. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassimatis D.C., Atwood J.E., Engler R.M., Linz P.E., Grabenstein J.D., Vernalis M.N. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43:1503–1510. doi: 10.1016/j.jacc.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Larson K.F., Ammirati E., Adler E.D., Cooper L.T., Hong K.N., Saponara G., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [CIRCULATIONAHA.121.055913] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. e2021052478. [DOI] [PubMed] [Google Scholar]
- 24.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickey J.B., Albert E, Badr M, Laraja K.M., Sena L.M., Gerson D.S., et al. A series of patients with myocarditis following SARS-CoV-2 vaccination with mRNA-1279 and BNT162b2. JACC Cardiovasc Imaging 2021;14: 1862–1863. 10.1016/j.jcmg.2021.06.003. [DOI] [PMC free article] [PubMed]
- 26.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour J., Short R.G., Bhalla S., Woodard P.K., Verma A., Robinson X., et al. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imaging. 2021;78:247–249. doi: 10.1016/j.clinimag.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Angelo T., Cattafi A., Carerj M.L., Booz C., Ascenti G., Cicero G., et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. 2021;37:1665–1667. doi: 10.1016/j.cjca.2021.05.010. [S0828-282X(21)00286-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habib M.B., Hamamyh T., Elyas A., Altermanini M., Elhassan M. Acute myocarditis following administration of BNT162b2 vaccine. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthukumar A, Narasimhan M, Li Q-Z, Mahimainathan L, Hitto I, Fuda F, et al. In depth evaluation of a case of presumed myocarditis following the second dose of COVID-19 mRNA vaccine. Circulation 2021;144:487-498. 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed]
- 31.Shaw K.E., Cavalcante J.L., Han B.K., Gössl M. Possible association between COVID-19 vaccine and myocarditis: clinical and CMR findings. JACC Cardiovasc Imaging. 2021;14:1856–1861. doi: 10.1016/j.jcmg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nevet A. Acute myocarditis associated with anti-COVID-19 vaccination. Clin Exp Vaccine Res. 2021;10:196–197. doi: 10.7774/cevr.2021.10.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidula M.K., Ambrose M., Glassberg H., Chokshi N., Chen T., Ferrari V.A., et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus. 2021;13:e15576. doi: 10.7759/cureus.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J, Brekke DR, Bratincsak A. Self-limited myocarditis presenting with chest pain and ST segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine. Cardiol Young. 2022; 32:146-149. 10.1017/S1047951121002547. [undefined/ed]. [DOI] [PubMed]
- 35.Ammirati E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F., Schroeder J.W., et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Heart Vasc. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García J.B., Ortega P.P., Antonio Bonilla Fernández J., León A.C., Burgos L.R., Dorta E.C. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol. 2021;74:812–814. doi: 10.1016/j.recesp.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deb A., Abdelmalek J., Iwuji K., Nugent K. Acute myocardial injury following COVID-19 vaccination: a case report and review of current evidence from vaccine adverse events reporting system database. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211029230. [21501327211029230] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh B., Kaur P., Cedeno L., Brahimi T., Patel P., Virk H., et al. COVID-19 mRNA vaccine and myocarditis. Eur J Case Rep Int Med. 2021;8:002681. doi: 10.12890/2021_002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minocha P.K., Better D., Singh R.K., Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. [S0022-3476(21)00617-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cereda A., Conca C., Barbieri L., Ferrante G., Tumminello G., Lucreziotti S., et al. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: serendipity or atypical causal relationship? Anatol J Cardiol. 2021;25:522–523. doi: 10.5152/AnatolJCardiol.2021.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watkins K., Griffin G., Septaric K., Simon E.L. Myocarditis after BNT162b2 vaccination in a healthy male. Am J Emerg Med. 2021;50:815.e1–815.e2. doi: 10.1016/j.ajem.2021.06.051. [S0735675721005362] [DOI] [PMC free article] [PubMed] [Google Scholar]