Summary
Hematopoietic stem cell transplantation (HSCT) is a frequent therapeutic approach to restore hematopoiesis in patients with hematologic diseases. Patients receive a hematopoietic stem cell (HSC)-enriched donor cell infusion also containing immune cells, which may have a beneficial effect by eliminating residual neoplastic cells. However, the effect that donor innate immune cells may have on the donor HSCs has not been deeply explored. Here, we evaluate the influence of donor natural killer (NK) cells on HSC fate, concluded that NK cells negatively affect HSC frequency and function, and identified interferon-gamma (IFNγ) as a potential mediator. Interestingly, improved HSC fitness was achieved by NK cell depletion from murine and human donor infusions or by blocking IFNγ activity. Thus, our data suggest that suppression of inflammatory signals generated by donor innate immune cells can enhance engraftment and hematopoietic reconstitution during HSCT, which is particularly critical when limited HSC numbers are available and the risk of engraftment failure is high.
Keywords: hematopoietic stem cell, hematopoietic stem cell transplantation, natural killer cell, C/EBPgamma, interferon-gamma
Graphical abstract
Highlights
-
•
NK cells negatively affect HSC function in culture and in vivo
-
•
NK cell depletion from mouse and human donor samples improves HSC fitness
-
•
C/EBPγ deficiency partially rescues NK cell detrimental effect on HSCs
-
•
IFNγ-neutralizing antibody diminishes the NK cell detrimental effect on HSC
The role of natural killer (NK) cells during bone marrow transplantation is not completely understood. Figueiredo-Pontes et al. demonstrate that NK cells impair hematopoietic stem cell (HSC) function via cytokine-mediated mechanisms, and show that NKs depletion from donor cell preparations or neutralizing IFNγ activity improves HSC function and transplantation outcome.
Introduction
Hematopoietic stem cell transplantation (HSCT) is the only curative therapy for many hematologic diseases where hematopoiesis needs to be replaced by healthy hematopoietic cells. The success of HSCT mostly depends on the speed and quality of the hematopoietic recovery in the early post-transplantation phase. Although the main goal of the procedure is to inject hematopoietic stem cells (HSCs) with high self-renewal potential to fully reestablish hematopoiesis, blood- or bone marrow-derived donor stem cell sources also contain committed progenitors and immune cells. Presence of immune cells in the donor sample has been associated with prevention of infections (Chen et al., 2006; Storek et al., 1997; Tomblyn et al., 2010) and graft-versus-tumor effects (Venstrom et al., 2010; Wagner et al., 2005). However, besides these well-known effects, it may be hypothesized that immune cells from the graft can also affect donor HSC properties and modify engraftment.
Recent evidence has elucidated that hematopoietic cells, particularly T lymphocytes, known for their ability to trigger inflammatory responses, may directly or indirectly affect the function of hematopoietic stem and progenitor cells (Geerman and Nolte, 2017). Hirata and colleagues showed that bone marrow (BM) cluster of differentiation (CD) 4 memory T cells and memory regulatory T cells (Tregs) coordinate each other to generate extracellular adenosine via CD39 and CD73, maintaining HSC quiescence (Hirata et al., 2018). Similarly, memory CD8+ T cells support the maintenance of HSCs in the BM (Geerman et al., 2018). Nevertheless, in a transplantation setting using a xenotransplant model, it was demonstrated that donor-derived activated memory T cells present in unfractionated umbilical cord blood (UCB) were associated with reduced HSC engraftment in comparison with CD34+ fractionated UCB. This effect was attributed to tumor necrosis factor alpha (TNFα) cytokine produced by these memory T cells and was reverted by TNFα receptor inhibition (Wang et al., 2017). On the contrary, several studies showed that, when HSCs are limiting, memory CD8+ T cells have a beneficial effect on HSPC engraftment, both in allogeneic and autologous transplantation (Russell et al., 2015; Touzot et al., 2015; Triplett et al., 2015). Altogether, these observations highlight the relevance of the immune cells present in the graft, and question their presence as an advantage or disadvantage in a transplantation setting.
Natural killer (NK) cells are lineage-specific lymphocytes with effector functions of cytotoxicity and cytokine production. NK cell activity depends on a dynamic balance between the expression of activating receptors (which recognize stress-induced ligands on infected or tumor cells), inhibitory receptors (which predominantly bind major histocompatibility complex [MHC] class I molecules), and cytokine receptors (Kim et al., 2005; Vivier et al., 2008). Upon stimulation, NK cells are also able to produce cytokines such as interleukin (IL)-1, IL-3, IL-4, IL-5, IL-6, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), TNFα, and IFNγ, which can potentially influence the properties of self-renewal, apoptosis, mobilization, and differentiation of HSCs and determine different outcomes to hematopoiesis (Baldridge et al., 2010; Buza-Vidas et al., 2006; Schuettpelz and Link, 2013; Zhang and Lodish, 2008). However, besides its cytotoxic effects mediated by HLA missense recognition signals in HSCT, a potential role in the regulation of HSC functions in transplantation has not been explored.
In the transplant setting, the immune content of the graft may be activated or modified by the pre-transplant conditioning regimen based on chemotherapy and/or radiotherapy (Joncker et al., 2010; Mehta et al., 2015). Both T and NK cells can recognize and kill residual tumor cells, but while the activation of T cells by the recipient's antigen-presenting cells often causes graft-versus-host disease (GvHD), a potentially lethal complication of the procedure, NK cells are capable of eliminating tumor cells without being involved in GvHD (Raziuddin et al., 2002; Ruggeri et al., 2002). Thus, differently from T cells, NK cells have been traditionally recognized as transplant helpers. However, the effects of donor NK cells based on their secretory potential on HSC function during transplantation so far remain unknown.
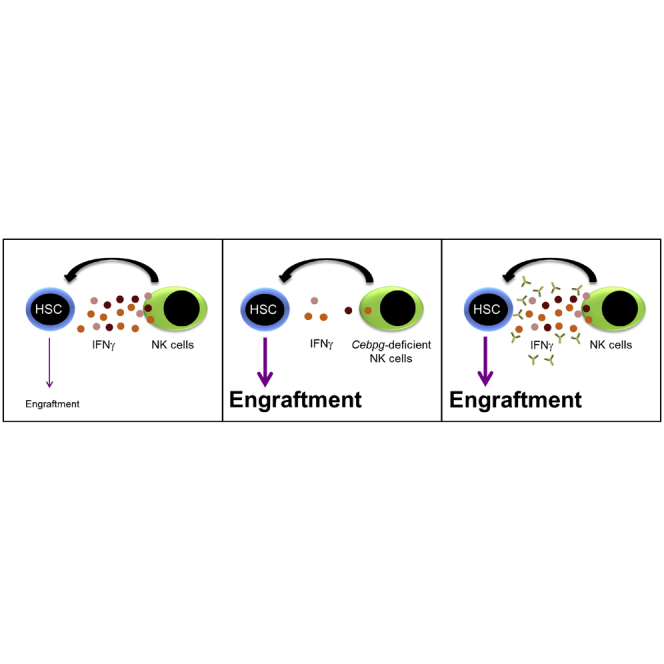
In the present study, we showed that NK cells impair HSC function in culture and in vivo. Our results demonstrated that NK cells from the graft negatively influence HSC engraftment and hematopoietic recovery upon BM transplantation. By using CCAAT-enhancer-binding protein gamma (Cebpg) conditional knockout (KO) mice, we revealed that the effect is mediated by the production of cytokines such as IFNγ. Importantly, addition of IFNγ-blocking antibodies to HSC-NK co-cultures prevented the NK detrimental effect on HSC, and administration of IFNγ-blocking antibodies during the early phase post transplant enhanced human HSC engraftment and hematopoietic reconstitution. Altogether, our study demonstrates that donor NK cells present in the graft can negatively affect the early post-transplantation phase, and that modulating inflammatory signals such as IFNγ can contribute to improved engraftment during HSCT.
Results
NK cells reduce HSC maintenance and function in culture
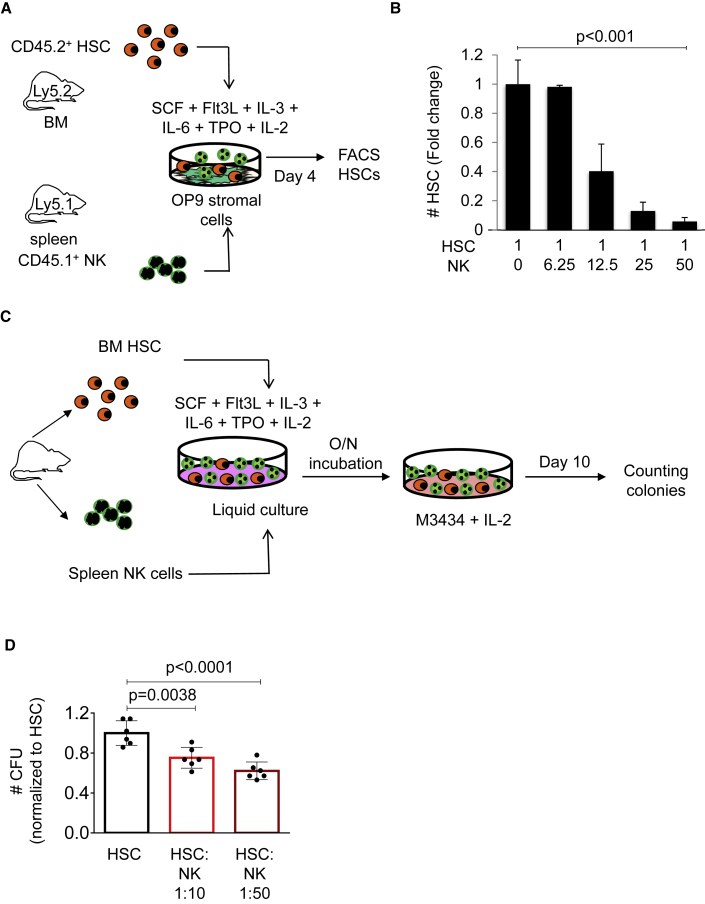
To investigate potential HSC alterations when exposed to NK cells, we developed a co-culture system of sorted murine HSCs and NK cells (Figure 1A). HSCs were defined by cell surface markers as lineage negative (Lin−), Sca-1+, c-kit+, CD48−, CD150+, and NK cells as Lin−, CD3−, and NK1.1+. Co-cultures were established on an OP9 stromal cell layer with appropriate HSC culture conditions in the presence of the NK cell activating cytokine IL-2 (Figure 1A). Using fluorescence-activated cell sorting (FACS) analysis, we observed a reduction of phenotypically defined HSCs after 4 days of culture when NK cells were present. This reduction was dependent on the NK/HSC ratio, and was more profound as the number of NK cells in culture increased (Figures 1B and S1). Next, we performed colony culture assays to assess whether the presence of NK cells would compromise HSC function in vitro (Figure 1C). Colony-forming units were enumerated at day 10 of culture, and we observed a significant reduction in the presence of NK cells. Similar to our previous result, the number of colonies was reduced in a dose-dependent manner according to the increasing number of NK cells (Figure 1D). Altogether, these experiments suggest that NK cells can negatively influence HSC frequency and function in culture.
Figure 1.
NK cells reduce HSC maintenance and function in culture
(A) Graphical representation of the experimental design. Co-cultures were established in stem cell medium containing stem cell factor (SCF), Flt3-ligand, IL-3, IL-6, thrombopoietin (TPO), and IL-2 over an OP9 stromal cell layer using sorted BM CD45.2+ HSCs (lin− c-Kit+ Sca-1− CD48− CD150+) and SP CD45.1+ NK cells (Ter119− CD19− CD4− CD8− CD3− NK1.1+). Days of culture are indicated. HSCs were enumerated by FACS analysis.
(B) Quantification of HSCs recovered from 4-day cultures by FACS analysis. Y axis indicates the absolute number of HSCs. X axis indicates presence (+) or absence (−) of NK cells in culture (ratios are indicated). Values and error bars indicate medians ±SEM. n = 3 biological samples in each condition. Jonckheere-Terpstra trend test was used to assess statistical significance (p value is indicated).
(C) Experimental design of in vitro colony culture assays.
(D) Quantification of colonies at day 10 of culture. Y axis indicates the number of CFU relative to the HSC counts. X axis indicates cells present in the semi-solid cultures. Two different HSC/NK cell ratios were used as indicated. Data indicate mean ± SD of three independent biological triplicates. Each dot represents one culture well. Two-tailed Student's t test was used to assess statistical significance (p values are indicated).
NK cells affect murine HSC repopulation capacity in vivo
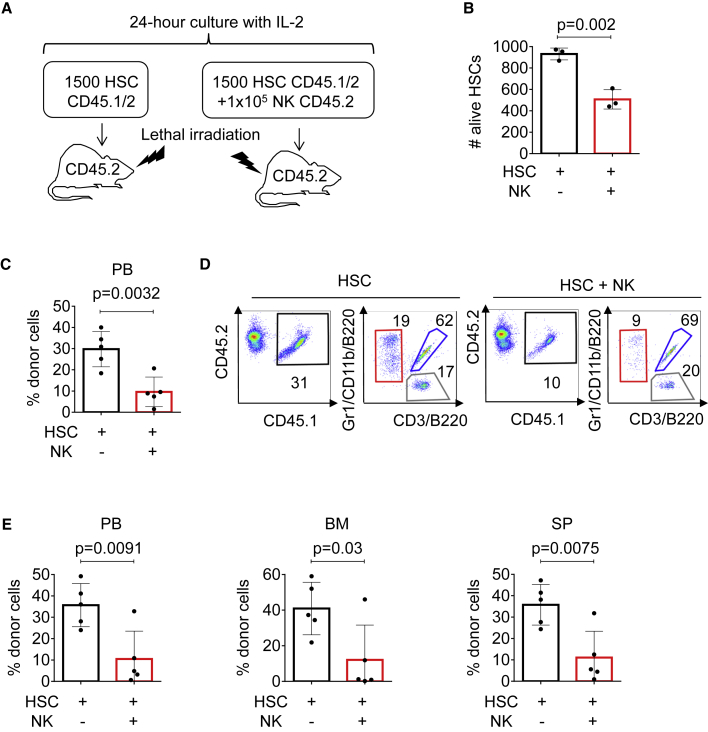
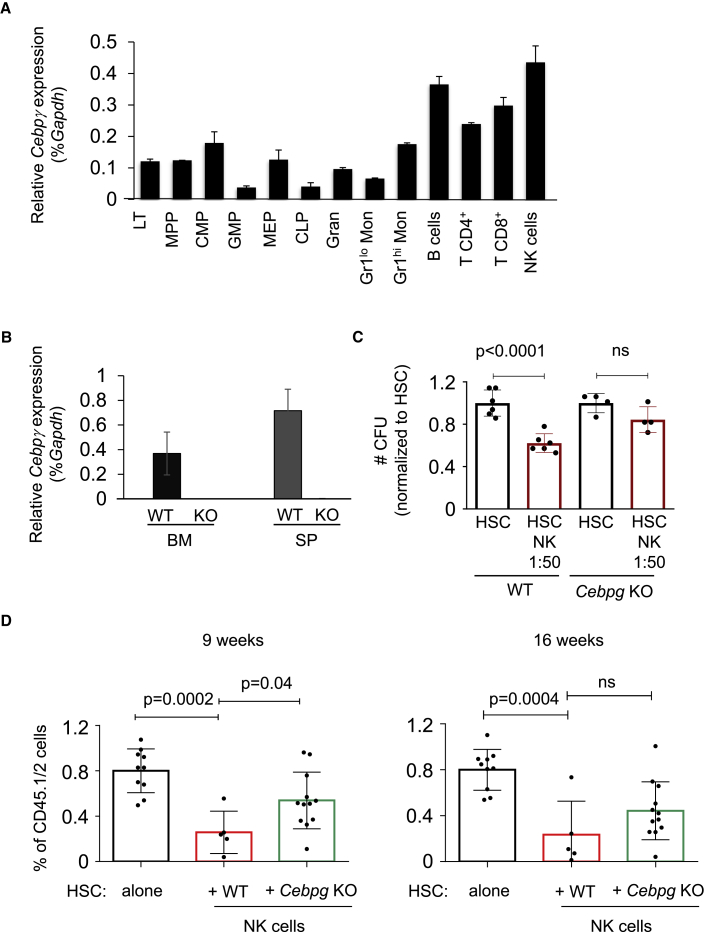
Next, we investigated whether NK cells alter HSC properties in vivo. Sorted CD45.1/2 HSCs were cultured alone or co-cultured with CD45.2 NK cells (ratio of 1 × 105 NK cells to 1,500 HSCs) overnight, and injected into lethally irradiated CD45.2 mice (Figure 2A). Importantly, we enumerated HSCs post overnight culture and prior to transplantation, and observed a reduced number of remaining viable HSCs in cell suspensions containing NK cells compared with suspensions without NK cells (Figure 2B). Nine weeks after transplantation, peripheral blood analysis demonstrated a reduction of CD45.1/2 cells when HSCs were exposed to NK cells prior to transplantation (Figure 2C). However, NK-exposed HSCs showed no skewing to myeloid, B, or T cell differentiation (Figures 2D and S2A). Next, we assessed the long-term effects of exposing HSCs to NK cells overnight, and observed a significantly reduced percentage of CD45.1/2 cells in blood, BM, and spleen (SP) of recipient mice 16 weeks after transplantation (Figure 2E). Importantly, homing assays demonstrated that addition of NK cells to the donor cell preparations did not alter HSC homing (Figure S2B). Together, these results demonstrated that NK cells negatively affect the numbers of functional HSCs, and, therefore, reduce their hematopoietic reconstitution ability compared with non-exposed HSCs.
Figure 2.
NK cell exposure compromises HSC engraftment in vivo
(A) Experimental strategy for NK and HSCT assays. HSCs from C57BL/6 CD45.1/2 mice were purified by sorting, cultured in the presence or absence of NK cells (Ter119− CD19− CD4− CD8− CD3− NK1.1+) obtained from C57BL/6 CD45.2 mice, and injected into lethally irradiated CD45.2 recipient animals in a ratio of 105 NK cells to 103 HSCs per mouse.
(B) Number of HSCs assessed by FACS. Y axis indicates number of alive Hoechst 33,258− HSCs after overnight incubation. X axis indicates culture conditions.
(C) Flow cytometry analysis of recipient mice peripheral blood 9 weeks after transplantation. Y axis indicates the percentage of donor-derived CD45.1/2+ cells. X axis indicates different culture conditions.
(D) Representative flow cytometry plots of recipient mice peripheral blood 9 weeks after transplantation. Plots show CD45.1 versus CD45.2 expression in HSC and HSC + NK transplanted mice. Black box indicates the percentage of CD45.1/2+ donor-derived cells and the gate used to analyze tri-lineage contribution: red box indicates the percentage of myeloid cells (Gr1/CD11b+), blue box the percentage of B cells (B220+), and gray box the percentage of T cells (CD3+). See also Figure S2.
(E) FACS analysis of peripheral blood (PB), BM, and SP isolated from recipient mice 16 weeks after transplantation. Y axes indicate the percentage of donor CD45.1/2+ cells and X axes indicate distinct culturing conditions. Five animals were included in each group. All data represent mean ± SD from one representative experiment out of three. Two-tailed Student's t test was used to assess statistical significance (p values are indicated).
NK cells present in murine BM grafts compromise stem cell function
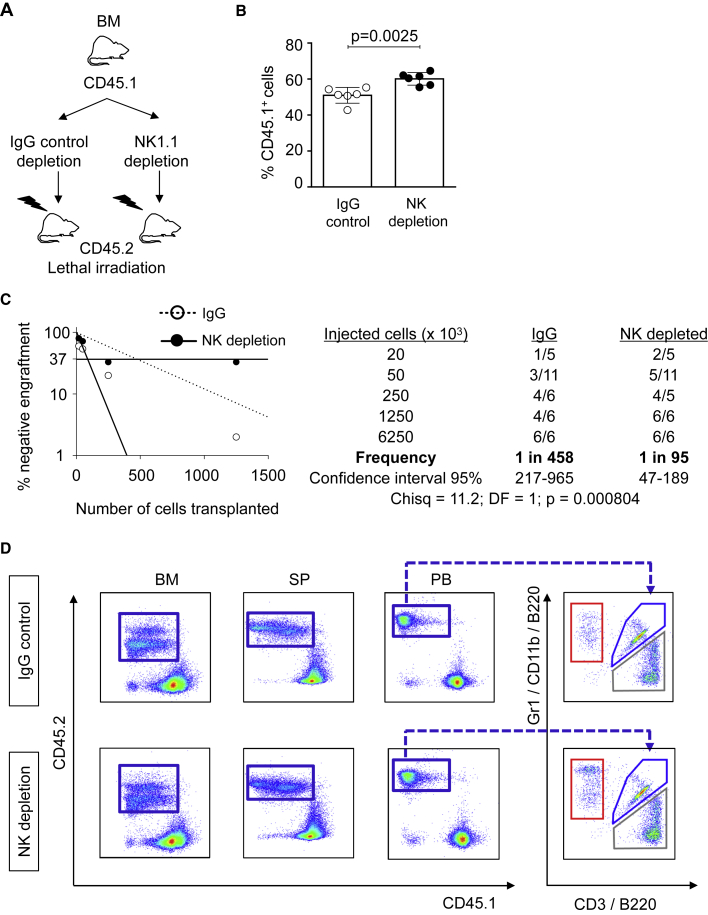
Since our previous results demonstrated that co-culturing NK cells and HSCs lead to HSC impaired repopulation capacity, we hypothesized that NK cells and HSC co-habitation in the BM donor samples could cause intrinsic changes to HSC function. To test this hypothesis, we first assessed whether removal of NK cells from total BM would improve short-term reconstitution. Whole BM from CD45.1 mice was submitted to immunomagnetic NK1.1 (NK cells) or immunoglobulin (Ig) G (control) depletion and injected into lethally irradiated CD45.2 mice (Figure 3A). NK cell depletion was verified by flow cytometry analysis prior to transplantation (Figure S3A). Donor chimerism after 8 weeks of transplant showed that NK cell depletion from the BM improved the short-term engraftment ability of HSC (Figure 3B). Next, we performed limiting dilution transplantation assays following the same NK cell depletion strategy (Figure 3A), and assessed whether the long-term HSC properties would be also affected by NK cells. Limiting dilution transplantation assays allowed us to measure the frequency of repopulating units, which reflects the number of functional HSCs. Positive engraftment was defined as >0.3% and at least two-lineage reconstitution. We observed a 5-fold increased number of functional HSCs when NK cells were removed prior to transplantation (p < 0.000804) (Figure 3C). Further, NK cell depletion from the BM graft did not affect the differentiation pattern of lymphoid or myeloid lineages (Figures 3D and S3B). Altogether, our results demonstrated that NK cell removal from donor BM in murine transplantation assays improves the short-term as well as long-term transplantation outcome without altering the lineage hematopoietic recovery.
Figure 3.
NK cell depletion from murine BM improves stem cell function
(A) Overview of the experimental strategy for the BM transplantation assay after NK cell depletion (NK1.1) or irrelevant IgG depletion (control). Upon depletion, cells were injected into lethally irradiated CD45.2 animals.
(B) FACS analysis of peripheral blood of recipient mice. Y axis indicates the percentage of donor CD45.1+ cells 9 weeks after transplantation. X axis indicates IgG control or NK cell depletion. In each group 6.25 × 106 cells were transplanted into recipient mice. Six animals were included in each group. All data represent mean ± SD from one representative experiment out of two. Two-tailed Student's t test was used to assess statistical significance (p value is indicated). See also Figure S3.
(C) Panels indicate results from limiting dilution competitive repopulation unit assays. (Left) Logarithmic plot showing the percentage of negative recipients transplanted with different cell doses of murine BM depleted with NK1.1 Ab (black dots) or control IgG Ab (white dots). Only recipients at 16 weeks with engraftment of CD45.1 cells ≥0.1% and contribution to all lineages (T cells, B cells, and granulocytes) higher than 1% were considered responders. (Right) Table showing the number of responders and the total number of recipients transplanted per cell dose. Frequencies of HSCs (1:95 in NK1.1-depleted BM transplants versus 1:458 in IgG-depleted control, p = 0.000804) were calculated according to Poisson statistics using ELDA software based on data from two independent experiments (Chisq, chi-square test).
(D) Representative flow cytometry dot plots showing the percentages of CD45.1+ donor cells (blue boxes, Y axes) and CD45.2+ host cells (X axes). Plots show BM, SP, and PB of mice injected with IgG-depleted control (upper panels) and NK-depleted (lower panels) BM cells 16 weeks after transplant. The panels on the right refer to gated CD45.1+ PB and indicate T (gray box), B (blue box), and myeloid (red box) cells, as determined by the use of antibodies against CD3, B220, and Gr1/CD11b, respectively.
NK cells negatively affect human CD34+ cells in vitro and in vivo
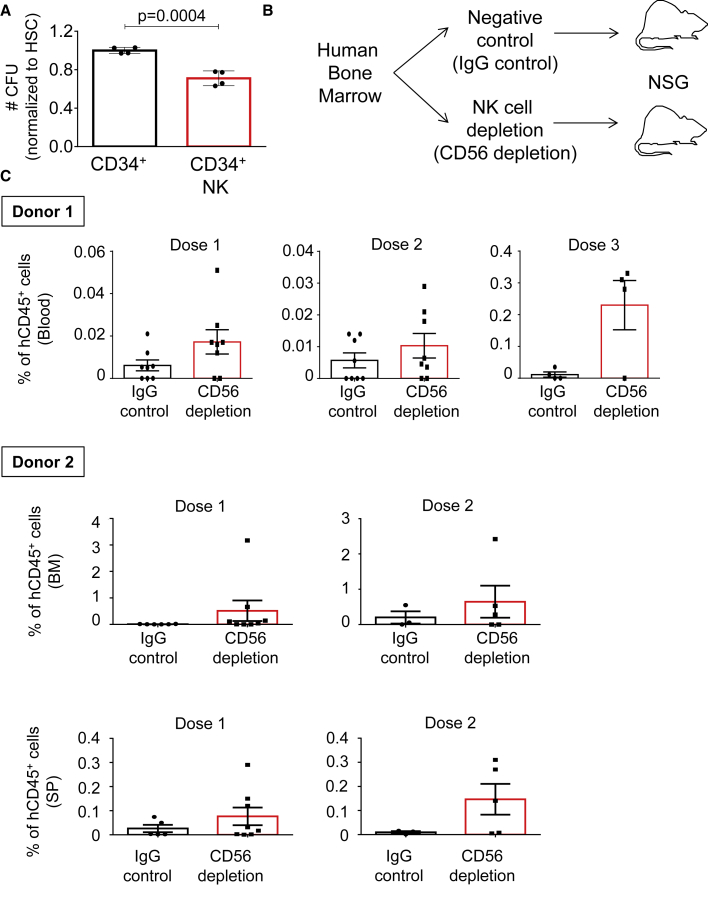
Next, we assessed whether the effects that NK cells have on murine HSCs would translate to human primary samples. Human HSCs were isolated from BM (CD34+), co-cultured overnight in the presence of human IL-2, with or without human NK cells (CD19− CD8− CD4− CD3− CD56+), and placed in semi-solid media supplemented with human IL-2 (same strategy as in Figure 1C). The number of colonies was enumerated at day 10 of culture, in which we observed colony-forming unit (CFU) reduction in the presence of NK cells, suggesting that NK cells can harm human HSCs’ ability to form colonies (Figure 4A). Since NK cell depletion during murine BM transplantation assays improved hematopoietic engraftment and reconstitution, we next performed experiments to determine how depletion of NK cells from human grafts could affect HSCT. NK cell-depleted (CD56) or non-depleted (IgG) human BM samples were transplanted into sublethally irradiated immunodeficient NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice at different doses (Figure 4B). CD3 antibodies were included in the depletion cocktail to remove human T cells from the graft, to prevent GvHD. The efficiency of immunomagnetic depletion of NK cells was demonstrated by flow cytometry analysis prior to BM transplantation (Figure S4). The engraftment and contribution of human BM cells to the hematopoietic system of recipient mice was determined by the percentage of human CD45+ cells in blood, BM, and SP 14 weeks after transplantation. We observed that ablation of NK cells from human BM grafts had a positive effect in the transplants, leading to better reconstitution and engraftment of human cells in recipient mice (Figure 4C). These positive effects after NK cell removal were visible in different cell doses and in two independent experiments using different healthy BM grafts; however, no statistical significance was reached and we could only observe a tendency in our human transplantation assays. Altogether, in vitro and in vivo experiments suggest that NK cells can impair HSC function, which can be restored by NK cell depletion.
Figure 4.
Engraftment of human BM stem cells is optimized by NK cell removal
(A) Human CFU assays of CD34+ cells with or without NK cells (ratio 1:10), after overnight culture in the presence of human IL-2. Y axis indicates the mean CFU number ±SD from two distinct human BM samples relative to CD34+ cells cultured alone. X axis indicates the distinct culture conditions. Each black dot indicates values for one culture well. Two-tailed Student's t test was used to assess statistical significance (p value is indicated).
(B) Illustration of the experimental strategy. Human BM samples depleted with antibodies against CD3 and IgG (negative control) or against CD3 and CD56 (NK cell depletion) were transplanted into sublethally irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice at different doses.
(C) The percentage of human CD45+ donor cells in murine blood, BM, and SP determined 14 weeks after transplantation. Results for two independent experiments using two healthy BM donors (donor 1 and donor 2) are shown. Y axes indicate the percentage of human CD45+ cells in the indicated tissues. Black boxes indicate animals transplanted with IgG-depleted control BM and red boxes indicate animals transplanted with NK-depleted (CD56 depletion) BM. Transplanted cell doses are indicated as dose 1 (0.5 × 106), dose 2 (1 × 106), and dose 3 (2.5 × 106). Data represent mean ± SD from two independent experiments. Two-tailed Student's t test was used to assess statistical significance, p values were not statistically significant.
See also Figure S4.
Cebpg KO NK cells exhibit milder effects than wild-type NK cells on HSC function
Next, since the transcription factor C/EBPγ was related to NK cell function and Cebpg-deficient NK cells were severely impaired in their cytotoxicity against target cells (Kaisho et al., 1999), we investigated the effect of Cebpg-deficient NK cells on HSC function. First, we analyzed Cebpg expression in distinct hematopoietic populations, and observed that Cebpg mRNA expression is higher in the lymphoid than myeloid lineage and BM HSPCs (Figure 5A). The highest Cebpg mRNA expression was detected in NK cells. Next, we took advantage of a Cebpg conditional KO mouse model generated in our laboratory (Kardosova et al., 2018). Cebpg conditional KO mice were crossed to Vav-iCre transgenic mice in order to induce Cebpg excision in the hematopoietic compartment. We generated Cebpgflox/flox Vav-iCre− (wild-type [WT]) and Cebpgflox/flox Vav-iCre+ (Cebpg KO) mice. Absence of Cebpg was confirmed by mRNA quantification in BM and SP (Figure 5B). Frequency of Cebpg KO NK cells was not altered, and expression of NK cell-activating receptors was similar in KO and WT mice (Figures S5A and S5B). However, purified Cebpg KO NK cells stimulated with IL-2 had lower cytotoxicity against murine YAC-1 lymphoma cells (Figure S5C), indicating that Cebpg-deficient NK cells exhibit reduced functionality. Next, we assessed whether Cebpg KO NK cells would be able to affect HSC colony-forming abilities. Interestingly, we observed that the deleterious effect of NK cells was abolished when Cebpg was absent in NK cells, demonstrating that non-functional NK cells do not harm HSCs in vitro (Figure 5C). Further, we investigated whether Cebpg KO NK cells would impair HSC function in vivo. Using similar HSC and NK cell co-culture conditions as previously described in Figure 2A, HSCs were isolated from WT CD45.1/2 mice and NK cells were isolated from either WT or Cebpg KO mice (CD45.2), co-cultured overnight, and were injected into lethally irradiated CD45.2 recipients. Prior to transplantation, we enumerated HSCs in the co-cultures and observed that Cebpg KO NK cells do not have the ability to reduce the number of viable HSCs, in contrast to WT NK cells (Figure S5D). Flow cytometric analysis of recipient mice showed that the effect of Cebpg-deficient NK cells was much milder than the effect of WT NK cells (Figure 5D). This partial rescue was present 9 and 16 weeks after transplantation, and the contribution to myeloid, B cells, and T cells was similar in all three groups (Figure S5D). Together, our in vitro and in vivo experiments demonstrated that Cebpg KO mice produce normal numbers of NK cells, which are functionally impaired, and do have milder effects than WT NK cells during HSCT.
Figure 5.
Defective Cebpg KO NK cells exhibit milder effects on HSC function than WT NK cells
(A) Relative Cebpg expression levels shown as the percentage of Gapdh (% Gapdh) in distinct sorted hematopoietic cell populations. LT (long-term HSC), MPP (multi-potent progenitor), CMP (common myeloid progenitor), GMP (granulocyte-macrophage progenitor), MEP (megakaryocyte-erythroid progenitor), CLP (common lymphoid progenitor), Gran (granulocyte), Gr1lo Mon (Gr1lo monocyte), Gr1hi Mon (Gr1hi monocyte), B cells, T CD4+ cells, T CD8+cells, and NK cells.
(B) Cebpg mRNA levels in BM and SP isolated from WT and Cebpg KO mice. Y axis indicates relative Cebpg expression levels relative to Gapdh (% Gapdh).
(C) Colony culture assays of murine cells. Y axis indicates the number of CFU relative to HSC condition. X axis indicates culture conditions: HSC alone or in the presence of WT or Cebpg KO NK cells. Cell ratio is indicated. Each dot represents one culture dish. Data represent mean ± SD from three independent experiments.
(D) Engraftment of CD45.1/2 cells in blood of recipient CD45.1 mice evaluated 9 (left) and 16 weeks (right) after transplantation. X axis indicates transplant conditions where purified HSCs alone or with WT or Cebpg KO NK cells were co-cultured overnight in the presence IL-2 prior to transplantation. Y axis demonstrates the percentage of CD45.1/2 cells. Each dot indicates values for one animal. All data represent mean ± SD from two independent experiments. For (C) and (D), two-tailed Student's t test was used to assess statistical significance (p values are indicated; ns, not significant).
See also Figure S5.
The regulatory effects of NK cells on stem cells are cytokine mediated
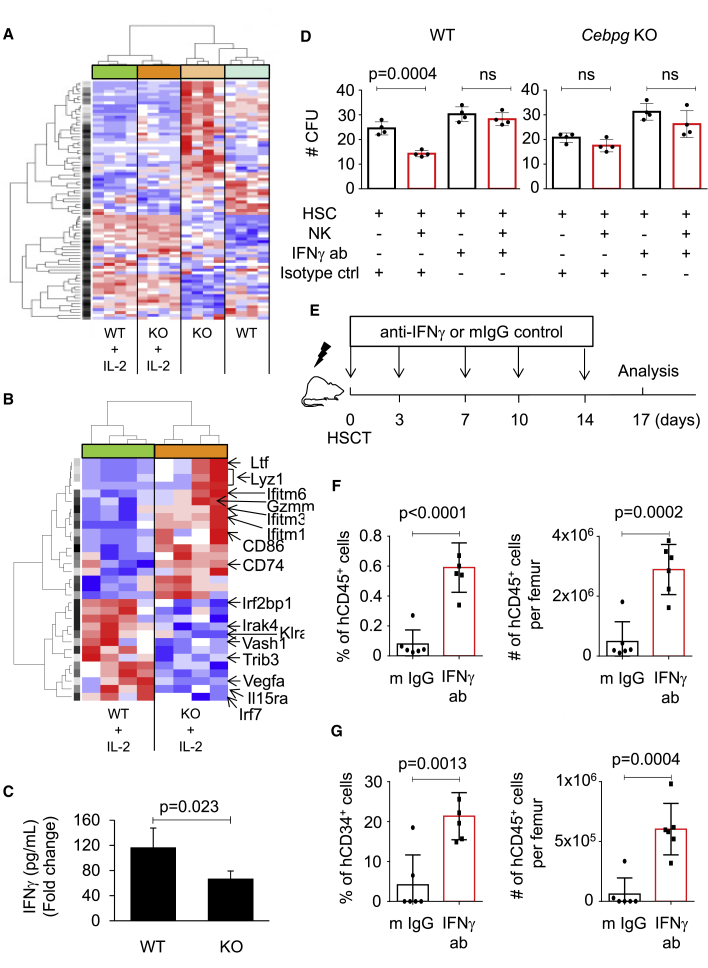
Since Cebpg-deficient NK cells were not able to affect the hematopoietic reconstitution of recipient mice, we investigated potential mediators of HSC regulation by NK cells by comparing the gene expression profile of WT and Cebpg KO NK cells. NK cells were sorted from WT and Cebpg KO mice, cultured overnight with IL-2 or vehicle control, and gene expression profiling was performed (E-MTAB-5604). Using the LIMMA package to assess differential gene expression, we identified 1,074 genes deregulated in WT versus Cebpg KO NK cells (p < 0.05), and 1,316 genes deregulated in IL-2-treated WT versus IL-2-treated Cebpg KO NK cells (p < 0.05). Using prediction analysis of microarrays, we identified and selected 84 probe sets, corresponding to 75 genes, significantly up- or downregulated in WT and Cebpg KO NK cells in the presence or absence of IL-2 (Table S1). Figure 6A shows a heatmap and hierarchical clustering according to expression of the 84 probe sets. These results identified an NK signature characterized by a set of genes targeted upon NK activation. Pathway analysis of differentially expressed mRNA demonstrated cell pathways associated to deregulated genes in WT versus Cebpg KO NK cells (Table S2). Since we observed more pronounced effects on HSC function when NK cells were exposed to IL-2, we further performed pathway analysis in IL-2 treated samples. Our results demonstrated that the IFNγ signaling pathway was the top pathway among deregulated genes, suggesting that it might contribute to NK cell effects on HSC (Table 1). To gain further insights into the mechanisms mediating the NK cell effects, we analyzed differentially expressed genes between WT and Cebpg-deficient NK cells treated with IL-2. Figure 6B shows a heatmap and hierarchical clustering according to expression of 29 genes present in the NK signature. Within the upregulated genes in the Cebpg KO NK cells, we identified lactotransferrin (Ltf), lysozyme 1 (Lyz1), granzyme M (Gzmm), and interferon-induced transmembrane proteins 1, 3, and 6 (Ifitm1, Ifitm3, and Ifitm6). Within the downregulated genes in the Cebpg KO NK cells, we identified interferon regulatory factor 2-binding protein (Irf2pb1), vascular endothelial growth factor A (Vegfa), and IL-15 receptor alpha chain (Il15rα).
Figure 6.
Cebpg deletion results in reduced IFNγ production in NK cells and blocking IFNγ signaling improves HSC fitness
(A) Heatmap and hierarchical clustering based on gene expression of 84 probe sets, which compose the NK signature. NK cells were isolated from WT and Cebpg KO murine SPs in non-stimulation conditions or upon stimulation with the NK cell-activating cytokine IL-2 (n = 4 for each condition). Data were normalized to z scores for each gene. Red/blue color indicates increase/decrease in gene expression relative to the universal mean for each gene. See also Table S1.
(B) Heatmap and hierarchical clustering according to expression of 29 genes present in the NK signature. WT and Cepbg KO NK cells were treated with IL-2 prior to gene expression profile analysis. Several genes deregulated between the two groups are indicated.
(C) IFNγ levels in supernatants after culturing overnight WT or Cebpg KO NK cells. Cultures were established in the absence or presence of IL-2. Y axes indicate cytokine levels (pg/mL) related to the condition without IL-2 for WT and KO NK cells (fold change). n = 4 mice per group, two independent experiments.
(D) Colony culture assays of murine cells. Y axes indicate the number of CFU. X axes indicate culture conditions: NK cells correspond to WT or Cebpg KO mice as indicated. Each dot represents one culture well, two independent experiments were performed.
(E) Illustration of the experimental scheme. Arrows and numbers indicate days when treatment was administered. Seventeen days after transplantation, recipient NSG mice were sacrificed and analyzed.
(F and G) Relative percentage (left graphics) and absolute number of cells per femur (right graphics). Y axes indicate the percentage (%) and numbers (#) of human CD45+ cells (F) and CD34+ cells (G) in BM. Black boxes indicate values for mice that received treatment with mouse IgG control antibodies, and red boxes indicate values for mice that received IFNγ-blocking antibody treatment. Each animal is indicated by a symbol (n = 5–6 animals per group). For (C), (D), (F) and (G), data represent mean ± SD, two-tailed Student's t test was used to assess statistical significance (p values are indicated; ns, not significant).
Table 1.
Cebpg ablation results in differential pathway enrichment analysis in WT and Cebpg KO NK cells stimulated with IL-2
| Index | Name | p value | Z score | Combined score |
|---|---|---|---|---|
| 1 | interferon-gamma signaling pathway | 0.01414 | −1.57 | 1.56 |
| 2 | insulin/IGF pathway- mitogen activated protein kinase kinase/MAP kinase cascade | 0.01625 | −1.47 | 1.46 |
| 3 | apoptosis signaling pathway | 0.01821 | −1.40 | 1.39 |
| 4 | oxidative stress response | 0.02026 | −0.78 | 0.77 |
| 5 | T cell activation | 0.02227 | −1.20 | 1.20 |
Results of Panther pathway analysis of IL-2-treated WT versus Cepbg KO NK cells with the threshold of p < 0.05.
In parallel, supernatants from the overnight cultures were analyzed by a flow cytometry-based cytokine bead array, which allows identification and quantification of a panel of cytokines. We observed that, upon NK cell activation, IFNγ production was diminished in Cebpg-deficient NK cells in comparison with WT NK cells (Figure 6C). Altogether, our findings identified a list of genes and pathways potentially mediating the negative NK cell effects on HSC function, and suggested that IFNγ production by NK cells may negatively affect HSCs during transplantation.
IFNγ-neutralizing antibody restores HSC function in the presence of WT NK cells in vitro and in vivo
Based on our observations, we first assessed whether IFNγ-neutralizing antibody would rescue the deleterious effect of NK cells on HSCs in culture. We observed that the number of colonies was restored when IFNγ-neutralizing antibody was added to the WT NK-containing cultures, while the presence of IFNγ-neutralizing antibody did not alter colony numbers with or without Cebpg KO NK cells (Figure 6D). Since humanized anti-IFNγ monoclonal antibodies are available and ready for clinical use, we next investigated whether inhibition of the IFNγ signaling pathway could improve short-term engraftment, a critical phase during human HSCT. G-CSF mobilized peripheral blood was transplanted into NSG mice, and, 4 h after transplantation, treatment with either IFNγ-blocking antibodies or IgG control was initiated and continued as indicated in Figure 6E. We observed that mice treated with IFNγ-blocking antibodies exhibited improved percentage and absolute numbers of human CD45+ cells in BM compared with mice treated with IgG control antibody (Figure 6F). Further, we observed that animals that received IFNγ-blocking treatment showed increased relative and absolute numbers of human CD34+ cells in the BM (Figure 6G). Together, our data suggest that blocking IFNγ improves HSC function in vitro and engraftment during the early phase of HSCT.
Discussion
Cellular crosstalk between distinct cell types occurs in our organism, including hematopoietic cells. Of particular interest is the relationship that HSCs establish with their neighbor cells (Guezguez et al., 2013; Hoggatt et al., 2016; Mirantes et al., 2014; Schuettpelz and Link, 2013; Schurch et al., 2014). During the last years it became evident that this crosstalk not only regulates normal hematopoiesis but also affects the outcome of certain clinical interventions, such as BM transplantation (Crippa and Bernardo, 2018; Russell et al., 2015; Touzot et al., 2015; Triplett et al., 2015). In the present study, we showed that NK cells, an important component of the innate immune system, negatively affect HSC function during transplantation, and that this detrimental effect can be ameliorated by either (1) removing NK cells from the donor graft, or (2) by blocking IFNγ signaling during HSCT. Previously, the role of NK cells in HSCT has largely been addressed, but with a different focus than ours. The majority of reports focused on the ability of donor NK cells to recognize and kill residual tumor cells; mediate innate immune responses to prevent post-transplant infections; facilitate adaptive immune responses mediated by B and T cells; lyse host dendritic cells, thus reducing the risk of GvHD; and contribute to epithelial regeneration (Leung, 2011; Montaldo et al., 2013; Palmer et al., 2013; Parham and McQueen, 2003; Passweg et al., 2004). In contrast to these studies supporting the influence of NK cells from BM grafts on host cells, our data show that donor NK cells can also directly target donor HSCs and affect their reconstitution potential.
C/EBPγ is a transcription factor known to participate in the maturation and function of NK cells (Di Santo, 2006; Kaisho et al., 1999). Recently, we generated a conditional KO mouse model with specific excision of Cebpg in hematopoietic cells (Kardosova et al., 2018). Here, we confirmed the previously reported functional defects in Cebpg KO NK cells (Kaisho et al., 1999), and used this model to investigate the potential mechanisms of NK-HSC regulation. Our data demonstrate that Cebpg-deficient NK cells, which do not harm HSC fitness, produce reduced levels of IFNγ in comparison with WT NK cells. Accordingly, the use of IFNγ-neutralizing antibodies in culture as well as during HSCT improved HSC function. Thus, our results suggest that the INFγ secreted by NK cells contributes to the detrimental effects on HSC. Accordingly, previous studies suggested negative effects of IFNγ on HSC function. For instance, enhanced proliferation of HSCs and reduction in long-term repopulating capacity was associated with IFNγ stimulation of murine cells in experimental models of bacterial infection (Baldridge et al., 2010; MacNamara et al., 2011). In agreement, Yang et al. (2005) showed that the ability of CD34+CD38− human cord blood cells to support hematopoiesis was inhibited by IFNγ treatment in a xenotransplant model. However, IFNγ is not exclusively produced by NK cells, and other immune cells, such as T cells, can also produce INFγ and, consequently, contribute to the deleterious effects on HSCs. In fact, T cells produce IFNγ and TNFα, and it was recently suggested that neutralizing T cell-mediated TNFα signaling was able to enhance engraftment and hematopoietic reconstitution upon HSCT (Wang et al., 2017). Together, these studies seed the path to potentially novel strategies directed to enhance and improve HSCT, in particular during the critical early stages upon transplantation. Further studies may address whether anti-inflammatory regimes shortly after transplantation, and for a limited period of time, may improve HSCT.
In our assays, since HSCs and mature NK cells were both isolated from the same mouse strain that differed only in CD45.1 or CD45.2 expression (congenic mice), no differential expression of MHC class I ligands was expected. Therefore, we assumed that the observed effects were associated with NK secretory function rather than being dependent on the classic NK missing self-recognition function. Accordingly, gene expression analysis of Cebpg-deficient NK cells, compared with controls, revealed deregulation of genes from several cytokine pathways. It was previously reported that, when BM cells were placed under ideal culture conditions, the addition of activated NK cells to the culture inhibited colony formation (Murphy et al., 1992). Our overnight co-cultures followed by frequency and functional assessment indicated reduced HSC numbers and activity in the presence of NK cells, which could be at least partially explained by a reduction in viability. However, these effects were lost when Cebpg KO NK cells were employed. Together, based on our observations on the crosstalk between HSCs and NK cells in culture and during HSCT, we hypothesize that the presence of NK cells in the BM microenvironment can modulate HSC activity. However, further studies will need to address whether the co-existence of these cell types in the BM niche during steady-state hematopoiesis may modulate HSC fitness.
It is important to consider that NK cell activity during HSCT may differ depending on the origin of the NK cells (i.e., recipient NK versus donor NK cells). Recipient NK cell subsets bearing MHC class I receptors are said to be licensed or educated, and rapidly respond to stimuli (Alvarez et al., 2016). While licensed recipient NK subsets have been implicated in inhibition of allogenic BM cells and consequent rejection, unlicensed recipient NK cells seem to be growth promoting, thus facilitating BM engraftment (Sun et al., 2012). In the clinical setting, it is undeniable that NK cells play a relevant role in promoting the clearance of malignant cells and avoiding HSCT complications, particularly in HLA-haploidentical transplants (Di Santo, 2006). Here, we describe a novel non-HLA-dependent 'graft NK' - 'graft HSC' regulation mechanism that may influence transplant outcome. The presence of NK cells in the graft, which are also transplanted and activated during the graft product infusion, may harm HSCs contained in the graft regardless of the 'missing self' recognition mechanism, and therefore impair engraftment. It is certainly beneficial to have NK donor cell-rich preparations for malignant diseases such as acute leukemias because of the desired graft-versus-leukemia (GvL) effect. However, we would like to propose that NK cell depletion may actually favor HSC donor engraftment in the setting of allogeneic transplantation for non-neoplastic diseases in selected patients. Such cases would include for instance, but are not limited to, patients with idiopathic severe aplastic anemia or sickle cell anemia. For those situations where GvL effect is not needed, having highly functional donor HSCs may accelerate blood recovery and increase the chances of sustained donor engraftment. NK depletion could gain great value when clinicians deal with limited donor cell numbers. Low number of HSCs in BM preparations can be a problem when insufficient HLA-matched cord blood sources are available for adult patients, such as in geographical regions where mixed ethnicity impairs HLA-matched donor availability. Also, peripheral blood mobilized cells (PBMCs) used for autologous transplantation in cancer patients undergoing aggressive therapy frequently have low numbers of HSCs.
Future prospective studies are needed to investigate whether NK frequency in HSC donor sources may be proved to be an additional non-HLA risk factor for BM transplantation outcomes together with recipient age, stage of disease, cytomegalovirus (CMV) serostatus, and comorbidities. If this is true, improving HSC function in bone marrow grafts by reducing the effect of NK cells can be clinically important to make BM transplantation feasible for individualized selected patients and to accelerate engraftment in adverse conditions for both allogeneic and autologous transplantation.
Experimental procedures
Mice
The cell subsets used for this study were obtained from WT C57BL/6 CD45.1, C57BL/6 CD45.2, or C57BL/6 CD45.1/CD45.2 congenic mouse strain. A Cebpg conditional KO mouse model was generated by our group and backcrossed into the C57BL/6 background (Kardosova et al., 2018). Cebpgflox/flox (Cebpgf/f) mice were bred to Vav-iCre transgenic mice (also in C57BL/6 background) to generate Cebpgf/f Vav-iCre− mice (control, WT) or Cebpgf/f Vav-iCre+ (KO with Cebpg specifically excised in the hematopoietic system), and used for experimental comparisons in this study. Transplantations of human samples were performed on NSG mice (Jackson Laboratory, stock no. 005557), which were maintained in specified pathogen-free conditions. All mice were housed in a sterile barrier facility.
Isolation of murine NK and HSC cells
NK cells were obtained from SP from WT C56BL/6, Cebpgf/f Vav-iCre−, and Cebpgf/f Vav-iCre+ (CD45.2+) mice. HSCs were obtained from WT C57BL/6 CD45.2 or C57BL/6 CD45.1/CD45.2 mice. SP cell suspensions were obtained and submitted to red blood cell (RBC) lysis, followed by immunomagnetic lineage depletion using Ter119, CD4, CD8, and CD19 biotinylated antibodies (BioLegend) and Anti-Biotin Microbeads Ultrapure (Miltenyi Biotec), and then sorted by flow cytometry as lineage negative, CD3 negative, and NK1.1 positive (Lin−, CD3−, NK1.1+). To obtain RNA and cell supernatants, 2.5 × 105 NK cells were cultured in RPMI1640 supplemented with 10% FBS and 1000 U/mL of IL-2 for 24 h. To isolate HSCs, BM samples were lysed by RBC lysis buffer and subsequently depleted using antibodies against Ter-119, CD19, B220, CD8, Gr1, and CD11b antigens by immunomagnetic separation, and then sorted as c-Kithi, Sca-1+, lineage-negative, CD48−, CD150+ cells. Culture conditions are detailed in the online supplemental information.
Flow cytometry
Single-cell suspensions from murine PB, BM, or SP were analyzed by flow cytometry using the following monoclonal antibodies conjugated with biotin (BIO), fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-Cy5, PE–Cy7, Pacific Blue (PB), allophycocyanin (APC), or APC–Cy7 and obtained from BioLegend or eBioscience: CD19 (MB19-1), B220 (RA3-6B2), CD4 (RM4-5), CD8 (53–6.7), Gr1 (RB6-8C5), CD11b (M1/70), TER119 (TER-119), Sca-1 (D7), streptavidin, c-Kit (2B8), CD48 (HM48-1), CD150 (TC15-12F12.2), CD3 (17A2), NK1.1 (PK136), NKG2A (20d5), NKG2D (CX5), Ly-49H (3D10), CD45.1 (A20), and CD45.2 (104). Stained cells were analyzed with an LSRII flow cytometer and sorted using a FACSAria II or Influx (BD Biosciences). Viable cells were identified by Hoechst 33258 exclusion. Diva (BD Biosciences) and FlowJo (Tree Star) software were used for data acquisition and analysis, respectively. CountBright Absolute Counting Beads (Molecular probes, Invitrogen) were used for HSC subpopulation quantification by flow cytometry.
Study approval
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the Beth Israel Deaconess Medical Center (Boston, United States) and by the Animal Ethical Committee at the Institute of Molecular Genetics (Prague, Czech Republic). Human BM samples were obtained after written informed consent was received from participants. Human studies were approved by the institutional review board at the First Faculty of Medicine, Charles University in Prague and General University Hospital (Prague, Czech Republic).
Statistical analysis
Statistical significance for indicated datasets was determined using two-sided, unpaired Student's t test, and p values < 0.05 were considered statistically significant. Scatter dot plots depict mean with error bars representing standard deviation (SD). Jonckheere-Terpstra trend tests (for group comparisons) using the SPSS software version 20 was employed when indicated. HSC frequencies were calculated with L-Calc software (StemCell Technologies, Vancouver, Canada) using Poisson statistics and the method of maximum likelihood to the proportion of negative recipients in a limiting dilution setting.
Data and code availability
The accession number for the microarray data reported in this paper is ArrayExpress: E-MTAB-5604.
Author contributions
Conceptualization, L.L.d.F-P., R.S.W., D.G.T., and M.A.-J.; methodology, L.L.d.F-P., R.S.W, S.L., and M.A.-J.; formal analysis, L.L.d.F-P., H.S., and M.A.-J.; investigation, L.L.d.F-P., R.S.W, M.K.A., S.G., M.K., I.A.L., A.F.d.O.C., H.Z., A.M., A.T.J., M.Z., and M.A.-J.; data curation, H.S.; writing, L.L.d.F-P. and M.A.-J.; visualization, L.L.d.F-P. and M.A.-J.; supervision, L.L.d.F-P., R.S.W., D.G.T., and M.A.-J.; funding acquisition, L.L.d.F-P., D.G.T., and M.A.-J.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
This work was supported by a grant from São Paulo Research Foundation (FAPESP; grant 2015/21866-1) to L.L.d.F.-P. M.A.-J. was supported by a GACR grant 18-08577S and institutional funding from the IMG CAS (RVO 68378050). D.G.T. was supported by a STaR Investigator Award, an RCE Core grant, and Tier 3 RNA Biology Center grant MOE2014-T3-1-006 from the NRF and MOE, Singapore, and NIH grants R35CA197697 and P01HL131477. The authors thank Prof. Vaclav Horejsi for providing us with the IFNγ and IgG1 antibodies.
Published: July 8, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.06.008.
Contributor Information
Daniel G. Tenen, Email: daniel.tenen@nus.edu.sg.
Meritxell Alberich-Jorda, Email: alberich@img.cas.cz.
Supplemental information
References
- Alvarez M., Sun K., Murphy W.J. Mouse host unlicensed NK cells promote donor allogeneic bone marrow engraftment. Blood. 2016;127:1202–1205. doi: 10.1182/blood-2015-08-665570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge M.T., King K.Y., Boles N.C., Weksberg D.C., Goodell M.A. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza-Vidas N., Antonchuk J., Qian H., Mansson R., Luc S., Zandi S., Anderson K., Takaki S., Nygren J.M., Jensen C.T. Cytokines regulate postnatal hematopoietic stem cell expansion: opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Busson M., Rocha V., Appert M.L., Lepage V., Dulphy N., Haas P., Socie G., Toubert A., Charron D. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38:437–444. doi: 10.1038/sj.bmt.1705468. [DOI] [PubMed] [Google Scholar]
- Crippa S., Bernardo M.E. Mesenchymal stromal cells: role in the BM niche and in the support of hematopoietic stem cell transplantation. Hemasphere. 2018;2:e151. doi: 10.1097/HS9.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo J.P. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- Geerman S., Brasser G., Bhushal S., Salerno F., Kragten N.A., Hoogenboezem M., de Haan G., Wolkers M.C., Pascutti M.F., Nolte M.A. Memory CD8(+) T cells support the maintenance of hematopoietic stem cells in the bone marrow. Haematologica. 2018;103:e230–e233. doi: 10.3324/haematol.2017.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerman S., Nolte M.A. Impact of T cells on hematopoietic stem and progenitor cell function: good guys or bad guys? World J. Stem Cells. 2017;9:37–44. doi: 10.4252/wjsc.v9.i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezguez B., Campbell C.J., Boyd A.L., Karanu F., Casado F.L., Di Cresce C., Collins T.J., Shapovalova Z., Xenocostas A., Bhatia M. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell. 2013;13:175–189. doi: 10.1016/j.stem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Furuhashi K., Ishii H., Li H.W., Pinho S., Ding L., Robson S.C., Frenette P.S., Fujisaki J. CD150(high) bone marrow Tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22:445–453. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J., Kfoury Y., Scadden D.T. Hematopoietic stem cell niche in health and disease. Annu. Rev. Pathol. 2016;11:555–581. doi: 10.1146/annurev-pathol-012615-044414. [DOI] [PubMed] [Google Scholar]
- Joncker N.T., Shifrin N., Delebecque F., Raulet D.H. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T., Tsutsui H., Tanaka T., Tsujimura T., Takeda K., Kawai T., Yoshida N., Nakanishi K., Akira S. Impairment of natural killer cytotoxic activity and interferon gamma production in CCAAT/enhancer binding protein gamma-deficient mice. J. Exp. Med. 1999;190:1573–1582. doi: 10.1084/jem.190.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardosova M., Zjablovskaja P., Danek P., Angelisova P., de Figueiredo-Pontes L.L., Welner R.S., Brdicka T., Lee S., Tenen D.G., Alberich-Jorda M. C/EBPgamma is dispensable for steady-state and emergency granulopoiesis. Haematologica. 2018;103:e331–e335. doi: 10.3324/haematol.2017.173781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Poursine-Laurent J., Truscott S.M., Lybarger L., Song Y.J., Yang L., French A.R., Sunwoo J.B., Lemieux S., Hansen T.H. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- Leung W. Use of NK cell activity in cure by transplant. Br. J. Haematol. 2011;155:14–29. doi: 10.1111/j.1365-2141.2011.08823.x. [DOI] [PubMed] [Google Scholar]
- MacNamara K.C., Jones M., Martin O., Winslow G.M. Transient activation of hematopoietic stem and progenitor cells by IFNgamma during acute bacterial infection. PLoS one. 2011;6:e28669. doi: 10.1371/journal.pone.0028669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R.S., Shpall E.J., Rezvani K. Cord blood as a source of natural killer cells. Front. Med. 2015;2:93. doi: 10.3389/fmed.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirantes C., Passegue E., Pietras E.M. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp. Cel. Res. 2014;329:248–254. doi: 10.1016/j.yexcr.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldo E., Vacca P., Moretta L., Mingari M.C. Understanding human NK cell differentiation: clues for improving the haploidentical hematopoietic stem cell transplantation. Immunol. Lett. 2013;155:2–5. doi: 10.1016/j.imlet.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Keller J.R., Harrison C.L., Young H.A., Longo D.L. Interleukin-2-activated natural killer cells can support hematopoiesis in vitro and promote marrow engraftment in vivo. Blood. 1992;80:670–677. [PubMed] [Google Scholar]
- Palmer J.M., Rajasekaran K., Thakar M.S., Malarkannan S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J. Cancer. 2013;4:25–35. doi: 10.7150/jca.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., McQueen K.L. Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nat. Rev. Immunol. 2003;3:108–122. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- Passweg J.R., Tichelli A., Meyer-Monard S., Heim D., Stern M., Kuhne T., Favre G., Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- Raziuddin A., Longo D.L., Bennett M., Winkler-Pickett R., Ortaldo J.R., Murphy W.J. Increased bone marrow allograft rejection by depletion of NK cells expressing inhibitory Ly49 NK receptors for donor class I antigens. Blood. 2002;100:3026–3033. doi: 10.1182/blood.V100.8.3026. [DOI] [PubMed] [Google Scholar]
- Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W.D., Tosti A., Posati S., Rogaia D., Frassoni F., Aversa F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Russell A., Malik S., Litzow M., Gastineau D., Roy V., Zubair A.C. Dual roles of autologous CD8+ T cells in hematopoietic progenitor cell mobilization and engraftment. Transfusion. 2015;55:1758–1765. doi: 10.1111/trf.13073. quiz 1757. [DOI] [PubMed] [Google Scholar]
- Schuettpelz L.G., Link D.C. Regulation of hematopoietic stem cell activity by inflammation. Front. Immunol. 2013;4:204. doi: 10.3389/fimmu.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurch C.M., Riether C., Ochsenbein A.F. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14:460–472. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Storek J., Gooley T., Witherspoon R.P., Sullivan K.M., Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am. J. Hematol. 1997;54:131–138. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Sun K., Alvarez M., Ames E., Barao I., Chen M., Longo D.L., Redelman D., Murphy W.J. Mouse NK cell-mediated rejection of bone marrow allografts exhibits patterns consistent with Ly49 subset licensing. Blood. 2012;119:1590–1598. doi: 10.1182/blood-2011-08-374314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblyn M., Young J.A., Haagenson M.D., Klein J.P., Trachtenberg E.A., Storek J., Spellman S.R., Cooley S., Miller J.S., Weisdorf D.J. Decreased infections in recipients of unrelated donor hematopoietic cell transplantation from donors with an activating KIR genotype. Biol. Blood Marrow Transplant. 2010;16:1155–1161. doi: 10.1016/j.bbmt.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot F., Neven B., Dal-Cortivo L., Gabrion A., Moshous D., Cros G., Chomton M., Luby J.M., Terniaux B., Magalon J. CD45RA depletion in HLA-mismatched allogeneic hematopoietic stem cell transplantation for primary combined immunodeficiency: a preliminary study. J. Allergy Clin. Immunol. 2015;135:1303–1309 e1301. doi: 10.1016/j.jaci.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Triplett B.M., Shook D.R., Eldridge P., Li Y., Kang G., Dallas M., Hartford C., Srinivasan A., Chan W.K., Suwannasaen D. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015;50:1012. doi: 10.1038/bmt.2015.139. [DOI] [PubMed] [Google Scholar]
- Venstrom J.M., Gooley T.A., Spellman S., Pring J., Malkki M., Dupont B., Petersdorf E., Hsu K.C. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2010;115:3162–3165. doi: 10.1182/blood-2009-08-236943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wagner J.E., Thompson J.S., Carter S.L., Kernan N.A. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- Wang W., Fujii H., Kim H.J., Hermans K., Usenko T., Xie S., Luo Z.J., Ma J., Celso C.L., Dick J.E. Enhanced human hematopoietic stem and progenitor cell engraftment by blocking donor T cell-mediated TNFalpha signaling. Sci. Transl. Med. 2017;9:eaag3214. doi: 10.1126/scitranslmed.aag3214. [DOI] [PubMed] [Google Scholar]
- Yang L., Dybedal I., Bryder D., Nilsson L., Sitnicka E., Sasaki Y., Jacobsen S.E. IFN-gamma negatively modulates self-renewal of repopulating human hemopoietic stem cells. J. Immunol. 2005;174:752–757. doi: 10.4049/jimmunol.174.2.752. [DOI] [PubMed] [Google Scholar]
- Zhang C.C., Lodish H.F. Cytokines regulating hematopoietic stem cell function. Curr. Opin. Hematol. 2008;15:307–311. doi: 10.1097/MOH.0b013e3283007db5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the microarray data reported in this paper is ArrayExpress: E-MTAB-5604.