Abstract
The colonic epithelium is the site of production and transport of many vasoactive metabolites and neurotransmitters that can modulate the immune system, affect cellular metabolism, and subsequently regulate blood pressure. As an important interface between the microbiome and its host, the colon can contribute to the development of hypertension. In this critical review, we highlight the role of colonic inflammation and microbial metabolites on the gut brain axis in the pathology of hypertension, with special emphasis on the interaction between tumor necrosis factor α (TNFα) and short chain fatty acid (SCFA) metabolites. Here, we review the current literature and identify novel pathways in the colonic epithelium related to hypertension. A network analysis on transcriptome data previously generated in spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY) rats reveals differences in several pathways associated with inflammation involving TNFα (TNF −> NF-κB Expression Targets; TNF −> STAT Expression Targets) as well as oxidative stress. We also identify down-regulation of networks associated with gastrointestinal function, cardiovascular function, enteric nervous system function, and cholinergic and adrenergic transmission. In addition, the analysis uncovered transcriptome responses related to glycolysis, butyrate oxidation, and mitochondrial function, in addition to gut neuropeptides that serve as modulators of blood pressure and metabolic function. We present a model for the role of TNFα in regulating bacterial metabolite transport and neuropeptide signaling in the gastrointestinal system, highlighting the complexity of host-microbiota interactions in hypertension.
Keywords: butyrate, blood pressure, microbiome, gene networks, Inflammation, hypertension
1. Hypertension: A continuing health concern
Hypertension remains one of the most concerning preventable conditions for the global human population. Based upon the updated 2017 definition of hypertension, the American Heart Association estimated that (1) 46% of the total adult population above the age of 20 (2013–2016) had some form of hypertension, (2) that estimates for the cost of treating high blood pressure was $55.9 billion dollars (2014–2015); and (3) that the total mortality attributed to high blood pressure alone was 90,098 (859,125 for cardiovascular disease) in 2017 (Virani et al. 2020). Most concerning is the realization that mortality rates for hypertension have been rising since 2013 (Nambiar et al. 2020), and account for about 9.4 million deaths globally (Vallianou et al. 2020). Moreover, between 2017–2018, estimates revealed that 22.4% of adults aged 18–39, 54.5% of adults 40–59, and 74.5% of adults over 60 presented with hypertension (Ostchega et al. 2020). This earlier onset of hypertension is especially troubling since it has been associated with higher risk of cardiovascular disease (CVD) and premature mortality (Wang et al. 2020).
The underlying causes of hypertension are many and include both genetic and environmental factors such as diet, lack of activity, pollution, drug abuse, smoking, in addition to other factors (Virani et al. 2020). In terms of underlying mechanism, hypertension is often associated with inflammation, oxidative stress, increased sympathetic activity, sodium retention, renin-angiotensin-aldosterone system (RAAS) over-activation, and endothelial cell dysfunction (Manrique et al. 2009). Due to these pro-hypertensive factors, many comorbidities such as metabolic syndrome, diabetes mellitus, dyslipidemia, stroke, congestive heart failure, chronic kidney disease, dementia, coronary heart disease, as well as auto-immune dysfunctions and gut dysbiosis occur in those patients with severe hypertension (Yang et al. 2015; Ventura andand Lavie, 2016; Wolf andand Ryan 2019; Virani et al. 2020). It is clear that hypertension exhibits complex etiology and is influenced by multiple factors, highlighting the need for continued efforts to develop new therapeutic interventions and treatment strategies. Here we conduct a comprehensive review to determine the role of TNF alpha in the gastrointestinal system and its relation to hypertension. To do this, our Google Scholar search terms included TNF-α, Colon, Colonocyte, Differentiation, Inflammation, Blood pressure, Hypertension, Vasodilation, Vasoconstriction, Gut permeability, Dysbiosis, Microbiome, Fermentation, Metabolism, Short Chain Fatty Acid, Branched SCFA, Transporter, Formate, Acetate, Propionate, Butyrate, Valerate, Isovalerate, Isobutyrate, 2-methylbutyrate, Concentration in Serum, and HDAC inhibition. The search was conducted between August, 2019 and July, 2020.
2. Inflammation is a hallmark of hypertension: A role for TNFα signaling
Hypertension is closely associated with a low grade chronic inflammatory response both in the periphery and within the central nervous system (CNS). Systemically, activation of innate immune system has been demonstrated in human hypertension and rodent models of hypertension (Barbaro andand Harrison 2019; Guzik et al. 2007; Kim et al. 2015), while activation of CNS glial cells and infiltration of peripheral immune cells in cardio-regulatory brain regions has been described (Santisteban et al. 2015; Shi et al. 2010; Stern et al. 2016). In addition, elevated serum and tissue levels of pro-inflammatory cytokines such as interleukins 1 and 6 (IL1 and IL6), and TNFα are reported in human and rodent hypertension studies (Segiet et al. 2019; Sriramula et al. 2008; Tanase et al. 2019; Mehaffey and Majid 2017; Navarro-Gonzalez et al. 2010). TNFα is a proinflammatory cytokine involved in acute phase response to tissue damage or infection (Gruys et al. 2005). The role of TNFα signaling is complex and cell type- and dose-dependent, regulating apoptosis, proliferation, cell survival, or differentiation (Aggarwal 2003), in addition to regulating the secretion of many secondary inflammatory cytokines. In humans, severity of resistant hypertension and an increased 6-year mortality odds ratio have both been linked to significantly higher levels of TNFα among other cytokines (Barbaro andand Harrison 2019), while TNFα inhibitors have been shown to lower blood pressure in both hypertensive humans and experimental models (Guzik et al. 2007; Yoshida et al. 2014). In salt-sensitive rodents, TNFα has also been shown to precede changes in blood pressure and was accompanied by reduced soluble tumor necrosis factor receptor 1 (TNFR1), which functions as a TNFα inhibitor, as well as elevated intracellular TNFR1 in hypertension-prone compared to hypertension-resistant rodents (Mazor et al. 2010). TNFα is produced by many different cells in the body; however, the main producers are monocyte/macrophages and other immune cells such as Natural Killer, T and B cells (Aggarwal, 2003). Once released from macrophages, TNFα can activate other immune cells (Gamble et al. 1985; Maini et al. 1995), as well as directly act on its receptors present in various tissues and cell types including those localized to the gastrointestinal (GI) system (Watson and Hughes, 2012). Considering the prominent role of monocytes and T and B cells in immune responses in hypertension including that observed in the GI tract (Kim et al. 2015; Santisteban et al. 2017), the evidence that elevated levels of TNFα are present in human and rodent hypertension (Navarro-Gonzalez et al. 2010; Yoshida et al. 2014; Yang et al. 2020; Silveira-Nunes et al. 2020), and data showing hypertension and gut microbiota can be modified with dietary interventions (Mazor et al. 2010), it becomes apparent there is a compelling rationale for further research into the role of TNFα in hypertension, with particular emphasis on the GI tract.
3. Inflammation, TNFα and the gastrointestinal microbiome in hypertension
The intersection between inflammation, diet, and microbiome in the onset of hypertension has received significant attention in recent years, with numerous reports linking gut dysbiosis to hypertension (e.g. Jose and Raj, 2015; Vallianou et al. 2020; Yang et al. 2015; Mell et al. 2015; Silveira-Nunes et al. 2020). Gut barrier function, microbial composition, and immune cell function are all important determinants of cardiovascular health. Animal models of hypertension are associated with gut inflammation, dysbiosis, and increased gut permeability (Richards et al. 2017, Santisteban et al. 2017), and 16S bacterial sequencing of human hypertensive microbiota and metabolomics analysis of human hypertensive plasma suggest gut dysbiosis and elevated circulating markers of gut permeability (Kim et al. 2018; Cheema and Pluznick, 2019; Karbach et al. 2016; Calderón-Pérez et al. 2020). Pathological levels of TNFα can increase cell shedding in the gut, leading to micro-erosions of multiple gut cells that negatively impact the epithelial barrier (Watson andand Hughes 2012), thus suggesting involvement of TNFα in gut permeability. Indeed, TNFα is critical for maintenance of intestinal homeostasis, and a fine balance between epithelial cell death and survival depends on the levels of TNFα and crosstalk between the TNFα receptors 1 and 2 (Ruder et al. 2019). Traditionally, microbiota is considered a crucial modulator of intestinal immune responses throughout one’s lifetime, and it involves TNFα signaling (Liebisch et al. 2019; Segain et al. 2000). Conversely, modulation of TNFα signaling can affect the gut microbiota composition (Busquets et al. 2015; Rooks et al. 2014), suggesting that reciprocal host-microbiota interactions can be modulated via TNFα signaling in the gut. Recently, our group demonstrated that reduced systemic and GI inflammation and decreased expression of TNFα signaling molecules such as membrane-spanning 4-domains subfamily A member 4B (Ms4a4b) was associated with beneficial shifts in the gut microbiota composition and reduced blood pressure (Yang et al. 2017; Ahmari et al. 2016). On the other hand, increased GI TNFα in rodent models of hypertension is associated with gut dysbiosis (Yang et al. 2020) and hypertensive individuals show increased TNFα to interferon γ (IFNγ) ratio and reduced fecal microbial diversity (Silveira-Nunes et al. 2020). Thus, TNFα may be an important mediator between host and microbiota in hypertension and related disorders characterized by inflammation and gut dysbiosis.
4. Microbial short chain fatty acids in regulation of inflammation and blood pressure in hypertension
Microbial enzymes in the gastrointestinal tract are critical moderators of human disease via the production of many vasoactive and immunomodulatory metabolites, including SCFAs. These microbial metabolites can modulate inflammation and gene expression (Venegas et al. 2019; Vinolo et al. 2011). For example, consumption of dietary fiber and supplementation with SCFAs such as acetate, butyrate, propionate, and valerate, or ample availability of their microbial producers in the GI tract have been shown to negatively associate with a variety of inflammatory conditions, including ulcerative colitis (Machiels et al. 2014), asthma (Arrieta et al. 2015), colorectal cancer (Chen et al. 2019; Hibberd et al. 2017; Yoon and Kim, 2018), and hypertension (Brown and Hazen, 2018; Chambers et al. 2018; Yang et al. 2019; Chang et al. 2020). On the other hand, elevated formate has been linked to gut dysbiosis and inflammation (Hughes et al. 2017; Jakobsdottir et al. 2013) while the branched chain SCFAS, isobutyrate and isovalerate, have been implicated in hypercholesterolemia (Granado-Serrano et al. 2019). Thus, the relative concentrations and ratios of the different SCFA metabolites may be important factors in the development and progression of many conditions characterized by inflammation, which have also been shown to be responsive targets of prebiotic and probiotic therapies (Ganesh et al. 2018; Robles-Vera et al. 2018; Robles-Vera et al. 2020a; Robles-Vera et al. 2020b; O’Connor et al. 2020). For instance, in an obstructive sleep apnea model of hypertension in which cecal acetate levels were reduced by 48%, hypertension was prevented by the probiotic Clostridium butyricum and prebiotic Hylon VII (both of which increase acetate and butyrate production) or by cecal acetate infusion (Ganesh et al. 2018). Another group has shown that acetate and butyrate supplementation, or some of their probiotic producers (Bifidobacterium breve CECT7263 or Lactobacillus fermentum CECT5716), can lower blood pressure in a variety of hypertensive models, including the SHR and Angiotensin II (Ang II) infusion, but not a chronic nitric oxide synthase inhibition model of hypertension despite improvements of many endpoints including reduced dysbiosis, inflammation, and oxidative stress (Robles-Vera et al. 2018; Robles-Vera et al. 2020a; Robles-Vera et al. 2020b).
SCFAs also exert direct effects on blood pressure (for review see Poll et al. 2020) either as vasodilators or vasoconstrictors. The effects of the smallest SCFA, formate (1C), on blood pressure is complicated by its role in chloride exchange in the kidneys. Administration of sodium formate at millimolar concentrations has produced vasoconstriction in canine basilar arteries (DeFelice et al. 1976). On the other hand, acetate (2C), propionate (3C), and butyrate (4C) are physiologically the most abundant SCFAs and exert well described anti-hypertensive effects (Bartolomaeus et al. 2019; Natarajan et al. 2016; Yang et al. 2019). Acetate and butyrate also have direct CNS effects (Soliman et al. 2012; Yang et al. 2019). Valerate (5C) is present in lower concentrations in the colon and has been less studied, however it appears to be at equivalent levels in the serum as propionate and butyrate (Jakobsdottir et al. 2013), and may favorably influence gut health and prevent certain types of hypertension (Chang et al. 2020; Onyszkiewicz et al. 2020; Li et al. 2020). For instance, intracolonic infusion of sodium valerate has been recently shown to lower blood pressure and heart rate in rats via a GPR41/43 dependent mechanism (Onyszkiewicz et al. 2020). However, increased plasma valerate has also been associated with preexisting cardiovascular disease in patients with chronic kidney disease (Jadoon et al. 2018). Due to these somewhat conflicting results, further study of the mechanisms of SCFA effects in hypertension is warranted.
5. Interaction between TNFα and SCFAs in the gut
Under physiological conditions, SCFAs can affect TNFα secretion and signaling, and conversely, TNFα has been shown to modulate the SCFA uptake in the gut. For example, a study in cardiomyocytes showed that histone deacetylase (HDAC) 3, a target for some SCFAs, mediates secretion of TNFα following lipopolysaccharide stimulation (Zhu et al. 2010), while mice treated with the HDAC6 specific inhibitor, tubastatin A, had lower TNFα levels than those without the inhibitor (Magupalli et al. 2020).
The extent to which TNFα secretion relies on HDAC-dependent mechanisms is not fully elucidated. However, the role of HDAC in TNFα signaling directly links inflammation to gut health, as many SCFAs, and particularly butyrate, are known HDAC inhibitors. Indeed, butyrate and other HDAC inhibitors have been shown by multiple groups to reduce inflammation in many organ systems. For a comprehensive review into the roles of SCFAs in inflammation, the reader is directed to Vinolo et. al. (2011) and Venegas et al. (2019). There are many examples of reciprocal regulation between butyrate and TNFα. Butyrate, for example, has been demonstrated to lower macrophage secretion of TNFα and other pro-inflammatory cytokines, while also increasing anti-inflammatory IL-10 secretion (Vinolo et al. 2011). Butyrate has also been shown to decrease TNFα production in intestinal biopsies from Crohn’s Disease patients through inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling (Segain et al. 2000). In an experimental colitis model, mice with a knockout to solute carrier family 5 member 8 (SLC5a8) (the high affinity transporter of butyrate and other SCFAs), which were fed low fiber diets, presented with decreased epithelial repair and increased inflammation compared to mice with functioning SLC5a8 (Sivaprakasam et al. 2019). We have recently shown a link between reduced colonic expression of Slc5a8 and reduced butyrate colonic transport in the SHR (Yang et al. 2019), a model also presenting with high colonic TNFα (Yang et al. 2020). In line with this, TNFα has been shown to influence SCFA uptake in the colon. For example, TNFα treatment decreased both the SLC5a8 mRNA expression levels and butyrate uptake transport in intestinal epithelial cell (IEC) 6 cells, while pretreatment with Lactobacilli plantarum exerted a positive effect on butyrate uptake and SLC5a8 expression (Borthakur et al. 2010). Thus, an important interaction between the SCFAs, TNFα and the gut epithelium may exist in hypertension. Figure 1a summarizes probable molecular interactions between gut epithelial SCFAs transporters and inflammatory cytokines (notably TNFα) as well as various cellular proteins. These were generated using Pathway Studio as previously described (Cao et al. 2019) and are based on gene and protein expression and their close relationships within the interactome developed from an extensive review of the current literature. As illustrated in Figure 1b, TNFα can regulate the expression of solute carrier family 16 member 1 (SLC16a1), a low affinity SCFA transporter, which is the predominant conveyor of butyrate and several other SCFAs under physiological conditions (Sivaprakasam et al. 2018) as well as SLC5a8, the high affinity SCFA transporter, which increases SCFA transport when concentrations are low. During pathological processes, such as lower SCFA production, the transport of butyrate and other SCFAs falls on Slc5a8 (Sivaprakasam et al. 2019), thus highlighting its importance in conditions such as hypertension where its expression is reduced while TNFα is high.
Fig. 1.
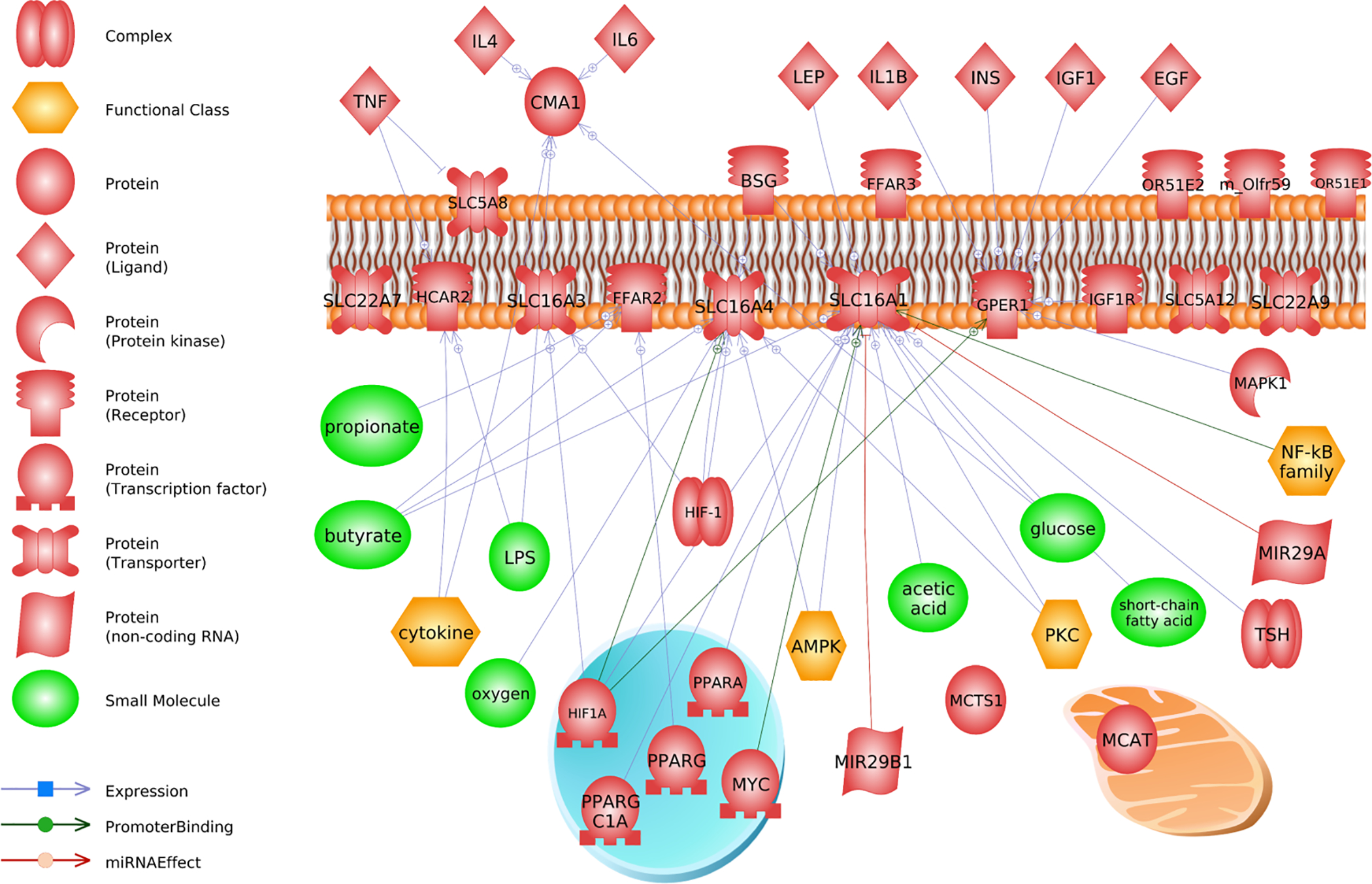
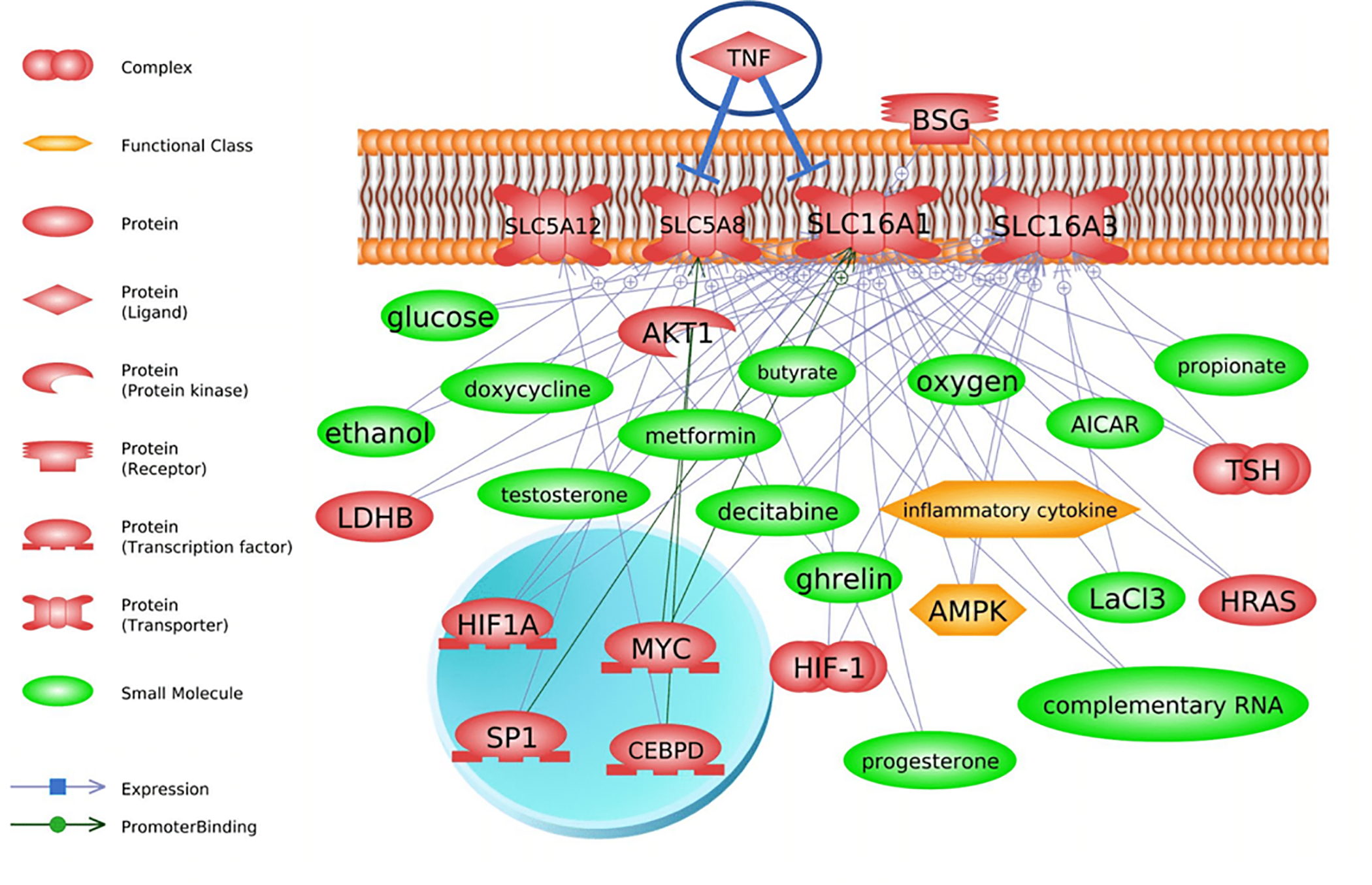
Literature connections established in Pathway Studio (Elsevier) between TNF-, SLC transporter transcriptional regulators, and downstream metabolites and hormones. The top graph (a) shows that TNF interacts at the level of the gene or protein with solute carriers on the plasma membrane. Cytokines also regulate these transporters which can affect production and synthesis of various short chain fatty acids (i.e. butyrate and propionate). There is direct evidence that TNF exerts regulatory control over SLC5A8 (bottom graph, b). This in turn can affect several small molecules, such as sex steroids and short chain fatty acids. Abbreviations are found is Supplemental Data 1
In addition to reducing TNFα secretion, SCFAs may also modulate downstream TNFα signaling and conversely, TNFα can subsequently influence the metabolism of SCFAs (i.e. butyrate, propionate) (Figure 1). We present in the figure those downstream proteins most likely involved in TNFα-mediated regulation of SCFAs, such as hypoxia-inducible factor 1 (HIF1) and peroxisome proliferator-activated receptors (PPARs). These are but a few examples of proteins that are putative mediators of such a pathway. Butyrate cotreatment can modulate pathways downstream of TNFα through transcription factors, reducing NF-κB signaling and secretion of factor B and IL-8, while increasing Complement component 3 (C3) secretion (Andoh et al. 1999). As indicated in Figure 1a,b, this may occur through specificity protein 1 (SP1), PPARs, or HIF1a signaling. However, these interactions and regulatory relationships remain to be rigorously tested in the gut epithelium.
It is important to note that the effect of butyrate on complex cellular responses is dependent upon the metabolic state of the cell (Ryu et al. 2018) as well as the gut luminal pH (Gálfi and Neogrády, 2001). As such, certain pathological conditions may exacerbate, rather than alleviate, the GI inflammatory responses. For instance, while the effects of butyrate are generally reported as beneficial to barrier function and inflammation, butyrate reportedly increases transepithelial electrical resistance in cells obtained from ulcerative colitis patients (a measure of membrane integrity and permeability) when applied alone, but can lead to a synergistic loss of membrane integrity when in the presence of TNFα and IFNγ, when TNFα and IFNγ applied alone did not significantly increase permeability (Vancamelbeke et al. 2019). Thus, although butyrate and TNFα may exert opposing effects under certain physiological conditions, during pathology they have been shown to determine gut permeability and the inflammatory state. The precise role of butyrate and other SCFAs and their interaction with TNFα and other inflammatory cytokines in regulation of homeostasis and barrier function of gut epithelial cells remains largely unanswered, especially in the pathophysiology of hypertension.
6. Microbial short chain fatty acids in regulation of epithelial cell homeostasis and cellular metabolism
SCFAs can be produced via multiple pathways, such as the catabolic fermentation of either dietary fiber or amino acids, the anabolic formation from smaller molecules such as pyruvate, lactate, carbon dioxide, or other SCFAs (Gonzalez-Garcia et al. 2017; Morrison and Preston, 2016; Oliphant and Allen-Vercoe, 2019), or during the metabolism of certain xenobiotic compounds such as ethanol (Pardo et al. 2013). Total SCFA concentrations vary by diet and microbial composition, and in physiological conditions, they are the highest in the proximal colon where they range between 70–140 mM, dropping to 20–70 mM in the distal colon (Topping and Clifton, 2001). Acetate, propionate, and butyrate absorption has been assessed in humans undergoing abdominal surgery and per hour account for roughly 34.9 μmol/kg body weight (Bloemen et al. 2009). In physiological conditions, the main function of many SCFAs is as an energy source/fuel for the host epithelial cells, with butyrate generally being the most potent and predominant energy source (Waldecker et al. 2008). SCFAs are metabolized in mitochondria through a β oxidation pathway to Acetyl-CoA (and methylmalonyl-CoA in the case of the odd carbon SCFAs), which then enter the tricarboxylic acid (TCA) cycle which functions to reduce NAD+ to NADH, culminating in ATP production via oxidative phosphorylation (Donohoe et al. 2011; Halarnkar and Blomquist, 1989). Far from being just an energy source, and as mentioned before, SCFAs are also HDAC inhibitors of varying potency (Waldecker et al. 2008). In line with this, Acetyl-CoA also supplies the acetyl group for histone acetylases (HATs) (Roth et al. 2001). Intriguingly, class III HDAC enzymes have an absolute requirement for NAD+ to remove the acetyl groups (Licciardi et al. 2011), suggesting that downstream TCA cycle activity might lower this class of HDAC activity in the presence of high SCFA levels. On the other hand, when butyrate and other SCFAs are low in abundance, such as the case in microbiome depleted or germ-free organisms (Hughes et al. 2017; Zarrinpar et al. 2018), the colonocytes exist in an energy-deprived condition, with a decrease in genes involved in the oxidation of butyrate, a lower NADH to NAD+ ratio, lower ATP levels, and increased AMP-activated protein kinase (AMPK) activation, cyclin-dependent kinase inhibitor 1B (P27KIP1) phosphorylation, and autophagy, which importantly, are rescued with butyrate supplementation (Donohoe et al. 2011). Thus, the presence or absence of specific SCFAs such as butyrate may lead to a switch in the metabolic functions of the colonic epithelial cells.
A shift in colonic butyrate metabolism has been shown in a number of inflammatory conditions of the gut such as colon cancer and ulcerative colitis (Chen et al. 2019; Sivaprakasam et al. 2018). Indeed, colon cancer cells are thought to maintain higher HDAC activity due to a shift from butyrate metabolism to glycolysis (Chen et al. 2019). Several butyrate transporters have been shown to be tumor-suppressor genes and have decreased expression levels in inflamed tissues in ulcerative colitis (Sivaprakasam et al. 2018). In both these conditions, decreased butyrate transport functionally leads to higher HDAC levels, lower acetylated histones and altered transcription patterns (Sivaprakasam et al. 2018; Chen et al. 2019). Thus, butyrate and other SCFAs represent major fuel source that maintain homeostasis of the gut epithelium, and absence of this fuel may lead to metabolic shifts in the GI tract. As these SCFAs are lower in hypertension, the metabolic function of colonic epithelia in hypertension may be affected.
Butyrate metabolism by enterocytes is also thought to protect intestinal stem cells in the crypt base (Rath et al. 2018). The intestinal epithelium has staggering regenerative abilities, and each crypt villus can shed almost the entire epithelial cell total daily, and a very small proportion of these are intestinal stem cells from which the villi regenerate (Nooteboom et al. 2010; Rath et al. 2018). These stem cells in the crypt base grow and differentiate into either secretory or absorptive lineages as they migrate upward, eventually undergoing senescence and a type of programmed cell death, anoikis, involving shedding into the lumen via tight junction reformation at the villus tip (Rath et al. 2018; Watson and Hughes, 2012). Mitochondrial function is a key determinant of IEC fate during differentiation (for review see Rath et al. 2018) and differentiated cells are also capable of dedifferentiation in response to damage and loss of columnar polarity (Weichselbaum and Klein, 2018).
IECs maintain a highly regulated process of cell division that may be dysregulated via chronic inflammation, including by TNFα (Thoo et al. 2019). Thus, in hypertension, elevated gut TNFα may result in decreased gut renewal and shifts in epithelial cell metabolism, and it is unclear whether butyrate mitigates or compounds this process. Increased luminal butyrate but lower circulating levels have been documented in the SHR (Yang et al. 2019) and lower circulating butyrate has also been shown in hypertensive patients (Calderón-Pérez et al. 2020), suggesting that altered butyrate transport and utilization is a feature of hypertension. However, whether there exists a shift in butyrate metabolism in the hypertensive colon, and what the consequence of that may be to hypertension, remains unknown.
7. Short chain fatty acids: effects on blood pressure
The relative abundance of SCFAs in circulation has been shown to vary considerably depending on diet, genetics, environment, and health condition, as well as the SCFA in question. Formate is physiologically in the range of 10 to 100 μM in the serum of healthy adults but has been reported to reach 300–400 μM in response to various vitamin deficiencies (Brosnan and Brosnan, 2016; Pietzke et al. 2020). Lower millimolar concentrations have been reported clinically in blood as a consequence of methanol poisoning, and at this concentration formate is mitotoxic, directly interfering with mitochondrial respiration (Zakharov et al. 2015). Branched chain amino acids can be catabolized in host tissues via cytosolic or mitochondrial Branched-chain amino acid aminotransferase (BCAT) enzymes (Zhang et al. 2018), though whether this contributes to circulating branched SCFA levels is poorly understood. Of the branched SCFAs isovalerate appears to be the highest in circulation, reported in the 30–40μM range, whereas isobutyrate is lower around 12 μM (Jakobsdottir et al. 2013). Less is known about 2-methylbutyric acid concentrations in circulation, although it has been reported around 220 nM in patients undergoing hemodialysis (Wu et al. 2019). Acetate is generally viewed as the most abundant SCFA produced in the colon, with human serum levels normally within the 25–200 μM range in healthy adults, with indication that higher serum concentrations are possible (Hosios and Vander Heiden, 2014; Zordoky et al. 2015; Verbeke, 2017) and acetate uptake to the brain appears to be saturated at 2–3 mM in rats (Deelchand et al. 2009). Propionate and butyrate undergo substantial metabolism in the liver and therefore are typically present in the higher nanomolar and lower μM range in the serum of healthy adults (Verbeke, 2017; Jakobsdottir et al. 2013). These concentrations may also be elevated in some pathological conditions such as propionic acidemia, where serum propionate concentrations have been reported in the high μM and low mM range (Thompson et al. 1990; Wolf et al. 1981) and are known to exert toxic effects (Wolf et al. 1978; Wolf et al. 1981). Thus, circulating quantities of SCFAs are quite specific to the SCFA in question but generally span the nM to μM range. Furthermore, they are sensitive to many environmental factors such as the health status and diet of the individual.
Reduced circulating levels of acetate, propionate, butyrate, and valerate in particular have been associated with human and rodent hypertension (Yang et al. 2019; Brown and Hazen, 2018; Huart et al. 2019; Chang et al. 2020). The reduced presence of beneficial SCFAs in circulation may partly be due to reduced absorption of these SCFAs in the proximal colon (Poll et al. 2020; Yang et al. 2019). Currently, it is unclear whether other factors such as increased liver metabolism, excretion by monocarboxylate efflux pumps, or decreased blood flow to the colon could also contribute to lower absorption into circulation. It is likely that the latter is involved to some degree as prolonged inflammation can lead to reduced blood supply (Rath et al. 2018) and low GI perfusion has been reported in the SHR (Santisteban et al. 2017). Butyrate and acetate can also directly affect the CNS cardio-regulatory regions and thereby modulate cardiovascular responses (Soliman et al. 2012; Yang et al. 2019). In addition, many SCFAs have a direct vasodilatory effect (Poll et al. 2020), while also having the ability to modify expression of genes such as Ang II receptors and other inflammatory markers (Yang et al. 2019). Thus, reduction of SCFAs in circulation partly due to reduced intestinal absorption can potentially exacerbate the pro-hypertensive responses.
8. Molecular markers for inflammatory, metabolic, and neuronally active gut peptides in SHR colonic epithelium: implications for hypertension
In previous sections, we outlined some published evidence in support of the TNFα -SCFA interaction in pathophysiology of the gut. To identify additional mechanisms in the gut that might be related to this interaction in hypertension, we utilized a computational method previously published by us (Zubcevic et al. 2017) and reanalyzed a recently published transcriptome data set from gut epithelial cells obtained from SHR and WKY rodents (Yang et al. 2020). In the study, epithelial cells were mechanically removed from adult male WKY and SHR, and differential gene expression analysis was conducted using RNA-seq. We downloaded the available published NCBI GEO dataset (GSE130658) and conducted the gene set enrichment and sub network enrichment analyses, two powerful approaches used to identify pathways and gene networks (Zubcevic et al. 2017). The analysis leverages literature information to build networks and to identify key pathways altered based on transcriptome data. Our reanalysis of these data identified intriguing new pathways not previously identified which supports our hypothesis of TNFα -SCFA nexus in hypertension. It is important to note that while WKY is considered to be a genetic control for SHR, there are limitations to this model as it has been linked to irritable bowel syndrome (O’Mahony et al. 2013), and thus may differ from other common normotensive control strains such as Wistar-Han or Sprague Dawley rats. Thus, this is one consideration when interpreting the present RNA-seq analysis. Nevertheless, such gene expression comparisons are relative and differences between WKY and SHR are expected to shed light into mechanisms and pathology of hypertension.
Indeed, several metabolic pathways are differentially expressed in the WKY and SHR, including those involved with xenobiotics, pterins, omega-3 fatty acids, estrogens, and pyrimidines (Supplemental Table 1). Within the Pterin family, folate metabolism has been linked to oxidative stress, elevated blood pressure, and insulin resistance in SHR (Pravenec et al. 2013). Folate also has a role in one carbon addition in nucleic and amino acid synthesis (Jamerson et al. 2013). Many genes in the folate cycle are differentially expressed in SHR colonic epithelium, including aminomethyltransferase (AMT), dihydrofolate reductase (DHFR), methylenetetrahydrofolate dehydrogenase 1 and 1-like (MTHFD1 and MTHFD1L), methylenetetrahydrofolate reductase (MTHFR), serine hydroxymethyltransferases 1 and 2 (SHMT1 and SHMT 2), and solute carrier family 19 member 1 (SLC19a1). Of note, MTHFR, the rate limiting enzyme of folate production, is expressed at higher levels in the SHR colonic epithelium. The vast majority of differentially expressed pyrimidine metabolic pathway genes have lower expression levels in the SHR, including carbamoyl-phosphate synthetase 2 (CAD), cytidine/uridine monophosphate kinase 1 (CMPK1), dihydroorotate dehydrogenase (DHODH), deoxythymidylate kinase (DTYMK), DUTP pyrophosphatase (DUT), ectonucleoside triphosphate diphosphohydrolase 1 and 4 (ENTPD1 andENTPD4), nucleoside diphosphate kinase A and B (NME1 and NME2), cytosolic 5’-nucleotidase 3 (NT5C3A), DNA polymerase alpha catalytic subunit (POLA1), DNA polymerase delta catalytic subunit and subunit 2 (POLD1 and POLD2), DNA polymerase epsilon catalytic subunit, subunit 2, and accessory subunit (POLE, POLE2, and POLE4), DNA-directed RNA polymerase III subunits RPC6, RPC8, and RPC10 (POLR3F, POLR3H, and POLR3K), DNA primase small subunit (PRIM1), Ribonucleoside-diphosphate reductase large and small subunits (RRM1 and RRM2), thymidine kinase 1 (TK1), and thymidylate synthase (TYMS), whereas guanine deaminase (GDA) and DNA polymerase delta subunit 4 (POLD4) are seemingly the only differentially expressed genes with higher expression. This is significant as nucleotide metabolism is an important component of both cell proliferation and differentiation in intestinal epithelial cells (Sanderson and He, 1994). Altered nucleic acid metabolism, the process by which DNA and RNA are synthesized and degraded, is a concern in metabolic disturbances, genetic mutation, and cancer.
Overall, there was overwhelming data to support a dysregulated immune system in the gut of SHR, specifically various downstream effectors of the TNFR1 signaling pathway (Table 1). In the hypertensive rats, this pathway was overexpressed, and gene expression of pro-apoptotic, pro-necrotic, and pro-inflammatory mediators appears to be higher in SHR rodents, suggesting that TNFα -related inflammation and epithelial damage may contribute to hypertension in this model. Caspase expression patterns favored the SHR, with Caspases 3, 4, 8, and 9 observed with higher expression levels in SHR, further demonstrating an association between hypertension and TNFα -related inflammation in the gut. These transcriptional responses in the gut epithelium also correspond to oxidative stress and dysfunction, such as apoptosis and oxidative stress (Table 2). Studies have pointed out that oxidative stress and reactive oxygen species (ROS) production underlie the intersection between TNFα, butyrate, and hypertension. For instance, in the Sabra salt-sensitive and resistant models of hypertension, the NADPH oxidase inhibitor, apocynin, exerts a protective effect on blood pressure in salt fed-rodents, while also significantly reducing the TNFα levels (Mazor et al. 2010). Several SCFAs have been shown to affect the production of ROS in rat endothelial cells. For example, when Ang II was co-incubated with acetate and butyrate, but not propionate, ROS levels were reduced in epithelial colonic cells (Robles-Vera et al. 2020b). Furthermore, for butyrate but not acetate, this effect was shown to be GPR41/43 dependent (Robles-Vera et al. 2020b), though, as β-hydroxybutyrate is a substrate of SCL5a8 and SLC16a1, this effect could also be mediated intracellularly.
Table 1.
Subnetwork enrichment analysis for metabolic processes that differ significantly between WKY and SHR. These pathways are those that include TNF or TNF receptor signaling. Provided in the table is the name of the metabolic pathway, a description of the pathway, the number of entities measured in the pathway, the median fold change, normalized enrichment score, and the P value. Transcriptome data for gut epithelial cells were obtained from Yang et al. 2020
| Name | Pathway Description | # of Entities | Expanded # of Entities | # of Measured Entities | Median change | NormalizedScore | p-value |
|---|---|---|---|---|---|---|---|
| IFNAl/lFNR Expression Targets | Interferons Expression Biomarkers; Interferons Expression Biomarkers (Cytokines Expression Biomarkers) | 40 | 49 | 22 | 1.05 | 1.5 | 0.04 |
| IL2 Expression Targets | Interleukins Expression Biomarkers; Interleukins Expression Biomarkers (Cytokines Expression Biomarkers) | 97 | 138 | 54 | −1.04 | 1.72 | 0 |
| IL13 Expression Targets | Interleukins Expression Biomarkers; Interleukins Expression Biomarkers (Cytokines Expression Biomarkers) | 66 | 73 | 30 | −1.03 | 1.65 | 0.01 |
| IL15 Expression Targets | Interleukins Expression Biomarkers; Interleukins Expression Biomarkers (Cytokines Expression Biomarkers) | 69 | 85 | 35 | 1.02 | 1.49 | 0.05 |
| Interleukin Receptors -> Expression Targets in Lymphoid System and Blood | Lymphoid System and Blood; Lymphoid System and Blood (Receptor Signaling with Elevated Expression in Anatomy); blood; hematological System; lymphatic System | 60 | 88 | 25 | −1.04 | 1.79 | 0 |
| CD40 -> Expression Targets in Lymph Node | Lymphoid System and Blood; Lymphoid System and Blood (Receptor Signaling with Elevated Expression in Anatomy); lymphatic System | 97 | 109 | 37 | −1.12 | 1.69 | 0.01 |
| TCR->NF-kB Expression Targets | T-cell Receptors Expression Biomarkers; T-cell Receptors Expression Biomarkers (CD Molecules Expression Biomarkers) | 62 | 73 | 31 | −1.25 | 1.73 | 0 |
| TCR -> STAT Expression Targets | T-cell Receptors Expression Biomarkers; T-cell Receptors Expression Biomarkers (CD Molecules Expression Biomarkers) | 41 | 49 | 24 | −1.03 | 1.54 | 0.03 |
| CD40LG -> STAT Expression Targets | TNF Receptor Superfamily Expression Biomarkers; TNF Receptor Superfamily Expression Biomarkers (Expression Biomarkers: Receptor-specific) | 45 | 61 | 24 | −1.11 | 1.78 | 0 |
| CD40LG -> NF-kB /ELK/SRF -> CREB/NFATC Expression Targets | TNF Receptor Superfamily Expression Biomarkers; TNF Receptor Superfamily Expression Biomarkers (Expression Biomarkers: Receptor-specific) | 88 | 101 | 52 | 1.03 | 1.64 | 0.01 |
| CD72 -> AP-1 Expression Targets | TNF Receptor Superfamily Expression Biomarkers; TNF Receptor Superfamily Expression Biomarkers (Expression Biomarkers: Receptor-specific) | 64 | 77 | 39 | −1.11 | 1.67 | 0.01 |
| TNF -> NF-kB Expression Targets | TNF Receptor Superfamily Expression Biomarkers; TNF Receptor Superfamily Expression Biomarkers (Expression Biomarkers: Receptor-specific) | 127 | 132 | 61 | −1.06 | 1.54 | 0.02 |
| TNF->STAT Expression Targets | TNF Receptor Superfamily Expression Biomarkers; TNF Receptor Superfamily Expression Biomarkers (Expression Biomarkers: Receptor-specific) | 83 | 98 | 46 | 1.03 | 1.47 | 0.03 |
| TLR1 -> 2/6 Expression Targets | Toll-like Receptors Expression Biomarkers; Toll-like Receptors Expression Biomarkers (CD Molecules Expression Biomarkers) | 77 | 88 | 39 | −1.1 | 1.55 | 0.02 |
| TLR4 -> AP- 1/EGR1/HIF1A Expression Targets | Toll-like Receptors Expression Biomarkers; Toll-like Receptors Expression Biomarkers (CD Molecules Expression Biomarkers) | 84 | 116 | 47 | −1.06 | 1.48 | 0.04 |
Table 2.
Sub-networks related to mitochondria and apoptosis. Provided in the table is the gene set seed, number of entities measured in the pathway, the median fold change, the P-value, and the activation score for the network. Transcriptome data for gut epithelial cells were obtained from Yang et al. 2020
| Gene Set Seed | Total # of Neighbors | # of Measured Neighbors | Median change | p-value | Activation Score |
|---|---|---|---|---|---|
| apoptosis | 9457 | 4780 | −1.04 | 0.00063 | 7.07 |
| apoptotic cell clearance | 310 | 157 | 1.02 | 0.028 | −2.36 |
| cell stress | 1226 | 722 | −1.03 | 0.00085 | 1.34 |
| extrinsic pathway of apoptosis | 221 | 137 | 1.01 | 0.002 | −0.24 |
| glucose import | 1304 | 679 | −1.02 | 0.00033 | 0.19 |
| intestinal D-glucose absorption | 74 | 36 | 1.05 | 0.0088 | 0.38 |
| intrinsic pathway of apoptosis | 386 | 232 | −1.01 | 0.014 | 1 |
| oxidative stress | 2889 | 1579 | −1.04 | 0.0012 | 1.06 |
| response to oxidative stress | 514 | 323 | −1.02 | 0.033 | 0 |
| ROS generation | 2188 | 1170 | −1.03 | 0.00019 | 0.82 |
| Superoxide anión generation | 707 | 349 | −1.03 | 0.019 | 0.26 |
Evidence from literature implicates that a shift in how the cells utilize SCFAs and particularly butyrate may be a marker of several inflammatory GI disorders. Here, lower butyrate utilization by the SHR gut epithelium may involve key transcription factors. For example, STAT3 and NF-κB are known to be downregulated by butyrate (Chen et al. 2019); however, our analysis shows increased STAT and NF-κB pathway expression in the SHR (Table 1, Figure 2). This may indicate a shift in metabolic capacity within the gut epithelium as a compensatory response to impaired oxidative phosphorylation. Genes involved in SCFA metabolism, acyl-CoA synthetase short-chain family members 1 and 3 (ACSS1 and ACSS3), are also differentially expressed in SHR colon, underscoring alterations to SCFA utilization. Additionally, several genes involved in glycolysis are differentially expressed, with aldehyde dehydrogenase 3 family member B1 (ALDH3B1), enolase 1 (ENO1), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4), Phosphofructokinase, platelet (PFKP) being expressed at higher levels in SHR and Phosphoenolpyruvate carboxykinase 2 (PCK2), aldehyde dehydrogenase 1 family member A7 (ALDH1A7), lactate dehydrogenase H subunit (LDHB), hexokinase-1 (HK1), enolase 3 (ENO3), aldo-keto reductase family 1 member A1 (AKR1A1) being expressed at lower levels. PFKP and PFKFB4 code isozymes of Phosphofructokinase 1 and 2 respectively, which are known to be key regulatory steps in pH-sensitive glycolysis (Ui, 1966).
Fig. 2.
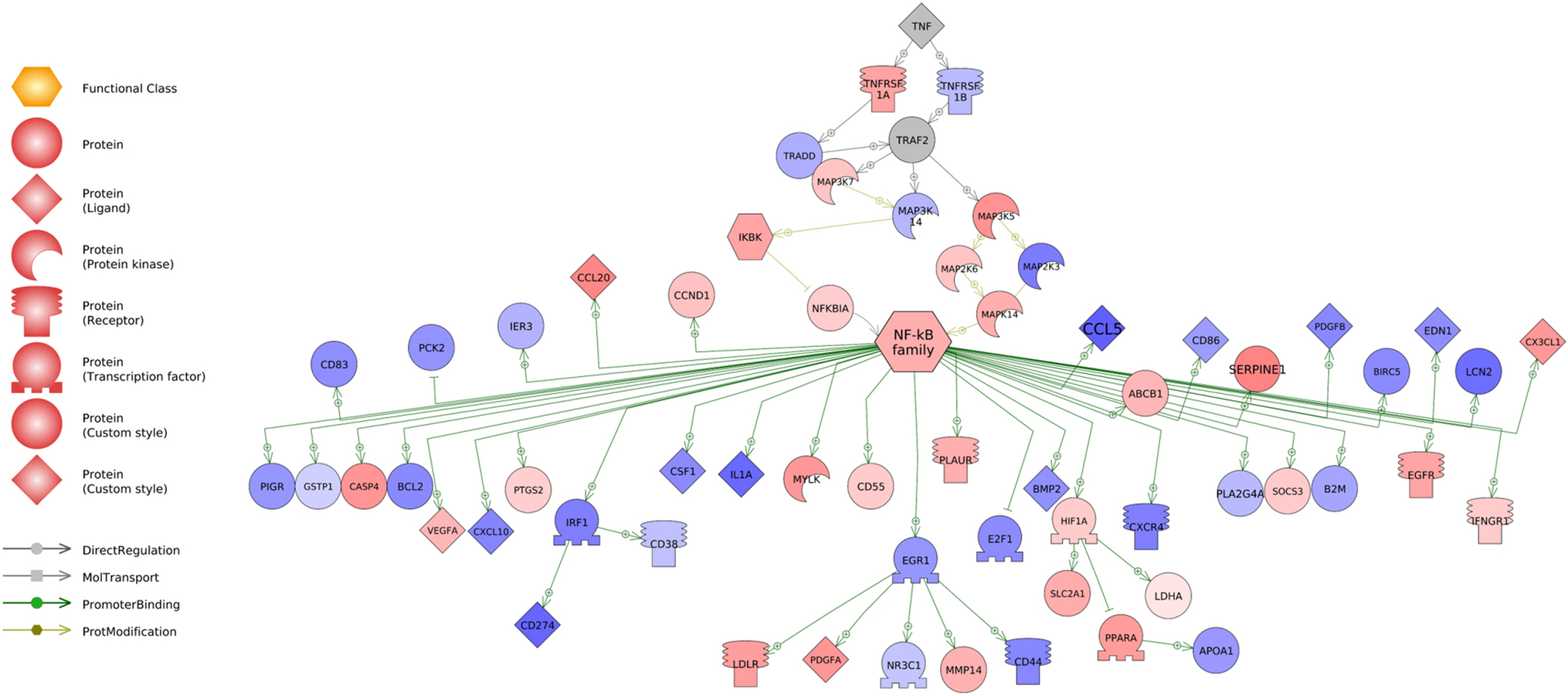
Relative expression levels of downstream targets of TNFα and NF-κB signaling molecules in the colonic epithelium of WKY and SHR rodents. Red corresponds to genes that are upregulated in SHR, while blue corresponds to genes with higher expression in WKY. Shade denotes degree the transcript is differentially expressed, with darker shades corresponding to higher level of differential expression. Grey denotes genes whose transcription levels were not assessed in this dataset. Transcriptome data for gut epithelial cells were obtained from Yang et al. 2020. Abbreviations are found is Supplemental Data 1
Lastly, our pathway analysis uncovered differentially expressed gene sets in the SHR epithelium that are associated with cardiovascular, GI and nervous system physiology and function, as well as with adrenergic, cholinergic, serotonergic and dopaminergic transmission (Table 3). Of particular importance may be those genes in the SHR epithelium that are related to neuro- and cardio-modulators such as serotonin (5HT), histamine, nesfatin-1, cholecystokinin (CCK), and glucagon-like peptides (GLP) 1 and 2 (Supplemental Table 2), with the last four also having a prominent role in metabolic functions. With regards to 5HT, the transcriptome data shows that the SHR express higher levels of the enteroendocrine cell differentiation marker, regenerating islet-derived protein 4 (REG4), aligning with previous reports that enteroendocrine cell numbers in the GI are elevated in hypertension (Niezgoda and Kasacka, 2017), during inflammation and in presence of high TNFα (Xiao et al. 2019). Enteroendocrine cells are responsible for 90% of total 5HT synthesis from tryptophan in the body, and their function can be modulated by both the host inflammatory responses as well as by microbiota and particularly butyrate (Reigstad et al. 2015). Indeed, we found that several genes involved in tryptophan metabolism were differentially expressed in the SHR epithelium, including a decrease in expression of tryptophan hydroxylase 1 (TPH1) and aromatic l-amino acid decarboxylase (DDC), enzymes responsible for 5HT synthesis. This may be significant for hypertension, as 5HT has been implicated regulation of blood pressure and in hypertension (Watts et al. 2012) as well as having an effect on the immune system, partially via its ability to block the inflammatory effects of TNFα through 5HT2A receptors (Pelletier and Siegel, 2009). Indeed, analysis of tryptophan metabolites in circulation of hypertensive patients suggests that these pathways may be important in hypertension (Qi et al. 2017). 5HT can also stimulate its 5HT3a receptors present on the vagal afferents (Li et al. 2000; Browning, 2015), and acute stimulation of 5HT3a receptors by selective agonists reportedly leads to decreased blood pressure (Ferreira et al. 2004; Urzedo-Rodrigues et al. 2011).
Table 3.
Sub-networks related to the nervous system, cardiovascular function, and the gastrointestinal system. Provided in the table is the gene set seed, number of entities measured in the pathway, the median fold change, the P-value, and the activation score for the network. Transcriptome data for gut epithelial cells were obtained from Yang et al. 2020
| Gene Set Seed | Total # of Neighbors | # of Measured Neighbors | Median change | p-value | Activation Score |
|---|---|---|---|---|---|
| adrenergic transmission | 58 | 13 | −1.05 | 0.0045 | −1.00 |
| cardiovascular deconditioning | 200 | 66 | 1.05 | 0.01 | −0.45 |
| cardiovascular function | 325 | 123 | 1.04 | 0.00038 | −0.45 |
| cardiovascular physiology | 61 | 22 | 1.04 | 0.036 | 1.00 |
| central nervous System function | 276 | 104 | 1.01 | 0.015 | 0.00 |
| cholinergic synaptic transmission | 125 | 40 | −1.01 | 0.00066 | 1.13 |
| dopaminergic activity | 99 | 29 | −1.02 | 0.036 | 0.58 |
| dopaminergic transmission | 140 | 51 | 1.04 | 0.0059 | −1.34 |
| enteric nervous System | 188 | 82 | −1.07 | 0.0001 | −1.00 |
| gastrointestinal function | 120 | 39 | −1.01 | 0.0078 | −1.00 |
| long-term synaptic depression | 439 | 206 | 1.02 | 0.02 | −1.28 |
| nerve regeneration | 498 | 216 | −1.03 | 2.30E-07 | 0.17 |
| nervous System physiology | 529 | 218 | −1.07 | 5.90E-09 | −1.00 |
| neuromodulation | 105 | 31 | −1.09 | 0.023 | unknown |
| neuron development | 370 | 143 | −1.03 | 0.00034 | −1.26 |
| neuron differentiation | 316 | 122 | −1.04 | 0.0043 | 0.00 |
| neuron sensitivity | 90 | 30 | 1.05 | 0.015 | 0.45 |
| neuronal activity | 699 | 275 | 1.02 | 1.40E-06 | −1.40 |
| neuronal death | 1535 | 828 | −1.03 | 0.0056 | 0.00 |
| renin-angiotensin System | 257 | 104 | 1.05 | 0.017 | −1.51 |
| synaptic plasticity | 1226 | 603 | 1.02 | 0.0006 | 0.17 |
In addition, both nesfatin-1 and histamine can regulate blood pressure via systemic and central action (Osaki and Shimizu, 2014; de Almeida et al. 2015; Yamanaka et al. 2017; Güneş et al. 2020). Nucleobindin-2 (NUCB2), the gene coding for nesfatin-1 has been shown to be primarily produced in the gastric mucosa and may be responsible for the secretion of incretins, such as GLP-1 and 2 and glucose dependent insulinotropic polypeptide (GIP), which further control meal-responsive insulin secretion (Ramesh et al. 2015). While the GIP gene does not appear in this dataset, pre-proglucagon (GCG),, which in L cells can be proteolytically cleaved to produce GLP-1 and 2, also appears to be downregulated in SHR gut epithelium, underscoring decreased insulin signaling in this model (Suzuki et al. 2018).
TNFα is also known to downregulate Gcg expression via G-protein coupled receptor 120 (Worthington et al. 2018). CCK is additionally expressed at lower levels in SHR, and apart from its known metabolic effects on protein and lipid metabolism, it may play a role in inflammation as it has been shown to promote a T helper 2 and regulatory T cell phenotype in vitro (Worthington et al. 2018). CCK and GLP-1 can also modulate the gut-brain neural axis involved in metabolic, digestive and behavioral functions by stimulating specific receptors on the vagal afferents in the gut (Krieger et al. 2016; Davis et al. 2020). Thus, a decrease in production of several gut peptides by the SHR epithelium may be important in pathophysiology of hypertension. Others have shown a potent anti-hypertensive effect of central injections of GLP-1 receptor agonist, which was reportedly mediated via activation of specific catecholaminergic neurons present in the nucleus of the solitary tract (NTS) (Katsurada et al. 2019). The NTS is the primary site of projection of gut vagal afferents and a major cardio-regulatory brain region, and the catecholaminergic neurons in the NTS can both suppress feeding via vagal activation in the gut (Chen et al. 2020) as well as restrain the development of hypertension (Duale et al. 2007). Moreover, while an antihypertensive effect of CCK has previously been reported in the SHR (Koyama et al. 2020), and reduced CCK levels were found in several SHR brain regions including the pituitary (Shulkes et al. 1989), the potential mechanistic link between hypertension, diabetes and obesity that lies within the gut needs to be further explored.
Indeed, diets high in sugars and fats (Areas et al. 1990; Reaven et al. 1990; Cao et al. 2011) can change the gut microbiota and exacerbate the SHR hypertension via several mechanisms of action. Conversely, inflammation can shift the colonic epithelial metabolism which in turn can reportedly modulate the composition of the gut bacteria towards dysbiosis (Litvak et al. 2018). Knowing what we know now regarding the role of microbiome in hypertension and the intricacies of the host-microbiota interactions, studies employing sugar and fat modifications in investigation of hypertension should be revisited. For example, our transcriptome pathway analysis identified differences in expression levels of genetic components of Omega-6-Fatty Acid Metabolism between WKY and SHR (Table 1), and this pathway could be explored as a therapeutic dietary intervention. To date, the role of Omega-6 fatty acids in hypertension is inconclusive. Several studies note that Omega-6 fatty acids do not appear to exert direct effects on blood pressure, but may lead to dyslipidemia and inflammation (Khandelwal et al. 2013). Thus, metabolic shifts in the gut epithelium that may arise due to inflammation and exacerbated by gut dysbiosis may impact production and release of several gut-associated neuropeptides that could contribute to the gut-brain axis dysfunction in hypertension.
9. Conclusions
A significant relationship exists between gut dysbiosis, GI inflammatory processes and hypertension. However, specific molecular mechanisms of these interactions in health and disease remain unknown. There are clearly defined changes in microbial metabolites throughout development of different inflammatory conditions, and a decrease in beneficial microbial metabolites such as SCFAs is a hallmark of hypertension. Here, we present the case for a potential TNFα-SCFA interaction in the hypertensive gut characterized by shifts in the gut epithelial cell metabolism, which may affect the production of metabolically active and neuroactive gut peptides within the gut-brain axis (Figure 3). We uncover specific transcriptome differences underlying hypertensive rodent models, which includes genes also involved in gastric inflammation and cancer (Supplemental Figure 1), and these proteins are proposed to contribute to hypertensive conditions within the gut. The comprehensive review of the literature and extensive pathway analysis reveals novel mechanisms and potential therapeutic targets in gut dysbiosis-associated hypertension.
Fig. 3.
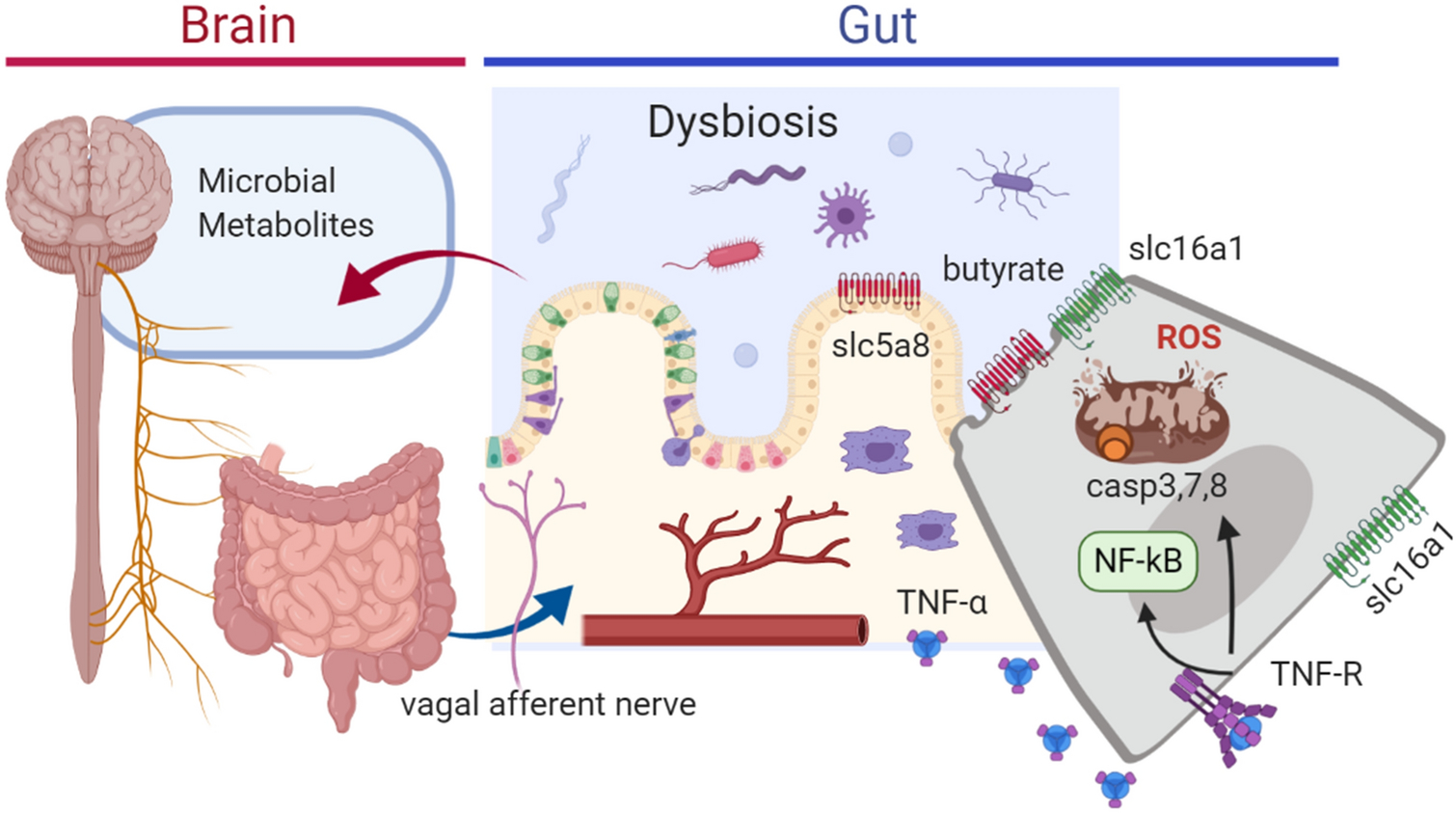
Conceptual model for the role of TNF-α in gastrointestinal dysbiosis and impacts on the gut-brain axis. Inflammation in the gut results in mitochondrial dysfunction and altered oxidative respiration, leading to oxidative stress and caspase-induced apoptosis. This in turn can impair butyrate uptake from the gastrointestinal tract, altering the expression of solute transporters. Gut dysbiosis can result in altered short chain fatty acids (e.g. butyrate, propionate) and neurotransmitter synthesis (serotonin, ghrelin, cholecystokinin and somatostatin) in gut epithelium. These mechanisms are proproposed to contribute to a hypertensive state
Supplementary Material
Acknowledgements
This work was supported by 1R56HL136692-01 to JZ and CJM, R21AT010192 Award to JZ and CJM, and University of Florida College of Veterinary Medicine (UFCVM) Start Up Funds to JZ and CJM. Funding also through University of Florida Graduate Student Fellowship (CLS).
Abbreviations
- 1C
1 Carbon
- 2C
2 Carbon
- 3C
3 Carbon
- 4C
4 Carbon
- 5C
5 Carbon
- PFKFB4
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4
- ACSS1
Acyl-CoA synthetase short-chain family member 1
- ACSS3
Acyl-CoA synthetase short-chain family member 3
- ALDH1A7
Aldehyde dehydrogenase 1 family member A7
- ALDH3B1
Aldehyde dehydrogenase 3 family member B1
- AKR1A1
Aldo-keto reductase family 1 member A1
- AMT
Aminomethyltransferase
- AMPK
AMP-activated protein kinase
- DDC
Aromatic l-amino acid decarboxylase
- BCAT
Branched-chain amino acid aminotransferase
- CAD
Carbamoyl-phosphate synthetase 2
- CVD
Cardiovascular disease
- CNS
Central nervous system
- CCK
Cholecystokinin
- C3
Complement component 3
- P27KIP1
Cyclin-dependent kinase inhibitor 1B
- CMPK1
Cytidine/Uridine Monophosphate Kinase 1
- NT5C3A
Cytosolic 5’-nucleotidase 3
- DTYMK
Deoxythymidylate Kinase
- DHFR
Dihydrofolate reductase
- DHODH
Dihydroorotate dehydrogenase
- POLA1
DNA polymerase alpha catalytic subunit
- POLD1
DNA polymerase delta catalytic subunit
- POLD2
DNA polymerase delta subunit 2
- POLD4
DNA polymerase delta subunit 4
- POLE
DNA polymerase epsilon catalytic subunit
- POLE2
DNA polymerase epsilon subunit 2
- PRIM1
DNA primase small subunit
- POLR3K
DNA-directed RNA polymerase III subunit RPC10
- POLR3F
DNA-directed RNA polymerase III subunit RPC6
- POLR3H
DNA-directed RNA polymerase III subunit RPC8
- DUT
DUTP pyrophosphatase
- ENTPD1
Ectonucleoside triphosphate diphosphohydrolase 1
- ENTPD4
Ectonucleoside triphosphate diphosphohydrolase 4
- ENO1
Enolase 1
- ENO3
Enolase 3
- GI
Gastrointestinal
- GLP
Glucagon-like peptide
- GIP
Glucose dependent insulinotropic polypeptide
- GDA
Guanine deaminase
- HK1
Hexokinase-1
- HAT
Histone acetylase
- HDAC
Histone deacetylase
- HIF1
Hypoxia-inducible factor 1
- IFNγ
Interferon gamma
- IL
Interleukin
- IEC
Intestinal epithelial cell
- LDHB
Lactate dehydrogenase H subunit
- Ms4a4b
Membrane-spanning 4-domains, subfamily A, member 4B
- MTHFD1
Methylenetetrahydrofolate dehydrogenase 1
- MTHFD1L
Methylenetetrahydrofolate dehydrogenase 1-like
- MTHFR
Methylenetetrahydrofolate reductase
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NUCB2
Nucleobindin-2
- NME1
Nucleoside diphosphate kinase A
- NME2
Nucleoside diphosphate kinase B
- NTS
Nucleus of the solitary tract
- PPARs
Peroxisome proliferator-activated receptors
- PCK2
Phosphoenolpyruvate carboxykinase 2
- PFKP
Phosphofructokinase, platelet
- POLE4
Polymerase (DNA-directed), epsilon 4, accessory subunit
- GCG
Pre-proglucagon
- ROS
Reactive oxygen species
- REG4
Regenerating islet-derived protein 4
- RAAS
Renin-angiotensin-aldosterone system
- RRM1
Ribonucleoside-diphosphate reductase large subunit
- RRM2
Ribonucleotide reductase small subunit
- SHMT1
Serine hydroxymethyltransferase 1
- SHMT2
Serine Hydroxymethyltransferase 2
- 5HT
Serotonin
- SCFA
Short chain fatty acid
- SLC16A1
Solute Carrier Family 16 Member 1
- SLC19A1
Solute Carrier Family 19 Member 1
- SLC5A8
Solute Carrier Family 5 Member 8
- SP1
specificity protein 1
- SHR
Spontaneously hypertensive rats
- TK1
Thymidine kinase 1
- TYMS
Thymidylate synthase
- TCA
Tricarboxylic acid
- TPH1
Tryptophan hydroxylase 1
- TNFR1
Tumor necrosis factor receptor 1
- TNFα
Tumor necrosis factor α
- WKY
Wistar-Kyoto
Footnotes
Declarations
The authors have no conflict of interest to disclose. We declare that this work is original and has not been considered for publication elsewhere. The computational analysis on the published dataset by Yang et al. is a new contribution and has not been published previously. CLS, JZ, and CJM all contributed to writing and editing of the article.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Aggarwal BB (2003). Signalling pathways of the TNF superfamily: A double-edged sword. Nature Reviews Immunology, Vol. 3, pp. 745–756. 10.1038/nri1184 [DOI] [PubMed] [Google Scholar]
- Ahmari N, Schmidt JT, Krane GA, Malphurs W, Cunningham BE, Owen JL, Martyniuk CM, and Zubcevic J (2016). Loss of bone marrow adrenergic beta 1 and 2 receptors modifies transcriptional networks, reduces circulating inflammatory factors, and regulates blood pressure. Physiological Genomics, 48(7), 526–536. 10.1152/physiolgenomics.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A, Fujiyama Y, Hata K, Araki Y, Takaya H, Shimada M, and Bamba T (1999). Counter-regulatory effect of sodium butyrate on tumour necrosis factor- alpha (TNF-α)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. Clinical and Experimental Immunology, 118(1), 23–29. 10.1046/j.1365-2249.1999.01038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areas MEZJ, Knapka J, Gleim G, Dipette D, Holland B, and Preuss HG (1990). Influence of oat bran of sucrose-induced blood pressure elevations in SHR. Life sciences, 47(13), 1121–1128. 10.1016/0024-3205(90)90171-M [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, and Subbarao P (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science translational medicine, 7(307), 307ra152–307ra152. 10.1126/scitranslmed.aab2271 [DOI] [PubMed] [Google Scholar]
- Barbaro NR, and Harrison DG (2019). Markers or makers: Inflammatory cytokines in treatment-resistant hypertension. Hypertension, Vol. 73, pp. 767–769. 10.1161/HYPERTENSIONAHA.119.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, and Haase N (2019). Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation, 139(11), 1407–1421. 10.1161/CIRCULATIONAHA.118.036652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, and Dejong CH (2009). Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clinical Nutrition, 28(6), 657–661. 10.1016/j.clnu.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Brosnan ME, and Brosnan JT (2016). Formate: The Neglected Member of One-Carbon Metabolism. A Annual Review of Nutrition, 36(1), 369–388. 10.1146/annurev-nutr-071715-050738 [DOI] [PubMed] [Google Scholar]
- Brown JM, and Hazen SL (2018). Microbial modulation of cardiovascular disease. Nature Reviews Microbiology, Vol. 16, pp. 171–181. 10.1038/nrmicro.2017.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN (2015). Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Frontiers in neuroscience, 9, 413. 10.3389/fnins.2015.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A, Anbazhagan AN, Kumar A, Raheja G, Singh V, Ramaswamy K, and Dudeja PK (2010). The probiotic Lactobacillus plantarum counteracts TNF-α-induced downregulation of SMCT1 expression and function. American Journal of Physiology-Gastrointestinal and Liver Physiology, 299(4), G928–G934. 10.1152/ajpgi.00279.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets D, Mas-de-Xaxars T, López-Siles M, Martínez-Medina M, Bahí A, Sàbat M, Louvriex R, Miquel-Cusachs JO, Garcia-Gil JL, Aldeguer X (2015). Anti-tumour Necrosis Factor Treatment with Adalimumab Induces Changes in the Microbiota of Crohn’s Disease. Journal of Crohn’s and Colitis, 9(10), 899–906. 10.1093/ecco-jcc/jjv119 [DOI] [PubMed] [Google Scholar]
- Calderón-Pérez L, Gosalbes MJ, Yuste S, Valls RM, Pedret A, Llauradó E, Jimenez-Hernandez N, Artacho A, Pla-Pagà L, Companys J, and Ludwig I (2020). Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Scientific Reports, 10(1), 1–16. 10.1038/s41598-020-63475-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Souders CL, Perez-Rodriguez V, and Martyniuk CJ (2019). Elucidating conserved transcriptional networks underlying pesticide exposure and parkinson’s disease: A focus on chemicals of epidemiological relevance. Frontiers in Genetics, Vol. 10. 10.3389/fgene.2018.00701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Sodhi K, Puri N, Monu SR, Rezzani R, and Abraham NG (2011). High fat diet enhances cardiac abnormalities in SHR rats: Protective role of heme oxygenase-adiponectin axis. Diabetology & metabolic syndrome, 3(1), 37. 10.1186/1758-5996-3-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Preston T, Frost G, and Morrison DJ (2018). Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Current Nutrition Reports, Vol. 7, pp. 198–206. 10.1007/s13668-018-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Chen Y, Zhou Q, Wang C, Chen L, Di W, and Zhang Y (2020). Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clinical Science, 134(2), 289–302. 10.1042/CS20191253 [DOI] [PubMed] [Google Scholar]
- Cheema MU and Pluznick JL (2019). Gut Microbiota Plays a Central Role to Modulate the Plasma and Fecal Metabolomes in Response to Angiotensin II. Hypertension, 74(1), 184–193. 10.1161/HYPERTENSIONAHA.119.13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cheng M, Wang L, Zhang L, Xu D, Cao P, Wang F, Herzog H, Song S and Zhan C (2020). A Vagal-NTS Neural Pathway that Stimulates Feeding. Current Biology. 10.1016/j.cub.2020.07.084 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao KN, and Vitetta L (2019). Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients, Vol. 11. 10.3390/nu11051026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EA, Wald HS, Suarez AN, Zubcevic J, Liu CM, Cortella AM, Kamitakahara AK, Polson JW, Arnold M, Grill HJ, and de Lartigue G (2020). Ghrelin Signaling Affects Feeding Behavior, Metabolism, and Memory through the Vagus Nerve. Current Biology. 10.1016/j.cub.2020.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida DO, Ferreira HS, Pereira LB, and Fregoneze JB (2015). Hypertensive response to stress: the role of histaminergic H1 and H2 receptors in the medial amygdala. Physiology and Behavior, 144, 95–102. 10.1016/j.physbeh.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Shestov AA, Koski DM, Uǧurbil K, and Henry PG (2009). Acetate transport and utilization in the rat brain. Journal of Neurochemistry, 109(SUPPL. 1), 46–54. 10.1111/j.1471-4159.2009.05895.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice A, Wilson W, and Ambre J (1976). Vasoactive effects of methanol and sodium formate on isolated canine basilar artery. Toxicology and Applied Pharmacology, 36(3), 595–601. 10.1016/0041-008X(76)90238-6 [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, and Bultman SJ (2011). The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabolism, 13(5), 517–526. 10.1016/j.cmet.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, and Paton JF (2007). Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovascular research, 76(1), 184–193. 10.1016/j.cardiores.2007.06.018 [DOI] [PubMed] [Google Scholar]
- Ferreira HS, e Silva EDC, Cointeiro C, Oliveira E, Faustino TN, and Fregoneze JB (2004). Role of central 5-HT3 receptors in the control of blood pressure in stressed and non-stressed rats. Brain research, 1028(1), 48–58. 10.1016/j.brainres.2004.08.063 [DOI] [PubMed] [Google Scholar]
- Furuoka M, Ozaki KI, Sadatomi D, Mamiya S, Yonezawa T, Tanimura S, and Takeda K (2016). TNF- α Induces Caspase-1 Activation Independently of Simultaneously Induced NLRP3 in 3T3-L1 Cells. Journal of Cellular Physiology, 231(12), 2761–2767. 10.1002/jcp.25385 [DOI] [PubMed] [Google Scholar]
- Gálfi P and Neogrády S (2001). The pH-dependent inhibitory action of n-butyrate on gastrointestinal epithelial cell division. Food Research International, 34(7), 581–586. 10.1016/S0963-9969(01)00075-8 [DOI] [Google Scholar]
- Gamble JR, Harlan JM, Klebanoff SJ, and Vadas MA (1985). Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proceedings of the National Academy of Sciences of the United States of America, 82(24), 8667–8671. 10.1073/pnas.82.24.8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM, and Durgan DJ (2018). Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension, 72(5), 1141–1150. 10.1161/HYPERTENSIONAHA.118.11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia R, McCubbin T, Navone L, Stowers C, Nielsen L, and Marcellin E (2017). Microbial Propionic Acid Production. Fermentation, 3(2), 21. 10.3390/fermentation3020021 [DOI] [Google Scholar]
- Granado-Serrano AB, Martín-Garí M, Sánchez V, Solans MR, Berdun R, Ludwig IA, Rubio L, Vilaprinyo E, Portero-Otín M, and Serrano JCE (2019). Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Scientific Reports, 9(1), 1–13. 10.1038/s41598-019-38874-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruys E, Toussaint MJM, Niewold TA, and Koopmans SJ (2005). Acute phase reaction and acute phase proteins. Journal of Zhejiang University: Science, 6 B(11), 1045–1056. 10.1631/jzus.2005.B1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güneş H, Baylan FA, Güneş H, and Temiz F (2020). Can Nesfatin-1 Predict Hypertension in Obese Children?. Journal of clinical research in pediatric endocrinology, 12(1), 29–36. 10.4274/jcrpe.galenos.2019.2019.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007). Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. Journal of Experimental Medicine, 204(10), 2449–2460. 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halarnkar PP and Blomquist GJ (1989). Comparative aspects of propionate metabolism. Comparative biochemistry and physiology. B, Comparative biochemistry, 92(2), 227. 10.1016/0305-0491(89)90270-8 [DOI] [PubMed] [Google Scholar]
- Hibberd AA, Lyra A, Ouwehand AC, Rolny P, Lindegren H, Cedgård L, and Wettergren Y (2017). Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterology, 4(1). 10.1136/bmjgast-2017-000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosios AM and Vander Heiden MG (2014). Acetate metabolism in cancer cells. Cancer and metabolism, 2(1), 27. 10.1186/s40170-014-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G, Krzesinski JM, Melin P, De Tullio P, and Jouret F (2019). Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension, 74(4), 1005–1013. 10.1161/HYPERTENSIONAHA.118.12588 [DOI] [PubMed] [Google Scholar]
- Hughes ER, Winter MG, Duerkop BA, Spiga L, de Carvalho TF, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, and Cherry S (2017). Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host and Microbe, 21(2), 208–219. 10.1016/j.chom.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoon A, Mathew AV, Byun J, Gadegbeku CA, Gipson DS, Afshinnia F, and Pennathur S (2018). Gut Microbial Product Predicts Cardiovascular Risk in Chronic Kidney Disease Patients. Translational Research Am J Nephrol, 48, 269–277. 10.1159/000493862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsdottir G, Bjerregaard JH, Skovbjerg H, and Nyman M (2013). Fasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: No difference compared with controls, but between genders. Scandinavian Journal of Gastroenterology, 48(6), 696–701. 10.3109/00365521.2013.786128 [DOI] [PubMed] [Google Scholar]
- Jamerson BD, Payne ME, Garrett ME, Ashley-Koch AE, Speer MC, and Steffens DC (2013). Folate metabolism genes, dietary folate and response to antidepressant medications in late-life depression. International Journal of Geriatric Psychiatry, 28(9), 925–932. 10.1002/gps.3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose PA and Raj D (2015). Gut microbiota in hypertension. Current Opinion in Nephrology and Hypertension, Vol. 24, pp. 403–409. 10.1097/MNH.0000000000000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, and Brandt M (2016). Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. Journal of the American Heart Association, 5(9). 10.1161/JAHA.116.003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsurada K, Nakata M, Saito T, Zhang B, Maejima Y, Nandi SS, Sharma NM, Patel KP, Kario K, and Yada T (2019). Central Glucagon-like Peptide-1 Receptor Signaling via Brainstem Catecholamine Neurons Counteracts Hypertension in Spontaneously Hypertensive Rats. Scientific Reports, 9(1), 1–13. 10.1038/s41598-019-49364-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney CJ, Cullen SP, Tynan GA, Henry CM, Clancy D, Lavelle EC, and Martin SJ (2015). Necroptosis suppresses inflammation via termination of TNF-or LPS-induced cytokine and chemokine production. Cell Death and Differentiation, 22(8), 1313–1327. 10.1038/cdd.2014.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal S, Kelly L, Malik R, Prabhakaran D, and Reddy S (2013). Impact of omega-6 fatty acids on cardiovascular outcomes: A review. Journal of preventive cardiology, 2(3), 325. [PMC free article] [PubMed] [Google Scholar]
- Kim S, Rodriguez V, Santisteban M, Yang T, Qi Y, Raizada M, and Pepine C (2015). Hypertensive Patients Exhibit Gut Microbial Dysbiosis and an Increase in TH17 Cells. Journal of Hypertension, 33, e77–e78. 10.1097/01.hjh.0000467562.03337.a5 [DOI] [Google Scholar]
- Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, and Raizada MK (2018). Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clinical Science, 132(6), 701–718. 10.1042/CS20180087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama D, Sasai M, Matsumura S, Inoue K, and Ohinata K (2020). A milk-derived pentapeptide reduces blood pressure in advanced hypertension in a CCK system-dependent manner. Food & Function, 11(11), 9489–9494. 10.1039/D0FO01122C [DOI] [PubMed] [Google Scholar]
- Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, and Lee SJ (2016). Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes, 65(1), 34–43. 10.2337/db15-0973 [DOI] [PubMed] [Google Scholar]
- Li Y, Hao Y, Zhu J, and Owyang C (2000). Serotonin released from intestinal enterochromaffin cells mediates luminal non–cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology, 118(6), 1197–1207. 10.1016/S0016-5085(00)70373-8 [DOI] [PubMed] [Google Scholar]
- Li Y, Dong J, Xiao H, Zhang S, Wang B, Cui M, and Fan S (2020). Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes, 11(4), 789–806. 10.1080/19490976.2019.1709387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi PV, Ververis K, and Karagiannis TC (2011). Histone Deacetylase Inhibition and Dietary Short-Chain Fatty Acids. ISRN Allergy, 2011, 1–8. 10.5402/2011/869647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebisch G, Ecker J, Roth S, Schweizer S, Öttl V, Schött HF, Yoon H, Haller D, Holler E, Burkhardt R, and Matysik S (2019). Quantification of Fecal Short Chain Fatty Acids by Liquid Chromatography Tandem Mass Spectrometry— Investigation of Pre-Analytic Stability. Biomolecules, 9(4), 121. 10.3390/biom9040121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y, Byndloss MX, and Bäumler AJ (2018). Colonocyte metabolism shapes the gut microbiota. Science, 362(6418). 10.1126/science.aat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZY, Zheng M, Li YM, Fan XY, Wang JC, Li ZC, Yang HJ, Yu JM, Cui J, Jiang JL, and Tang J (2019). RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics, 9(12), 3659–3673. 10.7150/thno.32126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, and Ferrante M (2014). A decrease of the butyrate-producing species roseburia hominis and faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. 10.1136/gutjnl-2013-304833 [DOI] [PubMed]
- Magupalli VG, Negro R, Tian Y, Hauenstein AV, Di Caprio G, Skillern W, Deng Q, Orning P, Alam HB, Maliga Z, and Sharif H (2020). HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science, 369(6510), eaas8995. 10.1126/science.aas8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini RN, Elliott MJ, Brennan FM, and Feldmann M (1995). Beneficial effects of tumour necrosis factor-alpha (TNF-α) blockade in rheumatoid arthritis (RA). Clinical and Experimental Immunology, Vol. 101, pp. 207–212. 10.1111/j.1365-2249.1995.tb08340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique C, Lastra G, Gardner M, and Sowers JR (2009). The Renin Angiotensin Aldosterone System in Hypertension: Roles of Insulin Resistance and Oxidative Stress. Medical Clinics of North America, Vol. 93, pp. 569–582. 10.1016/j.mcna.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor R, Itzhaki O, Sela S, Yagil Y, Cohen-Mazor M, Yagil C, and Kristal B (2010). Tumor necrosis factor-α: A possible priming agent for the polymorphonuclear leukocyte-reduced nicotinamide-adenine dinucleotide phosphate oxidase in hypertension. Hypertension, 55(2), 353–362. 10.1161/HYPERTENSIONAHA.109.144154 [DOI] [PubMed] [Google Scholar]
- Mehaffey E and Majid DSA (2017). Tumor necrosis factor-α, kidney function, and hypertension. American Journal of Physiology - Renal Physiology, Vol. 313, pp. F1005–F1008. 10.1152/ajprenal.00535.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, and Joe B (2015). Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiological genomics, 47(6), 187–197. 10.1152/physiolgenomics.00136.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Gonzalez J, Mora-Fernandez C, Gomez-Chinchon M, Muros M, Herrera H and Garcia J (2010). Serum and gene expression profile of tumor necrosis factor-α and interleukin-6 in hypertensive diabetic patients: effect of amlodipine administration. International Journal of Immunopathology and Pharmacology, 23(1), pp.51–59. 10.1177/039463201002300105 [DOI] [PubMed] [Google Scholar]
- Morrison DJ and Preston T (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. 10.1080/19490976.2015.1134082 [DOI] [PMC free article] [PubMed]
- Nambiar L, LeWinter MM, VanBuren PC, and Dauerman HL (2020). Decade-Long Temporal Trends in U.S. Hypertension-Related Cardiovascular Mortality. Journal of the American College of Cardiology, Vol. 75, pp. 2644–2646. 10.1016/j.jacc.2020.03.009 [DOI] [PubMed] [Google Scholar]
- Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, and Pluznick JL (2016). Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiological Genomics, 48(11), 826–834. 10.1152/physiolgenomics.00089.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niezgoda M, and Kasacka I (2017). Gastrointestinal neuroendocrine cells in various types of hypertension-a review. GIT Neuroendocrine Cells in Hypertension, 7(2), 2017–2117. 10.5604/01.3001.0010.7860 [DOI] [Google Scholar]
- Nooteboom M, Johnson R, Taylor RW, Wright NA, Lightowlers RN, Kirkwood TB, Mathers JC, Turnbull DM, and Greaves LC (2010). Age-associated mitochondrial DNA mutations lead to small but significant changes in cell proliferation and apoptosis in human colonic crypts. Aging Cell, 9(1), 96–99. 10.1111/j.1474-9726.2009.00531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KM, Lucking EF, Bastiaanssen TF, Peterson VL, Crispie F, Cotter PD, Clarke G, Cryan JF, and O’Halloran KD (2020). Prebiotic administration modulates gut microbiota and faecal short-chain fatty acid concentrations but does not prevent chronic intermittent hypoxia-induced apnoea and hypertension in adult rats. EBioMedicine, 59, 102968. 10.1016/j.ebiom.2020.102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant K and Allen-Vercoe E (2019). Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome, 7(1). 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, McKernan DP, Bravo JA, Dinan TG, and Cryan JF (2013). Differential visceral nociceptive, behavioural and neurochemical responses to an immune challenge in the stress-sensitive Wistar Kyoto rat strain. Behavioural brain research, 253, 310–317. 10.1016/j.bbr.2013.07.023 [DOI] [PubMed] [Google Scholar]
- Onyszkiewicz M, Gawrys-Kopczynska M, Sałagaj M, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M (2020). Valeric acid lowers arterial blood pressure in rats. European Journal of Pharmacology, 877, 173086. 10.1016/j.ejphar.2020.173086 [DOI] [PubMed] [Google Scholar]
- Osaki A and Shimizu H (2014). Peripheral administration of nesfatin-1 increases blood pressure in mice. Hypertens Res 37, 185–186. 10.1038/hr.2013.122 [DOI] [PubMed] [Google Scholar]
- Ostchega Y, Fryar CD, Nwankwo T, and Nguyen DT (2020). Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief, (364), 1–8. [PubMed] [Google Scholar]
- Pardo M, Betz AJ, San Miguel N, López-Cruz L, Salamone JD, and Correa M (2013). Acetate as an active metabolite of ethanol: studies of locomotion, loss of righting reflex, and anxiety in rodents. Frontiers in Behavioral Neuroscience, 7(JUN), 81. 10.3389/fnbeh.2013.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M and Siegel RM (2009). Wishing away inflammation? New links between serotonin and TNF signaling. Molecular Interventions, Vol. 9, pp. 299–301. 10.1124/mi.9.6.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzke M, Meiser J, and Vazquez A (2020). Formate metabolism in health and disease. Molecular Metabolism, Vol. 33, pp. 23–37. 10.1016/j.molmet.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiogalle E, Fontana M, Giusti AM, Pinto A, Iannucci G, Lenzi A, and Donini LM (2019). Amino acids and hypertension in adults. Nutrients, 11(7). 10.3390/nu11071459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll BG, Cheema MU, and Pluznick JL (2020). Gut microbial metabolites and blood pressure regulation: Focus on SCFAs and TMAO. Physiology, 35(4), 275–284. 10.1152/physiol.00004.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravenec M, Kožich V, Krijt J, Sokolová J, Zídek V, Landa V, Šimáková M, Mlejnek P, Šilhavý J, Oliyarnyk O, Kazdová L (2013). Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. American Journal of Hypertension, 26(1), 135–140. 10.1093/ajh/hps015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Kim S, Richards EM, Raizada MK, and Pepine CJ (2017). Gut Microbiota: Potential for a Unifying Hypothesis for Prevention and Treatment of Hypertension. Circulation Research, Vol. 120, pp. 1724–1726. 10.1161/CIRCRESAHA.117.310734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh N, Mortazavi S, and Unniappan S (2015). Nesfatin-1 stimulates glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide secretion from STC-1 cells in vitro. Biochemical and Biophysical Research Communications, 462(2), 124–130. 10.1016/j.bbrc.2015.04.100 [DOI] [PubMed] [Google Scholar]
- Rath E, Moschetta A, and Haller D (2018). Mitochondrial function — gatekeeper of intestinal epithelial cell homeostasis. Nature Reviews Gastroenterology and Hepatology, Vol. 15, pp. 497–516. 10.1038/s41575-018-0021-x [DOI] [PubMed] [Google Scholar]
- Reaven GM, Ho H, and Hoffman BB (1990) Effects of a fructose-enriched diet on plasma insulin and triglyceride concentration in SHR and WKY rats. Hormone and metabolic research, 22(07), 363–365. 10.1055/s-2007-1004922 [DOI] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, and Kashyap PC (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 29(4), 1395–1403. 10.1096/fj.14-259598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EM, Pepine CJ, Raizada MK, and Kim S (2017). The Gut, Its Microbiome, and Hypertension. Current Hypertension Reports, Vol. 19. 10.1007/s11906-017-0734-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, Gallini CA, Beal C, van Hylckama-Vlieg JE, Ballal SA, Morgan XC (2014). Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME Journal, 8(7), 1403–1417. 10.1038/ismej.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Vera I, Toral M, de la Visitación N, Sánchez M, Gómez-Guzmán M, Romero M, Yang T, Izquierdo-Garcia JL, Jiménez R, Ruiz-Cabello J, Guerra-Hernández E (2020a). Probiotics Prevent Dysbiosis and the Rise in Blood Pressure in Genetic Hypertension: Role of Short-Chain Fatty Acids. Molecular Nutrition and Food Research, 64(6), 1900616. 10.1002/mnfr.201900616 [DOI] [PubMed] [Google Scholar]
- Robles-Vera I, Toral M, de la Visitación N, Aguilera-Sánchez N, Redondo JM, Duarte J (2020b). Protective Effects of Short-Chain Fatty Acids on Endothelial Dysfunction Induced by Angiotensin II. Frontiers in Physiology, 11, 277. 10.3389/fphys.2020.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Vera I, Toral M, de la Visitación N, Sánchez M, Romero M, Olivares M, Jiménez R, Duarte J (2018). The probiotic Lactobacillus fermentum prevents dysbiosis and vascular oxidative stress in rats with hypertension induced by chronic nitric oxide blockade. Molecular nutrition and food research, 62(19), 1800298. 10.1002/mnfr.201800298 [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, and Allis CD (2001). Histone acetyltransferases. Annual review of biochemistry, 70(1), 81–120. 10.1146/annurev.biochem.70.1.81 [DOI] [PubMed] [Google Scholar]
- Ruder B, Atreya R, and Becker C (2019). Tumour necrosis factor alpha in intestinal homeostasis and gut related diseases. International Journal of Molecular Sciences, 20(8). 10.3390/ijms20081887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SH, Kaiko GE, and Stappenbeck TS (2018). Cellular differentiation: Potential insight into butyrate paradox? Molecular and Cellular Oncology, 5(3), 1212685. 10.1080/23723556.2016.1212685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson IR and He Y (1994). Nucleotide uptake and metabolism by intestinal epithelial cells. Journal of Nutrition, 124(1 SUPPL.), 131S–137S. 10.1093/jn/124.suppl_1.131s [DOI] [PubMed] [Google Scholar]
- Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, and Zubcevic J (2015). Involvement of bone marrow cells and neuroinflammation in hypertension. Circulation Research, 117(2), 178–191. 10.1161/CIRCRESAHA.117.305853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, and Raizada MK (2017). Hypertension-Linked Pathophysiological Alterations in the Gut. Circulation Research, 120(2), 312–323. 10.1161/CIRCRESAHA.116.309006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segain JP, De La Blétiere DR, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, and Galmiche JP (2000). Butyrate inhibits inflammatory responses through NF-κB inhibition: Implications for Crohn’s disease. Gut, 47(3), 397–403. 10.1136/gut.47.3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segiet A, Smykiewicz P, Kwiatkowski P, and Żera T (2019). Tumour necrosis factor and interleukin 10 in blood pressure regulation in spontaneously hypertensive and normotensive rats. Cytokine, 113, 185–194. 10.1016/j.cyto.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, and Raizada MK (2010). Brain microglial cytokines in neurogenic hypertension. Hypertension, 56(2), 297–303. 10.1161/HYPERTENSIONAHA.110.150409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulkes A, Lewis SJ, and Jarrott B (1989) Strain differences in central nervous system concentrations of cholecystokinin between normotensive Wistar Kyoto (WKY) and spontaneously hypertensive (SH) rats. Neuropeptides, 14(1), 59–64. 10.1016/0143-4179(89)90035-8 [DOI] [PubMed] [Google Scholar]
- Silveira-Nunes G, Durso DF, Cunha EH, Maioli TU, Vieira AT, Speziali E, Corrêa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, Franceschi C, and Rampelli S (2020). Hypertension Is Associated with Intestinal Microbiota Dysbiosis and Inflammation in a Brazilian Population. Frontiers in Pharmacology, 11, 258. 10.3389/fphar.2020.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprakasam S, Bhutia YD, Yang S, and Ganapathy V (2018). Short-chain fatty acid transporters: Role in colonic homeostasis. Comprehensive Physiology, 8(1), 299–314. 10.1002/cphy.c170014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprakasam S, Bhanapathy PK, Sikder MOF, Elmassry M, Ramachandran S, Kottapalli KR, and Ganapathy V (2019). Deficiency of Dietary Fiber in Slc5a8 -Null Mice Promotes Bacterial Dysbiosis and Alters Colonic Epithelial Transcriptome towards Proinflammatory Milieu. Canadian Journal of Gastroenterology and Hepatology, 2019. 10.1155/2019/2543082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman ML, Puig KL, Combs CK, and Rosenberger TA (2012). Acetate reduces microglia inflammatory signaling in vitro. Journal of Neurochemistry, 123(4), 555–567. 10.1111/j.1471-4159.2012.07955.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Haque M, Majid DSA, and Francis J (2008). Involvement of tumor necrosis factor-α in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension, 51(5), 1345–1351. 10.1161/HYPERTENSIONAHA.107.102152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, and Patel KP (2016). Astrocytes Contribute to Angiotensin II Stimulation of Hypothalamic Neuronal Activity and Sympathetic Outflow. Hypertension, 68(6), 1483–1493. 10.1161/HYPERTENSIONAHA.116.07747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Iwasaki K, Murata Y, Harada N, Yamane S, Hamasaki A, Shibue K, Joo E, Sankoda A, Fujiwara Y, and Hayashi Y (2018). Distribution and hormonal characterization of primary murine L cells throughout the gastrointestinal tract. Journal of Diabetes Investigation, 9(1), 25–32. 10.1111/jdi.12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase DM, Gosav EM, Radu S, Ouatu A, Rezus C, Ciocoiu M, Costea CF, and Floria M (2019). Arterial Hypertension and Interleukins: Potential Therapeutic Target or Future Diagnostic Marker? International Journal of Hypertension, Vol. 2019. 10.1155/2019/3159283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GN, Chalmers RA, Walter JH, Bresson JL, Lyonnet SL, Reed PJ, Saudubray JM, Leonard JV, and Halliday D (1990). The use of metronidazole in management of methylmalonic and propionic acidaemias. European Journal of Pediatrics, 149(11), 792–796. 10.1007/BF01957284 [DOI] [PubMed] [Google Scholar]
- Thoo L, Noti M, and Krebs P (2019). Keep calm: the intestinal barrier at the interface of peace and war. Cell Death and Disease, Vol. 10, pp. 1–13. 10.1038/s41419-019-2086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping DL and Clifton PM (2001). Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiological Reviews, Vol. 81, pp. 1031–1064. 10.1152/physrev.2001.81.3.1031 [DOI] [PubMed] [Google Scholar]
- Ui M (1966). A role of phosphofructokinase in pH-dependent regulation of glycolysis. Biochimica et Biophysica Acta (BBA)-General Subjects, 124(2), 310–322. 10.1016/0304-4165(66)90194-2 [DOI] [PubMed] [Google Scholar]
- Urzedo-Rodrigues LS, Ferreira HS, Almeida DO, Medeiros JP, Batista Á, e Silva EDC, and Fregoneze JB (2011). Blockade of 5-HT3 receptors at septal area increase blood pressure in unanaesthetized rats. Autonomic Neuroscience, 159(1–2), 51–61. 10.1016/j.autneu.2010.07.028 [DOI] [PubMed] [Google Scholar]
- Vallianou NG, Geladari E, and Kounatidis D (2020). Microbiome and hypertension: Where are we now? Journal of Cardiovascular Medicine, Vol. 21, pp. 83–88. 10.2459/JCM.0000000000000900 [DOI] [PubMed] [Google Scholar]
- Vancamelbeke M, Laeremans T, Vanhove W, Arnauts K, Ramalho AS, Farré R, Cleynen I, Ferrante M, and Vermeire S (2019). Butyrate Does Not Protect against Inflammation-induced Loss of Epithelial Barrier Function and Cytokine Production in Primary Cell Monolayers from Patients with Ulcerative Colitis. Journal of Crohn’s and Colitis, 13(10), 1351–1361. 10.1093/ecco-jcc/jjz064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJ, Faber KN, and Hermoso MA (2019). Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology, Vol. 10, p. 277. 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura HO and Lavie CJ (2016). Impact of comorbidities in hypertension. Current Opinion in Cardiology, 31(4), 374–375. 10.1097/HCO.0000000000000302 [DOI] [PubMed] [Google Scholar]
- Verbeke K (2017). Quantification of Plasma or Serum Short-Chain Fatty Acids: Choosing the Correct Blood Tube. Journal of Nutritional Health and Food Science, 5(6), 1–6. 10.15226/jnhfs.2017.001112 [DOI] [Google Scholar]
- Vinolo MAR, Rodrigues HG, Nachbar RT, and Curi R (2011). Regulation of inflammation by short chain fatty acids. Nutrients, Vol. 3, pp. 858–876. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, Wong SS, and Heard DG (2020). Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation, Vol. 141, pp. E139–E596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Waldecker M, Kautenburger T, Daumann H, Busch C, and Schrenk D (2008). Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. Journal of Nutritional Biochemistry, 19(9), 587–593. 10.1016/j.jnutbio.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, Li Y, Yao S, Chen S, Wu S, and Xue H (2020). Association of Age of Onset of Hypertension With Cardiovascular Diseases and Mortality. Journal of the American College of Cardiology, 75(23), 2921–2930. 10.1016/j.jacc.2020.04.038 [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, and Wang X (2008). TNF-α Induces Two Distinct Caspase-8 Activation Pathways. Cell, 133(4), 693–703. 10.1016/j.cell.2008.03.036 [DOI] [PubMed] [Google Scholar]
- Watson AJM and Hughes KR (2012). TNF-α-induced intestinal epithelial cell shedding: Implications for intestinal barrier function. Annals of the New York Academy of Sciences, 1258(1), 1–8. 10.1111/j.1749-6632.2012.06523.x [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, and Barman SM (2012). Serotonin and blood pressure regulation. Pharmacological reviews, 64(2), 359–388. 10.1124/pr.111.004697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum L and Klein OD (2018, October 1). The intestinal epithelial response to damage. Science China Life Sciences, Vol. 61, pp. 1205–1211. 10.1007/s11427-018-9331-y [DOI] [PubMed] [Google Scholar]
- Wolf B, Hsia YE, Tanaka K, and Rosenberg LE (1978). Correlation between serum propionate and blood ammonia concentrations in propionic acidemia. The Journal of pediatrics, 93(3), 471–473. 10.1016/S0022-3476(78)81167-6 [DOI] [PubMed] [Google Scholar]
- Wolf VL and Ryan MJ (2019, January 1). Autoimmune Disease-Associated Hypertension. Current Hypertension Reports, Vol. 21. 10.1007/s11906-019-0914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B, Hsia YE, Sweetman L, Gravel R, Harris DJ, and Nyhan WL (1981). Propionic acidemia: a clinical update. The Journal of pediatrics, 99(6), 835–846. 10.1016/S0022-3476(81)80004-2 [DOI] [PubMed] [Google Scholar]
- Worthington JJ, Reimann F, and Gribble FM (2018). Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunology, Vol. 11, pp. 3–20. 10.1038/mi.2017.73 [DOI] [PubMed] [Google Scholar]
- Wu PH, Chiu YW, Zou HB, Hsu CC, Lee SC, Lin YT, Tsai YC, Kuo MC, and Hwang SJ (2019). Exploring the benefit of 2-methylbutyric acid in patients undergoing hemodialysis using a cardiovascular proteomics approach. Nutrients, 11(12). 10.3390/nu11123033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Lu Y, Wang Y, Yan W, and Cai W (2019). Deficiency in intestinal epithelial Reg4 ameliorates intestinal inflammation and alters the colonic bacterial composition. Mucosal Immunology, 112(4), 919–929. 10.1038/s41385-019-0161-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yamanaka K, Gouraud SS, Takagishi M, Kohsaka A, Maeda M, and Waki H (2017). Evidence for a histaminergic input from the ventral tuberomammillary nucleus to the solitary tract nucleus involved in arterial pressure regulation. Physiological reports, 5(5), e13095. 10.14814/phy2.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Li H, Oliveira AC, Goel R, Richards EM, Pepine CJ, and Raizada MK (2020). Transcriptomic signature of gut microbiome-contacting cells in colon of spontaneously hypertensive rats. Physiological Genomics, 52(3), 121–132. 10.1152/physiolgenomics.00087.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Magee KL, Colon-Perez LM, Larkin R, Liao YS, Balazic E, Cowart JR, Arocha R, Redler T, Febo M, Vickroy T, Martyniuk CJ, Reznikov LR, and Zubcevic J (2019). Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiologica, 226(2). 10.1111/apha.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Ahmari N, Schmidt JT, Redler T, Arocha R, Pacholec K, Magee KL, Malphurs W, Owen JL, Krane GA, Li E, Wang GP, Vickroy TW, Raizada MK, Martyniuk CJ, and Zubcevic J (2017). Shifts in the gut microbiota composition due to depleted bone marrow beta adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Frontiers in Physiology, 8(APR). 10.3389/fphys.2017.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, and Mohamadzadeh M (2015). Gut Dysbiosis is Linked to Hypertension. Hypertension, 65(6), 1331–1340. 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K and Kim N (2018). The Effect of Microbiota on Colon Carcinogenesis. Journal of Cancer Prevention, 23(3), 117–125. 10.15430/jcp.2018.23.3.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, and Hanafusa T (2014). Infliximab, a TNF-α inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. Journal of Human Hypertension, 28(3), 165–169. 10.1038/jhh.2013.80 [DOI] [PubMed] [Google Scholar]
- Zakharov S, Kurcova I, Navratil T, Salek T, Komarc M, and Pelclova D (2015). Is the Measurement of Serum Formate Concentration Useful in the Diagnostics of Acute Methanol Poisoning? A Prospective Study of 38 Patients. Basic and Clinical Pharmacology and Toxicology, 116(5), 445–451. 10.1111/bcpt.12338 [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, and Panda S (2018). Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nature Communications, 9(1), 1–13. 10.1038/s41467-018-05336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Monleon D, Verhamme P, and Staessen JA (2018). Branched-chain amino acids as critical switches in health and disease. Hypertension, Vol. 72, pp. 1012–1022. 10.1161/HYPERTENSIONAHA.118.10919 [DOI] [PubMed] [Google Scholar]
- Zhu H, Shan L, Schiller PW, Mai A, and Peng T (2010). Histone deacetylase-3 activation promotes Tumor Necrosis Factor-α (TNF-α) expression in cardiomyocytes during lipopolysaccharide stimulation. Journal of Biological Chemistry, 285(13), 9429–9436. 10.1074/jbc.M109.071274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, and Dyck JR (2015). Metabolomic Fingerprint of Heart Failure with Preserved Ejection Fraction. PLOS ONE, 10(5), e0124844. 10.1371/journal.pone.0124844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Baker A, Martyniuk CJ (2017). Transcriptional networks in rodent models support a role for gut-brain communication in neurogenic hypertension: a review of the evidence. Physiological Genomics, 49(7), 327–338. 10.1152/physiolgenomics.00010.2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


