Abstract
Introduction
Only a few studies have comprehensively characterized default mode network (DMN) pathology on a structural and functional level, and definite conclusions cannot be drawn due to antipsychotic medication exposure and illness chronicity. The objective of this study was to characterize DMN pathology in medication-naïve first episode psychosis (FEP) patients, and determine if DMN structural and functional connectivity (FC) have potential utility as a predictor for subsequent antipsychotic treatment response.
Methods
Diffusion imaging and resting state FC data from 42 controls and 52 FEP were analyzed. Patients then received 16 weeks of antipsychotic treatment. Using region of interest analyses, we quantified FC of the DMN and structural integrity of the white matter tracts supporting DMN function. We then did linear regressions between DMN structural and FC indices and antipsychotic treatment response.
Results
We detected reduced DMN fractional anisotropy and axial diffusivity in FEP compared to controls. No DMN FC abnormalities nor correlations between DMN structural and FC were found. Finally, DMN fractional anisotropy and radial diffusivity were associated with response to treatment.
Conclusion
Our study highlights the critical role of the DMN in the pathophysiology suggesting that axonal damage may already be present in FEP patients. We also demonstrated that DMN pathology is clinically relevant, as greater structural DMN alterations were associated with a less favorable clinical response to antipsychotic medications.
Keywords: first episode psychosis, functional connectivity, diffusion weighted imaging, fractional anisotropy, medication-naïve, treatment response
INTRODUCTION
The default mode network (DMN) was first described in the literature in 2001 as the default functional brain state that is suspended during specific goal-directed behaviors.1–3 It is comprised of extensive portions of frontal and posterior midline regions and the inferior parietal lobe; and spontaneous brain activity across these areas is strongly and selectively correlated.4 The DMN is conceptualized as a fundamental neurobiological system with physiological and cognitive properties that distinguish it from other systems, and is considered a backbone of cortical integration.5 While the functional anatomy of this network does not observe the boundaries of traditionally defined cortical cytoarchitectonic maps, tract tracing studies in non-human primates show that projection zones of key network nodes spatially echo the regions of the DMN, suggesting that this network is supported by direct anatomical connectivity.4 Consistent with this, several human studies found that the strength of functional connectivity within the DMN reflects integrity of white matter tracts that structurally connect these regions.6–10
Dysfunction of the DMN is seen across a range of psychiatric disorders, and dysconnectivity is thought to represent a mechanism whereby clinical symptoms and cognitive deficits are induced or exacerbated.11 In schizophrenia spectrum disorders, disrupted functional connectivity within the DMN is frequently reported.12 However, only a few studies have integrated both functional and anatomical measures to provide a more comprehensive assessment of DMN pathology,13–15 all of which were conducted in chronic schizophrenia patients who were treated with antipsychotic medications at the time of assessment. These reports are consistent with the idea that a structural connectivity deficit may cause a change in functional connectivity, but because non-specific factors associated with the illness such as disease chronicity and antipsychotic medication exposure impact functional and structural connectivity,16–19 it is not possible to definitively determine that this mechanism plays a pivotal role in disease expression.
The DMN has also been identified as relevant for a number of clinical variables including symptom severity,20 long-term clinical outcomes,21 and response to antipsychotic treatment.22,23 Importantly, baseline functional connectivity of the DMN was found to be the single most positive predictor for subsequent response to antipsychotic treatment in an assessment of five key functional brain networks in early illness schizophrenia patients.22 Studies geared at elucidating neural signatures of antipsychotic treatment response not only have the potential to inform clinical decision making, but also set the stage to distinguish variations of the illness that may represent differences in the underlying pathophysiology.24 But because this type of study is not a trivial undertaking, the empirical literature on the topic remains sparse.
Here, we conducted a multimodal assessment of the DMN using resting state functional connectivity (FC) and diffusion weighted imaging (DWI) to quantitatively evaluate pathology of this network in antipsychotic medication-naïve first episode psychosis (FEP) patients. We specifically chose to quantify the four commonly reported white matter diffusion metrics25 in an attempt to generate a broad characterization of white matter integrity and, especially since there are only a few studies that have examined structural integrity of the DMN. We then assessed change in positive symptom severity following a 16-week antipsychotic treatment trial as a proxy for responsiveness to antipsychotic medication treatment. This time window was chosen because longer treatment trials are deemed more robust in first episode patients26 and early symptom reduction does not appear to be a clinically useful predictor of antipsychotic treatment response in this population.27 We had two main objectives for this study: (1) to characterize the DMN on a functional and structural level and examine putative relationships between functional and structural connectivity within the network in healthy subjects and those suffering from a first psychotic episode, and (2) to determine if a more comprehensive neurobiological assessment of DMN pathology can inform clinically relevant aspects of the illness such as predicting subsequent response to antipsychotic treatment in patients. We hypothesized that structural deficits and functional abnormalities in the DMN are present in FEP patients, and that a linear relationship between functional and structural variables is evident. We further hypothesized that greater DMN alterations at baseline would be associated with a less favorable subsequent response to antipsychotic treatment.
METHODS
Participants
FEP patients were recruited from outpatient, inpatient and emergency room settings at the University of Alabama at Birmingham (UAB). Healthy controls (HC) matched on age, sex and parental occupation were recruited by advertisements. Written informed consent was obtained (once FEP patients were deemed to have capacity to give consent28) prior to enrollment in this UAB Institutional Review Board approved study.
Patients and HC were excluded if they had major neurological or medical conditions, history of head trauma with loss of consciousness, substance use disorders (excluding nicotine and cannabis) within one month of imaging, were pregnant or breastfeeding, or had MRI contraindications. Patients were either medication-naïve or had no more than 5 days of lifetime antipsychotic exposure prior to study entry (83% of patients did not receive any antipsychotic prior to the initial scan). Healthy controls with a personal history of a mental illness or family history in a first-degree relative of a psychotic disorder were excluded.
Clinical Assessment
A consensus diagnosis was made by two board certified psychiatrists (NVK and ACL) who specialize in psychosis spectrum disorders. The diagnosis was based on all clinical information available including clinical interviews with the patient and family members as well as medical records (psychiatric assessment, review of systems, family history, laboratory workup) and clinical observations and assessments over several months of follow up as available. The Brief Psychiatric Rating Scale (BPRS) and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) were used to assess symptom severity and cognition, respectively.29,30 FEP patients enrolled in this longitudinal study entered into a 16-week trial of oral risperidone using a flexible dosing regimen. We chose risperidone because it is commonly prescribed, now available as generic medication and thus one of the more affordable second-generation antipsychotic medications in the United States, and is considered one first line medications for treatment in schizophrenia,31 specifically in FEP.32 Risperidone was started at 0.5–1 mg and titrated in 1–2 mg increments; dosing was based on therapeutic and side effects. In case of excessive side effect burden, as determined by a study physician, patients were switched to aripiprazole started at 2–5 mg and titrated in 2.5–10 mg increments (two patients were switched to aripiprazole; one developed hyperprolactinemia within the first week of using risperidone, the second was switched after 7 weeks of treatment due to irritability). Use of concomitant medication was permitted as clinically indicated. Treatment response was defined as the percentage of change on the BPRS positive subscale from baseline to 16 weeks of treatment, where a greater percentage indicates a greater reduction in positive symptoms: [(BPRSBaseline – BPRSWeek16)/(BPRSBaseline)] × (−100). Treatment response data were available for 43 patients.
Data Acquisition
Imaging was performed on a 3T Siemens Magnetom Prisma scanner equipped with a 20-channel head coil. T1 (MPRAGE: TR = 2400 ms; TE = 2.22 ms; inversion time = 1000 ms; flip angle = 8°; GRAPPA factor = 2; voxel size = 0.8 mm3) and T2 weighted scans (TR/TE: 3200/563 ms; GRAPPA factor 2; slice thickness 0.8 mm; 208 slices, voxel size 0.8 mm3) were acquired for anatomical reference. DWI data were acquired in opposing phase encoding directions (anterior > posterior, and posterior > anterior; [TR/TE: 3230 ms/89.20 ms; multiband acceleration factor 4, Flip angle: 84°; slice thickness 1.5 mm, 92 slices, voxel size 1.5 mm3, 92 diffusion weighted images distributed equally over 2 shells with b-values of ~1500 s/mm2 and ~3000 s/mm2, as well as 7 interspersed b = ~0 s/mm2 images]).
Resting state functional MRI (fMRI) data were also acquired in opposing phase encoding directions (anterior > posterior and posterior > anterior; TR = 1550 ms; TE = 37.80 ms; flip angle = 71°, FOV = 104 mm2; multi-band acceleration factor = 4; voxel size= 2 mm3; 225 volumes). Subjects were instructed to keep their eyes open and let their mind wander during this scan.
Data Preprocessing and Data Quality Control
DWI Data.
Preprocessing of DWI images was performed in TORTOISE (version 3.1.2). This included correction for thermal noise,33 Gibbs-ringing,34 high b-value based bulk motion and eddy-current distortions using a MAP-MRI model,35,36 resampling of images to 1 mm3 and rotation of gradient tables independently for each DWI phase encoding direction. Then, DR-BUDDI was used to correct EPI distortions with input from the T2 weighted anatomical image and to combine the two datasets. Tensors were computed with DIFF_CALC. To spatially normalize images to the Illinois Institute of Technology atlas (IIT4) space, we used a modified version of 3dQwarp in AFNI. Finally, we calculated voxelwise white matter fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) for each participant.
We visually inspected raw images for signal loss and presence of artifacts. We then assessed bulk motion and excluded datasets with an average root mean square of absolute displacement (RMSabs) of greater than the voxel edge length (1.5 mm) or root mean square of relative displacement (RMSrel) of >0.05 mm averaged across phase encoding directions from further analyses. DR_BUDDI outputs were assessed for quality of motion, eddy current and EPI distortion correction, and scalar diffusion maps were inspected for anomalies in parameters. DWI data from 3 HC and 10 FEP did not pass quality control and were excluded from analyses. Additionally, 4 FEP terminated the scan before DWI images were acquired. In summary, diffusion datasets from 52 FEP and 42 HC were included in the final analyses.
Resting State Functional Connectivity Data.
Data were analyzed using the CONN toolbox (version 19c).37 After discarding the first 10 volumes of each scan allowing for signal equilibration, susceptibility artifacts were corrected in FSL’s topup.38 Functional images were then slice-timing and motion-corrected using rigid-body realignment, co-registered to the structural image, normalized to Montreal Neurological Institute (MNI) space, bandpass filtered (0.008 < f < 0.08 Hz), and spatially smoothed with a 4-mm full width at half maximum (FWHM) Gaussian kernel.
Framewise displacement (FD) and percentage of censored data were then calculated.39 Motion outliers as detected by the artifact detection (ART) toolbox were censored (composite volume-to-volume motion > 0.9 mm and intensity >5 SDs), and the six motion parameters derived from rigid-body realignment and their derivatives, as well as the first five component time series derived from CSF and white matter using aCompCor and corresponding derivatives, were regressed out from the signal. In summary, FC datasets from 51 FEP and 42 HC were included in the final analyses.
DMN Regions of Interest
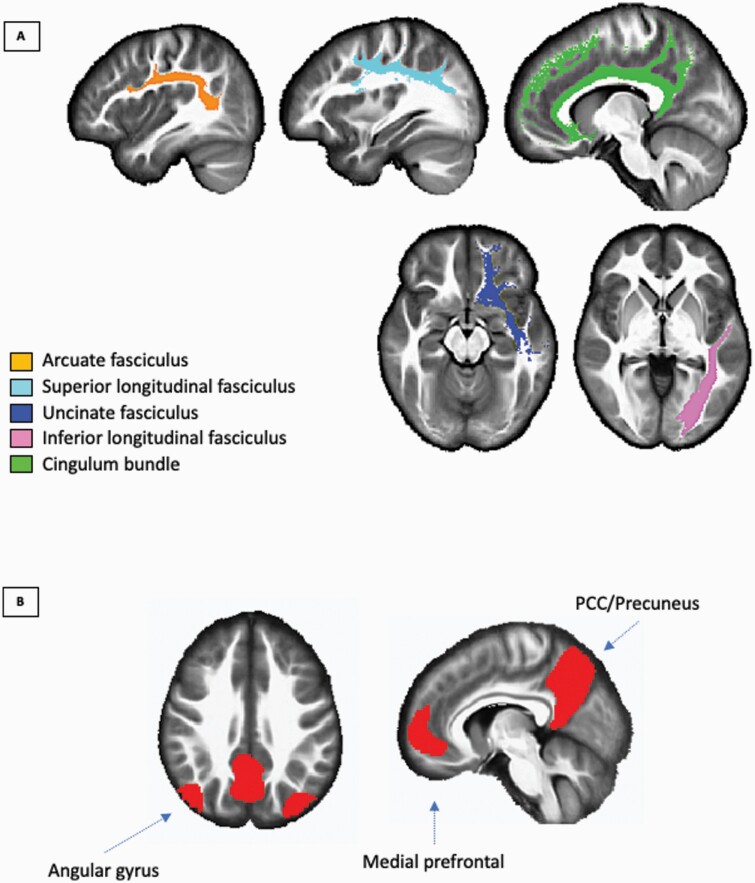
White matter tracts comprising the DMN were defined as outlined by Alves and colleagues.40 These tracts were obtained from the IIT4 atlas and were the following: L/R cingulum, L/R uncinate, L/R superior and L/R inferior longitudinal fasciculus, and L/R arcuate fasciculus (figure 1A). All tracts were binarized and combined to create a single DMN white matter mask for data extraction. We also used each unilateral binarized WM mask for post-hoc analyses. For FC analysis, we used the DMN mask defined from CONN’s independent component analysis of Human Connectome Project dataset (497 subjects). This mask included areas from the medial prefrontal cortex, bilateral angular gyri, and posterior cingulate cortex/precuneus (figure 1B).
Fig. 1.
ROIs used for (A) DMN WM tract (showing unilateral tract only) and (B) DMN ROIs for FC analysis rendered on a T1 IIT Human Brain Template. ROI, region of interest; DMN, default mode network; FC, functional connectivity; WM, white matter; and IIT, Illinois Institute of Technology.
Statistical Analyses
For structural connectivity analyses, the mean voxelwise FA, MD, RD, and AD for each subject from the whole DMN white matter mask was extracted. We also extracted WM measures from each unilateral individual tract and performed group analysis as post-hoc analyses. For FC analyses, DMN FC was calculated using the conn_withinbetweenROItest function implemented in CONN by averaging the BOLD signal across all voxels in all of the regions of interest (ROIs) within the DMN mask. This computes Pearson’s correlation across all voxels within the ROIs and then creates an average DMN FC value. The DMN FC value was z-transformed and group analyses (HC vs. FEP) were performed using separate ANCOVAs for WM and FC controlling for sex and motion parameters (RMSrel for white matter analysis and FD for functional connectivity). Given that age can potentially affect white matter diffusion indices, we also included it as a covariate in all of our analyses.41
Then, we examined the relationship between white matter and FC between groups using linear regressions on each group separately and by creating an interaction term between groups and FC and regressing the interaction term as well as age, sex, motion parameters (RMSrel and FD), group, and FC on each WM measure. Finally, we examined the relationship between treatment response % with WM and FC in FEP using a linear regression. Age, sex and motion parameters (RMSrel and FD) were included in the model. All analyses were controlled for multiple comparisons using the false discovery rate (FDR) correction method by Benjamin and Hochberg.42 All results are accompanied by effect sizes.
RESULTS
Demographics and Clinical Characteristics
Of the 183 patients assessed for eligibility to participate in this study between June 2016 and April 2019, 74 FEP were consented and 66 entered into the 16-week trial of risperidone, a total of 52 FEP completed the study. We also enrolled 45 HC, 42 were included in the final sample.
Groups did not differ in sex, age or parental socioeconomic status (table 1). In patients, the BPRS positive subscale (t42 = 11.06, Cohen’s d = 1.69, p < 0.001) and BPRS total scores (t42 = 10.71, Cohen’s d = 1.63, p < 0.001) significantly decreased after 16 weeks of treatment, but not for BPRS negative scale scores (t42 = 0.57, Cohen’s d = 0.09, p = 0.57). The average daily dose of risperidone at that time was 4.22 ± 2.70 mg.
Table 1.
Demographics, Clinical Measures, and Data Quality
| Groups (N = 94) | P Value | ||
|---|---|---|---|
| HC (n = 42) | FEP (n = 52) | ||
| Demographic variables | |||
| Age (in years) | 24.33 ± 5.93 (15–41) | 24.46 ± 6.34 (15–40) | 0.92 |
| Sex (M/F) | 28/14 | 33/19 | 0.75 |
| aParental occupation | 4.17 ± 4.11 (1–20) | 5.86 ± 5.12 (1–21) | 0.09 |
| Clinical variables | |||
| Diagnosis | |||
| Schizophrenia | - | 26 | - |
| Schizoaffective disorder | - | 12 | - |
| Bipolar disorder with psychosis | - | 3 | - |
| Schizophreniform disorder | - | 2 | - |
| Psychosis NOS | - | 7 | - |
| Brief psychotic disorder | - | 1 | - |
| Major depressive disorder w/psychosis | - | 1 | - |
| BPRS baseline | |||
| Positive | - | 11.85 ± 3.51 (3–20) | - |
| Negative | - | 6.17 ± 3.41 (3–16) | - |
| Total | - | 52.02 ± 11.97 (32–84) | - |
| bBPRS Week 16 | |||
| Positive | - | 4.63 ± 1.90 (3–9) | - |
| Negative | - | 5.53 ± 2.70 (3–12) | - |
| Total | - | 30.37 ± 6.00 (20–45) | - |
| cRBANS | |||
| Immediate memory | 100.08 ± 17.52 (72–130) | 82.64 ± 19.97 (44–125) | < 0.001 |
| Visuospatial/constructional | 85.72 ± 12.39 (62–117) | 79.02 ± 17.96 (44–125) | 0.06 |
| Language | 99.00 ± 14.97 (40–120) | 82.30 ± 15.82 (40–112) | < 0.001 |
| Attention | 100.61 ± 17.89 (72–141) | 80.47 ± 17.63 (43–109) | < 0.001 |
| Delayed memory | 91.56 ± 8.84 (77–122) | 79.85 ± 13.96 (44–108) | < 0.001 |
| Total index | 94.14 ± 11.13 (78–120) | 76.70 ± 16.69 (50–117) | < 0.001 |
| Scan quality data | |||
| DWI | |||
| RMS absolute motion (mm) | 0.34 ± 0.22 (0.14–1.37) | 0.40 ± 0.25 (0.13–1.10) | 0.25 |
| RMS relative motion (mm) | 0.01 ± 0.01 (.001–0.03) | 0.01 ± 0.01 (.001–.04) | 0.30 |
| FC | |||
| Power framewise displacement (mm) | 0.21 ± 0.10 (0.11–0.49) | 0.29 ± 0.20 (0.11–0.89) | 0.003 |
Note: mean ± standard deviation (range); data available for a91 subjects; b43 patients; c83 subjects; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; BPRS, Brief Psychiatric Rating Scale; DWI, diffusion weighted imaging; FC, functional connectivity; P values are from χ 2 and independent samples t-tests for differences between the groups. aRanks determined from the Diagnostic Interview for Genetic Studies; higher rank (lower numerical value) corresponds to higher socioeconomic status.
Structural and Functional Connectivity of the DMN
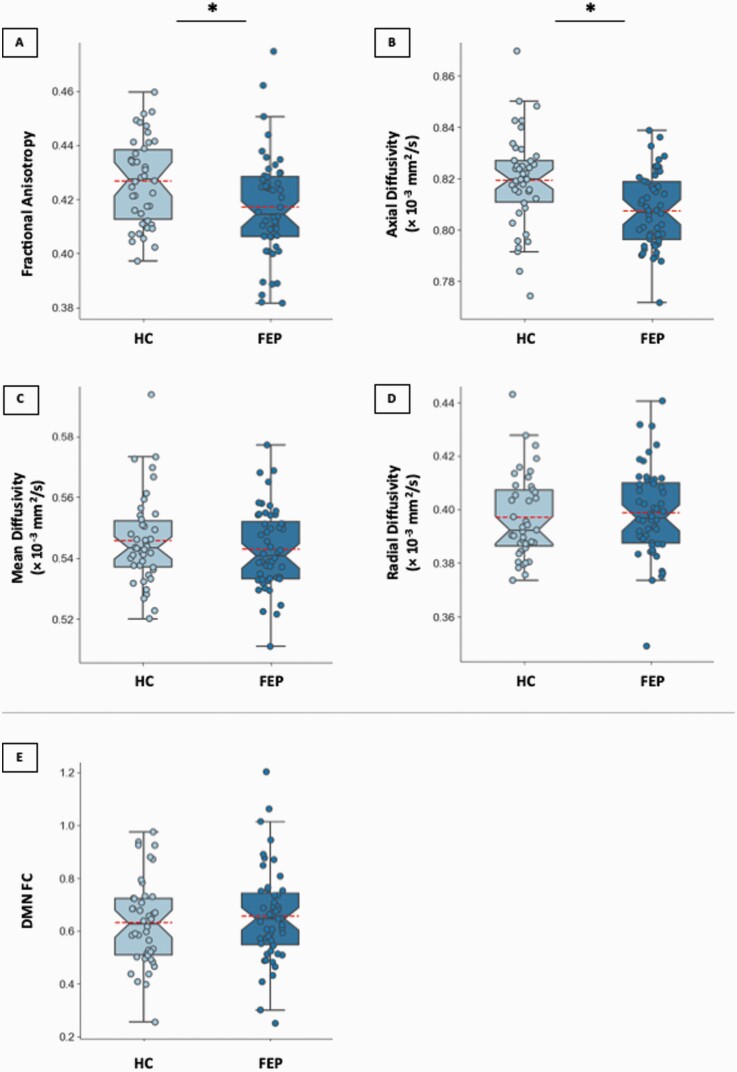
FEP showed lower DMN FA (F1, 89 = 6.64, partial η 2 = 0.07, p = 0.01, Figure 2A) and AD (F1, 89 = 11.42, partial η 2 = 0.11, p = 0.001, Figure 2B) compared to HC. We found no significant group differences for MD (F1, 89 = 0.85, partial η 2 = 0.01, p = 0.36, Figure 2C) or RD (F1, 88 = 0.22, partial η 2 = 0.002, p = 0.64, Figure 2D). Post-hoc analyses revealed significant group differences (HC > FEP) in FA in right inferior longitudinal fasciculus and AD in bilateral cingulum bundle (supplementary table 1). Functional connectivity of the DMN did not differ between groups (F1, 88 = 0.95, partial η 2 = 0.01, p = 0.33 , Figure 2E). All significant results survived FDR correction.
Fig. 2.
Boxplots depicting DMN DWI data for each group for (A) fractional anisotropy, (B) axial diffusivity, (C) mean diffusivity, and (D) radial diffusivity; and (E) boxplot depicting FC values for each group. Each individual dot is a data point from a single subject and red lines indicate average value for each group. HC, healthy control; FEP, first episode psychosis; DMN, default mode network; DWI, diffusion weighted imaging; and FC, functional connectivity. *p < 0.05, FDR corrected.
Relationships Between Structural and Functional Connectivity of the DMN
No significant associations between DMN WM indices and FC were found in HC or FEP nor significant group interactions.
Relationships of DMN Structure and Function With Treatment Response
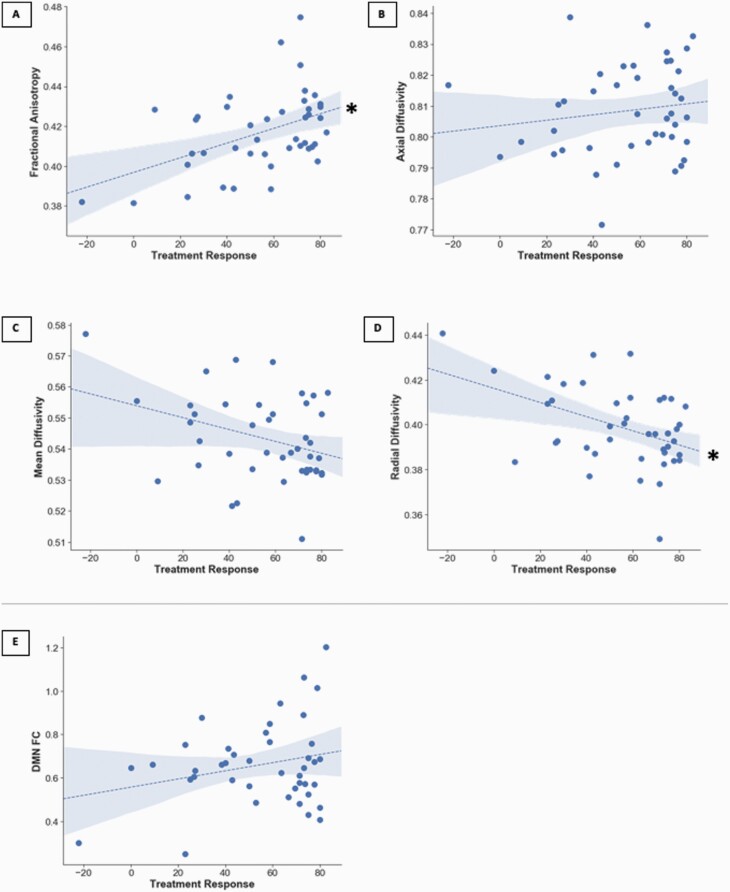
Two DMN structural connectivity indices, FA (b = 0.38, Cohen’s f2 = 0.51, p = 0.009, Figure 3A) and RD (b = −0.37, Cohen’s f2 = 0.44, p = 0.01, Figure 3D) were associated with response to antipsychotic treatment, however AD (b = 0.13, Cohen’s f2 = 0.04, p = 0.44, Figure 3B), MD, (b = −0.29, Cohen’s f2 = 0.23, p = 0.06, Figure 3C), and DMN FC (b = 0.28, Cohen’s f2 = 0.13, p = 0.09, Figure 3E) were not. All significant results survived FDR correction.
Fig. 3.
Scatterplots depicting associations between (A) DMN fractional anisotropy, (B) DMN axial diffusivity, (C) DMN medial diffusivity, (D) DMN radial diffusivity, and (E) DMN FC with treatment response. FEP, first episode psychosis; DMN, default mode network; and FC, functional connectivity. *p < 0.05, FDR corrected.
Sex and Age as Biological Variables
No significant effects of age or sex were found in any of our ANCOVA analyses (both WM and FC). Additionally, there were no significant effects in treatment response between the sexes in the regression analyses.
DISCUSSION
To our knowledge, this is the first study to comprehensively investigate DMN pathology on both a structural and functional level in medication-naïve FEP patients. Our results underscore the critical importance of this network for the pathophysiology of the disorder by showing that structural connectivity of the DMN is already impaired in the early illness stages. Findings also highlight the clinical relevance of DMN pathology, as we found that lower structural integrity in this network in medication-naïve patients was associated with poorer subsequent response to antipsychotic treatment.
Here, we report alterations in first episode patients across two diffusion indices, FA and AD, suggesting abnormalities in structure and organization in major white matter bundles providing the structural framework of the DMN.43 Our findings are consistent with diffusion imaging studies in schizophrenia reporting reduced white matter integrity in general25,44–46 and reduced integrity of the cingulum bundle, a white matter tract that structurally connects regions of the DMN, specifically.47 While FA is generally conceptualized as a non-specific marker of white matter integrity, other diffusion indices may yield additional information about the underlying pathophysiology. For example, preclinical studies have identified reduced AD in the corpus callosum as a potential marker of axonal damage.48,49 In contrast, demyelination is thought to be expressed as increased in RD without corresponding changes in AD.49 For example, an animal study conducted by Xie and colleagues found that initial stages of demyelination led to reduced axial diffusivity, but no change in radial diffusivity.50 In this context, reduced FA and AD in early stages of the illness in our study could be interpreted as evidence of axonal damage in structures supporting the DMN.
Surprisingly, we did not detect FC abnormalities of the DMN. This is in contrast with previous studies reporting increased or decreased functional connectivity of this network in chronic schizophrenia patients51–56 and medication-naïve first episode patients.57 However, a number of others also failed to detect functional DMN abnormalities in medication-naïve first-episode psychosis and chronic schizophrenia patients.58–60 These inconsistencies may be explained by methodological differences. For example, Gong and colleagues used a voxelwise approach where all analyses were performed in each voxel within a DMN mask and no white matter regressors or correction for micromovement (scrubbing or censoring) were implemented. It is important to note that the multimodal approach we employed here allows us to make a more nuanced interpretation of the apparent lack of functional connectivity alterations.
While we did not find group effects between DMN WM and FC metrics, there have been no studies that have specifically looked at DMN structural and functional connectivity in FEP.12 Our absence of results does not entirely represent a lack of DMN FC-WM abnormalities in FEP as more advanced techniques such as data fusion can help elucidate such potential abnormalities.61
Here, we report that greater FA and RD of the DMN in medication-naïve patients was associated with a more favorable subsequent response to antipsychotic treatment, which adds to the literature supporting the concept that white matter integrity has potential utility in predicting antipsychotic treatment response.62–64 Importantly, the DMN has been previously implicated as a pivotal functional network for clinical outcomes. Doucet and colleagues found that functional connectivity of the DMN was the single most important positive predictor of clinical outcome in schizophrenia spectrum patients who had with an illness duration of no more than 5 years.22 While our findings of decreased DMN FA and AD and DMN FA associations with treatment response are consistent with out previous work,45 our additional finding of treatment respond predicting DMN RD highlights the specificity of DMN in WM pathology and its susceptibility to response to antipsychotics.
One of the major strengths of our study was the enrollment of perhaps one of the largest groups of medication-naïve FEP in an imaging study followed longitudinally in the United States. This allowed us to mitigate effects from non-specific illness factors such as previous antipsychotic exposure and illness chronicity on neuroimaging variables. However, it is important to note that our group was heterogeneous in terms of diagnosis, which is to be expected in this patient population. Future larger scale studies that are adequately powered to discern if there are pathophysiological differences in functional and structural metrics will help shed light on how this affects treatment response in different groups of patients who present with a first psychotic episode. We treated patients with risperidone for a trial duration of 16 weeks, which diminishes the possibility that late responders are incorrectly characterized as poor responders. On the same note, the way treatment response was defined in our study was in the form of a spectrum, rather than a dichotomization of responders vs. non-responders. The former accurately reflects the clinical picture progression in various degrees. In addition, since FEP response rates are quite high, this create an uneven subgroup of responders compared to non-responders. Our retention rates were excellent for a clinical trial with antipsychotic medications, with only ~23% attrition.65 Another strength was that we used a multimodal approach, which by nature is more informative than unimodal studies and provides a more detailed description of the more complex picture of the neural correlates in FEP.12 It is possible that our approach for assessing structural connectivity using the tensor method may have underestimated the extent of demyelination and overestimated the axonal damage.66 Nonetheless, using biophysical diffusion models such as NODDI imaging64,67 could help characterize white matter pathology with more specificity. On the same note, our approach to capture functional connectivity of the DMN using region of interest analysis may not have captured functional connectivity abnormalities of the DMN that may have been evident with alternative approaches such as seed-based connectivity analyses. Even though cannabis, a major risk factor developing psychosis, may affect brain structure and function,68 we did not exclude patients with a history of cannabis use as this would have inadvertently biased our sample and limit the generalizability of our data. Another limitation of our study is that we did not examine possible confounding effects of tobacco smoking or body mass index.
Overall, our study highlights the critical role of the DMN in the underlying pathophysiology suggesting that axonal damage may already be present in FEP patients. We also demonstrated that DMN pathology is clinically relevant, as greater structural DMN alterations were associated with a less favorable clinical response to antipsychotic medications. Finally, our data underscore that a multimodal approach allows a more nuanced interpretation of the pathophysiology.
Supplementary Material
Acknowledgments
We would like to thank UAB IT Research Computing for providing the HPC resources (compute, storage, and networking) for this project. Special thanks to the patients and their families.
Role of the Sponsor: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Dr. Lahti had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: ACL.
Acquisition of data: BGR, ACL.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: JOM, NVK, BGR.
Statistical analysis: NVK, JOM.
Obtained funding: NVK, ACL.
Administrative, technical, or material support: ACL.
Study supervision: ACL.
Conflict of Interest: Dr. Kraguljac serves as consultant for Neurocrine Biosciences, Inc. All other authors declare no conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
Funding
This work was supported by the National Institutes of Health (R01MH102951 and R01MH113800, ACL; K23MH106683, NVK).
References
- 1.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. [DOI] [PubMed] [Google Scholar]
- 3.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner RL, DiNicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 2019;20(10):593–608. [DOI] [PubMed] [Google Scholar]
- 5.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 6.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43(3):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teipel SJ, Bokde AL, Meindl T, et al. . White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49(3):2021–2032. [DOI] [PubMed] [Google Scholar]
- 9.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30(10):3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28(43):10844–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen P, Sommer IE, Jardri R, Eysenck MW, Hugdahl K. Extrinsic and default mode networks in psychiatric conditions: relationship to excitatory-inhibitory transmitter balance and early trauma. Neurosci Biobehav Rev. 2019;99:90–100. [DOI] [PubMed] [Google Scholar]
- 12.Hu ML, Zong XF, Mann JJ, et al. . A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camchong J, MacDonald AW 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37(3):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ćurčić-Blake B, van der Meer L, Pijnenborg GH, David AS, Aleman A. Insight and psychosis: functional and anatomical brain connectivity and self-reflection in Schizophrenia. Hum Brain Mapp. 2015;36(12):4859–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skudlarski P, Jagannathan K, Anderson K, et al. . Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Tang W, Fan X, et al. . Resting-state functional connectivity changes within the default mode network and the salience network after antipsychotic treatment in early-phase schizophrenia. Neuropsychiatr Dis Treat. 2017;13:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraguljac NV, White DM, Hadley N, et al. . Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr Bull. 2016;42(4):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarpal DK, Robinson DG, Lencz T, et al. . Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szeszko PR, Robinson DG, Ikuta T, et al. . White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 2014;39(6):1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forlim CG, Klock L, Bächle J, et al. . Reduced resting-state connectivity in the precuneus is correlated with apathy in patients with schizophrenia. Sci Rep. 2020;10(1):2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Lee DK, Park K, Kim CE, Ryu S. Default mode network connectivity is associated with long-term clinical outcome in patients with schizophrenia. Neuroimage Clin. 2019;22:101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doucet GE, Moser DA, Luber MJ, Leibu E, Frangou S. Baseline brain structural and functional predictors of clinical outcome in the early course of schizophrenia. Mol Psychiatry. 2020;25(4):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maximo JO, Nelson EA, Armstrong WP, Kraguljac NV, Lahti AC. Duration of untreated psychosis correlates with brain connectivity and morphology in medication-naïve patients with first-episode psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farooq S, Agid O, Foussias G, Remington G. Using treatment response to subtype schizophrenia: proposal for a new paradigm in classification. Schizophr Bull. 2013;39(6):1169–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly S, Jahanshad N, Zalesky A, et al. . Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743–745. [DOI] [PubMed] [Google Scholar]
- 27.Gallego JA, Robinson DG, Sevy SM, et al. . Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter WT Jr, Gold JM, Lahti AC, et al. . Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry. 2000;57(6):533–538. [DOI] [PubMed] [Google Scholar]
- 29.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 30.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. [DOI] [PubMed] [Google Scholar]
- 31.Moore TA, Buchanan RW, Buckley PF, et al. . The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68(11):1751–1762. [DOI] [PubMed] [Google Scholar]
- 32.Robinson DG, Schooler NR, John M, et al. . Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magn Reson Med. 2016;76(5):1582–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76(5):1574–1581. [DOI] [PubMed] [Google Scholar]
- 35.Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51(1):103–114. [DOI] [PubMed] [Google Scholar]
- 36.Özarslan E, Koay CG, Shepherd TM, et al. . Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage. 2013;78:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. [DOI] [PubMed] [Google Scholar]
- 38.Glasser MF, Sotiropoulos SN, Wilson JA, et al. ; WU-Minn HCP Consortium . The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves PN, Foulon C, Karolis V, et al. . An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun Biol. 2019;2:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang AC, Tsai SJ, Liu ME, Huang CC, Lin CP. The association of aging with white matter integrity and functional connectivity hubs. Front Aging Neurosci. 2016;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 43.White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging. 2008;19(2):97–109. [DOI] [PubMed] [Google Scholar]
- 44.Karlsgodt KH. Diffusion imaging of white matter in schizophrenia: progress and future directions. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(3):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraguljac NV, Anthony T, Morgan CJ, Jindal RD, Burger MS, Lahti AC. White matter integrity, duration of untreated psychosis, and antipsychotic treatment response in medication-naive first-episode psychosis patients. Mol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid MA, White DM, Kraguljac NV, Lahti AC. A combined diffusion tensor imaging and magnetic resonance spectroscopy study of patients with schizophrenia. Schizophr Res. 2016;170(2-3):341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seitz J, Zuo JX, Lyall AE, et al. . Tractography analysis of 5 white matter bundles and their clinical and cognitive correlates in early-course schizophrenia. Schizophr Bull. 2016;42(3):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis KL, Stewart DG, Friedman JI, et al. . White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60(5):443–456. [DOI] [PubMed] [Google Scholar]
- 49.Song SK, Yoshino J, Le TQ, et al. . Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. [DOI] [PubMed] [Google Scholar]
- 50.Xie M, Tobin JE, Budde MD, et al. . Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J Neuropathol Exp Neurol. 2010;69(7):704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, Kaneko Y, Ouyang X, et al. . Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38(2):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bluhm RL, Miller J, Lanius RA, et al. . Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117(1):21–30. [DOI] [PubMed] [Google Scholar]
- 54.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. . Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4): 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130(1-3):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mannell MV, Franco AR, Calhoun VD, Cañive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31(3):424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong Q, Hu X, Pettersson-Yeo W, et al. . Network-level dysconnectivity in drug-naïve first-episode psychosis: dissociating transdiagnostic and diagnosis-specific alterations. Neuropsychopharmacology. 2017;42(4):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraguljac NV, White DM, Hadley JA, et al. . Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin. 2016;10:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf ND, Sambataro F, Vasic N, et al. . Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36(6):366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Q, Hu Y, Zeng B, et al. . Parietal memory network and default mode network in first-episode drug-naïve schizophrenia: associations with auditory hallucination. Hum Brain Mapp. 2020;41(8):1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calhoun VD, Sui J. Multimodal fusion of brain imaging data: a key to finding the missing link(s) in complex mental illness. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(3):230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reis Marques T, Taylor H, Chaddock C, et al. . White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 2014;137(Pt 1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crossley NA, Marques TR, Taylor H, et al. . Connectomic correlates of response to treatment in first-episode psychosis. Brain. 2017;140(2):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraguljac NV, Anthony T, Monroe WS, et al. . A longitudinal neurite and free water imaging study in patients with a schizophrenia spectrum disorder. Neuropsychopharmacology. 2019;44(11):1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabinowitz J, Levine SZ, Barkai O, Davidov O. Dropout rates in randomized clinical trials of antipsychotics: a meta-analysis comparing first- and second-generation drugs and an examination of the role of trial design features. Schizophr Bull. 2009;35(4):775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front Neurol. 2018;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodman AM, Allendorfer JB, Blum AS, et al. . White matter and neurite morphology differ in psychogenic nonepileptic seizures. Ann Clin Transl Neurol. 2020;7(10):1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cookey J, Bernier D, Tibbo PG. White matter changes in early phase schizophrenia and cannabis use: an update and systematic review of diffusion tensor imaging studies. Schizophr Res. 2014;156(2-3):137–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.