Abstract
Background
Previous studies have demonstrated the combination of epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) and other antitumor agents may delay drug resistance. In this study, we retrospectively reviewed the efficacy and safety of first‐line concurrent EGFR‐TKIs and platinum‐based doublet chemotherapy with or without an antiangiogenic agent for advanced lung adenocarcinoma patients in the real world.
Methods
A total of 30 patients with advanced lung adenocarcinoma and activating EGFR mutations concurrently received an EGFR‐TKI and platinum‐based doublet chemotherapy with or without bevacizumab. The safety profile and efficacy were retrospectively reviewed.
Results
At the median follow‐up time of 22.1 months, 18 patients had experienced disease progression, and six patients had died because of disease. The median progression‐free survival (mPFS) was 21.2 months (95% CI: 12.631–29.798). Of the 28 patients who had measurable lesions, the objective response rate and disease control rate were 71.4% and 96.4%, respectively (one patient achieved complete remission, 19 patients had a partial response and seven patients had stable disease). Male patients had significantly longer mPFS than female patients (32.6 vs. 14.6 months, HR = 3.593, 95% CI: 1.158–11.148, p = 0.027). The most frequently seen grade 3/4 adverse events were hematological toxicities, seen in three cases (10%). Three patients ceased bevacizumab due to vascular events, including hypertension (grade 2, 6.7%) and venous thrombosis (grade 2, 3.3%), and continued EGFR‐TKI and platinum‐based doublet chemotherapy.
Conclusions
The combination of first‐generation EGFR‐TKIs with platinum‐based chemotherapy may be a first‐line treatment for advanced lung adenocarcinoma patients harboring activated EGFR mutations and is well tolerated.
Keywords: advanced lung adenocarcinoma, concurrent therapy, epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitor (TKI), first‐line therapy, platinum‐based doublet chemotherapy
Previous studies have demonstrated that the combination of epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) and other antitumor agents may delay drug resistance. As results of phase III trial JMIT and NEJ009 indicated, the combination therapy of EGFR‐TKI and chemotherapy benefited NSCLC patients who harbored EGFR sensitive mutations as first‐line therapy. This study has provided further evidence for the efficacy and safety of concurrent use of EGFR‐TKI and platinum‐based chemotherapy as a first‐line therapy for advanced lung adenocarcinoma patients with EGFR 19 del or 21 L858R mutation in the real world. We also explored the relationship between EGFR mutation type and efficacy of the combination strategy. In conclusion, we determined that the combination strategy is promising as first‐line treatment for advanced lung adenocarcinoma patients harboring activated EGFR mutations, and is well‐tolerated.

Introduction
First‐generation oral epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs), including gefitinib, erlotinib, and icotinib, have been approved as standard first‐line therapies for non‐small cell lung cancer (NSCLC) patients harboring activating EGFR mutations, as they prolong progression‐free survival (PFS) compared to platinum‐based doublet chemotherapy according to multiple clinical studies.1, 2 Despite the related survival improvements in patients with advanced NSCLC, most develop resistance to TKIs after a median duration of 9–13 months, and about 60% of patients are found to have a p.Thr790Met (T790M) resistance mutation.3, 4 Therefore, studies are ongoing to investigate strategies, such as combining treatment with other antitumor agents to prolong the duration of response. Preclinical studies have shown that chemotherapy might have a synergistic effect with EGFR‐TKIs on the tumor growth of NSCLC in vitro.5 Clinical studies have shown controversial results, and therefore a combination strategy has not yet been proven as an effective standard first‐line treatment for advanced lung adenocarcinoma patients harboring EGFR‐activating mutations.
Most previous studies have failed to demonstrate the effect of a combination of chemotherapy and EGFR‐TKIs as first‐line treatment in unselected NSCLC patients.6, 7 However, for patients with EGFR mutations, recent studies have indicated the benefit of combining chemotherapy and EGFR‐TKIs in this population. The JMIT trial compared pemetrexed plus gefitinib versus gefitinib alone as first‐line therapy for EGFR‐mutated NSCLC, and the results showed significantly improved PFS8 and a numerically higher overall survival (OS) rate in the combination therapy group than in the TKI alone group.9 For the FASTACT‐2 study, platinum‐based chemotherapy plus first‐generation TKI erlotinib showed a benefit in terms of both PFS and OS compared to chemotherapy plus placebo, not only in stage IIIB/IV NSCLC patients with targetable EGFR mutations but also in selected patients with unknown EGFR mutations.10 Based on preclinical evidence and clinical data, it is possible that the combination therapy of chemotherapy and EGFR‐TKIs could be beneficial as first‐line therapy in advanced lung adenocarcinoma patients harboring an activating EGFR mutation.
However, the combination strategy has failed to prolong OS according to some studies. The NEJ009 study compared the efficacy of gefitinib to the concurrent strategy of gefitinib and pemetrexed plus carboplatin and found that although PFS and OS in the combination group were significantly longer than those in the monotherapy group, no significant difference was found in PFS2 considering the influence of the postprogressive disease period.11 More questions are still under investigation; for example, whether patients might benefit from maintenance treatment with chemotherapy and EGFR‐TKIs has not yet been fully studied, and whether the combination of antiangiogenic agents would increase efficacy has not yet been elucidated. In this real‐world study, we retrospectively reviewed and evaluated the effect and safety of concurrent therapy with chemotherapy and an EGFR‐TKI as first‐line therapy for patients with EGFR‐mutated advanced lung adenocarcinoma and provided more evidence of this combination regimen in clinical application.
METHODS
Patients
We retrospectively reviewed the records of patients diagnosed with advanced lung adenocarcinoma according to pathology and radiological results who received their first‐line therapy at the Cancer Hospital Chinese Academy of Medical Sciences (CAMS) from May 2014 to April 2020. All patients, including two patients who underwent palliative operation, were diagnosed by cytology or histology of at least one evaluable lesion. We collected clinicopathological features, including smoking history, family history, Eastern Cooperative Oncology Group performance status (ECOG PS), and oncogene mutation type. As EGFR‐TKIs must be administered after the gene test results are obtained, some patients received chemotherapy while the gene test was being performed. Therefore, we included patients who had concurrently received EGFR‐TKIs within four cycles of chemotherapy.
All methods were carried out in accordance with the relevant guidelines and regulations.
Clinical assessment
Radiographic assessments, including CT scans and brain MRI, were performed at baseline and after every two cycles of chemotherapy and were then performed every two to three months in the first two years and every six months thereafter until disease progression. Radiographic tests were also performed if the patient had worsening of symptoms. Further information, including survival and subsequent treatment, was collected every six months after disease progression following first‐line therapy. Response and PFS were assessed by investigators. The assessment of treatment efficacy was based on The Response Evaluation Criteria of Solid Tumors (RECIST) version 1.1.12 The tumor responses of target lesions were evaluated and categorized as complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD). The objective response rate (ORR) was defined as the sum of the CR and PR rates, and DCR was defined as the sum of the CR, PR, and SD rates. Progression‐free survival (PFS) was measured from the time of initiation of either treatment to PD or death from any cause, whichever came first, and overall survival (OS) was defined as the period from the initiation of treatment to death from any cause or the last follow‐up, and if lost to follow‐up, the case would be censored. Any adverse events (AEs) that occurred during treatment were recorded, and the grading of AEs was based on the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0). The highest toxicity grade for each patient in all cycles of chemotherapy was considered in the toxicity analysis.
Statistical analysis
The median PFS was calculated with the Kaplan–Meier product limit method. Risk factors for PFS were analyzed with the univariate Cox proportional hazards regression model. Patient clinical characteristics and responses to therapy were analyzed with descriptive methods. Continuous variables were compared using t‐tests, and categorial variables were compared using χ2 tests. All statistical analyses were performed using SPSS version 23.0, and differences with a p‐value <0.05 were considered statistically significant.
RESULTS
Patients
In total, 30 untreated patients with advanced adenocarcinoma who harbored sensitive EGFR mutations received platinum‐based doublet chemotherapy and an EGFR‐TKI concurrently as their first‐line therapy from May 2014 to April 2020. The median age of patients before therapy was 57 (25–70) years. Among them, 12 (40%) were male and 18 (60%) were female. Eight patients (26.7%) were current or former smokers, and 10 (33.3%) patients had a family history of cancer. Most patients (90%) had an excellent performance state (ECOG PS 0–1). Gene testing for tissue or pleural effusion indicated that 21 patients (70%) had EGFR exon 19 deletion and that the remaining nine patients (30%) had EGFR exon 21 deletion (L858R); 20 cases (66.7%) were tested by next‐generation sequencing (NGS) instead of amplification refractory mutation system (ARMS) real‐time polymerase chain reaction (PCR). In those 20 patients who underwent gene testing via NGS at baseline, analysis of a panel of at least 168 cancer‐related genes revealed that 19 patients had co‐occurring mutations in genes such as TP53, PIK3CA, and PTEN. Six patients (66.7%) harboring EGFR exon 21 deletion had known co‐occuring mutaions, and 13 patients (61.9%) harboring EGFR exon 19 deletion had co‐occuring mutations. Six patients (20%) were found to have brain metastases at baseline. No patient tested positive for the T790M mutation before treatment. The clinicopathological characteristics of these patients are listed in Table 1.
TABLE 1.
Clinicopathological features of all advanced lung adenocarcinoma patients harboring EGFR‐activating mutations who received first‐generation EGFR‐TKIs combined with platinum‐based doublet chemotherapy with or without an antiangiogenic agent as first‐line therapy
| Characteristics | n = 30 | Characteristics | n (%) |
|---|---|---|---|
| Age | Family history of tumor | ||
| Median | 57 | Yes | 10 (33.3) |
| Range | 25–70 | No | 20 (66.7) |
| Sex | n (%) | Baseline brain metastasis | |
| Male | 12 (40.0) | Yes | 6 (20.0) |
| Female | 18 (60.0) | No | 24 (80.0) |
| Location of primary tumor | n (%) | EGFR mutation | n (%) |
| Left | 15 (50.0) | Exon 19 deletion | 21 (70.0) |
| Right | 15 (50.0) | Exon 21 deletion (L858R) | 9 (30.0) |
| Smoking status | n (%) | Treatment strategy | n (%) |
| Never smoker | 22 (73.3) | EGFR‐TKI + bevacizumab + chemotherapy | 7 (23.3) |
| Current/former smoker | 8 (26.7) | EGFR‐TKI + chemotherapy | 23 (76.7) |
| Cycles of combination therapya | n (%) | Second‐line therapy | n (%) |
| ≤8 | 16 (53.3) | No | |
| >8 | 14 (46.7) | First‐line ongoing | 12 (40.0) |
| ECOG PS | n (%) | Death | 4 (13.3) |
| 0 | 10 (33.3) | Yes | n (%) |
| 1 | 17 (56.7) | Third‐generation EGFR‐TKI | 9 (30.0) |
| 2 | 3 (10.0) | Chemotherapy | 5 (16.7) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; n, number; TKI, tyrosine kinase inhibitors.
Since the median number of combination cycles was eight, effect in those who received more than or no less than eight cycles of combination therapy (including maintenance therapy) was compared.
Treatment
Among the 30 enrolled patients, 23 patients (76.7%) received a first‐generation EGFR‐TKI concurrently with platinum‐based doublet chemotherapy. In this group, 13 patients (56.5%) received icotinib, nine patients (39.1%) received gefitinib, and one patient (4.4%) received erlotinib. The remaining seven patients (23.3%) received a combination of EGFR‐TKIs and chemotherapy as well as bevacizumab, the antiangiogenic agent. In this group, six patients (85.7%) received icotinib and the other patient received gefitinib. Eighteen patients (60%) continued pemetrexed and EGFR‐TKIs with or without bevacizumab as maintenance therapy after six standard cycles of combination therapy, and the median number of combination therapy cycles was eight (range 2–24 cycles). As of the last day of follow‐up, 12 patients (40%) were still receiving first‐line treatment, and four patients (13.3%) had not received further treatment after disease progression. Among 18 patients who failed their first‐line treatment, eight patients received tissue or liquid biopsy and gene tests, and four patients (50.0%) were found to harbor EGFR T790M resistance mutations. Subsequent treatments are shown in Table 1.
Survival
Until January 21, 2021, the median follow‐up period was 22.1 months (range 4.6–76.2 months). Among the 30 patients enrolled, six patients (20%) died of disease, and 18 patients (60%) had disease progression after at least two cycles of concurrent chemotherapy and EGFR‐TKIs with or without bevacizumab. The median progression‐free survival (mPFS) was 21.2 months (95% CI: 12.631–29.798), as shown in Figure 1. The OS data had not yet matured as of the last day of follow‐up. The mPFS of 19 patients with known co‐occurring mutations was 21.2 months (95% CI: 13.023–29.397). The mPFS of other 11 patients with no co‐occuring mutations or unknown status was 21.9 months (95% CI: 12.276–31.504).
FIGURE 1.

Kaplan–Meier curves of progression‐free survival (PFS) for the entire population
Risk factors for PFS
The relationships between PFS and clinicopathological characteristics including age, sex, smoking history, family history of cancer, ECOG PS, and EGFR mutation type, were analyzed. The impact of bevacizumab was also taken into consideration during combination therapy. Patients harboring EGFR exon 19 deletion mutation might have a longer PFS than those with EGFR exon 21 L858R mutation, although no significant difference was found (21.4 vs. 15.6 months, p = 0.343), as shown in Figure 2. The results of univariate Cox proportional hazards regression model (Cox) analysis are shown in Figure 3. The results showed that sex was an independent risk factor for PFS in patients who received concurrent EGFR‐TKIs and chemotherapy as first‐line treatment. Male patients had significantly longer PFS than female patients (32.6 vs. 14.6 months, HR = 3.593, 95% CI: 1.158–11.148, p = 0.027). Although patients who received a combination of chemotherapy and EGFR‐TKIs with an antiangiogenic agent had a longer mPFS than those who received combination therapy without an antiangiogenic agent (21.4 vs. 15.6 months, HR = 0.827, 95% CI: 0.265–2.586, p = 0.744), the difference was not significant.
FIGURE 2.

Progression‐free survival (PFS) curves of advanced lung adenocarcinoma patients harboring EGFR exon 19 deletion mutation or EGFR exon 21 L858R mutation using univariate analysis with the Cox proportional hazards regression model
FIGURE 3.
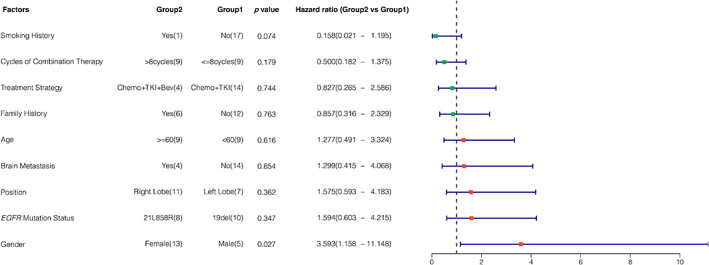
Hazard ratio of progression‐free survival in patients with different characteristics. A hazard ratio less than 1 implies a lower risk of disease progression or death in group 2 than in group 1. The number of cases that had progressive disease is shown in each group for every characteristic
Response to treatment
Two patients underwent lobe resection in palliative operations before other treatment, so 28 patients with target lesions were included for further analysis of treatment response. One patient (3.5%) had a complete response to concurrent therapy, 19 (67.9%) patients had a partial response to first‐line treatment, and seven (25%) had stable disease (SD). The ORR and DCR were 71.4% and 96.4%, respectively, as shown in Table 2, and the best overall response of each individual is shown in Figure 4.
TABLE 2.
Treatment outcomes of 28 advanced lung adenocarcinoma patients harboring EGFR‐activating mutations with at least one measurable lesion who received concurrent EGFR‐TKI and platinum‐based doublet chemotherapy as first‐line therapy
| Overall best response | n (%) |
|---|---|
| CR | 1 (3.6) |
| PR | 19 (67.8) |
| SD | 7 (25) |
| PD | 1 (3.6) |
| ORR | 20 (71.4) |
| DCR | 27 (96.4) |
Abbreviations: CR, complete remission; DCR, disease control rate (DCR = CR + PR + SD); n, number; ORR, objective response rate (ORR = CR + PR); PD, progressive disease; PR, partial response; SD, stable disease.
FIGURE 4.

Maximum tumor size change from baseline by the best overall response, as per response evaluation criteria in solid tumors (RECIST) version 1.1, in 28 patients with at least one measurable target lesion who received first‐line concurrent therapy of chemotherapy and first‐generation EGFR‐TKI treatment for advanced lung adenocarcinoma. Each bar represents the maximum change in the sum of the diameters of the target lesions of an individual patient
The ORR in patients with co‐occuring mutations and with no co‐occuring mutations or unknown status was 70.6% and 72.7%, respectively, and there was no significant difference (p = 1.000). The ORR in patients who received the regimen with an antiangiogenic agent and without an antiangiogenic agent was 85.7% and 66.7%, respectively, and no significant difference was observed (p = 0.633).
Safety and tolerability
Table 3 lists the incidence of hematological and nonhematological toxicities observed in the study. During combination therapy with EGFR‐TKIs and chemotherapy, with or without bevacizumab, the most frequently seen grade 3/4 AEs were hematological toxicities, including neutropenia and leukopenia, which were seen in three cases (10%). One patient had grade 4 neutropenia during chemotherapy and continued treatment after proper supportive treatment. Other grade 1/2 toxicities included hepatic toxicity (3/30, 7.4%) and diarrhea (9/30, 29.6%), fatigue (2/30, 6.7%), and rash (2/30, 6.7%). Vascular events, such as hypertension (grade 2, 2/30, 6.7%) and venous thrombosis (grade 2, 1/30, 3.3%), were seen in patients treated with the antiangiogenic agent bevacizumab, and these patients ceased bevacizumab due to toxicities.
TABLE 3.
Treatment‐related adverse events (AEs) in all advanced lung adenocarcinoma patients harboring EGFR‐activating mutations who received first‐generation EGFR‐TKIs combined with platinum‐based doublet chemotherapy as first‐line therapy
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| General disorders | ||||
| Fatigue | 2 (6.7) | |||
| Hematological toxicity | ||||
| Leukopenia | 2 (6.7) | 2 (6.7) | 2 (6.7) | |
| Neutropenia | 2 (6.7) | 2 (6.7) | 1 (3.3) | |
| Vascular disorders | ||||
| Hypertension | 2 (6.7) | |||
| Thromboembolic event | 1 (3.3) | |||
| Dermatological toxicity | ||||
| Rash | 2 (6.7) | |||
| Gastrointestinal toxicity | ||||
| Diarrhea | 5 (16.7) | 4 (13.3) | ||
| Hepatic toxicity | 2 (6.7) | 1 (3.3) | ||
Abbreviation: n, number.
DISCUSSION
Our study retrospectively reviewed the efficacy and safety profile of this combined strategy as first‐line therapy in patients diagnosed with advanced lung adenocarcinoma harboring sensitive EGFR mutations in real‐world situations. The ORR and DCR values in our study were 71.4% and 96.4%, respectively. We observed a mPFS of 21.2 months, while the OS data were immature at the end of the analysis, with only six events occurring. Grade 3/4 hematological toxicities, including neutropenia and leukopenia, were most common (11.1%, seen in 3/27 patients). Other nonhematological toxicities included gastrointestinal toxicities and dermatological toxicity, which were well tolerated after local therapy.
The results showed little difference in the disease control rate from the results of EGFR‐TKI monotherapy in historic studies (73.7%–77.8%)13, 14 however, the PFS time in our study was comparable to, and even numerically longer than, that of previous trials in which combination therapy was administered (17.5–20.9 months).11, 15 Additionally, our results showed that patients who received combination therapy with an antiangiogenic agent had longer mPFS and better ORR than those who did not receive an antiangiogenic agent, although no significant difference were found. Most patients maintained EGFR‐TKIs combined with monoagent chemotherapy, such as pemetrexed, with or without bevacizumab after four to six cycles of first‐line combination therapy. Among 18 patients who progressed after first‐line combination therapy, eight patients received tissue or liquid biopsy for another oncogene test, and four (50%) showed EGFR exon 20 T790M point mutations. The incidence of the EGFR T790M resistance mutation was also parallel to that in a previous study concerning resistance to first‐ or second‐generation EGFR‐TKIs, being around 50%–60%.3 These four patients then received osimertinib, a third‐generation EGFR‐TKI targeting the resistance mutation as second‐line therapy, and had not shown disease progression as of the last follow‐up.
EGFR‐TKIs have been proven to be beneficial for selected NSCLC patients who harbor sensitive EGFR mutations, such as EGFR exon 19 deletion mutation and EGFR exon 21 L858R mutation, and patients harboring EGFR 21 L858R mutation were deemed to benefit less from EGFR‐TKIs, possibly due to the higher rate of comutations, such as TP53.16 An in vivo and in vitro study has shown that first‐line EGFR‐TKI may work synergistically with pemetrexed by downregulating thymidylate synthase, the target of pemetrexed.17 On the other hand, combination therapy may prevent resistance of EGFR‐TKI mediated by EGFR T790M mutation or the epithelial‐to‐mesenthymal transition (EMT) process.18 As investigated in preclinical and clinical trials, first‐generation EGFR‐TKI therapy is inevitably followed by acquired resistance; thus, novel strategies are required to delay drug resistance. For example, the combination of EGFR‐TKIs and antiangiogenic therapy has been considered promising in prolonging PFS compared to that with monotherapy with EGFR‐TKIs.19, 20 Recently, studies showed that the combination strategy of chemotherapy and EGFR‐TKIs in treatment‐naive patients with advanced lung adenocarcinoma harboring sensitive EGFR mutations prolonged PFS and OS and also improved the response rate compared to that with chemotherapy or TKIs alone,15, 21 which indicates the promising benefit of this combination regimen. Although multiple trials have failed to present improved benefit from the concurrent use of chemotherapy and EGFR‐TKIs, these trials have not taken population selection into consideration under the circumstance that the EGFR driver gene was the essential predictor of efficacy. In subgroup studies, however, the combination strategy proved to be effective in patients harboring sensitive EGFR mutations.22 For example, in a subgroup analysis of the FASTACT‐2 trial, the mPFS of the EGFR‐TKI and chemotherapy combination group was significantly longer than chemotherapy (16.8 months vs. 6.9 months, p < 0.0001), and a significant benefit was also seen in terms of OS (31.4 months vs. 20.6 months, p = 0.0092).10 The phase III study NEJ009 also reported significantly longer PFS and OS in the EGFR‐TKI and chemotherapy combination group than in the TKI monotherapy group, with mPFS times of 20.9 months versus 11.2 months (p < 0.001) and OS times of 52.2 months versus 38.8 months (p = 0.013).11 Third‐generation osimertinib is an effective strategy as first‐line therapy, with a mPFS of 18.9 months.23 However, the mechanism of acquired resistance has proved to be more complicated than first‐ or second‐generation EGFR‐TKIs, which makes it more difficult to choose a subsequent strategy after disease progression. Since third‐generation osimertinib is also effective for EGFR‐mutated NSCLC patients with T790M mutation which exists in half of patients who progress after first‐ or second‐line therapy,24 the first‐line EGFR‐TKI combined with chemotherapy followed by osimertinib may be an optional strategy. As for other combination regimens, combining EGFR‐TKIs with antiangiogenic agents has also been reported to prolong PFS in EGFR‐mutated NSCLC patients, while no difference has been observed in OS after long‐term follow‐up.25, 26 Therefore, more randomized clinical trials should be designed to determine the optimal strategy for advanced EGFR‐mutated NSCLC patients.
According to previous studies, the addition of EGFR‐TKIs adds to the possibility and severity of adverse events from platinum‐based therapy, especially hematological and gastrointestinal toxicity.27 Some studies altered the administration of the regimen to avoid such adverse events; for example, platinum‐based chemotherapy was administered every four weeks instead of every three weeks, and EGFR‐TKIs were administered on Days 5–21.15 Despite the positive data provided by a growing number of clinical trials, first‐line treatment with platinum‐based doublet chemotherapy combined with EGFR‐TKIs has not been widely used in clinical work; in addition, whether chemotherapy should be maintained after standard cycles of therapy and whether it is safe to maintain combination therapy remain controversial. In our study, no grade 4 nonhematological toxicity was observed, and no novel AEs or treatment‐related deaths were observed. All AEs were related to chemotherapy, EGFR‐TKIs, and the antiangiogenic drug, and had mostly been reported previously and were controllable. The longest time of maintenance therapy in our study was 28 months, which indicated that combination therapy is tolerable and that maintenance therapy with chemotherapy and EGFR‐TKIs might be beneficial.
There are several limitations to this study. First, it was retrospective with a relatively small sample size, and thus the results may not comprehensively reflect the efficacy and safety of the combination strategy in the real world. Due to the limited number of patients who received NGS gene testing, we failed to further study the relationship of comutations and the efficacy of the combination strategy. Second, since the overall survival data were not sufficient, further follow‐up of these patients is required to provide further evidence of the survival benefits of this combination regimen. Moreover, as a descriptive study, this study failed to demonstrate the optimal administration schedule, and further studies should be designed to compare the sequential, concurrent, or intercalating combination of EGFR‐TKIs and chemotherapy. A randomized, large‐sample phase III clinical trial is required in the future to further address these unknown questions.
In conclusion, combination therapy with first‐generation EGFR‐TKIs and platinum‐based chemotherapy may be a first‐line treatment for advanced lung adenocarcinoma patients harboring activating EGFR mutations and is well tolerated.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Xu Z, Hao X, Lin L, Li J, Xing P. Concurrent chemotherapy and first‐generation epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) with or without an antiangiogenic agent as first‐line treatment in advanced lung adenocarcinoma harboring an EGFR mutation. Thorac Cancer. 2021;12:2233–2240. 10.1111/1759-7714.14057
Ziyi Xu and Xuezhi Hao contributed equally to this manuscript as cofirst authors.
Contributor Information
Junling Li, Email: lijunling@cicams.ac.cn.
Puyuan Xing, Email: xingpuyuan@cicams.ac.cn.
REFERENCES
- 1.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol. 2015;26:1883–9. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Waltman BA, Dias‐Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CY, Chang YL, Shih JY, Lin JW, Chen KY, Yang CH, et al. Thymidylate synthase and dihydrofolate reductase expression in non‐small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer. 2011;74:132–8. [DOI] [PubMed] [Google Scholar]
- 6.Giaccone G, Herbst RS, Manegold C, Scagliotti GV, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non‐small‐cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol. 2004;22:777–84. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI‐774) combined with carboplatin and paclitaxel chemotherapy in advanced non‐small‐cell lung cancer. J Clin Oncol. 2005;23:5892–9. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first‐line therapy in patients with advanced nonsquamous non‐small‐cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol. 2016;34:3258–66. [DOI] [PubMed] [Google Scholar]
- 9.Yang JCH, Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, et al. Gefitinib with or without pemetrexed in nonsquamous (NS) non‐small cell lung cancer (NSCLC) with EGFR mutation (Mut): final overall survival (OS) results from a randomized phase II study. Ann Oncol. 2018;29:viii495–viii6. [Google Scholar]
- 10.Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non‐small‐cell lung cancer (FASTACT‐2): a randomised, double‐blind trial. Lancet Oncol. 2013;14:777–86. [DOI] [PubMed] [Google Scholar]
- 11.Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib alone versus Gefitinib plus chemotherapy for non‐small‐cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–23. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 13.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Qi Q, Zhang Y, Cui J, Liu R, Li Y. Combination of icotinib and chemotherapy as first‐line treatment for advanced lung adenocarcinoma in patients with sensitive EGFR mutations: a randomized controlled study. Lung Cancer. 2019;133:23–31. [DOI] [PubMed] [Google Scholar]
- 15.Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, et al. Combination of chemotherapy and gefitinib as first‐line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int J Cancer. 2017;141:1249–56. [DOI] [PubMed] [Google Scholar]
- 16.Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR‐mutant advanced non‐small cell lung cancer. JAMA Oncol. 2018;4:739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okabe T, Okamoto I, Tsukioka S, Uchida J, Iwasa T, Yoshida T, et al. Synergistic antitumor effect of S‐1 and the epidermal growth factor receptor inhibitor gefitinib in non‐small cell lung cancer cell lines: role of gefitinib‐induced down‐regulation of thymidylate synthase. Mol Cancer Ther. 2008;7:599–606. [DOI] [PubMed] [Google Scholar]
- 18.La Monica S, Madeddu D, Tiseo M, Vivo V, Galetti M, Cretella D, et al. Combination of gefitinib and pemetrexed prevents the acquisition of TKI resistance in NSCLC cell lines carrying EGFR‐activating mutation. J Thorac Oncol. 2016;11:1051–63. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Wu YL, Cheng Y, Liu Y, Chen G, Cui J, et al. 1480O ‐ CTONG 1509: phase III study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR‐mutated NSCLC. Ann Oncol. 2019;30:v603. [Google Scholar]
- 20.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR‐positive advanced non‐squamous non‐small‐cell lung cancer (NEJ026): interim analysis of an open‐label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–35. [DOI] [PubMed] [Google Scholar]
- 21.Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR‐mutated lung cancer. J Clin Oncol. 2020;38:124–36. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non‐small‐cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–94. [DOI] [PubMed] [Google Scholar]
- 23.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 24.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maemondo M, Fukuhara T, Saito H, Furuya N, Watanabe K, Sugawara S, et al. NEJ026: final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR‐mutations. J Clin Oncol. 2020;38:9506. [Google Scholar]
- 26.Yamamoto N, Seto T, Nishio M, Goto K, Okamoto I, Yamanaka T, et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first‐line treatment for advanced EGFR mutation–positive non‐squamous non–small‐cell lung cancer (NSCLC): survival follow‐up results of JO25567. J Clin Oncol. 2018;36:9007. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura N, Kudoh S, Mitsuoka S, Yoshimoto N, Oka T, Nakai T, et al. Phase II study of a combination regimen of gefitinib and pemetrexed as first‐line treatment in patients with advanced non‐small cell lung cancer harboring a sensitive EGFR mutation. Lung Cancer. 2015;90:65–70. [DOI] [PubMed] [Google Scholar]


