Abstract
While infection by Neisseria gonorrhoeae is often asymptomatic in women, undetected infections can ascend into the upper genital tract to elicit an inflammatory response that manifests as pelvic inflammatory disease, with the outcomes depending on the intensity and duration of inflammation and whether it is localized to the endometrial, fallopian tube, ovarian, and/or other tissues. This review examines the contribution of N. gonorrhoeae versus other potential causes of pelvic inflammatory disease by considering new insights gained through molecular, immunological, and microbiome-based analyses, and the current epidemiological burden of infection, with an aim to highlighting key areas for future study.
Keywords: Neisseria gonorrhoeae, gonorrhea, pelvic inflammatory disease (PID), cervicitis, endometrial infection, endometritis, salpingitis, oophoritis, neutrophil
Gonorrhea has long plagued humans, although it was not until 1879 that Neisseria gonorrhoeae was determined as the infectious cause [1]. Gonococcal pelvic inflammatory disease (PID) is a complication of cervical infection that has substantial immediate and long-term consequences for women, including chronic pain, ectopic pregnancy, and infertility. PID is an umbrella term describing inflammation of the female upper genital tract (UGT), including salpingitis, endometritis, oophoritis, tubo-ovarian abscess, pelvic peritonitis, and perihepatitis [2], and it has been used to generally encompass these conditions in any combination. For the purposes of this review, we will focus on acute PID (≤30 days), because chronic PID (>30 days) is not typically associated with gonococcal infection [3].
PID is a result of microbes ascending the UGT, triggering host inflammation and subsequent tissue damage. Because sampling of the UGT and laparoscopy are not routinely performed due to the invasive nature of such procedures, many PID diagnoses make the supposition that N. gonorrhoeae is the cause if pelvic or lower abdominal pain is accompanied by gonococcal recovery from the cervix or urine. We will refer to such cases as “N. gonorrhoeae–associated PID” when it is not explicit that N. gonorrhoeae was isolated from the UGT.
HOW DOES N. gonorrhoeae CONTRIBUTE TO PID?
While Koch’s postulates have been fulfilled for gonococcal urethritis in men [4] and Chlamydia trachomatis salpingitis in female monkeys [5], this has not been the case for gonococcal PID. The reasons for this include the difficulty of modeling PID in animals, because N. gonorrhoeae is a very fastidious and human-specific pathogen, and because it is highly unethical to infect a woman with N. gonorrhoeae owing to the risk of severe sequelae. However, N. gonorrhoeae has long been suspected of causing PID. In 1886, shortly after the discovery of N. gonorrhoeae, gonococci were isolated from the fallopian tubes (FTs) of a woman with acute salpingitis [5], leading to the conclusion that N. gonorrhoeae was the etiological agent for PID. Later, salpingitis cases became subcategorized as gonococcal or nongonococcal in origin. N. gonorrhoeae has since been isolated from the endometrium, FTs, and peritoneal fluid of women with PID [6–17]; notably, UGT recovery is not always accompanied by detectable cervical infection in these studies.
Aside from classic sexually transmitted infections (STIs), respiratory pathogens and anaerobes can also be detected in the UGT of women with PID, suggesting that the clinical outcome may be a general response to ascending infection, and there may be a polymicrobial cause [6]. In this context, the fact that STIs are strongly associated with PID and account for the majority of cases [3] may be a matter of infection location and opportunistic spread into the UGT, rather than a site-specific tropism. This is muddled by the uncertain role that bacterial vaginosis plays in PID development [18], as well as the ongoing uncertainty regarding the existence of a normal UGT microbiome [19]. It is therefore clear that bacterial seeding of the uterus is more commonplace than once assumed, but it remains difficult to make conclusions about the relative contribution of bacteria other than N. gonorrhoeae and C. trachomatis to pathogenesis without more comprehensive PID-focused microbiome analysis.
Aside from the microbial composition, the factors that determine whether they are tolerated or drive inflammation within the UGT remain unknown. It has been proposed that salpingitis could be temporally polymicrobial—with N. gonorrhoeae or another inflammatory STI first ascending and triggering damage that “primes” the tissue, so that it may be colonized by anaerobic or other opportunistic bacteria [20]. However, this hypothesis has not been subjected to rigorous examination and thus remains an important focus for future study.
HOW DOES N. gonorrhoeae ENTER THE UGT?
Since no experimental model can recapitulate all aspects of gonococcal disease, we must learn from studies using different systems. N. gonorrhoeae and host factors responsible for the establishment of lower genital tract colonization have been expertly reviewed elsewhere [21, 22]. Here we discuss ascending infections and UGT-specific findings.
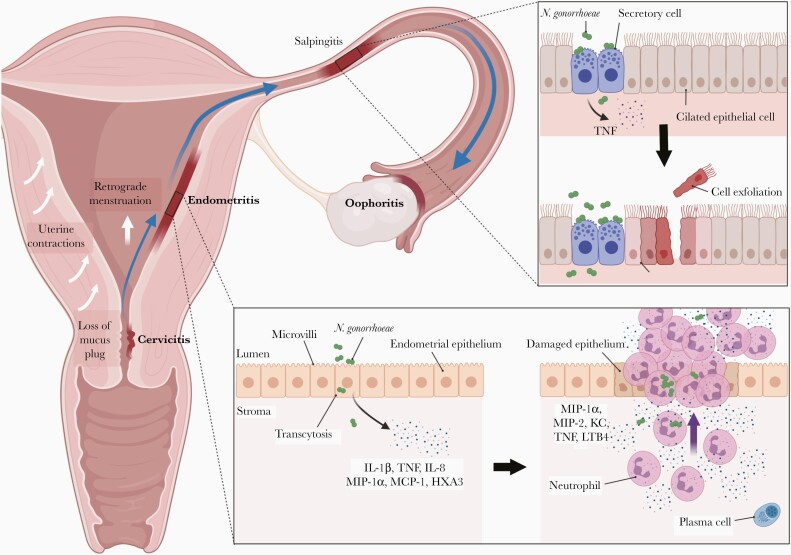
Fulminant abdominal pain during, or shortly after, menses has classically indicated PID [18]. While this is often correlated with N. gonorrhoeae–associated PID [23, 24], it is not gonococcal specific [25]. Bacterial recovery is most common in the follicular/proliferative phases [2, 24, 25], which coincide with estrogen-controlled changes in mucus viscosity and uterine contractions that move fluid upward [2, 26]. These normal physiological changes, in additional to sexual intercourse and retrograde menstruation, can provide opportunities for N. gonorrhoeae and other microbes to ascend, with the outcome dependent on the load and virulence of the microbe as well as the resulting immune response [2, 26]. Figure 1 shows the canalicular flow of infection after gonococcal colonization of the endocervix.
Figure 1.
Ascending infection and mechanisms of inflammatory damage leading to pelvic inflammatory disease after endocervical infection by Neisseria gonorrhoeae. Endocervical infection by N. gonorrhoeae may or may not manifest as cervicitis. After infection is established, host physiological and physical factors, including hormone-controlled loss of the mucus plug, uterine contractions, sexual intercourse, and retrograde menstruation, can lead to ascent of bacteria into the endometrium [2, 26]. N. gonorrhoeae–specific virulence factors may also be involved, although their actual role during ascent have yet to be demonstrated in an appropriate model. Infection and subsequent inflammation of the endometrium (endometritis), fallopian tubes (FTs) (salpingitis), or ovaries (oophoritis) can occur. Inset (bottom), Endometrial damage occurs when N. gonorrhoeae interacts with the epithelial lining, leading to bacterial transcytosis and epithelial responses including release of proinflammatory cytokines interleukin 1β (IL-1β) and tumor necrosis factor (TNF); neutrophil chemokine interleukin 8 (IL-8), and the eicosanoid hepoxilin A3 (HXA3) [27, 28]. On recruitment to the site of infection, neutrophils enter a positive feedback loop by producing additional neutrophil chemotactic factors, including leukotriene B4 (LTB4), KC, and macrophage inflammatory protein (MIP) 2 (unpublished observations) [28, 29]. This results in a secondary wave of neutrophil migration into the uterine lumen, resulting in tissue damage [30]. Abbreviations: KC, keratinocyte-derived chemokine; MCP, monocyte chemoattractant protein. Inset (top), N. gonorrhoeae binding to nonciliated secretory cells and release of peptidoglycan and lipo-oligosaccharide fragments stimulate a potent inflammatory response from the epithelia of the FTs [31, 32]. The TNF produced leads to death and sloughing of ciliated cells [33], which can lead to irreversible scarring, deciliation and impaired fertility. (Illustration created using BioRender.com.)
Ascending infections occur in approximately 20% of estradiol-treated, wild-type mice infected with N. gonorrhoeae vaginally [34]. Because mice do not express epithelial receptors required for N. gonorrhoeae attachment, this supports the premise that basic physiological processes can carry the bacteria upward, even in the absence of more specialized virulence factors. The utility of this lower genital tract infection model to study ascending infection is unclear because the original study had only 2 mice with recoverable bacteria in the UGT [34]. Another study detected uterine bacteria in all infected mice, although this protocol described a large volume injected against the cervix, which may reflect a direct uterine inoculation [35]. However, no other model has been described to model bacterial ascent, and it remains to be seen whether transgenic mice expressing human factors that facilitate N. gonorrhoeae infection show increased ascent of N. gonorrhoeae into the UGT after their introduction into the vagina.
WHAT HAPPENS WHEN NGO REACHES THE UGT?
The endometrial epithelia represents a critical line of defense against infections [36]. In vitro cell culture model systems of human endometrium epithelial cells, including organ cultures and 3-dimensional cell models [27, 37], have demonstrated proinflammatory responses to N. gonorrhoeae infection. Notably, endometrial cells can differentiate between bacterial species since they produce inflammatory cytokines in response to N. gonorrhoeae infection but not commensal Lactobacillus crispatus or even bacterial vaginosis–associated Gardnerella vaginalis [27]. Therefore, there are clearly pathogen-specific cues with the potential to trigger inflammation and, potentially, development of PID. A cell culture model system, combining epithelial cells and neutrophils to study N. gonorrhoeae–induced neutrophil transcytosis across the epithelia, demonstrated coordinated eicosanoid-driven signaling between these cell types to enact the characteristic robust inflammation [28]. This work reinforced the observation that neutrophils have a self-perpetuating feedback loop that drives the N. gonorrhoeae immunopathogenic response [29]. Future work must aim to understand whether these effects contribute to the infiltration of neutrophils and plasma cells into the endometrium, since this typifies acute endometriosis in humans [13] (Figure 1, inset).
To put these in vitro findings into physiological context, mouse modeling can be used to study the onset of endometritis by directly infecting the uterus. Transcervical infection of progestin-treated mice leads to a rapid proinflammatory cytokine response and massive purulent influx of neutrophils into the uterine lumen, disrupting the epithelial layer and causing tissue damage [30]. Transcriptomic analysis reveals that infections in both estrus and diestrus stages of the reproductive cycle result in up-regulation of similar host response pathways, although diestrus infection has neutrophil-related signals that are magnitudes higher [38]. Interestingly, N. gonorrhoeae infection of mice expressing human carcinoembryonic antigen–related cell adhesion molecule 1 (CEACAM1) during estrus resulted in greater bacterial association and penetration of endometrial tissue than in wild-type mice, although tissue invasion did not affect bacterial clearance nor was there increased tissue damage [39].
To date, no animal model has described gonococcal FT involvement or postinfection sequelae such as tissue scarring or infertility, perhaps attributable to the specific adaptation of N. gonorrhoeae for humans. Interestingly, the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) clinical trial also failed to find a connection between endometritis and infertility in the absence of salpingitis [8]. This suggests that murine uterine infection may remain a valid model for studying acute endometritis and the onset of host inflammatory responses in the uterus.
HOW DOES GONORRHEA CAUSE TISSUE DAMAGE AND PID SEQUELAE?
PID morbidity arises primarily as a result of the tissue repair process after infection-triggered inflammatory damage to epithelium. Of particular importance to fertility are the ciliated cells of the FTs, which are crucial to transferring the ovum to the uterus [40]. Fibroblast replacement of damaged cells results in scarring, tubal occlusions, deciliation, pelvic adhesions and, potentially, irreversible tubal function loss, which can lead to ectopic pregnancy and infertility [2].
Unlike mouse infection with Chlamydia, N. gonorrhoeae uterine infection does not result in the development of hydrosalpinx [41], so an animal model for gonococcal salpingitis and sequelae is lacking. Experiments using human FT explants have shown that ciliated cells are particularly susceptible to N. gonorrhoeae–induced death, despite the fact that N. gonorrhoeae primarily interact with nonciliated secretory cells [40, 42]. Because N. gonorrhoeae have no cytolytic toxins to cause direct cell death, ciliated cells instead undergo tumor necrosis factor–induced apoptosis as a result of host detection of peptidoglycan monomers and lipo-oligosaccharide, both of which are abundantly released by growing N. gonorrhoeae [31–33, 40].
Pain, a common PID morbid effect, can also be caused by inflammation. Lipid mediators produced by neutrophils, such as prostaglandin E2 and leukotriene B4, are important mediators in models of inflammatory hypernociception [43, 44]. Neutrophils produce leukotriene B4 [28] and dendritic cells produce prostaglandin E2 in response to N. gonorrhoeae in vitro [45], and elevated levels of both mediators have been found in the peritoneal fluid of women with acute PID [46]. It is enticing to consider that N. gonorrhoeae–induced neutrophilic responses could be the source of pain in women with PID, and that targeting these responses could help provide pain relief. The trigger for these lipid mediator–based responses remains unclear. However, when considered along with the cytokine and neutrophil responses, it seems clear that the exuberant response to N. gonorrhoeae infection, rather than a direct effect of the bacteria itself, leads to the tissue damage that typifies PID.
WHY IS PID SO DIFFICULT TO STUDY?
Clinical manifestations of PID vary widely, making diagnoses challenging and surveillance data difficult to interpret. A PID diagnosis is based on clinical evidence but cannot be made from the results of any single or specific physical, laboratory, or patient history finding [47]. It typically depends on pelvic pain and signs of lower genital tract infection, which can be subtle, vague, or asymptomatic. Laparoscopic visualization of salpingitis is considered the reference standard for diagnosis, although it may miss early signs of inflammation or endometritis. In addition, it is an expensive and invasive procedure, with variable user interpretation, and is not available in all healthcare settings [3]. Furthermore, the treatment of PID is dependent on accurate and timely detection of microbes, but N. gonorrhoeae has historically been considered short-lived in the FT and peritoneal cavity [5], making it difficult to distinguish from other triggers.
WHAT IS THE IMPACT OF ASYMPTOMATIC N. gonorrhoeae INFECTION AND SUBCLINICAL PID?
Asymptomatic rates of gonorrhea are thought to be high, especially in women, although data remain sparse and depend on the population studied. Random sampling revealed more cases of untreated gonorrhea than cases diagnosed and reported [48], while another US study estimated 45% of gonorrhea cases to be asymptomatic [49]. This study also found that most cases of untreated gonococcal infections were due to a lack of symptoms [49]. More recently, Detels et al [50] found that 67%–100% of N. gonorrhoeae–infected individuals reported no symptoms in some populations. However, the reason why some infections remain asymptomatic while others are pathogenic remains poorly understood.
The concept of subclinical PID (also called atypical or silent PID) is that uncomplicated or asymptomatic cervical infection can be accompanied by inflammation of the UGT, without incurring symptoms of acute PID. A cohort of women with subclinical PID, defined by histological endometritis, had a significant risk of infertility compared with women with normal histological findings. Importantly, cervical infection with either C. trachomatis or N. gonorrhoeae without UGT involvement was not itself a risk factor for infertility [51]. However, up to half of women with apparently uncomplicated N. gonorrhoeae or C. trachomatis cervicitis have histological signs of subclinical PID [52], and women with infertility due to bilateral tubal occlusion were frequently found to have serological evidence of past N. gonorrhoeae or C. trachomatis infection despite not having a clinical history of salpingitis [53].
Early PID diagnosis is imperative to prevent sequelae, because delayed treatment is linked to worsened fertility outcomes [54]. This may explain why endometritis in subclinical PID (for which women do not seek medical attention) is linked to decreased fertility, unlike symptomatic endometritis in mild to moderate acute PID cases [8]. The varying sequelae, even among clinically inapparent infections, make it difficult to reveal a causal link between discrete attributes of infection and disease, particularly when considering the diversity among gonococcal strains that might also contribute to the outcome.
HOW HAS GONOCOCCAL-ASSOCIATED PID PREVALENCE CHANGED OVER THE YEARS?
Table 1 lists select studies conducted in the last 5 decades, indicating the proportion of acute PID associated with N. gonorrhoeae. These clinical studies reveal enormous variations in N. gonorrhoeae–associated PID, ranging from 1% to 74% of all patients with acute PID, making interpretations difficult. Certainly, some of these differences can be explained by the geographic location (eg, Israel having a low prevalence of gonorrhea [55]) and the time period (eg, gonorrhea epidemic in industrialized countries in the 1960s and 1970s [47]). However, small study sizes, variance in sampling and culture methods, differences in access to technology, and variation in clinical case definitions heavily affect the estimates of N. gonorrhoeae–associated PID. The results of several large-scale and long-term studies from different countries are briefly summarized below.
Table 1.
Prevalence of Neisseria gonorrhoeae–Associated Acute Pelvic Inflammatory Disease in Selected Studies by Country and Year
| Country | Site of Specimen Collection | Laparoscopy- Confirmed PID | Patients With Neisseria gonorrhoeae–Associated PID, No. (%) | Years of Study | Authors (Year of Publication) | |
|---|---|---|---|---|---|---|
| LGT and UGTa | UGT Onlyb | |||||
| Denmark | LGT | No | 9/166 (5) | NA | 1979–1980 | Møller et al (1981) [76] |
| Denmark | LGT and UGT | Yes | 8/46 (17) | Unclear | Kristensen et al (1985) [64] | |
| Sweden | LGT and UGT | Yes | 5/65 (5) | 0/65 (0) | Gjønnaess et al (1982) [77] | |
| Sweden | LGT | Yes | 41/209 (20) | NA | 1979–1980 | Osser and Persson (1982) [78] |
| Finland | LGT and UGT | Yes | 18/72 (25) | 7–9/72 (10–13)c | 1983–1988 | Heinonen and Miettinen (1994) [15] |
| USA | LGT and UGT | No | 91/204 (45) | 7/54 (13) | 1972–1974 | Eschenbach et al (1975) [6] |
| USA | LGT and UGT | No | 133/197 (68) | 12/197 (6) | 1976 | Cunningham et al (1977) [7] |
| USA | LGT | No | 41/83 (49) | NA | McCormack et al (1977) [23] | |
| USA | LGT | Retrospective, unspecified | 70/114 (61) | NA | 1978–1979 | Tavelli (1986) [79] |
| USA | LGT and UGT | Yes | 11/23 (48) | 6/23 (26) | 1982–1983 | Wasserheit et al (1986) [11] |
| USA | LGT | No | 27/42 (64) | NA | Dodson and Faro (1986) [80] | |
| USA | LGT and UGT | Yes | 23–30/46 (50–65)d | 22/46 (48) | 1982–1986 | Kiviat et al (1990) [13] |
| USA | LGT | No | 69/93 (74) | NA | 1985–1988 | Golden et al (1989) [81] |
| USA | LGT and UGT | Yes | 55/82 (67) | 41/78 (53) | 1982–1988 | Eschenbach et al (1997) [17] |
| USA | LGT | Retrospective, unspecified | 24/343 (7) | NA | 2007–2010 | Burnett et al (2011) [82] |
| USA | LGT | No | 27/271 (10) | NA | 2012–2016 | Trent et al (2019) [83] |
| USA | LGT and UGT | No | 17/233 (7) | 12/233 (5) | 2010–2015 | Wiesenfeld et al (2021) [9] |
| Canada | LGT | No | 15/43 (35) | NA | 1978–1980 | Bowie and Jones (1981) [84] |
| Canada | LGT and UGT | Yes | 21/50 (42) | 9/50 (18) | 1983–1987 | Brunham et al (1988) [12] |
| Canada | LGT | No | 19/100 (19) | NA | 2004–2014 | Chen et al (2018) [73] |
| United Kingdom | LGT and UGT | Yes | 7/23 (30) | 2/23 (9) | 1984–1987 | Stacey et al (1992) [14] |
| Israel | UGT | Yes | NA | 0/40 (0) | 1987–1989 | Dan et al (1993) [55] |
| Kenya | LGT and UGT | Yes | 83/133 (62) | Unspecified | 1994–1996 | Cohen et al (1998) [85] |
| Cameroon | LGT | No | 1/70 (1) | NA | 2013–2014 | Nkwabong and Dingom (2015) [86] |
Abbreviations: LGT, lower genital tract; NA, not available; PID, pelvic inflammatory disease; UGT, upper genital tract; USA, United States of America.
aIncluding coinfections and any LGT sites (cervical, vaginal, rectal, and urinary tract).
bUGT sites included endometrium, fallopian tubes, and peritoneal fluid. Percentages represent the percentage of all PID cases.
cData were reported as numbers of patient with N. gonorrhoeae isolated from fallopian tubes or endometrium but without specifying whether any patients had N. gonorrhoeae isolated from both sites.
dAn additional 8 women had cervical N. gonorrhoeae and/or Chlamydia trachomatis infection without UGT infection, but numbers were not delineated further.
The 2 largest and longest running studies on gonococcal-associated PID come from Sweden. Both cohorts of approximately 2500 patients, staggered by 10 years, showed similar rates of N. gonorrhoeae–associated PID (48.7% in Lund [56] and 42% in Örebro [16]) at the start of each study period, followed by a steady decline over the following 25 years. In the United States, the PEACH trial examined mild to moderate PID from multiple US sites between 1996–1999; a total of 831 patients were enrolled in this study, with 20% being N. gonorrhoeae associated [57]. A large retrospective ecological study was performed in a cohort of Australian women from 2009 to 2014. Of the total 14 271 PID admissions, only 0.1% were N. gonorrhoeae related, while 5.3% were C. trachomatis related, suggesting that N. gonorrhoeae was a minor cause of PID in this population. However, it should be noted that 22.6% of these admissions were categorized as chronic PID, and 65.8% were considered unspecified PID [58]. For the latter, it is unknown whether another etiological agent (such as Mycoplasma genitalium) is prevalent, or whether diagnoses were simply miscategorized, since this study did not link to test results for N. gonorrhoeae or C. trachomatis.
These studies support an overall consensus that N. gonorrhoeae–associated PID rates have dropped in industrialized countries since their peak in the 1960s and 1970s but that rates differ significantly between populations. More recent studies suggest that N. gonorrhoeae–related PID have remained below their former high levels, and overall rates of PID have been observed to decrease in the United Kingdom, the United States, and Australia [59]. These results have been attributed to aggressive public health campaigns that not only educate but also actively screen for N. gonorrhoeae and C. trachomatis, higher adoption rates of safe sex practices in response to human immunodeficiency virus, and the practice of treating infected partners to reduce PID recurrence [59–61]. Whether they begin to escalate again with recent increases in N. gonorrhoeae infection rate remains to be seen.
DO GONOCOCCI STILL CAUSE A SIGNIFICANT BURDEN OF PID?
The global decrease in N. gonorrhoeae–related PID begs the question of whether N. gonorrhoeae is still an important cause of PID. C. trachomatis infection remains the most prevalent bacterial STI worldwide, and is responsible for a higher proportion of PID cases than N. gonorrhoeae. There is also growing concern about the role of M. genitalium or other bacteria that are not monitored [3].
However, N. gonorrhoeae clearly remains a substantial problem. There is a strong correlation between gonorrhea prevalence and negative reproductive outcomes, with secondary and tertiary waves of PID and ectopic pregnancy following increases in gonorrhea incidence [47, 60, 62]. Meta-analysis shows that gonococcal infections are many times more prevalent in infertile populations than in the general population [63]. Patients with N. gonorrhoeae–associated PID are more febrile and ill than those with nongonococcal PID [23, 64, 65], consistent with the PEACH trial finding that women with N. gonorrhoeae–associated PID sought care more quickly than women with C. trachomatis or M. genitalium infections [66]. An Australian study also found that hospitalization rates are higher for N. gonorrhoeae–associated than for C. trachomatis–associated PID [67], suggesting increased disease severity.
Unfortunately, gonococcal surveillance indicates that incidence rates are growing around the world [68–71]. It is suggested that 10%–20% of untreated gonococcal infections develop into PID [1, 60]. However, a small study from 1983 found rates as high as 47% in women who contracted gonorrhea from their infected partners [72], while a more recent Canadian study from 2004–2014 found PID complications in only 1.5% of gonorrhea cases [73]. A 2012 study alarmingly found that after treatment for C. trachomatis or N. gonorrhoeae, acute PID still developed in 13% of patients [74]. The recent emergence of gonococcal resistance to last-line antibiotics raises new concerns relevant to the PID discussion. The first reported case of ceftriaxone-resistant case of N. gonorrhoeae in Canada was asymptomatic and was discovered only because of STI screening [75]. The combination of increasing incidence rates and the possibility of untreatable gonococcal infections makes for a particularly urgent issue.
Conclusions
Because PID primarily strikes sexually active women during their reproductive years, it represents a terrible personal, economic, and societal burden. Public health–based interventions to reduce N. gonorrhoeae infection rates are clearly effective at reducing PID incidence. Indeed, preventing cervical infection in the first place, either by safe sex barrier methods or vaccines once they become available, will remain the most effective way to avoid the consequences of PID. However, beyond this, progress is beginning to be made toward understanding the intimate relationship between N. gonorrhoeae and humans. While this stealthy pathogen can persist undetected, the vigorous immunopathogenic response once it is recognized suggests that therapeutic immune modulation may help clear the infection and help suppress the emergence of sequelae. Given the inherent difficulties in studying gonococcal PID in humans, future work must aim to use clinical studies and primary cell or mouse-based modeling to reveal what processes drive the immunopathology associated with PID, so that new approaches to mitigate this damage can be developed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Owing to the restriction on references allowable, references 51–86 are cited but provided as supplementary content.
Notes
Financial support: This work was supported by the Canadian Institutes of Health Research (grants PJT-153177, PJT-392076, and MFE-152579) and the National Institutes of Health (grants 2R01-AI103400-05 and 1U19AI144182-01).
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. Both authors: No reported conflicts. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hook EW 3rd, Holmes KK. Gonococcal infections. Ann Intern Med 1985; 102:229–43. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Westrõm L, Eschenbach D. Pelvic inflammatory disease. In: Holmes KK, Sparling FP, Stamm WE, et al. , eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 2008. [Google Scholar]
- 3.Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med 2015; 372:2039–48. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Cannon JG. Human experimentation with Neisseria gonorrhoeae: progress and goals. J Infect Dis 1999; 179(suppl 2):S375–9. [DOI] [PubMed] [Google Scholar]
- 5.Mårdh PA. An overview of infectious agents of salpingitis, their biology, and recent advances in methods of detection. Am J Obstet Gynecol 1980; 138:933–51. [DOI] [PubMed] [Google Scholar]
- 6.Eschenbach DA, Buchanan TM, Pollock HM, et al. . Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med 1975; 293:166–71. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham FG, Hauth JC, Strong JD, et al. . Evaluation of tetracycline or penicillin and ampicillin for treatment of acute pelvic inflammatory disease. N Engl J Med 1977; 296:1380–3. [DOI] [PubMed] [Google Scholar]
- 8.Haggerty CL, Ness RB, Amortegui A, et al. . Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol 2003; 188:141–8. [DOI] [PubMed] [Google Scholar]
- 9.Wiesenfeld HC, Meyn LA, Darville T, Macio IS, Hillier SL. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis 2021; 72:1181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham FG, Hauth JC, Gilstrap LC, Herbert WN, Kappus SS. The bacterial pathogenesis of acute pelvic inflammatory disease. Obstet Gynecol 1978; 52:161–4. [PubMed] [Google Scholar]
- 11.Wasserheit JN, Bell TA, Kiviat NB, et al. . Microbial causes of proven pelvic inflammatory disease and efficacy of clindamycin and tobramycin. Ann Intern Med 1986; 104:187–93. [DOI] [PubMed] [Google Scholar]
- 12.Brunham RC, Binns B, Guijon F, et al. . Etiology and outcome of acute pelvic inflammatory disease. J Infect Dis 1988; 158:510–7. [DOI] [PubMed] [Google Scholar]
- 13.Kiviat NB, Wølner-Hanssen P, Eschenbach DA, et al. . Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol 1990; 14:167–75. [DOI] [PubMed] [Google Scholar]
- 14.Stacey CM, Munday PE, Taylor-Robinson D, et al. . A longitudinal study of pelvic inflammatory disease. Br J Obstet Gynaecol 1992; 99:994–9. [DOI] [PubMed] [Google Scholar]
- 15.Heinonen PK, Miettinen A. Laparoscopic study on the microbiology and severity of acute pelvic inflammatory disease. Eur J Obstet Gynecol Reprod Biol 1994; 57:85–9. [DOI] [PubMed] [Google Scholar]
- 16.Kamwendo F, Forslin L, Bodin L, Danielsson D. Decreasing incidences of gonorrhea- and chlamydia-associated acute pelvic inflammatory disease: a 25-year study from an urban area of central Sweden. Sex Transm Dis 1996; 23:384–91. [DOI] [PubMed] [Google Scholar]
- 17.Eschenbach DA, Wölner-Hanssen P, Hawes SE, Pavletic A, Paavonen J, Holmes KK. Acute pelvic inflammatory disease: associations of clinical and laboratory findings with laparoscopic findings. Obstet Gynecol 1997; 89:184–92. [DOI] [PubMed] [Google Scholar]
- 18.Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis 2013; 40:117–22. [DOI] [PubMed] [Google Scholar]
- 19.Baker JM, Chase DM, Herbst-Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol 2018; 9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weström L. Pelvic inflammatory disease: bacteriology and sequelae. Contraception 1987; 36:111–28. [DOI] [PubMed] [Google Scholar]
- 21.Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 2018; 16:226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 2004; 17:965–81, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormack WM, Nowroozi K, Alpert S, et al. . Acute pelvic inflammatory disease: characteristics of patients with gonococcal and nongonococcal infection and evaluation of their response to treatment with aqueous procaine penicillin G and spectinomycin hydrochloride. Sex Transm Dis 1977; 4:125–31. [PubMed] [Google Scholar]
- 24.McCormack WM, Reynolds GH. Effect of menstrual cycle and method of contraception on recovery of Neisseria gonorrhoeae. JAMA 1982; 247:1292–4. [PubMed] [Google Scholar]
- 25.Sweet RL, Blankfort-Doyle M, Robbie MO, Schacter J. The occurrence of chlamydial and gonococcal salpingitis during the menstrual cycle. JAMA 1986; 255:2062–4. [PubMed] [Google Scholar]
- 26.Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am 2013; 27:793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laniewski P, Gomez A, Hire G, So M, Herbst-Kralovetz MM. Human three-dimensional endometrial epithelial cell model to study host interactions with vaginal bacteria and Neisseria gonorrhoeae. Infect Immun Am Soc Microbiol 2017; 85:e01049–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens JS, Gray MC, Morisseau C, Criss AK. Endocervical and neutrophil lipoxygenases coordinate neutrophil transepithelial migration to Neisseria gonorrhoeae. J Infect Dis 2018; 218:1663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sintsova A, Sarantis H, Islam EA, et al. . Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog 2014; 10:e1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam EA, Shaik-Dasthagirisaheb Y, Kaushic C, Wetzler LM, Gray-Owen SD. The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice. Mucosal Immunol 2016; 9:1051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melly MA, McGee ZA, Rosenthal RS. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis 1984; 149:378–86. [DOI] [PubMed] [Google Scholar]
- 32.Gregg CR, Melly MA, Hellerqvist CG, Coniglio JG, Mc Gee ZA. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis 1981; 143:432–9. [DOI] [PubMed] [Google Scholar]
- 33.McGee ZA, Clemens CM, Jensen RL, Klein JJ, Barley LR, Gorby GL. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb Pathog 1992; 12:333–41. [DOI] [PubMed] [Google Scholar]
- 34.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun 1999; 67:5699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imarai M, Candia E, Rodriguez-Tirado C, et al. . Regulatory T cells are locally induced during intravaginal infection of mice with Neisseria gonorrhoeae. Infect Immun 2008; 76:5456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 2005; 206:306–35. [DOI] [PubMed] [Google Scholar]
- 37.Timmerman MM, Shao JQ, Apicella MA. Ultrastructural analysis of the pathogenesis of Neisseria gonorrhoeae endometrial infection. Cell Microbiol 2005; 7:627–36. [DOI] [PubMed] [Google Scholar]
- 38.Francis IP, Islam EA, Gower AC, Shaik-Dasthagirisaheb YB, Gray-Owen SD, Wetzler LM. Murine host response to Neisseria gonorrhoeae upper genital tract infection reveals a common transcriptional signature, plus distinct inflammatory responses that vary between reproductive cycle phases. BMC Genomics 2018; 19:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam EA, Anipindi VC, Francis I, et al. . Specific binding to differentially expressed human carcinoembryonic antigen-related cell adhesion molecules determines the outcome of Neisseria gonorrhoeae infections along the female reproductive tract. Infect Immun 2018; 86:e00092-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenz JD, Dillard JP. Pathogenesis of Neisseria gonorrhoeae and the host defense in ascending infections of human fallopian tube. Front Immunol 2018; 9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun 2013; 81:3060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor-Robinson D, Whytock S, Green CJ, Carney FJ. Effect of Neisseria gonorrhoeae on human and rabbit oviducts. Br J Vener Dis 1974; 50:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunha TM, Verri WA, Schivo IR, et al. . Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 2008; 83:824–32. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero ATG, Verri WA, Cunha TM, et al. . Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol 2008; 83:122–30. [DOI] [PubMed] [Google Scholar]
- 45.Stefanelli P, Teloni R, Carannante A, Mariotti S, Nisini R, Gagliardi MC. Neisseria gonorrhoeae triggers the PGE2/IL-23 pathway and promotes IL-17 production by human memory T cells. Prostaglandins Other Lipid Mediat 2012; 99:24–9. [DOI] [PubMed] [Google Scholar]
- 46.Heinonen PK, Aine R, Seppälä E. Peritoneal fluid leukotriene B4 and prostaglandin E2 in acute salpingitis. Gynecol Obstet Invest 1990; 29:292–5. [DOI] [PubMed] [Google Scholar]
- 47.Simms I, Stephenson JM. Pelvic inflammatory disease epidemiology: what do we know and what do we need to know? Sex Transm Infect 2000; 76:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner CF, Rogers SM, Miller HG, et al. . Untreated gonococcal and chlamydial infection in a probability sample of adults. JAMA 2002; 287:726–33. [DOI] [PubMed] [Google Scholar]
- 49.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36:502–9. [DOI] [PubMed] [Google Scholar]
- 50.Detels R, Green AM, Klausner JD, et al. . The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis 2011; 38:1. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.