Abstract
目的
总结临床T1(cT1)期肾细胞癌的临床影像学特点,探究影响cT1期肾细胞癌患者发生肾窦侵犯的危险因素。
方法
回顾性分析2016年1月至2019年8月于北京大学第三医院泌尿外科住院行肾部分切除术或根治性肾切除术,术前诊断为cT1期肾细胞癌的患者,收集患者的临床、影像学和病理学资料。采用卡方检验、Mann-Whitney U检验、多因素Logistic回归,对cT1期肾细胞癌发生肾窦侵犯的相关危险因素进行分析。
结果
共纳入507例患者,其中男性354例(69.8%),女性153例(30.2%),中位年龄59岁,中位体重指数25.5 kg/m2。术前有肉眼血尿者18例(3.6%),中位肿瘤直径3.5 cm。cT1a期322例(63.5%),cT1b期185例(36.5%),中位R.E.N.A.L.评分为8分。肿瘤边界规则者359例(70.8%),肿瘤边界不规则者148例(29.2%)。所有患者均行手术治疗,包括186例(36.7%)肾部分切除术和321例(63.3%)根治性肾切除术。术后病理提示肾窦侵犯75例(14.8%),其中cT1a期侵犯肾窦18例,占cT1a期总数的5.6%;cT1b期侵犯肾窦57例,占cT1b期总数的30.8%。单因素分析发现,年龄(P=0.02)、R.E.N.A.L.评分(P < 0.001)、肿瘤边界状态(P < 0.001)是cT1期肾细胞癌发生肾窦侵犯的相关危险因素。多因素Logistic回归显示,R.E.N.A.L.评分(P≤0.020)和肿瘤边界状态(P=0.001)是cT1期肾细胞癌发生肾窦侵犯的独立危险因素。
结论
对cT1期肾细胞癌,术后接近15%的患者存在肾窦侵犯现象; R.E.N.A.L.高评分和肿瘤边界不规则,均可以提示cT1期肾细胞癌有发生肾窦侵犯的风险。
Keywords: 肾细胞癌, 肿瘤浸润, 肾窦, 肿瘤分期, 危险因素
Abstract
Objective
To summarize the clinicoradiological characteristics of clinical T1 renal cell carcinoma patients and to investigate the risk factors of renal sinus invasion in cT1 renal cell carcinoma patients undergoing nephrectomy.
Methods
A retrospective study was conducted in cT1 renal cell carcinoma patients from January 2016 to August 2019 in Department of Urology, Peking University Third Hospital, who underwent partial or radical nephrectomy by analyzing clinicopathological and radiological data. The influencing factors of renal sinus invasion for cT1 renal cell carcinoma were determined by χ2 test, Mann-Whitney U test and Logistic regression analysis.
Results
A total of 507 patients were enrolled, including 354 males (69.8%) and 153 females (30.2%). The median age was 59 years and the median body mass index (BMI) was 25.5 kg/m2. Eighteen patients (3.6%) had gross hematuria preoperatively. The median tumor diameter was 3.5 cm. Three hundred twenty-two patients (63.5%) were staged clinical T1a and 165 cases (36.5%) were staged clinical T1b. The median R.E.N.A.L. score was 8. Three hundred fifty-nine patients (70.8%) had regular tumor border and 148 (29.2%) irregular. All the patients underwent surgical treatment, including 186 (36.7%) partial nephrectomy and 321 (63.3%) radical nephrectomy. Postoperative pathology showed seventy-five patients (14.8%) had renal sinus invasion, including 18 in cT1a (5.6%) and 57 in cT1b (30.8%). Univariate analysis showed that age (P=0.020), R.E.N.A.L. score (R value, E value, N value, P < 0.001) and tumor border (P < 0.001) were associated risk factors for cT1 renal cell carcinoma with renal sinus invasion. On multivariate binary Logistic analysis, R.E.N.A.L. score (P≤0.020) and irregular tumor border (P=0.001) were independent risk factors.
Conclusion
For cT1 renal cell carcinoma patients undergoing nephrectomy, about 15% had renal sinus invasion postoperatively. High R.E.N.A.L. score and irre-gular tumor border help predicting cT1 renal cell carcinoma renal sinus invasion.
Keywords: Renal cell carcinoma, Neoplasm invasiveness, Renal sinus, Neoplasm staging, Risk factors
肾细胞癌是泌尿系统第三大恶性肿瘤,据国家癌症中心统计,我国2014年肾细胞癌发病率约为4.99例/10万[1],且发病率仍在逐年增加。肾细胞癌治疗方案及预后与TNM分期密切相关,根据第8版美国癌症联合委员会(American Joint Committeeon Cancer,AJCC)肾细胞癌TNM分期标准,T1a期定义为肿瘤局限于肾实质且直径≤4 cm,T1b期定义为肿瘤局限于肾实质且4 cm<直径≤7 cm[2]。手术切除是当前治疗肾细胞癌的最主要手段。目前,欧洲泌尿外科协会(European Association of Urology,EAU)和美国国家综合癌症网络(National Comprehensive Cancer Network,NCCN)指南均推荐将保留肾单位的肾部分切除术(partial nephrectomy,PN)作为cT1a期肾细胞癌的标准治疗手段,cT1b期的患者则主要推荐行根治性肾切除术(radical nephrectomy,RN)[3-4]。
但术前基于临床及影像信息诊断的T分期并不完全准确[5],根据文献报道,约有3.1%~12.4%的临床T1期肾细胞癌术后病理提示肿瘤侵犯肾窦脂肪[5-7],即从临床T1期升级为病理T3a期[8-9],而这部分接受了肾部分切除术的患者,其总生存(overall survival,OS)、肿瘤特异性生存(cancer specific survival,CSS)、肿瘤无复发生存(relapse-free survival,RFS)均更差[10-11]。既往文献报道,未发生肾窦侵犯的cT1期肾细胞癌的5年CSS为97%,5年OS为80%,5年RFS为75%;而发生肾窦侵犯的cT1期肾细胞癌5年CSS为90%,5年OS为71%,5年RFS为58%[12]。
因此,在现有的影像学评估体系基础上,探索更多的临床或影像指标,以更为准确地预测肾窦侵犯的风险,具有十分明确的临床意义。本研究旨在通过分析cT1期肾细胞癌的临床影像病理特点,探究cT1期肾细胞癌患者发生肾窦侵犯的危险因素。
1. 资料与方法
1.1. 研究对象
本研究为回顾性研究,研究对象为2016年1月至2019年8月于北京大学第三医院泌尿外科住院行肾部分切除术或根治性肾切除术的肾细胞癌病例。纳入标准为:(1)根据第8版AJCC肾细胞癌分期, 为临床T1期; (2)年龄≥18岁; (3)术前1个月内行肾增强CT检查,且至少包含平扫期、动脉期、实质期、排泄期序列; (4)术后病理证实为肾细胞癌。排除标准为:(1)孤立肾、多灶性肾细胞癌、双侧肾细胞癌; (2)多囊肾、肾萎缩; (3)临床关键信息缺失; (4)术前存在远处转移或淋巴结转移者。
1.2. 研究方法
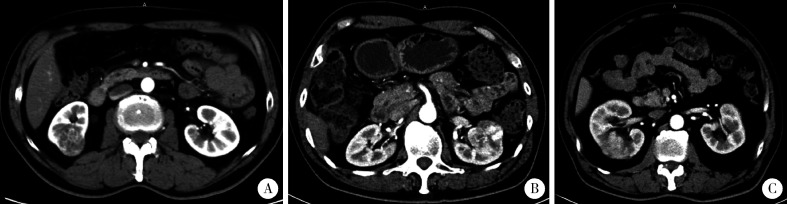
回顾性收集患者的临床信息、肿瘤影像特征及病理资料等。临床信息包括:性别、年龄、身高、体质量、有无肉眼血尿; 肿瘤影像学资料包括:肿瘤侧别、最大径、R.E.N.A.L.单项及总评分、肿瘤边界状态、有无淋巴结肿大; 手术病理资料包括:术式、组织病理类型、世界卫生组织(World Health Organization,WHO)/国际泌尿病理学会(International Society of Urological Pathology,ISUP)分级、有无肾窦侵犯、有无肾周脂肪侵犯。R.E.N.A.L.评分由5个单项组成:R表示肿瘤最大径,反映肿瘤的大小; E表示肿瘤外生或内生性; N表示肿瘤最深处距离集合系统或肾窦的距离; A表示肿瘤位于肾脏的前后位置; L表示肿瘤与肾脏极线的关系[13]。肾窦及肾周脂肪侵犯以术后病理结果为最终判定。肿瘤边界不规则定义为肿瘤肾窦侧边缘不清晰或肿瘤分叶(即肿瘤边界切线与肿瘤自身相交)[6],示意图见图 1。
图 1.
肿瘤边界状态示意图
Example of tumor border definition
A: This tumor in the right kidney had a clear border without tangent lines across tumor, which was defined as regular border. Pathological reports revealed a clear cell carcinoma without renal sinus invasion. B: This tumor in the left kidney had a clear border with lobulation, which was defined as re-gular border. Pathological reports confirmed tumor invasion into the renal sinus. C: This tumor in the right kidney had an unclear border whose tangent lines were hard to determine, which was defined as irregular border. Pathological reports confirmed clear cell carcinoma with macro tumor invasion into the renal sinus.
1.3. 统计学方法
采用SPSS 18.0软件进行统计分析。所有指标均进行正态检验及方差齐性分析。非正态分布计量资料以中位数(四分位数间距)表示,两组间比较采用Mann-Whitney U非参数检验; 计数资料以个数(百分比)表示,组间比较采用卡方检验。将患者的各项临床参数与是否存在肾窦侵犯进行单因素分析,将单因素分析中有统计学意义的变量纳入多因素Logistic回归分析,以评估cT1期肾细胞癌肾窦侵犯的危险因素,结果表示为优势比(odds ratio,OR)及95%置信区间(confidence interval,CI),P < 0.05为差异有统计学意义。
2. 结果
2.1. 一般资料
本研究共纳入507例患者,其中男性354例(69.8%),女性153例(30.2%),中位年龄59(48~65)岁,中位体重指数25.5 kg/m2。术前有肉眼血尿者18例(3.6%),中位肿瘤直径为3.5(2.5~4.6) cm,中位R.E.N.A.L.评分为8分(6~9分)。其中,临床T1a期322例(63.5%),临床T1b期185例(36.5%); 肾透明细胞癌431例(85.0%),肾乳头状细胞癌22例(4.3%),肾嫌色细胞癌21例(4.1%)。术后病理证实肾窦侵犯75例(14.8%),其中cT1a期肾窦侵犯18例(5.6%),cT1b期肾窦侵犯57例(30.8%),肾周脂肪侵犯19例(3.7%)。T1a与T1b两组间年龄(P=0.027)、BMI(P=0.042)、肿瘤大小(P < 0.001)、R.E.N.A.L.评分(P < 0.001)、肿瘤边界状态(P=0.013)、手术方式(P < 0.001)、WHO/ISUP分级(P=0.013)、肾窦侵犯(P < 0.001)、肾周脂肪侵犯(P=0.014)差异有统计学意义(表 1)。
表 1.
患者的基线临床、病理和影像学资料
Baseline clinical, pathological and radiological information of the patients
| Variables | Total (n=507) | cT1a (n=322) | cT1b (n=185) | Z/χ2 | P |
| cT1a, clinical T1a; cT1b, clinical T1b; BMI, body mass index; LN, lymph node; RN, radical nephrectomy; PN, partial nephrectomy; WHO, World Health Organization; ISUP, International Society of Urological Pathology; RSI, renal sinus invasion; PFI, perinephric fat invasion. R.E.N.A.L., radius, exophytic/endophytic, nearness, anterior/posterior, location. | |||||
| Age/years, M (P25, P75) | 59.0 (48.0, 65.0) | 56.5 (47.0, 64.3) | 60.0(51-66) | -2.206 | 0.027 |
| Gender, n(%) | 0.001 | 0.972 | |||
| Male | 354 (69.8) | 225 (69.9) | 129 (69.7) | ||
| Female | 153 (30.2) | 97 (30.1) | 56 (30.3) | ||
| BMI/(kg/m2), M (P25, P75) | 25.5(23.2, 27.9) | 25.1(22.8, 27.9) | 26.0(24.1-27.9) | -2.033 | 0.042 |
| Complains, n(%) | 1.399 | 0.237 | |||
| Hematuria | 18 (3.6) | 9 (2.8) | 9 (4.9) | ||
| Non-hematuria | 482 (96.4) | 309 (97.2) | 175 (95.1) | ||
| Tumor side, n(%) | 0.001 | 0.981 | |||
| Right | 247 (48.7) | 157 (48.8) | 90 (48.6) | ||
| Left | 260 (51.3) | 165 (51.2) | 95 (51.4) | ||
| Tumor size/cm, M (P25, P75) | 3.5 (2.5, 4.6) | 2.7 (2.1, 3.4) | 5.0(4.4-5.9) | -18.761 | < 0.001 |
| R.E.N.A.L. score, M (P25, P75) | 8 (6, 9) | 6 (5, 8) | 9 (8-10) | 169.419 | < 0.001 |
| Border, n(%) | -2.483 | 0.013 | |||
| Regular | 359 (70.8) | 272 (84.5) | 87 (47.0) | ||
| Irregular | 148 (29.2) | 50 (15.5) | 98 (53.0) | ||
| LN enlargement,n(%) | 1.008 | 0.315 | |||
| No | 488 (96.3) | 312 (96.9) | 176 (95.1) | ||
| Yes | 19 (3.7) | 10 (3.1) | 9 (4.9) | ||
| Surgical strategy, n(%) | 185.390 | < 0.001 | |||
| RN | 186 (36.7) | 47 (14.6) | 139 (75.1) | ||
| PN | 321 (63.3) | 275 (85.4) | 46 (24.9) | ||
| Histology, n(%) | 4.735 | 0.860 | |||
| Clear cell | 431 (85.0) | 275 (85.4) | 156 (84.3) | ||
| Papillary | 22 (4.3) | 11 (3.4) | 11 (6.0) | ||
| Chromophobe | 21 (4.1) | 13 (4.0) | 8 (4.3) | ||
| Others | 33 (6.5) | 23 (7.1) | 10 (5.4) | ||
| WHO/ISUP grade, n(%) | 6.106 | 0.013 | |||
| Ⅰ-Ⅱ | 393 (84.1) | 256 (87.4) | 137 (78.7) | ||
| Ⅲ-Ⅳ | 74 (15.8) | 37 (12.6) | 37 (21.3) | ||
| RSI, n(%) | 59.293 | < 0.001 | |||
| Yes | 75 (14.8) | 18 (5.6) | 57 (30.8) | ||
| No | 432 (85.2) | 304 (94.4) | 128 (69.2) | ||
| PFI, n(%) | 6.058 | 0.014 | |||
| Yes | 19 (3.7) | 7 (2.2) | 12 (6.5) | ||
| No | 488 (96.3) | 315 (97.8) | 173 (93.5) | ||
2.2. 肾窦侵犯相关危险因素分析
根据术后病理结果,将入组患者分为肾窦侵犯(renal sinus invasion,RSI)组和非肾窦侵犯(non-renal sinus invasion,NRSI)组,根据R.E.N.A.L.评分定义,N=1为肿瘤距离肾窦>7 mm,肿瘤距离肾盂肾窦集合系统较远时,肿瘤理论上无法侵犯到肾窦组织,本组数据实际上RSI组中N=1占比为0,与预期相符。因而,进一步将R.E.N.A.L.评分中N=1的病例203例剔除后进行单因素分析。结果提示,NRSI与RSI两组间年龄(P=0.020)、R.E.N.A.L.评分(P<0.001)、R评分(P<0.001)、E评分(P<0.001)、N评分(P<0.001)、肿瘤边界(P<0.001)差异具有统计学意义。发生肾窦侵犯的患者年龄更大,R.E.N.A.L.评分更高(肿瘤更大,更倾向于内生性生长,距离集合系统距离更近),边界更不规则(表 2)。
表 2.
肾窦侵犯组和非肾窦侵犯组间比较(排除N=1)
Comparison between RSI group and NRSI group (N=1 excluded)
| Variables | NRSI (n=229) | RSI (n=75) | Z/χ2 | P |
| RSI, renal sinus invasion; NRSI, non-renal sinus invasion; BMI, body mass index. Other abbreviations as in Table 1. | ||||
| Age/ years, M (P25, P75) | 59.0 (47.0, 65.0) | 63.0 (55.0, 66.0) | -2.325 | 0.020 |
| BMI/(kg/m2),M (P25, P75) | 25.7 (23.4, 27.8) | 24.9 (22.7, 27.5) | -1.108 | 0.268 |
| Complains, n(%) | 0.264 | 0.853 | ||
| Hematuria | 9 (3.9) | 4 (5.3) | ||
| Non-hematuria | 219 (96.1) | 71 (94.7) | ||
| Tumor size/cm, M (P25, P75) | 4.0 (3.2, 5.1) | 4.8 (4.1, 5.7) | -3.782 | <0.001 |
| R.E.N.A.L. score, M (P25, P75) | 9 (8, 9) | 9 (9, 10) | 27.509 | <0.001 |
| R value, n(%) | 16.285 | <0.001 | ||
| 1 | 116 (50.7) | 18 (24.0) | ||
| 2 | 113 (49.3) | 57 (76.0) | ||
| E value, n(%) | 7.908 | 0.019 | ||
| 1 | 82 (35.8) | 14 (18.7) | ||
| 2 | 94 (41.0) | 41 (54.7) | ||
| 3 | 53 (23.1) | 20 (26.7) | ||
| N value, n(%) | 20.335 | <0.001 | ||
| 2 | 67 (29.3) | 3 (4.0) | ||
| 3 | 162 (70.7) | 72 (96.0) | ||
| A, n(%) | 0.042 | 0.853 | ||
| Ventricle | 110 (48.0) | 35 (46.7) | ||
| Dorsal | 119 (52.0) | 40 (53.3) | ||
| L value, n(%) | 0.408 | 0.815 | ||
| 1 | 33 (14.0) | 9 (12.0) | ||
| 2 | 78 (34.1) | 24 (32.0) | ||
| 3 | 119 (52.0) | 42 (56.0) | ||
| Border, n(%) | 20.867 | <0.001 | ||
| Regular | 151 (65.9) | 27 (36.0) | ||
| Irregular | 78 (34.1) | 48 (64.0) | ||
| LN enlargement, n(%) | 0.034 | 1.000 | ||
| No | 218 (95.2) | 71 (94.7) | ||
| Yes | 11 (4.8) | 4 (5.3) | ||
| Histology, n(%) | 7.950 | 0.931 | ||
| Clear cell | 195 (85.2) | 65 (86.7) | ||
| Non-clear cell | 34 (14.8) | 10 (13.3) | ||
| WHO/ISUP grade, n(%) | 0.275 | 0.600 | ||
| Ⅰ-Ⅱ | 168 (80.0) | 58 (81.7) | ||
| Ⅲ-Ⅳ | 42 (18.3) | 12 (16.9) | ||
将年龄、R评分、E评分、N评分、肿瘤边界状态纳入多因素二元Logistic回归分析。结果显示,R评分、E评分和N评分增加,均会增加T1期肾细胞癌发生肾窦侵犯的风险(OR分别为2.34、2.19、5.48,P分别为0.020、0.001、0.007);肿瘤边界不规则也会增加肾窦侵犯的风险(P=0.001,表 3)。
表 3.
T1期肾细胞癌肾窦侵犯相关因素的二元Logistic回归分析
Binary Logistic regression analysis of associated factors of cT1 renal cell carcinoma with renal sinus invasion
| Items | OR (95%CI) | P value |
| Age | 1.02 (1.00-1.05) | 0.062 |
| R value | 2.34 (1.15-4.78) | 0.020 |
| E value | 2.19 (1.39-3.44) | 0.001 |
| N value | 5.48 (1.59-18.91) | 0.007 |
| Border | 2.72 (1.47-5.05) | 0.001 |
3. 讨论
肾细胞癌约占成人恶性肿瘤的2%~3%。近年来随着我国人均期望寿命的提高以及超声、CT等影像检查的普及应用,早期肾细胞癌检出率逐渐增加。大部分文献报道cT1期肾细胞癌占肾细胞癌患者的67.8%~70.3%,其中cT1a期约占42.9%~47.5%,cT1b期约占20%~25%[14-15]。肾细胞癌的T分期是决定治疗方案、预测肿瘤预后及生存的主要指标之一,主要依赖术前增强CT或磁共振成像确定[5]。肾窦位于肾实质与肾盂集合系统之间,由于富含丰富的淋巴和血管,且肾被膜在此处中断,故肿瘤易发生肾窦侵犯[16]。但显微镜下能观察到的肾窦浸润在术前CT检查中难以被发现,因而,临床上会出现部分cT1期肾细胞癌患者术后病理提示肿瘤侵犯肾窦脂肪,即从临床T1期升级为病理T3a期。文献报道,cT1期肾细胞癌术后病理肾窦侵犯比例为3.1%~12.4%[5-7],由于pT3a期还包括肾周脂肪侵犯及脉管侵犯,所以cT1期肾细胞癌术后病理升级为pT3a期的比例更高,为4.8%~22.5%[6-7, 17-21]。本研究回顾性分析的507例cT1期肾细胞癌患者,术后病理证实有14.8%存在肾窦侵犯,其中cT1a期病理升级比例为5.6%,cT1b期病理升级比例为30.8%。
近年,关于肾细胞癌肾窦侵犯的研究主要集中于相关危险因素及肿瘤学预后方面。回顾文献发现,肾细胞癌发生肾窦侵犯的相关危险因素包括年龄[7-8, 10, 19, 22-23]、肿瘤直径[6-8, 10, 17, 19-24]、肿瘤位置[7, 17, 20-21]、外凸率[7, 20-21]、不规则的肾窦边界[6, 18, 25]、不规则的肿瘤形态[6, 18]、肿瘤坏死[6, 21, 25]、肿瘤病理类型[8, 24]、肿瘤病理分级[8, 22-23]。本研究也通过单因素和多因素分析探究了可能提示肾窦侵犯的潜在危险因素,我们发现,cT1期肾细胞癌发生肾窦侵犯的独立危险因素为R.E.N.A.L.评分中的高R评分、高E评分、高N评分以及肿瘤边界不规则。高R评分表明肿瘤体积更大、分期更高,由于本研究主要探索cT1期肾细胞癌发生肾窦侵犯的危险因素,R评分为1即cT1a期,R评分为2即cT1b期,这也与前述cT1a期病理升级比例为5.6%,cT1b期病理升级比例为30.8%的结果相一致; 高E评分表明肿瘤更偏向内生性生长; 高N评分表明肿瘤更贴近肾盂、肾窦或集合系统; 肿瘤边界不规则表明肿瘤可能缺乏假包膜的约束,更易发生侵袭性生长。上述四项危险因素都衍生于肿瘤形态学特征,因此,肿瘤的影像学指标对预测cT1期肾细胞癌发生肾窦侵犯具有重要意义。患者年龄虽与肾细胞癌发生肾窦侵犯相关,但多因素分析结果提示其并非独立危险因素(P值接近显著性水平0.05),这可能与本研究纳入样本量有限相关。既往文献报道肾细胞癌病理类型及病理分级也是肿瘤发生肾窦侵犯的危险因素,但本研究并未得出阳性结果,需要更大样本量或前瞻性研究以探究其对肾细胞癌发生肾窦侵犯的影响。
R.E.N.A.L.评分纳入了五项肾肿瘤影像指标,反映了肿瘤的解剖学特性,最初被用于预测保留肾单位手术困难程度以及对围手术期结局进行评价[13, 26],而肿瘤肾窦侵犯反映了肿瘤的生物学行为,在局部影像上也有所表现。本研究发现高R评分、高E评分、高N评分是临床T1期肾细胞癌病理升级的独立危险因素,有助于术前评估T1期肾细胞癌是否发生肾窦侵犯,这与既往的诸多研究具有内在一致性。Tay等[17]研究发现,R.E.N.A.L.评分中的R评分和L评分是T1期肾细胞癌病理升级的独立危险因素,Veccia等[10]、Gorin等[20]、Mouracade等[21]也同样报道高R.E.N.A.L.评分与肿瘤病理升级相关。
Ni等[6]研究发现,CT或MRI显示肿瘤延伸到窦内,肿瘤-肾窦界限不规则以及肿瘤形状不规则是RSI的独立危险因素。Teishima等[18]研究发现,不规则的肿瘤类型是RSI的独立危险因素,说明肿瘤的边界状态也反映了肿瘤的侵袭能力。肿瘤边界状态一定程度上受肿瘤周围假包膜的影响,假包膜是早期肾细胞癌常见的组织病理特征,是肿瘤向外生长过程中压迫周围组织,导致局部缺血、坏死、纤维化而形成的纤维结缔组织层,具有限制肿瘤外生扩张的作用[27]。假包膜在CT上通常表现为高密度的环周带,尽管CT检测肾细胞癌假包膜的诊断性能较弱,但假包膜的存在限制了肿瘤向外侵袭生长,其边界通常更为清晰规则。随着肿瘤增大,突破假包膜的限制,肿瘤组织生长失去限制,表现出侵袭的特性,肿瘤形态也更多变,同时边界也变得不规则。因此,肿瘤边界状态一定程度上反映了肿瘤的侵袭能力。
作为回顾性研究,本研究存在一定的局限性。第一,本研究数据均来自于同一中心,样本量有限,所得出的结果缺乏多中心复杂条件的验证; 第二,本研究所纳入的指标仍不够全面,例如未纳入患者血液化验指标; 第三,本研究未进一步阐述肾窦侵犯与肿瘤学预后的观测情况,这也是做预测研究最核心的临床价值所在。后续的研究还需进一步扩大样本量,纳入更多的临床指标,获得更为确切的证据。
总之,基于临床和影像学信息判定的cT1期肾细胞癌患者中,有一部分被术后病理证实存在肿瘤肾窦侵犯,分期升级至pT3a期。更大的肿瘤直径(高R评分)、偏内生性生长倾向(高E评分)、肿瘤距离肾窦更近(高N评分)、肿瘤边界不规则可以预测T1期肾细胞癌发生肾窦侵犯的风险。将来需进一步探索上述危险因素与肾窦侵犯对患者远期肿瘤学预后的影响。
References
- 1.Chen WQ, Sun KX, Zheng RS, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Jonasch E, Michaelson MD, et al. NCCN guidelines insights: Kidney cancer, version 2.2020. J Natl Compr Canc Netw. 2019;17(11):1278–1285. doi: 10.6004/jnccn.2019.0054. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol. 2019;75(5):799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Renard AS, Nedelcu C, Paisant A, et al. Is multidetector CT-scan able to detect T3a renal tumor before surgery? Scand J Urol. 2019;53(5):350–355. doi: 10.1080/21681805.2019.1675756. [DOI] [PubMed] [Google Scholar]
- 6.Ni D, Ma X, Li HZ, et al. Factors associated with postoperative renal sinus invasion and perinephric fat invasion in renal cell can-cer: Treatment planning implications. Oncotarget. 2018;9(11):10091–10099. doi: 10.18632/oncotarget.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nocera L, Stolzenbach LF, Ruvolo CC, et al. Predicting the risk of pT3a stage in cT1 clear cell renal cell carcinoma. Eur J Surg Oncol. 2021;47(5):1187–1190. doi: 10.1016/j.ejso.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Nayak JG, Patel P, Saarela O, et al. Pathological upstaging of clinical T1 to pathological T3a renal cell carcinoma: A multi-institutional analysis of short-term outcomes. Urology. 2016;94:154–160. doi: 10.1016/j.urology.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Deng W, Liu X, et al. Impact of pathological T3a upstaging on oncological outcomes of clinical T1 renal cell carcinoma: A meta-analysis. J Cancer. 2019;10(20):4998–5006. doi: 10.7150/jca.32859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veccia A, Falagario U, Martini A, et al. Upstaging to pT3a in patients undergoing partial or radical nephrectomy for cT1 renal tumors: A systematic review and meta-analysis of outcomes and predictive factors [J/OL]. Eur Urol Focus, (2020-06-19) [2021-03-02]. https://linkinghub.elsevier.com/retrieve/pii/S2405-4569(20)30148-6
- 11.Shah PH, Moreira DM, Patel VR, et al. Partial nephrectomy is associated with higher risk of relapse compared with radical nephrectomy for clinical stage T1 renal cell carcinoma pathologically up staged to T3a. J Urol. 2017;198(2):289–296. doi: 10.1016/j.juro.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Russell CM, Lebastchi AH, Chipollini J, et al. Multi-institutional survival analysis of incidental pathologic T3a upstaging in clinical T1 renal cell carcinoma following partial nephrectomy. Urology. 2018;117:95–100. doi: 10.1016/j.urology.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Patel HD, Gupta M, Joice GA, et al. Clinical stage migration and survival for renal cell carcinoma in the United States. Eur Urol Oncol. 2019;2(4):343–348. doi: 10.1016/j.euo.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Laguna MP, Algaba F, Cadeddu J, et al. Current patterns of pre-sentation and treatment of renal masses: A clinical research office of the endourological society prospective study. J Endourol. 2014;28(7):861–870. doi: 10.1089/end.2013.0724. [DOI] [PubMed] [Google Scholar]
- 16.Krishna S, Schieda N, Flood TA, et al. Magnetic resonance imaging (MRI) of the renal sinus. Abdom Radiol (NY) 2018;43(11):3082–3100. doi: 10.1007/s00261-018-1593-1. [DOI] [PubMed] [Google Scholar]
- 17.Tay MH, Thamboo TP, Wu FM, et al. High R.E.N.A.L. nephrometry scores are associated with pathologic upstaging of cli-nical T1 renal-cell carcinomas in radical nephrectomy specimens: Implications for nephron-sparing surgery. J Endourol. 2014;28(9):1135–1142. doi: 10.1089/end.2014.0123. [DOI] [PubMed] [Google Scholar]
- 18.Teishima J, Hayashi T, Kitano H, et al. Impact of radiological morphology of clinical T1 renal cell carcinoma on the prediction of upstaging to pathological T3. Jpn J Clin Oncol. 2020;50(4):473–478. doi: 10.1093/jjco/hyz154. [DOI] [PubMed] [Google Scholar]
- 19.de la Barra CC, Gonzalez PG, Baeza MA, et al. A preoperative model to predict pT3 upstaging in clinically localized renal cell carcinoma. Cent European J Urol. 2020;73(2):173–177. doi: 10.5173/ceju.2020.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: A multi-institutional analysis. J Urol. 2013;190(5):1907–1911. doi: 10.1016/j.juro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Mouracade P, Kara O, Dagenais J, et al. Perioperative morbidity, oncological outcomes and predictors of pT3a upstaging for patients undergoing partial nephrectomy for cT1 tumors. World J Urol. 2017;35(9):1425–1433. doi: 10.1007/s00345-017-2004-x. [DOI] [PubMed] [Google Scholar]
- 22.Ghanie A, Formica MK, Wang D, et al. Pathological upstaging of clinical T1 renal cell carcinoma: An analysis of 115, 835 patients from National Cancer Data Base, 2004-2013. Int Urol Nephrol. 2018;50(2):237–245. doi: 10.1007/s11255-017-1768-7. [DOI] [PubMed] [Google Scholar]
- 23.Jeong SH, Kim JK, Park J, et al. Pathological T3a upstaging of clinical T1 renal cell carcinoma: Outcomes according to surgical technique and predictors of upstaging. PLoS One. 2016;11(11):e0166183. doi: 10.1371/journal.pone.0166183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramaswamy K, Kheterpal E, Pham H, et al. Significance of pathologic T3a upstaging in clinical T1 renal masses undergoing nephrectomy. Clin Genitourin Cancer. 2015;13(4):344–349. doi: 10.1016/j.clgc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Sokhi HK, Mok WY, Patel U. Stage T3a renal cell carcinoma: Staging accuracy of CT for sinus fat, perinephric fat or renal vein invasion. Br J Radiol. 2015;88(1045):20140504. doi: 10.1259/bjr.20140504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin D, Zhang J, Zhang Y, et al. A combination of the Mayo adhesive probability score and the R.E.N.A.L. score to predict intraoperative complications in small renal masses. Urol Int. 2020;104(1/2):142–147. doi: 10.1159/000504767. [DOI] [PubMed] [Google Scholar]
- 27.Tsili AC, Argyropoulou MI. Advances of multidetector computed tomography in the characterization and staging of renal cell carcinoma. World J Radiol. 2015;7(6):110–127. doi: 10.4329/wjr.v7.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]