Abstract
目的
评估癌栓粘连静脉壁对肾细胞癌伴下腔静脉癌栓患者手术难度和预后的影响。
方法
对北京大学第三医院泌尿外科于2017年1月至2020年6月收治的94例非转移性肾细胞癌合并下腔静脉癌栓患者进行回顾性队列研究,收集患者的一般情况、临床病理特征、手术及生存信息。按术中发现癌栓粘连静脉壁为标准将患者分为两组,其中64例为癌栓粘连静脉壁组(deep invasive tumor thrombus, DITT),30例为非粘连组(non-invasive tumor thrombus, NITT)。分别采用卡方检验、t检验和Mann-Whitney U检验进行两组间分类变量和连续变量的单因素比较,绘制Kaplan-Meier曲线并进行多变量Cox回归分析以评估癌栓粘连静脉壁对患者预后的影响。
结果
与NITT组相比,DITT组患者的手术难度明显增加,主要表现为手术时间更长(362.5 vs. 307.5 min,P=0.010),手术出血量更多(1 200 vs. 450 mL,P=0.006),围术期输血量更多(800 vs. 0 mL,P=0.021),血浆输注量更多(200 vs. 0 mL,P=0.001),开放手术占比更高(70.3% vs. 36.7%,P=0.002),术后住院时间更长(9.5 vs. 8.0 d,P=0.036),且发生术后并发症的比例更高(46.9% vs. 13.8%,P=0.002)。DITT与患者的总生存期更差呈正相关(P=0.022),即使在多因素分析中,DITT仍是影响肾细胞癌伴下腔静脉癌栓患者术后总生存率的不良预后因素[HR: 4.635 (1.017~21.116),P=0.047]。
结论
对于非转移性肾细胞癌合并下腔静脉癌栓的患者而言,癌栓粘连静脉壁会明显增加其手术难度,并与患者的不良预后相关。
Keywords: 肾细胞癌, 下腔静脉, 癌栓, 预后
Abstract
Objective
To evaluate the impact of deep invasive tumor thrombus on the surgical complexity and prognosis of patients with renal cell carcinoma complicated with inferior vena cava tumor thrombus.
Methods
We retrospectively reviewed the clinical data of 94 patients with non-metastatic renal cell carcinoma complicated with inferior vena cava tumor thrombus, who underwent surgical treatment in Peking University Third Hospital from January 2017 to June 2020. The patient's general condition, clinicopathological characteristics, surgery and survival information were collected. The patients were divided into two groups based on the intra-operative findings of tumor thrombus adhesion to the venous wall, of which 64 cases were in the deep invasive tumor thrombus (DITT) group and 30 cases were in the non-invasive tumor thrombus (NITT) group. Chi-square, t test and Mann-Whitney U test were used for categorical and continuous variables respectively. Kaplan-Meier plots and multivariable Cox regressions were performed to evaluate the influence of DITT on the prognosis of the patients with renal cell carcinoma with inferior vena cava tumor thrombus.
Results
DITT significantly increase the difficulty of surgery for the patients with renal cell carcinoma with venous tumor thrombus, which was mainly reflected in the longer operation time (362.5 vs. 307.5 min, P=0.010), more surgical bleeding (1 200 vs. 450 mL, P=0.006), more surgical blood transfusion (800 vs. 0 mL, P=0.021), more plasma transfusion (200 vs. 0 mL, P=0.001), a higher proportion of open surgery (70.3% vs. 36.7%, P=0.002), a longer post-operative hospital stay (9.5 vs. 8 days, P=0.036), and a higher proportion of post-operative complications (46.9% vs. 13.8%, P=0.002). DITT was associated with worse overall survival of the patients with renal cell carcinoma with inferior vena cava tumor thrombus (P=0.022). Even in the multivariate analysis, DITT was still a poor prognostic factor for the overall survival of these patients [HR: 4.635 (1.017-21.116), P=0.047].
Conclusion
For patients with non-metastatic renal cell carcinoma with inferior vena cava tumor thrombus, DITT will significantly increase the difficulty of surgery, and may lead to poor prognosis.
Keywords: Renal cell carcinoma, Inferior vena cava, Tumor thrombus, Prognosis
肾细胞癌(renal cell carcinoma, RCC)是常见的泌尿系统恶性肿瘤,占成人恶性肿瘤的2%~3%[1],其中有4%~10%的患者会形成下腔静脉癌栓[2]。根治性肾切除术和癌栓取出术可以有效改善患者预后,手术完全切除肿瘤及癌栓后可实现50%以上的5年存活率,而切除不完全的5年存活率只有约10%[3]。根据临床经验,癌栓粘连静脉壁(deep invasive tumor thrombus, DITT)可显著增加手术难度,甚至需要部分或节段性地切除下腔静脉[4],但评价DITT对手术难度具体影响的相关研究并不多见。DITT是否会导致RCC伴下腔静脉癌栓患者预后不良,目前尚存在争议,本文的目的是评估DITT对RCC伴下腔静脉癌栓患者的手术难度和预后的影响,以指导临床治疗和随访。
1. 资料与方法
1.1. 研究对象
北京大学第三医院泌尿外科于2017年1月至2020年6月收治的RCC合并下腔静脉癌栓患者。纳入标准:(1)术前检查发现有下腔静脉癌栓; (2)在我院行肾根治性切除及下腔静脉癌栓取出术; (3)术后组织病理为RCC。排除标准:(1)术前检查发现远处转移; (2)术后组织病理为T4期; (3)信息记录不全及失访的患者。经筛选后共94例患者纳入本研究。
1.2. 研究方法
本研究为回顾性研究。以手术中发现癌栓是否粘连静脉壁为标准,将患者分为DITT组与癌栓不粘连静脉壁(non-invasive tumor thrombus, NITT)组,分析两组患者在临床资料和预后上的差异。
收集患者的围术期临床资料,包括:年龄、性别、肿瘤侧别、体重指数(body mass index,BMI)、血红蛋白(hemoglobin,Hb)、中性粒细胞计数(neutrophil count,Neu)、血小板计数(platelet count,Plt)、白蛋白(albumin,Alb)、血清钙(calcium,Ca)、碱性磷酸酶(alkaline phosphatase,ALP)、术前血清肌酐(serum creatinine,SCr)、术后1周内SCr、美国麻醉医师学会(American Society of Anesthesiologists,ASA)分级、淋巴结转移、组织病理类型、Fuhrman核分级等临床病理特征,进行统计分析比较两组的基线特征; 收集患者手术时间、术中出血和输血等术中资料以及术后并发症、术后住院日等术后资料,进行统计分析比较DITT对手术复杂性的影响。对所有患者进行门诊或电话随访获取预后信息,分析比较DITT对患者预后的影响。
1.3. 统计学分析
使用SPSS 24.0统计软件(IBM公司,美国)进行统计分析。正态分布的计量资料以平均值±标准差表示,进行独立样本t检验; 非正态分布的计量资料以中位数(四分位数)表示,进行非参数检验; 计数资料以百分比表示,进行卡方检验。生存时间是从手术日期到死亡或最后一次随访(确认患者存活)的日期,绘制Kaplan-Meier生存曲线,进行对数秩检验(Log-rank test)分析差异。将基线有差异的因素和临床上重要的预后相关因素纳入多变量Cox回归分析,进一步评估DITT对预后的影响。P < 0.05为差异有统计学意义。
2. 结果
94例患者中,64例(68.1%)为DITT组,30例(31.9%)为NITT组,两组的典型CT影像见图 1。两组在年龄、性别、BMI、肿瘤侧别、临床症状、ASA分级、淋巴结分期、组织病理类型、核分级等临床特征及术后靶向治疗上均无明显差异。
图 1.
DITT组和NITT组的典型CT影像
Typical CT image appearance of DITT group and NITT group
DITT, deep invasive tumor thrombus; NITT, non-invasive tumor thrombus.
与NITT组患者相比,DITT组患者的基线血红蛋白明显降低(P=0.030),癌栓的Mayo分级更高(P=0.033)。在对手术复杂性和术后恢复的影响方面,DITT组与NITT组相比,手术时间更长(362.5 vs. 307.5 min,P=0.010),手术出血量更多(1 200 vs. 450 mL,P=0.006),手术输血量更多(800 vs. 0 mL,P=0.021),血浆输注量更多(200 vs. 0 mL,P=0.001),开放手术的患者占比更高(70.3% vs. 36.7%,P=0.002),下腔静脉节段性切除的患者占比更高(34.4% vs. 0,P < 0.001),术后住院时间更长(9.5 vs. 8.0 d,P=0.036),发生术后并发症的比例更高(46.9% vs. 13.8%,P=0.002),具体见表 1。
表 1.
DITT组和NITT组临床和病理特征比较
Comparison of clinical and pathologic features between DITT group and NITT group
| Items | DITT (n=64) | NITT (n=30) | t/U/χ2 | P value |
| Data are presented as x±s, n (%) or M (P25, P75). DITT, deep invasive tumor thrombus; NITT, non-invasive tumor thrombus; BMI, body mass index; Hb, hemoglobin; Neu, neutrophil count; Plt, platelet count; ALP, alkaline phosphatase; Alb, albumin; Ca, calcium; SCr, serum creatinine; ASA, American Society of Anesthesiologists; IVC, inferior vena cava. | ||||
| Age/years | 60.4±8.9 | 59.4±10.9 | 0.453 | 0.652 |
| Gender | 3.548 | 0.060 | ||
| Male | 52 (81.3%) | 19 (63.3%) | ||
| Female | 12 (18.8%) | 11 (36.7%) | ||
| Side | 0.121 | 0.728 | ||
| Left | 17 (26.6%) | 9 (30.0%) | ||
| Right | 47 (73.4%) | 21 (70.0%) | ||
| Clinical symptoms | 1.294 | 0.523 | ||
| No clinical symptoms | 13 (20.3%) | 7 (23.3%) | ||
| Local symptoms | 36 (56.3%) | 19 (63.3%) | ||
| Systemic symptoms | 15 (23.4%) | 4 (13.3%) | ||
| BMI/(kg/m2) | 24.7±3.5 | 24.0±2.8 | 0.893 | 0.374 |
| Tumor diameter/cm | 8.1±2.9 | 9.1±2.8 | 1.684 | 0.096 |
| Hb/(g/L) | 120.3±20.4 | 130.3±20.7 | 2.203 | 0.030 |
| Neu/(×109/L) | 4.8±1.7 | 4.2±1.1 | 1.784 | 0.078 |
| Plt/(×109/L) | 255.0±118.6 | 262.7±77.8 | 0.325 | 0.746 |
| ALP/(U/L) | 91.6±37.6 | 85.6±22.5 | 0.802 | 0.424 |
| Alb/(g/L) | 39.1±5.1 | 40.5±4.4 | 1.257 | 0.212 |
| Ca/(mg/L) | 2.3±0.2 | 2.2±0.1 | 0.552 | 0.583 |
| SCr/(μmol/L) | 100.2±25.3 | 90.8±20.4 | 1.766 | 0.081 |
| SCr after surgery/(μmol/L) | 116.5±54.2 | 101.9±37.7 | 1.259 | 0.211 |
| ASA score | 5.876 | 0.053 | ||
| 1 | 3 (4.7%) | 2 (6.7%) | ||
| 2 | 50 (78.1%) | 28 (93.3%) | ||
| 3 | 11 (17.2%) | 0 (0) | ||
| pN stage | 4.099 | 0.052 | ||
| N0 | 56 (87.5%) | 30 (100.0%) | ||
| N1 | 8 (12.5%) | 0 (0) | ||
| Mayo classification | 8.755 | 0.033 | ||
| Ⅰ | 11 (17.2%) | 12 (40.0%) | ||
| Ⅱ | 40 (62.5%) | 15 (50.0%) | ||
| Ⅲ | 8 (12.5%) | 0 (0) | ||
| Ⅳ | 5 (7.8%) | 3 (10.0%) | ||
| Pathology type | 0.424 | 0.769 | ||
| Clear cell carcinoma | 52 (81.3%) | 26 (86.7%) | ||
| Non clear cell carcinoma | 12 (18.7%) | 4 (13.3%) | ||
| Fuhrman grade | 0.419 | 0.518 | ||
| Ⅰ-Ⅱ | 27 (42.9%) | 15 (50.0%) | ||
| Ⅲ-Ⅳ | 36 (57.1%) | 15 (50.0%) | ||
| Surgical approach | 9.601 | 0.002 | ||
| Laparoscopic surgery | 19 (29.7%) | 19 (63.3%) | ||
| Open surgery | 45 (70.3%) | 11 (36.7%) | ||
| IVC resection | 13.464 | < 0.001 | ||
| No | 42 (65.6%) | 30 (100.0%) | ||
| Yes | 22 (34.4%) | 0 (0) | ||
| Operative time/min | 362.5 (305.3, 429.8) | 307.5 (254.5, 374.0) | 644.0 | 0.010 |
| Surgical bleeding volume/mL | 1 200 (325, 2 500) | 450 (200, 800) | 621.0 | 0.006 |
| Surgical blood transfusion volume/mL | 800 (0, 1 600) | 0 (0, 600) | 689.0 | 0.021 |
| Plasma transfusion volume/mL | 200 (0, 750) | 0 (0, 0) | 598.0 | 0.001 |
| Post-operative hospital stay/d | 9.5 (7.0, 13.0) | 8.0 (6.0, 9.3) | 702.5 | 0.036 |
| Post-operative adjuvant targeted therapy | 2.546 | 0.111 | ||
| No | 23 (35.9%) | 16 (53.3%) | ||
| Yes | 41 (64.1%) | 14 (46.7%) | ||
| Post-operative complications | 9.417 | 0.002 | ||
| No | 34 (53.1%) | 25 (86.2%) | ||
| Yes | 30 (46.9%) | 4 (13.8%) | ||
癌栓分级对手术难度有显著影响,由于DITT组的癌栓分级更高,因此为进一步独立评估DITT对手术难度的影响,我们对Mayo Ⅱ级癌栓进行了亚组分析(表 2)。在同一癌栓分级下,DITT组与NITT组相比,仍旧表现为手术时间更长(368.5 vs. 302.0 min,P=0.002),术中出血更多(1 450 vs. 400 mL,P=0.005),血浆输注量更多(400 vs. 0 mL,P=0.003),开放手术的患者占比更高(75% vs. 26.7%,P=0.001),术后住院时间更长(11 vs. 8 d,P=0.036),术后并发症更多(55.0% vs. 13.3%,P=0.006),术中输血量也多于NITT组(800 vs. 0 mL,P=0.054,差异无统计学意义)。Ⅰ级和Ⅲ~Ⅳ级的癌栓患者相对较少,无法进行亚组分析。DITT组在术中需要切除粘连的血管壁,而NITT组可直接将癌栓拖拽出来,在腔静脉的缝合上,DITT组同样更加复杂,由于DITT对手术的影响与癌栓长度无明显关系,因此我们合理推测,在扩大样本量之后,Ⅰ级和Ⅲ~Ⅳ级癌栓的结果应与Ⅱ级癌栓相似。在排除了癌栓分级的影响后,DITT仍明显增加了RCC伴下腔静脉癌栓患者的手术难度。
表 2.
Mayo Ⅱ级亚组中DITT组和NITT组的手术相关特征比较
Comparison of surgical features between DITT group and NITT group in Mayo Ⅱ level tumor thrombus subgroup
| Items | DITT (n=40) | NITT (n=15) | U/χ2 | P value |
| Data are presented as n (%) or M (P25, P75). DITT, deep invasive tumor thrombus; NITT, non-invasive tumor thrombus. | ||||
| Operative time/min | 368.5 (306.5, 525.0) | 302.0 (258.0, 355.0) | 139 | 0.002 |
| Surgical bleeding volume/mL | 1 450 (525, 2 925) | 400 (200, 800) | 152.5 | 0.005 |
| Surgical blood transfusion volume/mL | 800 (0, 1 600) | 0 (0, 400) | 202 | 0.054 |
| Plasma transfusion volume/mL | 400 (0, 800) | 0 (0, 0) | 155 | 0.003 |
| Post-operative hospital stay/d | 11.0 (7.0, 13.0) | 8.0 (6.0, 9.0) | 190 | 0.036 |
| Open surgery | 30 (75.0%) | 4 (26.7%) | 10.8 | 0.001 |
| Post-operative complications | 22 (55.0%) | 2 (13.3%) | 7.7 | 0.006 |
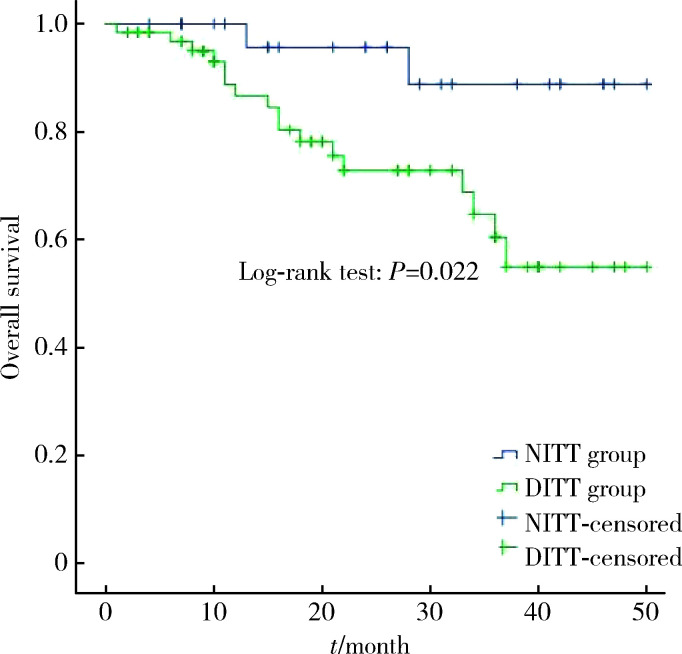
所有患者的中位随访时间21.0个月(1.0~50.0个月),DITT组的术后平均总生存时间少于NITT组[(37.5±2.4)个月vs. (46.9±2.1)个月,P=0.022,图 2]。为进一步研究DITT对患者预后的影响,我们将基线有差异的因素(血红蛋白、癌栓分级)和临床上重要的预后相关因素(核分级、组织病理类型)纳入多变量Cox回归分析,结果见表 3。即使排除了血红蛋白、核分级、组织病理类型和癌栓分级的影响后,DITT仍是影响RCC伴下腔静脉癌栓患者总生存率的不良预后因素[HR: 4.635 (1.017~21.116),P=0.047],可见,DITT是非转移性RCC伴下腔静脉癌栓患者的不良预后因素。
图 2.
DITT组和NITT组患者的总生存率
Overall survival time between DITT group and NITT group
DITT, deep invasive tumor thrombus; NITT, non-invasive tumor thrombus.
表 3.
非转移性肾细胞癌伴下腔静脉癌栓预后危险因素的多因素回归分析
Multivariate Cox regression analysis of prognosis risk factors for patients with non-metastatic renal cell carcinoma and inferior vena cava tumor thrombus
| Items | HR | 95%CI | P value |
| DITT, deep invasive tumor thrombus; Hb, hemoglobin. | |||
| DITT | 4.635 | 1.017-21.116 | 0.047 |
| Mayo classification | 2.046 | 1.161-3.604 | 0.013 |
| Hb | - | - | 0.484 |
| Non-clear cell carcinoma | 4.275 | 1.495-12.229 | 0.007 |
| Fuhrman grade (Ⅲ-Ⅳ vs. Ⅰ-Ⅱ) | - | - | 0.719 |
3. 讨论
术前影像学检查预测癌栓是否粘连静脉壁,对于合理规划手术以及合理评估患者预后都有很大帮助。通常认为,增强MRI有助于术前诊断静脉壁是否存在粘连,主要的预测特征包括血管壁不连续和下腔静脉完全闭塞等,具有高灵敏度(92%)和特异性(86%)[5]。以往研究报道,静脉壁最易受侵的部位是肾静脉开口处,当肾静脉开口处的下腔静脉直径≥24.0 mm时,癌栓粘连静脉壁的概率较大[6]。此外,下腔静脉最大冠状径也被认为是癌栓粘连静脉壁的主要特征之一[7]。一项超声造影研究显示,下腔静脉壁的连续性中断被认为是静脉壁受侵的表现,其预测准确性可达93.3%[8]。需要注意的是,各种影像学检查都高度依赖于阅片者的经验性判断,并且受限于检查设备参数的一致性水平,因此其准确度和可重复性是有限的。本研究中DITT的判断是以术中所见为标准,相对于影像学检查具有较高水平的可靠性。我们同时回顾了所有患者的术前影像学检查,有57例(89.1%)DITT与影像学检查一致。
术前血液学参数可反映患者的炎症状态和一般健康状况。本研究中DITT的患者具有血红蛋白降低的特征,与NITT组相比差异有统计学意义,且DITT组有白蛋白低、中性粒细胞增高的趋势(尽管差异没有统计学意义)。低血红蛋白、低白蛋白代表了长期不良饮食或失血的肿瘤高消耗状况,中性粒细胞升高代表着相对较高的免疫炎症状态,由于癌栓直接与血液接触,因此我们推测此类患者肿瘤的理化性质差异更容易引起血液学指标的变化。既往研究中,血红蛋白、中性粒细胞等指标已被纳入转移性RCC的预后模型[9]。有研究报道在非转移性RCC中,血红蛋白、中性粒细胞、白蛋白等这些血液指标也与预后相关[10]。我们认为血液指标的变化一定程度上反映了DITT的不良生物学行为。
根治性肾切除术和癌栓取出术能有效改善RCC伴下腔癌栓患者的预后。外科手术治疗的目的是完全消除肿瘤负担,而DITT则显著增加了完全切除癌栓的手术难度。若DITT程度较深、范围较大,难以剥离,则有必要去除被癌栓浸润的血管壁,甚至需要节段性下腔静脉切除及使用补片移植来重建血管。既往研究报道,约6%~8%的患者需要节段性下腔静脉切除[6]。因此,DITT会导致手术时间延长、术中出血增多,需要更高的手术技术和更多的血管处理经验[11]。本研究纳入的病例中,有68.1%为DITT患者,其中34.4%需要下腔静脉节段性切除。与NITT患者相比,DITT患者的手术难度明显增加,需要开放手术的比例更高,手术时间更长,术中出血更多,围术期输红细胞及血浆的总量也更多。术后DITT患者的恢复也更慢,体现在术后住院日更长、术后并发症更多,这与我们的以往经验及既往研究报道相符[12]。即使在排除了癌栓分级的影响后,DITT依旧显著增加了RCC伴下腔癌栓患者的手术难度及导致了更多的术后并发症。
关于DITT对患者预后的影响尚存争议。一项系统综述显示,临床症状、癌栓分级、Fuhrman分级、肾上腺浸润、肿瘤坏死是影响非转移性RCC伴癌栓患者预后的主要因素[12],DITT对患者预后的影响较小。但也有研究持相反意见,认为DITT可导致RCC伴癌栓患者的肿瘤特异性生存率(cancer specific survival,CSS)显著降低[13]。DITT可以为血凝块黏附以及血栓形成提供合适的基质,从而有利于血栓形成,而血栓形成与RCC伴癌栓患者的不良预后相关[14]。根据既往研究经验,肿瘤远处转移及局部进展对患者的远期生存有显著影响,因此,为了确切地研究DITT对患者预后的影响,我们将术前存在肿瘤远处转移及术后组织病理判定为T4期的患者排除在本研究之外,结果表明,DITT患者的总生存期明显少于NITT患者(P=0.022)。即使在排除了血红蛋白、组织病理类型、核分级和癌栓分级的影响后,DITT仍是RCC伴下腔静脉癌栓患者总生存率的不良预后因素[HR: 4.635 (1.017~21.116),P=0.047]。我们认为,DITT一方面意味着肿瘤的侵袭性更强,生物学行为更差,另一方面,粘连静脉壁对应着手术难度更高,更难做到完全切干净肿瘤,因此会导致患者更差的预后。本研究结果表明,DITT会明显增加RCC伴下腔静脉癌栓患者的手术难度,并会导致非转移性RCC伴下腔癌栓患者的不良预后。
本研究存在一些局限性,首先为回顾性研究,且为单中心经验,存在选择偏倚; 其次,DITT的判定标准为术中发现,具有一定主观性,有时并不完全是癌栓侵犯静脉壁,也有可能是肿瘤诱导的严重炎症所引起的粘连; 第三,由于术后患者接受的辅助、一线或后线治疗的种类繁多,时程不一致,我们很难量化评估后线治疗对患者总生存期的影响程度; 最后,本研究的主要评价指标为总生存期,未对无复发生存期进行考察。后续的研究需要在进一步扩大样本量的同时,设计前瞻性研究,涵盖总生存期、无病生存期等指标,同时兼顾平衡后线治疗的影响,为临床工作提供更充分的证据。
Funding Statement
国家自然科学基金(82070778)
Supported by the National Natural Science Foundation of China (82070778)
Contributor Information
刘 承 (Cheng LIU), Email: chengliu@bjmu.edu.cn.
马 潞林 (Lu-lin MA), Email: malulinpku@163.com.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Lardas M, Stewart F, Scrimgeour D, et al. Systematic review of surgical management of nonmetastatic renal cell carcinoma with vena caval thrombus. Eur Urol. 2016;70(2):265–280. doi: 10.1016/j.eururo.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Quencer KB, Friedman T, Sheth R, et al. Tumor thrombus: incidence, imaging, prognosis and treatment. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S165–S177. doi: 10.21037/cdt.2017.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González J, Gorin MA, Garcia-Roig M, et al. Inferior vena cava resection and reconstruction: Technical considerations in the surgical management of renal cell carcinoma with tumor thrombus. Urol Oncol. 2014;32(1):34.e19–26. doi: 10.1016/j.urolonc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Adams LC, Ralla B, Bender YY, et al. Renal cell carcinoma with venous extension: prediction of inferior vena cava wall invasion by MRI. Cancer Imaging. 2018;18(1):17. doi: 10.1186/s40644-018-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psutka SP, Boorjian SA, Thompson RH, et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus. BJU Int. 2015;116(3):388–396. doi: 10.1111/bju.13005. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Li L, Hong P, et al. A predictive model for tumor invasion of the inferior vena cava wall using multimodal imaging in patients with renal cell carcinoma and inferior vena cava tumor thrombus. Biomed Res Int. 2020;2020:9530618. doi: 10.1155/2020/9530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li QY, Li N, Huang QB, et al. Contrast-enhanced ultrasound in detecting wall invasion and differentiating bland from tumor thrombus during robot-assisted inferior vena cava thrombectomy for renal cell carcinoma. Cancer Imaging. 2019;19(1):79. doi: 10.1186/s40644-019-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: A population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao R, Xu C, He W, et al. Preoperative anaemia and thrombocytosis predict adverse prognosis in non-metastatic renal cell carcinoma with tumour thrombus. BMC Urol. 2021;21(1):31. doi: 10.1186/s12894-021-00796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du S, Huang Q, Yu H, et al. Initial series of robotic segmental inferior vena cava resection in left renal cell carcinoma with caval tumor thrombus. Urology. 2020;142:125–132. doi: 10.1016/j.urology.2020.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Gu L, Li H, Wang Z, et al. A systematic review and meta-analysis of clinicopathologic factors linked to oncologic outcomes for renal cell carcinoma with tumor thrombus treated by radical nephrectomy with thrombectomy. Cancer Treat Rev. 2018;69:112–120. doi: 10.1016/j.ctrv.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez Faba O, Linares E, Tilki D, et al. Impact of microscopic wall invasion of the renal vein or inferior vena cava on cancer-specific survival in patients with renal cell carcinoma and tumor thrombus: A multi-institutional analysis from the International Renal Cell Carcinoma-Venous Thrombus Consortium. Eur Urol Focus. 2018;4(3):435–441. doi: 10.1016/j.euf.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Li X, Huang Q, et al. Prognostic role of bland thrombus in patients treated with resection of renal cell carcinoma with infe-rior vena cava tumor thrombus. Urol Oncol. 2021;39(5):302.e1–e7. doi: 10.1016/j.urolonc.2021.02.005. [DOI] [PubMed] [Google Scholar]