Abstract
The adaptive immune system has the enormous challenge to protect the host through the generation and differentiation of pathogen-specific short-lived effector T cells while in parallel developing long-lived memory cells to control future encounters with the same pathogen. A complex regulatory network is needed to preserve a population of naïve cells over lifetime that exhibit sufficient diversity of antigen receptors to respond to new antigens, while also sustaining immune memory. In parallel, cells need to maintain their proliferative potential and the plasticity to differentiate into different functional lineages. Initial signs of waning immune competence emerge after 50 years of age, with increasing clinical relevance in the 7th –10th decade of life. Morbidity and mortality from infections increase, as drastically exemplified by the current COVID-19 pandemic. Many vaccines, such as for the influenza virus, are poorly effective to generate protective immunity in older individuals. Age-associated changes occur at the level of the T cell population as well as the functionality of its cellular constituents. The system highly relies on the self-renewal of naïve and memory T cells, which is robust but eventually fails. Genetic and epigenetic modifications contribute to functional differences in responsiveness and differentiation potential. To some extent, these changes arise from defective maintenance; to some, they represent successful, but not universally beneficial adaptations to the aging host. Interventions that can compensate for the age-related defects and improve immune responses in older adults are increasingly within reach.
Keywords: Immunosenescence, adaptive immunity, T cell aging, T cell homeostasis, T cell differentiation, cellular senescence
The T cell system has to meet the challenge to preserve a diverse population capable of self-renewal while rapidly responding and differentiating upon pathogen encounter. T cell aging is a combination of failure to maintain this balance and lack of adaptation to a changing environment in the host while dealing with aging-related cellular defects. Their molecular definition has laid the foundation to explore targeted interventions to improve immune function.
Graphical Abstract

Introduction
The lymphoid population is a dynamic system of high complexity that is under constant pressure to replenish its cellular constituents. In parallel, it has to deal with frequent disturbances, both from endogenous signals such as tissue injury as well as from exogenous stimuli such as infections that trigger a burst of expanding antigen-specific T cells. After resolution of the insult, it has to regain a new balance that is upheld by homeostatic mechanisms. One important function of the adaptive system is to preserve a memory of previous antigenic encounters. Memory again is systemic and dynamic, as it is not retained by the longevity of singular memory cells, but by the division of their progeny. The complexity of the system and in particular its high reliance on replication of its constituents makes it highly susceptible to changes with age. These changes are in part a consequence of pathways that have been invoked in the aging process, to some degree they represent successful adaptations to changing environment and needs in older individuals (1). The clinical consequences of immune aging are striking. Pathogenic infections, in particular influenza and pneumococcal diseases, are among the leading causes of hospitalization and death in the aging population. The case fatality rate of the more recent COVID-19 pandemic SARS-CoV-2 is strictly correlated with age after the age of 50 years and is increased 10- to 100-fold in elderly compared to middle-age adults (2). Likewise, responses to most vaccines are reduced with age (3). For example, for influenza vaccination in the 2017/2018 season, clinical effectiveness of the influenza vaccine, i.e., the prevention of influenza illness, as reported by the CDC, dropped from 68% in children to 30–40% in middle-aged adults and 17% for adults ≥65 years (4). For H3N2, the most virulent subtype of seasonal influenza strains, it was even only 10%. Attempts to improve the efficacy of influenza vaccination had only limited success. However, considerable population heterogeneity exists. Frail elderly are generally more immunocompromised. Conversely, some older adults remain relatively immune competent into older age. Moreover, certain vaccines can be very effective in older age. The prime example is shingles vaccination, where the adjuvanted component vaccine Shingrix proved to have a higher efficacy in older age than the vaccination with an attenuated life virus, Zostavax (5). Here, we review recent progress of the mechanisms how the human T cell system ages and how recent insights provide a better understanding on which interventions might prevent immune decline or might compensate for immune defects. For an overview of T cell aging in the mouse, we refer to recent excellent reviews (6, 7); we focus here on human studies, only contrasting them with findings of T cell aging in the mouse when explicitly stated.
Naïve T cell generation – the challenge to maintain a dynamic and diverse system
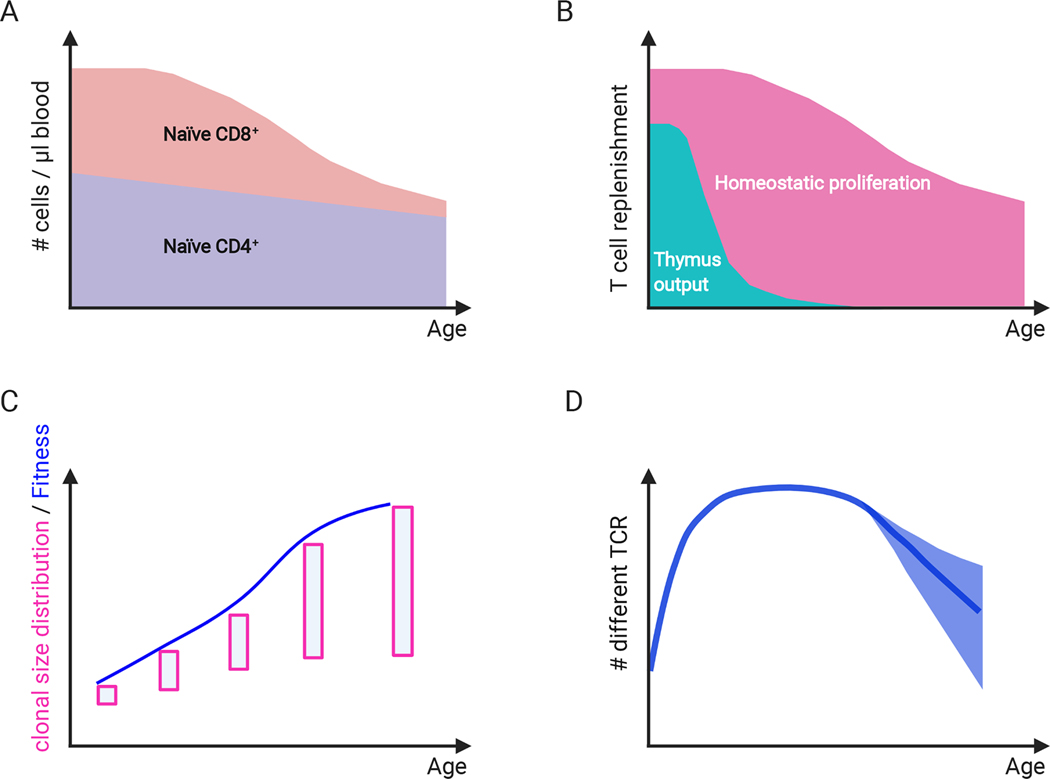
One of the greatest challenges of the aging process is to replenish cells while keeping the integrity of the organ. The lymphoid system is fundamentally dynamic, employing a vast number of T cells (>1011) (8) and trying to maintain a balance between cell production, death, and differentiation. Replenishment of T cells comes from two sources, generation of new T cells in the thymus and homeostatic proliferation of peripheral T cells (Figure 1). Two principal subsets of T cells can be distinguished that are fundamentally different not only in their antigen recognition structures but also in their homeostatic control mechanisms. T cells expressing a γδ T cell receptor (TCR) undergo dramatic changes in the first years of life. In contrast to early developmental studies, their evolution in the later aging process has been less well explored (9, 10). Here, we focus on T cells expressing the αβ TCR. The relative contribution of their generation in the thymus changes over lifetime in a species-specific manner, e.g., it is very different between mouse and humans. In the young adult mouse, the contribution of thymic production is about 2 to 5 fold higher than that of peripheral proliferation (11). Conversely in the young adult human, less than 20% of nascent T cells are produced from the thymus, which dwindles to less than 1% after the age of 50 years (12). Furthermore, naïve T cells in humans are long-lived, with lifespans of several years, while naïve T cells in mice only live 6–11 weeks, significantly shorter than their time between divisions from homeostatic proliferation (13). Given these species differences, data on T cell homeostasis in the mouse over cannot not be easily extrapolated to humans, complicating mechanistic studies. Human T cell turnover rates remain stable over adult lifetime, consistent with the notion that even in the young adult, the majority of T cells are produced in the periphery (14). Only in late life, a pickup in proliferation has been described in humans as well as non-human primates, possibly as a consequence of increased cell death and evolving lymphopenia (15–17). Naïve T cells decline with age both in absolute as well as relative numbers, likely due to insufficient homeostatic proliferation (Figure 1); this decline is only modest for naïve CD4+ T cells but very striking for naïve CD8+ T cells even in healthy elderly (18, 19). The reduction in absolute numbers of naïve CD8+ T cells is one of the most significant hallmarks of T cell aging (20).
Figure 1. Naïve T cell homeostasis.
A. Both, naïve CD4+ and naïve CD8+ T cells decrease in absolute numbers with age with the latter decreasing more drastically. B. Naïve T cell replenishment is maintained by thymic output and homeostatic proliferation in neonates. At age 20 years, thymic output has dropped to less than 20% with further decline to <1%; homeostatic proliferation accounts for the majority of naïve T cell generation throughout adulthood. C. The distribution of clonal sizes (illustrated by boxes) widens with age. Occurrences of larger clones in the naïve T cell compartment increase, associated with increased fitness selection over lifetime. D. The number of TCR in the T cell repertoire rapidly increases within the first decade of life and declines with older age. Computer simulations predict that the confidence interval of this decline is large.
Naïve T cell homeostasis is maintained within secondary lymphoid tissues (SLT) by a specialized stromal cell population, fibroblastic reticular cells (FRC) that produce the survival cytokine IL-7 and other mediators (21–23). The abundance of IL-7 influences the overall size of the T-cell compartment mainly through changes in peripheral dynamics. So far, there has not been any evidence for a defect in IL-7 production by FRC with age. Instead, SLT exhibit structural changes with age that may account for the reduced efficiency of naïve T cell homeostasis (24–27). Moreover, SLT fibrosis is induced by chronic or recurrent inflammation, which reduces the niches for T cells and may further accelerate age-associated defects in T cell generation. Inflammation-induced SLT fibrosis is associated with CD4+ T cell lymphopenia and reduced vaccine responses (28). These structural changes should equally affect naïve CD4+ and CD8+ T cells and cannot explain the preferential loss of naïve CD8+ T cells. Therefore, T cell-intrinsic differences may account for the lower resilience of CD8+ T cells to aging. Indeed, as described below in more detail, transcriptional and epigenetic studies indicated that age-associated intrinsic changes in naïve CD8+ T cells were not or much less present in CD4+ T cells of older adults (29–31).
Maintaining compartment size is only one out of three elements that successful T cell replenishment has to accomplish, with TCR diversity and cell functionality the others. An enormous TCR diversity is required to be able to respond to the universe of possible peptides (>209), even when taking into consideration the high degree of TCR cross-reactivity (32, 33). Obviously, only T cell generation in the thymus can add new TCR specificities, while homoeostatic proliferation at best maintains diversity. With the progress in next generation sequencing and with new analytical approaches to overcome the problem of subsampling of the human naïve T cell pool, diversity estimates have become more accurate over the last two decades increasing from 106 and 107 to now >108 unique TCR in a given adult (34–36). These high estimates provide a large cushion for an age-related decline before being of functional significance. The number of unique TCR β-chain sequences (i.e. TCR richness) for naïve T cells has been estimated to shrink by a factor of 2 to 5 between young adults and adults older than 60 years. This reduction represents a substantial decline, but still leaves a diverse repertoire without major holes in the ability to respond to the universe of foreign peptides (Figure 1). It should, however, be noted that these repertoire studies were done on old healthy blood donors to be able to sample a sufficiently large number of cells to obtain a robust estimate; it cannot be excluded that the results will be different in frail adults. In addition, environmental as well as genetic confounding factors likely influence these findings. In the study by Qi et al, a similar loss in TCR richness was seen for CD4+ and CD8+ T cells (36). However, a recent study by Krishna et al showed that the best model predicting TCR richness includes age as well as the number of different MHC class I molecules (37). The finding that MHC class II polymorphisms did not matter suggest that the repertoire diversity in CD8+ T cells may be more variable than that of CD4+ T cells; repertoire contraction in CD8+ T cells with age could therefore be biologically more relevant, in particular in adults with homozygous MHC class I alleles.
Computational modeling supported the notion that the demise of thymic output is not sufficient to cause repertoire contraction, even if the compartment size declines as is the case for CD8+ T cells (38). Indeed, experimental estimates found a similar contraction in naïve CD4+ and CD8+ T cells suggesting that a smaller number of different TCR is not correlated with the smaller pool of CD8+ T cells (36). Rather, growth behavior changes of single clones could cause a rapid loss in diversity, suggesting that fitness selection has a negative impact on T cell homeostasis with aging. In independent simulations, such a loss randomly occurred and was essentially unpredictable. Interestingly, diversity could not be restored by the virtual introduction of thymic activity at older age in the simulation, consistent with increased fitness of the existing repertoire. Experiments in old mice have confirmed that restoring thymic activity is not sufficient to rebuild the repertoire, likely due to a competitive disadvantage of recent thymic emigrants as well as age-associated changes in the SLT (26).
Recent studies have shown that the naïve TCR repertoire has an unexpectedly broad distribution of clonal sizes (39). The clonal size distribution best fitted a power law with one to five percent of clones having a size of >105 cells. Aging is associated with a shift in clonal size distribution with a larger frequency of large clones within the naïve compartment (Figure 1). Because the definition of naïve T cells in these studies relied on the phenotype, some of these clones may represent memory cells that have re-assumed a naïve phenotype. Indeed, stem-like memory cells are difficult to phenotypically distinguish from truly naïve T cells. Moreover, in tetramer studies, virus-specific memory CD8+ T cells had a naïve phenotype although carrying an epigenetic profile of effector T cells (40). In contrast, virus-specific CD4+ memory T cells express a central memory phenotype and are not found in the phenotypically naïve CD45RA+ T cell population (41). Taken together, contamination with memory T cells cannot fully account for clonal expansion within naïve CD8+ and even less so within naïve CD4+ T cells. The uneven clonal size distributions may reflect a fitness selection in homeostatic proliferation over lifetime that increases with age (Figure 1). In addition, endogenous changes in growth behavior such as they occur due to somatic mutations in clonal hematopoiesis could contribute (see genome stability section). Clonal sizes of naïve T cells are of potential functional importance, because the size determines the probability of a response. At the extreme, clonal expansion due to fitness selection by recognition of peripheral self-MHC molecules could increase the risk of autoimmunity (42).
In the absence of an appropriate animal model, T cell reconstitution after medically induced lymphopenia may be informative to study aging-associated changes in T cell homeostasis. In the extreme form, hematopoietic stem cell (HSC) transplantation, T cell numbers drastically drop after conditioning. The subsequent increased proliferation and clonal expansion of surviving and/or donor T cells results in contraction of TCR diversity (43). Similar results were obtained after antibody-mediated T cell depletion, excluding possible confounding effects from the conditioning regimen (44). Full reconstitution, if it occurred at all, took months to years, with CD8+ T cells recovering faster than CD4+ T cells, very much different from the natural aging process. These observations are consistent with mouse models where lymphopenia-induced proliferation of CD8+ T cells by far outpaces that of CD4+ T cells (45, 46). The rapid proliferation of CD8+ T cells is associated with their differentiation into virtual memory T cells that also accumulate with age in mice (47). Less drastic lymphopenia is seen with adjuvant chemotherapy of breast cancer patients (48). Here again, CD8+ T cell recovery is superior to that of CD4+ T cells, however, this time without phenotypic changes and without contraction of diversity. Taken together, accelerated homeostatic proliferation only partially resembles the aging process.
Influence of age on the functional heterogeneity of the naïve T cell population
Naïve T cells generated at different stages in life are functionally different, causing age-specific changes in the composition of naïve T cell compartment. Very early in life, T cells are produced into an empty environment. The frequency of dividing T cells at the late fetal stage is high, suggesting a major contribution of lymphopenia-induced proliferation (11, 49). Newborn T cells display many innate immune features, including production of IL-8, responsiveness to TLR, and ready induction of effector functions (50). Some descendants of these early T cells may survive into late adult life, as shown in monozygotic twins for T cells generated during prenatal life when circulatory systems are connected (51). Also, modeling studies suggested that the largest T cell clones emerge from clonal growth during repertoire formation; i.e. early founded clones are highly enriched among the largest clones even after decades of human aging (52). However, functional activities resembling those of the innate immune system and exhibited by these early T cells are mostly lost in the second decade of life.
Recent thymic emigrants, i.e., cells that have recently egressed from the thymus and are defined by the expression of CD31 and PTK7 are also functionally different. They are sensitive to danger signals and display innate-like effector functions, which have the advantage of a rapid response at an age when memory is not fully established. However, they have a reduced ability to form high-avidity, long-lived memory responses (53–55). The CD31+PTK7+ fraction of peripheral lymphocytes is lost with age, which may represent a true loss or maturation of recent thymic emigrants with homeostatic proliferation (50, 56). Taken together, the functional composition of naïve T cells changes with age and T cell populations with more innate response patterns present in early life are lost with adulthood. It is remarkable that T cells in older individuals again acquire innate and effector functions while losing the ability to generate memory, mimicking functionality during early life (see below).
Immune memory – the challenge to make it last
While most attention in cell replenishment in immune aging is on naïve T cells, likely due to the impressive decline in thymic function in the first decades of life, a decline in immune memory is of no less importance. A typical example is shingles, a reactivation of varicella zoster virus that occurs in up to 50% of the population by the age of 80 years. Reduced memory function accounts for the increased incidence and severity of common childhood infections including pneumococcal disease, infections with the respiratory syncytial virus or with the influenza virus. At the same time, memory cell function to other pathogens can be highly protective. For example, symptomatic reactivation of Epstein Barr (EBV) or cytomegalovirus (CMV) is rarely seen with normal aging. Antigenic restimulation in latent EBV and CMV infections may be important for maintaining antigen-specific memory T cells. But even in settings, where re-exposure is unlikely, immune memory can last for more than four to six decades (57, 58). Why T cell memory to certain pathogens is better maintained than to others remains elusive. Immunological memory is a dynamic process and is not determined by the longevity of individual cells, but by persistence of their progeny. Longitudinal studies have demonstrated the persistence of clonal TCRs over years (59). While turnover of memory cells is much higher than that of naïve T cells and in the order of months (60), memory T cells that revert back to a naïve phenotype such as CD8+ stem-like memory cells divide about once a year (40, 61).
Immunological memory typically refers to T cells that have been primed by the recognition of a pathogen before and therefore have entered a more differentiated state. Distinct from these primed T cells, naïve T cells may also enter differentiation just based on cytokine stimulation. These cells are referred to as “virtual” and “innate” memory T cells (Figure 2). They are rapidly generated in states of lymphopenia, but can also derive from normal homeostatic proliferation. Virtual CD8+ T cells accumulate with age and are dysfunctional in older mice (62, 63). A human equivalent has been less studied in the context of aging, also because their phenotypic definition is still vague.
Figure 2. Fates of memory T cells with age.
The antigen-specific memory T cells adopt several fates with age, including increase in NK cell-like TEMRA, short-lived effector memory T cells, exhausted T cells, decrease in stem-like memory T cells and decrease in tissue-residing T memory cells. Virtual memory T cells without prior experience of antigen encounter also increase with age.
Beyond virtual memory T cells, memory T cells are a heterogeneous population, as has been increasingly shown with the progress in single cell cytometric and RNA-seq analysis (Figure 2) (64, 65). One of the first classifications has been to discriminate central and effector memory T cells based on their trafficking patterns and their effector functions (66). Effector T cells accumulate with age, in part as terminally differentiated effector T cells (TEMRA) that have lost the expression of CD27 and CD28 and instead have gained various NK receptors, exposing them to regulatory influence by distinct environmental cues (67). Expansion of TEMRAs is mostly driven by CMV infection, with age being only a secondary factor (19). Expression of CD57 on these cells is frequently considered as evidence of senescence or end-differentiation (68); still, CD57+CD28+ T cells can be polyfunctional (69). These population shifts are less seen for CD4+ than CD8+ T cells in vivo (70), however, even CD4+ T cells from old adults are poised to develop into effector T cells rather than memory or T follicular helper cells after in vitro activation (71, 72). More granular approaches to define the impact of age on memory cell heterogeneity are only in the beginning (65). In particular, tissue-resident memory T cells (TRM) are an important subset that are trapped in the tissue where they function as a first line of defense against pathogen infection, in particular in skin and at mucosal surfaces (73, 74). A reduced number or function of TRMs may contribute to the preferential involvement of the respiratory tract by infections in the elderly. We have recently identified a subpopulation of peripheral T cells that are greatly enriched for virus-specific memory T cells and excel through their durability, their poised effector function and their ability to differentiate into TRMs (75). This population declines with age in the blood, which may impair the replenishment of tissue-residing T cells and increase the susceptibility to skin and mucosal infections in the elderly.
So far, identification of T cell heterogeneity relies on phenotypic marker profiles. While different in young and old adults, they alone are not sufficient to predict immunological age, in part due to high inter-individual variability. Yet, Alpert et al. proposed a metric of age based on such marker profiles (76). These authors determined the intra-individual frequency changes of cell surface markers, including those discussed for T cells above, in a longitudinal study over several years. Based on the baseline frequencies and the longitudinal dynamics, they identified steady state levels toward which cell subsets converged. Data for different subsets form a high-dimensional trajectory of immune aging, designated as IMM-AGE that correlates with survival and is therefore biologically meaningful.
The challenge to maintain genome stability in a highly replicating system
Maintaining genome integrity and stability is one of the major challenges evoked during aging (77). Somatic mutations accumulate with age in various tissues (78, 79). When mutations sprouting from a stem cell impart a survival and proliferative advantage, the mutated stem cell outgrows the population and undergoes clonal expansion. This phenomenon has been extensively studied in the hematopoietic system and termed clonal hematopoiesis (CH) (80). CH is rarely detected in humans under age 40 but observed in 10–20% of humans beyond age 70 (81). Many CH occur without a known driver mutation (82), thus clonal hematopoiesis of indeterminate potential (CHIP) was coined to distinguish the individuals carrying a cancer-associated mutation without manifest malignancy (80). The current threshold for CHIP is set at a variant allele fraction of 2% and is associated with a ten-fold increased risk of hematopoietic malignancies (81). Several CHIP-associated genes have been identified, with TET2 and DNMT3A the most frequently reported (83, 84). TET2 and DNMT3A carry out the function of removing or adding the methyl moiety of cytosine, eliciting the decade-long conundrum how two enzymes with opposing functions lead to common phenotypes. A recent single cell study sheds some light on this enigma; while the methylation by DNMT3A is genome-wide, the demethylation by TET2 is more focal, indicating a regional differential regulation of the genome by the two enzymes (85). Supporting this notion, a treatment study on T cells expressing a chimeric antigen receptor (CAR) discovered that biallelic dysfunctional mutations in TET2 conferred a skewed expansion of central memory T cells with downregulated effector differentiation programs and upregulated cell cycle and TCR signaling programs (86).
In healthy adults, T lymphocytes, compared to other hematopoietic descendants, are largely spared from CH-derived mutations (87, 88), possibly attributed to the minimal thymic activity in older adults and the stringent thymic selection process. Consistent with post-thymic origin, mutations if present do not co-occur in CD4+ and CD8+ T cells (89), but are especially enriched in clonally expanded CD8+ T cells (90–92). The association with clonal expansion may indicate that these mutations have occurred during an antigen-specific T cell response or that mutations at an earlier stage confer increased proliferative potential. In support of the latter, gain-of-function mutations of STAT3 inducing constitutive activation could explain the abnormal expansion of cytotoxic CD8+ T cells (90). Such somatic mutations may have implications for T cell homeostasis. In computational simulation studies of T cell homeostasis with age, a clonal progeny with an abnormal growth potential resulted in an abrupt contraction of the T cell receptor diversity (38).
In general, somatic mutations arise from several common sources, including spontaneous cytosine deamination, errors from DNA double strand break repair, replication errors and large structural variations, all of which increase with age (80). Mutation spectra vary across tissue types (78, 79), suggesting that each tissue is subject to unique mutation mechanisms. In T cells, several master components of the DNA repair machinery can be dysregulated, including loss of MRE11 and ATM (93, 94) and gain of DNA-PKcs (95). Since MRE11 and ATM promote the homologous recombination repair while DNA-PKcs accounts for the error-prone non-homologous end joining repair (96), a nontrivial portion of the mutations in aged T cells probably stems from inaccurately repaired DNA breaks. Increased replication stress in proliferating T cells from old adults is another potential source for DNA lesions (97). Taken together, in addition to CHIP mutations of prethymic cells, impaired DNA repair machinery and increased replication stress could be a major source of somatic mutations in T cells, detectable in clonally expanded populations. Single cell studies may therefore uncover a substantial number of mutations in T cells. Although not yet examined in T cells, this prediction is corroborated by a recent single-cell whole-genome sequencing study of B cells (98). Whether and how such mutations drive clonal expansion or have other consequences remains to be investigated.
Unlike the sporadic somatic mutations across the entire genome, erosion of the telomeres is a consistent feature of aging (99). Due to their extensive replication history, the telomere length is shorter in naïve as well as memory T cells in older individuals (100). In T cells from patients with rheumatoid arthritis, shortening is further aggravated by the accumulation of structural changes indicative of increased telomeric damage (94). Telomere attrition is associated with cellular senescence in TEMRA T cells including the expression of p16 (101). Telomeric erosion alone is sufficient to induce features reminiscent of T cell aging (102). Patients with genetic short telomere syndrome exhibited a primary T cell immunodeficiency with a marked attrition of the naïve T cell population and increased frequencies of opportunistic infection. However, the reduced frequency of naïve T cells was mainly due to a decline in thymic production and increased intrathymic cell death rather than a failure of homeostatic mechanisms, in contrast to age-associated mechanisms. In parallel, the TEMRA population in these patients was expanded, possibly due to reactivation of latent viral infections. Consistent with telomeric erosion, the p53 pathway was activated resulting in increased apoptosis. In contrast, in spite of telomeric erosion, the vast majority of naïve and memory T cells except TEMRAs in healthy older adults do not express p16 or have other evidence of cellular senescence such as senescence-associated β-galactosidase activity or induction of senescence-associated secretory patterns. Activation of the p53 pathway in T cells from older adults is seen only under conditions of proliferative stress (97) and may then contribute to the increased T cell attrition with age after antigen-induced expansion (103). Nevertheless, telomeric erosion can cause increased apoptosis when telomerase activity is inhibited, as shown in patients with rheumatoid arthritis (104).
Taken together, replication-associated telomere erosion is inevitable with age and T cells are not exempt. However, telomeric erosion is not sufficient to induce cellular senescence except in terminally differentiated T cells, possibly due to the co-existing telomerase activity in T cells. It is, however, possible that telomeric erosion contributes to the increased DNA damage responses in older individuals during the clonal T cell burst of an antiviral response and thereby contribute to higher attrition of effector cells and failure to generate long-lived memory cells.
Age-associated alterations in the epigenome - Setting the stage for altered function
The epigenome embraces several layers of alterations to the chromatin from DNA methylation and histone modification to high-dimensional organization, all of which are associated with progressive changes with age (105). In T cells, the age-associated alterations in the epigenome is different between CD4+ and CD8+ cells (31). While it is ostensibly stable with age for CD4+ T cells, CD8+ naïve and memory T cells are shifted in chromatin accessibility patterns towards a more differentiated and effector state that is exemplified by the repression of IL-7 receptor signaling pathway genes (29, 106). Mechanistically, CD8+ T cells may be more susceptible to activation, possibly due to the more widespread expression of MHC class I vs class II molecules or an intrinsic limitation in maintaining quiescence (107). Additional epigenetic hallmarks of CD8+ T cell aging are the inaccessibility to regulatory regions of genes involved in basic cellular functions, such as the mitochondrial respiratory genes (106). This bias in epigenetic changes may explain the preferential loss of naïve CD8+ T cells and the accumulation of CD8+ TEMRAs with age. Nevertheless, the CD4+ T cell epigenome is not unaffected by age, as discussed below for recent studies on age-associated changes of DNA methylation and histone modifications in T cells.
DNA methylation at subsets of CpGs was shown to be remarkably predictive of chronological age (108, 109), as well as phenotypic age (110, 111) irrespective of tissue types. However, the full spectrum of methylation sites with age-associated variation can be highly tissue-specific, especially at sites associated with functional gene expression (112). DNA methylation in T cells co-evolves with TCR activation, differentiation, and lineage commitment (113–115). Over ten thousand age-associated variations of CpG sites in naïve CD4+ T cell were found, mostly enriched in pathways related to TCR signaling, apoptosis, mTOR signaling and MAPK signaling (116). The environmental burden on the T cell aging process also manifests at the level of DNA methylation. For example, smoking-associated reduction of DNA methylation at the aryl hydrocarbon receptor repressor gene in CD8+ T cells was shown to be a strong indicator of age acceleration (117). For most part, the functional consequences of these age-associated epigenetic alterations await further investigation.
Histone modifications, intertwined with DNA methylation, play critical roles in the regulation of T cell-mediated immunity (118, 119). Evident imbalance of histone modifications with age were reported in many organisms and tissues (105). In T cells, the heterogeneity of the histone modification profile is markedly increased with age. Also, most histone modifications show increased cell to cell variability in T cells from older individuals, corresponding to higher single-cell transcriptional variability. Specifically, in naïve CD4+ T cells, the H3K27me3-marked genes exhibit significantly higher transcriptional variability with age despite no difference of gene expression at the population level (120). In summary, zooming in on the details of chromatin marks, the above studies suggest that the epigenetic alterations of CD4+ T cells with age is nontrivial, albeit more subtle than those in CD8+ T cells. These alterations may poise the response pattern and get propagated and more overt after T cell activation. In support of this notion are recent longitudinal ATAC-seq data aiming to capture the trajectory of TCR activation-associated changes in CD4+ T cells from old adults (Zhang and Goronzy, unpublished data).
Noncoding RNAs, extending epigenetic regulation, are implicated in T cell function and aging (121, 122). Numerous microRNAs (miRNAs) show altered expression with age in T cells (30, 123). Some have clear functional implications. For example, loss of miR-181a blunts TCR signaling in aged naïve CD4+ T cells through upregulation of several phosphatases including DUSP6 as well as the deacetylase SIRT1 (Figure 3) (124, 125). Upregulation of miR-24 is associated with impaired DNA damage response in the age-expanded population of CD28- T cells (126). Of particular interest is miR-21, which is upregulated with age in activated naïve CD4+ T cells and thereby inhibits negative regulatory control mechanisms of activation pathways including of AKT-mTORC1. The outcomes of increased miR-21 are notably different for naïve and memory CD4+ T cell subsets. While in naïve CD4+ T cells miR-21 antagonizes the development of a memory cell signature in favor of short-lived effector T cells (Figure 4) (127), it confers a survival advantage in memory CD4+ T cells through downregulation of apoptosis (128). These age-related changes in microRNA expressions account for functional differences in T cell activation as well as differentiation as discussed below.
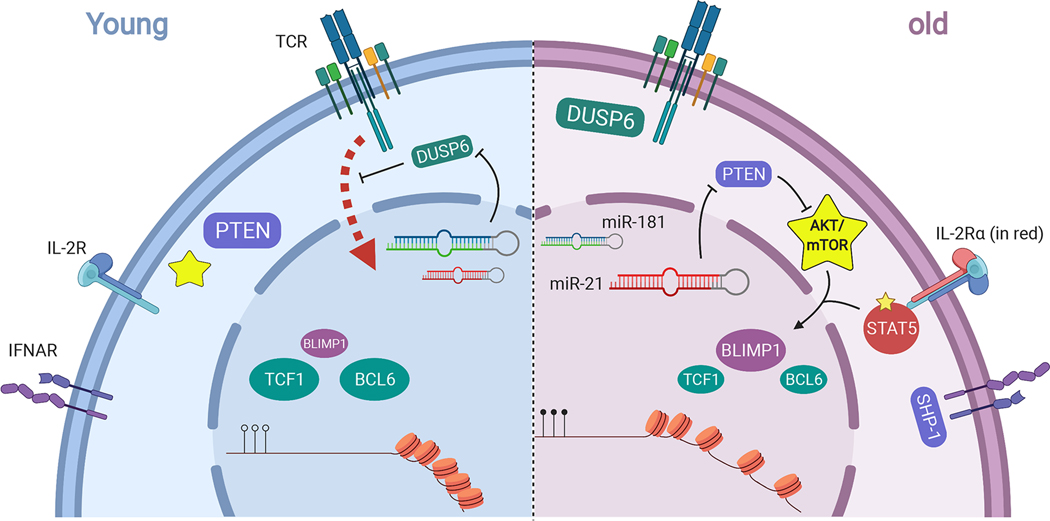
Figure 3. Molecular mechanisms underlying age-related naïve T cell functionality.
Selected age-related changes at the cell surface and cytosolic level: TCR signaling is blunted with age through upregulation of DUSP6 due to loss of miR-181. Type I interferon response is weakened with age due to accumulation of SHP-1 at IFNα receptors (IFNAR). IL-2/STAT5 signaling is augmented with age due to the upregulation of CD25 aka IL-2 receptor α subunit (IL2R α). AKT/mTOR signaling is increased with age due to downregulation of PTEN by miR-21. At the nuclear level: In old T cells, chromatin accessibility shifts towards more differentiated states (illustrated by more opened chromatin) coupled with alterations in DNA methylation patterns. Transcription factors (TFs) driving effector differentiation such as BLIMP-1 increase with age due to upregulation of AKT/mTOR signaling and STAT5 signaling, whereas TFs driving memory and TFH differentiation such as TCF1 and BCL6 decline.
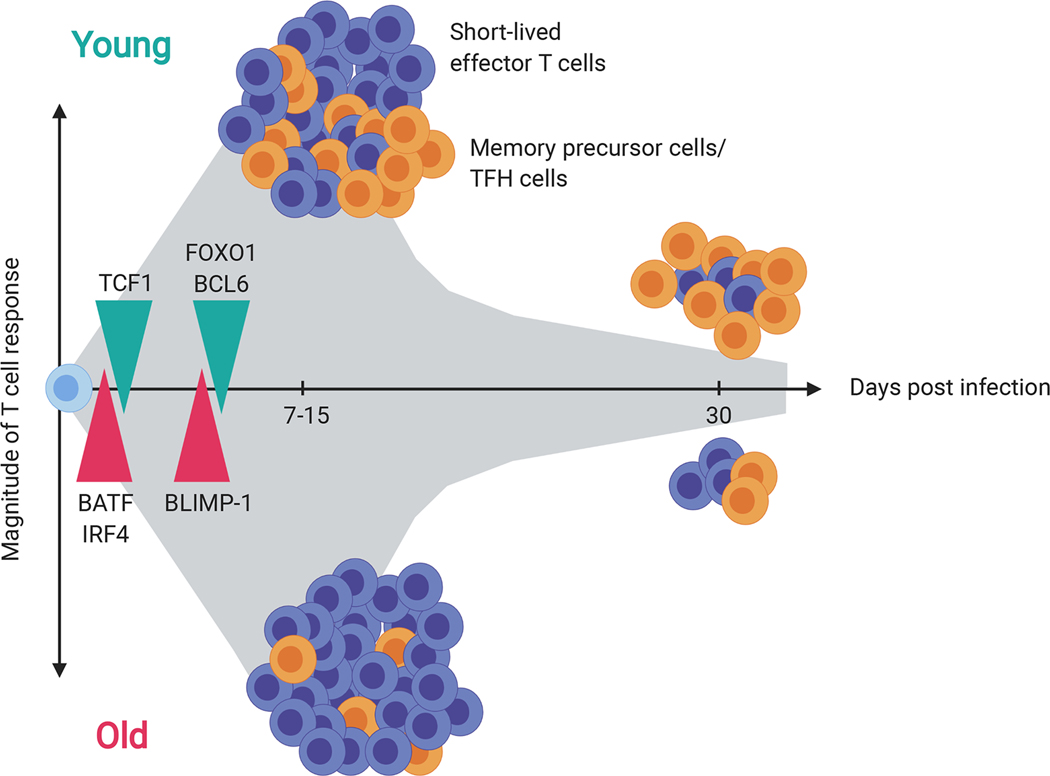
Figure 4. Skewed differentiation of naïve T cells after antigen encounter.
Naïve CD4+ T cells in older individuals tend to differentiate into short-lived effector T cells after antigen stimulation coupled with an impaired development of memory and TFH cells. This results in a curtailed memory population after infection or vaccination in older individuals. Mechanistically, the skewed differentiation is partially due to an early shift in transcription networks including upregulation of BATF, IRF4 and BLIMP-1 and loss of TCF1, FOXO1 and BCL6.
Changes in T cell activation – Age-associated failure or active recalibration?
Efficient and effective onset of adaptive immune response requires optimal TCR signaling (129). In healthy adults, TCR signaling is tuned down with age in virtually every subset of T cells, but through a variety of mechanisms (Figure 3). In naïve CD4+ T cells, TCR signaling is blunted in older adults due to upregulation of DUSP6 and other phosphatases by diminished miR-181 (124). Loss of miR-181a with age is less significant in memory CD4+ T cells that already have lower expression compared to naïve T cells, but other negative regulatory pathways blunt TCR signaling including the upregulation of DUSP4 (130). TCR signaling is complemented and propagated by CD28 costimulation (131). Both in CD4+ and CD8+ T cells, CD28 is lost with age on a subset of effector T cells (18), coupled with expression of natural killer (NK) receptors and increased cytotoxic activity (132–134). Mechanistically, the stress-sensing protein sestrins are gained with age in TEMRA cells, which in the absence of proximal TCR and CD28 signaling upregulate basal activity of mitogen-activated protein kinases and promote expression of the NK T cell adaptor module DAP12 (135, 136).
From the developmental point of view, TCR signaling is constitutively downregulated from thymocyte to mature T cells and in naïve T cells from neonate to adult, and to older age (124, 137). This increased TCR activation threshold in aged T cells might represent an adaptive adjustment of naïve T cells to the changing conditions in the host. Homeostatic proliferation might induce the selection of a repertoire with higher affinity to self with the risk of autoimmunity (138), and the tissue environment is getting more stimulatory due to enhanced activation of antigen-presenting cells, to the increase in tissue damage and repair and to post-translational modifications of self-antigens (139, 140). This calibration process can benefit the host in several aspects. First, it can protect the host from unwanted immune responses. In line with this, failure of TCR threshold calibration has been reported in several mouse models of autoimmunity and plays a role in rheumatoid arthritis (141). Secondly, it can limit homeostatic clonal expansion, which is prevalent in older adults both in peripheral blood and in tissues (23, 36, 142). Lastly, weak TCR signals intrinsically favor long-term memory development (143), thereby potentially compensating for the defects of naïve T cells from older individuals in memory formation, such as by dysregulated miR-21 (127). Of note, this feature of TCR signal calibration has recently been adopted to generate long-term memory CAR T cells targeting B cell lymphoma (144). Despite the aforementioned benefits, blunted TCR signaling in aged T cells certainly compromises the effectiveness in host defense against infection. However, the model that aging attenuates responsiveness is not correct in this simplicity. For example, CD25 is upregulated in aged naïve T cells (145). Costimulation from upregulated IL-2 signaling after TCR stimulation induces an augmented response of transcription factor networks in spite of reduced TCR signaling (Zhang and Goronzy 2020, unpublished), These observations provide an elegant example of how T cells maintain the balance of tolerance and immunity in adaptation to the aging host environment.
T cell responsiveness to cytokines generally declines with age, particularly the response of both CD4+ and CD8+ T cells to IFN-α and IL-6 (146–148). The mechanisms underlying the hyporesponsiveness are largely unknown and may include the activation of negative regulatory pathways due to the inflammatory environment. In case of activated CD4+ T cells, SHP-1 is retained in the IFN-α receptor signaling complex, which potentially dephosphorylates STAT1 and STAT5 (Figure 3) (146). Taken together, curtailing signaling to stimuli is a general strategy for T cells to adapt to the aging host environment, which may be a double‐edged sword, resulting in less coordinated adaptive immune responses to acute infection.
Age-related changes in mitochondrial function – a checkpoint for metabolic interventions.
Mitochondrial dysfunction is considered a driver of aging in various organ systems. Age-related metabolic changes in T cells have been implicated in defective proliferative responses as well as contributors to inflammaging. However, a unifying concept of the findings has so far been elusive, likely also because of the complexity of the metabolic regulation. The metabolic system undergoes extensive rewiring upon normal T cell activation and differentiation, complicating the interpretation of data on aged T cells generated under different conditions. Upon activation, T cells change their metabolic program. They upregulate glucose and one-carbon metabolism to provide precursor molecules enabling cell building programs (149). Conversely, memory cell differentiation depends on the reinstitution of preferential mitochondrial respiration. Mitochondrial mass is higher in memory cells than naïve cells. Against this background, results from aging studies have to be interpreted.
Bektas et al. found increased proteins of the electron transport chain, but reduced respiratory activity in total CD4+ T cells from old human adults (150). Based on an increase in autophagosomes, in part engulfing mitochondrial fragments, the authors postulated a distal autophagic defect that result in accumulation of dysfunctional mitochondria and ROS production. Increased ROS may explain the increased NF-κB activity that was previously described by the same authors and could contribute to inflammaging (151). In contrast, Bharath et al. examined total CD4+ T cells from young and around 60 year-old adults 40 hours after CD3/CD28 stimulation, i.e., at a time point when activated T cells have expanded their mitochondria and are switching to a more anabolic state to be able to proliferate. These authors found an increase in mitochondrial mass associated with increased respiratory activity and increased ROS production in older adults (152). This increase was associated with reduced autophagy and increased production of cytokines including IL-17 that could contribute to inflammaging. Both, autophagy and IL-17 production were corrected by metformin, although T cells in general lack the organic cation transporters OCT1 and OCT3 that are needed to take up metformin as a hydrophilic cation (153). A similar increase in activation-induced oxygen consumption and ROS production was observed for activated CD4+ memory T cells from older adults by Yanes et al. Increased mitochondrial activity was functional, as demonstrated by increased ATP production (154). In contrast, in patients with rheumatoid arthritis, who have accelerated T cell aging, mitochondrial respiration was reduced in five-day activated CD4+ naïve T cells, while the pentose phosphate pathway (PPP) was enhanced, poising the cell to produce excessive TH1 as well as TH17 cytokines upon restimulation (155, 156). In this setting, metformin was ineffective (157). Finally, in old mice, activation of T cells induced mitochondrial biogenesis, but yielded smaller mitochondria with lower respiratory capacity compared to young animals (158). Here, glycolysis was also decreased, and carbon intermediates from the PPP as well as the tricarbon acid cycle were reduced. Replenishing one-carbon metabolites improved the function of aged T cells. Taken together these data illustrate the complexity of metabolic networks, but also the potential of metabolic intervention in T cell aging and inflammaging.
Differentiation skewed towards effector function – Costs and benefits
Asymmetric division during differentiation enables a stem cell to simultaneously self-renew and execute a particular cellular function; this ability is gradually lost with age in HSCs (159, 160). Naïve T cells, similar to HSCs, exhibit stem cell features by maintaining homeostasis with symmetric division and acquiring effector functions vs developing into long-lived memory cells with asymmetric division (161, 162). Reminiscent of the aging HSC compartment, naïve T cells in older individuals show reduced capacity of asymmetric division upon TCR stimulation by preferentially differentiating into effector T cells coupled with compromised memory T cell formation (Figure 4).
Mechanistically, shifted metabolic programs, redistribution of cellular organelles, and skewed expression of lineage-specifying transcription factors collectively drive the aged naïve T cells towards an effector-like state after activation (163).The mammalian target of rapamycin (mTOR) is a metabolic rheostat that regulates effector vs. memory T cell fate decisions during infection (164, 165). miR-21 was shown to be upregulated in old naïve CD4+ T cells after activation, sustaining mTORC1 activation and thus inhibiting memory T cell development (127). Sustained signaling favors the upregulation of the transcription factor BLIMP1, which cross-regulates BCL6. The loss of BCL6 releases the expression of the ecto-NTPDase CD39, which is increased in T cells from old adults after activation (71). CD39, through its ATPase activity, inhibits memory and follicular helper T (TFH) cell differentiation through the generation of adenosine and activation of the cAMP/PKA pathway (72). Moreover, altered transcription factor networks in aged naïve T cells after activation include enhanced BATF and IRF4 and reduced ID3 and BCL6 expression, biasing the differentiation potential of old naïve T cells, for example to the TH9 lineage (166). Memory T cells can also switch to an effector state with age through metabolic reprogramming. Terminally differentiated CD8+C28- T cells drastically expand with age (67) and show enhanced cytotoxic functions through upregulated glycolytic capacity due to loss of SIRT1 (167). Another feature that routes old naïve T cells to a more effector-like state after activation is the skewed distribution of cellular organelles, manifested as downregulated lysosome biogenesis coupled with expansion of multivesicular bodies due to a failure to restore FOXO1 expression (168).
Unlike aged naïve CD8+ T cells, which exhibit more differentiated effector states already at resting state (106), naïve CD4+ T cells from old adults are prone to acquire an effector-like state only after activation. Several lines of evidence suggest that the age-associated propensity to effector differentiation is cell intrinsic in both naïve CD4+ and CD8+ T cells, reflecting increased heterogeneity of the naïve T cell compartment with age. The high-affinity IL-2 receptor α subunit CD25 is upregulated in naïve CD4+ T cells with age (145). Considering the critical role of IL-2 in balancing effector and memory cell differentiation (169), the increased CD25 expression contributes to the differentiation bias of naïve CD4+ T cells from old adults. TCF1, a master transcription factor maintaining stem-like properties in both naïve and memory T cells, is reduced in naïve CD4+ T cells from older adults (170); moreover, the activation-induced decline of TCF1 is more sustained, which probably tempers their potential to develop into memory T cells (127, 171). Thus, the naïve T cells generated at older ages may constitute another layer of the naïve T cell compartment, reminiscent of the neonatal layer. Davenport, Smith and Rudd proposed that in early life, the T cell system is poised to exhibit effector function to fight the infections with pathogens encountered for the first time (172). In contrast, it is more important throughout adult life to develop long-lived memory cells rather than short-lived effector T cells upon pathogen encounter. It is tempting to extend this interpretation to the later stages of life, when the function of the immune system is compromised and effector functions are getting again more important than generation of immune memory. In this model, the favored differentiation into short-lived effector T cells, often combined with the acquisition of innate immune functions (133, 136) could be interpreted as a successful adaptation to changing needs.
Conclusion
Major inroads have been made to understand the complex organization regulating cell replenishment and homeostasis that T cells rely on. Unfortunately, rodent model systems are quite different in how a T cell population is maintained; research therefore has to rely on human studies and computational simulations. With few exceptions, in particular the frequency of naïve CD8+ T cells, the human T cell system has been shown to be relatively robust to agng, although changes are clearly evident (Table 1); however, studies so far have mostly relied on healthy elderly and the conclusions may be very different in frail adults. Strategies aiming at rebuilding a diverse T cell repertoire had very limited success; interventions therefore have to be preventive, leaning on insights on how the T cell system resist aging-related changes in some healthy elderly. Fortunately, the TCR diversity is enormous and it may be more important keeping up total lymphocyte numbers and preventing clonal expansion, rather than generating T cells with novel TCRs.
Table 1.
Hallmarks of human T cell aging
| Findings | Mechanisms | Functional Consequences | Ref | |
|---|---|---|---|---|
| Failure in T cell maintenance | Decline in T cell numbers | Reduced T cell generation | Reduced T cell responses | (12, 13) |
| Loss in T cell niches | ||||
| Decline in naïve T cell diversity | Thymic involution | Contracted antigen-specific TCR repertoire in a T cell response | (15, 16, 36) | |
| Uneven homeostatic proliferation | ||||
| Preferential loss in CD8+ naïve T cells | Unknown | Reduced generation of cytotoxic T cells to new antigenic encounters | (20) | |
| Expansion of TEMRA (mostly CD8+ T cells) | Mostly chronic viral infection (CMV), autoreactive clonal expansion | Chronic inflammatory responses | (19, 67) | |
| Virtual memory cells | Homeostatic proliferation | Cellular dysfunction | (62, 63) | |
| Failure in DNA integrity | Impaired DNA repair | Loss of ATM/MRE11, gain in DNA-PKcs | Increased inflammatory responses in rheumatoid arthritis | (93, 95) |
| Telomeric erosion | History of replication | Replicative stress, reduced T cell survival | (94, 97) | |
| Loss of MRE11 | ||||
| Dysregulated histone expression | Increased SIRT1 expression | Replicative stress, ATR activation, p21 expression | (97) | |
| Somatic mutations | Clonal hematopoiesis (stem cells) | Increase in TCR clonality | (80) | |
| Epigenetic changes | Chromatin accessibility patterns indicating differentiation | Failure to maintain quiescence | Increased effector cell generation | (29, 106) |
| DNA methylation patterns | Unknown | Altered gene expression | (112, 116) | |
| Histone modification patterns | Unknown | (120) | ||
| p16 expression (TEMRA) | Cellular senescence | Cell cycle block, inflammatory mediators | (101) | |
| Cell surface molecules | Reduced IL-7R | Reduced chromatin accessibility (CD8+ T cells) | Reduced survival | (29) |
| Increased CD39 | Reduced BCL6 activity, increased RUNX3 | Increased adenosine signaling, reduced TFH generation | (71, 72) | |
| Loss of CD28 | Gain in sestrins | Reduced costimulation | (135) | |
| Expression of NK cell-related receptors (KIR, ILT, etc), mostly on TEMRAs | Gain in sestrins | Upregulated negative regulatory signals on T cell proliferation and function | (136) | |
| Signaling | Blunted TCR signaling | Reduced miR-181a, increased DUSP6 | Increased threshold of T cell activation | (124, 125) |
| Sustained mTORC activity | Increased miR-21 suppressing negative regulators, increased mTORC activation at expanded multivesicular bodies | Preferred effector and reduced TFH and memory cell differentiation | (127, 168) | |
| Reduced type I IFN signaling | Increased recruitment of SHP-1 to receptor complex | Reduced T cell retention due to shorter CD69 expression | (146) | |
| Metabolism | Increased ROS production | Mitochondrial dysfunction, reduced autophagy | Increased production of IL-17 and NF-kB-dependent mediators | (152) |
| Defective one-carbon metabolism (mice) | Mitochondrial dysfunction | Reduced proliferation | (158) | |
| Lysosome dysfunction, expansion of multivesicular bodies | Repressed FOXO1 activity, increased mTORC activity | Exosome secretion, generation of inflammatory effector cells | (168) |
Genomic and cellular pathways implicated in the general aging process are highly pertinent for the T cell system, and age-associated changes are observed at all different levels (Table 1). It is striking that many of these changes are reminiscent of physiological pathways that are activated during normal development or activation. Typical senescence or exhaustion features are not the norm for T cell aging. Again, one limitation is that many of these studies have been done in relatively healthy elderly who in general are also clinically more immunocompetent. Nevertheless, age-associated changes in these adults are clearly functionally important; it frequently depends on the setting whether they are useful adaptations or whether they are inappropriate deviations. E.g., an already more differentiated naïve T cell, poised to develop into an effector T cell, may be useful in an acute infection but would also take away from the plasticity that is seen in a primary response and would not be useful for a vaccine response geared at inducing memory or protective antibodies. The acquisition of innate function of end-differentiated T cells will allow them to sense the environment independent of the inciting antigen, but also impose a lack of selectivity and the triggering of non-specific inflammation. This fall back on evolutionarily more primitive systems is reminiscent of the antagonistic pleiotropy hypothesis. If these age-associated changes are useful adaptations, they should not universally be prevented, but their molecular characterization will allow the design of interventions that are tailored to the particular setting. For example, in the case of vaccination it can be useful to inhibit effector cell generation to favor memory cell generation.
Acknowledgements
This work was supported by the National Institutes of Health (R01 AR042527, R01 HL117913, R01 AI108906, R01 HL142068, and P01 HL129941 to C.M.W and R01 AI108891, R01 AG045779, U19 AI057266, and R01 AI129191 to J.J.G).
Abbreviations
- TCRT
cell receptor
- SLT
secondary lymphoid tissue
- FRC
fibroblastic reticular cell
- TEMRA
terminally differentiated effector T cell
- TRM
tissue-resident memory T cell
- CH
clonal hematopoiesis
- CHIP
clonal hematopoiesis of indeterminate potential
- NK
natural killer
- CAR
chimeric antigen receptor
- PPP
pentose phosphate pathway
- HSC
hematopoietic stem cell
- mTOR
mammalian target of rapamycin
- TFH
follicular helper T
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Goronzy JJ and Weyand CM (2017) Successful and Maladaptive T Cell Aging. Immunity 46, 364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallapaty S. (2020) The coronavirus is most deadly if you are older and male - new data reveal the risks. Nature 585, 16–17 [DOI] [PubMed] [Google Scholar]
- 3.Gustafson CE, Kim C, Weyand CM, and Goronzy JJ (2020) Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol 145, 1309–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seasonal Influenza Vaccine Effectiveness, 2017-2018. | CDC. [Google Scholar]
- 5.Levin MJ and Weinberg A. (2019) Immune responses to zoster vaccines. Hum. Vaccin. Immunother 15, 772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, and Haynes L. (2009) T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 30, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes L. and Swain SL (2012) Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin. Immunol 24, 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DR, de Boer RJ, Wolthers KC, and Miedema F. (1999) T cell dynamics in HIV-1 infection. Adv Immunol 73, 301–327 [DOI] [PubMed] [Google Scholar]
- 9.Clark BL and Thomas PG (2020) A Cell for the Ages: Human γδ T Cells across the Lifespan. Int. J. Mol. Sci 21, 8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, Lau ZWX, Fulop T, and Larbi A. (2020) The Aging of γδ T Cells. Cells 9, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon B. and Yates AJ (2018) The natural history of naive T cells from birth to maturity. Immunol Rev 285, 218–232 [DOI] [PubMed] [Google Scholar]
- 12.Bains I, Antia R, Callard R, and Yates AJ (2009) Quantifying the development of the peripheral naive CD4+ T-cell pool in humans. Blood 113, 5480–5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, Bregje de Boer A, Willems N, Schrijver EHR, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AFC, Ackermans MT, Miedema F, Borghans JAM, de Boer RJ, and Tesselaar K. (2012) Maintenance of Peripheral Naive T Cells Is Sustained by Thymus Output in Mice but Not Humans. Immunity 36, 288–297 [DOI] [PubMed] [Google Scholar]
- 14.Westera L, van Hoeven V, Drylewicz J, Spierenburg G, van Velzen JF, de Boer RJ, Tesselaar K, and Borghans JAM (2015) Lymphocyte maintenance during healthy aging requires no substantial alterations in cellular turnover. Aging Cell 14, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, and Goronzy JJ (2005) The influence of age on T cell generation and TCR diversity. J Immunol 174, 7446–7452 [DOI] [PubMed] [Google Scholar]
- 16.Sauce D, Larsen M, Fastenackels S, Roux A, Gorochov G, Katlama C, Sidi D, Sibony-Prat J, and Appay V. (2012) Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol 189, 5541–5548 [DOI] [PubMed] [Google Scholar]
- 17.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, and Nikolich-Zugich J. (2007) Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A 104, 19960–19965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, and Goronzy JJ (2008) T cell subset-specific susceptibility to aging. Clin. Immunol 127, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Žugich D, Kaye J, and Nikolich-Žugich J. (2014) Aging and Cytomegalovirus Infection Differentially and Jointly Affect Distinct Circulating T Cell Subsets in Humans. J. Immunol 192, 2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiting CC, Siebert J, Newman AM, Du HW, Alizadeh AA, Goronzy J, Weyand CM, Krishnan E, Fathman CG, and Maecker HT (2015) Large-Scale and Comprehensive Immune Profiling and Functional Analysis of Normal Human Aging. PLoS One 10, e0133627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, and Luther SA (2007) Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 8, 1255–1265 [DOI] [PubMed] [Google Scholar]
- 22.Fletcher AL, Acton SE, and Knoblich K. (2015) Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol 15, 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thome JJC, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, Sempowski GD, Shen Y, and Farber DL (2016) Long-term maintenance of human naïve T cells through in situ homeostasis in lymphoid tissue sites. Sci. Immunol 1, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masters AR, Haynes L, Su DM, and Palmer DB (2017) Immune senescence: significance of the stromal microenvironment. Clin Exp Immunol 187, 6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masters AR, Hall A, Bartley JM, Keilich SR, Lorenzo EC, Jellison ER, Puddington L, and Haynes L. (2019) Assessment of Lymph Node Stromal Cells as an Underlying Factor in Age-Related Immune Impairment. J Gerontol A Biol Sci Med Sci 74, 1734–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson HL, Smithey MJ, Uhrlaub JL, Jeftic I, Jergovic M, White SE, Currier N, Lang AM, Okoye A, Park B, Picker LJ, Surh CD, and Nikolich-Zugich J. (2019) Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates. Aging Cell 18, e12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becklund BR, Purton JF, Ramsey C, Favre S, Vogt TK, Martin CE, Spasova DS, Sarkisyan G, LeRoy E, Tan JT, Wahlus H, Bondi-Boyd B, Luther SA, and Surh CD (2016) The aged lymphoid tissue environment fails to support naive T cell homeostasis. Sci. Rep 6, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kityo C, Makamdop KN, Rothenberger M, Chipman JG, Hoskuldsson T, Beilman GJ, Grzywacz B, Mugyenyi P, Ssali F, Akondy RS, Anderson J, Schmidt TE, Reimann T, Callisto SP, Schoephoerster J, Schuster J, Muloma P, Ssengendo P, Moysi E, Petrovas C, Lanciotti R, Zhang L, Arevalo MT, Rodriguez B, Ross TM, Trautmann L, Sekaly RP, Lederman MM, Koup RA, Ahmed R, Reilly C, Douek DC, and Schacker TW (2018) Lymphoid tissue fibrosis is associated with impaired vaccine responses. J Clin Invest 128, 2763–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ucar D, Márquez EJ, Chung CH, Marches R, Rossi RJ, Uyar A, Wu TC, George J, Stitzel ML, Karolina Palucka A, Kuchel GA, and Banchereau J. (2017) The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med 214, 3123–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafson CE, Cavanagh MM, Jin J, Weyand CM, and Goronzy JJ (2019) Functional pathways regulated by microRNA networks in CD8 T-cell aging. Aging Cell 18, e12879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, Le Saux S, Gustafson CE, Ye Z, Jadhav RR, Li X, Tian L, Weyand CM, and Goronzy J. (2020) Distinct age-related epigenetic signatures in CD4 and CD8 T cells. Front. Immunol 11, 2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason D. (1998) A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today 19, 395–404 [DOI] [PubMed] [Google Scholar]
- 33.Sewell AK (2012) Why must T cells be cross-reactive? Nat Rev Immunol 12, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, and Kourilsky P. (1999) A direct estimate of the human alphabeta T cell receptor diversity. Science (80-. ). 286, 958–961 [DOI] [PubMed] [Google Scholar]
- 35.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, and Carlson CS (2009) Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 114, 4099–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, and Goronzy JJ (2014) Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. U. S. A 111, 13139–13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishna C, Chowell D, Gönen M, Elhanati Y, and Chan TA (2020) Genetic and environmental determinants of human TCR repertoire diversity. Immun. Ageing 17, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson PL, Yates AJ, Goronzy JJ, and Antia R. (2012) Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc Natl Acad Sci U S A 109, 21432–21437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Greef PC, Oakes T, Gerritsen B, Ismail M, Heather JM, Hermsen R, Chain B, and de Boer RJ (2020) The naive T-cell receptor repertoire has an extremely broad distribution of clone sizes. Elife 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, Alexe G, Nagar S, McCausland MM, Gupta S, Tata P, Haining WN, McElrath MJ, Zhang D, Hu B, Greenleaf WJ, Goronzy JJ, Mulligan MJ, Hellerstein M, and Ahmed R. (2017) Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552, 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim C, Jadhav RR, Gustafson CE, Smithey MJ, Hirsch AJ, Uhrlaub JL, Hildebrand WH, Nikolich-Žugich J, Weyand CM, and Goronzy JJ (2019) Defects in Antiviral T Cell Responses Inflicted by Aging-Associated miR-181a Deficiency. Cell Rep. 29, 2202–2216.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goronzy JJ and Weyand CM (2012) Immune aging and autoimmunity. Cell. Mol. Life Sci 69, 1615–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alho AC, Kim HT, Chammas MJ, Reynolds CG, Matos TR, Forcade E, Whangbo J, Nikiforow S, Cutler CS, Koreth J, Ho VT, Armand P, Antin JH, Alyea EP, Lacerda JF, Soiffer RJ, and Ritz J. (2016) Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood 127, 646–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jendro MC, Ganten T, Matteson EL, Weyand CM, and Goronzy JJ (1995) Emergence of oligoclonal T cell populations following therapeutic T cell depletion in rheumatoid arthritis. Arthritis Rheum 38, 1242–1251 [DOI] [PubMed] [Google Scholar]
- 45.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, and Surh CD (2002) Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med 195, 1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ernst B, Lee DS, Chang JM, Sprent J, and Surh CD (1999) The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 [DOI] [PubMed] [Google Scholar]
- 47.Quinn KM, Fox A, Harland KL, Russ BE, Li J, Nguyen THO, Loh L, Olshanksy M, Naeem H, Tsyganov K, Wiede F, Webster R, Blyth C, Sng XYX, Tiganis T, Powell D, Doherty PC, Turner SJ, Kedzierska K, and La Gruta NL (2018) Age-Related Decline in Primary CD8+ T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8+ T Cells. Cell Rep. 23, 3512–3524 [DOI] [PubMed] [Google Scholar]
- 48.Gustafson CE, Jadhav R, Cao W, Qi Q, Pegram M, Tian L, Weyand CM, and Goronzy JJ (2020) Immune cell repertoires in breast cancer patients after adjuvant chemotherapy. JCI Insight 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyand CM, Schonberger J, Oppitz U, Hunder NN, Hicok KC, and Goronzy JJ (1994) Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med 179, 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Broek T, Borghans JAM, and van Wijk F. (2018) The full spectrum of human naive T cells. Nat Rev Immunol 18, 363–373 [DOI] [PubMed] [Google Scholar]
- 51.Tanno H, Gould TM, McDaniel JR, Cao W, Tanno Y, Durrett RE, Park D, Cate SJ, Hildebrand WH, Dekker CL, Tian L, Weyand CM, Georgiou G, and Goronzy JJ (2020) Determinants governing T cell receptor alpha/beta-chain pairing in repertoire formation of identical twins. Proc Natl Acad Sci U S A 117, 532–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaimann MU, Nguyen M, Desponds J, and Mayer A. (2020) Early life imprints the hierarchy of T cell clone size. arXiv 2007, 11113v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, and Rudd BD (2014) Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol 193, 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M, and Farber DL (2017) Reduced generation of lung tissue-resident memory T cells during infancy. J Exp Med 214, 2915–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudd BD, Venturi V, Smith NL, Nzingha K, Goldberg EL, Li G, Nikolich-Zugich J, and Davenport MP (2013) Acute neonatal infections “lock-in” a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog 9, e1003572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohler S. and Thiel A. (2009) Life after the thymus: CD31+ and CD31 human naive CD4+ T-cell subsets. Blood 113, 769–774 [DOI] [PubMed] [Google Scholar]
- 57.Crotty S. and Ahmed R. (2004) Immunological memory in humans. Semin. Immunol 16, 197–203 [DOI] [PubMed] [Google Scholar]
- 58.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, and Slifka MK (2003) Duration of antiviral immunity after smallpox vaccination. Nat Med 9, 1131–1137 [DOI] [PubMed] [Google Scholar]
- 59.Britanova OV, Shugay M, Merzlyak EM, Staroverov DB, Putintseva EV, Turchaninova MA, Mamedov IZ, Pogorelyy MV, Bolotin DA, Izraelson M, Davydov AN, Egorov ES, Kasatskaya SA, Rebrikov DV, Lukyanov S, and Chudakov DM (2016) Dynamics of Individual T Cell Repertoires: From Cord Blood to Centenarians. J Immunol 196, 5005–5013 [DOI] [PubMed] [Google Scholar]
- 60.Borghans JAM, Tesselaar K, and de Boer RJ (2018) Current best estimates for the average lifespans of mouse and human leukocytes: reviewing two decades of deuterium-labeling experiments. Immunol. Rev 285, 233–248 [DOI] [PubMed] [Google Scholar]
- 61.del Amo PC, Beneytez JL, Boelen L, Ahmed R, Miners KL, Zhang Y, Roger L, Jones RE, Marraco SAF, Speiser DE, Baird DM, Price DA, Ladell K, Macallan D, and Asquith B. (2018) Human T SCM cell dynamics in vivo are compatible with long-lived immunological memory and stemness. PLoS Biol. 16, e2005523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprent J. and Surh CD (2011) Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol 12, 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu BC, Martin BE, Stolberg VR, and Chensue SW (2013) Cutting edge: Central memory CD8 T cells in aged mice are virtual memory cells. J Immunol 191, 5793–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jameson SC and Masopust D. (2009) Diversity in T cell memory: an embarrassment of riches. Immunity 31, 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jameson SC and Masopust D. (2018) Understanding Subset Diversity in T Cell Memory. Immunity 48, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sallusto F, Geginat J, and Lanzavecchia A. (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22, 745–763 [DOI] [PubMed] [Google Scholar]
- 67.Weng N. ping, Akbar AN, and Goronzy J. (2009) CD28- T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, and Koup RA (2003) Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 [DOI] [PubMed] [Google Scholar]
- 69.Pera A, Vasudev A, Tan C, Kared H, Solana R, and Larbi A. (2017) CMV induces expansion of highly polyfunctional CD4 + T cell subset coexpressing CD57 and CD154. J. Leukoc. Biol 101, 555–566 [DOI] [PubMed] [Google Scholar]
- 70.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, and Pawelec G. (2008) Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang F, Yu M, Cavanagh MM, Hutter Saunders J, Qi Q, Ye Z, Le Saux S, Sultan W, Turgano E, Dekker CL, Tian L, Weyand CM, and Goronzy JJ (2016) Expression of CD39 on Activated T Cells Impairs their Survival in Older Individuals. Cell Rep. 14, 1218–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao W, Fang F, Gould T, Li X, Kim C, Gustafson C, Lambert S, Weyand CM, and Goronzy JJ (2020) Ecto-NTPDase CD39 is a negative checkpoint that inhibits follicular helper cell generation. J Clin Invest 130, 3422–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schenkel JM and Masopust D. (2014) Tissue-resident memory T cells. Immunity 41, 886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mueller SN and Mackay LK (2016) Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16, 79–89 [DOI] [PubMed] [Google Scholar]
- 75.Fang F, Cao W, Zhu W, Lam N, Li L, Gaddam S, Wang Y, Kim C, Lambert S, Zhang H, Hu B, Farber DL, Weyand CM, and Goronzy JJ (2021) Influence of age on functional memory T cell diversity. Submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, Rabani H, Starosvetsky E, Kveler K, Schaffert S, Furman D, Caspi O, Rosenschein U, Khatri P, Dekker CL, Maecker HT, Davis MM, and Shen-Orr SS (2019) A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med 25, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G. (2013) The hallmarks of aging. Cell 153, 1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blokzijl F, De Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, Huch M, Boymans S, Kuijk E, Prins P, Nijman IJ, Martincorena I, Mokry M, Wiegerinck CL, Middendorp S, Sato T, Schwank G, Nieuwenhuis EES, Verstegen MMA, Van Der Laan LJW, De Jonge J, Ijzermans JNM, Vries RG, Van De Wetering M, Stratton MR, Clevers H, Cuppen E, and Van Boxtel R. (2016) Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoang ML, Kinde I, Tomasetti C, McMahon KW, Rosenquist TA, Grollman AP, Kinzler KW, Vogelstein B, and Papadopoulos N. (2016) Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing. Proc. Natl. Acad. Sci. U. S. A 113, 9846–9851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaiswal S. and Ebert BL (2019) Clonal hematopoiesis in human aging and disease. Science (80-. ). 366, eaan4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, and Ebert BL (2014) Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med 371, 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J, Jonasson JG, Tryggvadottir L, Jonsson T, Helgason A, Gylfason A, Sulem P, Rafnar T, Thorsteinsdottir U, Gudbjartsson DF, Masson G, Kong A, and Stefansson K. (2017) Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quivoron C, Couronné L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern MH, Godley L, Opolon P, Tilly H, Solary E, Duffourd Y, Dessen P, Merle-Beral H, Nguyen-Khac F, Fontenay M, Vainchenker W, Bastard C, Mercher T, and Bernard OA (2011) TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer Cell 20, 25–38 [DOI] [PubMed] [Google Scholar]
- 84.Couronné L, Bastard C, and Bernard OA (2012) TET2 and DNMT3A mutations in human T-Cell lymphoma. N. Engl. J. Med 366, 95–96 [DOI] [PubMed] [Google Scholar]
- 85.Izzo F, Lee SC, Poran A, Chaligne R, Gaiti F, Gross B, Murali RR, Deochand SD, Ang C, Jones PW, Nam AS, Kim KT, Kothen-Hill S, Schulman RC, Ki M, Lhoumaud P, Skok JA, Viny AD, Levine RL, Kenigsberg E, Abdel-Wahab O, and Landau DA (2020) DNA methylation disruption reshapes the hematopoietic differentiation landscape. Nat. Genet 52, 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, Cogdill AP, Morrissette JJD, DeNizio JE, Reddy S, Hwang Y, Gohil M, Kulikovskaya I, Nazimuddin F, Gupta M, Chen F, Everett JK, Alexander KA, Lin-Shiao E, Gee MH, Liu X, Young RM, Ambrose D, Wang Y, Xu J, Jordan MS, Marcucci KT, Levine BL, Garcia KC, Zhao Y, Kalos M, Porter DL, Kohli RM, Lacey SF, Berger SL, Bushman FD, June CH, and Melenhorst JJ (2018) Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young AL, Challen GA, Birmann BM, and Druley TE (2016) Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee-Six H, Øbro NF, Shepherd MS, Grossmann S, Dawson K, Belmonte M, Osborne RJ, Huntly BJP, Martincorena I, Anderson E, O’Neill L, Stratton MR, Laurenti E, Green AR, Kent DG, and Campbell PJ (2018) Population dynamics of normal human blood inferred from somatic mutations. Nature 561, 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Savola P, Martelius T, Kankainen M, Huuhtanen J, Lundgren S, Koski Y, Eldfors S, Kelkka T, Keränen MAI, Ellonen P, Kovanen PE, Kytölä S, Saarela J, Lähdesmäki H, Seppänen MRJ, and Mustjoki S. (2019) Somatic mutations and T-cell clonality in patients with immunodeficiency. Haematologica haematol.2019.220889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koskela HLM, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ, Olson T, Jalkanen SE, Majumder MM, Almusa H, Edgren H, Lepistö M, Mattila P, Guinta K, Koistinen P, Kuittinen T, Penttinen K, Parsons A, Knowles J, Saarela J, Wennerberg K, Kallioniemi O, Porkka K, Loughran TP, Heckman CA, Maciejewski JP, and Mustjoki S. (2012) Somatic STAT3 Mutations in Large Granular Lymphocytic Leukemia. N. Engl. J. Med 366, 1905–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savola P, Kelkka T, Rajala HL, Kuuliala A, Kuuliala K, Eldfors S, Ellonen P, Lagström S, Lepistö M, Hannunen T, Andersson EI, Khajuria RK, Jaatinen T, Koivuniemi R, Repo H, Saarela J, Porkka K, Leirisalo-Repo M, and Mustjoki S. (2017) Somatic mutations in clonally expanded cytotoxic T lymphocytes in patients with newly diagnosed rheumatoid arthritis. Nat. Commun 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valori M, Jansson L, Kiviharju A, Ellonen P, Rajala H, Awad SA, Mustjoki S, and Tienari PJ (2017) A novel class of somatic mutations in blood detected preferentially in CD8 + cells. Clin. Immunol 175, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, and Weyand CM (2009) Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med 206, 1435–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Shen Y, Hohensinner P, Ju J, Wen Z, Goodman SB, Zhang H, Goronzy JJ, and Weyand CM (2016) Deficient Activity of the Nuclease MRE11A Induces T Cell Aging and Promotes Arthritogenic Effector Functions in Patients with Rheumatoid Arthritis. Immunity 45, 903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shao L, Goronzy JJ, and Weyand CM (2010) DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol. Med 2, 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shrivastav M, De Haro LP, and Nickoloff JA (2008) Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–147 [DOI] [PubMed] [Google Scholar]
- 97.Kim C, Jin J, Ye Z, Jadhav RR, Gustafson CE, Hu B, Cao W, Weiskopf D, Grifoni A, Sette A, and Weyand CM (2020) Histone loss and replication stress in the age-related decline of T cell immunity. Submitted. [Google Scholar]
- 98.Zhang L, Dong X, Lee M, Maslov AY, Wang T, and Vijg J. (2019) Single-cell whole-genome sequencing reveals the functional landscape of somatic mutations in B lymphocytes across the human lifespan. Proc. Natl. Acad. Sci. U. S. A 116, 9014–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blackburn EH, Greider CW, and Szostak JW (2006) Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med 12, 1133–1138 [DOI] [PubMed] [Google Scholar]
- 100.Weng NP, Levine BL, June CH, and Hodes RJ (1995) Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc. Natl. Acad. Sci. U. S. A 92, 11091–11094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akbar AN, Beverley PCL, and Salmon M. (2004) Will telomere erosion lead to a loss of T-cell memory? Nat. Rev. Immunol 4, 737–743 [DOI] [PubMed] [Google Scholar]
- 102.Wagner CL, Hanumanthu VS, Conover Talbot C, Abraham RS, Hamm D, Gable DL, Kanakry CG, Applegate CD, Siliciano J, Brooks Jackson J, Desiderio S, Alder JK, Luznik L, and Armanios M. (2018) Short telomere syndromes cause a primary T cell immunodeficiency. J. Clin. Invest 128, 5222–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qi Q, Cavanagh MM, Le Saux S, Wagar LE, Mackey S, Hu J, Maecker H, Swan GE, Davis MM, Dekker CL, Tian L, Weyand CM, and Goronzy JJ (2016) Defective T Memory Cell Differentiation after Varicella Zoster Vaccination in Older Individuals. PLOS Pathog. 12, e1005892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujii H, Shao L, Colmegna I, Goronzy JJ, and Yand CM (2009) Telomerase insufficiency in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A 106, 4360–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sen P, Shah PP, Nativio R, and Berger SL (2016) Epigenetic Mechanisms of Longevity and Aging. Cell 166, 822–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moskowitz DM, Zhang DW, Hu B, Le Saux S, Yanes RE, Ye Z, Buenrostro JD, Weyand CM, Greenleaf WJ, and Goronzy JJ (2017) Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krishna C, Chowell D, Gönen M, Elhanati Y, and Chan TA (2020) Genetic and environmental determinants of human TCR repertoire diversity. Immun. Ageing 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda SV, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, and Zhang K. (2013) Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 49, 359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horvath S. (2013) DNA methylation age of human tissues and cell types. Genome Biol. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Wilson R, Heiss J, Breitling LP, Saum KU, Schöttker B, Holleczek B, Waldenberger M, Peters A, and Brenner H. (2017) DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun 8, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner1 AP, Aviv1 A, Lohman K, Liu Y, Ferrucci L, and Horvath S. (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany. NY). 10, 573–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reynolds LM, Taylor JR, Ding J, Lohman K, Johnson C, Siscovick D, Burke G, Post W, Shea S, Jacobs DR, Stunnenberg H, Kritchevsky SB, Hoeschele I, McCall CE, Herrington DM, Tracy RP, and Liu Y. (2014) Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat. Commun 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilson CB, Makar KW, Shnyreva M, and Fitzpatrick DR (2005) DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin. Immunol 17, 105–119 [DOI] [PubMed] [Google Scholar]
- 114.Komori HK, Hart T, LaMere SA, Chew PV, and Salomon DR (2015) Defining CD4 T Cell Memory by the Epigenetic Landscape of CpG DNA Methylation. J. Immunol 194, 1565–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, Triplett BM, Sekaly RP, and Youngblood B. (2017) Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J. Exp. Med 214, 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dozmorov MG, Coit P, Maksimowicz-McKinnon K, and Sawalha AH (2017) Age-associated DNA methylation changes in naive CD4 + T cells suggest an evolving autoimmune epigenotype in aging T cells. Epigenomics 9, 429–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martos SN, Campbell MR, Lozoya OA, Wang X, Bennett BD, Thompson IJB, Wan M, Pittman GS, and Bell DA (2020) Single-Cell Analyses Identify Dysfunctional CD16+ CD8 T Cells in Smokers. Cell Reports Med. 1, 100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ellmeier W. and Seiser C. (2018) Histone deacetylase function in CD4+ T cells. Nat. Rev. Immunol 18, 617–634 [DOI] [PubMed] [Google Scholar]
- 119.Cuddapah S, Barski A, and Zhao K. (2010) Epigenomics of T cell activation, differentiation, and memory. Curr. Opin. Immunol 22, 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheung P, Vallania F, Warsinske HC, Donato M, Schaffert S, Chang SE, Dvorak M, Dekker CL, Davis MM, Utz PJ, Khatri P, and Kuo AJ (2018) Single-Cell Chromatin Modification Profiling Reveals Increased Epigenetic Variations with Aging. Cell 173, 1385–1397.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kroesen BJ, Teteloshvili N, Smigielska-Czepiel K, Brouwer E, Boots AMH, van den Berg A, and Kluiver J. (2015) Immuno-miRs: Critical regulators of T-cell development, function and ageing. Immunology 144, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou Z, Sun B, Huang S, and Zhao L. (2019) Roles of circular RNAs in immune regulation and autoimmune diseases. Cell Death Dis. 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hackl M, Brunner S, Fortschegger K, Schreiner C, Micutkova L, Mück C, Laschober GT, Lepperdinger G, Sampson N, Berger P, Herndler-Brandstetter D, Wieser M, Kühnel H, Strasser A, Rinnerthaler M, Breitenbach M, Mildner M, Eckhart L, Tschachler E, Trost A, Bauer JW, Papak C, Trajanoski Z, Scheideler M, Grillari-Voglauer R, Grubeck-Loebenstein B, Jansen-Dürr P, and Grillari J. (2010) miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell 9, 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, and Goronzy JJ (2012) Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med 18, 1518–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye Z, Li G, Kim C, Hu B, Jadhav RR, Weyand CM, and Goronzy JJ (2018) Regulation of miR-181a expression in T cell aging. Nat. Commun 9, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brunner S, Herndler-Brandstetter D, Arnold CR, Wiegers GJ, Villunger A, Hackl M, Grillari J, Moreno-Villanueva M, Bürkle A, and Grubeck-Loebenstein B. (2012) Upregulation of miR-24 is associated with a decreased DNA damage response upon etoposide treatment in highly differentiated CD8 + T cells sensitizing them to apoptotic cell death. Aging Cell 11, 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim C, Hu B, Jadhav RR, Jin J, Zhang H, Cavanagh MM, Akondy RS, Ahmed R, Weyand CM, and Goronzy JJ (2018) Activation of miR-21-Regulated Pathways in Immune Aging Selects against Signatures Characteristic of Memory T Cells. Cell Rep. 25, 2148–2162.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smigielska-Czepiel K, van den Berg A, Jellema P, Slezak-Prochazka I, Maat H, van den Bos H, van der Lei RJ, Kluiver J, Brouwer E, Boots AMH, and Kroesen BJ (2013) Dual Role of miR-21 in CD4+ T-Cells: Activation-Induced miR-21 Supports Survival of Memory T-Cells and Regulates CCR7 Expression in Naive T-Cells. PLoS One 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Courtney AH, Lo WL, and Weiss A. (2018) TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem. Sci 43, 108–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, and Goronzy JJ (2012) Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc. Natl. Acad. Sci. U. S. A 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Michel F, Attal-Bonnefoy G, Mangino G, Mise-Omata S, and Acuto O. (2001) CD28 as a molecular amplifier extending TCR ligation and signaling capabilities. Immunity 15, 935–945 [DOI] [PubMed] [Google Scholar]
- 132.Snyder MR, Nakajima T, Leibson PJ, Weyand CM, and Goronzy JJ (2004) Stimulatory Killer Ig-Like Receptors Modulate T Cell Activation through DAP12-Dependent and DAP12-Independent Mechanisms. J. Immunol 173, 3725–3731 [DOI] [PubMed] [Google Scholar]
- 133.Warrington KJ, Takemura S, Goronzy JJ, and Weyand CM (2001) CD4+,CD28- T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 44, 13–20 [DOI] [PubMed] [Google Scholar]
- 134.Snyder MR, Lucas M, Vivier E, Weyand CM, and Goronzy JJ (2003) Selective activation of the c-Jun NH2-terminal protein Kinase signaling pathway by stimulatory KIR in the absence of KARAP/DAP12 in CD4+ T cells. J. Exp. Med 197, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lanna A, Gomes DCO, Muller-Durovic B, McDonnell T, Escors D, Gilroy DW, Lee JH, Karin M, and Akbar AN (2017) A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol 18, 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pereira BI, De Maeyer RPH, Covre LP, Nehar-Belaid D, Lanna A, Ward S, Marches R, Chambers ES, Gomes DCO, Riddell NE, Maini MK, Teixeira VH, Janes SM, Gilroy DW, Larbi A, Mabbott NA, Ucar D, Kuchel GA, Henson SM, Strid J, Lee JH, Banchereau J, and Akbar AN (2020) Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol 21, 684–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Palin AC, Ramachandran V, Acharya S, and Lewis DB (2013) Human Neonatal Naive CD4 + T Cells Have Enhanced Activation-Dependent Signaling Regulated by the MicroRNA miR-181a. J. Immunol 190, 2682–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nikolich-Žugich J, Slifka MK, and Messaoudi I. (2004) The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol 4, 123–132 [DOI] [PubMed] [Google Scholar]
- 139.Agrawal A, Tay J, Ton S, Agrawal S, and Gupta S. (2009) Increased Reactivity of Dendritic Cells from Aged Subjects to Self-Antigen, the Human DNA. J. Immunol 182, 1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clarke S. (2003) Aging as war between chemical and biochemical processes: Protein methylation and the recognition of age-damaged proteins for repair. Ageing Res. Rev 2, 263–285 [DOI] [PubMed] [Google Scholar]
- 141.Singh K, Deshpande P, Pryshchep S, Colmegna I, Liarski V, Weyand CM, and Goronzy JJ (2009) ERK-Dependent T Cell Receptor Threshold Calibration in Rheumatoid Arthritis. J. Immunol 183, 8258–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, George BM, Boutet SC, Hebestreit K, Pluvinage JV, Wyss-Coray T, Weissman IL, Vogel H, Davis MM, and Brunet A. (2019) Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Snook JP, Kim C, and Williams MA (2018) TCR signal strength controls the differentiation of CD4+ effector and memory T cells. Sci. Immunol 3, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feucht J, Sun J, Eyquem J, Ho YJ, Zhao Z, Leibold J, Dobrin A, Cabriolu A, Hamieh M, and Sadelain M. (2019) Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med 25, 82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pekalski ML, Ferreira RC, Coulson RMR, Cutler AJ, Guo H, Smyth DJ, Downes K, Dendrou CA, Castro Dopico X, Esposito L, Coleman G, Stevens HE, Nutland S, Walker NM, Guy C, Dunger DB, Wallace C, Tree TIM, Todd JA, and Wicker LS (2013) Postthymic Expansion in Human CD4 Naive T Cells Defined by Expression of Functional High-Affinity IL-2 Receptors. J. Immunol 190, 2554–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li G, Ju J, Weyand CM, and Goronzy JJ (2015) Age-Associated Failure To Adjust Type I IFN Receptor Signaling Thresholds after T Cell Activation. J. Immunol 195, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Longo DM, Louie B, Putta S, Evensen E, Ptacek J, Cordeiro J, Wang E, Pos Z, Hawtin RE, Marincola FM, and Cesano A. (2012) Single-Cell Network Profiling of Peripheral Blood Mononuclear Cells from Healthy Donors Reveals Age- and Race-Associated Differences in Immune Signaling Pathway Activation. J. Immunol 188, 1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shen-Orr SS, Furman D, Kidd BA, Hadad F, Lovelace P, Huang YW, Rosenberg-Hasson Y, Mackey S, Grisar FAG, Pickman Y, Maecker HT, Chien Y. hsiu, Dekker CL, Wu JC, Butte AJ, and Davis MM (2016) Defective Signaling in the JAK-STAT Pathway Tracks with Chronic Inflammation and Cardiovascular Risk in Aging Humans. Cell Syst. 3, 374–384.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Klein Geltink RI, Kyle RL, and Pearce EL (2018) Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu. Rev. Immunol 36, 461–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bektas A, Schurman SH, Gonzalez-Freire M, Dunn CA, Singh AK, Macian F, Cuervo AM, Sen R, and Ferrucci L. (2019) Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging (Albany. NY). 11, 9234–9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bektas A, Zhang Y, Lehmann E, Wood WH, Becker KG, Madara K, Ferrucci L, and Sen R. (2014) Age-associated changes in basal NF-κB function in human CD4+ T lymphocytes via dysregulation of PI3 kinase. Aging (Albany. NY). 6, 957–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM, Snyder-Cappione J, Hawk GS, Fleeman RM, Pihl RMF, Thompson K, Belkina AC, Cui L, Proctor EA, Kern PA, and Nikolajczyk BS (2020) Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 32, 44–55.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liang X. and Giacomini KM (2017) Transporters Involved in Metformin Pharmacokinetics and Treatment Response. J. Pharm. Sci 106, 2245–2250 [DOI] [PubMed] [Google Scholar]
- 154.Yanes RE, Zhang H, Shen Y, Weyand CM, and Goronzy JJ (2019) Metabolic reprogramming in memory CD4 T cell responses of old adults. Clin. Immunol 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weyand CM and Goronzy JJ (2020) The immunology of rheumatoid arthritis. Nat. Immunol 22, 10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weyand CM and Goronzy JJ (2020) Immunometabolism in the development of rheumatoid arthritis. Immunol. Rev 294, 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wen Z, Jin K, Shen Y, Yang Z, Li Y, Wu B, Tian L, Shoor S, Roche NE, Goronzy JJ, and Weyand CM (2019) N-myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation. Nat. Immunol 20, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ron-Harel N, Notarangelo G, Ghergurovich JM, Paulo JA, Sage PT, Santos D, Kyle Satterstrom F, Gygi SP, Rabinowitz JD, Sharpe AH, and Haigis MC (2018) Defective respiration and one-carbon metabolism contribute to impaired naïve T cell activation in aged mice. Proc. Natl. Acad. Sci. U. S. A 115, 13347–13352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morrison SJ and Kimble J. (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068–1074 [DOI] [PubMed] [Google Scholar]
- 160.Geiger H, De Haan G, and Carolina Florian M. (2013) The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol 13, 376–389 [DOI] [PubMed] [Google Scholar]
- 161.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, and Reiner SL (2007) Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science (80-. ). 315, 1687–1691 [DOI] [PubMed] [Google Scholar]
- 162.Chang JT and Reiner SL (2008) Asymmetric division and stem cell renewal without a permanent niche: Lessons from lymphocytes. Cold Spring Harb. Symp. Quant. Biol 73, 73–79 [DOI] [PubMed] [Google Scholar]
- 163.Goronzy JJ and Weyand CM (2019) Mechanisms underlying T cell ageing. Nat. Rev. Immunol 19, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chi H. (2012) Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol 12, 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, and Ahmed R. (2009) mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu B, Li G, Ye Z, Gustafson CE, Tian L, Weyand CM, and Goronzy JJ (2019) Transcription factor networks in aged naïve CD4 T cells bias lineage differentiation. Aging Cell 18, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jeng MY, Hull PA, Fei M, Kwon H-S, Tsou C-L, Kasler H, Ng C-P, Gordon DE, Johnson J, Krogan N, Verdin E, and Ott M. (2018) Metabolic reprogramming of human CD8+ memory T cells through loss of SIRT1. J. Exp. Med 215, 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jin J, Li X, Hu B, Kim C, Cao W, Zhang H, Weyand CM, and Goronzy JJ (2020) FOXO1 deficiency impairs proteostasis in aged T cells. Sci. Adv 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ross SH and Cantrell DA (2018) Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol 36, 411–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ye Z, Gould TM, Zhang H, Jin J, Weyand CM, and Goronzy JJ (2021) The GSK3β-β-catenin-TCF1 pathway improves naive T cell activation in old adults by upregulating miR-181a. npj Aging Mech. Dis 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim C, Jin J, Weyand CM, and Goronzy JJ (2020) The Transcription Factor TCF1 in T Cell Differentiation and Aging. Int. J. Mol. Sci 21, 6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davenport MP, Smith NL, and Rudd BD (2020) Building a T cell compartment: how immune cell development shapes function. Nat. Rev. Immunol 20, 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]